Abstract
Herbivores that forage on chemically defended plants consume complex mixtures of plant secondary metabolites (PSMs). However, the mechanisms by which herbivores tolerate mixtures of PSMs are relatively poorly understood. As such, it remains difficult to predict how PSMs, singly or as complex mixtures, influence diet selection by herbivores. Although relative rates of detoxification of PSMs have been used to explain tolerance of PSMs by dietary specialist herbivores, few studies have used the rate of detoxification of individual PSMs to understand dietary preferences of individual herbivores for individual versus mixtures of PSMs. We coupled in vivo experiments using captive feeding trials with in vitro experiments using enzymatic detoxification assays to evaluate the dietary preferences and detoxification capacities of pygmy rabbits (Brachylagus idahoensis), dietary specialists on sagebrush (Artemisia spp.), and mountain cottontails (Sylvilagus nuttallii), dietary generalists. We compared preference for five single PSMs in sagebrush compared to a mixture containing those same five PSMs. We hypothesized that relative preference for individual PSMs would coincide with faster detoxification capacity for those PSMs by specialists and generalists. Pygmy rabbits generally showed little preference among individual PSMs compared to mixed PSMs, whereas mountain cottontails exhibited stronger preferences. Pygmy rabbits had faster detoxification capacities for all PSMs and consumed higher concentrations of individual PSMs versus a mixture than cottontails. However, detoxification capacity for an individual PSM did not generally coincide with preferences or avoidance of individual PSMs by either species. Cottontails avoided, but pygmy rabbits preferred, camphor, the PSM with the slowest detoxification rate by both species. Both species avoided β-pinene despite it having one of the fastest detoxification rate. Taken together our in vivo and in vitro results add to existing evidence that detoxification capacity is higher in dietary specialist than generalist herbivores. However, results also suggest that alternative mechanisms such as absorption and the pharmacological action of individual mixtures of PSMs may play a role in determining preference of PSMs within herbivore species.
Keywords: Specialist, generalist, plant secondary metabolite, monoterpenes, Pygmy rabbit, Cottontail rabbit, sagebrush
INTRODUCTION
Plant secondary metabolites (PSMs) influence the foraging behavior of herbivores and may affect patterns of habitat selection at multiple scales (Duncan and Gordon 1999; Frye et al. 2013; Lawler et al. 2000; Moore and Foley 2005; Ulappa et al. 2014). High concentrations of PSMs often have deleterious effects on foraging herbivores (Degabriel et al. 2009; Estell 2010; Guglielmo et al. 1996; Sorensen et al. 2005a), and selective foraging is one mechanism to limit exposure to those PSMs (Frye et al. 2013; Moore and Foley 2005; Ulappa et al. 2014; Wiggins et al. 2006). Plants often contain complex mixtures of PSMs, the identities and concentrations of which can vary among taxa, populations, and individual plants within populations (Frye et al. 2013; Julkunen-Tiitto 1986; Hemming and Lindroth 1995; Lawler et al. 1998; Nyman and Julkunen-Tiitto 2005; O’Reilly-Wapstra et al. 2013; Richards et al. 2015; Thoss et al. 2007; Ulappa et al. 2014). This diversity of PSMs has wide-ranging physiological effects on vertebrate herbivores including reduced digestion, interference with cellular processes, and compromised energy budgets and reproductive success (Degabriel et al. 2009; Estell 2010; Guglielmo et al. 1996; Kohl et al. 2015; Sorensen et al. 2005a). Animals also cope with absorbed PSMs via different detoxification strategies (Sorensen et al. 2006; Sorensen and Dearing 2006), with specialist herbivores generally relying on faster and less expensive detoxification systems than their generalist counterparts (Boyle et al. 1999; Shipley et al. 2012; Sorensen and Dearing 2003a; Sorensen et al. 2004). The complexities of chemicals mixtures in plants and variable capacity of herbivores to detoxify PSMs make it difficult to identify which specific compounds, combinations, and concentrations drive observed patterns in diet selection by herbivores.
Three general approaches – field observations, in vivo captive studies, and in vitro enzymatic assays – have been used to understand how PSMs influence the foraging behavior of herbivores. Field-based, observational studies maintain the complexity inherent in natural systems while sacrificing a degree of causality in the relationships observed. These studies often identify correlations between intake and the concentration of individual PSMs and broad classes of PSMs (e.g., total monoterpenes or polyphenolics) that are thought to be representative of more complex mixtures of PSMs (Duncan et al. 1994; Moore and Foley 2005; Moore et al. 2010; Frye et al. 2013; Ulappa et al. 2014). The patterns that emerge from these studies may help predict habitat selection and foraging behavior, but are correlative, and must be considered in light of other habitat parameters (e.g., nutritional quality, predation risk, microclimate) that may complicate or obscure the interpretation of observed patterns.
In vivo laboratory studies address the mechanisms by which PSMs directly affect diet selection by manipulating concentrations of specific compounds and measuring food intake by captive animals (Dziba and Provenza 2008; Farentinos et al. 1981; Kirmani et al. 2010; Kimball et al. 2012; Shipley et al. 2012). Although better suited to establish causal relationships between PSMs and diet selection than field-based studies, captive studies often sacrifice natural chemical complexity by focusing on a single compound rather than complex mixtures of PSMs found in whole plants (McLean et al. 2007; Kirmani et al. 2010; Shipley et al. 2012; Wiggins et al. 2003). Captive studies that use artificial diets that contain whole plants or extracts from plants can preserve the chemical complexity of natural forage (Kohl et al. 2015; McIlwee et al. 2001; Sorensen et al. 2005a), but do not help identify which specific PSMs or combination of PSMs explain dietary preferences of herbivores. Additionally, many herbivores respond differently to diets containing individual versus mixtures of PSMs (Bernays et al. 1994; Dyer et al. 2003; Marsh et al. 2006; Richards et al. 2010, 2012; Wiggins et al. 2003). Generalist herbivores restricted to a single PSM may overload a specific detoxification pathway and consequently consume less food than when offered a diet containing an equivalent concentration of a mixture of PSMs (Burritt and Provenza 2000; Dearing and Cork 1999; Marsh et al. 2006; Wiggins et al. 2003). While the diversity and evenness of PSMs absorbed by specialist and generalist herbivores consuming natural plant diets has not, to our knowledge, been evaluated, greater PSM diversity can be inferred from studies demonstrating that the diversity of plants consumed is higher in generalists than specialists when both have equal access to plant communities (Crowell et al. 2018; Dial 1988). Specialist herbivores may show relatively higher tolerances for the PSMs they regularly encounter consuming primarily one plant species (Shipley et al. 2012; Sorensen et al. 2004, 2005a), but may have reduced tolerance for novel PSMs (Sorensen et al. 2005b). Captive feeding trials focused on individual PSMs do not capture the additive, synergistic, or potential inhibitory effects of consuming mixtures of PSMs. Likewise, trials employing artificial diets containing whole plants or plant extracts do not capture which combination or individual compound explain diet selection by herbivores nor do they reveal the mechanisms for variable tolerance among PSMs within a species or among species of herbivores.
In vitro pharmacological assays that quantify rates of enzymatic detoxification can provide insight into the mechanisms for variable tolerance of PSMs by herbivores. The majority of these studies focus on comparing enzymatic activity of microsomes from herbivores that vary in dietary selection using standard substrates (Dermauw and Van Leeuwen 2014; Green et al. 2004; Kumar et al. 2014; Labbé et al. 2011; Li et al. 2004; Skopec et al. 2007). Unfortunately, the majority of these assays only assess the rates of detoxification of standard substrates developed for use in model organisms or humans and do not assess how specific enzymes of herbivores detoxify the PSMs they encounter in natural forage. Even in human pharmacology, in vitro assays often do not predict in vivo outcomes (Karlsson et al. 2013; Tan et al. 2017). To our knowledge, no study has assessed whether the rate of detoxification of individual PSMs by metabolizing enzymes from wild vertebrate species can explain in vivo dietary preferences observed in the same species.
Incorporating biologically relevant mixtures of PSMs into captive feeding trials and coupling those trials with mechanistic understanding of the rates of detoxification of PSMs within the same mixture by specialists and generalists may lead to better predictions of diet selection in the field. To do this, we investigated the relationship between: 1) the relative preference of specialist (pygmy rabbit, Brachylagus idahoensis) and generalist (mountain cottontail, Sylvilagus nuttallii) mammalian herbivores for individual and mixtures of monoterpenes, a class of PSMs, in sagebrush (Artemisia tridentata spp.) and 2) the relative rates of detoxification of a mixture of the same individual monoterpenes by enzymes isolated from the specialist and generalist herbivores. Monoterpenes are a class of volatile PSMs that comprise approximately 2.5% of the dry weight (DW) of sagebrush leaves on plants browsed naturally by pygmy rabbits and cottontails (Crowell 2015). High concentrations of both total monoterpenes and specific individual monoterpenes have been correlated with reduced intake among a variety of free-ranging (Frye et al. 2013; Ulappa et al. 2014) and captive (Dziba and Provenza 2008; Kirmani et al. 2010; Lamb et al. 2004; Shipley et al. 2012) mammalian herbivores. Pygmy rabbits have a higher tolerance of sagebrush and specific monoterpenes than mountain cottontails (Camp et al. 2015; Camp et al. 2017; Shipley et al. 2012;). However, plant selection in the field and daily intake in laboratory studies by pygmy rabbits is compromised, at least in part, by increasing concentrations of monoterpenes (Camp et al. 2015; Camp et al. 2017; Ulappa et al. 2014; Utz et al. 2016;). The prevalence and variability of monoterpenes in sagebrush (Kelsey et al. 1982), their putative, differential, and dose-dependent effects on feeding behavior by a variety of specialist and generalist herbivores (Boyle et al. 1999; Lawler et al. 1998; Shipley et al. 2012; Wiggins et al. 2003), and commercial availability of pure forms of monoterpenes make them an ideal class of PSMs to assess the link between selection of individual versus mixtures of PSMs by herbivores and enzymatic detoxification rates of PSMs.
We first conducted in vivo assays to compare the relative preference of pygmy rabbits and cottontail rabbits between individual monoterpenes and mixtures. We also conducted in vitro enzymatic assays to compare the relative rate of detoxification of individual monoterpenes within a mixture using microsomal enzymes isolated from a pygmy rabbit (n = 1) and cottontail rabbits (n = 2). The relative proportions of monoterpenes in the mixture used in both in vivo and in vitro assays was representative of the composition and relative ratio of monoterpenes quantified in Wyoming big sagebrush (A. t. wyomingensis) from field sites where both pygmy rabbits and mountain cottontail rabbits forage. We hypothesized that specialists would be less selective between individual PSMs and a mixture of PSMs than their generalist counterparts due, in part, to faster rates of detoxification for all monoterpenes in sagebrush than generalists. In contrast, because PSMs consumed individually could overwhelm any single detoxification pathway (Estell 2010), we predicted that generalists would show stronger preferences for the mixture of monoterpenes which contained lower concentrations of any one monoterpene than specialists. Finally, we hypothesized that individual PSMs that were preferred compared to mixtures by a species would have the fastest rates of detoxification in that species.
By providing captive herbivores with a mixture of PSMs, we assessed how potential synergistic, antagonistic or neutral interactions among multiple PSMs influence diet selection by herbivores. By controlling the identities, concentrations, and ratios of PSMs within this mixture, we minimized the potentially confounding natural variation in concentrations and ratios of nutrients and PSMs found within whole plants. We propose that comparing preferences of herbivores between concentrations of mixtures of PSMs and equivalent concentrations of the individual PSM isolated from the mixtures occurring in whole plants would help identify which individual PSMs are most likely to influence foraging under natural conditions. Specifically, preference for a mixture over an equivalent concentration of an individual PSM might suggest selection and intake of whole plants is limited by concentrations of the avoided individual PSM. In contrast, preference for an individual PSM compared to a mixture may suggest relatively fast detoxification, low potential for toxic consequences, or high potential for beneficial consequences of that individual PSM. Although a simplified mixture is incapable of representing the full complexity of PSMs produced by wild plants, the individual compounds selected or avoided using this method could be targeted to establish and test hypotheses related to both the pattern and mechanism by which PSMs influence diet selection by wild herbivores.
METHODS AND MATERIALS
Animal Capture and Care.
We captured adult pygmy rabbits from sagebrush-dominated sites in Blaine, Camas, and Lemhi Counties in Idaho (Idaho Department of Fish and Game collection permits 100310 and 01813) and Beaverhead County, Montana (Montana Department of Fish, Wildlife, and Parks scientific collection permit 2014–062). We captured mountain cottontail rabbits in Pullman, Washington (Washington Department of Fish and Wildlife Scientific Collection Permit #14–206). When not undergoing trials, all animals were housed indoors in individual 1.2 × 1.8 m mesh cages at the Small Mammal Research Facility at Washington State University (Boise State University Institutional Animal Care and Use Committee Protocol # 006-AC12–009, Washington State University Institutional Animal Care and Use Committee Protocol # 04513–001). Animals not in trials were provided with ad libitum pelleted commercial rabbit chow (Purina Professional Rabbit Chow, Purina Mills LLC, St. Louise, MO) and fresh water and approximately 15 g/day of fresh mixed greens and greenhouse-grown basin big sagebrush (A. t. tridentata). The rabbit chow was the same used throughout experimental trials and was similar in fiber (36% by dry weight (DW)) and nitrogen (3.4% by DW) to sagebrush leaves (30% fiber and 2.5–4.5% nitrogen by DW, Camp et al. 2015). Rabbits were maintained at an average temperature of 7.66 °C (average minimum 1.58 °C, average maximum 13.42 °C) throughout trial period from 28 March through 16 April 2014.
Identification of Monoterpenes for In Vivo Feeding Studies and In Vitro Enzymatic Assays.
To create a mixture of PSMs for in vivo feeding trials and in vitro enzymatic assays that mimicked the natural concentration of monoterpenes in sagebrush, we first analyzed the monoterpene profile of 420 individual Wyoming big sagebrush plants (Table 1). Plants were selected within a ~ 1000 ha area with evidence of browsing by both pygmy rabbits and mountain cottontails in southern Blaine County, Idaho (43°14’ N, 114°19’ W; elevation: 1470 m). Browsed plants were selected because previous work indicated that although the composition of monoterpenes does not differ between individual sagebrush within a species and within foraging patches browsed by vertebrate herbivores, including pygmy rabbits, the concentrations of individual monoterpenes can differ (Frye et al. 2013; Ulappa et al. 2014). As such, monoterpene profiles of browsed plants more accurately represent profiles that pygmy rabbits and cottontails would naturally consume. The monoterpene profile was analyzed from frozen leaf and stem material from each plant that was coarsely ground (< 2 mm particle size) in liquid nitrogen with a mortar and pestle. Relative concentrations of each monoterpene from each sample (100 mg wet weight) were determined using headspace gas chromatography. All samples were analyzed using an Agilent 6890N gas chromatograph (GC, Santa Clara, CA) coupled with a Hewlett-Packard HP7694 headspace autosampler (Palo Alto, CA). The headspace program was as follows: 100 °C oven temperature, 110 °C loop temperature, and 120 °C transfer line temperature. The vial equilibrium and pressurization times were each 0.20 minutes, the loop fill time was 0.50 minutes, the loop equilibrium time was 0.20 minutes, and the injection time was 0.50 minutes. One mL of headspace gas from each sample was injected into an Agilent J&W DB-5 capillary column (30 m × 250 μm × 0.25 μm, Santa Clara, CA) with helium as the carrier gas at a constant flow of 1.0 mL.min−1 and splitless injector temperature of 250 °C. The temperature program for the GC was as follows: 40 °C for 2.0 minutes, then increased by 3 °C.min−1 to 60 °C, then by 5 °C.min−1 to 120 °C and finally by 20 °C.min−1 to 300 °C where final temperature was held for seven minutes. Inlet pressure was 80 KPa and we used a flame ionization detector set at 300 °C. Retention times of individual monoterpenes and individual areas under the curve (AUC) were quantified using Hewlett-Packard ChemStation software version B.01.00 (Palo Alto, CA). Peaks were identified using co-chromatography with known standards. Samples were then dried at 60° C for 24 hours to correct for water content of sample and to calculate AUC per 100 μg of DW of sagebrush. Relative concentrations (AUC/100 μg DW) of individual monoterpenes were then averaged across all plants and divided by the total concentration of monoterpenes to obtain ratios among constituent compounds. To create a monoterpene mixture that represented whole sagebrush, we determined the proportions of the top five most prevalent individual monoterpenes in sagebrush based on relative AUC (α-pinene, β-pinene, camphene, camphor, 1,8-cineole at 99% purity or greater, Sigma Aldrich, St. Louis, MO, Table 1). These five compounds were added to food (in vivo assays) or microsomes (in vitro enzymatic assays) in the same average proportions in which they occurred naturally in sagebrush (Table 1).
Table 1.
The average percent composition (± SE) and estimated concentration (mg/g dry weight) of the five most abundant monoterpenes (α-pinene, 13.00 min; β-pinene, 14.70 min; camphene, 13.58 min; camphor, 21.15 min; and 1,8-cineole, 16.81 min) in Wyoming big sagebrush (Artemisia tridentate subsp. wyomingensis) and a mixture added to commercial rabbit chow and offered to captive pygmy rabbits (Brachylagus idahoensis) and mountain cottontails (Sylvilagus nuttallii, in vivo) or added to liver microsomes (in vitro).
| Monoterpene (retention time) | Proportion of total monoterpenes in sagebrush leavesa | Estimated concentration (mg/g DW) in sagebrush leavesb | Equivalent proportion of mixture of five monoterpenes | Actual proportion of five monoterpenes in mixture used in vivoac | Estimated concentration (mg/g DW) in 1% DW mixture used in vivod | Actual proportion of five monoterpenes in mixture used in vitroae |
|---|---|---|---|---|---|---|
| α-pinene (13.00 min) |
2.2 ± 0.2% | 0.55 | 2.5% | 1.6 ± 0.3% | 0.16 | 1.7 ± 0.13% |
| β-pinene (14.70 min) |
1.7 ± 0.1% | 0.43 | 2% | 1.8 ± 0.3% | 0.18 | 1.5 ± 0.10% |
| Camphene (13.58 min) |
19 ± 0.8% | 4.75 | 22% | 32.7 ± 5.8% | 3.27 | 11.0 ± 1.07% |
| Camphor (21.15 min) |
56.5 ± 1.7% | 14.13 | 65% | 55.4 ± 5.4% | 5.54 | 73.2 ± 1.11% |
| 1,8-cineole (16.81 min) |
7.5 ± 0.6% | 1.86 | 8.5% | 8.5 ± 2.3% | 0.85 | 12.6 ± 0.17% |
| Total | 87 ± 2.9%f | 25.0 | 100% | 100% | 10.0 | 100% |
Concentrations were determined using co-chromatography with known standards using headspace gas chromatography.
Estimated concentration of each monoterpene in sagebrush leaves was calculated as the product of the average total monoterpene oil extracted by hydrodistillation from fresh sagebrush leaves (2.5% by dry weight (DW) = 25 mg/g DW, Crowell 2015) and the proportion of each individual monoterpene in whole leavesa.
Actual proportion was measured in frozen diets consisting of commercial rabbit chow treated with the mixture of commercially available monoterpenes.
Actual concentration of each monoterpene in the mixture was calculated as the product of the total monoterpenes added to the commercial rabbit chow (1% by dry weight (DW) = 10 mg/g DW) and the actual proportion of each individual monoterpene in the mixtureac. Concentrations in the 1% mixture are lower than in the sagebrush leaves because leaves contain a higher percentage (2.5%, Crowell 2015) of total monoterpenes than the artificial diets.
Actual proportion was measured at time zero in the reaction vial just after the monoterpene mixture was added to each microsome sample (n = 3).
Total does not equal 100% because other monoterpenes comprise the remaining portion in whole sagebrush.
In Vivo Feeding Studies – Artificial Diets.
For artificial diets, individual monoterpenes or the monoterpene mixture was added to pelleted rabbit chow at 1% of DW. Camphor and camphene are solids at room temperature and cannot be added homogenously to rabbit chow, whereas α-pinene, β-pinene, and 1,8-cineole are liquid and can be directly added to rabbit chow. Pure camphor (260 mg/mL) and camphene (248 mg/mL) were therefore dissolved together in methylene chloride (≥ 99.8% pure, Sigma Aldrich, St. Louis, MO). The methylene chloride mixture was thoroughly mixed with rabbit chow in a glass jar at a concentration of 25 μg/g DW of chow. The treated rabbit chow was then spread in a single layer in a fume hood for six hours to allow the highly volatile solvent to evaporate. The time needed for evaporation of the solvent relative to individual monoterpenes was determined by analyzing the concentration of methylene chloride and the camphor and camphene dissolved in methylene chloride added to rabbit chow over time until concentrations of methylene chloride were less than 1.0 μg/g DW of chow. The evaporation of camphor and camphene during the six hour period was minimal relative to the solvent, resulting in the desired final concentrations of monoterpenes (Table 1). In a preliminary study, we determined that pygmy rabbits and mountain cottontails did not discriminate between control rabbit chow and chow that was mixed with methylene chloride only (no camphor and camphene) and allowed to evaporate for six hours (Nobler 2016). After the solvent was evaporated, the remaining liquid monoterpenes were thoroughly mixed with the rabbit chow already treated with camphor and camphene in a glass jar. To prevent the volatilization of monoterpenes, all treated chow was stored at −20° C until offered to rabbits. Samples of treated rabbit chow were saved in sealed scintillation vials at −20° C before being analyzed for concentrations of monoterpenes via gas chromatography.
In Vivo Feeding Studies – Feeding Trials.
Before beginning feeding trials with monoterpene diets, all animals were acclimated to receiving commercial rabbit chow offered in equal portions at two feeding stations equal distances from a nest box over a period of three days. After acclimation, rabbits were offered a choice between rabbit chow treated with either 1% by DW of each individual monoterpene or 1% by DW monoterpene mixture (Table 1). This concentration represents the lower end of the range of monoterpene concentration by weight in sagebrush (Kelsey et al. 1982), and corresponds with concentrations at which individual monoterpenes reduce intake by mountain cottontails (Shipley et al. 2012). Individual monoterpene treatments that were paired with the mixture were administered sequentially, but in a randomly-determined order. Animals were also given rest periods of three to five days between treatments to prevent habituation. The mixture was first offered on a randomly determined side of the nest box, followed by alternating sides relative to the individual monoterpene treatment for three days to avoid directional bias (Utz 2012). We recorded the amount of food offered and remaining (orts) after 24 hours from each choice (individual monoterpene versus mixture) in each feeding trial (encompassing both diurnal and nocturnal intake), and corrected for DW by drying the orts and a sample of the treated rabbit chow offered at 100° C for ≥ 24 hrs. Five feeding trials were conducted (three days/trial) in which the monoterpene mixture was paired with each of the five individual monoterpenes.
In Vitro Enzymatic Detoxification Assays.
Microsomes from a pygmy rabbit (n = 1) and mountain cottontails (n = 2) were prepared from livers obtained from freshly euthanized animals that had been in captivity for at least one year. Pygmy rabbits are a species of conservation concern with one population listed as endangered under the Endangered Species Act. Therefore, agencies were reluctant to issue permits that involved terminal outcomes for this species. Thus, the euthanasia of additional animals to increase sample sizes for rabbits was not possible. Tissues from euthanized animals were collected on dry ice and immediately transferred and stored at −70° C. All steps involved with sample preparation were carried out on ice. Partially thawed livers were cut into small pieces (< 3 mm2) and approximately 1.0 g of chopped tissue was combined with 3–4 mL of cold homogenizing buffer (150 mM KCl, 10 mM EDTA, 0.10 M Tris, pH 7.4). Tissue was homogenized with 5–8 short bursts using the probe of an Omni Tissue Master. The liver homogenates were then centrifuged at 12,500 × g for 15 minutes at 4 °C. The resulting supernatants were collected, then centrifuged at 105,000 × g for 70 minutes at 4 °C. Supernatants were discarded, and pellets were re-suspended in the original volume of homogenizing buffer. These samples were centrifuged again at 105,000 × g for 40 minutes at 4 °C. Supernatants were discarded, and the final pellet re-suspended in cold microsome buffer (10 mM EDTA, 20% glycerol, 0.050 M Tris, pH 7.5). The total protein concentration of the microsome suspensions was determined using a Biorad DC Protein assay kit according to manufacturer’s directions and suspensions were adjusted to a final concentration of 20 mg/mL total protein prior to conducting enzymatic assays used to measure rates of detoxification of individual monoterpenes. Microsome suspensions were stored at −70 °C until use.
Rates of detoxification of individual monoterpenes within a mixture using microsomal enzymes isolated from a pygmy rabbit and mountain cottontails were monitored in vitro by measuring the percent difference in monoterpene concentration between paired enzyme reactions at time zero and at 15 minutes using headspace GC analysis. Concentrations of monoterpenes (α-pinene, β-pinene, camphene, camphor, 1,8-cineole) that represented proportions in whole sagebrush (Table 1) were dissolved as a mixture in DMSO at 50X final reaction concentrations. Assay tubes contained 864 μL of phosphate buffered saline solution (0.137 M NaCl, 0.01 M K2HPO4, 0.0027 M KCl, pH 7.4); 100 μL of 10 mM NADPH, and 26 μL of microsome (20 mg/mL in PBS). To start the reaction, 10 μL of the monoterpene mixture was added to microsomes in pairs. One paired reaction was incubated at 37 °C for zero minutes and the other paired reaction was incubated at 37 °C for 15 minutes. To terminate the reaction at zero or 15 minutes, the mixture was transferred to a 20 mL headspace vial containing 0.5 g NaCl, sealed, and heated for 1.0 minute at 200 °C. Rate of detoxification was determined as the percent difference in concentration of each monoterpene in the mixture between the enzyme reactions terminated at zero minutes and the reactions terminated at 15 minutes. Assays for each paired reaction for each microsomal enzyme sample were run in triplicate and thus represent pseudoreplication due to limited sample size of animals used to obtain microscomes. Negative control reactions included reactions that contained all components of enzyme reactions, but did not contain either NADPH nor microsomes or contained heat-denatured microsomes. Control reactions were used to confirm that loss of monoterpenes from assay tubes was only associated with microsomal enzyme activity.
Statistical Analysis.
To determine preferences for or against individual monoterpenes compared to a mixture, we divided the amount of each treatment consumed (i.e., individual monoterpene versus mixture) by the total amount of food consumed from both choices each day. The calculated proportion of total intake constituting a single monoterpene was averaged across the three day choice trial for each treatment for each animal. Preferences for the single monoterpene (compared to the mixture) are reported as the three-day mean proportion (± standard error) of the total food consumed constituting the individual monoterpene. Preferences were reported separately for each treatment comparison (n = 5), and for each rabbit species (i.e., pygmy rabbits and mountain cottontails). To evaluate the rabbits’ preference for each treatment, we compared the proportion consumed of each treatment to 0.50 using a one sample t-test. Animals consuming an equal proportion (0.50) from the feeding station with the individual monoterpene and the feeding station with the monoterpene mixture were considered to have no preference between the treatments. To evaluate if the type of individual monoterpene influenced the proportion of the mixture, we used a mixed-effects linear model with the proportion of the individual monoterpene consumed as the response variable and rabbit species and treatment (i.e., type of individual monoterpene offered), and the interaction of species and treatment as fixed effects, and individual rabbit as a random effect. To investigate a potential relationship between preference and total daily intake, we added intake (total daily g DW consumed from both choices/g body mass) and the interaction between species and intake as fixed effects to the mixed-effects linear model with the proportion of the individual monoterpene consumed as the response variable and rabbit species and treatment (i.e., type of individual monoterpene offered), and the interaction between species and treatment as fixed effects, and individual rabbit as a random effect. To evaluate differences between species, we followed significant results with pairwise comparisons using a Tukey’s HSD test adjusted p-value.
To compare rates of detoxification for monoterpenes, we used a generalized linear model with individual monoterpene and rabbit species, and the interaction of monoterpene and species as fixed effects. We used a Tukey’s HSD test to compare rates of detoxification among monoterpenes within each species. All statistical analyses were conducted using R version 3.2.0 (R Foundation for Statistical Computing 2015) and JMP Pro 11.0 (SAS Institute Inc. 2013).
RESULTS
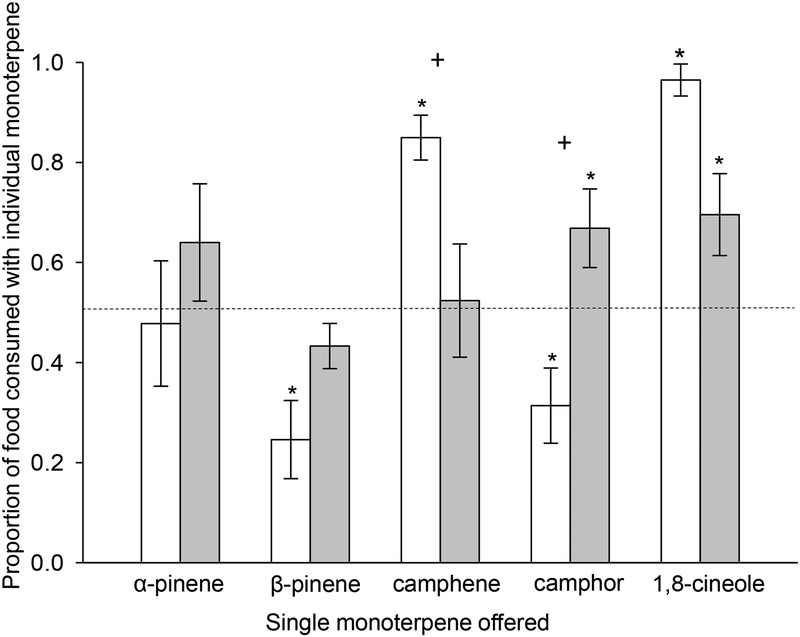
Both rabbit species responded to choices between individual monoterpenes and mixtures, but their preferences varied among individual monoterpenes. The proportion of individual monoterpenes consumed did not differ between species (F1,28 = 0.26, P > 0.05), but did differ with treatment (i.e., individual monoterpene offered, F4,28 = 18.04, P < 0.0001), and species × treatment interaction (F4,28 = 11.68, P < 0.0001). When offered choices between one of five individual monoterpenes compared to mixed monoterpenes, pygmy rabbits showed no preference when α-pinene (64% ± 0.11, t4 = −1.80, P > 0.05), β-pinene (43% ± 0.04, t4 = 2.06, P > 0.05), or camphene (52% ± 0.11, t4 = −0.27, P > 0.05) were paired with the mixture. However, pygmy rabbits preferred camphor (t4 = −4.37, P = 0.01) and 1,8-cineole (t4 = −4.93, P = 0.008) compared to the mixture (Fig. 1). The percentage of camphor (66% ± 0.08) or 1,8-cineole (70% ± 0.08) in the diet of pygmy rabbits was twice that of the monoterpene mixture (Fig. 1). The effect of total intake (daily g DW consumed from both choices/g body mass) on preferences was not significant (F1,26= 0.55, P = 0.46), nor was the interaction between species and total intake (F1,26 = 0.43, P = 0.51).
Fig. 1.
Mean proportions (± SE) of total mass consumed by mountain cottontails (Sylvilagus nuttalli, white bars) and pygmy rabbits (Brachylagus idahoensis, grey bars) from a feeding station consisting of a diet of commercial rabbit chow containing a single monoterpene paired with a diet containing a mixture of monoterpenes. When the single monoterpene constitutes a 0.50 proportion of total food consumed, rabbits are considered to have no preference. An asterisk above bars denotes proportions consumed of the single monoterpene that were significantly different from 0.5 for each species with α = 0.05. A plus sign above sets of bars denotes a significant difference between species in the proportion consumed of the single monoterpene
Similar to pygmy rabbits, mountain cottontails showed no significant preference between α-pinene (48% ± 0.13) and the monoterpene mixture (t3 = 0.20, P > 0.05). However, generalists showed significant preferences for both camphene (t3 = −9.77, P = 0.002) and 1,8-cineole (t3 = −23.81, P = 0.002), consuming more than five times as much camphene (85% ± 0.04) as the monoterpene mixture, and 24 times as much 1,8-cineole (96% ± 0.03) as the monoterpene mixture. Mountain cottontails consumed three times as much monoterpene mixture as β-pinene (25% ± 0.08, t11 = 0.643, P < 0.001) and twice as much monoterpene mixture as camphor (31% ± 0.08, t11 = 4.991, P < 0.001, Fig. 1).
Pygmy rabbits and cottontails did not differ in their preference for α-pinene (t28 = 1.81, P > 0.05, pygmy rabbit, 64% ± 0.11; cottontail, 48% ± 0.13), β-pinene (t28 = 2.08, P > 0.05, pygmy rabbit, 43% ± 0.04; cottontail, 25% ± 0.08), or 1,8-cineole (t28 = −3.00 P > 0.05, pygmy rabbit, 70% ± 0.08; cottontail, 96% ± 0.03, Fig 1) compared to the monoterpene mixture. However, the preferences between species differed significantly for camphene (t28 = −3.63, P = 0.03, Fig. 1), which was preferred by cottontails (85% ± 0.04) compared to the monoterpene mixture, but consumed in similar proportions (52% ± 0.11) to the monoterpene mixture by pygmy rabbits. Pygmy rabbits preferred camphor (66% ± 0.08) relative to the monoterpene mixture, and cottontails preferred the mixture relative to camphor (31% ± 0.08). Pygmy rabbits consumed twice the proportion of camphor as cottontails (t28 = 3.95, P = 0.01, Fig. 1).
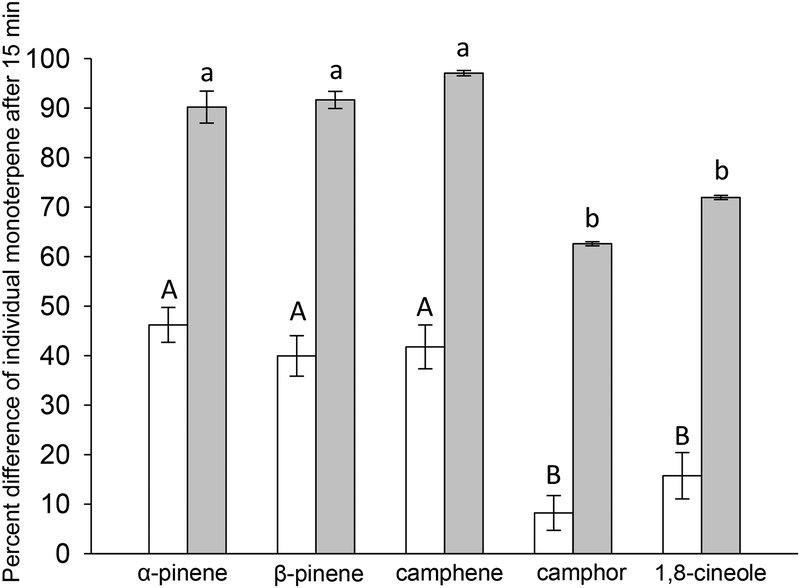
Rates of detoxification were faster in the pygmy rabbit microsomes than in cottontails microsomes for all monoterpenes within the mixture (F1,35 = 371.6, P < 0.0001), and rates differed among individual monoterpenes (F4,35 = 27.0, P < 0.0001). The monoterpene by species interaction was removed because it was not significant (F4,35 = 0.66, P > 0.05). The percent difference for α-pinene (pygmy rabbit, 90.18% ± 3.23; cottontail, 31% ± 0.08), β-pinene (pygmy rabbit, 91.63% ± 1.74; cottontail, 39.9% ± 4.09), camphene (pygmy rabbit, 97.04% ± 0.54; cottontail, 41.76% ± 4.43), camphor (pygmy rabbit, 62.58% ± 0.42; cottontail, 8.22% ± 3.5), and 1,8-cineole (pygmy rabbit, 71.93% ± 0.43; cottontail, 15.74% ± 4.69) during a 15 minute reaction compared to a zero minute reaction was 2.0, 2.3, 2.3, 7.6, and 4.6 fold faster, respectively, for pygmy rabbit microsomes than for cottontail microsomes (Fig 2). In both pygmy rabbit and cottontail microsomes, camphor and 1,8-cineole did not differ from each other and had significantly slower rates of detoxification than α-pinene, β-pinene, and camphene which did not differ from each other (Fig. 2). After a 15 minute reaction with microsomes from a pygmy rabbit, there was only a 63% decline of camphor and 72% decline of 1,8-cineole compared to a decline of more than 90% for α-pinene, β-pinene, and camphene. Similarly, there was only an 8% decline of camphor and 16% decline of 1,8-cineole after reacting with cottontail microsomes compared to a decline of approximately 40% for α-pinene, β-pinene and camphene.
Fig. 2.
Mean percent difference (± SE) of single monoterpenes after 15 minutes of reaction with microsomal enzymes isolated from mountain cottontails (Sylvilagus nuttalli, white bars) and pygmy rabbits (Brachylagus idahoensis, grey bars) compared to the paired reaction at zero minutes. Rates of detoxification differed significantly between species for all five single monoterpenes. Different letters denote significantly different rates of detoxification among single monoterpenes within a single species
DISCUSSION
Dietary preferences of herbivores have long been hypothesized to be dictated by the physiological capacity of herbivores to process absorbed PSMs (Freeland and Janzen 1974; Freeland 1991; Foley et al. 1999). Specifically, faster rates of detoxification should increase tolerance and therefore relative intake of PSMs by herbivores. In support of expectations, the microsomes from specialist herbivores (pygmy rabbit) had faster rates of detoxification for all monoterpenes than the generalists (Fig 1) and are consistent with higher daily intake of single monoterpenes in captivity (cineole, Shipley et al. 2012) and higher proportion of sagebrush in the diet (Crowell et al. 2018) by specialist pygmy rabbits compared to generalist cottontails. In contrast, relative differences in detoxification rates among monoterpenes were not consistent with patterns of diet selection for individual monoterpenes within species in our study. For generalists, the hypothesis that monoterpenes with the fastest detoxification rates would be preferred over mixtures that contain monoterpenes with slower detoxification rates was only partially supported. Consistent with predictions, camphor had with the slowest detoxification rate in mountain cottontails and was associated with avoidance relative to the mixture. However, both mountain cottontails and pygmy rabbits preferred 1,8-cineole despite it having one of the slowest detoxification rates. In contrast to cottontails and in opposition to predictions, pygmy rabbits preferred camphor which had the slowest rate of detoxification. For both species, β-pinene had one of the fastest rates of detoxification, yet was associated with the lowest proportional intake of any individual monoterpene.
Preferences are likely a function of the dose-dependent pharmacological consequences of PSMs (Forbey et al. 2011; Kohl et al. 2015) that can be influenced by a variety of mechanisms. Limitations to enzymatic detoxification has received the most attention as an explanation limiting intake of any one plant by generalist herbivores like mountain cottontails (Dearing and Cork 1999; Dearing et al. 2000; Freeland and Janzen 1974; Shipley et al. 2009). Assuming different plants contain different types of PSMs that use different detoxification pathways, generalists are thought to avoid overwhelming a single detoxification pathway by mixing their diet. Diet mixing results in the intake of smaller amounts of any one plant and therefore smaller concentrations of any one PSM. In support, several generalist herbivores do consume more food when offered a diet containing mixed PSMs than when restricted to an individual PSM (Burritt and Provenza 2000; Dearing and Cork 1999; Wiggins et al. 2003). This pattern remains even when the diets are identical nutritionally (Bernays et al. 1994), supporting the hypothesis that saturated detoxification pathways can play a role in limiting intake (Freeland and Janzen 1974). The hypothesis that diet mixing by generalists minimizes saturation of detoxification pathways assumes that generalists have reduced capacity (lower diversity or expression) in the enzymes responsible for detoxifying individual PSMs compared to specialists and that individual PSMs use different detoxification pathways. Recent genomic studies provide evidence that insect (Calla et al. 2017) and vertebrate (Johnson et al. 2018; Kitanovic et al. 2018) specialists may have higher capacity to detoxify PSMs in host plants through relatively high diversification and duplication of the cytochrome P450 (CYP) enzymes. Although detoxification enzymes generally have broad substrate affinity, CYPs do have differential substrate selectivity for particular monoterpenes (Hernandez-Ortega et al. 2018) and affinity for one monoterpene can be shifted to another structurally similar monoterpene by mutations in the CYP enzyme (Bell et al. 2003). As such, genetic diversity of detoxification enzymes could result in differential capacity to detoxify individual monoterpenes.
Under the assumption that detoxification pathways are rate limited, dietary specialists have a greater diversity of detoxification pathways for PSMs in their host plant, and that individual monoterpenes have higher affinity for specific detoxification pathways, we expected mountain cottontails to prefer the monoterpene mixture that contained lower absolute concentrations of any individual monoterpene than diets containing a single monoterpene at higher concentrations (Table 1). However, cottontails preferred the monoterpene mixture only when paired with camphor and β-pinene, consumed equal proportions of the mixture and α-pinene, and preferred camphene and 1,8-cineole more than the mixture. Like cottontails, pgymy rabbits preferred 1,8-cineole more than the mixture, but also preferred camphor more than the mixture. However, pygmy rabbits did not demonstrate a preference for or against α-pinene, β-pinene, or camphene. A lack of preference for α-pinene by both specialists and generalists could indicate that the dose-dependent pharmacology of α-pinene is equivalent to that of a mixture of monoterpenes. Preference for individual monoterpenes relative to a mixture could indicate that 1% DW of the individual monoterpene was not at a high enough dose to have a negative pharmacological effect regardless of detoxification rate. Alternatively, preference for individual monoterpenes may indicate that the mixture at 1% DW had synergistic negative effects or contained individual compounds that are biologically active even at relatively low doses.
In vivo dietary preferences that are inconsistent with in vitro detoxification rates of liver microsomes may suggest differential rates of absorption among individual monoterpenes. Diet selection may be dependent on rates of detoxification by host and microbial enzymes in the intestine prior to absorption and mechanisms regulating the absorption of PSMs Cui, 2018, Kohl and Dearing 2017; Peters et al., 2016). Evidence exists that tolerance of PSMs by herbivores is linked to the functional attributes of microbial communities (Kohl et al. 2014) and mechanisms that limit absorption of ingested PSMs. For example, specialist woodrats absorbed five times less of the most abundant monoterpene in juniper (α-pinene) than generalist counterparts after receiving identical doses (Sorensen and Dearing 2003b) and specialist sage-grouse excrete PSMs from their diet of sagebrush unchanged in feces (Frye 2012, Thacker et al. 2012). In addition, inhibition of lymphatic absorption resulted in greater intake of PSMs in whole plants by generalist woodrats (Kohl and Dearing 2017). These studies provide examples of how in vivo experiments can be used to assess how intestinal absorption can explain tolerance of PSMs by herbivores. In addition, in vitro assays of efflux transporters and their substrates (see Sorensen et al. 2006) can be used to compare mechanisms that regulate absorption among taxa.
Evaluation of the matches and mismatches between in vitro rates of detoxification and in vivo diet selection from this study, coupled with physio-chemical properties of PSMs (e.g., tissue/blood partition coefficients, Daina et al., 2017) may help focus attention on particular PSMs most likely to influence foraging by vertebrate herbivores. For example, PSMs that are avoided at low concentrations by herbivores and have molecular structures that indicate high absorption may be particularly bioactive even at low concentrations in mixtures and could therefore serve as valuable predictors of intake by herbivores. For example, in vivo and in vitro results demonstrate that β-pinene comprised the lowest proportion of the total intake in both pygmy rabbits and mountain cottontails (Fig 1) despite it having one of the fastest rates of detoxification (Fig 2). In contrast, 1,8-cineole comprised the highest proportion of the total intake in both pygmy rabbits and mountain cottontails (Fig 1) despite it having one of the slowest rates of detoxification (Fig 2). Based on structural properties of β-pinene (lower molecular weight, lack of oxygen atom), this PSM is predicted to be more lipophilic and less water soluble and therefore has lower absorption than 1,8-cineole and is more likely to be an inhibitor of detoxification enzymes than1,8-cineole (from SwissADME, Daina et al., 2017). The predicted pharmacokinetic properties may explain the avoidance of individual β-pinene at 1% DW (10 mg/g DW) and why higher concentrations of cineole (at 1% DW, 10 mg/g DW) was preferred compared to low concentrations of β-pinene in the mixture (0.018% DW, 0.18 mg/g DW). The pharmacodynamic properties of PSMs may also explain preference patterns. For example, preference of pygmy rabbits and avoidance of cottontails for camphor may reflect differences in pharmacological mechanisms of action of this PSM. For example, camphor reduced digestive enzyme activity in a generalist more than in a specialist avian folivore (Greater sage-grouse, Centrocercus urophasianus, Kohl et al. 2015). We propose that pygmy rabbits may be more resistant to the pharmacological affects of camphor than cottontails. Although not tested in specialist pygmy rabbits, avian herbivores that specialize on sagebrush are more resistant to concentration-dependent inhibition of digestive enzymes by camphor than a generalist (Kohl et al. 2015). Similar resistance to this mechanism of action by pygmy rabbits may explain why pygmy rabbits can subsist almost entirely on sagebrush (Crowell et al. 2018) containing monoterpenes dominated by camphor (Table 1). The relatively high absolute concentration of camphor in the individual diet (10 mg/g DW) may also provide a more realistic olfactory cue for pygmy rabbits that naturally consume sagebrush containing camphor at similar concentrations (estimated at 14 mg/g DW of leaves, Table 1, Crowell 2015). Combined in vivo and in vitro assays could help isolate the olfactory cues that explain pre-ingestive diet selection (Finnerty et al. 2017, Schmitt et al. 2018) from the post-ingestive pharmacokinetic (absorption and detoxification, Kohl and Dearing 2017, Sorensen et al. 2006. Sorensen and Dearing 2006) and pharmacodynamic (mechanisms of action, Forbey et al. 2011, Kohl et al. 2015) consequences of subsequent dietary choices.
The role of PSMs in influencing patterns of foraging and habitat selection is slowly becoming better understood (Denno 2012; Frye et al. 2013; Lawler et al. 1998; Moore and Foley 2005; Moore et al. 2010; Rosenthal and Berenbaum 2012; Ulappa et al. 2014). However, the complexity of PSMs and the diverse effects PSMs have on the physiology and behavior of herbivores has made it difficult to identify the compounds and combinations of compounds most likely to drive complex patterns of foraging. When forced to choose at random from hundreds of potentially influential PSMs, chemical ecologists and physiologists have been hard pressed to narrow their focus and determine mechanistic relationships between compounds and the animals that consume them. Field-based studies can be used to identify and quantify the most common PSMs thought to influence habitat selection. Those data in turn, can inform the hybrid approach we present in this paper, in which simplified mixtures of PSMs can be used in in vivo and in vitro assays to identify the few compounds most likely to influence diet selection, either singly or in combinations. Moreover, combining in vivo captive studies, in vitro enzymatic assays, and predicted pharmacology based on the structure of PSMs could help establish and test a priori predictions of how specific mixtures of PSM influence both specialist and generalist herbivores in the field.
ACKNOWLEDGEMENTS
We thank S. Berry, B. Davitt, J. Fluegel, L. McMahon, the volunteers at the Small Mammal Research Facility, and the APE lab at University of Nevada Reno. We also thank two anonymous reviewers for their suggestions to improve the manuscript. This research was funded by the National Science Foundation (DEB-1146194, IOS-1258217 and OIA-1826801, J.S. Forbey; NSF; DEB-1146368, L.A. Shipley; DEB-1146166, J.L. Rachlow), Washington State University, Bureau of Land Management (BLM; #L09AC16253, J.S. Forbey; #L09AC15391, J.L. Rachlow), the USDA National Institute of Food and Agriculture (NIFA; Hatch Project 1005876, L.A. Shipley) and the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408 and P20GM109095, National Science Foundation Grant Nos. 0619793 and 0923535; the MJ Murdock Charitable Trust; and the Idaho State Board of Education (C. Dadabay, L. James, J.S. Forbey).
REFERENCES
- Bernays EA, Bright KL, Gonzalez N, Angel J (1994) Dietary mixing in a generalist herbivore: tests of two hypotheses. Ecology 75:1997–2006 [Google Scholar]
- Bell SG, Chen X, Sowden RJ (2003) Molecular recognition in (+)-α-pinene oxidation by cytochrome P450cam. J Am Chem Soc 125: 705–714. [DOI] [PubMed] [Google Scholar]
- Boyle R, McLean S, Foley WJ, Davies NW (1999) Comparative metabolism of dietary terpene, p-cymene, in generalist and specialist folivorous marsupials. J Chem Ecol 25:2109–2126 [Google Scholar]
- Burritt EA, Provenza FD (2000) Role of toxins in intake of varied diets by sheep. J Chem Ecol 26:1991–2005 [Google Scholar]
- Calla B, Noble K, Johnson RM, et al. (2017) Cytochrome P450 diversification and hostplant utilization patterns in specialist and generalist moths: Birth, death and adaptation. Mol Ecol 26:6021–6035 [DOI] [PubMed] [Google Scholar]
- Camp MJ, Shipley LA, Johnson TR, et al. (2017) The balancing act of foraging: mammalian herbivores trade-off multiple risks when selecting food patches. Oecologia 1–13. doi: 10.1007/s00442-017-3957-6 [DOI] [PubMed] [Google Scholar]
- Camp MJ, Shipley LA, Johnson TR, et al. (2015) Modeling trade-offs between plant fiber and toxins: a framework for quantifying risks perceived by foraging herbivores. Ecology 96:3292–3302. doi: 10.1890/14-2412.1 [DOI] [PubMed] [Google Scholar]
- Crowell Miranda Maurine. Food and fearscapes: responses of specialist and generalist rabbits to food and predation risks. Diss. Washington State University, 2015.
- Crowell MM, Shipley LA, Forbey JS, et al. (2018) Dietary partitioning of toxic leaves and fibrous stems differs between sympatric specialist and generalist mammalian herbivores. J. Mammal doi: 10.1093/jmammal/gyy018 [DOI] [Google Scholar]
- Cui JY (2018) Understanding the GUT microbiome-liver axis in xenobiotic biotransformation. Drug Metab and Pharmacok 33:S10 [Google Scholar]
- Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep-UK 7: 42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing MD, Cork S (1999) Role of detoxification of plant secondary compounds on diet breadth in a mammalian herbivore, Trichosurus vulpecula. J Chem Ecol 25:1205–1219 [Google Scholar]
- Dearing MD, Mangione AM, Karasov WH (2000) Diet breadth of mammalian herbivores: nutrient versus detoxification constraints. Oecologia 123:397–405 [DOI] [PubMed] [Google Scholar]
- Degabriel JL, Moore BD, Foley WJ, Johnson CN (2009) The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 90:711–719. doi: 10.1890/08-0940.1 [DOI] [PubMed] [Google Scholar]
- Denno R (2012) Variable plants and herbivores in natural and managed systems. Elsevier [Google Scholar]
- Dermauw W, Van Leeuwen T (2014) The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem Mol Biol 45:89–110. doi: 10.1016/j.ibmb.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Dial KP (1988) Three sympatric species of Neotoma: dietary specialization and coexistence. Oecologia 76:531–537 [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Hartley SE, Iason GR (1994) The effect of monoterpene concentrations in Sitka spruce (Picea sitchensis) on the browsing behaviour of red deer (Cervus elaphus). Can J Zool 72:1715–1720 [Google Scholar]
- Dyer LA, Dodson CD, Stireman Iii JO, et al. (2003) Synergistic effects of three Piper amides on generalist and specialist herbivores. J Chem Ecol 29:2499–2514 [DOI] [PubMed] [Google Scholar]
- Dziba LE, Provenza FD (2008) Dietary monoterpene concentrations influence feeding patterns of lambs. Appl Anim Behav Sci 109:49–57 [Google Scholar]
- Estell RE (2010) Coping with shrub secondary metabolites by ruminants. Small Rumin Res 94:1–9. doi: 10.1016/j.smallrumres.2010.09.012 [DOI] [Google Scholar]
- Farentinos RC, Capretta PJ, Kepner RE, Littlefield VM (1981) Selective herbivory in tassel-eared squirrels - role of monoterpenes in Ponderosa pines chosen as feeding trees. Science 213:1273–1275. doi: 10.1126/science.213.4513.1273 [DOI] [PubMed] [Google Scholar]
- Finnerty PB, Stutz R, Price CJ, et al. (2017) Leaf odour cues enable non-random foraging by mammalian herbivores. J Anim Ecol 86(6): 1317–1328 [DOI] [PubMed] [Google Scholar]
- Foley WJ, Iason GR, McArthur C (1999) Role of plant secondary metobolites in the nutritional ecology of mammalian herbivores: How far have we come in 25 years? In: 5th International Symposium on the Nutrition of Herbivores. pp 130–209 [Google Scholar]
- Forbey JS, Pu X, Xu D, et al. (2011) Inhibition of snowshoe hare succinate dehydrogenase activity as a mechanism of deterrence for papyriferic acid in birch. J Chem Ecol 37:1285–1293 [DOI] [PubMed] [Google Scholar]
- Freeland WJ (1991) Plant secondary metabolites: biochemical coevolution with herbivores. Plant Def Mamm Herbiv CRC Press Boca Raton FL; 61–81 [Google Scholar]
- Freeland WJ, Janzen DH (1974) Strategies in herbivory by mammals: the role of plant secondary compounds. Am Nat 269–289 [Google Scholar]
- Frye GG, Connelly JW, Musil DD, Forbey JS (2013) Phytochemistry predicts habitat selection by an avian herbivore at multiple spatial scales. Ecology 94:308–314 [DOI] [PubMed] [Google Scholar]
- Green AK, Haley SL, Dearing MD, et al. (2004) Intestinal capacity of P-glycoprotein is higher in the juniper specialist, Neotoma stephensi, than the sympatric generalist, Neotoma albigula. Comp Biochem Physiol A Mol Integr Physiol 139:325–333. doi: 10.1016/j.cbpb.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Guglielmo CG, Karasov WH, Jakubas WJ (1996) Nutritional costs of a plant secondary metabolite explain selective foraging by ruffed grouse. Ecology 77:1103–1115. doi: 10.2307/2265579 [DOI] [Google Scholar]
- Hemming JD, Lindroth RL (1995) Intraspecific variation in aspen phytochemistry: effects on performance of gypsy moths and forest tent caterpillars. Oecologia 103:79–88 [DOI] [PubMed] [Google Scholar]
- Hernandez-Ortega A, Vinaixa M, Zebec Z (2018) A toolbox for diverse oxyfunctionalisation of monoterpenes. Sci Rep-UK 8: 14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RN, O’Meally D, Chen Z, et al. (2018) Adaptation and conservation insights from the koala genome. Nat Genet 50:1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkunen-Tiitto R (1986) A chemotaxonomic survey of phenolics in leaves of northern Salicaceae species. Phytochemistry 25:663–667 [Google Scholar]
- Karlsson FH, Bouchene S, Hilgendorf C, et al. (2013) Utility of In vitro Systems and Preclinical Data for the Prediction of Human Intestinal First-Pass Metabolism during Drug Discovery and Preclinical Development. Drug Metab Dispos 41:2033–2046. doi: 10.1124/dmd.113.051664 [DOI] [PubMed] [Google Scholar]
- Kelsey RG, Stephens JR, Shafizadeh F (1982) The chemical-constituents of sagebrush foliage and their isolation. J Range Manag 35:617–622. doi: 10.2307/3898650 [DOI] [Google Scholar]
- Kimball BA, Russell JH, Ott PK (2012) Phytochemical variation within a single plant species influences foraging behavior of deer. Oikos 121:743–751. doi: 10.1111/j.1600-0706.2011.19515.x [DOI] [Google Scholar]
- Kirmani SN, Banks PB, McArthur C (2010) Integrating the costs of plant toxins and predation risk in foraging decisions of a mammalian herbivore. Oecologia 164:349–356. doi: 10.1007/s00442-010-1717-y [DOI] [PubMed] [Google Scholar]
- Kitanovic S, Orr TJ, Spalink D et al. (2018) Role of cytochrome P450 2B sequence variation and gene copy number in facilitating dietary specialization in mammalian herbivores. Mol Ecol 27: 723–736 [DOI] [PubMed] [Google Scholar]
- Kohl KD, Pitman E, Robb BC, et al. (2015) Monoterpenes as inhibitors of digestive enzymes and counter-adaptations in a specialist avian herbivore. J Comp Physiol B 185:425–434 [DOI] [PubMed] [Google Scholar]
- Kohl KD, Weiss RB, Cox J, et al. (2014) Gut microbes of mammalian herbivores facilitate intake of plant toxins. Ecol Lett 17(10): 1238–1246 [DOI] [PubMed] [Google Scholar]
- Kohl KD, Varner J, Wilkening JL, et al. (2017) Patterns of host gene expression associated with harboring a foregut microbial community. BMC Genomics 18(1): 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Rathi P, Schöttner M, et al. (2014) Differences in nicotine metabolism of two Nicotiana attenuata herbivores render them differentially susceptible to a common native predator. PLoS ONE 9:e95982. doi: 10.1371/journal.pone.0095982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé R, Caveney S, Donly C (2011) Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol Biol 20:243–256 [DOI] [PubMed] [Google Scholar]
- Lamb JG, Marick P, Sorensen J, et al. (2004) Liver biotransforming enzymes in woodrats Neotoma stephensi (Muridae). Comp Biochem Physiol C-Toxicol Pharmacol 138:195–201. doi: 10.1016/j.cca.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Lawler IR, Foley WJ, Eschler BM, et al. (1998) Intraspecific variation in Eucalyptus secondary metabolites determines food intake by folivorous marsupials. Oecologia 116:160–169 [DOI] [PubMed] [Google Scholar]
- Lawler IR, Foley WJ, Eschler BM (2000) Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology 81:1327–1338 [Google Scholar]
- Li X, Baudry J, Berenbaum MR, Schuler MA (2004) Structural and functional divergence of insect CYP6B proteins: from specialist to generalist cytochrome P450. Proc Natl Acad Sci U S A 101:2939–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh KJ, Wallis IR, McLean S, et al. (2006) Conflicting demands on detoxification pathways influence how common brushtail possums choose their diets. Ecology 87:2103–2112. doi: 10.1890/0012-9658(2006)87[2103:cdodpi]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- McIlwee AM, Lawler IR, Cork SJ, Foley WJ (2001) Coping with chemical complexity in mammal-plant interactions: near-infrared spectroscopy as a predictor of Eucalyptus foliar nutrients and of the feeding rates of folivorous marsupials. Oecologia 128:539–548 [DOI] [PubMed] [Google Scholar]
- McLean S, Boyle RR, Brandon S, et al. (2007) Pharmacokinetics of 1, 8-cineole, a dietary toxin, in the brushtail possum (Trichosurus vulpecula): significance for feeding. Xenobiotica 37:903–922 [DOI] [PubMed] [Google Scholar]
- Moore BD, Foley WJ (2005) Tree use by koalas in a chemically complex landscape. Nature 435:488–490 [DOI] [PubMed] [Google Scholar]
- Moore BD, Lawler IR, Wallis IR, et al. (2010) Palatability mapping: a koala’s eye view of spatial variation in habitat quality. Ecology 91:3165–3176. doi: 10.1890/09-1714.1 [DOI] [PubMed] [Google Scholar]
- Nyman T, Julkunen-Tiitto R (2005) Chemical variation within and among six northern willow species. Phytochemistry 66:2836–2843 [DOI] [PubMed] [Google Scholar]
- O’Reilly-Wapstra JM, Miller AM, Hamilton MG, et al. (2013) Chemical variation in a dominant tree species: population divergence, selection and genetic stability across environments [DOI] [PMC free article] [PubMed]
- Peters SA, Jones CR, Ungell A-L and Hatley OJD (2016) Predicting drug extraction in the human gut wall: Assessing contributions from drug metabolizing enzymes and transporter proteins using preclinical models. Clin Pharmacokinet 55: 673–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LA, Dyer LA, Forister ML, et al. (2015) Phytochemical diversity drives plant–insect community diversity. Proc Natl Acad Sci 112:10973–10978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LA, Dyer LA, Smilanich AM, Dodson CD (2010) Synergistic effects of amides from two Piper species on generalist and specialist herbivores. J Chem Ecol 36:1105–1113 [DOI] [PubMed] [Google Scholar]
- Richards LA, Lampert EC, Bowers MD, et al. (2012) Synergistic effects of iridoid glycosides on the survival, development and immune response of a specialist caterpillar, Junonia coenia (Nymphalidae). J Chem Ecol 38:1276–1284 [DOI] [PubMed] [Google Scholar]
- Rosenthal GA, Berenbaum MR (2012) Herbivores: Their interactions with secondary plant metabolites: Ecological and evolutionary processes. Academic Press [Google Scholar]
- Schmitt MH, Shuttleworth A, Ward D, and Shrader AM (2018) African elephants use plant odours to make foraging decisions across multiple spatial scales. Anim Behav 141: 17–27 [Google Scholar]
- Shipley LA, Davis EM, Felicetti LA, et al. (2012) Mechanisms for eliminating monoterpenes of sagebrush by specialist and generalist rabbits. J Chem Ecol 38:1178–1189. doi: 10.1007/s10886-012-0192-9 [DOI] [PubMed] [Google Scholar]
- Shipley LA, Forbey JS, Moore BD (2009) Revisiting the dietary niche: When is a mammalian herbivore a specialist? Integr Comp Biol 49:274–290. doi: 10.1093/icb/icp051 [DOI] [PubMed] [Google Scholar]
- Skopec MM, Haley S, Dearing MD (2007) Differential hepatic gene expression of a dietary specialist (Neotoma stephensi) and generalist (Neotoma albigula) in response to juniper (Juniperus monosperma) ingestion. Comp Biochem Physiol Part D Genomics Proteomics 2:34–43. doi: 10.1016/j.cbd.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Sorensen J, Dearing M (2003) Elimination of plant toxins by herbivorous woodrats: revisiting an explanation for dietary specialization in mammalian herbivores. Oecologia 134:88–94 [DOI] [PubMed] [Google Scholar]
- Sorensen JS, Dearing MD (2006) Efflux transporters as a novel herbivore countermechanism to plant chemical defenses. J Chem Ecol 32:1181–1196. doi: 10.1007/s10886-006-9079-y [DOI] [PubMed] [Google Scholar]
- Sorensen JS, McLister JD, Dearing MD (2005a) Plant secondary metabolites compromise the energy budgets of specialist and generalist mammalian herbivores. Ecology 86:125–139. doi: 10.1890/03-0627 [DOI] [Google Scholar]
- Sorensen JS, McLister JD, Dearing MD (2005b) Novel plant secondary metabolites impact dietary specialists more than generalists (Neotoma spp.). Ecology 86:140–154. doi: 10.1890/03-0669 [DOI] [Google Scholar]
- Sorensen JS, Skopec MM, Dearing MD (2006) Application of pharmacological approaches to plant-mammal interactions. J Chem Ecol 32:1229–1246. doi: 10.1007/s10886-006-9086-z [DOI] [PubMed] [Google Scholar]
- Sorensen JS, Turnbull CA, Dearing MD (2004) A specialist herbivore (Neotoma stephensi) absorbs fewer plant toxins than does a generalist (Neotoma albigula). Physiol Biochem Zool 77:139–148. doi: 10.1086/378923 [DOI] [PubMed] [Google Scholar]
- Tan BH, Pan Y, Dong AN, Ong CE (2017) In vitro and in silico approaches to study cytochrome P450-mediated interactions. J Pharm Pharm Sci 20:319–328 [DOI] [PubMed] [Google Scholar]
- Thoss V, O’Reilly-Wapstra J, Iason GR (2007) Assessment and implications of intraspecific and phenological variability in monoterpenes of Scots pine (Pinus sylvestris) foliage. J Chem Ecol 33:477–491 [DOI] [PubMed] [Google Scholar]
- Ulappa AC, Kelsey RG, Frye GG, et al. (2014) Plant protein and secondary metabolites influence diet selection in a mammalian specialist herbivore. J Mammal 95:834–842. doi: 10.1644/14-mamm-a-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz JL (2012) Understanding the tradeoff between safety and food quality in a mammalian herbivore specialist, the pygmy rabbit. Boise State University [Google Scholar]
- Utz JL, Shipley LA, Rachlow JL, et al. (2016) Understanding tradeoffs between food and predation risks in a specialist mammalian herbivore. Wildl Biol 22:167–173. doi: 10.2981/wlb.00121 [DOI] [Google Scholar]
- White SM, Welch BL, Flinders JT (1982) Monoterpenoid content of pygmy rabbit stomach ingesta. J Range Manag 35:107–109. doi: 10.2307/3898533 [DOI] [Google Scholar]
- Wiggins NL, McArthur C, Davies NW, McLean S (2006) Behavioral responses of a generalist mammalian folivore to the physiological constraints of a chemically defended diet. J Chem Ecol 32:1133–1147. doi: 10.1007/s10886-006-9076-1 [DOI] [PubMed] [Google Scholar]
- Wiggins NL, McArthur C, McLean S, Boyle R (2003) Effects of two plant secondary metabolites, cineole and gallic acid, on nightly feeding patterns of the common brushtail possum. J Chem Ecol 29:1447–1464. doi: 10.1023/A:1024221705354 [DOI] [PubMed] [Google Scholar]