Abstract
Programmed cell death 1 (PD-1) and its ligand (PD-L1) are key physiologic suppressors of the cytotoxic immune reaction. However, to date, the combination of PD1/PD-L1 expression and tumor-infiltrating lymphocytes (TILs) and antigen-presenting cells has been only minimally reported in breast carcinoma, in particular in relation to HER2-positive cases. The goal of this study was to evaluate both cellular tumoral immune reaction and PD-L1/PD1 distribution in HER2-positive cases, as well as any associations with clinical outcome using conventional chemotherapy combined with HER2 blocking. Multicolor immunohistochemical multiplex assays simultaneously demonstrating PD1, PD-L1, and CD8 or PD-L1, CD3, and CD163 were performed on tissue microarrays (TMA) representing 216 pretreatment cases of HER2-positive invasive breast carcinoma. PD-L1 expression was identified in 38 cases (18%), including 12 cases (6%) with PD-L1 labeling of tumor cells and 26 cases (12%) with PD-L1 labeling of immune cells only. Ten of 12 cases with PD-L1 staining of tumor cells showed staining of associated immune cells as well. With this assay method, PD1 was detectable in many fewer cases (6 cases or 3%). PD-L1 expression was positively associated with high Nottingham grade, negative ER and PR, the absence of lymph node metastasis, and high levels of CD8+ cells. The overall survival by univariate analysis was positively associated with lower tumor stage, the absence of lymph node metastasis, PD-L1 expression, and high levels of CD8+ cells. Therefore, our data suggest cytotoxic immune reaction mediated by CD8-positive T cells and PD-L1 expression may predict a better outcome in patients with HER2-positive breast carcinoma managed with conventional chemotherapy and HER2-blocking therapy. These findings recommend clinical trials utilizing checkpoint blocking immunotherapy in some form for HER2-positive breast cancer.
Keywords: breast carcinoma, cytotoxic T cells, HER2, PD1, PD-L1
1 |. INTRODUCTION
Approximately 15%–20% of breast cancers demonstrate HER2 (ERBB2) gene amplification and/or protein overexpression.1–3 Anti-HER2 therapy, such as trastuzumab, is effective against HER2+ tumors and has been incorporated within standard therapy of HER2+ tumors over the past 15 years.4,5 However, both primary and secondary resistances to anti-HER2 agents have been observed in up to 50% of HER2-postive patients.6–8
One key immune response modifier is the PD-1 (programmed cell death 1)-PD-L1 axis, which plays an integral physiologic role in limiting the primary cytotoxic immune response. PD-L1 expression by tumor cells, or the tumor microenvironment, has been reported in a variety of tumors. Results from preclinical studies support the idea that inhibition of PD-L1 and PD-1 axis in the tumor microenvironment may promote tumor regression, and in clinical trials, various agents targeting PD-1 or PD-L1 have demonstrated robust response rates in a variety of tumor types.9–14
Limited data have been reported on the expression of PD-L1 in tumor cells and/or immune cells in breast cancer, but preliminary reports are divergent.15–18 There has been no study to date investigating PD-L1 expression in a pure cohort of HER2-positive breast carcinoma, although published studies have included patients with HER2-positive breast carcinoma in their cohorts. In general, there is agreement that PD-L1 is expressed in higher percentage of HER2-positive and triple-negative breast cancer (TNBC) cases as compared to other breast cancer types.17–19
Recently, the combination of PD-L1 and tumor-infiltrating lymphocytes (TILs) has been investigated in TNBC, and the reported findings confirm the importance of examining both PD-L1 and TILs for clinical outcome prediction.17,20,21 In the current study, we evaluated PD-L1 and a set of other relevant immune markers in relation to their association with clinical outcome in a series of HER2-positive cases.
2 |. MATERIALS AND METHODS
2.1 |. Patients and specimens
This study was approved by the Ohio State University Institutional Review Board. The cohort included 216 HER2-positive cases of operable invasive breast carcinoma treated initially with surgical resection. Clinical and pathological characteristics, including patient’s age, tumor grade, tumor size, lymph node status, hormonal receptor status, HER2 amplification status, and clinical outcome, were collected. All patients were treated with standard adjuvant chemotherapy and HER2-blocking therapy following surgery.
HER2 status was determined by HER2 immunohistochemistry (IHC) and/or HER2 fluorescence in situ hybridization (FISH). The FISH analysis with CEN17 probe was performed using the dual-color Vysis FDA-approved PathVysion HER2 DNA Probe Kit (Abbott Molecular, Des Plaines, IL, USA). The signals for HER2 gene and CEN17 were visualized under a fluorescence microscope using appropriate filters. The average numbers of HER2 and CEN17 signals per cell were recorded for at least 50 cells, and the HER2:CEN17 ratio was calculated for each case. The HER2 IHC results were interpreted by subspecialist breast pathologists, and HER2 FISH results were interpreted by specialist molecular pathologists.
2.2 |. Tissue microarrays (TMAs)
A tumor block representative of primary resected tumor specimen was collected from each case through Tissue Archive Services. A pair of TMAs representative of each tumor was constructed with core size of 1.5 mm at our pathology core facility.
2.3 |. Multicolor multiplex immunohistochemistry and assessment of checkpoint immune system
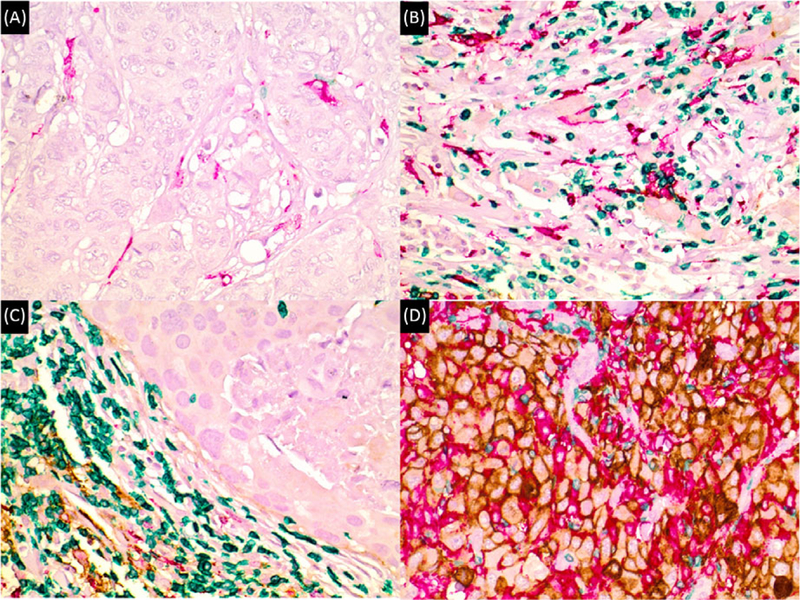
Multicolor multiplex immunohistochemical assays capable of demonstrating colocalization of PD-L1 with either PD1 and CD8 or CD163 and CD3 were performed on TMA sections according to manufacturers’ protocol. The antibodies used were as follow: for PD-L1 clone SP263, rabbit, Ventana; for PD1 clone NAT105, mouse, Ventana; for CD8 clone MRQ26, mouse, Ventana; for CD163 clone SP57, rabbit, Ventana; for CD3 clone 2GV6, rabbit, Ventana. The immunohistochemistry was evaluated with consensus viewing by two pathologists (YH and ZL). A membranous PD-L1 staining of tumor cells or immune cells was considered as specific staining and a membranous PD1 staining in immune cells was considered as specific staining. A positive PD-L1 expression was defined as any membranous staining in ≥1% of tumor cells or immune cells in order to maximize the assay sensitivity for PD-L1-positive cases. Similarly, a positive PD1 expression was also defined as any membranous staining in ≥1% of immune cells. The parameters assessed were as follows: PD-L1 expression in tumor cells (PD-L1 TC), PD-L1expression in immune cells (PD-L1 IC), PD1 expression in immune cells, intratumoral CD8+ immune cells (IT-CD8+), peritumoral CD8+ immune cells (PT-CD8+), intratumoral CD163+ macrophages (IT-CD163+), and peritumoral CD163+ macrophages (PT-CD163+). The cutoff percentage for CD8+ cells and CD163+ cells was set at 10%. The term “macrophage” was used as a surrogate for CD163-positive cells, with the acknowledgment that this marker is sensitive for all antigen-presenting cells, including specialized accessory cells as well as macrophages. Some representative images with different combinations of CD8, CD163, CD3, and PD1/PD-L1 expression are illustrated in Figures 1 and 2.
Figure 1.
Distinct patterns of immune system activity in HER2-positive breast carcinoma, as detected with anti-PD-L1 multiplex immunohistochemical assay 1 (CD8—green; PD1—red; PD-L1—brown). A, “Immune desert” with very rare or no cytotoxic T cells (in green). Note the absence of PD-L1 staining (in brown). B, Primary cytotoxic immune response without PD-L1 expression. Note abundance of cytotoxic T cells (green) within tumor cells. C, Peritumoral immune response (“halo” pattern) with abundant cytotoxic T cells (green) and variable PD-L1 (brown) expression in immune cells at the periphery. D, Peritumoral and intratumoral immune response with strong PD-L1 expression (brown) among tumor cells and cytotoxic T cells (green). Note the absence of PD1 staining in A, B, and D, and very rare PD1 staining in C
Figure 2.
Distinct patterns of immune system activity in HER2-positive breast carcinoma, as detected with anti-PD-L1 multiplex immunohistochemical assay 2 (CD3: green; CD163: red; PD-L1: brown). A, “Immune desert” with few macrophages (red) and only very rare T-cells (green). Note the absence of PD-L1 staining (in brown). B, Primary cytotoxic immune response without PD-L1 expression. Note abundance of T-cells (green) and macrophages (red) associated with tumor cells. C, Peritumoral immune response (“halo” pattern) with abundant T-cells (green), scattered macrophages (red) and variable PD-L1 (brown) expression in immune cells at the periphery. D, Intratumoral immune response with strong PD-L1 expression (brown) among tumor cells, abundant macrophages (red) and T-cells (green)
2.4 |. Statistical analyses
All data were analyzed using SAS version 9.4 for Windows (SAS Institute, Cary, NC, USA). Patient clinical and pathologic characteristics were summarized using descriptive statistics. The relationship between checkpoint immune system and clinicopathological features/patient outcome was assessed using Fisher’s exact test or chi-square test, except that age and tumor size were assessed using Wilcoxon test. Overall survival (OS) was calculated from the date of diagnosis to death from any cause or the date of last follow-up. Patients who were still alive were censored at the last follow-up. Survival curves were estimated using the method of Kaplan-Meier. For the univariate analysis, the association between OS and categorical variables is studied using log-rank test. The association between OS and age was studied using Cox regression model. P values <0.05 were considered statistically significant. The median survival has not been reached overall and in many of the subgroups as well due to the extended survival time of breast cancer patients managed with current modalities. In particular, several subgroups (eg, patients with PD-L1 expression) have not yet suffered a single event (death). Therefore, a multivariate analysis with Cox regression model could not yet be carried out.
3 |. RESULTS
3.1 |. Patient characteristics
A total of 216 surgically resected FFPE primary HER2-positive breast carcinomas were included in the study. There was a median follow-up of 73 months (range 7–162 months) and a median age at diagnosis of 53 years (range 27–88 years). The majority of tumors (63%) were grade 3, while 47% of patients had lymph node metastasis (Table 1).
TABLE 1.
Clinical and pathological characteristics and immune reaction in 216 HER2-positive breast cancers
| Case No. | % | |
|---|---|---|
| Total case # | 216 | |
| Age (years) (median, range) | 53 | 27–88 |
| Race | ||
| Black | 14 | 6.4 |
| Caucasian | 191 | 88.4 |
| Other | 11 | 5.1 |
| Nottingham grade | ||
| 3 | 135 | 62.5 |
| 1/2 | 79 | 36.6 |
| Unknown | 2 | 0.9 |
| Hormone receptor | ||
| ER-positive | 126 | 58.3 |
| PR-positive | 99 | 45.8 |
| T stage | ||
| 1 | 116 | 53.7 |
| 2 | 83 | 38.4 |
| 3 | 13 | 6.0 |
| 4 | 4 | 1.9 |
| Lymph node | ||
| Positive | 102 | 47.2 |
| Surgery | ||
| Lumpectomy | 93 | 43.1 |
| Mastectomy | 123 | 56.9 |
| Margin status | ||
| Positive | 12 | 5.6 |
| PD-L1 | ||
| All PD-L1 | 38 | 17.6 |
| PD-L1 TC | 12 | 5.6 |
| PD-L1 IC only | 26 | 12.0 |
| PD1 | 6 | 2.9 |
| IT-CD8+ | 49 | 22.7 |
| IT-CD163+ | 50 | 23.2 |
| PT-CD8+ | 69 | 31.9 |
| PT-CD163+ | 89 | 41.2 |
ER: estrogen receptor; PR: progesterone receptor; TC: tumor cells; IC: immune cells; IT: intratumoral; PT: paratumoral.
3.2 |. Assessment of PD-L1 expression and immune reaction in 216 HER2-positive breast carcinomas
Among all 216 cases, PD-L1 expression was identified in 38 cases (18%) including 12 cases (6%) with PD-L1 expressed by tumor cells (PD-L1 TCs) and 26 cases (12%) with PD-L1 expressing immune cells (PD-L1 ICs) only. Ten cases showed both PD-L1 TCs and PD-L1 ICs, including 7 cases with PD-L1 TCs, intratumoral PD-L1 ICs and peritumoral PD-L1 ICs, and 3 cases with PD-L1+ TCs and peritumoral PD-L1 ICs. Among 26 cases with PD-L1 ICs only, 24 cases showed peritumoral PD-L1 ICs as well, and two cases showed intratumoral PD-L1 ICs. However, PD1 was expressed in many fewer cases (6 or only 3%) (Table 1).
Next, we examined the association between PD-L1 expression and clinical/pathologic characteristics. PD-L1 expression was positively associated with higher Nottingham grade, negative ER and PR status, and the absence of lymph node metastasis. Furthermore, PD-L1 expression was positively associated with high levels of intratumoral/peritumoral CD8+ and peritumoral CD163+ cells, but not intratumoral CD163+ cells (Table 2).
TABLE 2.
PD-L1 expression and its association with clinical/pathologic characteristics in 216 HER2-positive breast carcinomas
| PD-L1-negative (n = 178) | Total PD-L1+ (n = 38) | P value | |||
|---|---|---|---|---|---|
| Age (years) (median, range) | 54.1 | 27–86 | 53.7 | 28–88 | NS |
| Race | |||||
| Caucasian | 159 | 89.3% | 32 | 84.2% | NS |
| Black | 11 | 6.2% | 3 | 7.9% | |
| Other | 8 | 4.5% | 3 | 7.9% | |
| Tumor type | |||||
| Ductal | 163 | 91.6% | 37 | 97.4% | NS |
| Lobular | 7 | 3.9% | 0 | 0.0% | |
| Other | 8 | 4.5% | 1 | 2.6% | |
| Nottingham grade | |||||
| 1 or 2 | 76 | 42.7% | 3 | 7.9% | <0.0001 |
| 3 | 100 | 56.2% | 35 | 92.1% | |
| Unknown | 2 | 1.1% | 0 | 0.0% | |
| Tumor size (cm) (median, range) | 2.518 | 0.3–13 | 2.08 | 0.7–5 | NS |
| Hormone receptor | |||||
| ER+ | 114 | 64.0% | 12 | 31.6% | 0.0002 |
| PR+ | 89 | 50.0% | 10 | 26.3% | 0.0160 |
| T staging | |||||
| 1 | 94 | 52.8% | 22 | 57.9% | NS |
| 2 | 67 | 37.6% | 16 | 42.1% | |
| 3 | 13 | 7.3% | 0 | 0.0% | |
| 4 | 4 | 2.2% | 0 | 0.0% | |
| Lymph node+ | 90 | 50.6% | 12 | 31.6% | 0.0333 |
| Lymphovascular space invasion+ | 74 | 41.6% | 12 | 31.6% | NS |
| Immune cells | |||||
| IT-CD8+ | 18 | 10.1% | 31 | 81.6% | <0.0001 |
| IT-CD163+ | 37 | 20.8% | 13 | 34.2% | NS |
| PT-CD8+ | 32 | 18.0% | 37 | 97.4% | <0.0001 |
| PT-CD163+ | 55 | 30.9% | 34 | 89.5% | <0.0001 |
| PD1+ | 1 | 0.6% | 5 | 13.2% | <0.0001 |
ER: estrogen receptor; PR: progesterone receptor; IT: intratumoral; PT: paratumoral.
In addition, we compared PD-L1 TC+ cases (n = 12) with only PD-L1 IC+ cases (n = 26). There was no significant difference between these two groups in regard to clinical/pathologic factors, with the exception of age (older in PD-L1 TC+ cases), positive lymph nodes (fewer in PD-L1 TC+ cases), and intratumoral CD−163+ cells (more in PD-L1 TC+ cases; Table 3).
TABLE 3.
Comparison between 12 cases with PD-L1 expression in tumor cells and 26 cases with PD-L1 expression in immune cells only
| PD-L1-TC+a (n = 12) | PD-L1-IC+ only (n = 26) | P value | |||
|---|---|---|---|---|---|
| Age (years) (median, range) | 59.5 | 48–88 | 51 | 28–68 | 0.0337 |
| Race | |||||
| Caucasian | 10 | 83.3% | 22 | 84.6% | NS |
| Black | 1 | 8.3% | 2 | 7.7% | |
| Other | 1 | 8.3% | 2 | 7.7% | |
| Tumor type | |||||
| Ductal | 11 | 91.7% | 26 | 100.0% | NS |
| Lobular | 0 | 0.0% | 0 | 0.0% | |
| Other | 1 | 8.3% | 0 | 0.0% | |
| Nottingham grade | |||||
| 1 or 2 | 1 | 8.3% | 2 | 7.7% | NS |
| 3 | 11 | 91.7% | 24 | 92.3% | |
| Tumor size (cm) (median, range) | 2.31 | 0.7–5 | 1.98 | 0.7–4.2 | NS |
| Hormone receptor | |||||
| ER+ | 3 | 25.0% | 9 | 34.6% | NS |
| PR+ | 2 | 16.7% | 8 | 30.8% | NS |
| T staging | |||||
| 1 | 5 | 41.7% | 17 | 65.4% | NS |
| 2 | 7 | 58.3% | 9 | 34.6% | |
| Lymph node+ | 0 | 0.0% | 12 | 46.2% | 0.0071 |
| Lymphovascular space invasion+ | 3 | 25.0% | 9 | 34.6% | NS |
| Immune cells | |||||
| IT-CD8+ | 10 | 83.3% | 21 | 80.8% | NS |
| IT-CD163+ | 8 | 66.7% | 5 | 19.2% | 0.0133 |
| PT-CD8+ | 12 | 100.0% | 25 | 96.2% | NS |
| PT-CD163+ | 11 | 91.7% | 23 | 88.5% | NS |
| PD1+ | 2 | 16.7% | 3 | 11.5% | NS |
TC: tumor cells; IC: immune cells; ER: estrogen receptor; PR: progesterone receptor; IT: intratumoral; PT: paratumoral.
Two cases with PD-L1 expression in tumor cells (PD-L1 TC), but no PD-L1 expression in immune cells (PD-L1 IC).
3.3 |. The association of PD-L1 expression and immune reaction with overall survival among HER2-positive breast carcinomas
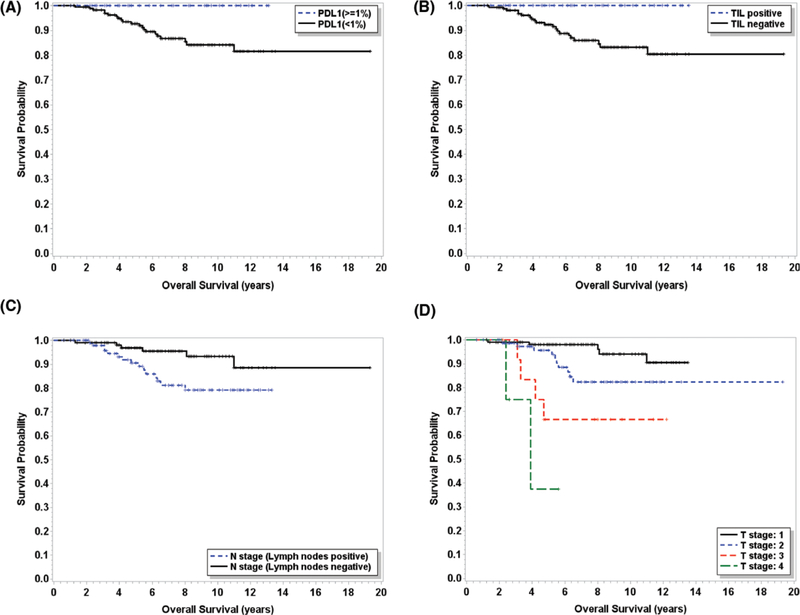
First, univariate analysis was performed to examine the associations between OS and clinical and pathologic characteristics, including age, histologic grades, ER and PR status, T stages, lymph node status, surgery type, surgical margin status, and immune checkpoint expression parameters. The OS was positively associated with lower T stages, the absence of lymph node metastasis, overall PD-L1 expression, intratumoral CD8+ cells, peritumoral CD8+ cells, and the presence of immune reaction, but not other factors such as race, age, histologic grade, surgical margin status, ER/PR status, surgery type, or CD163+ cells (Table 4). Kaplan-Meier curves of OS were also plotted with several significantly associated factors including PD-L1, intratumoral CD8+ cells lymph node status, and T stages, as shown in Figure 3. Median survival has not been reached for many subgroups; therefore, a multivariate analysis with Cox regression model was not performed.
TABLE 4.
Univariate analysis of factors associated with overall survival in 216 HER2-positive breast carcinomas
| Case No. | Death No. | P value | |
|---|---|---|---|
| Race | |||
| Caucasian | 191 | 20 | 0.527 |
| Black | 14 | 0 | |
| Other | 11 | 1 | |
| Age | 216 | 21 | 0.646 |
| Nottingham grade | |||
| 3 | 135 | 12 | 0.992 |
| 1/2 | 79 | 8 | |
| Unknown | 2 | 1 | |
| T stage | |||
| 1 | 116 | 5 | <0.001 |
| 2 | 83 | 10 | |
| 3 | 13 | 4 | |
| 4 | 4 | 2 | |
| Lymph node | |||
| Negative | 114 | 6 | 0.020 |
| Positive | 102 | 15 | |
| Margin | |||
| Negative | 204 | 20 | 0.945 |
| Positive | 12 | 1 | |
| ER | |||
| Negative | 90 | 7 | 0.456 |
| Positive | 126 | 14 | |
| PR | |||
| Negative | 117 | 12 | 0.795 |
| Positive | 99 | 9 | |
| Surgery | |||
| Lumpectomy | 93 | 8 | 0.486 |
| Mastectomy | 123 | 13 | |
| PD-L1 | |||
| Negative | 178 | 21 | 0.045 |
| Positive | 38 | 0 | |
| PD-L1 TC | |||
| Negative | 204 | 21 | 0.383 |
| Positive | 12 | 0 | |
| PD-L1 ICa | |||
| Negative | 182 | 20 | 0.221 |
| Positive | 36 | 1 | |
| PT-CD8 | |||
| Negative | 147 | 19 | 0.036 |
| Positive | 69 | 2 | |
| IT-CD8 | |||
| Negative | 167 | 21 | 0.016 |
| Positive | 49 | 0 | |
| PT-CD163 | |||
| Negative | 127 | 13 | 0.687 |
| Positive | 89 | 8 | |
| IT-CD163 | |||
| Negative | 166 | 18 | 0.509 |
| Positive | 50 | 3 | |
ER: estrogen receptor; PR: progesterone receptor; TC: tumor cells; IC: immune cells; IT: intratumoral; PT: paratumoral.
These 36 cases include 10 cases with both PD-L1+ TCs and PD-L1+ ICs and 26 cases with PD-L1+ ICs only.
Figure 3.
Overall survival plots with different factors. A, Overall survival plot with PD-L1 expression; B, Overall survival plot with intratumoral CD8+ cells (TILs); C, Overall survival plot with lymph node status; and D, Overall survival plot with T stages
4 |. DISCUSSION
To our knowledge, this is the first large study focusing solely on HER2-positive breast carcinomas to assess both tumor cell and stromal immune cell expression of PD-L1 together with other immune biomarkers (PD1, CD8, CD3, and CD163). In our cohort, PD-L1 expression, high CD8+ cell levels, the absence of lymph node metastasis, and lower T stages were all significantly correlated with better OS.
The frequency of PD-L1 expression has not previously been investigated in a cohort of exclusively HER2-positive cases. The reported frequency of PD-L1 expression in HER2-positive breast cancers from diverse breast cancer subtype studies varies from 3.7% to 50% in tumor cells and from 11.0% to 86% in immune cells, using two anti-PD-L1 antibodies (rabbit monoclonal antibody E1L3N from Cell Signaling Technology; rabbit monoclonal antibody SP142 from Spring Bioscience; clone 5H1).19,22 Our study cohort was composed of 216 cases, all HER2-positive. In this cohort, 18% of cases had PD-L1 expression, including 6% of patients with PD-L1 expression in tumor cells and 12% with PD-L1 expression in immune cells but not in tumor cells. The anti-PD-L1 antibodies used in previously reported studies differed from that used in our analysis (SP263 from Ventana). The cutoff for positive PD-L1 expression in tumor cells was chosen at ≥1% for all previous studies, with the exception of one study which used 5%22; and the cutoff for positive PD-L1 expression in immune cells was 5% except for one study which used 1%.23 In our earlier pilot study24 with a different cohort of 123 HER2-positive breast cancers, which consisted of larger samplings (whole section slides), a higher percentage of PD-L1+ cases was encountered (up to 17% in tumor cells and 55% in immune cells). This disparity between our two studies is likely due to a higher likelihood of detecting positive cellularity in much larger samples, as the distribution of PD-L1 staining is often somewhat focal or multifocal rather than uniform.
In our cohort, PD-L1 expression was positively associated with higher Nottingham grade, negative ER/PR, and the absence of lymph node metastases. The positive association between PD-L1 expression and higher Nottingham grade suggests high-grade tumors may harbor higher level of tumor neoantigens, resulting in increased immune response and PD-L1 expression. PD-L1 was also positively associated with the absence of lymph node metastases, suggesting immune reaction with PD-L1 expression may play a role against lymph node metastasis.
In our current study, the PD-L1/PD1 expression was examined in relation to markers for T cells and antigen-presenting cells by using two multicolor multiplex immunohistochemical assays capable of demonstrating colocalization of PD-L1 and PD1 with other cell type-specific markers (CD8, CD3, and CD163). This novel technique has allowed us to accurately localize PD-L1 expression in tumor cells and/or immune cells and PD1 in immune cells, as well to assess CD8+ or CD3+ T-lymphocytes and CD163+ antigen-presenting cells. Our observations confirm the presence of antigen-presenting cells in the vast majority of cases with PD-L1 expression (Tables 1 and 3). This supports recent experimental evidence indicating a pivotal role for macrophage amplification of the PD-L1 immune checkpoint system.25 The low frequency of PD1 staining among immune cells probably relates to the assay method. It has been shown by flow cytometry studies that CD8-positive T cells express PD1 in cell surface distribution, and that this is much weaker expression than that seen in (follicular) helper T cells.26 Such a type of antigen distribution and level of expression is difficult to detect in tissue sections with immunohistochemistry.
There have been conflicting results in the literature as to whether PD-L1 expression is a favorable or adverse prognostic factor for breast cancer patients managed with standard therapy. Most of the published studies have dealt with patients with diverse subtypes of breast cancer, with the exception of several studies which focused on TNBC exclusively.15–22 The discordance of reported results may relate to differences in methods of assessment, numeric thresholds, antibody clones or composition of cohorts. Our data demonstrate that PD-L1 expression is significantly correlated with better OS in univariate analysis, although a multivariate analysis has not been performed in lieu of attainment of median survival. Since PD-L1 mediates checkpoint immune evasion by suppressing the immune response to tumor cells, it has been anticipated that PD-L1 expression might be associated with a poorer prognosis. However, studies of breast carcinoma, along with many other neoplasms including lung carcinoma and melanoma, have demonstrated that PD-L1 expression can be associated with a more favorable prognosis.20,27,28 Intriguingly, in our study, while PD-L1 expression was associated with a better prognosis in the univariate analysis, its expression was strongly associated with high levels of intratumoral/peritumoral CD8+ cells. This is in keeping with the findings from previous studies which have described PD-L1 expression as being associated with prominent TILs.20,27,28 Therefore, PD-L1 expression in the predominance of cases could reflect a strong primary immune response, rather than successful immune evasion. One likely explanation for such findings is that, in these studied tumors, PD-L1 expression by both tumor and immune cells represents an adaptive response to an intensive primary cytotoxic immune attack on tumor neoantigens, rather than a primary, constitutive tumor cell production of PD-L1 based upon genetically determined activation pathways.20 This would render these neoplasms all the more sensitive to either driver pathway (eg, HER2) blocking therapy or to checkpoint immune pathway blocking therapy. The significance of the various patterns of immune response, that is, intratumoral versus peritumoral (“halo”) pattern in relation to checkpoint blocking therapy must await outcome data from immune therapy trials.
The greatest limitation of this study is perhaps the use of TMAs, which may result in a relatively lower percentage of PD-L1-positive cases in our cohort as compared to previously published studies19,22 and to our own recent, yet unpublished study in which PD-L1 expression was examined on the basis of whole section slides and PD-L1 expression was identified in up to 17% of HER2-positive breast cancer cases in tumor cells and 55% in immune cells.24 It has been shown that PD-L1 is expressed variably within and around tumor cellularity, and thus, TMAs may not be representative, raising the possibility of false-negative results. A strength of the study is its relatively large sample size (216 cases). A second limitation is this study’s reliance on a single PD-L1 antibody clone (SP263; Roche Ventana). There are multiple anti-PD-L1 antibodies available commercially; the cutoff values and PD-L1 expressing cells are different among these antibodies. It will be interesting to examine PD-L1 expression in these same specimens using different anti-PD-L1 antibodies. Additionally, the clinical data associated with our cohort were limited to overall survival only. Distal metastasis or local recurrence data were not available for analysis. In our future study, it would be interesting to investigate PD-L1/PD1’s association with distal metastasis or local recurrence to further understand their clinical impact.
In conclusion, our data indicate that both primary immune reaction with CD8+ cells and PD-L1 expression are predictive of outcome for HER2-positive breast cancer managed with standard therapy. Supported by other studies as well, these findings raise hope that such evaluation may play an important role in predicting response to immune checkpoint therapy.17,20,21
Funding information
This study is partly supported by The Ohio State University FAME program.
Footnotes
ETHICAL APPROVAL
This article does not contain any studies with animals performed by any of the authors.
INFORMED CONSENT
Informed consent was obtained from all individual patients included in the study.
CONFLICT OF INTEREST
Y. Hou, L, Wei, M. Lustberg, R. Wesolowski, B. Ramaswamy, A. Parwani, and Z. Li have no financial relationship to disclose. H. Nitta and P. Banks are employees of Ventana Medical Systems, Inc
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 2.Press MF, Pike MC, Chazin VR, et al. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 3.Tandon AK, Clark GM, Chamness GC, et al. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989;7:1120–1128. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. [DOI] [PubMed] [Google Scholar]
- 5.Olson EM. Maximizing human epidermal growth factor receptor 2 inhibition: a new oncologic paradigm in the era of targeted therapy. J Clin Oncol. 2012;30(14):1712–1714. [DOI] [PubMed] [Google Scholar]
- 6.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2- overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 8.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366 (26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribas A Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughn CP, Zobell SD, Furtado LV, et al. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50:307–312. [DOI] [PubMed] [Google Scholar]
- 15.Baptista MZ, Sarian LO, Derchain SF, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84. [DOI] [PubMed] [Google Scholar]
- 16.Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckers RK, Selinger CI, Vilain R, et al. Programmed death ligand 1-expression in triple negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69:25–34. [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dill EA, Gru AA, Atkins KA et al. PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: an assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol. 2017;41(3):334–342. [DOI] [PubMed] [Google Scholar]
- 20.Bae SB, Cho HD, Oh MH, et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer. 2016;19(3):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori H, Kubo M, Yamaguchi R, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8(9):15584–15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7–H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–1493. [DOI] [PubMed] [Google Scholar]
- 24.Hou Y, Nitta H, Wei L, Banks PM, Parwani AV, Li Z. Evaluation of immune reaction and PD-L1 expression with multiplex immunohistochemistry in HER2-positive breast cancer: the association with response to anti-HER2 neoadjuvant therapy. Clin Breast Cancer 2018;18(2):e237–e244. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beatson R, Tajadura-Ortega V, Achkova D, et al. MUC1 modulates the tumor immune microenvironment through the engagement of Siglec-9. Nat Immunol. 2016;17:1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosario Quinones F, Dogan A, Roshal M.Flow characterization ofPD1 expression in tumor infiltrating T-cells in B-cell lymphoproliferative disorders identifies distinct, disease specific patterns. Abstract 1474. United States and Canadian Academy of Pathology Meeting, Seattle, WA, 2016. [Google Scholar]
- 27.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Invest 2014;94:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7–H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med 2012;4(127):127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]