Abstract
Chemical permeation enhancers (CPEs) can enable antibiotic flux across the tympanic membrane. Here we study whether combinations of CPEs (sodium dodecyl sulfate, limonene, and bupivacaine hydrochloride) are synergistic and whether they could increase the peak drug flux. Synergy is studied by isobolographic analysis and combination indices. CPE concentration-response (i.e. trans-tympanic flux of ciprofloxacin) curves are demonstrated for each CPE, isobolograms constructed for pairs of CPEs, and synergy demonstrated for all three pairs. Synergy is much greater at earlier (6 h) than later (48 h) time points, although the effect sizes are greater later. Synergy is also demonstrated with the three-drug combination. Combinations of CPEs also greatly enhance the maximum drug flux achievable over that achieved by individual CPEs.
Keywords: Chemical permeation enhancers, Trans-tympanic permeation, Otitis media
1. Introduction
Ototopical drug delivery presents a promising alternative to oral therapeutics for drug administration to the middle ear. Localized delivery of therapeutics across the intact tympanic membrane (TM) and directly to the middle ear could minimize adverse systemic effects (diarrhea, rashes, and perhaps antibiotic resistance caused by oral antibiotics for the treatment of otitis media [OM] [1]), improve patient adherence with therapy (due to reduced side effects and obviation of the need for extended treatment of often uncooperative toddlers), and therefore possibly achieve better therapeutic outcomes. However, non-invasive trans-tympanic delivery has seldom been explored until recently [2, 3] due to the impermeability of the TM [4,5]. The TM is a 100 μm-thick trilayer membrane whose outer layer, the stratum corneum (SC), is a stratified squamous keratinizing epithelium continuous with the skin of the external auditory canal, and is structurally similar to that in skin.
Chemical permeation enhancers (CPEs) are an effective means of enhancing the flux of small-molecule therapeutics across the TM [2, 3]. Moreover, the enhancement can be increased by increasing the concentration of CPEs [6, 7]. CPEs are known to disrupt the structural integrity of the lipid bilayers in the stratum corneum, enhancing the diffusion of therapeutics [6]. We have previously demonstrated that OM can be treated by the trans-tympanic delivery of ciprofloxacin (Cip) enabled by a combination of CPEs [2, 3]. However, the benefits of combinations of CPEs remain to be demonstrated formally, specifically whether their effects are truly synergistic, or simply additive. Synergistic interactions hold the potential to reduce the amount of CPEs needed to achieve a given effect, thus potentially also reducing toxicity.
A related important issue is whether combinations of CPEs can be used to maximize peak effect, i.e. the maximum drug flux across a barrier. The magnitude of drug flux is particularly important in treating OM, as relatively high antibiotic concentrations are needed to treat some bacteria, such as the common OM pathogen Streptococcus pneumoniae [8, 9].
Pioneering work on interactions of CPEs has demonstrated the possibility of achieving higher than expected permeation enhancement when CPEs were combined [10–15]. Here, we have used formal pharmacological approaches to establish whether the CPE interactions noted here are synergistic [16] and also whether CPE combinations could be used to increase the peak effects that could be achieved. We investigate potentially synergistic effects among three CPEs delivered in a polymer matrix (Supplementary Fig. S1) in enhancing trans-tympanic permeation using isobole analysis [17, 18] and combination indices [16, 19, 20]
Sodium dodecyl sulfate (SDS), a surfactant, and limonene (LIM), a terpene, were chosen because they are both CPEs approved by the FDA for topical use [21]. SDS (an anionic surfactant) can enhance SC permeability by extracting lipids from the SC and altering the protein structure of keratin in corneocytes, [22] while LIM (a terpene) can partition into the SC lipids, forming a pathway for drug molecules [23]. Synergistic effects are often found with processes that act on a common phenomenon by different mechanisms [10, 24–26]. The clinically-used local anesthetic bupivacaine hydrochloride (BUP) was studied because it may reduce pain associated with OM.
The effect of SDS, LIM, BUP, and their combinations on permeation enhancement was elucidated by measuring their effect on the permeability of Cip across the TMs of healthy chinchillas. Cip was selected because it is FDA-approved to be administered locally to the middle ear for the treatment of OM [27]. Cip and the CPEs were delivered from a hydrogel reported previously, poloxamer 407-polybutylphosphoester (P407-PBP) (Supplementary Fig. S1) [2] P407-PBP was used here because of its robust reverse thermal gelation behavior [2]. The hydrogel-based formulation is an easy-to-apply liquid at room temperature, and gels quickly and firmly upon contacting the warm TM, holding the antibiotic and CPEs in place (i.e. on the TM) throughout the permeability measurements.
Chinchilla TMs were used as the model system here, because of their well-established structural similarity to human TMs [28]. The principal difference between chinchilla and human TMs is that the latter are much thicker [2, 29]. Nonetheless, the conclusions reached here are likely to bear on human TMs as well because a) the TMs in the two species are structurally similar and b) CPEs can affect much thicker structures, such as human skin.
2. Materials and methods
2.1. Materials
2-chloro-2-oxo-1,3,2-dioxaphospholane (COP), 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU), n-butanol, diethyl ether, acetic acid, anhydrous dichloromethane, anhydrous tetrahydrofuran, SDS, LIM, and US pharmaceutical grade Cip and BUP were used as received from Sigma-Aldrich (St. Louis, MO). Kolliphor® P407 micro-prilled (pelletized into micro-particles), received from BASF (Florham Park, NJ).
2.2. Animal maintenance
Healthy adult male chinchillas weighting 500 to 650 g were purchased from Ryerson Chinchilla Ranch (Plymouth, OH) and cared for in accordance with protocols approved institutionally and nationally. Experiments were carried out in accordance with the Boston Children’s Hospital Animal Use Guidelines and approved by the Animal Care and Use Committee.
2.3. Synthesis of butoxy-2-oxo-1,3,2-dioxaphospholane (BP)
BP was prepared by condensation reaction of COP and n-butanol. COP (5.0 g, 35 mmol) in anhydrous THF (50 mL) was added to a stirring solution of n-butanol (2.6 g, 35 mmol) and trimethylamine (3.9 g, 39 mmol) in anhydrous THF (100 mL) at 0 °C dropwise. The reaction mixture was stirred in an ice bath for 12 h upon completed addition of COP in THF. Upon complete conversion of COP, the reaction mixture was filtered and the filtrate was concentrated. The concentrated filtrate was purified by vacuum distillation under reduced vacuum to yield a viscous colorless liquid.
2.4. Synthesis of P407-PBP
P407-PBP was synthesized by ring opening polymerization (ROP) of BP with P407 as the macroinitiator in the presence of an organocatalyst, DBU at −20°C [30]. P407 (8.1 g, 0.56 mmol) and BP (1.0 g, 5.6 mmol) in anhydrous dichloromethane (DCM, 0.5 mL) was added to a flame dried Schlenk flask (10 mL) equipped with a stir bar. The reaction mixture was flushed with nitrogen gas for 5 min while immersed in an ice bath with saturated NaCl solution. A solution of DBU in anhydrous DCM (0.13 g, 0.84 mmol) was added to the stirring solution via a syringe dropwise while maintaining the reaction under nitrogen gas atmosphere. Upon completion of the reaction, excess amount of acetic acid in DCM was added to the reaction mixture to quench the reaction. The product was purified by precipitation into ether (3 times) and dried under vacuum to obtain a white powder product.
2.5. Hydrogel formation
Hydrogel solutions of 12% (w/v) P407-PBP hydrogel formulations were made by addition of powdered polymers to aqueous solutions of 4% (w/v) Cip (pH = 3.3–3.9) and simple dissolution in a cold room to allow better solubility of P407-PBP. SDS, and/or LIM, and/or BUP were added to the solution of 4% (w/v) Cip and 12% (w/v) P407-PBP and allowed to dissolve in a cold room for at least 4 h.
2.6. In vitro release studies
The release of Cip from each formulation was measured using a diffusion system. Transwell® membrane inserts (0.4 μm pore size, 1.1 cm2 area; Costar, Cambridge, MA) and 24-well culture plates were employed as the donor and acceptor chambers, respectively. 200 μL of each formulation was pipetted directly onto pre-warmed filter inserts to obtain a solid hydrogel. Filter inserts (donor compartments) with formed gels were suspended in wells (acceptor compartments) filled with pre-warmed phosphate buffered saline (PBS) and the plates then kept in a 37 °C incubator. At each time point (0.5, 1, 2, 6, 12, 24, 48 h), 1 mL aliquots of the PBS receiving media were sampled and inserts sequentially moved into a new well with fresh PBS. Aliquots were suspended in 70:30 acetonitrile/PBS to ensure total drug dissolution. Sample aliquots were chromatographically analyzed with high-performance liquid chromatography (HPLC) to determine Cip concentrations (absorption at the wavelength λ = 275 nm). More details regarding the Cip measurement and HPLC conditions can be found in reference [3]. Experiments were performed in quadruplicate.
2.7. Ex vivo permeation experiment
The trans-tympanic permeation rate of Cip was determined with auditory bullae harvested from healthy chinchillas. Chinchillas were placed under deep general anesthesia by the intramuscular administration of ketamine (30 mg/kg) and xylazine (4 mg/kg), and then euthanized with intracardiac administration of pentobarbital (100 mg/ kg). Euthanized animals were decapitated and the auditory bullae removed undamaged, with the tympanic ring still attached. Their integrity was assessed by measuring their electrical impedance (indicated by a resistivity ≥18kΩ*cm2; a value previously determined [3]) in a setup where TMs were placed horizontally in a 12-well plate with donor solution above and recipient solution below. The same setup was used to measure drug flux, in lieu of a conventional diffusion cell - which would deform or rupture the TM. All formulations were applied into the bullae kept at 37 °C and deposited onto the TMs. The volume applied was 200 μL, which translates to 8 mg Cip. Permeation of Cip across TM into the receiving chamber was quantified using HPLC. Detailed information regarding TM harvesting, TM electrical resistance measurement, and configuration of the ex vivo permeation experiment can be found in reference [3].
2.8. Statistical analysis
Data which were normally distributed were described with means and standard deviations (calculated using Microsoft® Excel®) and compared by unpaired Student t-tests (using Origin® 8, OriginLab). Otherwise, data were presented as median ± quartiles (using Microsoft® Excel ®).
3. Results
3.1. Overview and nomenclature of the formulation
Hydrogel formulations were formulated with the antibiotic Cip at 4% (w/v), the penta-block copolymer P407-PBP at 12% (w/v), and CPEs at various concentrations; the gels are referred to as CPPB-x%LIM-y%SDS-z%BUP, where CPPB represents the invariant 4%Cip-12%[P407-PBP]; x, y, z are weight by volume percentage concentrations of LIM, SDS, and BUP respectively. Twelve percent P407-PBP was used throughout this work as it was easily extruded from a syringe at room temperature and gelled rapidly at body temperature [2]. (The latter property would be important when applying the materials in toddlers who prefer not to stay still. The hydrogel itself would maintain the antibiotic and CPEs at the TM in vivo. P407-PBP is necessary for the continuous exposure of TMs to CPEs and antibiotics [2]).
P407-PBP was synthesized by ring-opening polymerization, as reported [2]. Nuclear magnetic resonance (NMR) confirmed the presence of the PBP moieties and determined the degree of polymerization of the PBP moieties to be 5 (Supplementary Fig. S2). Fourier transform infrared spectroscopy (FTIR) confirmed the successful synthesis of the penta-block copolymer P407-PBP (Supplementary Fig. S2).
If a component was absent from a formulation, it was omitted from the above nomenclature. A previously reported combination of three CPEs, [2] i.e., 2%LIM, 1%SDS, and 0.5%BUP is denoted as 3CPE. Unless specified otherwise, all percentages are weight by volume percent.
The cumulative amount of Cip that permeated across excised TM in ex vivo experiments was represented as VCIPt, where t is the time in hours over which cumulative permeation of Cip was measured. Specifically, VCIP6 and VCIP48 represent the cumulative amount of Cip that permeated across the TM within 6 and 48 h in ex vivo experiments, respectively.
3.2. In vitro drug release from hydrogels
The release of Cip from each formulation was measured using Transwell® membrane inserts. Cip release from 200 μL of CPPB gels containing 8.0 mg of drug with or without CPEs was measured at 37 °C (Fig. 1). Drug release slowed down significantly after roughly 12 h for Cip solution, and roughly 24 h for CPPB gels with or without CPEs. In 48 h, CPPB released almost the entirety of the loaded Cip (7.7 mg), while CPPB-3CPE released approximately three quarters (5.9 mg).
Fig. 1.
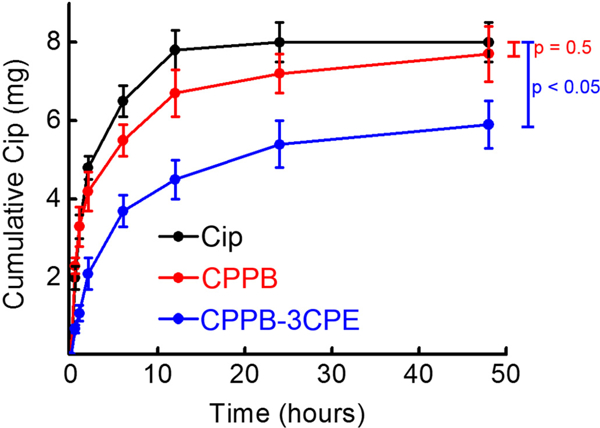
Cumulative in vitro release of Cip from the hydrogel formulations under infinite sink conditions. Eight milligrams of Cip were contained in each gel and solution at time zero. Data are means ± SD (n = 4).
3.3. Synergistic interactions among CPEs
3.3.1. Isobolographic analysis
A key concept in comparing interactions of drug doses is that of dose equivalence [17, 18]. One rigorous way of establishing equivalence is in terms of a dose that affects a given percentage of a population or has a given percentage of a maximal effect (both of these have been defined as, for example, the EC50 [half maximal effect concentration]). In such cases, the effects of doses can be compared by isobolographic analysis.
The following steps are followed to perform the isobolographic analysis. Concentration-response curves are constructed for drugs X and Y, and the equivalent concentration (or dose) to achieve a given effect (e.g., the VCIP48 of 0.4 mg) is determined for each (Fig. 2A). An isobologram (Fig. 2B) is constructed where the concentration of drug X to achieve that given effect is plotted on the x-axis and the equivalent for drug Y on the y-axis. A line connecting the two (the isobole) is the line of additivity; the effect of combinations of fractions of the equivalent doses for drugs X and Y are then plotted on the graph. If, for example, a combination of 10% of the equivalent dose of X and 90% of the equivalent dose of Y (i.e. a total of 100% of an equivalent dose) achieves the given effect, then X and Y are simply additive. If only 10% of the equivalent dose of X and 10% of the equivalent dose of Y (i.e. 20% of an equivalent dose) achieve the given effect, they are synergistic. If a combination of 90% of the equivalent dose of X and 90% of the equivalent dose of Y (i.e. 180% of an equivalent dose) have the given effect they are antagonistic.
Fig. 2.
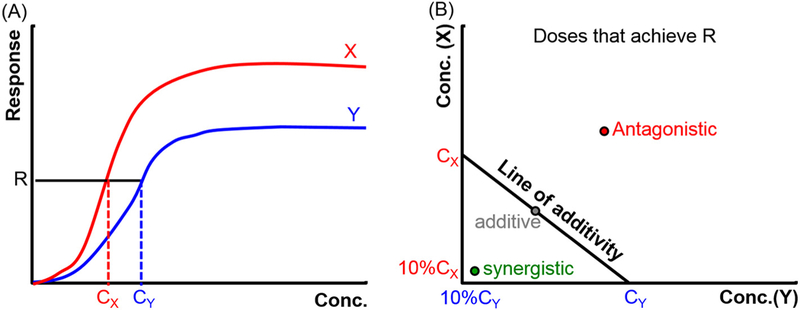
Construction of an isobologram. (A) Concentration (Conc.)-response curves are used to identify isoboles, i.e. concentrations achieving the same effect (R). In this work, the principal R is VCIP48. (B) Isobolographic analysis. See text for explanation. Cx and Cy are the equivalent doses for drugs X and Y. The diagonal line is the line of additivity, also known as the isobole.
3.3.2. Concentration-response curves for single CPEs
To produce the isobolographic analysis, we generated curves (analogous to Fig. 2) relating the effect of concentrations of single CPEs to trans-tympanic drug permeation of Cip. These curves were subsequently used to construct isobolograms [18] to assess whether the effects of combinations of CPEs were additive, synergistic, or possibly antagonistic.
Drug transport across the TM was studied ex vivo in auditory bullae excised from healthy chinchillas at 37 °C. 200 μL of CPPB gels (donor solution) containing 8.0 mg of drug with or without various concentrations of SDS, LIM, or BUP was placed on one surface of the TM (see Methods for details) and flux into 3 mL of PBS (recipient solution) was measured (Fig. 3). Curves relating CPE concentration (x-axis) to VCIP6 and VCIP48 were constructed for each CPE.
Fig. 3.
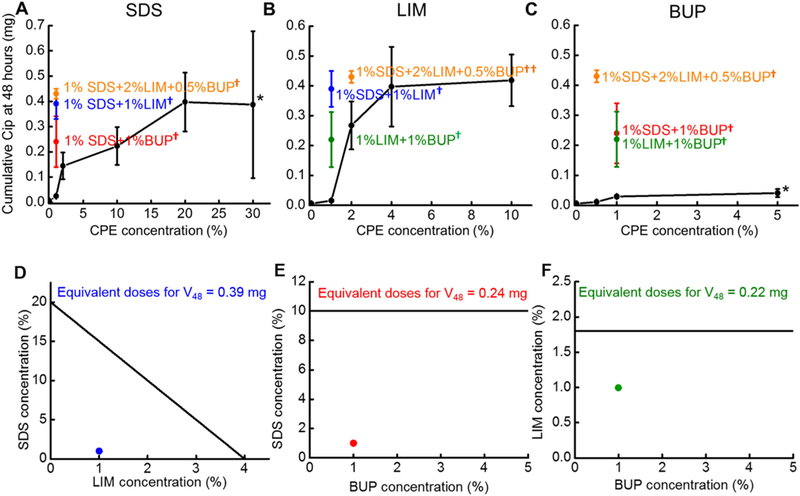
Performance of single and pairs of CPEs. (A-C) Cumulative Cip permeation across the TM over 48 h (VCIP48) from CPPB containing varying concentrations of CPEs, singly (black curves), or in combination with other CPEs (colored points on the graphs). The colored points represent the same data in all panels. Data are means ± SD (n = 4). *5% BUP and 30% SDS were suspensions, not homogeneous solutions. †p < 0.05 for the comparisons between CPE combinations and the single CPE that is the subject of the panel; ††p < 0.1. (D-F) Isobolograms for combinations of (D) SDS and/or LIM that achieved VCIP48 = 0.39 mg, (E) SDS and/or BUP that achieved VCIP48 = 0.24mg, and (F) BUP and/or LIM that achieved VCIP48 = 0.22 mg. The data are derived from (A-C).
Cip flux across the TM from CPPB-SDS was studied in the SDS concentration range of 0 to 20% because 20% was the solubility limit for SDS in water [31]. (Although the FDA-approved concentration limit for topical application is 40% for SDS, [21] formulations with > 20% SDS were suspensions not solutions.) Cip flux increased continuously with increasing SDS concentration. At 6h (Fig. 4), Cip permeation across the TM in the absence of CPEs was below the detection limit of HPLC (about 1 μg/mL). Introducing 1%SDS to the hydrogel (Fig. 4A) increased VCIP6 to about 0.001 ± 0.0002 mg (p < 0.001); increasing the SDS concentration from 1% to 20% roughly doubled the VCIP6 (0.002 ± 0.002 mg) at 6 h (p = 0.29). At 48 h (Fig. 3A), increasing the SDS concentration from 1% to 20% increased the VCIP48 from 0.03 ± 0.004 mg to 0.39 ± 0.11 mg (p < 0.001), a 13-fold enhancement. Further increasing the SDS concentration to 30% did not further increase VCIP6 and VCIP48 [0.002 ± 0.001 mg (p = 0.83) and 0.39 ± 0.29 mg (p = 0.94) respectively], presumably because SDS was not soluble beyond 20%.
Fig. 4.
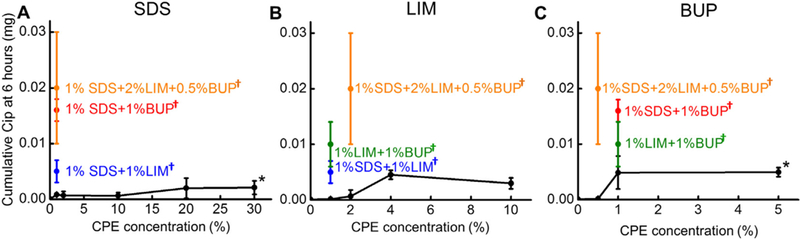
Cumulative Cip permeation across the TM over 6 h (VCIP6) from CPPB containing varying concentrations of CPEs, singly (black curves), or in combination with other CPEs (colored points on the graphs). The colored points represent the same data in all panels. Data are means ± SD (n = 4). *5% BUP and 30% SDS were suspensions, not homogeneous solutions. †p < 0.05 for the comparisons between CPE combinations and the single CPE that is the subject of the panel; ††p < 0.1.
The effect of LIM on VCIP6 and VCIP48 from CPPB-LIM hydrogels was studied in the LIM concentration range of 0 to 10%, as 10% is the highest LIM concentration approved by the US FDA for topical applications [21]. With the addition of 1% LIM, VCIP6 remained below the HPLC detection limit (Fig. 4B); with 4% LIM it was 0.004 ± 0.001 mg, and did not increase further with 10% LIM (0.004 ± 0.001 mg, p = 0.51). VCIP48 (Fig. 3B) increased ~25 fold (from 0.02 ± 0.004 mg to 0.40 ± 0.13 mg, p = 0.001) as the concentration of LIM increased from 1% to 4%; there was no further increase at 10% LIM (0.42 ± 0.09 mg, p = 0.73).
VCIP6 and VCIP48 plateaued at a BUP concentration of 1%; the flux was very similar at 5%, a supersaturated concentration that was a slurry. VCIP6 (Fig. 4C) was about 2 ± 2 μg at 0.5% BUP, and VCIP6 5 ± 3 μg at 1% and 5% BUP (Fig. 3C). Although the maximal VCIP6 with BUP was comparable to that of the other CPEs, the VCIP48 with BUP was much less than those from LIM or SDS. VCIP48 was 0.03 mg at 1% BUP and 0.04 ± 0.01 mg at 5%.
One interesting observation was that the combination effects among CPEs change over time. The degree of enhancement from combining CPEs was much greater at 6 h than 48 h, even though the net drug permeation rates involved were much smaller. For example, VCIP6 achieved by the 3CPE combination was 20 fold that of 1% SDS, 10 fold that of 0.5% BUP, and infinite fold that of 2% LIM (the latter was below the HPLC detection limit), whereas VCIP48 with 3CPE was 17, 2, and 37 fold that of 1%SDS, 0.5% BUP, and 2% LIM respectively.
In fact the effect of the CPE combinations are so much in excess of the peak effects (determined by concentration-response curves) of individual CPEs, it is impossible to construct an isobologram.
Isobolograms were constructed using VCIP48. The CPE concentration-VCIP48 curves (Fig. 3A-C) were fitted with a three-parameter hyperbolic function (the logistic function most commonly used for concentration-response curves [30]) to determine the peak effect Emax, with the equation below: [18, 32].
| (1) |
where VCIP48 is the measured response; C is a concentration of a CPE that resulted in the VCIP48; Emax is the response for an infinite concentration (i.e., maximal response); EC50 is the concentration resulting in a response half that of Emax; p is a constant that determines the steepness of the hyperbolic curve for each CPE, often called a Hill’s coefficient [18]. Hill’s coefficients derived from concentration-response curves of pharmaceuticals represent the number of interacting sites (e.g. number of bound ligands to a receptor) [33]. In the context of CPEs, the molecular correlate of Hill’s coefficient is unclear, but it can be determined by fitting data to Eq. (1).
The Emax values were obtained for SDS, LIM, and BUP by fitting the CPE concentration-VCIP48 curves to (Eq. 1) (Fig. 5 and Table 1) using nonlinear least squares regression. SDS had an Emax of 0.65 mg, indicating the maximum VCIP48 that can be achieved by SDS is ~0.65 mg. However, SDS at 20% and 30% achieved similar VCIP48, ~0.4mg, and the concentration at which the calculated Emax occurred is a slurry. Consequently, we used the experimentally determined peak effect of 0.4 mg for the Emax for SDS.
Fig. 5.
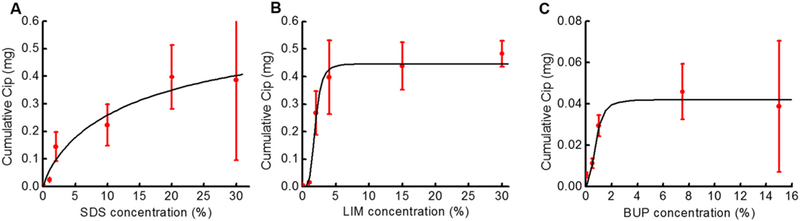
Concentration-response curves for ciprofloxacin permeation across the TM after 48 h (i.e. VCIP48) from CPPB containing (A) SDS, (B) LIM, and (C) BUP. Data were fitted to a three-parameter hyperbolic function model (black line) using (Eq. 1). The fitting parameters are listed in Table 1. Data points (red dots) originate from Fig. 2. Note that the y-axis scale for (C) is different from those for (A) and (B). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Concentration-response curve fitting parameters for SDS, LIM, and BUP.
Derived from Eq. (1).
Derived experimentally.
LIM had an Emax of 0.41 mg. Its permeation enhancement effect plateaued at a LIM concentration around 4%. BUP had the smallest Emax (0.04 mg). Bupivacaine’s Emax was 9.76% that of LIM, and 6.15% that of SDS. The effect of BUP on Cip permeation plateaued at a concentration ~1%.
3.3.3. Combinations of two CPEs
To assess whether synergy occurred between CPEs, their effects on drug flux across the TM were analyzed by the isobolographic method. The concentration-response curves above identified two factors complicating the use of this approach: 1) for some of the CPEs, physicochemical factors (e.g. solubility) that limited CPE concentrations that could be achieved might have prevented determination of the peak effect, 2) the maximal effects of the individual CPEs were very different. In such circumstances, isobolograms can be constructed using specific absolute effects, (e.g. a given drug permeation rate) [18]. If a drug with low maximal effect is compared with one with a large maximal effect (e.g. BUP and LIM in this case, or glucosamine and ibuprofen [34]), the line of additivity would be parallel to the axis representing the drug with lesser maximal effect [18, 34] (i.e. no concentration of that drug would achieve the given absolute effect).
A VCIP48 of 0.39 mg was used as the “effect” for the isobole analysis of synergistic effects among CPEs. Both CPPB-4%LIM and CPPB-20% SDS resulted in that VCIP48 (Fig. 3A and B, p = 0.96), and thus 4% LIM and 20% SDS were considered equivalent doses. An isobologram (Fig. 3D) was constructed as discussed previously, with the concentration of LIM on the x-axis and that of SDS on the y-axis, and the equivalent doses of each (4% LIM and 20% SDS) plotted on their respective axes. A line connecting the two is the line of additivity (the isobole); which can be described using the following equation, [17, 18].
| (2) |
where dLIM is the weight by volume percentage of LIM in a given formulation and dSDS the weight by volume percentage of SDS. The “4%” on the right hand of the equation indicated that combinations of dLIM and dSDS would achieve the same response as 4% LIM if SDS and LIM were additive. Rearranging Eq. (2) gave the linear isobole equation:
| (3) |
The line connecting the axes in the isobole graph (Fig. 3D), plotted based on (Eq. 3), represented all of the LIM and SDS combinations that would yield a response of VCIP48 = 0.39 mg if the effects of LIM and SDS were additive. Experimentally, CPPB-1%SDS-1%LIM, i.e. the combination of 5% of the SDS equivalent dose (i.e. 5% of 20% SDS) and 25% of the LIM equivalent dose (25% of 4% LIM) achieved a VCIP48 of ~ 0.4 mg, i.e. 14 fold the response of 1% SDS and 28 fold the response of 1% LIM. The point representing this combination fell below the line of additivity (i.e. dLIM < < 4% ‒ dSDS ), indicating synergistic effects between LIM and SDS.
SDS and BUP also interacted synergistically (Fig. 3E). Similar calculations to (Eqs. 1)–(3), were applied to combinations of SDS and BUP (see Supporting Information Section S1). To reduce the number of animals required to identify equivalent doses and combinations, we first measured the response achieved using formulation CPPB-1%SDS-1%BUP, and then identified the equivalent doses of individual CPEs using the concentration-response curves (Fig. 3A and C). VCIP48 for CPPB-1%SDS-1%BUP was 0.24 mg. From the concentration-response (i.e. CPE-drug flux) curve for SDS (Fig. 3A), it was interpolated that 10% SDS (in CPPB-10%SDS) achieved a VCIP48 of 0.24 ± 0.07 mg (Fig. 3A). The concentration of BUP required to achieve VCIP48 = 0.24 mg was infinite (Emax[BUP] =0.04 mg, Table 1). The combination of 1%SDS and 1%BUP, containing 10% of the SDS equivalent dose (10% of 10% SDS) and 0% of the BUP equivalent dose resulted in VCIP48 = ~0.24 mg, 8 fold the response of 1% SDS and 8 fold the response of 1%BUP.
The isobole (i.e. the line of additivity) for combinations of SDS and BUP to achieve 0.24 mg VCIP48 was a straight line parallel to the BUP axis, intersecting the SDS axis at 10% (Fig. 3E), [18]. The point representing the combination of SDS and BUP that achieved VCIP48 of 0.24 mg (CPPB-1%SDS-1%BUP) was far below the isobole, indicating strong synergistic effects between SDS and BUP.
LIM and BUP also had synergistic effects. Similar calculations to (Eqs. 1)–(3) were applied to combinations of LIM and BUP (see Supporting Information Section S1). Again, we first measured the response achieved using formulation CPPB-1%LIM-1%BUP, and then identified the equivalent doses of individual CPEs using the concentration-response curves. VCIP48 for CPPB-1%LIM-1%BUP was 0.22 mg. To achieve VCIP48 = 0.22 mg, ~1.8% LIM was required (Fig. 3B). The amount of BUP required to achieve 0.22 mg VCIP48 was infinite (Emax[BUP] = 0.04 mg, Table 1). Therefore, the isobole line for LIM and BUP was a line parallel to the BUP axis, intersecting the LIM axis at 1.8% (Fig. 3F). The formulation CPPB-1%LIM-1%BUP, containing 56% of the LIM equivalent dose (56% of 1.8% LIM) and 0% of the BUP equivalent dose, achieved VCIP48 = 0.22 mg, 16 fold the response of 1% LIM, and 7 fold the response of 1%BUP. The point representing the combination of LIM and BUP was below the isobole line, indicating synergy.
As a further demonstration of synergy, we calculated the combination index (CI), defined as in Eqs. (4)–(7). The CI compares the doses of two drugs producing a given effect in combination measured experimentally (numerator) to the doses expected to produce the same effect if there were additivity (denominator) [16, 19, 20]. A CI < 1 indicates synergy; the lower the CI the greater the synergy.
For the combination of SDS and LIM:
| (4) |
where dLIMeqv. and dSDSeqv. are the equivalent doses of LIM and SDS respectively that achieved VCIP48 of ~0.4 mg; and dLIMexp. and dSDSexp. are the combination of LIM and SDS that achieved VCIP48 of ~0.4 mg experimentally. Therefore,
| (5) |
For the combination of SDS and BUP:
| (6) |
For the combination of LIM and BUP:
| (7) |
The CIs for all pairs of CPEs indicated strong synergistic effects.
3.3.4. Combinations of three CPEs
Synergy among three components is rarely analyzed; here we extended the concept of synergy from two-component systems (e.g. between LIM and BUP) to three components by plotting the isobologram as a plane (Fig. 6A). The concentrations of CPEs required to achieve a VCIP48 of 0.4 mg when they were used singly was ~20% for SDS (Fig. 3A), ~4% for LIM (Fig. 3B), and infinite for BUP (Emax[BUP] = 0.04 mg, Table 1). Therefore, the isobole plane crossed the axes representing SDS and LIM at 20% and 4%, and was parallel to the BUP axis (Fig. 6A). The combination of three CPEs, CPPB-3CPE (i.e. 2%LIM, 1%SDS, and 0.5%BUP), corresponding to 5% of the SDS equivalent dose (5% of 20% SDS), 50% of the LIM equivalent dose (50% of 4% LIM), and 0% of the BUP equivalent dose, achieved a Cip flux of 0.43 ± 0.02 mg. The point representing CPPB-3CPE at VCIP48 = 0.43 mg was well below the isobole plane (Fig. 6A), suggesting synergy. The CI for the 3CPE combination was not calculated, as a CI value < 1 could indicate synergistic effects between two out of the three CPEs, rather than between all three CPEs.
Fig. 6.
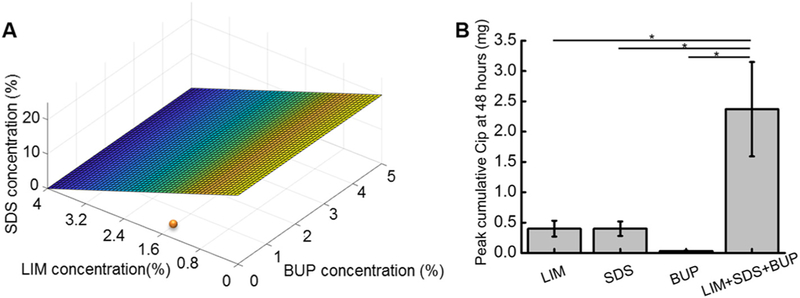
(A) Isobologram plot for combinations of SDS and/or LIM and/or BUP that achieved VCIP48 = 0.4 mg. The surface is derived from the isobole for the three CPEs, from 3 A-C. The orange dot is the measured VCIP48 from a combination of all three CPEs. (B) Effect of CPE combinations on the peak VCIP48. The peak flux for CPEs happened at 4%, 20%, and 1% for LIM, SDS, and BUP respectively; the combination column included 4% LIM, 1% BUP, and 20% SDS. Data are means ± SD (n = 4).
3.4. Effect of CPE combinations on the peak effect
The study of synergy by the isobolographic method is concerned with determining the interactions between pharmacological agents and establishing whether, for example, a given effect can be achieved with a lesser amount of two drugs rather than one drug. A related but different question is whether the use of combinations of agents can achieve a greater peak effect than could ever be achieved by either single agent alone. In the context of trans-tympanic delivery of antibiotics using CPEs, the maximal achievable peak effect is of great interest for the fast elimination of infections [35].
To address this issue, we studied whether combining the concentrations of individual CPEs that provided the maximal flux (i.e. plateau) would increase maximal flux (Fig. 6B). From Fig. 3, the peak VCIP48 for LIM, SDS, and BUP was achieved at 4% LIM (0.40 ± 0.13 mg), 20% SDS (0.39 ± 0.11 mg), and 1% BUP (0.03 ± 0.01 mg) respectively. The concentrations of the three CPEs that provided their greatest respective VCIP48 were combined. That combination, CPPB-4%LIM-1%BUP-20%SDS, achieved a VCIP48 of 2.37 ± 0.78 mg, 6 fold greater than that of the highest Emax from any individual CPE (Fig. 6B).
4. Discussion
We have formally demonstrated that CPEs have synergistic effects on drug flux across the TM, and that combination of CPEs can increase the maximal flux beyond what could be achieved by any concentration of a single CPE. We postulate that similar phenomena would be observed in skin, which is structurally similar, and in other settings where CPEs have been shown to be effective [36].
There were two principal barriers to transport for this trans-tympanic drug delivery platform: 1) diffusion through the bulk hydrogel matrix and 2) permeation across the TM. The similarity between the release profiles of Cip from aqueous solution and from CPPB (Fig. 1) indicated minimal diffusion resistance within the bulk hydrogel matrix. Incorporation of 3CPE slowed the diffusion, suggesting the possibility of additional physical cross-linking as a result of the interactions between SDS/LIM and the PBP end groups.
SDS, LIM, and BUP enhanced TM permeability (Fig. 3), in proportion to CPE concentration. Interestingly, the enhancement effects for each CPE relative to the others were different at 6 h than at 48 h. For example, BUP had approximately twice the maximal VCIP6 achieved by SDS or LIM. However, the maximal VCIP48 from SDS or LIM was roughly 10 fold that of BUP. The contrast between short-term (6 h) and long-term (48 h) permeation enhancement effects implied that BUP may have a different permeation enhancement mechanism from traditional CPEs such as SDS and LIM.
There was marked synergy between CPEs. Synergistic effects can be used to reduce the total amount of CPEs used, which might achieve some desirable goal (such as reducing tissue irritation, or reducing formulation viscosity) while maintaining the same or greater permeation enhancement. BUP, although not the most effective CPE by itself, dramatically increased the permeation enhancement of SDS and LIM. Interestingly, the synergistic effects of CPEs changed over time, i.e. the 3CPE combination increased drug flux to a greater degree at 6 h than 48 h. The greatly enhanced drug flux during the early phase of the antibiotic treatment is likely important in accelerating the time course of cure. Clinical evidence has shown that early eradication of pathogens from the middle ear improves clinical outcome [37].
A related but different need is to achieve a greater peak effect than could be achieved by any single agent alone. A greater peak effect is particularly desirable in the context of trans-tympanic drug delivery of antibiotics, to improve the therapeutic effect. Combination of the three CPEs at the concentrations that provided the largest possible effect when used singly, achieved a marked enhancement of drug permeation.
5. Conclusions
In summary, strong synergistic effects among SDS, BUP, and LIM were demonstrated by isobolographic analysis and combination indices. The analysis was extended to demonstrate strong synergistic effects when all three CPEs were used together. The CPE combinations could also improve the peak effect on drug flux.
Supplementary Material
Acknowledgements
This work was financially supported by NIH DC015050 to D.S.K., and Trailblazer Research Grant by the Department of Anesthesia at Boston Children’s Hospital and Charles H. Hood Foundation Child Health Research Award to R.Y.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2018.06.019.
References
- [1].Dagan R, Barkai G, Givon-Lavi N, Sharf AZ, Vardy D, Cohen T, Lipsitch M, Greenberg D, Seasonality of antibiotic-resistant streptococcus pneumoniae that causes acute otitis media: a clue for an antibiotic-restriction policy? J. Infect. Dis. 197 (2008) 1094–1102, 10.1086/528995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang R, Sabharwal V, Okonkwo OS, Shlykova N, Tong R, Lin LY, Wang W, Guo S, Rosowski JJ, Pelton SI, Kohane DS, Treatment of otitis media by transtympanic delivery of antibiotics, Sci. Transl. Med. 8 (2016), 10.1126/scitranslmed.aaf4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khoo X, Simons EJ, Chiang HH, Hickey JM, Sabharwal V, Pelton SI, Rosowski JJ, Langer R, Kohane DS, Formulations for trans-tympanic antibiotic delivery, Biomaterials 34 (2013) 1281–1288, 10.1016/j.biomaterials.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Uhde GI, The problem of permeability and anesthesia of the tympanic membrane, AMA. Arch. Otolaryngol. 66 (1957) 391–407. [DOI] [PubMed] [Google Scholar]
- [5].Doyle WJ, Alper CM, Seroky JT, Karnavas WJ, Exchange rates of gases across the tympanic membrane in rhesus monkeys, Acta Otolaryngol. 118 (1998) 567–573. [DOI] [PubMed] [Google Scholar]
- [6].Williams AC, Barry BW, Penetration enhancers, Adv. Drug Deliv. Rev. 56 (2004) 603–618, 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- [7].Notman R, Anwar J, Briels WJ, Noro MG, Den Otter WK, Simulations of skin barrier function: free energies of hydrophobic and hydrophilic transmembrane pores in ceramide bilayers, Biophys. J. 95 (2008) 4763–4771, 10.1529/biophysj.108.138545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Littorin N, Ahl J, Udden F, Resman F, Riesbeck K, Reduction of Streptococcus pneumoniae in upper respiratory tract cultures and a decreased incidence of related acute otitis media following introduction of childhood pneumococcal conjugate vaccines in a Swedish county, BMC Infect. Dis. 16 (2016), 10.1186/s12879-016-1750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Van Dyke MK, Pircon J-Y, Cohen R, Madhi SA, Rosenblut A, Macias Parra M, Al-Mazrou K, Grevers G, Lopez P, Naranjo L, Pumarola F, Sonsuwan N, Hausdorff WP, Etiology of acute otitis Media in Children Less than 5 years of age: a pooled analysis of 10 similarly designed observational studies, Pediatr. Infect. Dis. J. 36 (2017) 274–281, 10.1097/INF.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karande P, Jain A, Mitragotri S, Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery, J. Control. Release 115 (2006) 85–93, 10.1016/j.jconrel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [11].Karande P, Mitragotri S, Enhancement of transdermal drug delivery via synergistic action of chemicals, Biochim. Biophys. Acta Biomembr. 1788 (2009) 2362–2373, 10.1016/j.bbamem.2009.08.015. [DOI] [PubMed] [Google Scholar]
- [12].Kim YC, Ludovice PJ, Prausnitz MR, Transdermal delivery enhanced by magainin pore-forming peptide, J. Control. Release 122 (2007) 375–383, 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barry BW, Novel mechanisms and devices to enable successful transdermal drug delivery, Eur. J. Pharm. Sci. 14 (2001) 101–114, 10.1016/S0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- [14].Prausnitz MR, Mitragotri S, Langer R, Current status and future potential of transdermal drug delivery, Nat. Rev. Drug Discov. 3 (2004) 115–124, 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- [15].Lee PJ, Ahmad N, Langer R, Mitragotri S, Prasad Shastri V, Evaluation of chemical enhancers in the transdermal delivery of lidocaine, Int. J. Pharm. 308 (2006) 33–39, 10.1016/j.ijpharm.2005.10.027. [DOI] [PubMed] [Google Scholar]
- [16].Chou TC, Drug combination studies and their synergy quantification using the chou-talalay method, Cancer Res. 70 (2010) 440–446, 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- [17].Tallarida RJ, Quantitative methods for assessing drug synergism, Genes Cancer 2 (2011) 1003–1008, 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tallarida RJ, Drug combinations: tests and analysis with isoboles, Curr. Protoc. Pharmacol. 2016 (2016), 10.1002/0471141755.ph0919s72. (9.19.1-9.19.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chou TC, Talalay P, Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors, Adv. Enzym. Regul. 22 (1984) 27–55, 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [20].Foucquier J, Guedj M, Analysis of drug combinations: current methodological landscape, Pharmacol. Res. Perspect. 3 (2015), 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].H. Food And Drug Administration, Inactive Ingredient Search for Approved Drug Products-Titanium, Fda, 2015, pp. 1–4 http://www.fda.gov/Drugs/Information0nDrugs/ucm080123.htm#what.is.inactive.ing.
- [22].Tupker RA, Pinnagoda J, Nater JP, The transient and cumulative effect of sodium lauryl sulphate on the epidermal barrier assessed by transepidermal water loss: inter-individual variation, Acta Derm. Venereol. 70 (1990) 1–5. [PubMed] [Google Scholar]
- [23].Cornwell PA, Barry BW, Bouwstra JA, Gooris GS, Modes of action of terpene penetration enhancers in human skin; differential scanning calorimetry, small-angle X-ray diffraction and enhancer uptake studies, Int. J. Pharm. 127 (1996) 9–26, 10.1016/0378-5173(95)04108-7. [DOI] [Google Scholar]
- [24].Kohane DS, Kuang Y, Lu NT, Langer R, Strichartz GR, Berde CB, Vanilloid receptor agonists potentiate the in vivo local anesthetic activity of percutaneously injected site 1 sodium channel blockers, Anesthesiology 90 (1999) 524–534, 10.1097/00000542-199902000-00029. [DOI] [PubMed] [Google Scholar]
- [25].Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB, A re-examination of tetrodotoxin for prolonged duration local anesthesia, Anesthesiology 89 (1998) 119–131, 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- [26].Barnet CS, Tse JY, Kohane DS, Site 1 sodium channel blockers prolong the duration of sciatic nerve blockade from tricyclic antidepressants, Pain 110 (2004) 432–438, 10.1016/j.pain.2004.04.027. [DOI] [PubMed] [Google Scholar]
- [27].Food and Drug Administration, Approved drug products with therapeutic equivalence evaluations, Dep. Heal. Hum. Serv. (2017) 1346. [Google Scholar]
- [28].Suckow M, Stevens K, Wilson R, The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, (2012), 10.1016/C2009-0-30495-X. [DOI]
- [29].Dear SP, Saunders JC, Middle ear structure in the chinchilla: a quantitative study, Am. J. Otolaryngol. 9 (1988) 58–67, 10.1016/S0196-0709(88)80009-7. [DOI] [PubMed] [Google Scholar]
- [30].Iwasaki Y, Yamaguchi E, Synthesis of well-defined thermoresponsive polyphosphoester macroinitiators using organocatalysts, Macromolecules 43 (2010) 2664–2666, 10.1021/ma100242s. [DOI] [Google Scholar]
- [31].Wisniewski JR, Zougman A, Nagaraj N, Mann M, Wi JR, Universal sample preparation method for proteome analysis, Nat. Methods 6 (2009), 10.1038/nmeth.1322.(377–362) [DOI] [PubMed] [Google Scholar]
- [32].Delean A, Munson PJ, Rodbard D, Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves, Am. J. Phys. 235 (1978) E97, 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- [33].Prinz H, Hill coefficients, dose-response curves and allosteric mechanisms, J. Chem. Biol. 3 (2010) 37–44, 10.1007/s12154-009-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tallarida RJ, Antinociceptive synergy, additivity, and subadditivity with combinations of oral glucosamine plus nonopioid analgesics in mice, J. Pharmacol. Exp. Ther. 307 (2003) 699–704, 10.1124/jpet.103.054320. [DOI] [PubMed] [Google Scholar]
- [35].Craig WA, Choosing an antibiotic on the basis of pharmacodynamics, Ear Nose Throat J. 77 (1998) 7–12. [PubMed] [Google Scholar]
- [36].Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS, Getting drugs across biological barriers, Adv. Mater. 29 (2017), 10.1002/adma.201606596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dagan R, Leibovitz E, Greenberg D, Yagupsky P, Fliss DM, Leiberman A, Early eradication of pathogens from middle ear fluid during antibiotic treatment of acute otitis media is associated with improved clinical outcome, Pediatr. Infect. Dis. J. 17 (1998) 776–782 http://www.ncbi.nlm.nih.gov/pubmed/9779760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


