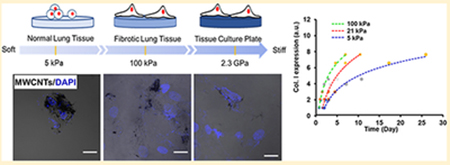
Abstract
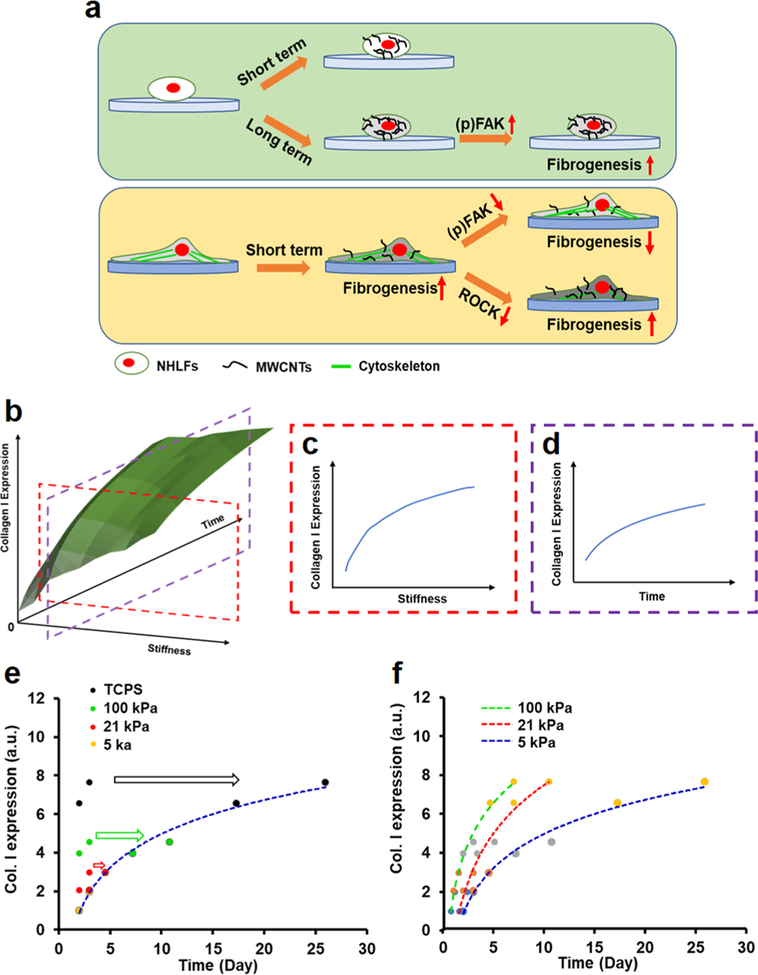
Most living tissues exhibit the specific stiffness, which has been known to have profound influence on cell behaviors, yet how the stiffness affects cellular responses to engineered nanomaterials has not been elucidated. Particularly, discrepancies exist between in vitro and in vivo nanotoxicological studies. Here, we investigated the effects of substrate stiffness on the fibrogenic responses of normal human lung fibroblasts (NHLFs) to multiwalled carbon nanotubes (MWCNTs). NHLFs were grown on polyacrylamide (PAAm) hydrogels with the stiffness comparable to that of human normal and fibrotic lung tissues, and treated with MWCNTs for various time. The fibrogenic responses, including cell proliferation, reactive oxygen species production, and collagen I expression, of NHLFs to MWCNTs were observed to be regulated by substrate stiffness in a time-dependent manner. NHLFs generally were rounded on soft hydrogels and required a long treatment time to exhibit fibrogenic responses, while on stiff hydrogels the cells were well-spread with defined stress fibers and short-time MWCNTs treatment suffciently induced the fibrogenic responses. Mechanistic studies showed that MWCNTs induced fibrogenesis of NHLFs through promoting expression and phosphorylation of focal adhesion kinase (FAK), while attenuating intracellular tension in the cells on stiff gels could increase MWCNTs uptake and thus elevate the induced fibrogenic responses. Moreover, we proposed a time-stiffness superposition principle to describe the equivalent effects of treatment time and substrate stiffness on nanomaterials-induced fibrogenesis, which suggested that increasing substrate stiffness expedited fibrogenesis and shed light on the rational design of in vitro models for nanotoxicological study.
Keywords: nanotoxicity, stiffness, carbon nanotubes, lung fibrogenesis, focal adhesion kinase, intracellular tension
Graphical Abstract
Nanometer-sized materials have been widely applied in electronics, energy, life science, and many other fields, yet the toxicity assessment of the engineered nanomaterials is still challenging. For example, carbon nanotubes (CNTs), a major class of engineered nanomaterials, have been used to develop various types of sensors,1,2 drug delivery carriers,3,4 and tissue engineering scaffolds.5 The occupational and consumer exposure to CNTs can produce diverse biological and toxicological effects and thus result in public health problems.6–8 In vivo studies have shown that CNTs can enter the respiratory tract by inhalation and cause potential damage to lung tissue.9,10 The inhaled CNTs rapidly penetrate the lung lining of mice and induce lung fibrogenesis by stimulating the overproduction of collagen.11 In vitro studies using tissue culture plastic substrates have demonstrated that CNTs can promote the differentiation of fibroblasts to myofibroblasts and induce the fibrogenic responses of fibroblasts.12–14 Although both in vivo and in vitro studies have shown that CNTs can induce lung fibrogenesis, discrepancies exist in the assessment of CNTs toxicity between in vivo and in vitro studies.15 For example, the fibrogenic responses of lung fibroblasts to multiwalled CNTs (MWCNTs) are observed 2 days in vitro,16,17 but it takes weeks or months to develop fibrosis in vivo18 after the MWCNTs exposure. Predictive in vitro models are thus necessary to assess the toxicity of nanomaterials in vivo.15
The significant deviation of the in vitro cellular responses to nanomaterials from the in vivo events is attributed to the failure to recapitulate the in vivo microenvironmental characteristics in current in vitro models. Among the microenvironmental characteristics, matrix stiffness plays a critical role in the development and maintenance of the normal function of tissues and organs,19,20 the alteration in tissue stiffness could be associated with certain types of diseases.21,22 For instance, the Young’s modulus of the normal lung tissues is 1–5 kPa, which is elevated to 20–100 kPa in the fibrotic lung tissues.23–25 The substrate stiffness has also been shown to be a potent regulator of cell behaviors in vitro. For example, NIH 3T3 fibroblasts are well-spread on stiff (>180 kPa) hydrogels with mature stress fibers, whereas they exhibit a rounded shape with less prominent actin bundles on soft (~5 kPa) gels.26,27 On the stiff (100 kPa) gels, the fibroblasts also exhibit higher contractility, proliferation rate, and myofibroblast differentiation effciency than those on the soft (5 kPa) gels.28 Although previous studies have suggested that the CNTs could induce substantial lung fibrogenesis,12,16,29–31 less effort has been made to explore the stiffness effects on CNTs-induced lung fibrogenesis. It is worth mentioning that current in vitro models are mainly based on supraphysiologically stiff tissue culture polystyrene (TCPS) of 2.3 GPa in stiffness, which is several orders of magnitude higher than the stiffness of most human tissues.32 Therefore, it is highly desirable to assess the toxicity of nanomaterials using the substrate of physiologically relevant stiffness.
In this study, we investigated the effects of substrate stiffness on the fibrogenic responses of normal human lung fibroblasts (NHLFs) to MWCNTs. Polyacrylamide (PAAm) gels with the stiffness comparable to the normal and fibrotic lung tissues as well as TCPS as a control were used as cell culture substrates. NHLFs are associated with collagen production during lung fibrogenesis,12,33 and hence, their fibrogenic responses to MWCNTs on these substrates were assessed. Moreover, we explored the underlying mechanism of how the substrate stiffness affected cellular responses to MWCNTs. We further proposed a time-stiffness superposition principle to describe the equivalent effects of substrate stiffness and treatment time on the fibrogenic responses of the fibroblasts to MWCNTs.
Results and Discussion.
Substrate Stiffness Altered Cell Spreading and MWCNTs Uptake of NHLFs.
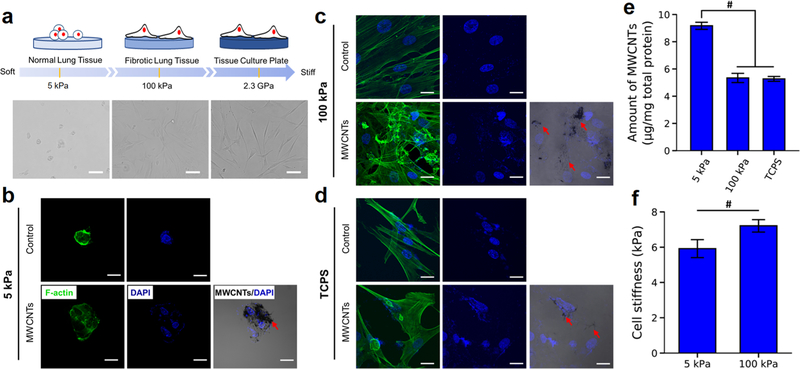
The stiffness of PAAm gels was controlled by the ratio of acrylamide to bis-acrylamide, and measured by using the atomic force microscopy (AFM) (Table S1). The soft (4.9 ± 0.5 kPa, labeled as 5 kPa) and stiff (100.8 ± 2.1 kPa, labeled as 100 kPa) gels were used to mimic the normal (1–5 kPa) and fibrotic (20–100 kPa) lung tissues, respectively. NHLFs displayed a round shape on 5 kPa gels with the significantly smaller size and lower aspect ratio than the cells on 100 kPa gels and the TCPS, where the cells were spread out with distinct stress fibers (Figures 1a-d and S1). The cell morphologies were similar to what were observed in the fibroblasts grown on the polydimethylsiloxane (PDMS) and PAAm substrates with the stiffness closed to 5 and 100 kPa.26,27
Figure 1.
Substrate stiffness effects on the morphology, MWCNTs uptake, and cell stiffness of NHLFs. (a) Illustration and bright field images of NHLFs on substrates of various stiffnesses. The scale bars are 100 μm. (b−d) Confocal fluorescent images of NHLFs on 5 kPa (b) and 100 kPa (c) gels and TCPS (d) with or without MWCNTs treatment. Green, F-actin; blue, nuclei. The overlay images showed the MWCNTs (indicated by the red arrows) localized in the cells. The scale bars are 20 μm. (e) Quantification of MWCNTs uptake in NHLFs on different substrates (n = 3). The total amount of MWCNTs was normalized by total protein concentration. (f) AFM measurement of the stiffness of NHLFs on soft and stiff gels (n > 50). #p < 0.05.
The cells were treated with MWCNTs at the concentration of 1 μg/mL for 3 days. The MWCNTs at this dosage induced collagen I overexpression of NHLFs without causing cell death, as shown in Figure S2and our previous work.17 The MWCNTs used in this study were characterized and reported previously.17,31 The MWCNTs were around 12 nm in diameter and several micrometers in length with less than 1% of contaminants. The major type of metal residues was iron (Fe), which was about 0.32% (w/w). MWCNTs were dispersed in phosphate buffered saline containing bovine serum albumin at the concentration of 1 mg/mL and further diluted in the culture medium to obtain the desired concentrations. The MWCNTs treatment did not result in significant change in the cell morphology, compared with the control−no MWCNTs treatment (Figures 1b-d and S1). Interestingly, the overlay images in the MWCNTs groups exhibited a higher MWCNTs uptake in the cells on the soft gels than those on the stiff gels and the TCPS. The cellular uptake of MWCNTs was further quantified by measuring the turbidity of the cell lysate with referring to the standard curve (Figure S3).34 The results verified that the MWCNTs uptake in the cells on 5 kPa gels was significantly higher than those of the cells grown on 100 kPa gels and TCPS (Figure 1e).
Moreover, the AFM measurement of the stiffness of NHLFs on these gels revealed that the cells on 100 kPa gels were significantly stiffer than the cells on 5 kPa gels (Figure 1f). We postulated that the softer cells had higher MWCNTs accessibility than stiffer cells. Our postulation was supported by previous studies,35,36 which suggested that the higher membrane tension of the cells on stiff substrates resisted nanoparticles from entering the cells compared to the cells on soft substrates, and the soft cytoskeleton facilitated nanoparticle uptake.
Substrate Stiffness Modulated MWCNTs-Induced Fibro-genesis of NHLFs.
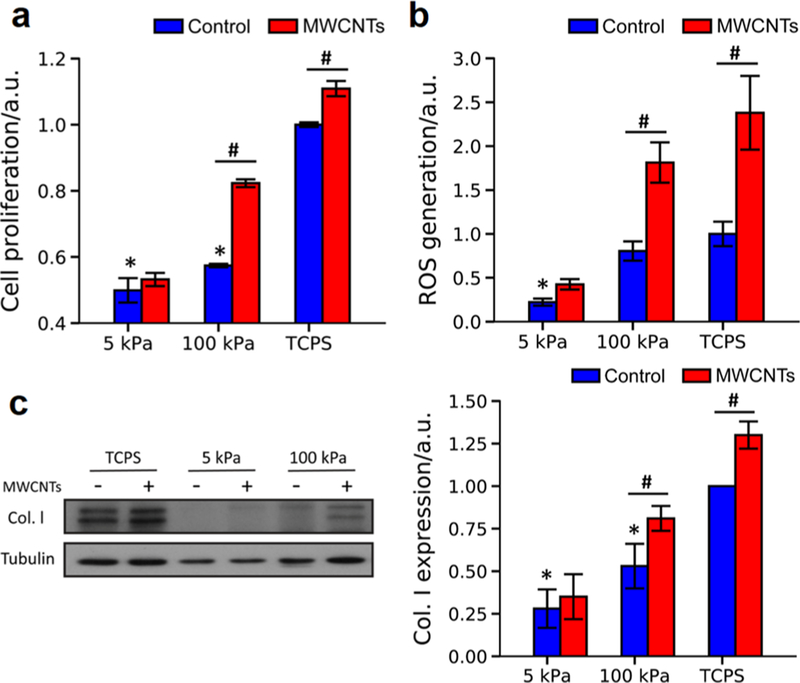
Increased cell proliferation, reactive oxygen species (ROS) generation, and collagen I expression are the hallmarks of lung fibrosis, we herein examined these events to evaluate the effects of substrate stiffness on MWCNTs-induced fibrogenesis of NHLFs. In the absence of MWCNTs (i.e., the control groups), the cell proliferation, ROS generation, and collagen I expression of NHLFs increased with the increase in the substrate stiffness (Figure 2). Evidently, the behaviors of the fibroblasts grown on TCPS dramatically deviated from those of the cells on the gels of pathophysiologically relevant stiffness.
Figure 2.
Fibrogenic responses of NHLFs to MWCNTs on PAAm gels and TCPS. (a) Cell proliferation (n = 3), (b) ROS generation (n = 10), and (c) collagen I expression (n = 3) were measured to assess the fibrogenic responses of NHLFs to MWCNTs. Control: without MWCNTs treatment. MWCNTs: with MWCNTs treatment. All the data were normalized to the mean value of the TCPS group without MWCNTs treatment. Negative and positive signs in (c) indicated the cells were treated without and with MWCNTs, respectively. *significant difference (p < 0.05) from the TCPS controls (no MWNCTs treatment); #significant difference (p < 0.05) between groups.
Treated with MWCNTs, the cells also exhibited the stiffness-dependent fibrogenic responses to MWCNTs. Strikingly, the cells grown on 5 kPa gels did not show a significant increase in the proliferation, ROS generation, or collagen I expression with MWCNTs exposure. Yet, on 100 kPa gels and TCPS, these fibrogenic responses of NHLFs significantly increased with the MWCNTs treatment. Notably, the MWCNTs-induced fibrogenic responses on the 100 kPa gels were more profound than the TCPS controls. The MWCNTs treatment increased cell proliferation and collagen expression on 100 kPa gels by 43.4% and 51.5%, respectively, higher than those on TCPS (10.9% and 29.6%, respectively; Figure 2a,c). The MWCNTs-induced increases in ROS generation of NHLFs on 100 kPa gels, and TCPS were similar (Figures 2b and S4).
Our finding that the increase in substrate stiffness enhanced the cell proliferation, ROS generation, and collagen expression of lung fibroblasts agreed with the previous study,37 which showed that the mRNA expression of collagen I α1 in the lung fibroblasts cultured on PAAm gels of fibrotic lung tissue-like stiffness was significantly higher than that of the cells on the gels of normal lung tissue-like stiffness. As expected, MWCNTs-induced fibrogenesis of NHLFs was also regulated by the substrate stiffness. On 5 kPa gels, despite the higher level of MWCNTs uptake observed, the fibrogenic responses of NHLFs were not sensitive to MWNCTs. However, NHLFs grown on 100 kPa gels displayed more severe fibrogenic responses to MWCNTs than those on TCPS. Previously, we evaluated the effects of substrate nanotopography on the fibrogenic response of NHLFs to the MWCNTs and found that the cells on PDMS nanopillars of 500 nm in diameter and 560 nm in height exhibited more pronounced fibrogenic responses than the cells on PDMS flat substrates.17,38 The nanopillars had a calculated stiffness of about 280 kPa,39 comparable to the 100 kPa gels in the present study. The cells on PDMS flat substrates showed stiffer cytoskeleton and nucleus, accompanied by elevated fibrogenic responses; and the MWCNTs treatment only showed limited increases in induced fibrogenic responses of NHLFs.17 While the distinct MWCNTs-induced fibrogenic responses of NHLFs were observed on the substrates of pathophysiologically different stiffness, it was important to understand how mechanotransduction affected the induced fibrogenesis of the fibroblasts.
FAK Regulated MWCNTs-Induced Fibrogenic Responses of NHLFs.
Substrate stiffness regulates cell behaviors through mediating integrin activation, focal adhesion assembly, cytoskeleton organization, and nuclear deformation.40 We herein examined focal adhesion kinase (FAK, a key signaling kinase in focal adhesions that link integrins and cytoskeleton), F-actin, and intracellular localization of yes-associated protein (YAP, a recognized cellular mechanosensor).
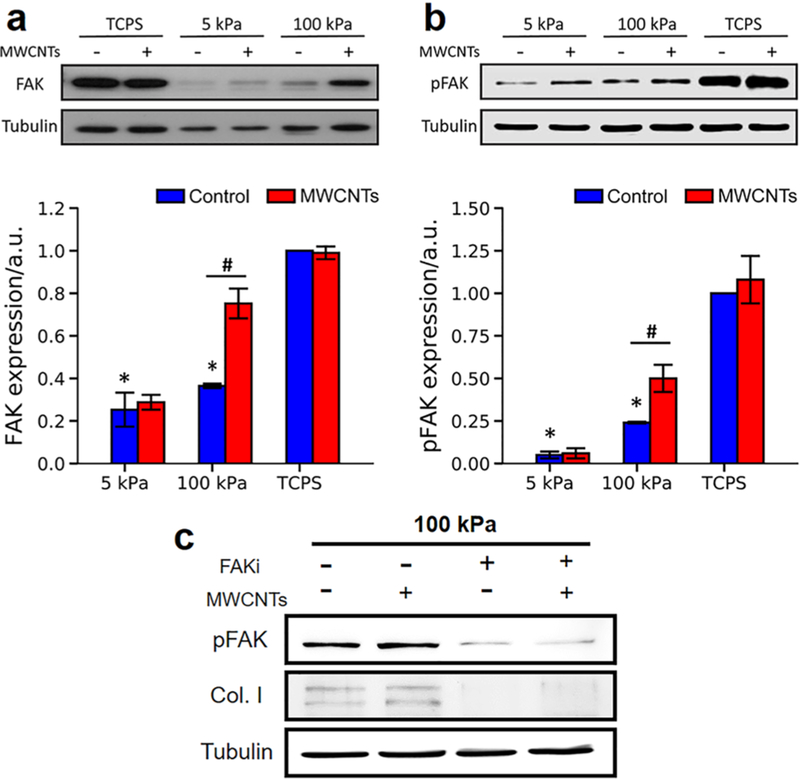
Similar trends were observed in F-actin expression and YAP intracellular localization in NHLFs on these substrates in response to the MWCNTs treatment. NHLFs on 5 kPa gels expressed a significantly lower level of F-actin than those on 100 kPa gels and TCPS, whereas the MWCNTs treatment did not significantly influence the F-actin expression for all these substrates (Figure S5). The percentage of cells with nuclear YAP on 5 kPa gels was also significantly lower than that of the cells on 100 kPa gels and TCPS, and no significant change in YAP intracellular localization was observed in all groups with or without MWCNTs treatment (Figure S6). Intriguingly, our Western blot analysis showed that, without the MWCNTs treatment, the expression and phosphorylation (tyrosine-397) of FAK increased when the substrate stiffness increased, and the levels of FAK and phosphorylated FAK (pFAK) in the cells on 5 and 100 kPa gels were significantly lower than those on on the substrate stiffness. No significant change in the FAK expression and phosphorylation was detected in the cells on 5 kPa gels or TCPS after MWCNTs treatment; however, MWCNTs significantly upregulated the FAK expression and phosphorylation in NHLFs on 100 kPa gels. To further verify the role of FAK in stiffness effects on the MWCNTs-induced fibrogenic responses of NHLFs, we treated the cells with FAK inhibitor (FAK Inhibitor 14, 5 μM) and found that the FAK inhibitor treatment profoundly downregulated the level of pFAK and collagen I, compared to the nontreated groups (Figure 3c). This indicated that inhibition of FAK expression and phosphorylation attenuated the MWCNTs-induced fibro-genesis.
Figure 3.
Role of FAK in MWCNTs-induced fibrogenic responses of NHLFs. Expression of (a) FAK (n = 3) and (b) phosphorylated FAK (pFAK) (n = 3) in NHLFs on different substrates in response to the MWCNTs treatment. Control: without MWCNTs treatment. MWCNTs: with MWCNTs treatment. All the data were normalized to the mean value of the TCPS group without MWCNTs treatment. (c) Expression of pFAK and collagen I in NHLFs on 100 kPa gels with MWCNTs and FAK inhibitor treatment. Tubulin was used as the loading control. Negative and positive signs indicated the cells were treated without and with MWCNTs or FAK inhibitor. *significant difference (p < 0.05) from the TCPS controls (no MWNCTs treatment); #significant difference (p < 0.05) between groups.
The expression and phosphorylation of FAK are associated with the mechanotransduction of cells in response to the extracellular physical signals such as stiffness and regulate many cell behaviors such as cell proliferation and differentiation.41,42 It has been reported that substrate stiffness regulated the idiopathic pulmonary fibrogenesis through activating the FAK/ Akt pathway,37 and increasing substrate stiffness enhanced the FAK expression and phosphorylation in different types of cells.42–44 Our results showed that the FAK expression and phosphorylation in NHLFs increased with the increased substrate stiffness (Figure 3), concurring with previous studies. Moreover, our results demonstrated that the MWCNTs treatment significantly upregulated FAK expression and phosphorylation in the cells cultured on 100 kPa gels, but not on 5 kPa gels or TCPS. Similarly, a previous study showed that single-walled CNTs (SWCNTs) treatment for 5 days did not alter the FAK expression or phosphorylation in human epithelial cells cultured on TCPS.45 Yet, the MWCNTs treatment at a high concentration (50 μg/mL) was shown to reduce the mRNA expression of FAK in human dermal fibroblasts on TCPS.46 The difference between this study and our observation was likely to be caused by a much lower MWCNTs concentration (1 μg/mL) in the present study. Our results manifested the important role of FAK expression and phosphorylation in the mechanosensitive fibrogenic responses of NHLFs to MWCNTs. Further, we explored the underlying mechanism of how the MWCNTs-induced fibrogenic responses were affected by the substrate stiffness at the pathophysiological range.
Time-Dependent MWCNTs-Induced Lung Fibrogenesis.
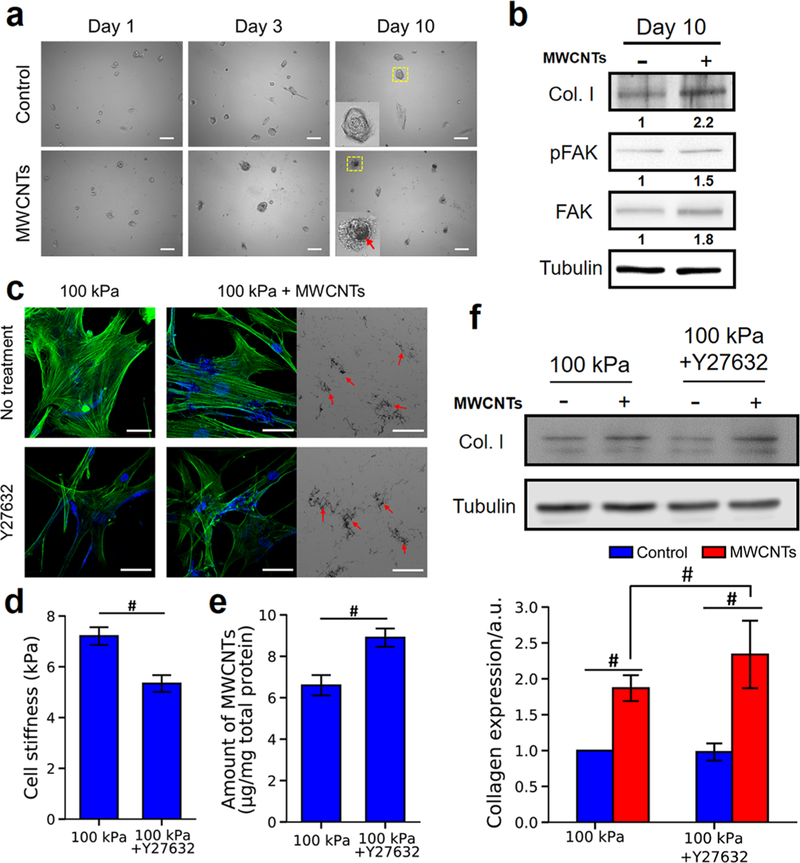
Animal studies showed that the lung fibrogenesis induced by the inhaled MWCNTs was a subchronic or chronic process.11,18,47 Although MWCNTs treatment did not induce significant fibrogenic responses of NHLFs on the normal lung-mimicking (5 kPa) substrates after 3 days of exposure (Figure 2), we extended the treatment time to 10 days without noticing evident cell death and evaluated the expression of collagen I, FAK, and pFAK in NHLFs on 5 kPa gels in response to MWCNTs. Shown in Figure 4a, increased MWCNTs uptake was observed in NHLFs over time. At 10 days post-MWCNTs treatment, NHLFs expressed a higher level of collagen I compared with the control (i.e., without MWCNTs treatment). Moreover, the increased levels of both expression and phosphorylation of FAK were detected as well (Figure 4b), which further confirmed that FAK played an important role in regulating the fibrogenic responses of NHLFs to MWCNTs.
Figure 4.
Effects of treatment time and ROCK inhibition on MWCNT-induced fibrogenesis of NHLFs. (a) Bright field images of NHLFs on 5 kPa gels with or without MWCNTs treatment at Days 1, 3, and 10. The insets were enlarged boxed areas, and the red arrow pointed to the MWCNTs. Scale bars are 100 μm. (b) Expression of collagen I, pFAK, and FAK in NHLFs on 5 kPa gels with (+) or without (−) MWCNTs treatment for 10 days. Tubulin was used as the loading control. The relative protein band intensities were normalized to the tubulin control and presented underneath the protein bands. (c) Confocal images of NHLFs on 100 kPa gels with or without Y27632 treatment. Y27632 (10 μM) was added 2 h before NHLFs were treated with MWCNTs for 3 days. Green, F-actin; blue, nuclei. The bright field images showed the MWCNTs localized in the cells. The red arrows indicated the MWCNTs. Scale bars are 50 μm. (d) Stiffness of NHLFs on 100 kPa gels with or without Y27632 treatment (n > 50). (e) Quantification of the MWCNTs uptake of NHLFs on 100 kPa gels with or without Y27632 treatment (n = 3). (f) Western blot analysis of collagen I in NHLFs (n = 3). Tubulin was used as the loading control. Negative and positive signs indicated the cells were treated without and with MWCNTs. #significant difference (p < 0.05) between groups.
Intracellular Tension Modulated Fibrogenic Responses of NHLFs to MWCNTs.
Because the fibroblasts grown on 100 kPa gels were stiffer and exhibited a lower MWCNTs uptake (Figure 1e,f), yet more significant fibrogenic responses to MWCNTs than the cells on 5 kPa gels (Figure 2), we postulated that the intracellular tension influenced the fibrogenic responses of NHLFs to MWCNTs. We therefore treated the cells on 100 kPa gels with a Rho kinase (ROCK) inhibitor (Y27632, 10 μM) to assess the effects of intracellular tension on MWCNTs-induced fibrogenic responses. After treated with Y27632, the actin stress fibers were attenuated, and the cells exhibited a lower stiffness (Figure 4c,d), both suggesting a lower intracellular tension after Y27632 treatment. A higher MWCNTs uptake was observed in the Y27632 treated cells (Figure 4c) compared to the no-treatment group, which was further confirmed quantitatively (Figure 4e). The observations suggested that a lower intracellular tension favored MWCNTs uptake, being consistent with the previous finding (Figure 1). Intriguingly, while the Y27632 treatment did not cause any evident change in collagen I expression in NHLFs in the control groups, the MWCNTs exposure resulted in ~2.4-fold increase in collagen I expression in the Y27632-treated cells, significantly higher than the ~1.8-fold increase in the cells without the ROCK inhibition (Figure 4f). ROCK was recently shown to be related to the uptake effciency of cells.48,49 For example, ROCK inhibition by Y27632 promoted the microglial uptake of dextran.48 Our results suggested that reducing the ROCK activity and consequently lowering the intracellular tension could enhance the cellular accessibility to MWNCTs and thus elevate the fibrogenic responses of NHLFs to MWCNTs.
Time-Stiffness Superposition of MWCNTs-Induced Lung Fibrogenesis.
Our results suggested the equivalent effects of substrate stiffness and MWCNTs treatment time on the fibrogenic responses of the fibroblasts to MWCNTs. As illustrated in Figure 5a, on the normal lung-mimicking (5 kPa) substrates a short-term MWCNTs treatment did not induce evident fibrogenic responses, yet extending the treatment time increased the MWCNTs uptake and subsequently induced the fibrogenesis through promoting expression and phosphorylation of FAK. These observations were in line with the subchronic or chronic process of MWCNTs-induced fibro-genesis in the animal studies. By contrast, on the fibrotic lung-mimicking (100 kPa) substrates a short-term MWCNTs treatment was sufficient to induce the acute fibrogenic responses of NHLFs through the FAK and ROCK signaling cascades. Attenuating intracellular tension could increase the MWCNTs uptake and thus elevate the fibrogenic responses. It was reasonable to speculate that, while MWCNTs induced collagen overproduction and consequently stiffened the substrate, the fibrogenesis would be expedited through the positive feedback, by which the stiffer substrate accelerated collagen production.
Figure 5.
Time-stiffness superposition of MWCNTs-induced lung fibrogenesis. (a) Mechanosensitive fibrogenic responses of NHLFs to MWCNTs. (b) Three-dimensional plot of the stiffness and time effects on MWCNTs-induced collagen I expression. Collagen I expression of NHLFs increased as the function of (c) substrate stiffness while the treatment time was fixed and (d) MWCNTs treatment time while the substrate stiffness was fixed. (e) Generation of a master curve referring to the 5 kPa gel. (f) Master curves generated by referring to the substrate of a specific stiffness.
The fibrogenic responses such as collagen I expression were observed as the functions of substrate stiffness and treatment time as illustrated in Figure 5b. Upon the same MWCNTs treatment time, increasing the substrate stiffness would enhance collagen I expression of NHLFs (Figure 5c). Similarly, when the cells were grown on the substrates of the same stiffness, increasing the MWCNTs treatment time would increase the collagen I expression (Figure 5d). Both the mechanistic understanding and experimental assessment suggested that the MWCNTs treatment time and substrate stiffness had equivalent influences on the MWCNTs-induced fibrogenesis of NHLFs.
To correlate the equivalent effects of MWCNTs treatment time and substrate stiffness on the fibrogenic responses of NHLFs to MWCNTs, we proposed a time-stiffness superposition principle. This principle is analogous to the time− temperature superposition principle in polymer rheology, which has been applied to describe the equivalent effects of time and temperature on the viscoelastic properties of polymers, and thus the viscoelastic data measured at one temperature could be superimposed on the data measured at a different temperature by shifting one set of data along the time axis.50 By the proposed time-stiffness superposition principle, a set of MWCNTs-induced fibrogenic responses of the fibroblasts measured on one substrate of stiffness E could be horizontally shifted by using a shift factor αE upon a reference stiffness E0 as follows:
where C1 and C2 were constants. The superposition process from the normalization of fibrogenic response data to the estimation and determination of αE as well as C1 and C 2 were described in Chart S1and the “Time-stiffness superposition of MWCNT-induced fibrogenic responses of NHLFs” section in the Supporting Information. After determining αEs, C1, and C 2, a “master curve” could be generated to predict the MWCNTs-induced fibrogenic response of interest at a specific stiffness over a broad time scale.
We herein demonstrated the time-stiffness superposition principle by taking example of the collagen I production as the fibrogenic response of interest. In addition to the PAAm gels of 5 and 100 kPa in stiffness and TCPS, NHLFs were also grown on 21 kPa gels that mimicked the fibrotic lung tissue at the early stage. The cells were treated with MWCNTs for 2 and 3 days, and the collagen I expression was measured by Western blotting. MWCNTs treatment for 3 days induced a higher level of collagen I expression than that of 2 days of treatment on all substrates (Figure 5e). By taking the collagen I production on the 5 kPa gels (E0 = 4.9 kPa) as the reference, the measurement on different substrates was shifted with a shift factor specific to the substrate E along the time axis. By applying the least-squares method, the αEs were determined (see detailed description in the Supporting Information, Figures S7-10and Tables S3,S4), and then the fibrogenesis data on the reference substrate and the shifted data sets from other substrates formed a master curve as indicated by the broken curve (Figure 5e). By following the same process, the master curves that referred to the substrate of a different stiffness, for instance 21 and 100 kPa gels, were generated (Figure 5f). Figure 5f showed that, at the same MWCNTs treatment time, increasing substrate stiffness would enhance collagen I production, and a longer time was required to reach the same level of collagen I expression on a softer substrate than a stiffer substrate.
While the time-stiffness superposition principle was proposed to correlate the time- and stiffness-dependent MWCNTs-induced lung fibrogenesis, it was likely to be applicable to other engineered nanomaterials and stiffness relevant fibrosis. Moreover, other cell types such as lung epithelial cells and macrophages were involved in lung fibrogenesis upon MWCNTs exposure. The lung epithelial cells underwent the epithelial−mesenchymal transition (EMT) after MWCNTs treatment and transdifferentiated to myofibroblasts, therefore promoting fibrogenesis.13,51 The EMT could be driven by increasing substrate stiffness.52 The macrophages could also be activated by MWCNTs treatment and stiff substrates to promote pulmonary fibrosis.53,54 It is reasonable to speculate that the time-stiffness superposition principle is applicable to those events. The validation of the principle in those applications required further investigation.
Conclusion.
This study investigated the effects of substrate stiffness in the pathophysiological range on the fibrogenic responses of NHLFs to MWCNTs. The substrate stiffness profoundly affected cell spreading and cell stiffness and, consequently, the uptake of MWCNTs. The MWCNTs-induced fibrogenic responses were not evident on the normal lung tissue mimicking hydrogels (5 kPa) upon short-term exposure, yet gradually increased for extended time. However, the induced fibrogenesis was significant on the fibrotic lung tissue-mimicking gels (100 kPa). The mechanical study suggested that the MWCNTs induced lung fibrogenesis through the FAK and ROCK signaling cascades. Based on the demonstrated equivalent effects of substrate stiffness and treatment time on the fibrogenic responses of the fibroblasts to MWCNTs, we proposed a time-stiffness superposition principle to correlate these dependences and to predict MWCNTs-induced fibrogenic responses over a broad time scale. Moreover, the superposition principle provided insight into the design of pathophysiologically relevant in vitro platforms for nanotoxicology study.
Supplementary Material
ACKNOWLEDGMENTS
This work is partly supported by National Institutes of Health R15GM122953 (to Y.Y.) and R01-EB018857 (to Y.R.).
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the offcial position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications websiteat DOI: 10.1021/acs.nanolett.9b01943.
Materials and methods. Table S1: Preparation and characterization of PAAm hydrogels. Table S2: Information and dilution of antibodies. Figure S1: Influence of substrate stiffness on the morphology of NHLFs with or without MWCNTs treatment. Figure S2: Influence of MWCNTs dosage on the proliferation of NHLFs. Figure S3: Standard curve of O.D. (640 nm) value of MWCNTs at different concentrations. Figure S4: Fluorescent images of NHLFs on different substrates with the staining of DCFDA to detect the generated ROS inside the cells with or without MWCNTs treatment. Figure S5: Western blot analysis of the F-actin expression in NHLFs on different substrates with or without MWCNTs treatment. Figure S6: YAP intracellular localization in NHLFs on different substrates. Detailed explanation of the time-stiffness superposition principle of MWCNT-induced fibrogenic responses of NHLFs (Tables S3, S4 and Figures S7-S10) (PDF)
REFERENCES
- (1).Yang N; Chen X; Ren T; Zhang P; Yang D Carbon Nanotube Based Biosensors. Sens. Actuators, B 2015, 207, 690–715. [Google Scholar]
- (2).Schroeder V; Savagatrup S; He M; Lin S; Swager TM Carbon Nanotube Chemical Sensors. Chem. Rev 2019, 119 (1), 599–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Liu Z; Tabakman S; Welsher K; Dai H Carbon Nanotubes in Biology and Medicine: In Vitro and in Vivo Detection, Imaging and Drug Delivery. Nano Res 2009, 2 (2), 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wang K; Huang Q; Qiu F; Sui M Non-Viral Delivery Systems for the Application in P53 Cancer Gene Therapy. Curr. Med. Chem 2015, 22 (35), 4118–4136. [DOI] [PubMed] [Google Scholar]
- (5).Gorain B; Choudhury H; Pandey M; Kesharwani P; Abeer MM; Tekade RK; Hussain Z Carbon Nanotube Scaffolds as Emerging Nanoplatform for Myocardial Tissue Regeneration: A Review of Recent Developments and Therapeutic Implications. Biomed. Pharmacother 2018, 104, 496–508. [DOI] [PubMed] [Google Scholar]
- (6).Helland A; Wick P; Koehler A; Schmid K; Som C Reviewing the Environmental and Human Health Knowledge Base of Carbon Nanotubes. Environ. Health Perspect 2007, 115 (8), 1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Shvedova AA; Kisin ER; Porter D; Schulte P; Kagan VE; Fadeel B; Castranova V Mechanisms of Pulmonary Toxicity and Medical Applications of Carbon Nanotubes: Two Faces of Janus? Pharmacol. Ther 2009, 121 (2), 192–204. [DOI] [PubMed] [Google Scholar]
- (8).He X; Kiratipaiboon C; Porter DW; Rojanasakul LW; Dinu CZ; Wang K; Yang Y; Rojanasakul Y Predicting Nanotube Fibrogenicity through Stem Cell-Mediated Fibroblast Focus and Spheroid Formation. Nano Lett. 2018, 18 (10), 6500–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Firme CP III; Bandaru PR Toxicity Issues in the Application of Carbon Nanotubes to Biological Systems. Nano-medicine 2010, 6 (2), 245–256. [DOI] [PubMed] [Google Scholar]
- (10).Muller J; Huaux F; Moreau N; Misson P; Heilier J-F; Delos M; Arras M; Fonseca A; Nagy JB; Lison D Respiratory Toxicity of Multi-Wall Carbon Nanotubes. Toxicol. Appl. Pharmacol 2005, 207 (3), 221–231. [DOI] [PubMed] [Google Scholar]
- (11).Ryman-Rasmussen JP; Cesta MF; Brody AR; Shipley-Phillips JK; Everitt JI; Tewksbury EW; Moss OR; Wong BA; Dodd DE; Andersen ME Inhaled Carbon Nanotubes Reach the Subpleural Tissue in Mice. Nat. Nanotechnol 2009, 4 (11), 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang L; Mercer RR; Rojanasakul Y; Qiu A; Lu Y; Scabilloni JF; Wu N; Castranova V Direct Fibrogenic Effects of Dispersed Single-Walled Carbon Nanotubes on Human Lung Fibroblasts. J. Toxicol. Environ. Health, Part A 2010, 73 (5−6), 410–422. [DOI] [PubMed] [Google Scholar]
- (13).Wang P; Wang Y; Nie X; Braïni C; Bai R; Chen C Multiwall Carbon Nanotubes Directly Promote Fibroblast−Myofibroblast and Epithelial−Mesenchymal Transitions through the Activation of the Tgf-B/Smad Signaling Pathway. Small 2015, 11 (4), 446–455. [DOI] [PubMed] [Google Scholar]
- (14).He X; Kiratipaiboon C; Porter DW; Rojanasakul LW; Dinu CZ; Wang K; Yang Y; Rojanasakul Y Predicting Nanotube Fibrogenicity through Stem Cell-Mediated Fibroblast Focus and Spheroid Formation. Nano Lett 2018, 18 (10), 6500–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Clippinger AJ; Ahluwalia A; Allen D; Bonner JC; Casey W; Castranova V; David RM; Halappanavar S; Hotchkiss JA; Jarabek AM Expert Consensus on an in Vitro Approach to Assess Pulmonary Fibrogenic Potential of Aerosolized Nanomaterials. Arch. Toxicol 2016, 90 (7), 1769–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mishra A; Rojanasakul Y; Chen BT; Castranova V; Mercer RR; Wang L Assessment of Pulmonary Fibrogenic Potential of Multiwalled Carbon Nanotubes in Human Lung Cells. J. Nanomater 2012, 2012, 4. [Google Scholar]
- (17).Wang K; He X; Linthicum W; Mezan R; Wang L; Rojanasakul Y; Wen Q; Yang Y Carbon Nanotubes Induced Fibrogenesis on Nanostructured Substrates. Environ. Sci.: Nano 2017, 4 (3), 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Porter DW; Hubbs AF; Mercer RR; Wu N; Wolfarth MG; Sriram K; Leonard S; Battelli L; Schwegler-Berry D; Friend S Mouse Pulmonary Dose-and Time Course-Responses Induced by Exposure to Multi-Walled Carbon Nanotubes. Toxicology 2010, 269 (2–3), 136–147. [DOI] [PubMed] [Google Scholar]
- (19).Handorf AM; Zhou Y; Halanski MA; Li W-J Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).White ES Lung Extracellular Matrix and Fibroblast Function. Ann. Am. Thorac. Soc 2015, 12 (Supplement1), S30–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Paszek MJ; Zahir N; Johnson KR; Lakins JN; Rozenberg GI; Gefen A; Reinhart-King CA; Margulies SS; Dembo M; Boettiger D; Hammer DA; Weaver VM Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8 (3), 241–254. [DOI] [PubMed] [Google Scholar]
- (22).Sigal IA; Flanagan JG; Ethier CR Factors Influencing Optic Nerve Head Biomechanics . Invest. Ophthalmol. Visual Sci 2005, 46 (11), 4189–99. [DOI] [PubMed] [Google Scholar]
- (23).Navajas D; Alcaraz J; Peslin R; Roca J; Farre R Evaluation of a Method for Assessing Respiratory Mechanics During Noninvasive Ventilation. Eur. Respir. J 2000, 16 (4), 704–709. [DOI] [PubMed] [Google Scholar]
- (24).Lai-Fook SJ; Hyatt RE Effects of Age on Elastic Moduli of Human Lungs. J. Appl. Physiol 2000, 89 (1), 163–168. [DOI] [PubMed] [Google Scholar]
- (25).Liu F; Mih JD; Shea BS; Kho AT; Sharif AS; Tager AM; Tschumperlin DJ Feedback Amplification of Fibrosis through Matrix Stiffening and Cox-2 Suppression. J. Cell Biol 2010, 190 (4), 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Prager-Khoutorsky M; Lichtenstein A; Krishnan R; Rajendran K; Mayo A; Kam Z; Geiger B; Bershadsky AD Fibroblast Polarization Is a Matrix-Rigidity-Dependent Process Controlled by Focal Adhesion Mechanosensing. Nat. Cell Biol 2011, 13 (12), 1457. [DOI] [PubMed] [Google Scholar]
- (27).Sunyer R; Jin AJ; Nossal R; Sackett DL Fabrication of Hydrogels with Steep Stiffness Gradients for Studying Cell Mechanical Response. PLoS One 2012, 7 (10), No. e46107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Balestrini JL; Chaudhry S; Sarrazy V; Koehler A; Hinz B The Mechanical Memory of Lung Myofibroblasts . Integr. Biol 2012, 4(4), 410–421. [DOI] [PubMed] [Google Scholar]
- (29).He X; Young S-H; Schwegler-Berry D; Chisholm WP; Fernback JE; Ma Q Multiwalled Carbon Nanotubes Induce a Fibrogenic Response by Stimulating Reactive Oxygen Species Production, Activating Nf-Kb Signaling, and Promoting Fibroblastto-Myofibroblast Transformation. Chem. Res. Toxicol 2011, 24 (12), 2237–2248. [DOI] [PubMed] [Google Scholar]
- (30).Hussain S; Sangtian S; Anderson SM; Snyder RJ; Marshburn JD; Rice AB; Bonner JC; Garantziotis S Inflammasome Activation in Airway Epithelial Cells after Multi-Walled Carbon Nanotube Exposure Mediates a Profibrotic Response in Lung Fibroblasts . Part. Fibre Toxicol 2014, 11 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Luanpitpong S; Wang L; Manke A; Martin KH; Ammer AG; Castranova V; Yang Y; Rojansakul Y Induction of Stemlike Cells with Fibrogenic Properties by Carbon Nanotubes and Its Role in Fibrogenesis. Nano Lett 2014, 14 (6), 3110–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sahin O; Magonov S; Su C; Quate CF; Solgaard O An Atomic Force Microscope Tip Designed to Measure Time-Varying Nanomechanical Forces. Nat. Nanotechnol 2007, 2 (8), 507. [DOI] [PubMed] [Google Scholar]
- (33).Wynn TA Integrating Mechanisms of Pulmonary Fibrosis. J. Exp. Med 2011, 208 (7), 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hirano S; Fujitani Y; Furuyama A; Kanno S Uptake and Cytotoxic Effects of Multi-Walled Carbon Nanotubes in Human Bronchial Epithelial Cells. Toxicol. Appl. Pharmacol 2010, 249 (1), 8–15. [DOI] [PubMed] [Google Scholar]
- (35).Wang J; Li L Coupled Elasticity−Diffusion Model for the Effects of Cytoskeleton Deformation on Cellular Uptake of Cylindrical Nanoparticles. J. R. Soc., Interface 2015, 12 (102), 20141023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Huang C; Butler PJ; Tong S; Muddana HS; Bao G; Zhang S Substrate Stiffness Regulates Cellular Uptake of Nano-particles. Nano Lett 2013, 13 (4), 1611–5. [DOI] [PubMed] [Google Scholar]
- (37).Gimenez A; Duch P; Puig M; Gabasa M; Xaubet A; Alcaraz J Dysregulated Collagen Homeostasis by Matrix Stiffening and Tgf-B1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of Fak/Akt. Int. J. Mol. Sci 2017, 18 (11), 2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Wang K; Bruce A; Mezan R; Kadiyala A; Wang L; Dawson J; Rojanasakul Y; Yang Y Nanotopographical Modulation of Cell Function through Nuclear Deformation. ACS Appl. Mater. Interfaces 2016, 8 (8), 5082–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Rasmussen CH; Reynolds PM; Petersen DR; Hansson M; McMeeking RM; Dufva M; Gadegaard N Enhanced Differentiation of Human Embryonic Stem Cells toward Definitive Endoderm on Ultrahigh Aspect Ratio Nanopillars. Adv. Funct. Mater 2016, 26 (6), 815–823. [Google Scholar]
- (40).Yang Y; Wang K; Gu X; Leong KW Biophysical Regulation of Cell Behavior Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3 (1), 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Seong J; Tajik A; Sun J; Guan J-L; Humphries MJ; Craig SE; Shekaran A; García AJ; Lu S; Lin MZ Distinct Biophysical Mechanisms of Focal Adhesion Kinase Mechanoactivation by Different Extracellular Matrix Proteins. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (48), 19372–19377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Shih YRV; Tseng KF; Lai HY; Lin CH; Lee OK Matrix Stiffness Regulation of Integrin-Mediated Mechanotransduction During Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Bone Miner. Res 2011, 26 (4), 730–738. [DOI] [PubMed] [Google Scholar]
- (43).Hui L; Zhang J; Ding X; Guo X; Jiang X Matrix Stiffness Regulates the Proliferation, Stemness and Chemoresistance of Laryngeal Squamous Cancer Cells. Int. J. Oncol 2017, 50 (4), 1439–1447. [DOI] [PubMed] [Google Scholar]
- (44).Provenzano PP; Inman DR; Eliceiri KW; Keely PJ Matrix Density-Induced Mechanoregulation of Breast Cell Phenotype, Signaling and Gene Expression through a Fak−Erk Linkage. Oncogene 2009, 28 (49), 4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kaiser J-P; Buerki-Thurnherr T; Wick P Influence of Single Walled Carbon Nanotubes at Subtoxical Concentrations on Cell Adhesion and Other Cell Parameters of Human Epithelial Cells. J. King Saud Univ., Sci 2013, 25 (1), 15–27. [Google Scholar]
- (46).Zhang Y; Wang B; Meng X; Sun G; Gao C Influences of Acid-Treated Multiwalled Carbon Nanotubes on Fibroblasts: Proliferation, Adhesion, Migration, and Wound Healing. Ann. Biomed. Eng 2011, 39 (1), 414–426. [DOI] [PubMed] [Google Scholar]
- (47).Rahman L; Jacobsen NR; Aziz SA; Wu D; Williams A; Yauk CL; White P; Wallin H; Vogel U; Halappanavar S Multi-Walled Carbon Nanotube-Induced Genotoxic, Inflammatory and Pro-Fibrotic Responses in Mice: Investigating the Mechanisms of Pulmonary Carcinogenesis. Mutat. Res., Genet. Toxicol. Environ. Mutagen 2017, 823, 28–44. [DOI] [PubMed] [Google Scholar]
- (48).Fu P; Tang R; Yu Z; Li C; Chen X; Xie M; Wang W; Luo X Rho-Associated Kinase Inhibitors Promote Microglial Uptake Via the Erk Signaling Pathway. Neurosci. Bull 2016, 32 (1), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Street CA; Routhier AA; Spencer C; Perkins AL; Masterjohn K; Hackathorn A; Montalvo J; Dennstedt EA; Bryan BA Pharmacological Inhibition of Rho-Kinase (Rock) Signaling Enhances Cisplatin Resistance in Neuroblastoma Cells. Int. J. Oncol 2010, 37 (5), 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Williams ML; Landel RF; Ferry JD The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc 1955, 77 (14), 3701–3707. [Google Scholar]
- (51).Chen T; Nie H; Gao X; Yang J; Pu J; Chen Z; Cui X; Wang Y; Wang H; Jia G Epithelial−Mesenchymal Transition Involved in Pulmonary Fibrosis Induced by Multi-Walled Carbon Nanotubes Via Tgf-Beta/Smad Signaling Pathway. Toxicol. Lett 2014, 226 (2), 150–162. [DOI] [PubMed] [Google Scholar]
- (52).Wei SC; Fattet L; Tsai JH; Guo Y; Pai VH; Majeski HE; Chen AC; Sah RL; Taylor SS; Engler AJ Matrix Stiffness Drives Epithelial−Mesenchymal Transition and Tumour Metastasis through a Twist1−G3bp2Mechanotransduction Pathway. Nat. Cell Biol 2015, 17 (5), 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Wang P; Nie X; Wang Y; Li Y; Ge C; Zhang L; Wang L; Bai R; Chen Z; Zhao Y Multiwall Carbon Nanotubes Mediate Macrophage Activation and Promote Pulmonary Fibrosis through Tgf-B/Smad Signaling Pathway. Small 2013, 9 (22), 3799–3811. [DOI] [PubMed] [Google Scholar]
- (54).Blakney AK; Swartzlander MD; Bryant SJ The Effects of Substrate Stiffness on the in Vitro Activation of Macrophages and in Vivo Host Response to Poly (Ethylene Glycol)-Based Hydrogels. J. Biomed. Mater. Res., Part A 2012, 100 (6), 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.