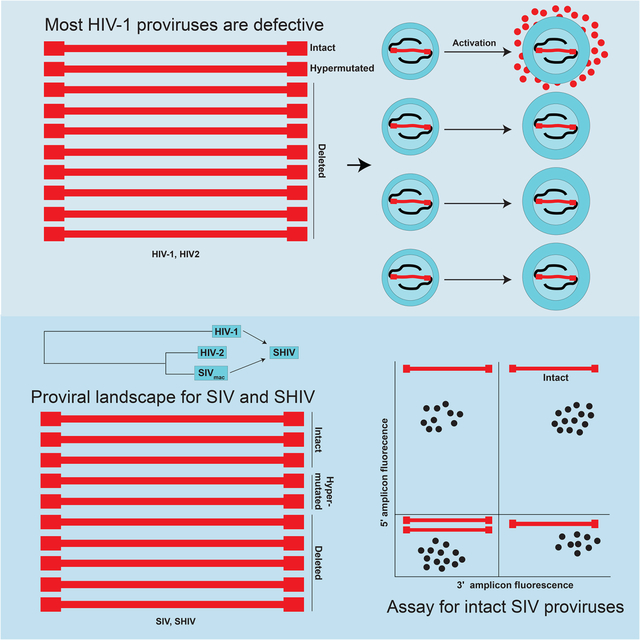
Summary
Evaluation of HIV cure strategies is complicated by defective proviruses that persist in ART treated patients but are irrelevant to cure. Nonhuman primates (NHP) are essential for testing cure strategies. However, the persisting proviral landscape in ART treated NHPs is uncharacterized. Here we describe viral genomes persisting in ART treated, simian immunodeficiency virus (SIV) infected NHPs, simian-human immunodeficiency virus (SHIV) infected NHPs, and humans infected with HIV-2, an SIV-related virus. The landscapes of persisting SIV, SHIV, and HIV-2 genomes are also dominated by defective sequences. However, there was a significantly higher fraction of intact SIV proviral genomes compared to ART treated HIV-1 or HIV-2 infected humans. Compared to humans with HIV-1, SIV-infected NHPs had more hypermutated genomes, a relative paucity of clonal SIV sequences, and a lower frequency of deleted genomes. Finally, we report an assay for measuring intact SIV genomes which may have value in cure research.
Graphical Abstract
eTOC Paragraph:
Understanding the proviral landscape in ART treated nonhuman primates (NHP) is crucial before using them to test HIV cure strategies. Bender et al. report that while most proviruses persisting in ART-treated SIV-infected NHPs are defective, there is a significantly higher fraction of intact genomes compared to ART-treated HIV-1-infected humans.
Introduction
Antiretroviral therapy (ART) does not cure HIV-1 infection due to persistence of a latent form of the virus (Chun et al., 1995; Chun, Carruth et al., 1997; Chun, Stuyver et al., 1997; Finzi et al., 1997; Finzi et al., 1999; Wong et al., 1997). A small pool of latently infected resting CD4+ T cells (the latent reservoir) persists even with optimal treatment, necessitating lifelong adherence to ART (Crooks et al., 2015; Finzi et al., 1997; Finzi et al., 1999; Siliciano et al., 2003; Wong et al., 1997). The remarkable stability of the reservoir (Siliciano et al., 2003) is due in part to the proliferation of infected cells (Bailey et al., 2006; Tobin et al., 2005 Maldarelli et al., 2014; Wagner et al., 2014). At any given time, most of the cells in the reservoir are generated by proliferation rather than de novo infection (Bui et al., 2017; Hosmane et al., 2017; Lorenzi et al., 2016; Simonetti et al., 2016).
Interventions that purge the reservoir would be of great value (Deeks et al., 2016; Richman et al., 2009). Following pharmacologic reversal of latency, viral cytopathic effects or immune effector mechanisms might eliminate infected cells (Archin et al., 2012; Borducchi et al., 2016). Bringing this approach to the clinic requires 1) an accurate means to measure the reservoir and 2) an animal model in which to test this approach. Replication-competent HIV-1 proviruses can be detected with the quantitative viral outgrowth assay (QVOA) Crooks et al., 2015; Finzi et al., 1997; Laird et al., 2016; Siliciano et al., 2003). The QVOA gives a definitive minimal estimate of the frequency of latently infected cells but may underestimate reservoir size because not all cells with intact proviruses are induced by a single round of T cell activation in this assay (Ho, Y. C. et al., 2013; Hosmane et al., 2017). PCR assays for proviral DNA are much simpler but dramatically overestimate reservoir size due to detection of defective proviruses with deletions and/or APOBEC3G/F-mediated G-to-A hypermutation (Bruner et al., 2016; Eriksson et al., 2013; Hiener et al., 2017; Ho, Y. C. et al., 2013; Imamichi et al., 2016). Recently, we described an intact proviral DNA assay (IPDA) that selectively measures intact proviruses lacking common defects (Bruner et al., 2019).
Non-human primate (NHP) models involving macaques infected with simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV) allow investigation of viral persistence and cure strategies (Borducchi et al., 2016; Cartwright et al., 2016; Del Prete, Shoemaker et al., 2014; Del Prete et al., 2015; Del Prete and Lifson, 2018; Dinoso et al., 2009; Kumar et al., 2016; Mavigner et al., 2014; McGary et al., 2017; Okoye et al., 2018; Shen et al., 2003; Swanstrom et al., 2018; Whitney et al., 2014). ART suppresses SIV replication (Del Prete et al., 2016) but is not curative because SIV also establishes a latent reservoir in resting CD4+ T cells (Dinoso et al., 2009; Shen et al., 2003). Little is known about the SIV genomes that persist in the setting of ART.
Here, we characterize the SIV and SHIV genomes that persist in treated macaques. We also examine the viral genomes that persist in humans infected with HIV-2, a virus phylogenetically related to SIVs used in cure studies (Clavel et al., 1986; Gao et al., 1999; Guyader et al., 1987). Our results provide new insights critical for understanding reservoir assays and cure interventions in NHP models.
Results
Defective viral genomes predominate in treated SIVmac infection
To characterize SIV genomes that persist despite ART, we studied 7 rhesus macaques infected with an SIVmac251 swarm (Del Prete et al., 2013) by the IR route. After 95 weeks, an ART regimen of tenofovir disoproxil fumarate, emtricitabine, and dolutegravir (TDF/FTC/DTG) was started (Del Prete et al., 2016) (Fig. 1a). At this time, all animals were viremic (plasma SIV RNA levels 9170 – 2,850,000 copies/ml, geometric mean 385,000 copies/ml). ART suppressed viremia to <50 copies/ml by week 16 in all but one animal. That animal achieved suppression by week 20. CD4+ T cells were purified from peripheral blood mononuclear cells (PBMC) 36 weeks after initiation of ART. Using a limiting-dilution, near full genome sequencing method that covers all ORFs and part of the LTR (Fig.1b), we obtained 267 independent sequences (Fig. 1c). The majority (72%) had obvious fatal defects, including large internal deletions (39%), G→A hypermutation (13%), or both (19%) (Fig. 1d). The remainder (28%) were intact, defined here as lacking obvious fatal defects such as large deletions, hypermutation, or other sequence abnormalities that disrupt gene function. Most intact HIV-1 genomes identified in the same manner show normal in vitro replication (Ho, Y. C. et al., 2013), but unapparent minor defects affecting viral fitness cannot be excluded. The proportions of intact, deleted, and hypermutated genomes were similar in each animal (Fig. 1d).
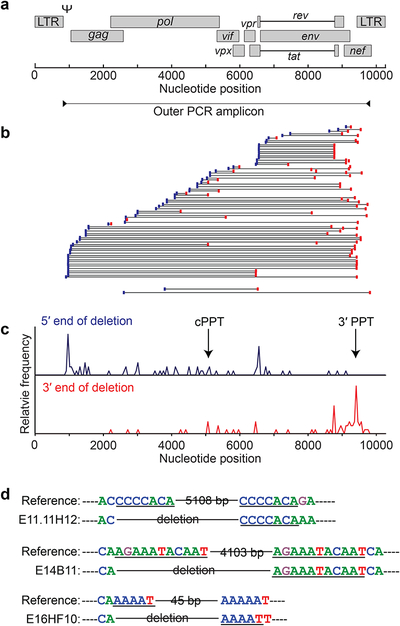
Fig. 1. Landscape of SIV genomes persisting in treated macaques is dominated by defective sequences.
(a) Time line of infection, treatment and sampling. Animals were infected with SIVmac251 for 95 weeks (red) before ART (blue box). All animals achieved and maintained suppression of viremia to <50 copies/ml of SIV RNA by week 20 of ART (dark blue box). Small arrows indicate sampling times. Samples for near full genome sequencing were obtained 36 weeks after ART initiation (red arrow). (b) Method for near full length, single genome analysis. Positions of PCR amplicons are shown in relation to a map of the SIV genome (top). See Methods for details, (c) Individual genomes from treated animals. Each horizontal bar represents a single genome. 221 genomes for which >1 inner PCR reaction was positive are shown. An additional 46 genomes were detected by near full genome length outer and nested gag inner PCR, but could not be fully sequenced due to large unmapped deletions (n=32) and/or extensive hypermutation (n=14). (d) Number of genomes with the indicated types of defects in each animal, (e) Comparison of 267 SIV sequences from 7 animals and 152 previously published (Bruner et al., 2016) HIV-1 proviral sequences from 10 individuals initiating ART during chronic infection. Significant differences between SIV and HIV-1 in the fraction of different categories of genomes are indicated (**, P < 0.01; ****, P < 0.0001). (f) Comparison of the average time periods of infection and ART between the SIV-infected macaques studied here and HIV-1-infected patients analyzed by Bruner et al., 2016.
These SIV genomes were compared to HIV-1 genomes persisting in patients on long term ART (Fig. 1e). Defective sequences predominate in both cases, but for SIV there was a higher fraction of intact sequences and of hypermutated sequences, and a lower fraction of deleted sequences. These differences could reflect biological differences in the viruses or the hosts or differences in the time scales of the infections (Fig. 1f). In a single round of SIV infection, deletions and hypermutation can arise but intact genomes predominate (Fig. S1a,b). The same is true for HIV-1 infections, and during periods of active HIV-1 replication in vivo, intact proviruses also predominate (Bruner et al., 2016). However, following initiation of ART, time dependent changes in the proportions of intact and defective genomes occur as a result of selection against infected cells capable of expressing viral proteins (Pollack et al., 2017), accumulation of cells carrying defective forms (Bruner et al., 2016), decay of labile cell populations (Blankson et al., 2000), and decay of labile unintegrated DNA forms (Bukrinsky et al., 1991; Pierson, Zhou et al., 2002; Zack et al., 1990). Thus time scale is important. Another potential explanation for the higher fraction of intact SIV genomes is the higher pre-ART level of viremia relative to HIV-1 infection. The mean pre-ART SIV viral load in this cohort was 1.2 × 106 copies/ml. However, there was no relationship between the set point or pre-ART SIV viral load and the fraction of intact proviruses on ART (Fig. S1c, d).
Analysis of deletions and hypermutation.
As with HIV-1, deletions were the most common type of defect identified (58%). Deletions were generally large, encompassing on average 41% of the genome (Figs. 1c, 2). Deletions were distributed non-randomly across the SIV genome (Fig. 2b,c). Apparent deletion hot spots represent independent deletion events and not clonal expansion of cells carrying deleted proviruses (see below). One group of deletions extended from the 3’ polypurine tract (PPT) variable distances toward the 5’ end of the genome. As with HIV-1, regions of sequence homology were found at the junctions in a subset of deleted sequences (Fig. 2d), consistent with copy choice recombination (Delviks-Frankenberry et al., 2011; Ho, Y. C. et al., 2013; Sanchez et al., 1997; Sharaf et al., 2018).
Fig. 2. Deletions in the SIV genome.
(a) SIV genome and outer PCR amplicon used for near full genome sequencing, (b) Position of deletions. Each horizon line represents a mapped deletion. The 5’ and 3’ ends of the deletion are indicated by blue and red boxes respectively. Also shown are the maximum and minimum possible sizes of deletions in 32 genomes for which only the inner gag PCR was positive (gag only), (c) Histograms indicating the relative frequency of sequences with deletions with 5’ and 3 ’ boundaries at the indicated positions in the genome. It is not clear if any deletions extend into the 5’ LTR, although this is not expected based on the mechanism of reverse transcription, (d) Examples of regions of homology at the deletion junctions for genomes with a very large internal deletion (E11.11H12), a 3’ deletion (E14B11), and a 5’ deletion (E16HF10).
One feature that distinguishes the SIV sequences is the degree of APOBEC3-mediated hypermutation (Fig.1c–e). Compared to HIV-1, a higher fraction of SIV sequences were hypermutated (Fig. 1e, P=0.006). G→A mutations were present throughout the genomes of all hypermutated sequences (Fig. 3a), and at some positions a high fraction of sequences (>80%) have G→A mutations (Fig. 3b). As previously described for HIV-1 and consistent with the current model (Yu et al., 2004), there were two 5’→3’ gradients of hypermutation separated by the central polypurine tract (cPPT) (Fig. 3b). The extent of hypermutation varied greatly between individual hypermutated sequences and for some genomes approached 50% of all G’s (Fig. 3c). The extent of hypermutation within hypermutated SIV genomes was significantly greater than for HIV-1 (P < 0.00001, Fig. 3d). The sequence context in which the mutations occurred was also different. All hypermutated SIV sequences had statistically confirmed hypermutation in the GA→AA context (Fig. 3e). This is the preferred sense strand context for APOBEC3F and APOBEC3H (Harris and Liddament, 2004). Half of these sequences also had hypermutation in the GG→AG context, consistent with APOBEC3G-mediated cytidine deamination. HIV-1 genomes predominantly have GG→GA mutations. The occurrence of multiple adjacent G→A mutations complicates analysis of context because it is unclear in which order mutations occur. Nevertheless, the nucleotide immediately 3’ to sense strand G→A mutations in SIV tends to be A (Fig. 3f).
Fig. 3. Hypermutation in SIV infection.
(a) Maps of hypermutated SIV genomes. Vertical slashes indicate positions of G→A mutations that are not present in non-hypermutated genomes from the same animals. Hypermutated genomes with unmapped deletions are not shown. Boxed area is expanded to show individual sites of mutation, (b) Extent of hypermutation at individual positions in hypermutated genomes. Vertical bars indicate the fraction of hypermutated genomes with a G→A mutation at the indicated genomic positions. Values are normalized for deletions and missing sequence. cPPT, central polypurine track, (c) Fraction of sequenced positions in individual hypermutated genomes at which a G in the reference sequence is mutated to A. (d) Histograms showing the fraction of hypermutated SIV (top) and HIV-1 (bottom) genomes with the indicated level of hypermutation expressed as the fraction of G’s mutated to A. Mutation was assessed relative to non-hypermutated sequences from the same infected animal or patient. Fractions are normalized for deletions and missing sequence. For SIV, 86 genomes from 7 treated animals were analyzed. For HIV-1, 47 sequences from 9 patients treated during chronic infection (Bruner et al., 2016) were included, (e) Context of SIV hypermutation. Fraction of genomes with statistically confirmed hypermutation in the sense strand context GG→AG or GA→AA. (f) Sequence context of individual G→A mutations in hypermutated SIV genomes.
The predicted effect of observed G→A mutations on viral fitness was profound, with altered start codons (ATG→ATA) for many ORFs, especially tat and rev, and multiple internal stop codons in most ORFs (Fig. S2a–c). Gene by gene analysis showed that most defective SIV genomes are defective in most SIV genes (Fig. S2d). Genes encoding Tat and Rev, which are important for SIV gene expression, are defective in >80% of defective genomes. Because cure strategies depend on induced gene expression from latent proviruses, cells with intact and defective proviruses may be affected differently. Thus, separate enumeration of intact SIV proviruses will be important (see below).
Frequency of identical sequences in the SIV reservoir.
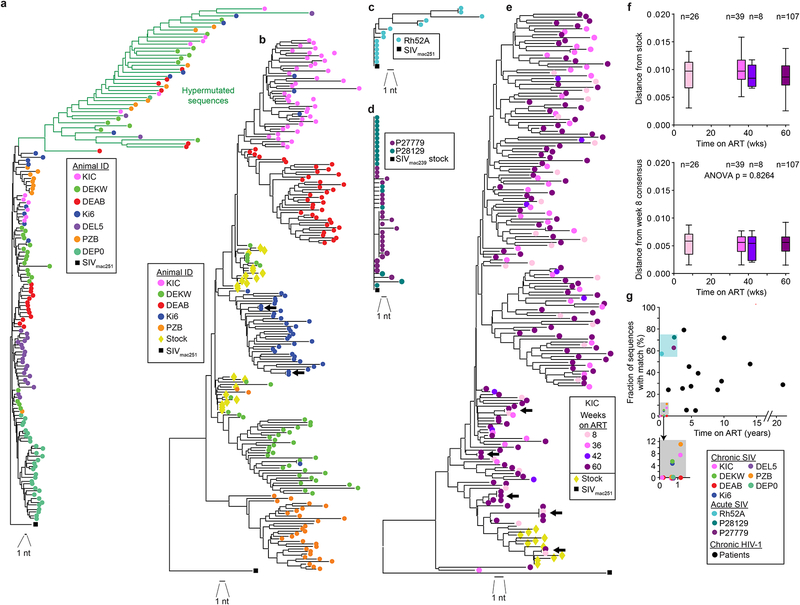
An important feature of the HIV-1 reservoir is the prominence of large clonal populations of infected cells. Despite extensive viral diversification prior to treatment (Keele et al., 2008; Shankarappa et al., 1999), 50–60% of replication-competent HIV-1 isolates from treated patients are identical to other isolates from the same blood sample (Bui et al., 2017; Hosmane et al., 2017; Lorenzi et al., 2016). This identity generally reflects the proliferation of infected cells rather than infection of multiple cells by a dominant viral species (Cohn et al., 2015; Hosmane et al., 2017; Laskey et al., 2016; Maldarelli et al., 2014; Simonetti et al., 2016; Wagner et al., 2014). To detect clonal expansion in the SIV model, we compared the 267 near full-length SIV genomes at 36 weeks after ART initiation. Diversification from the stock was evident, and all sequences were unique. Analysis of 146 proviruses with intact env genes showed no identical sequences (Fig. 4a). The 121 proviruses lacking an intact env gene were also compared, and no identical sequences were found. We then used single-genome amplification (SGA) to obtain 236 additional env sequences at the 36 week time point (Fig. 4b). Two pairs of identical sequences from a single animal were observed (1.7%). To verify that identical sequences can be detected, by our sequencing methods, we studied animals treated during acuteinfection prior to sequence diversification. Using near full genome sequencing (Fig. 4c) or env SGA (Fig. 4d), multiple identical sequences matching the stock were obtained.
Fig. 4. Identical sequences in SIVmac infection.
(a) Phylogenetic tree of SIV env sequences obtained by near full-genome sequencing from 7 treated animals infected with SIVmac251 and sampled 36 weeks after ART initiation and the SIVmac251 reference sequence (black square). Sequence analysis includes 2044 nt. Hypermutated sequences from each animal clustered together due to commonly mutated positions, (b) Composite phylogenetic tree of env sequences obtained by full-genome sequencing and env SGA along with SIVmac251 stock sequences (yellow diamonds) and the SIVmac251 reference sequence (black square). Hypermutated sequences were omitted. Two pairs of identical sequences potentially representing expanded cellular clones were identified (arrows). Three identical sequences from animal DEKW are intermingled with the stock sequences and may represent infection of multiple cells with the same variant present in the stock, (c) Tree of env sequences from full-genome sequencing on CD4+T cells from a rhesus macaque treated with ART 2 weeks after infection with SIVmac251 and sampled after 19 weeks of treatment. (d) Tree of env sequences from SGA on CD4+ T cells from 2 rhesus macaques treated with ART 6 weeks after infection with SIVmac239 and sampled after 120 weeks of treatment, (e) Representative phylogenetic tree of env sequences obtained by SGA from animal KIC at multiple time points. Arrows indicated identical sequences, (f) Root-to-tip distances for the phylogenetic trees shown in (e), calculated using the stock consensus sequence (top) or the week 8 consensus (bottom) at the root. Data for animal KIC are shown here. Similar plots for animals DEAB, PZB, and DEKW are shown in Fig. S3e–g. (g) Relationship between fraction of clonal sequences and time on ART. Data are from studies using env SGA to analyze viral genomes in SIV-infected macaques (colored symbols) or HIV-1-infected patients on ART for the indicated times. HIV-1 data are from published studies (Bailey et al., 2006; Bar et al., 2016; Bruner et al., 2016; Wang et al., 2018).
Since studies in humans have shown that the fraction of identical HIV-1 sequences increases progressively over time on ART (Cohn et al., 2015; Wagner et al., 2013), we we examined env sequences obtained by SGA fat 8, 42, and 60 weeks after initiation of ART. Rare identical sequences were found in 3 of 4 animals at the 60 week time point (Figs. 4e, S4a–c).
TWe also studied a separate cohort of 4 rhesus macaques infected with SIVmac239x (Del Prete, Park et al., 2014), treated with ART starting at 33–56 weeks post infection, and sampled after 53–58 weeks of suppressive ART (Fig. S3d). Of 133 SGA envsequences Among sequences that had diverged significantly from the stock, we found three pairs and one triplet of identical sequences.
One potential explanation for the relative paucity of clonal sequences is ongoing viral replication despite ART. This could allow continuous evolution of new variants, obscuring clonal populations. To exclude this possibility, we examined env sequences in longitudinal samples from the SIVmac251-infected animals (Fig. 1a) and calculated root-to-tip distances using as a baseline the consensus sequence of the SIVmac251 viral stock (Del Prete et al., 2013). We found no increase in distances over time, consistent with complete or near complete suppression of ongoing cycles of viral replication on ART (Fig. 4f, Fig. S3e–g). A more likely explanation is that clonal expansions occur over months to years and are easier to observe in patients on long term ART than in SIV-infected monkeys that are typically studied after <1–2 years of infection/treatment (Fig. 4g).
Viral genomes persisting in HIV-2 and SHIV infection
To understand viral and host contributions to differences between persistent viral genomes in HIV-1 and SIV infections, we characterized viral genomes in humans infected with HIV-2. We identified 3 HIV-2-infected individuals from Senegal who had viral suppression on ART for 3.1–4.3 years. The landscape of persistent HIV-2 genomes is also dominated by defective sequences (Figs. 5a, S6a). Although HIV-2 is genetically related to SIVsm, the genomic landscape of HIV-2 more closely resembled HIV-1, suggesting a dominant influence of host factors including the time scale issue discussed above. Only 1 of 41 HIV-2 sequences (2.4%) was intact. Large deletions were mapped in 66% of viral genomes (Fig. 5a). Another 24% likely had deletions that affected some inner PCRs and prevented precise mapping. Hypermutation was observed in a smaller fraction of genomes (12%). A complete picture viral genomes persisting in HIV-2-infected individuals will require more extensive analysis of samples from these rare patients.
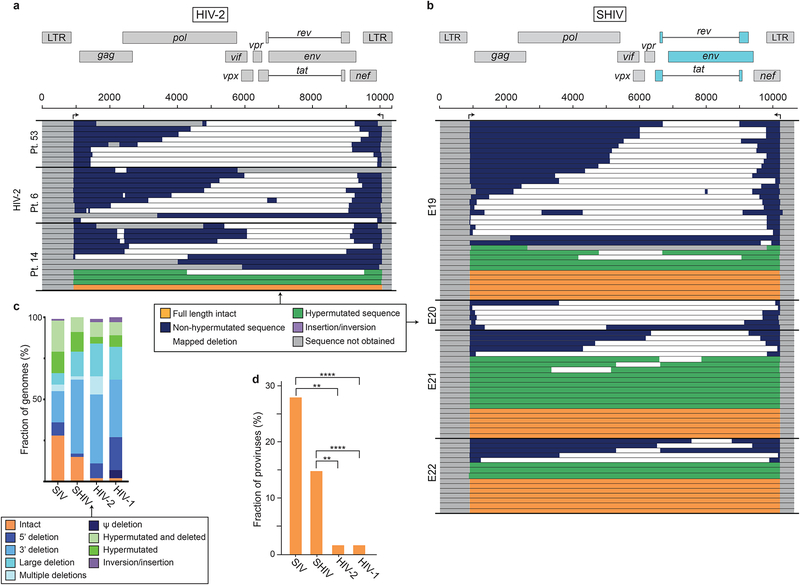
Fig. 5. HIV-2 and SHIV genomes that persist in the setting of ART are largely defective.
(a) Maps of genomes amplified from PBMC of three individuals with treated HIV-2 infection. Arrows indicate positions of outer primers used in near full genome amplification. Mapping strategy and primer sequences are in described in Fig. S6a and Table S1, respectively. Of 41 genomes, 8 had deletions that could not be precisely mapped due to large deletions affecting multiple inner PCRs and are not shown, (b) Maps of SHIV genomes from treated macaques. Arrows indicate positions of outer primers used in near full genome amplification. Mapping strategy and primer sequences are in described in Fig. S6b and Table S1, respectively. Of 131 genomes analyzed, 49 gave bands only with the gag inner PCR. These were considered to have unmapped deletions and are not shown, (c) Summary of the frequency of intact and defective genomes persisting in the setting of ART for HIV-1, HIV-2, SIV, and SHIV. Data for HIV-1 are from Bruner et al. 2016. (d) Fraction of intact proviruses for HIV-1, HIV-2, SIV, and SHIV. Significance of differences was determined by multiple unpaired T tests corrected for multiple comparisons using the Holm-Sidak method (**, p ≤ 0.01; ****, p ≤ 0.0001).
We also analyzed persistent viral genomes in one of the important SHIV models (Ambrose et al., 2007; Balzarini et al., 1995; Borducchi et al., 2018; Del Prete and Lifson, 2018; Liu et al., 2016; Luciw et al., 1995; North et al., 2010). SHIVSF162P3 was derived from SIVmac239 by replacement of a region including SIV tat, rev, and env with a corresponding sequence from HIV-1SF162 (Luciw et al., 1995). SHIVSF162P3-infected animals were treated with ART 89 weeks post infection and sampled 36 weeks later. We obtained 131 near full genome sequences from 4 animals (Fig. S6b). As with HIV-1, HIV-2, and SIV, the majority of SHIV genomes were grossly defective (85%, Fig. 5b,c). The fractions of genomes that were intact (15%), hypermutated (22%), and deleted (63%) were similar to those observed with SIV (Fig. 5b,c). Deletions in the 3’ half of the genome encompassing HIV-1 env were common (44%).
A comparison of viral gnomes persisting in treated infections with HIV-1, HIV-2, SIVmac251/239, and SHIVSF162P3 is in Fig. 5c. Higher frequencies of intact genomes were observed for SIV and SHIV (Fig. 5d). The shorter time scale of experimental SIV and SHIV infections may leave less time for accumulation of defective forms or selection against cells with intact viral genomes (Bruner et al., 2016; Pollack et al., 2017).
We also examined SHIV and HIV-2 sequences for evidence of clonal expansion of infected cells. For 3 of the 4 SHIV-infected animals analyzed after 36 weeks of ART, there were no identical sequences among 92 analyzed (Fig. S5a). However, one animal had 3 pairs of identical sequences among 39 analyzed (7.7%). However, for these sequences, we only captured a small part of the genome (~1700 bases), likely due to large 3’ deletions. For HIV-2, fewer sequences were available (average 15/patient), one set of identical sequences was found in one patient (Fig. S5b).
An assay for intact SIV genomes
Commonly used SIV DNA assays amplify small genomic regions and miss defects outside of the region amplified. We compiled 636 SIVmac sequences (267 obtained by full-genome sequencing, 369 by SGA) and used them to develop a digital droplet PCR (ddPCR) assay that is more specific for intact SIV genomes. DNA from infected cells is distributed into nanoliter size droplets such that individual droplets are unlikely to contain >1 genome. Multiplex PCR reactions occurring within droplets interrogate individual genomes at informative positions to distinguish intact genomes from those with common fatal defects, deletions and hypermutation. Because deletions are generally large (Fig. 1c, Fig. 2b)) and hypermutation is extensive (Fig. 2 a–d), intact and defective viral genomes can be distinguished using a small number of strategically placed amplicons, as i with HIV-1 (Bruner et al., 2019). Some genomes defined as intact in this way may have minor defects affecting viral fitness. Nevertheless, this approach allows us exclusion of most genomes with overt fatal defects.
To exclude deleted genomes, we identified 2 amplicons, in pol and env, that together correctly excluded >90% of deleted genomes (Fig. 6a). Probes were chosen to include bases frequently mutated in hypermutated genomes. For each amplicon, we designed a labeled probe that hybridizes with unmutated sequence and an unlabeled competition probe that binds hypermutated sequence. Based on sequence analysis, over 90% of the hypermutated SIV genomes are correctly identified as defective by these primer/probe combinations.
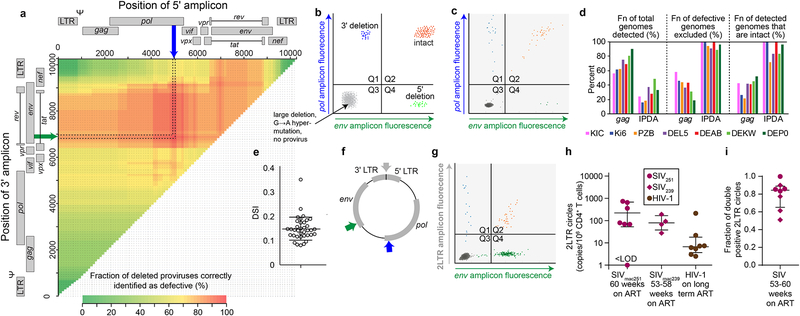
Fig. 6. ddPCR assay for intact SIV genomes.
(a) Sliding window analysis of optimal amplicon positioning to detect deletions. Optimal discrimination between intact and deleted sequences is obtained with a 5’ pol amplicon (blue arrow) and a 3’ env amplicon in (green arrow). Colors in grid indicate fraction of deleted genomes correctly identified as defective based on overlap of the indicated 5’ and/or 3’ amplicons and mapped deletions. Based on 267 independent near full genome sequences including 64 that contain mapped deletions, (b) Schematic of ddPCR assay output. Types of genomes detected in each quadrant are indicated. Many defective SIV genomes fail to amplification with either primer set and are present in quadrant 3 (Q3) along with droplets containing no SIV DNA. (c) Representative dot plot from analysis of CD4+ T cells from a treated macaque, (d) Bioinformatic comparison of standard SIV gag PCR (Gama et al., 2017) and the ddPCR assay for intact SIV genomes with respect to % of genomes amplified, % of defective genomes excluded, and % of amplified genomes that are intact. Based on 267 near full genome sequences from treated macaques (Fig. 1). (e) DNA shearing index (DSI) for representative study samples. Samples with high DSI (>25%) were excluded from further analysis. Bars show mean ± SD. (f) Position of amplicons used in ddPCR assay for 2LTR circles. Droplets are analyzed for the 2LTR junction and either pol or env. The pol and env amplicons are the same as those in a. (g) Representative 2LTR assay using on DNA from a treated macaque. A minority of the viral genomes detected are circles (Q1 and Q2), and some of the circles have 3’ deletions (Q1). Similar assays using the pol as the second amplicon give similar results as expected based on the distribution of mapped deletions, (h) 2LTR circles in treated SIV and HIV-1 infection. Total 2LTR circles (Q1+Q2) were measured in the infected macaques describe above after ~1 year of ART. Previous measurements (Bruner et al., 2016) of 2LTR circles in HIV-1 infected patients on long term ART are shown for comparison, (i) The fraction of double positive SIV 2LTR circles for the animals shown in g.
These considerations allowed development of an assay for intact SIV genomes. Intact genomes are detected as double positive droplets resulting from successful amplification with both pol and env primer sets while hypermutated and deleted genomes give double negative or single positive droplets (Fig. 6b). Control experiments with plasmids and synthetic double stranded DNA fragments representing intact genomes and genomes with deletions or hypermutation gave droplets in expected quadrants (Fig. S6a, b). Representative results from analysis of a sample from a treated macaque are in Fig. 6c. Using 267 near full genome sequences from treated macaques (Fig. 1c), we compared this assay to a widely used SIV gag qPCR assay PCR (Gama et al., 2017) with respect to critical assay parameters (Fig. 6d). The standard gag PCR detects a higher fraction of SIV genomes, but most genomes detected are defective. The ddPCR assay for intact genomes excludes a much higher fraction of detective sequences, and as a result, an average of 90% of the sequences detected are intact compared to only 41% for the gag assay (Fig. 6d). Analysis of samples from SIVmac251-infected animals at the 36 week time point gave fractions of intact genomes consistent with near full genome sequencing results (R2 = 0.867 by linear regression, p = 0.0003). DNA shearing between amplicons can reduce the number of intact genomes detected. Therefore, duplex ddPCR for rhesus macaque RPP30 was performed on each sample. The RPP30 amplicons were spaced the same distance apart as the SIV amplicons, allowing correction for shearing (Bruner et al, 2019) as well as quantitation of input cellular DNA. Because SIV pol and env amplicons are relatively close (Fig. 6a), shearing between amplicons occurs only ~15% of the time, based on analysis of RPP30 shearing in the same sample (Fig. 6e).
The ddPCR assay for intact SIV genomes could be affected by unintegrated viral DNA (Chan et al., 2016; Pierson, Zhou et al., 2002; Trinite et al., 2013; Zack et al., 1990). Linear unintegrated forms of HIV-1 DNA are labile () and therefore unlikely to persist with long term ART. However, 2LTR circles resulting from end-to-end ligation of viral genomes are more stable (Butler et al., 2002; Pierson, Kieffer et al., 2002)and might be detected by PCR, thereby complicating analysis of SIV persistence. Therefore, we developed a ddPCR assay for SIV 2LTR circles. The assay utilizes two amplicons, one spanning the 2LTR circle junction (Policicchio et al., 2018), and one in either pol or env, (Fig. 6f). Droplets positive for the 2LTR amplicon but negative for pol (or env) likely represent deleted viral genomes that circularized while double positive droplets may represent intact genomes that circularized. We detected both types circles in SIV-infected macaques on ART for 53–60 weeks (Fig. 6g). Average levels were 100/106 CD4+ T cells, higher than levels in HIV-1-infected individuals on long term ART (<10 per 106 CD4+ T cells, Fig. 6h). Most circles (>80%) were double positive, indicating the - absence of large deletions (Fig. 6i). It is therefore important to account for these forms in SIV reservoir analysis. Consequently, a ddPCR assay for 2LTR circles is also run on each sample allowing quantitation intact linear SIV genomes, with the potential caveat that 1-LTR circles, if present, would be included.
SIV dynamics in treated macaques.
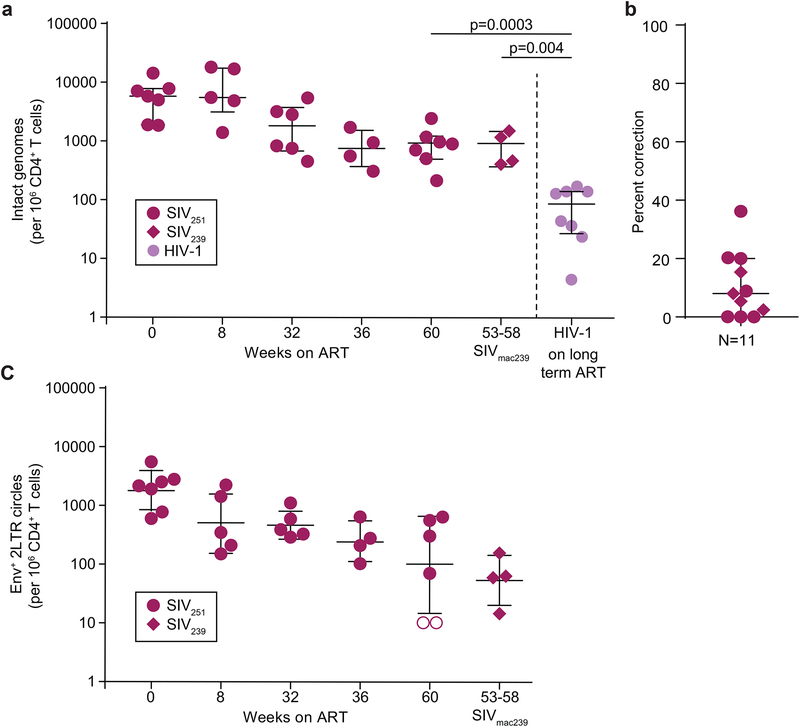
Using the ddPCR assay for intact SIV genomes corrected for 2-LTR circles, we examined decay of intact viral genomes following initiation of ART in the SIVmac251-infected macaques described above (Fig. 1a). At treatment initiation, the level of intact genomes was ~10,000/106 CD4+ T cells (Fig. 7a). Many of these were likely present in recently infected cells not destined to become part of the stable reservoir. Over the next 36 weeks, levels declined to ~1000 copies/106 CD4+ T cells, approximately 10 fold higher than the level of intact HIV-1 proviruses in patients on long term ART (Fig. 7a) but similar to levels in in the SIVmac239x-infected macaques on ART (Fig. 7a). Even after ~1 year of ART, 2LTR circles were readily detectable in some animals (Fig. 7b,c). Thus although 2LTR circles decline over the first year of ART (Fig. 7c), they should be excluded from PCR-based measures of the reservoir.
Fig. 7. Frequency of cells with intact SIV genomes.
(a) Frequency of intact SIV genomes in longitudinal samples before and after initiation of ART. Intact SIV DNA levels from the latest available time point (~1 year of ART) are compared to intact HIV-1 DNA in patients on long term ART (Bruner et al., 2019). The frequencies have been corrected for double positive 2-LTR circles. Bars on graphs represent median and interquartile range, (b) Percentage of 2-LTR correction. The frequency of 2 LTR circles retaining internal env or pol regions was used to correct intact DNA copies measured by ddPCR. Bars on graphs represent median and interquartile range, (c) Decay of double positive 2-LTR circles in animais on ART. Bars represent geometric mean and standard deviation.
Discussion
We characterized the viral DNA genomes that persist in the setting of suppressive ART in SIV, SHIV, and HIV-2 infections. We show that for all three infections, most genomes are highly defective, as for HIV-1 (Bruner et al., 2016; Hiener et al., 2017; Ho, Y. C. et al., 2013; Imamichi et al., 2016; Lee et al., 2017). Our findings provide insights into how viral reservoirs should be measured in these infections. Standard PCR assays that fail to distinguish intact and defective viral genomes cannot accurately measure replication-competent viral reservoirs in NHP models.
Although defective genomes predominate in both HIV-1 and SIV infections, differences were noted. Some reflect biological differences between the host species, while others reflect the shorter time scale of SIV studies. The clearest biological difference involved the extent of G→A hypermutation. Many SIV genomes were grossly hypermutated. Not only was the fraction of hypermutated genomes higher than for HIV-1, but across each hypermutated SIV genome, an extraordinarily high fraction of Gs were mutated to As (as high as 47%, Fig. 3c,d). These mutations can have profound functional consequences since they introduce multiple internal stop codons, alter start codons, and cause missense mutations (Fig. S2).
Another interesting difference is in the sequence context in which the hypermutation occurs. Members of the APOBEC3 family differ in the consensus target site — APOBEC3G acts at (−) strand 5’-CC dinucleotides producing (+) strand GG→AG hypermutation while other family members, including APOBEC3F and APOBEC3H, act at 5’-TC dinucleotides and produce AG→AA hypermutation (Desimmie et al., 2014; Harris et al., 2003; Huthoff and Towers, 2008; Malim, 2009; Wiegand et al., 2004). Hypermutated HIV-1 proviruses all show hypermutation at GG consensus sites or at both the GG and GA consensus sites. In contrast, all hypermutated SIV genomes showed AG→AA hypermutation, and some also showed GG→AG hypermutation (Fig. 3e,f). This may reflect differences in expression and activity of APOBEC3 family members as well as differences Vif- induced degradation. In any event, hypermutated SIV genomes are clearly replication-incompetent and should be excluded from reservoir measurements.
The other major class of defective SIV genomes were those with large internal deletions. These wereless frequent than in HIV-1 infection (39% vs 80%, Fig. 1e). We identified regions of homology at deletion junctions (Fig. 2d), similar to those seen in HIV-1 (Bruner et al., 2016; Ho, Y. C. et al., 2013; Sanchez et al., 1997). The lower fraction of deleted SIV genomes may reflect a lower propensity of SIV RT to undergo template switching. It is also possible that the fraction of highly deleted sequences increases over time due to lack of immune selection (Pollack et al., 2017). The shorter time scale of SIV studies does not allow this process to proceed to the same extent as in patients on long term ART.
Although most SIV genomes sequenced were defective, an average of 28% of sequences from SI Vmac251-infected macaques (Fig. 1a) were intact at the 36 week time point (Fig.1 c–e). For SHIV, the average value was 15%. These values are significantly higher than those observed in HIV-1- or HIV-2-infected humans on long term ART, in whom <2% of proviruses are intact (Fig. 5d). Several factors may explain the higher level of intact SIV genomes. The first is the time scale of SIV studies (Fig. 1f). Time-dependent changes in the pool of persisting genomes may occur following the initiation of ART. In untreated individuals with HIV-1 infection (Bruner et al., 2016) and in single round in vitro infections with SIV (Fig. S1), intact genomes in recently infected cells predominate. Some of these are linear or circular unintegrated forms. Linear unintegrated forms decay rapidly (Bukrinsky et al., 1991; Pierson, Zhou et al., 2002; Zack et al., 1990). Integrated proviruses are stable, but most productively infected cells do not survive to enter the stable reservoir (Ho, D. D. et al., 1995; Perelson et al., 1997; Wei et al., 1995). In HIV-1 infected individuals, latently infected cells detected by the QVOA decline after initiation of ART, reaching a plateau at 6–12 months (Blankson et al., 2000). Therefore, reservoir measurement in HIV-1 infection are generally done only after 6 months of ART. We show here that the frequency of intact SIV genomes, corrected for 2-LTR circles, declines during the 9 months of ART (Fig. 7a). Whether further changes occur cannot be ascertained without SIV treatment studies that extend over longer time scales.
Another explanation for the higher fraction of intact SIV proviruses is that the well-established ART regimens do not fully suppress SIV replication. However, the animals studied consistently maintained plasma virus levels below 15 copies/ml. In previous studies, no additional benefit was seen with of a fourth drug (Del Prete et al., 2016). In addition, we found no evidence for ongoing sequence evolution in treated macaques (Fig. 4f, Fig. S3c,d). The latent reservoir is a diverse collection of variants generated by pre-ART viral evolution (Nickle et al., 2003), and thus additional sampling typically reveals previously unencountered sequences. However, new sequences identified at later time points on ART did not show increased phylogenetic distance from the inoculum than variants detected at the first on-ART time point. In addition, distance from a consensus sequence generated from variants present at the first on-ART time point does not increase over time (Fig. 4f and Fig. S3e–g).
Another interesting finding was the limited number of identical SIV sequences in treated animals (Figs. 4a,b,e, S3a–d). Identical sequences were readily detected in animals treated during the acute phase of infection before significant viral diversification had occurred (Fig. 4c,d). Thus, the sequencing methods used can detect identical sequences if present. These results contrast with findings in HIV-1-infected individuals on ART in whom 50–60% of the independent isolates of replication competent virus have viral sequence identity to other isolates in the same sample (Bui et al., 2017; Hosmane et al., 2017; Lorenzi et al., 2016; Wang et al., 2018). Detection of a clonal population of infected cells within a single sample requires that the progeny of a single infected cell proliferate to comprise a detectable fraction of a feasibly sampled portion of the total population of infected cells. The proportion of the infected cell population comprised of expanded cellular clones increases over intervals longer than the time scale of most SIV experiments (Cohn et al., 2015; Wagner et al., 2013). Individual clones of cells carrying replication-competent HIV-1 wax and wane on a time scale of months to years (Wang et al., 2018). Thus, clones of SIV-infected cells may not expand to comprise a major fraction of the total population of infected cells during the time scale of current SIV studies. However, we detected a small number of identical sequences after ~1 year of ART (Figs. 4b,e and S3b,c,d). With the greater sampling and definitive assessment of clonality afforded by integration site sequencing, clones should be apparent in NHP models. Integration site analysis of treated SIVmac239-infected rhesus macaques in a separate study has provided evidence for expanded clones of CD4+ T cells harboring SIV proviruses. Clonal sequences represented approximately 20% of the total integrations identified after ~1 year of ART (S. Hughes, personal communication).
We also observed that in treated SHIV and HIV-2infections, the viral genomes that persist are dominated by defective sequences (Fig. 5). The patterns of defects segregate largely by host species.
The quantitative dominance of defective SIV genomes led us to develop a scalable method to directly enumerate intact SIV genomes. Unlike standard PCR, this assay signal is not dominated by defective sequences (Fig. 6d) and may therefore be of particular value in cure research. Although not all genomes detected by this method may represent replication-competent inducible proviruses, it excludes most genomes with deletions or hypermutation while also correcting for 2LTR circles. Thus the method is much more likely to detect reductions in the latent reservoir than assays dominated by the defective sequences which we have shown are prevalent in NHP models.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Robert F. Siliciano (rsiliciano@jhmi.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals.
All animal work was approved by the Institutional Animal Care and Use Committees of Bioqual, Johns Hopkins, and the NIH and determined to be in accordance with the guidelines outlines in the Animal Welfare Act and Regulation (USDA) & the Guide for the Care & Use of Laboratory Animals, 8th Edition (NIH). All experiments involving laboratory animals conformed to the regulatory standards used by these committees. Indian-origin rhesus macaques (Macaca mulatta) were used in these studies. All animals were negative for the protective alleles Mamu-A*01(DB and JC), Mamu-B*08, and Mamu-B*17. Animals were tested for antibodies to SIV, herpes simian B-virus, SRV, STLV-1, Measles, and B-virus. Near full genome sequencing was carried out on a cohort of 7 macaques infected with repetitive IR inoculations of 500 TCID50 of an SIVmac251 swarm challenge stock (Del Prete et al., 2013). There were 3 female and 4 male animals in this cohort (aged 3.2—4.7 years at time of SIV infection). Other details of this macaque cohort will be presented elsewhere. Another cohort of male animals aged 3.6—6.7 years at the time of SIV infection (T028, T154, T158, and T159) were housed at the National Institutes of Health and cared for in accordance with American Association for the Accreditation of Laboratory Animal Care (AAALAC) standards in an AAALAC-accredited facility (Animal Welfare Assurance number A4149–01). All work involving these animals was conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Cancer Institute and adhered to the standards of the Guide for the Care and Use of Laboratory Animals (National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC) in accordance with the Animal Welfare Act. These animals were infected intrarectally with a limiting dose of SIVmac239x (Del Prete, Park et al., 2014), a molecularly-tagged clonal synthetic swarm challenge stock containing 10 clones that are isogenic to SIVmac239 apart from two or three synonymous mutations in the integrase gene. All animals were treated with a suppressive ART regimen of TDF/FTC/DTG as previously described (plus DRV for JL animals).
HIV-2 study participants.
Blood samples were obtained from HIV-2-infected subjects on ART in Senegal, West Africa as part of ongoing studies of long-term outcomes of ART for HIV-2 infection. All subjects were on an ART regimen of LPV/r, IDV, AZT, and 3TC. Patient viral loads at ART initiation were 223, 3008, and 3776. Patients were on ART for 224, 208.5, and 161.6 weeks at sampling. PBMCs were isolated from whole blood and cryo-preserved at −80°C. The study was conducted according to procedures approved by the University of Washington Institutional Review Board and the Senegalese National Ethics Committee (CNERS); all participants provided written informed consent.
METHOD DETAILS
Isolation of macaque CD4+ T lymphocytes.
PBMCs previously isolated from whole blood and cryo-preserved in liquid nitrogen were thawed and CD4+ T cells were isolated using negative selection (CD4+T Cell Isolation Kit, non-human primate, Miltenyi Biotec).
DNA isolation.
For near full-genome sequencing, DNA was isolated from 2×106 CD4+ T cells using the Gentra Puregene Cell Kit (Qiagen) which gives molecular weight DNA (100–200kb) to minimize shearing of proviral genomes. For digital droplet PCR (ddPCR), DNA was extracted using the QIAamp DNA Mini kit (Qiagen), which produces smaller fragments averaging 50kb, which provide optimal balance between requirements for proviral integrity and droplet formation. DNA concentration was measured by NanoDrop 2000 and/or Qubit 4 Fluorometer (ThermoFisher Scientific).
Limiting dilution PCR and full-genome sequencing.
DNA was subjected to limiting dilution, nested PCR as previously described for HIV-1 (Bruner et al., 2016) using either Platinum Taq DNA Polymerase High Fidelity or Platinum SuperFi DNA Polymerase (ThermoFisher Scientific). The outer PCR uses a touchdown method and extends from just downstream of the 5’ LTR (position 854 of SIVmac251) to the middle of the 3’ LTR (position 9871 of SIVmac251)· All wells were diluted 1:3 and 1 μl of each diluted outer well product was subjected to inner PCRs that amplify the gag and/or env genes to determine clonality. Clonal dilutions were determined using Poisson statistics. For plates with probability of clonality ≥ 0.85, all outer wells, regardless of gag or env positivity, were subjected to 7 inner PCRs for SIVmac251, 5 inner PCRs for HIV-2, or 2 inner PCRs for SHIVSF162. PCR primer and probe locations and sequences and PCR conditions are in Table S1. PCR products were visualized using a 0.75%–1% agarose gel and DNA was extracted for direct sequencing (QIAquick Gel Extract Kit, Qiagen). Sanger sequencing was used for bands <3000bp in size, were sequenced using Sanger sequencing; illumina MiSeq 2×300 (Nextera DNA Library Preparation Kit, illumina) sequencing was used for bands >3000bp. illumina reads were compiled and analyzed using an in-house pipeline (CLC Genomics Workbench, Qiagen). Sanger sequencing reads were aligned to reference in CodonCode Aligner Software.
Defective genomes were identified as follows. Genomes for which one or more inner PCR products had a contiguous stretch of missing nucleotides not attributable to normal length polymorphism were classified as deleted. In the case of genomes for which full sequences could not be obtained, we assumed that a deletion was present only if the following conditions were met
Successful amplification was obtained with at least one set of inner primers.
One or more inner primer sets failed to give amplification, leaving a region of unknown sequence.
Successful amplification was obtained with each inner primer set on other genomes from the same animal.
Multiple attempts to obtain sequence in the missing region with alternative primers were unsuccessful.
Hypermutation was determined using the reconstructed full-length sequence of each provirus and the Los Alamos Hypermut algorithm (Rose and Korber, 2000). Two sequences that did not reach statistical significance but that had numerous G to A mutations at consensus sites leading to introduction of premature stop codons were also called hypermutated. In the case of hypermutated proviruses for which we did not obtain full sequences, deletions were not presumed since the extensive mutation could affect primer binding. These sequences were classified as hypermutated.
Single-genome amplification of SIVmac251 and SIVmac239 env.
Extracted DNA (above) was subjected to limiting dilution nested PCR of near-full-length env as previously described (Stone et al., 2010), using the Taq High Fidelity DNA Polymerase. The outer reverse primer was modified for use with SIVmac239 such that 200nM of 239envR1 (5’-GAG TAT CCA TCT TCC ACC TCT CCT AAG AGT-3’) was used in place of 251 envR1. Outer PCRs were performed with the following cycles: 94°C for 2 minutes, 44 cycles of: 94°C for 30 seconds, 50°C for 30 seconds, 72°C for 2 minutes 30 seconds, and 72°C for 3 minutes. Inner PCRs were performed with the following cycles: 94°C for 2 minutes, 41 cycles of: 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 2 minutes, and 72°C for 3 minutes.
In vitro infections.
Frozen rhesus PBMCs were rapidly thawed and cultured in RPMI + 10% FBS. After 24 hours of rest, cells were activated in media containing 5μg/mL phytohemagglutinin (PHA, Fisher Scientific) + 2ng/mL IL-2. After 21 hours, activating mitogen was removed, and cells were resuspended in RPMI + 10% FBS (R10) at 2×106/mL. Cells were infected by spinoculation (2 hours, 1200xg, 30°C) at a MOI of 0.01 in 96-well plate format. At 15 hours after spinoculation, 10μM emtricitabine (FTC) and 1μM tenofovir (TDF) were added to cultures to limit infection to a single round. After 48 hours, cells were lysed and DNA extracted using the Puregene Cell Kit as described above.
Shearing Controls.
DNA shearing between RPP30 amplicons was evaluated using intentionally sheared DNA extracted from PBMC of uninfected rhesus macaques using the QIAamp kit. DNA was sheared to different lengths using g-TUBEs for DNA Shearing (Covaris). Targeted DNA fragments of 10kb were centrifuged at 6,000 RPM for 60 seconds. Targeted DNA fragments of 6kb were centrifuged at 11,000 RPM for 30 seconds. The DNA used for the degradation condition was pulse-vortexed for 60 seconds and incubated with shaking at 98°C for 30 m in.
2-LTR ddPCR.
2-LTR circles were quantified using primers and probes adapted from (Policicchio et al., 2018). For each ddPCR reaction, 5.5 μl of eluted DNA from each time point was added to 16.5 μl master mix containing 10μl of 2x ddPCR Supermix for Probes (no dUTP) (Bio-rad) and primers and probes for detection of 2LTR circle and an internal pol or env region as described below for the SIV IPDA. Droplets were made using the manual Droplet Generator (Bio-Rad). Droplets underwent thermocycling of 10 minutes at 95°C, 45 cycles of (30 seconds at 94°C, 60 seconds at 56°C), and 10 minutes at 98°C. Droplets were read by the QX100 Droplet Reader (Bio-Rad).
gag qPCR.
Proviral DNA was quantified using gag qPCR as previously described (Gama et al., 2017). Briefly, the following primer/probe set was used for SIV gag: SIV21F: 5’-GTCTGCGTCATCTGGTGCATTC-3’, SIV22R: 5’-CACTAGGTGTCTCTGCACTATCTGTTTTG-3’ AND SIV23: FAM-5’-CTTCCTCAGTGTGTTTCACTTTCTCTTCTG-3’-BH1 (Integrated DNA Technologies). Reactions were performed with the following cycles: 95°C for 10 minutes, 45 cycles of: 95°C for 15 seconds, 55°C for 15 seconds, 60°C for 30 seconds. Cell equivalents were determined by PCR analysis of the interferon β gene (Gama et al., 2017).
ddPCR assay for intact SIV genomes.
Intact SIV genomes were quantitated using a method based on the HIV-1 IPDA (Bruner et al., 2019). 8.5μl DNA (200–1000 ng) from each time point was added to a master mix containing 10 μl of 2x ddPCR Supermix for Probes (no dUTP, Bio-rad), 600 nM of each primer and 200 nM of each probe per 22 μl reaction. Primer and probe locations and sequences and PCR conditions are detailed in Table S1. A second-tier probe with an R base was designed for the 3’ amplicon at a position that can be, rarely, an A instead of a G. ddPCR output was analyzed using QuantaSoft Analysis-Pro (Bio-Rad).
RPP30 ddPCR assay.
For all SIV ddPCR samples, input cell number was quantitated using a ddPCR assay for a rhesus macaque gene (RPP30). This assay also allowed us to exclude samples with excessive DNA shearing which could reduce the number of double positive IPDA droplets. Two RPP30 amplicons spaced at the same distance as the IPDA amplicons were amplified in duplex ddPCR reactions. An aliquot of each DNA sample (3 ng) was added to a master mix consisting of 10 μl of 2x ddPCR Supermix for Probes (no dUTP) (Bio-rad), 500nM of each primer, and 250nM of each probe per 22μl reaction. Details of primer and probe sequences and PCR conditions are in Table S1. A DNA Shearing Index (DSI) was calculated as the reciprocal of the ratio between double positive events and total positive events by the RPP30 assay. Output of intact and defective genomes was normalized to copies per million cells (determined by RPP30), and samples with excessive DNA shearing between amplicons were excluded from further analysis.
QUANTIFICATION AND STATISTICAL ANALYSIS
Phylogenetic Analysis.
Full genome and env sequences were aligned using Ciustal W (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and G → A hypermutants were identified with Hypermut 2.0 (Rose and Korber, 2000). Sequences with stop codons due to hypermutation were excluded from downstream evolutionary analyses. Neighbor-joining trees based on p-distance were constructed in MEGA 7.0 (http://meqasoftware.net). The consensus sequence of the relevant reference virus was used as the outgroup to root the trees (SIVmac251, M19499.1; SIVmac239, M33262.1; HIV-2, M15390.1; SHIV SF162, KF042063.1). When available, single genome sequences from the relevant viral stock were included in the alignment (Del Prete et al., 2013). Phylogenetic structure was tested by bootstrap analysis (1,000 replicates). Population genetic diversity was calculated as the average pairwise distance (APD) using MEGA 7.0. Accumulation of genetic change over time was tested by comparing the root-to-tip distances to the viral stock. Root-to-tip distance was measured using the p-distance of each taxa from the consensus sequence of the viral stock (MEGA 7.0), with variance estimation performed using 1,000 bootstrap replicates.
DATA AND SOFTWARE AVAILABILITY
All sequences have been deposited in GenBank with accession numbers MK686073 - MK686202 for SHIV, MK686203 - MK686247 for HIV-2, and MK686248 - MK686564 and MK686635 - MK687385 for SIV.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| SIVmac251 | NIH AIDS Reagent Program | Cat # 253 |
| SHIVSF162P3 | NIH AIDS Reagent Program | Cat # 6526 |
| SIVmac239 SpX | NIH AIDS Reagent Program | Cat # 12249 |
| Biological Samples | ||
| DB SIVmac251-infected RM PBMCs | Dr. Dan Barouch, BIDMC | N/A |
| JL SIVmac239X-infected RM PBMCs | Dr. Jeff Lifson, NCI | N/A |
| JC SIVmac251-infected RM PBMCs | Dr. Janice Clements, JHMI | N/A |
| LP SIVmac239X-infected RM PBMCs | Dr. Louis Picker, OHSU | N/A |
| HIV-2 Patient PBMC samples | Dr. Geoffrey Gottlieb, UW | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Platinum Taq DNA Polymerase High Fidelity | ThermoFisher Scientific | Cat # 11304011 |
| Platinum SuperFi DNA Polymerase | ThermoFisher Scientific | Cat # 12351010 |
| Critical Commercial Assays | ||
| Gentra Puregene Cell Kit | Qiagen | Cat # 158388 |
| QIAamp DNA Mini kit | Qiagen | Cat # 51304 |
| Nextera DNA Library Preparation Kit | Illumina | FC-131–1096 |
| Nextera XT Index Kit v2 Set A | Illumina | FC-131–2001 |
| CD4+ T Cell Isolation Kit, non-human primate | Miltenyi Biotec | 130–092-144 |
| Deposited Data | ||
| SIV, SHIV, HIV-2 sequences | This paper | MK686073 - MK686202 for SHIV, MK686203 - MK686247 for HIV-2, and MK686248 - MK686564 and MK686635 - MK687385 for SIV. |
| Software and Algorithms | ||
| CodonCode Aligner Software | CodonCode | https://www.codoncode.com/aligner/download.htm |
| CLC Genomics Workbench | Qiagen | https://www.qiagenbioinformatics.com/products/clc-genomics-workbench/ |
| QuantaSoft Analysis-Pro | Bio-Rad | http://www.bio-rad.com/en-us/product/qx200-droplet-digital-pcr-system?ID=MPOQQE4VY |
| Clustal W | EMBL-EBI | http://www.ebi.ac.uk/Tools/msa/clustalw2/ |
| MEGA 7.0 | MEGA | https://www.megasoftware.net/ |
| Experimental Models: Organisms/Strains | ||
| Rhesus macaques (Macaca mulatta) infected with SIVmac251 | Indian origin | Animals KIC, Ki6, PZB, DEL5, DEAB, DEKW and DEP0 |
| Rhesus macaques (Macaca mulatta) infected with SIVmac251 | Indian origin | Rh52A |
| Rhesus macaques (Macaca mulatta) infected with SIVmac239X | Indian origin | T028, T154, T158, and T159 |
| Rhesus macaques (Macaca mulatta) infected with SIVmac239 | Indian origin | P27779 and P28129 |
| Rhesus macaques (Macaca mulatta) infected with SHIVSF162P | Indian origin | MH8, PNP, PZN, and MC4 |
| Oligonucleotides | ||
| Please refer to Table S1 | ||
Highlights:
Defective viral genomes predominate in treated SIVmac and HIV-2 infection
Significantly more SIV proviruses are intact compared to HIV-1 in treated humans
Compared to HIV, clonal sequences and deleted genomes are less frequent in SIV infection
An assay to directly enumerate intact SIV genomes was developed
Acknowledgements
We thank Dr. Stephen Hughes (NCI) for valuable. This work was supported by the NIH Martin Delaney I4C (UM1 AI126603), Beat-HIV (UM1 AI126620) and DARE (UM1 AI12661) Collaboratories, the Howard Hughes Medical Institute, an unrestricted research grant from Gilead, and the Bill and Melinda Gates Foundation (OPP1115715) to R.F.S. Animal studies at Johns Hopkins were supported by NCRR and the Office of Research Infrastructure Programs (ORIP) of the NIH grant P40 OD013117. Additional support for animal studies came from NIH grants PPG MH070306, NS077869, NS076357, U19–0AI076113, R56 AI118753, 1R01AI127142, and in part with federal funds from the National Cancer Institute, NIH contract HSN261200800001E. The authors thank Gilead and ViiV for providing antiretrovirals. The funders had no role in study design, data collection and interpretation, or publication. E.J.F. was supported by NIH T32 GM007445. A.A.R.A was supported by NIH T32 AI007291–27. HIV-2 studies were supported by grants to G.S.G. from the NIH/NIAID (AI120765 and AI060466). We thank the patients and staff of the UW-Senegal Research Collaboration (http://www.uwseneqalresearch.com).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
Aspects of HIV-1 IPDA are subject of a patent application PCT/US16/28822 filed by Johns Hopkins University. K.M.B. and R.F.S. are inventors on this application. Accelevir Diagnostics holds an exclusive license for this patent application. G.M.L. is an employee of and shareholder in Accelevir Diagnostics. R.F.S. holds no equity interest in Accelevir Diagnostics. R.F.S is a consultant on cure-related HIV research for Merck and Abbvie.
Reference List
- Ambrose Z, Palmer S, Boltz VF, Kearney M, Larsen K, Polacino P, Flanary L, Oswald K, Piatak M Jr, Smedley J, et al. (2007). Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J. Virol 81, 12145–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. (2012). Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, et al. (2006). Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol 80, 6441–6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Weeger M, Camarasa MJ, De Clercq E, and Liberia K (1995). Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1-specific non-nucleoside reverse transcriptase inhibitors. Biochem. Biophys. Res. Commun 211, 850–856. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, et al. (2016). Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N. Engl. J. Med 375, 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankson JN, Finzi D, Pierson TC, Sabundayo BP, Chadwick K, Margolick JB, Quinn TC, and Siliciano RF (2000). Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J. Infect. Dis 182, 1636–1642. [DOI] [PubMed] [Google Scholar]
- Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, Nkolola JP, Brinkman AL, Peter L, Lee BC, et al. (2016). Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, et al. (2018). Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, et al. (2016). Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med 22, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, Fray EJ, Beg SA, Antar AAR, Jenike KM, et al. (2019). A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui JK, Sobolewski MD, Keele BF, Spindler J, Musick A, Wiegand A, Luke BT, Shao W, Hughes SH, Coffin JM, Kearney MF, and Mellors JW (2017). Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 13, e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Stanwick TL, Dempsey MP, and Stevenson M (1991). Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254, 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Johnson EP, and Bushman FD (2002). Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J. Virol 76, 3739–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, Lawson BO, Nega M, Easley K, Schmitz JE, et al. (2016). CD8(+) Lymphocytes Are Required for Maintaining Viral Suppression in SIV-infected Macaques Treated with Short-Term Antiretroviral Therapy. Immunity 45, 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CN, Trinité B, Lee CS, Mahajan S, Anand A, Wodarz D, Sabbaj S, Bansal A, Goepfert PA, and Levy DN (2016). HIV-1 latency and virus production from unintegrated genomes following direct infection of resting CD4 T cells. Retrovirology 13, 1–015-0234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. (1997). Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188. [DOI] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, and Siliciano RF (1995). In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med 1,1284–1290. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, and Fauci AS (1997). Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A 94, 13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M Jr, Coffin JM, and Mellors JW (2014). Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A 111, 7078–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F, Guyader M, Guetard D, Salle M, Montagnier L, and Alizon M (1986). Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature 324, 691–695. [DOI] [PubMed] [Google Scholar]
- Cohn LB, Silva IT, Oliveira TY, Rosales RA, Parrish EH, Learn GH, Hahn BH, Czartoski JL, McElrath MJ, Lehmann C, et al. (2015). HIV-1 integration landscape during latent and active infection. Cell 160, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, Kuruc JD, Gay CL, Eron JJ, Margoiis DM, Bosch RJ, and Archin NM (2015). Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. J. Infect. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo YR, et al. (2016). International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med 22, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, and Lifson JD (2018). Nonhuman primate models for studies of AIDS virus persistence during suppressive combination antiretroviral therapy. Curr. Top. Microbiol. Immunol 417, 69–109. [DOI] [PubMed] [Google Scholar]
- Del Prete GQ, Oswald K, Lara A, Shoemaker R, Smedley J, Macallister R, Coalter V, Wiles A, Wiles R, Li Y, et al. (2015). Elevated Plasma Viral Loads in Romidepsin-Treated Simian Immunodeficiency Virus-Infected Rhesus Macaques on Suppressive Combination Antiretroviral Therapy. Antimicrob. Agents Chemother 60, 1560–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Park H, Fennessey CM, Reid C, Lipkey L, Newman L, Oswald K, Kahl C, Piatak M Jr, Quinones OA, et al. (2014). Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J. Virol 88, 8077–8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Scarlotta M, Newman L, Reid C, Parodi LM, Roser JD, Oswald K, Marx PA, Miller CJ, Desrosiers RC, et al. (2013). Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J. Virol 87, 4584–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Shoemaker R, Oswald K, Lara A, Trubey CM, Fast R, Schneider DK, Kiser R, Coalter V, Wiles A, et al. (2014). Effect of suberoylanilide hydroxamic acid (SAHA) administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected indian rhesus macaques. Antimicrob. Agents Chemother. 58, 6790–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Smedley J, Macallister R, Jones GS, Li B, Hattersley J, Zheng J, Piatak M Jr, Keele BF, Hesselgesser J, Geleziunas R, and Lifson JD (2016). Short Communication: Comparative Evaluation of Coformulated Injectable Combination Antiretroviral Therapy Regimens in Simian Immunodeficiency Virus-Infected Rhesus Macaques. AIDS Res. Hum. Retroviruses 32, 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delviks-Frankenberry K, Galli A, Nikolaitchik O, Mens H, Pathak VK, and Hu WS (2011). Mechanisms and factors that influence high frequency retroviral recombination. Viruses 3, 1650–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimmie BA, Delviks-Frankenberrry KA, Burdick RC, Qi D, Izumi T, and Pathak VK (2014). Multiple APOBEC3 restriction factors for HIV-1 and one Vif to rule them all. J. Mol. Biol 426,1220–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinoso JB, Rabi SA, Blankson JN, Gama L, Mankowski JL, Siliciano RF, Zink MC, and Clements JE (2009). A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J. Virol 83, 9247–9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, et al. (2013). Comparative Analysis of Measures of Viral Reservoirs in HIV-1 Eradication Studies. PLoS Pathog. 9, e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. (1999). Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med 5, 512–517. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. (1997). Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, Pate KA, Wietgrefe SW, O’Connor SL, Pianowski L, et al. (2017). Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. Aids 31, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, Sharp PM, and Hahn BH (1999). Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436–441. [DOI] [PubMed] [Google Scholar]
- Guyader M, Emerman M, Sonigo P, Clavel F, Montagnier L, and Alizon M (1987). Genome organization and transactivation of the human immunodeficiency virus type 2. Nature 326, 662–669. [DOI] [PubMed] [Google Scholar]
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, and Malim MH (2003). DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809. [DOI] [PubMed] [Google Scholar]
- Harris RS, and Liddament MT (2004). Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol 4, 868–877. [DOI] [PubMed] [Google Scholar]
- Hiener B, Horsburgh BA, Eden JS, Barton K, Schlub TE, Lee E, von Stockenstrom S, Odevall L, Milush JM, Liegler T, et al. (2017). Identification of Genetically Intact HIV-1 Proviruses in Specific CD4(+) T Cells from Effectively Treated Participants. Cell. Rep 21, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, and Markowitz M (1995). Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373, 123–126. [DOI] [PubMed] [Google Scholar]
- Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom D.i., Lai J, Blankson JN, Siliciano JD, and Siliciano RF (2013). Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DI, Keele BF, Ho YC, Siliciano JD, and Siliciano RF (2017). Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med 214, 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huthoff H, and Towers GJ (2008). Restriction of retroviral replication by APOBEC3G/F and TRIM5alpha. Trends Microbiol. 16, 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi H, Dewar RL, Adelsberger JW, Rehm CA, O’Doherty U, Paxinos EE, Fauci AS, and Lane HC (2016). Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. (2008). Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Chahroudi A, and Silvestri G (2016). Animal models to achieve an HIV cure. Curr. Opin. HIV. AIDS 11, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird GM, Rosenbloom DI, Lai J, Siliciano RF, and Siliciano JD (2016). Measuring the Frequency of Latent HIV-1 in Resting CD4(+) T Cells Using a Limiting Dilution Coculture Assay. Methods Mol. Biol 1354, 239–253. [DOI] [PubMed] [Google Scholar]
- Laskey SB, Pohlmeyer CW, Bruner KM, and Siliciano RF (2016). Evaluating Clonal Expansion of HIV-Infected Cells: Optimization of PCR Strategies to Predict Clonality. PLoS Pathog. 12, e1005689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GQ, Orlova-Fink N, Einkauf K, Chowdhury FZ, Sun X, Harrington S, Kuo HH, Hua S, Chen HR, Ouyang Z, et al. (2017). Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J. Clin. Invest 127, 2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, et al. (2016). Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353, 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi JC, Cohen YZ, Cohn LB, Kreider EF, Barton JP, Learn GH, Oliveira T, Lavine CL, Horwitz JA, Settler A, et al. (2016). Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc. Natl. Acad. Sci. U. S. A 113, E7908–E7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw PA, Pratt-Lowe E, Shaw KE, Levy JA, and Cheng-Mayer C (1995). Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. U. S. A 92, 7490–7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, and Hughes SH (2014). HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH (2009). APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavigner M, Watkins B, Lawson B, Lee ST, Chahroudi A, Kean L, and Silvestri G (2014). Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog. 10, e1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, Kuri-Cervantes L, Benne C, Ryan ES, Balderas R, et al. (2017). CTLA-4(+)PD-1(-) Memory CD4(+) T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-infected Rhesus Macaques. Immunity 47, 776–788.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle DC, Jensen MA, Shriner D, Brodie SJ, Frenkel LM, Mittler JE, and Mullins JI (2003). Evolutionary indicators of human immunodeficiency virus type 1 reservoirs and compartments. J. Virol 77, 5540–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, and Luciw PA (2010). Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol 84, 2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye AA, Hansen SG, Vaidya M, Fukazawa Y, Park H, Duell DM, Lum R, Hughes CM, Ventura AB, Ainslie E, et al. (2018). Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med 24, 1430–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, and Ho DD (1997). Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387, 188–191. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, and Siliciano RF (2002). Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J. Virol 76, 4138–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C, and Siliciano RF (2002). Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol 76, 8518–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policicchio BB, Cardozo EF, Sette P, Xu C, Haret-Richter G, Dunsmore T, Apetrei C, Pandrea I, and Ribeiro RM (2018). Dynamics of Simian Immunodeficiency Virus Two-Long-Terminal-Repeat Circles in the Presence and Absence of CD8(+) Cells. J. Virol 92, 10.1128/JVI.02100-17. Print 2018 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack RA, Jones RB, Pertea M, Bruner KM, Martin AR, Thomas AS, Capoferri AA, Beg SA, Huang SH, Karandish S, et al. (2017). Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell. Host Microbe 21, 494–506.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procopio FA, Fromentin R, Kulpa DA, Brehm JH, Bebin AG, Strain MC, Richman DD, O’Doherty U, Palmer S, Hecht FM, et al. (2015). A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine 2, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, and Pomerantz RJ (2009). The challenge of finding a cure for HIV infection. Science 323, 1304–1307. [DOI] [PubMed] [Google Scholar]
- Rose PP, and Korber BT (2000). Detecting hypermutations in viral sequences with an emphasis on G --> A hypermutation. Bioinformatics 16, 400–401. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Xu X, Chermann JC, and Hirsch I (1997). Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J. Virol 71, 2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Mailliard RB, Rinaldo CR, Ratner D, Ding M, Chen Y, Zerbato JM, Giacobbi NS, Venkatachari NJ, Patterson BK, et al. (2017). Novel assay reveals a large, inducible, replication-competent HIV-1 reservoir in resting CD4+ T cells. Nat. Med 23, 885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankarappa R, Margolick JB, Gange SJ, Rodrigo AG, Upchurch D, Farzadegan H, Gupta P, Rinaldo CR, Learn GH, He X, Huang XL, and Mullins J.l. (1999). Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol 73, 10489–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf R, Lee GQ, Sun X, Etemad B, Aboukhater LM, Hu Z, Brumme ZL, Aga E, Bosch RJ, Wen Y, et al. (2018). HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J. Clin. Invest 128, 4074–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A, Zink MC, Mankowski JL, Chadwick K, Margolick JB, Carruth LM, Li M, Clements JE, and Siliciano RF (2003). Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J. Virol 77,4938–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, and Siliciano RF (2003). Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med 9, 727–728. [DOI] [PubMed] [Google Scholar]
- Simonetti FR, Sobolewski MD, Fyne E, Shao W, Spindler J, Hattori J, Anderson EM, Watters SA, Hill S, Wu X, et al. (2016). Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc. Natl. Acad. Sci. U. S. A 113, 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, and Miller CJ (2010). A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginaiiy inoculated with SIVmac251. J. Virol 84, 7083–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom AE, Del Prete GQ, Deleage C, Elser SE, Lackner AA, and Hoxie JA (2018). The SIV Envelope Glycoprotein, Viral Tropism, and Pathogenesis: Novel Insights from Nonhuman Primate Models of AIDS. Curr. HIV. Res 16, 29–40. [DOI] [PubMed] [Google Scholar]
- Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, and Frenkel LM. (2005). Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J. Virol 79, 9625–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinité B, Ohlson EC, Voznesensky I, Rana SP, Chan CN, Mahajan S, Alster J, Burke SA, Wodarz D, and Levy DN (2013). An HIV-1 replication pathway utilizing reverse transcription products that fail to integrate. J. Virol 87, 12701–12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stockenstrom S, Odevall L, Lee E, Sinclair E, Bacchetti P, Killian M, Epling L, Shao W, Hoh R, Ho T, et al. (2015). Longitudinal Genetic Characterization Reveals That Cell Proliferation Maintains a Persistent HIV Type 1 DNA Pool During Effective HIV Therapy. J. Infect. Dis 212, 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TA, McKernan JL, Tobin NH, Tapia KA, Mullins JI, and Frenkel LM. (2013). An increasing proportion of monotypic HIV-1 DNA sequences during antiretroviral treatment suggests proliferation of HIV-infected cells. J. Virol 87, 1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TA, McLaughlin S, Garg K, Cheung CY, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, and Frenkel LM (2014). HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345, 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gurule EE, Brennan TP, Gerold JM, Kwon KJ, Hosmane NN, Kumar MR, Beg SA, Capoferri AA, Ray SC, et al. (2018). Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc. Natl. Acad. Sci. U. S. A 115, E2575–E2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, and Hahn BH (1995). Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373, 117–122. [DOI] [PubMed] [Google Scholar]
- Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, et al. (2014). Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand HL, Doehle BP, Bogerd HP, and Cullen BR (2004). A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EmboJ. 23, 2451–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, and Richman DD (1997). Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295. [DOI] [PubMed] [Google Scholar]
- Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, and Landau NR (2004). Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat. Struct. Mol. Biol 11, 435–442. [DOI] [PubMed] [Google Scholar]
- Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, and Chen IS (1990). HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61, 213–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequences have been deposited in GenBank with accession numbers MK686073 - MK686202 for SHIV, MK686203 - MK686247 for HIV-2, and MK686248 - MK686564 and MK686635 - MK687385 for SIV.