Abstract
Mutations in natriuretic peptide receptor 2 (Npr2) gene cause a rare form of short-limbed dwarfism, but its physiological effects have not been well studied. Human and mouse genetic data suggest that Npr2 in the kidney plays a role in salt homeostasis. Herein, we described anatomic changes within renal papilla of Npr2 knockout (Npr2−/−) mice. Dramatic reduction was found in diuresis, and albuminuria was evident after administration of 1% NaCl in drinking water in Npr2−/− and heterozygous (Npr2+/−) mice compared with their wild-type (Npr2+/+) littermates. There was indication of renal epithelial damage accompanied by high numbers of red blood cells and inflammatory cells (macrophage surface glycoproteins binding to galectin-3) and an increase of renal epithelial damage marker (T-cell Ig and mucin domain 1) in Npr2−/− mice. Addition of 1% NaCl tended to increase apoptotic cells (cleaved caspase 3) in the renal papilla of Npr2−/− mice. In vitro, genetic silencing of the Npr2 abolished protective effects of C-type natriuretic peptide, a ligand for Npr2, against death of M-1 kidney epithelial cells exposed to 360 mmol/L NaCl. Finally, significantly lower levels of expression of the NPR2 protein were detected in renal samples of hypertensive compared with normotensive human subjects. Taken together, these findings suggest that Npr2 is essential to protect renal epithelial cells from high concentrations of salt and prevent kidney injury.
The natriuretic peptide (NP) family in mammals comprises three structurally homologous but genetically distinct peptides, the atrial NP, brain NP, and C-type NP (CNP). These peptides have been suggested to be involved in blood pressure (BP) regulation, fluid and electrolyte balance, and cardiovascular homeostasis.1, 2, 3, 4, 5 Both atrial NP and brain NP are mainly produced in atrial and ventricular cardiomyocytes, whereas CNP is found in a variety of tissues and acts locally in an autocrine and paracrine manner.6 Atrial NP and brain NP have high affinity for NP receptor A (Npr1; alias NPR-A), whereas CNP binding is limited to NP receptor B (Npr2; alias NPR-B).7, 8 Binding of CNP to Npr2 increases the level of a second messenger, cGMP, which, in turn, activates protein kinase GI and phosphorylates target proteins, ultimately leading to regulation of a variety of physiological processes, including smooth muscle cell relaxation.9
Homozygous loss of function in the human NPR2 has been identified in patients with a rare form of short-limbed dwarfism called acromesomelic dysplasia, type Maroteaux.10 Homozygous deletion of Npr2 in mice (Npr2−/−) caused dwarfism and female sterility.11 Mutations in the human NPR2 gene were associated with essential hypertension in a Japanese population.12 Genetic studies between C57BL/6J and A/J inbred mouse strains mapped a Bpq3 locus on chromosome 4, and suggested Npr2 as a candidate for 1% NaCl–induced BP variation.13 Endothelial-specific deletion of CNP in mice demonstrated a pivotal role for CNP in BP homeostasis, although it appeared that signaling did not involve Npr2 receptor.14 It is likely that renal Npr2 plays an important role in kidney functions because it is widely expressed in several renal structures, including the glomeruli, tubules, and microvasculature.15, 16 In this study, we investigated the effects of Npr2 gene deletion on renal structure and function in response to 1% NaCl water intake.
Materials and Methods
Animals
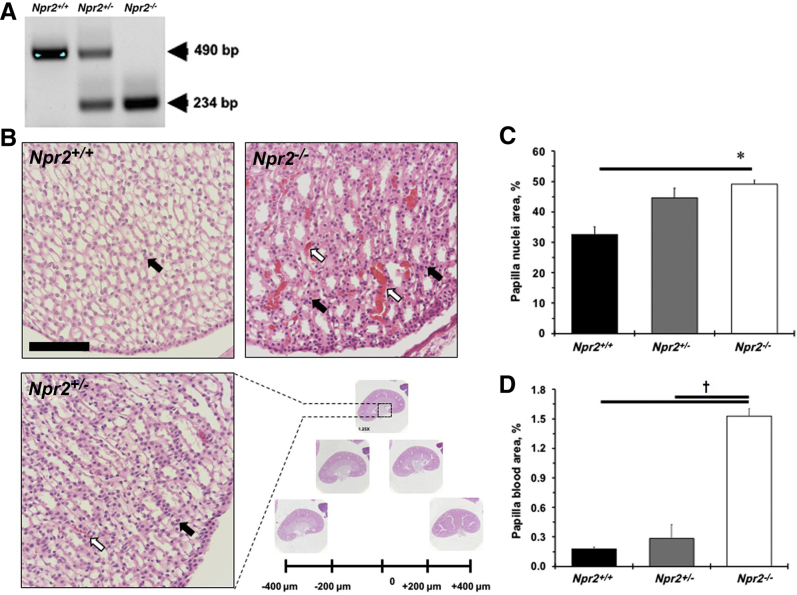
The Npr2tm1Gar/J breeding pair (stock number 007658) was obtained from The Jackson laboratory (Bar Harbor, ME). Genotyping was performed as described before.11 Briefly, mice that carry heterozygous alleles (Npr2+/−) had two bands, whereas one band was detected in wild-type (Npr2+/+; 490 bp) or knockout (Npr2−/−; 234 bp) littermates (Figure 1A) with a robust dwarfism phenotype.11 A 12-hour light/dark cycle was maintained (lights on at 6 am, lights off at 6 pm) for animal housing. Mice had free access to chow and water. Experimental mice were given regular chow and 1% NaCl in drinking water for 2 weeks, as originally reported.13 The study was approved by the University of Rochester (Rochester, NY) Animal Care Committee in accordance with the Guide for the Care and Use of Laboratory Animals.17
Figure 1.
Deletion of the Npr2 gene leads to renal papilla abnormality. A: A representative gel shows results of genotyping Npr2 wild-type (Npr2+/+), heterozygous (Npr2+/−), and knockout (Npr2−/−) littermates. Teal markings are PCR products in bp. B: A quantification strategy of series of kidney sections every 200 μm. Dotted boxed area highlights a portion of a magnified renal papilla. White arrows point to blood in renal papilla. Black arrows point to nuclei in renal papilla. C: Quantification of nuclei area in renal papilla. D: Quantification of blood area in renal papilla. Black bars are Npr2+/+ mice. Gray bars are Npr2+/− mice. White bars are Npr2−/− mice. Data are expressed as means ± SEM (C and D). n = 5 per group (C and D). ∗P < 0.05 versus Npr2+/+; †P < 0.05 versus Npr2−/−. Scale bar = 100 μm (B).
Renal Cell Culture
The M-1 ATCC cell line (ATCC, Manassas, VA) was maintained at 70% to 80% confluency in 1:1 Dulbecco's modified Eagle's and Ham's F12 medium with 120 mmol/L NaCl.18 M-1 cells were exposed to various concentrations of NaCl (120 to 600 mmol/L) in the medium for 1 hour, as before.19 The Npr2 gene was silenced with siRNA, or a negative control confirmed expression of the Npr2 and Gapdh using mouse primers (Integrated DNA Technologies, Skokie, IL) in the M-1 cells by real-time quantitative RT-PCR (CFX Connect; Bio-Rad, Hercules, CA). M-1 cells were pretreated with 100 nmol/L CNP (Sigma-Aldrich, St. Louis, MO) or phosphate-buffered saline (PBS) and exposed to 360 mmol/L NaCl medium for 1 hour. Dead cells were stained with trypan blue, and images were captured by the EVOS FL Auto Imaging System (Thermo Fisher Scientific, Waltham, MA). Relative expression of live over dead cells was measured using ImagePro Analyzer software version 6.2.1 (Media Cybernetics, Rockville, MD).
Hematology
Peripheral blood was collected via orbital bleeding into EDTA-coated tubes under isoflurane anesthesia, as before.20 Hematological parameters and peripheral blood cell count were measured in Npr2 mice using an automated cell counter (VetScan HM5; Abaxis, Union City, CA).
Ultrasound Measurement of Kidneys in Npr2 Mice
Renal artery and heart hemodynamic profiles and three-dimensional imaging of the right kidney were acquired with a high-resolution Vevo2100 ultrasound system (FUJIFILM VisualSonics, Toronto, ON, Canada), as described before.21, 22 FUJIFILM VisualSonics Vevo LAB analysis software version 1.6.0 was used to calculate hemodynamic volume and percentage vasculature measurements.
Blood Pressure Measurements in Npr2 Mice
BP was measured in isoflurane anesthetized Npr2 mice, as recently reported.23 Briefly, a 1F Mikro-tip transducer (Millar Instruments, Houston, TX) was placed in the left femoral artery and advanced into the descending aorta for recordings of the BP and heart rate. Parameters were analyzed using LabChartPro software version 7 (AD Instruments, Sydney, Australia).
Kidney Functions in Npr2 Mice
Details of urine collection, measurements of urine albumin, and fluid intake were previously described.21 Briefly, fluid intake and urination were measured for 24 hours in metabolic cages (Nalgene, North Las Vegas, NV) at baseline and 2 weeks after 1% NaCl. Proteinuria was determined by the ratio of albumin (μg)/creatinine (mg), as was standardized for mice.24 Peripheral blood was collected via cardiac puncture of anesthetized animals. The level of cGMP (Enzo Life Sciences, Farmingdale, NY) or creatinine (Abcam, Cambridge, UK) in the plasma was quantified by using enzyme-linked immunosorbent assay kits. FluostarOptima version 2.20R2 (BMG LabTech, Cary, NC) was used to measure urine and plasma samples, and concentrations were calculated on the basis of a standard curve.
Histology and Immunohistochemistry in Npr2 Mice
At the time of termination, mice were perfused and fixed with 10% paraformaldehyde in sodium phosphate buffer (pH 7.0). Bones were also collected and underwent decalcification with 0.5 mol/L EDTA before tissue processing, as before.25 Paraffin-embedded kidney sections were cut in series every 200 μm through the tip of the renal pelvis (Figure 1B). For general histology, cross-sections of bones and kidneys were stained with hematoxylin and eosin in autostainer XL (Leica, Wetzlar, Germany). For immunohistologic evaluation, cross-sections of kidneys were incubated with hydrous 3% H2O2 and followed by an antigen retrieval in a Decloaker buffer (pH 6.0; Biocare, Pacheco, CA) with high temperature and pressure. The sections were double stained with rat anti-mouse macrophage surface glycoproteins binding to galectin-3 (Mac-2; dilution 1:15,000; Cedarlane, Burlington, ON, Canada) and rabbit anti-mouse T-cell Ig and mucin domain 1 (TIM-1; dilution 1:5000; Thermo Fisher Scientific) antibodies, as recently reported.21 Rabbit antibodies against cleaved caspase 3 (dilution 1:100; Cell Signaling, Danvers, MA) and Npr2 (dilution 1:100; Abcam) were used, incubated overnight at 4°C, and followed by application of horseradish peroxidase or alkaline-phosphate polymers (Biocare). A secondary goat anti-rabbit antibody (dilution 1:400; Vector Laboratories, Burlingame, CA) with ABC kit (Vector Laboratories) was used to detect NPR2 in humans. Samples were counterstained with hematoxylin or methyl green. Images were captured by SPOT INSIGHT FireWire camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed in ImagePro Analyzer software (Media Cybernetics). A percentage of positive cells or staining was determined in relation to counterstained cells or background area within defined area of the kidney (renal medulla or papilla), as reported before.26
Human Samples
Cross-sections of deidentified human kidney biopsies from hypertensive or normotensive subjects were obtained with the approval from the University of Rochester School of Medicine and Dentistry Research Subjects Review Board (RSRB00073722).
Statistical Analysis
Results are reported as means ± SEM. Statistical tests were performed using JMP13.0.0 (SAS, Cary, NC). Differences between three or more groups were analyzed by one-way analysis of variance, followed by post-hoc Tukey-Kramer honestly significant difference test. P < 0.05 was regarded as significant.
Results
Characterization of the Npr2−/− Mouse Reveals a Significant Kidney Phenotype
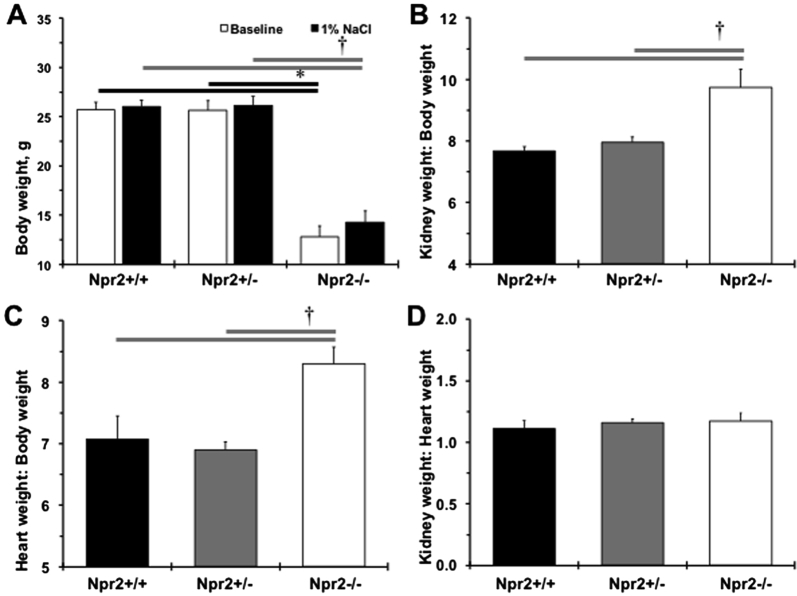
The importance of the Npr2 gene in the regulation of body weight was confirmed (Supplemental Figure S1A). A significant reduction in left ventricular mass and anterior wall diameter in systole was also observed in small Npr2−/− mice compared with their littermates (Supplemental Table S1). However, reductions in stroke volume, left ventricular anterior wall diameter, internal diameter, and posterior wall diameter in diastole were found in Npr2−/− compared with Npr2+/+ or Npr2+/− mice (Supplemental Table S1). Relative kidney weight/body weight, but not to heart weight, was increased in Npr2−/− mice after 1% NaCl intake for 2 weeks (Supplemental Figure S1, B–D). The most prominent changes were in the kidneys of the Npr2−/− mice (Figure 1B). Specifically, higher blood cell deposits were observed in the renal papilla of Npr2−/− mice (Figure 1, B and D). There was an increase in papilla cell nuclear number in relation to Npr2 gene depletion (Figure 1, B and C). The renal cortex histomorphometry was similar across Npr2 genotypes (data not shown). The increased nuclei and red blood cell deposits suggest an inflammatory and prothrombotic environment in kidneys of Npr2−/− mice.
Low Platelet Numbers in the Peripheral Blood in Npr2−/− Mice
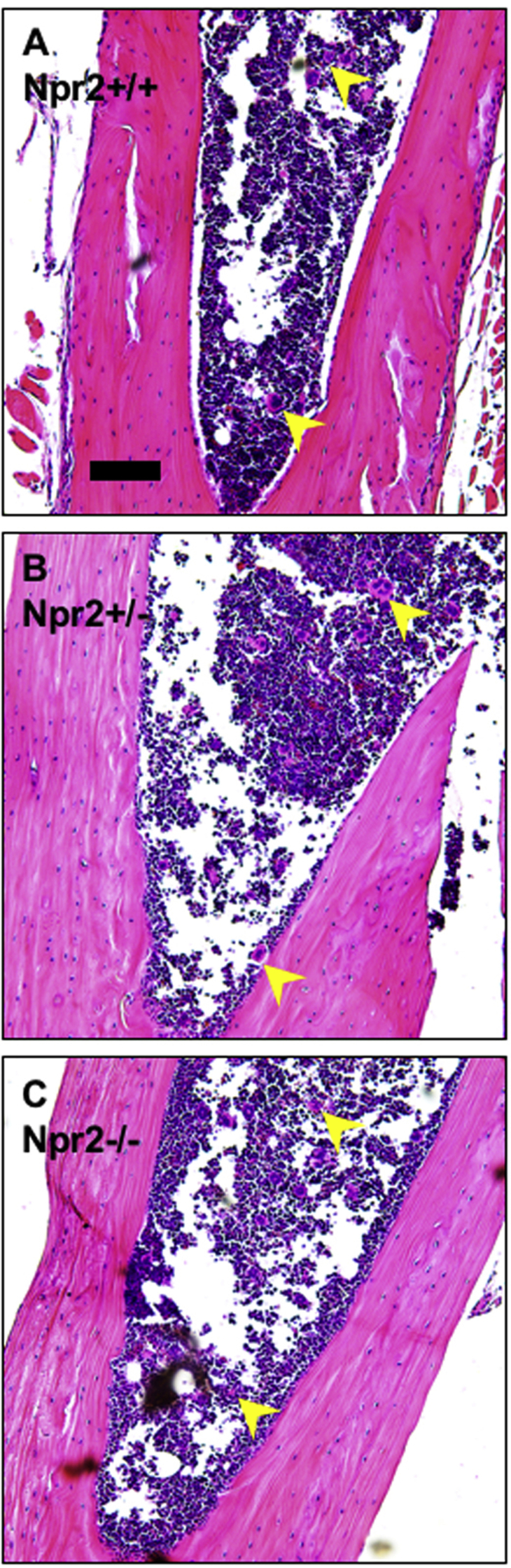
Alterations in circulating cells could lead to blood congestion in the kidneys. Npr2−/− mice had normal numbers of white and red blood cells as well as other rheological parameters in the peripheral blood (Table 1). There were significantly lower (approximately 20%) platelet numbers in the peripheral blood of Npr2−/− compared with Npr2+/+ mice (Table 1). However, a similar distribution of megakaryocytes in the bone marrow indicates normal platelet development regardless of Npr2 genotype (Supplemental Figure S2). These data suggest that lack of Npr2 results in a small reduction of platelets in the peripheral blood.
Table 1.
Hematological Parameters across Npr2+/+, Npr2+/−, and Npr2−/− Mice
| Genotype parameter | Npr2+/+ mice (n = 10) | Npr2+/− mice (n = 14) | Npr2−/− mice (n = 8) |
|---|---|---|---|
| White blood cells, ×109/L | 8.9 ± 0.6 | 7.5 ± 0.5 | 8.4 ± 0.8 |
| Lymphocytes, ×109/L | 8.8 ± 0.7 | 7.4 ± 0.5 | 7.5 ± 0.7 |
| Neutrophils, ×109/L | 0.48 ± 0.07 | 0.44 ± 0.07 | 0.57 ± 0.11 |
| Monocytes, ×109/L | 0.23 ± 0.05 | 0.16 ± 0.02 | 0.21 ± 0.03 |
| Red blood cells, ×109/L | 10.8 ± 0.4 | 10.8 ± 0.1 | 10.8 ± 0.2 |
| Platelets, ×109/L | 595 ± 50 | 578 ± 22 | 484 ± 38∗ |
| Hematocrit, % | 46.6 ± 1.3 | 46.0 ± 0.6 | 46.4 ± 1.1 |
| Hemoglobin, g/dL | 15.2 ± 0.4 | 15.2 ± 0.2 | 15.7 ± 0.1 |
| Plateletcrit, % | 0.39 ± 0.03 | 0.38 ± 0.02 | 0.31 ± 0.02 |
| Mean corpuscular volume, fL | 43.7 ± 0.3 | 43.1 ± 0.3 | 43.3 ± 0.7 |
| Mean corpuscular hemoglobin, pg | 14.2 ± 0.3 | 14.3 ± 0.3 | 14.7 ± 0.3 |
| Mean corpuscular hemoglobin concentration, g/dL | 32.7 ± 0.9 | 33.2 ± 0.8 | 34.0 ± 0.8 |
| Red blood cell distribution width, % | 18.3 ± 0.1 | 18.2 ± 0.1 | 18.6 ± 0.3 |
| Mean platelet volume, fL | 6.5 ± 0.1 | 6.3 ± 0.1 | 6.3 ± 0.1 |
| Platelet distribution width, % | 30.0 ± 0.3 | 29.4 ± 0.2 | 30.0 ± 0.5 |
Parameters are shown as means ± SEM.
Npr2+/+, Npr2 wild type; Npr2+/−, Npr2 heterozygous; Npr2−/−, Npr2 knockout.
P < 0.05 versus Npr2+/+.
Kidney Dysfunction after Salt Load in Npr2−/− Mice
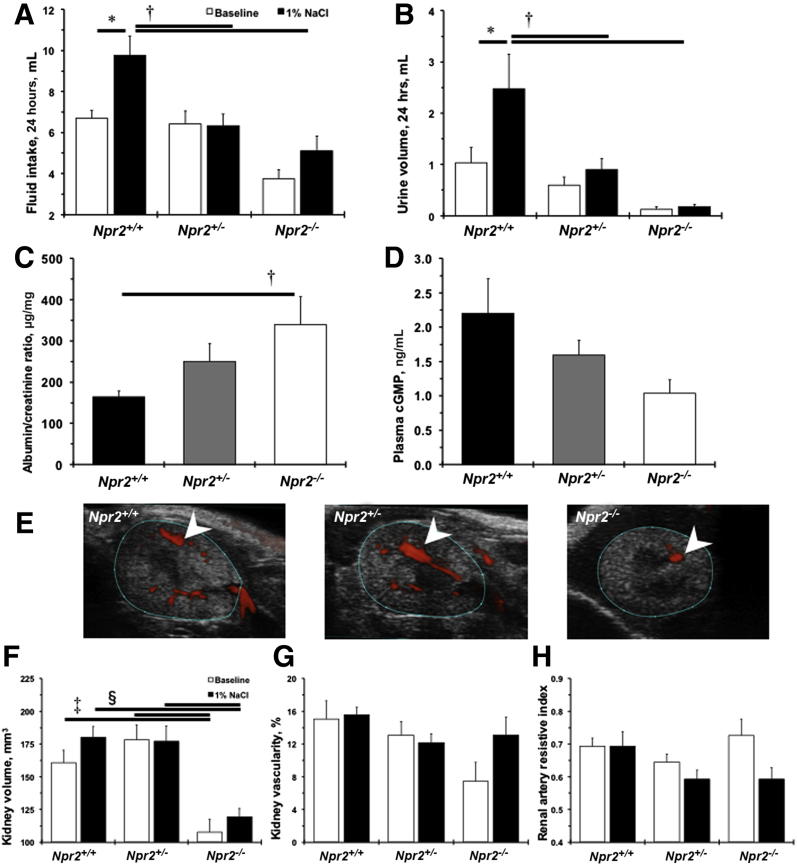
Npr2 was discovered as a candidate gene for BP variation after 1% NaCl intake in a cross between C57BL/6J and A/J inbred mouse strains.13 No differences were observed in BP or heart rate among Npr2 mice genotypes at baseline or after giving them 1% NaCl for 2 weeks (Table 2). Two weeks of 1% NaCl water doubled fluid intake and urination in Npr2+/+ mice (Figure 2, A and B). These functional responses to 1% NaCl were diminished in Npr2+/− and Npr2−/− mice (Figure 2, A and B). Significant albuminuria was detected in Npr2−/− compared with Npr2+/+ mice after 1% NaCl (Figure 2C). Plasma levels of cGMP did not reach statistical significance between Npr2−/− and Npr2+/+ mice after 2 weeks of 1% NaCl intake (Figure 2D). Ultrasound measurements revealed smaller kidney volumes in Npr2−/− mice compared with Npr2+/+ and Npr2+/− mice at baseline (Figure 2, E–G). Treatment with 1% NaCl water had little effect on kidney volume or renal artery hemodynamics in Npr2 mice (Figure 2, F–H, and Supplemental Table S2). Taken together, these data show that the Npr2 gene is protective against kidney dysfunction in response to a 1% salt load.
Table 2.
Hemodynamic Changes across Npr2+/+, Npr2+/−, and Npr2−/− Mice after 1% NaCl Intake for 2 Weeks
| Parameter | Genotype group | Npr2+/+ mice | Npr2+/− mice | Npr2−/− mice |
|---|---|---|---|---|
| Systolic BP, mmHg | Baseline | 98 ± 4 | 97 ± 7 | 91 ± 4 |
| 1% NaCl | 91 ± 2 | 95 ± 2 | 89 ± 7 | |
| Diastolic BP, mmHg | Baseline | 66 ± 5 | 71 ± 5 | 66 ± 3 |
| 1% NaCl | 62 ± 2 | 65 ± 2 | 65 ± 5 | |
| Pulse pressure, mmHg | Baseline | 33 ± 4 | 26 ± 2 | 25 ± 1 |
| 1% NaCl | 29 ± 2 | 29 ± 2 | 25 ± 2 | |
| Mean arterial pressure, mmHg | Baseline | 79 ± 6 | 84 ± 6 | 78 ± 3 |
| 1% NaCl | 76 ± 2 | 79 ± 2 | 76 ± 6 | |
| Heart rate, beats/minute | Baseline | 515 ± 56 | 490 ± 54 | 483 ± 45 |
| 1% NaCl | 463 ± 51 | 443 ± 39 | 462 ± 15 |
Parameters are shown as means ± SEM. n = 3 to 5 per group.
BP, blood pressure; Npr2+/+, Npr2 wild type; Npr2+/−, Npr2 heterozygous; Npr2−/−, Npr2 knockout.
Figure 2.
Lack of Npr2 worsens kidney dysfunction in response to salt. A: Fluid intake for 24 hours across Npr2 mice. B: Urine volume for 24 hours across Npr2 mice. White bars are baseline values. Black bars are values after 2 weeks of intake of 1% NaCl in drinking water. C and D: A ratio of urine albumin/plasma creatinine (C) and plasma cGMP (D) across Npr2 genotypes after 2 weeks of 1% NaCl intake in drinking water. Black bars are Npr2 wild-type (Npr2+/+) mice. Gray bars are Npr2 heterozygous (Npr2+/−) mice. White bars are Npr2 knockout (Npr2−/−) mice. E: Representative ultrasound images of a transverse plane of kidneys across Npr2 mice. Blue outlined areas define kidney boundaries. White arrowheads point to vascularity. F: Kidney volumes based on 3-dimensional (3D) imaging of the kidneys across Npr2 genotypes. G: Percentages of the kidney vasculature based on 3D imaging of the kidneys across Npr2 genotypes. H: Renal artery resistive index based on ultrasound imaging across Npr2 genotypes. White bars are baseline values. Black bars are values after 2 weeks of 1% NaCl intake in drinking water. Data are expressed as means ± SEM. n = 7 to 11 per group (A, B, and F–G); n = 5 to 9 per group (C and D). ∗P < 0.05 versus Npr2+/+ (baseline); †P < 0.05 versus Npr2+/+ (1% NaCl); ‡P < 0.05 versus Npr2−/− (baseline); §P < 0.05 versus Npr2−/− (1% NaCl).
Deletion of Npr2 Gene Leads to Kidney Injury after Salt Intake
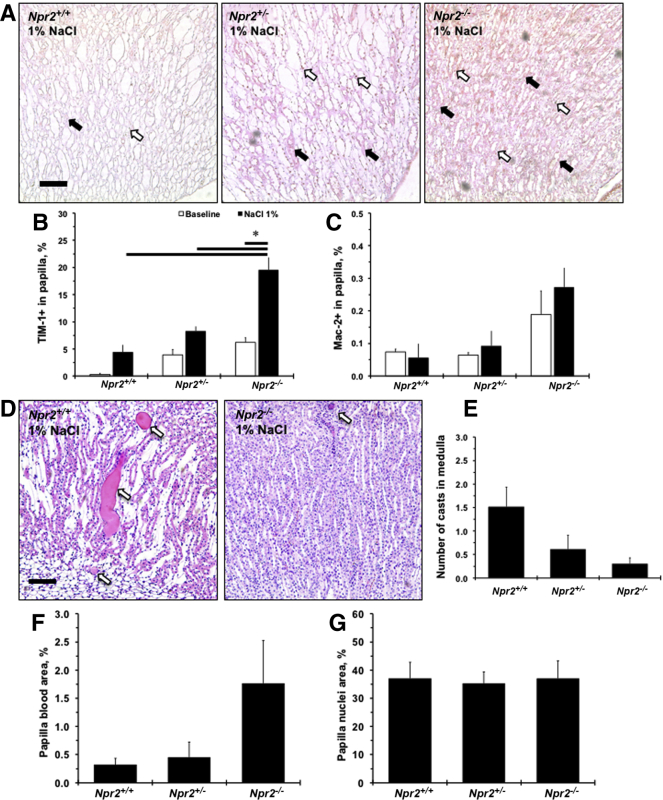
Expression of an epithelial damage marker, TIM-1, was weak and tended to increase after 1% NaCl in renal papilla in Npr2+/+ mice (P < 0.1) (Figure 3, A and B). The strongest TIM-1 staining was in Npr2−/− after 1% NaCl intake compared with Npr2+/+ and Npr2+/− mice (Figure 3, A and B). There was a trend (P < 0.1) toward increased presence of Mac-2+ cells in the renal papilla in Npr2−/− mice after NaCl (Figure 3, A and C). Of interest, fewer protein casts were observed in the renal medulla of Npr2−/− mice compared with Npr2+/− and Npr2+/+ mice after NaCl (Figure 3, D and E). In the renal papilla of Npr2−/− mice, there was a trend (P < 0.1) toward increased red blood cell aggregates after NaCl (Figure 3F). Finally, there were no differences in cell nuclei in renal papilla across Npr2 genotypes after salt exposure (Figure 3G). Loss of the Npr2 gene causes renal papilla injury, which is exacerbated by salt.
Figure 3.
Histologic evaluation of renal papillary injury across Npr2 genotypes in response to salt. A: Representative images of double-stained [macrophage surface glycoproteins binding to galectin-3 (Mac-2) and T-cell Ig and mucin domain 1 (TIM-1)] kidney papilla across Npr2 genotypes after 1% NaCl intake in drinking water for 2 weeks. White arrows show Mac-2+ cells (brown). Black arrows show TIM-1+ staining (pink). Counterstain is green. B and C: Quantitative analysis of the TIM-1 (B) and Mac-2 (C) expression in the renal papilla in Npr2 mice. D: Representative images of hematoxylin and eosin–stained renal medulla of Npr2 wild-type (Npr2+/+) and Npr2 knockout (Npr2−/−) mice after 1% NaCl intake in drinking water for 2 weeks. White arrows point to protein casts. E: Quantitative analysis of protein casts in the renal medulla in Npr2 mice. No protein casts were detected at baseline. F: Quantification of blood in renal papilla in Npr2 mice. G: Quantification of nuclei area in renal papilla in Npr2 mice. White bars are baseline values. Black bars are values after 2 weeks of 1% NaCl intake in drinking water. Data are expressed as means ± SEM. n = 3 per group (B and C); n = 3 to 5 per group (E–G). ∗P < 0.05 versus Npr2−/− (1% NaCl). Scale bars = 100 μm (A and D). Npr2+/−, Npr2 heterozygous.
Protective Effects of the CNP/Npr2 Pathway in Renal Epithelial Cells
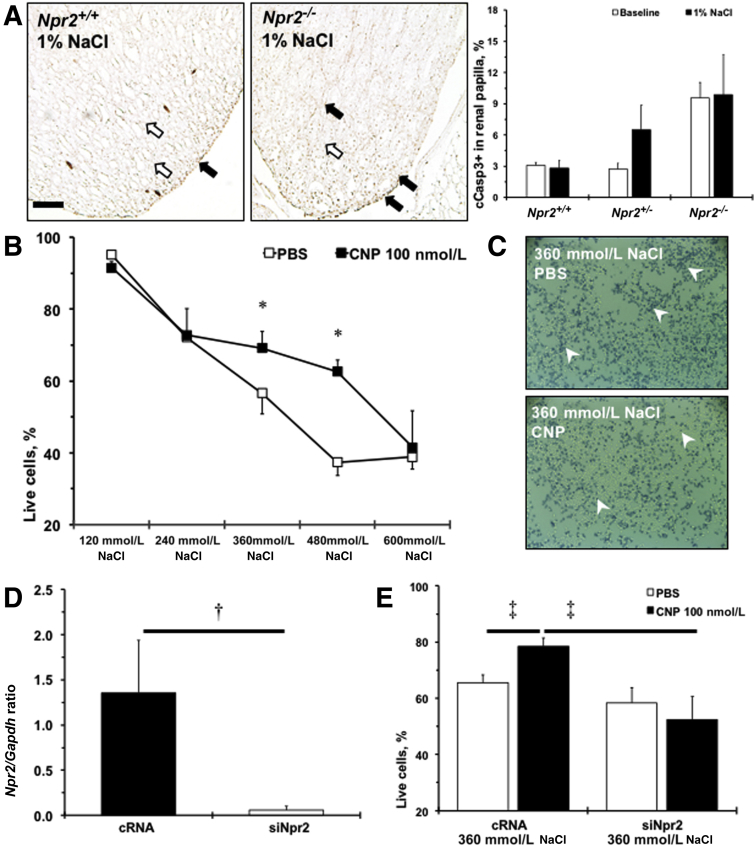
Administration of the Npr2 ligand, CNP, was shown to reduce cell death in the medulla after kidney damage.27 Two weeks of 1% NaCl tended (P < 0.1) to increase cleaved caspase 3–positive cells at the tip of renal papilla in Npr2+/− and remained elevated in Npr2−/− compared with Npr2+/+ mice (Figure 4A). The number of apoptotic cells measured by cleaved caspase 3 immunohistochemistry within the renal cortex was similar across Npr2 genotypes (data not shown). Previous data showed that renal epithelial cells could withstand high levels of NaCl in vitro, which reflects the in vivo fivefold increase in osmolality from renal cortex to papilla.19, 28 A significant reduction of survived M-1 cells to 40% with increased concentration of NaCl from 120 to 600 mmol/L was observed (Figure 4B). However, pretreatment with CNP significantly increased numbers of surviving M-1 cells at 360 and 480 mmol/L of NaCl (Figure 4, B and C). Npr2 was expressed in M-1 cells, and Npr2 siRNA drastically reduced its expression (Figure 4D). Knocking down of Npr2 gene through Npr2 siRNA abolished the protective effects of the CNP against 360 mmol/L NaCl-induced M-1 cell death (Figure 4E). These findings suggest that activation of Npr2 by CNP is important in protecting renal epithelial cells against high salt.
Figure 4.
Npr2 protects renal epithelial cells against apoptosis in response to high salt. A: Representative images of cleaved caspase 3 staining (cCasp3; brown; black arrows) of renal papilla of Npr2 wild-type (Npr2+/+) and Npr2 knockout (Npr2−/−) mice after 1% NaCl intake in drinking water for 2 weeks. Counterstained nuclei are indicated by white arrows. Bar graph shows a quantitative analysis of cCasp3 expression in the renal papilla across experimental mice. White bars are baseline values. Black bars are values after 2 weeks of 1% NaCl intake in drinking water. B: C-type natriuretic peptide (CNP) protects M-1 cells from higher concentrations of NaCl in the medium. White squares show phosphate-buffered saline (PBS) treatment. Black squares show CNP (100 nmol/L) treatment. C: Representative images of trypan blue staining (white arrowheads) of treated M-1 cells at 360 mmol/L NaCl. D: Relative expression of Npr2 on gene silencing in M-1 cells. E:Npr2 depletion abolishes protective effects of CNP in M-1 cells at 360 mmol/L NaCl. White bars show PBS treatment. Black bars show CNP (100 nmol/L) treatment. Data are expressed as the means ± SEM. n = 3 per group (A and B); n = 3 to 4 per group (D and E). ∗P < 0.05 versus PBS treatment; †P < 0.05 versus complementary RNA (cRNA); ‡P < 0.05 versus cRNA (CNP, 100 nmol/L). Scale bar = 100 μm (A). siNpr2, silencing of Npr2.
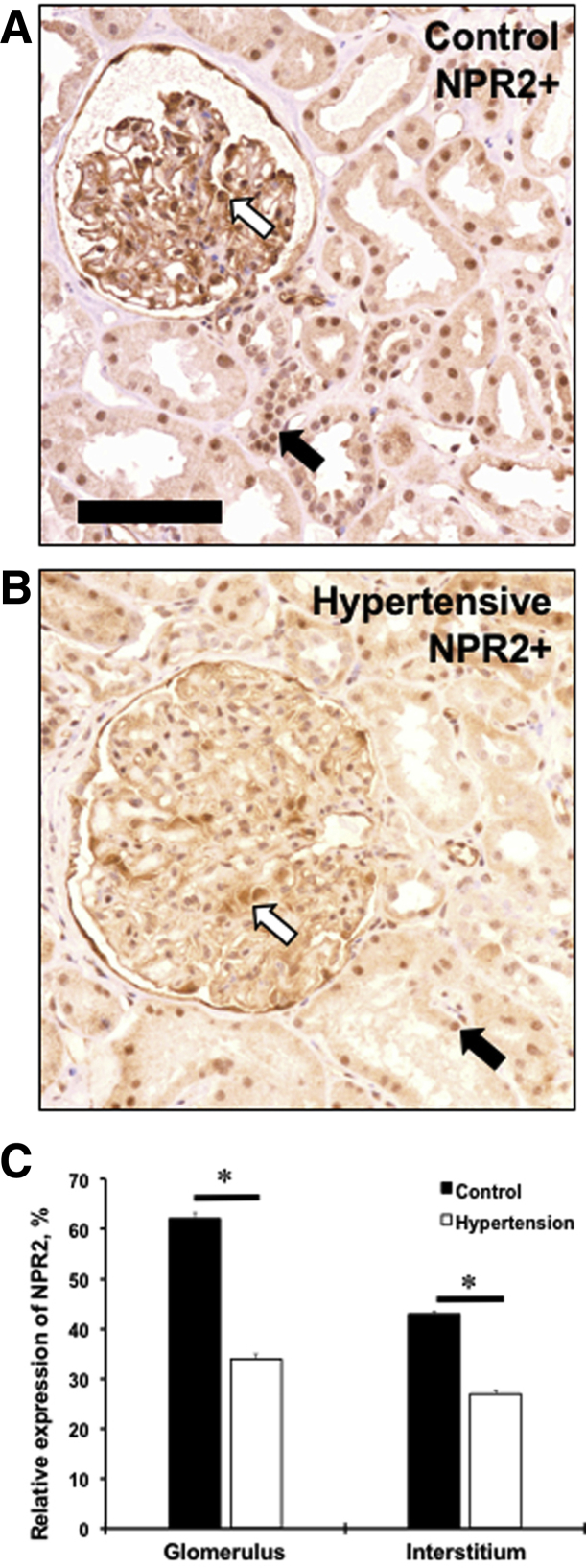
NPR2 Expression Is Decreased in Human Hypertensive Kidney
A human genetic study suggested that mutations in NPR2 gene are associated with essential hypertension.12 As in mouse kidneys, expression of the NPR2 was evident in the interstitial compartment with a greater intensity in a glomerular area of the normotensive human kidney (Figure 5, A and C). There was a significant decrease in relative expression of the NPR2 in hypertensive kidney compared with normotensive controls (Figure 5, B and C). Thus, a decreased expression of NPR2 in human disease supports our findings on the protective role of the receptor in kidney dysfunction in mice.
Figure 5.
NPR2 expression is decreased in kidneys of hypertensive patients. A and B: Renal expression of NPR2 (brown staining) in control (A) and hypertensive (B) kidney samples. White arrows point to NPR2+ cells in glomerulus. Black arrows point to NPR2+ cells in interstitium. C: Quantification of NPR2 immunoreactivity in kidney compartments on x axis. Values are expressed as means ± SEM. n = 5 per group (C). ∗P < 0.05 versus control. Scale bar = 100 μm (A and B).
Discussion
A major finding in the present study is that the Npr2 gene prevents renal dysfunction by protecting renal papilla cells against salt-induced damage. A short-limbed dwarfism in Npr2−/− mice in our colony was confirmed, as was originally reported.11 For the first time, we described a phenotype of blood cell deposits in renal papilla with a slight reduction of platelets in blood in Npr2−/− mice. Npr2−/− and Npr2+/− mice showed reduced diuresis, and 1% NaCl in drinking water significantly worsened albuminuria and renal dysfunction in these mice. There was an increase in renal epithelial damage (TIM-1+ staining) with blood cell deposits and inflammatory cells (Mac-2+) within renal papilla in mice with Npr2 depletion. Both Npr2−/− and Npr2+/− mice exhibited worse responses to salt, likely because of augmentation in cell death in renal papilla. In fact, there was a trend toward increased apoptotic cells (cleaved caspase 3 positive) near the tip of the renal papilla in Npr2−/− mice. A knockdown of the Npr2 with siRNA in M-1 cells abolished beneficial effects of CNP after exposure to 360 mmol/L of NaCl. Finally, significantly lower levels of NPR2 protein were detected in renal samples of hypertensive patients.
The NPR2 gene is associated with essential hypertension in the Japanese population in addition to a genetic link to a rare form of dwarfism.12 A study with a majority of European ancestry patients with coronary artery disease showed that a minor allele of NPR2 (rs10758325) was significantly associated with a lower rate of cardiovascular outcomes but not with BP.29 Intriguingly, another report suggested that shorter individuals are more prone to cardiovascular disease than their taller counterparts.30 Npr2 was implicated as a candidate gene within the Bpq3 locus in salt-induced BP variation in a mouse genetic cross between C57BL/6J and A/J inbred mouse strains.13 Recent studies with cell-specific gene targeting in mice or spontaneously hypertensive rats suggested that BP homeostasis is primarily regulated by CNP and Npr3 (not Npr2) that is produced by the endothelium.14, 31, 32 A genome-wide association study showed that genetic variations in NPPA, NPPB, and NPR3 genes affected BP, but not kidney dysfunction.33 Similar to the original findings after Npr2 deletion on BP,11 the same BP was also observed across all Npr2 genotypes. In contrast, decreased expression of Npr2 was associated with renal kidney dysfunction, which was augmented by 1% NaCl in drinking water. A recent report showed that a release of CNP from endothelial cells primarily relaxes precapillary arterioles and capillaries through activation of the Npr2/cGMP axis in pericytes.34 These data suggest additional roles for the CNP/Npr2 pathway in the autocrine regulation of renal epithelium that contribute to response to salt.
A decrease in diuresis was observed after Npr2 gene depletion, which correlated well with anatomic changes in renal medulla. These pathologic alterations in the kidney worsened after 2 weeks of 1% NaCl intake. However, renal cortex appeared to be normal in Npr2−/− mice. The affected compartment of the kidney medulla, renal papilla, is responsible for transporting urine produced in the renal cortex to the cup-shaped cavity where the urine accumulates before passing through the ureter into the bladder.35 Sodium concentration gradually increases from the base to the tip of the renal pelvis.36 Dehydration of rats significantly increased Na+ and urea in the interstitial fluid in renal papilla.28 An Npr2 gene-titration effect was also observed on fluid intake associated with dehydration, especially after 1% NaCl intake in Npr2+/− and Npr2−/− mice. High levels of apoptosis in renal papilla in vivo and an increase in M-1 cell death in vitro with Npr2 depletion support the prosurvival effects of CNP/Npr2 signals in epithelial cells under high concentrations of NaCl. It has been shown that renal epithelial cells resist elevated Na+ compared with other cell types.19 In addition, the effects of Npr2 on platelet count may exacerbate epithelial apoptosis by increasing parenchymal bleeding in Npr2−/− mice. The deposition of iron from red blood cells would increase reactive oxygen species, which could cause apoptosis. It is likely that reduced platelet count in Npr2−/− mice is due to increase in platelet clearance rather than a production issue as no expression of NPRs in human platelets has been reported.37 Herein, it was confirmed that Npr2 is a critical receptor for CNP-dependent renal protection against high salt.
Renal papilla has low blood supply, which might make it vulnerable to ischemia and necrosis. In fact, CNP administration inhibited oxidative and apoptotic pathways and ameliorated acute kidney injury in a rat model of renal ischemia/reperfusion injury.38 High concentrations of drugs and their metabolites in the renal medulla have been shown to contribute to renal papilla damage.39, 40 For example, cisplatin is a potent chemotherapeutic agent but is highly toxic to renal tubular cells.41 Administration of cisplatin to rats induced nephropathy, which was accompanied by a significant reduction in the levels of cGMP in renal papilla and decreased expression of Nprs.42 A coadministration of cisplatin with CNP reduced cisplatin-induced nephropathy in mice.27 The authors found that CNP prevented decline in Npr2 expression with significant reduction of markers of renal tubular damage (apoptosis and inflammation) compared with mice that received cisplatin alone. In this study, depletion of Npr2 significantly increased apoptosis, inflammation, and tubular injury after 1% NaCl intake. These findings suggest that CNP and Npr2 are important survival factors for renal papillary cells, especially in response to pathophysiological insults to the kidney medulla.
One of the limitations of this study is related to the confounding effects of the small size of mice lacking Npr2. For example, heart and kidney sizes were significantly smaller. Furthermore, BP could be directly measured under anesthesia only, which was lower compared with BP values in response to 1% NaCl in conscious C57BL/6J mice.13 Nevertheless, these findings are in line with an original report suggesting a minimal role for Npr2 in BP increase after salt.11 In contrast, renal phenotypes were augmented not only in Npr2−/− but also in Npr2+/− mice compared with Npr2+/+ littermates after salt intake. Although M-1 cells were originally derived from cortical collecting ducts of mouse kidney, these cells are accepted as a model for studies of renal epithelial cells. Known profiles of the expression of Npr2 within kidneys make our experiments relevant to functions of renal epithelium.15, 16 Although limited data are presented in human kidneys, a lower immunoreactivity to NPR2 protein in hypertensive patients supports a paradigm of decreased expression of the receptor under pathologic conditions.42 Future studies evaluating CNP and NPR2 are warranted in humans with renal damage.
In summary, the present study is the first to report on the importance of the Npr2 gene in kidney function. These findings highlight effects of CNP/Npr2-mediated protection of renal papilla cells under high concentrations of NaCl. These results could lead to new therapeutic approaches in patients with salt-sensitive hypertension and other kidney disorders.
Acknowledgments
We thank Qian Zhou and Kathy Donlon for help with animal handling and histologic processing of mouse kidneys.
V.A.K. and B.C.B. conceived and managed the project; G.J.D., B.Q., L.L., D.M.M., S.K.T., and V.A.K. performed experiments; G.J.D., B.Q., D.M.M., and V.A.K. analyzed data; G.J.D., B.Q., and V.A.K. prepared figures; C.N.M., J.C., M.M.D., and C.Y. interpreted results; G.J.D. wrote the manuscript; G.J.D., L.L., D.M.M., C.N.M., J.C., M.M.D., C.Y., B.C.B., and V.A.K. edited and revised the manuscript; and G.J.D., B.Q., L.L., D.M.M., S.K.T., C.N.M., J.C., M.M.D., C.Y., B.C.B., and V.A.K. approved the final version of the manuscript.
Footnotes
Supported in part by the University of Rochester Award 2016 (V.A.K. and M.M.D.) and NIH grants R01 HL134910 (C.Y.) and R01 HL062826 (B.C.B.).
Disclosures: V.A.K. received research support from Novartis Pharmaceuticals Corp.
Current address of G.J.D., Department of Pharmacology and Toxicology, School of Pharmacy, College of Health Sciences, University of Ghana, Accra, Ghana.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2019.05.020.
Contributor Information
Bradford C. Berk, Email: bradford_berk@urmc.rochester.edu.
Vyacheslav A. Korshunov, Email: slava_korshunov@urmc.rochester.edu.
Supplemental Data
Supplemental Figure S1.
Changes in weights of Npr2 mice after 1% NaCl intake in drinking water for 2 weeks. A: Body weights across Npr2 genotypes. White bars show body weights at the baseline. Black bars show body weight after 2 weeks of 1% NaCl. B–D: Relative kidney weight/body weight (B), heart weight/body weight (C), kidney weight/heart weight (D) ratios after 2 weeks of 1% NaCl administration. Black bars show Npr2 wild-type (Npr2+/+) mice. Gray bars show Npr2 heterozygous (Npr2+/−) mice. White bars show Npr2 knockout (Npr2−/−) mice. Data are expressed as means ± SEM (A–D). n = 5 to 9 per group. ∗P < 0.05 versus Npr2−/− (baseline); †P < 0.05 versus Npr2−/− (1% NaCl).
Supplemental Figure S2.
Bone marrow sections in Npr2 mice. Representative images of bone marrow across Npr2 wild-type (Npr2+/+; A), heterozygous (Npr2+/−; B), and knockout (Npr2−/−; C) mice. Yellow arrowheads point to megakaryocytes. Scale bar = 100 μm (A–C).
References
- 1.Kinnunen P., Vuolteenaho O., Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzweig A., Seidman C.E. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 3.Brenner B.M., Ballermann B.J., Gunning M.E., Zeidel M.L. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 4.Inagami T. Atrial natriuretic factor. J Biol Chem. 1989;264:3043–3046. [PubMed] [Google Scholar]
- 5.de Bold A.J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 6.Suga S.-I., Nakao K., Itoh H., Komatsu Y., Ogawa Y., Hama N., Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor B. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsukawa N., Grzesik W.J., Takahashi N., Pandey K.N., Pang S., Yamauchi M., Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suga S., Nakao K., Hosoda K., Mukoyama M., Ogawa Y., Shirakami G., Arai H., Saito Y., Kambayashi Y., Inouye K., Imura H. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 9.Potter L.R., Abbey-Hosch S., Dickey D.M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 10.Bartels C.F., Bukulmez H., Padayatti P., Rhee D.K., van Ravenswaaij-Arts C., Pauli R.M., Mundlos S., Chitayat D., Shih L.Y., Al-Gazali L.I., Kant S., Cole T., Morton J., Cormier-Daire V., Faivre L., Lees M., Kirk J., Mortier G.R., Leroy J., Zabel B., Kim C.A., Crow Y., Braverman N.E., van den Akker F., Warman M.L. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura N., Doolittle L.K., Hammer R.E., Shelton J.M., Richardson J.A., Garbers D.L. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci U S A. 2004;101:17300–17305. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehemudula D., Nakayama T., Soma M., Takahashi Y., Uwabo J., Sato M., Izumi Y., Kanmatsuse K., Ozawa Y. Structure of the type B human natriuretic peptide receptor gene and association of a novel microsatellite polymorphism with essential hypertension. Circ Res. 1999;84:605–610. doi: 10.1161/01.res.84.5.605. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama F., Churchill G.A., Higgins D.C., Johns C., Makaritsis K.P., Gavras H., Paigen B. Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics. 2001;71:70–77. doi: 10.1006/geno.2000.6401. [DOI] [PubMed] [Google Scholar]
- 14.Moyes A.J., Khambata R.S., Villar I., Bubb K.J., Baliga R.S., Lumsden N.G., Xiao F., Gane P.J., Rebstock A.S., Worthington R.J., Simone M.I., Mota F., Rivilla F., Vallejo S., Peiro C., Sanchez Ferrer C.F., Djordjevic S., Caulfield M.J., MacAllister R.J., Selwood D.L., Ahluwalia A., Hobbs A.J. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. doi: 10.1172/JCI74281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terada Y., Tomita K., Nonoguchi H., Yang T., Marumo F. PCR localization of C-type natriuretic peptide and B-type receptor mRNAs in rat nephron segments. Am J Physiol. 1994;267:F215–F222. doi: 10.1152/ajprenal.1994.267.2.F215. [DOI] [PubMed] [Google Scholar]
- 16.Dean A.D., Vehaskari V.M., Ritter D., Greenwald J.E. Distribution and regulation of guanylyl cyclase type B in the rat nephron. Am J Physiol. 1996;270:F311–F318. doi: 10.1152/ajprenal.1996.270.2.F311. [DOI] [PubMed] [Google Scholar]
- 17.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council . National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 18.Ilatovskaya D.V., Chubinskiy-Nadezhdin V., Pavlov T.S., Shuyskiy L.S., Tomilin V., Palygin O., Staruschenko A., Negulyaev Y.A. Arp2/3 complex inhibitors adversely affect actin cytoskeleton remodeling in the cultured murine kidney collecting duct M-1 cells. Cell Tissue Res. 2013;354:783–792. doi: 10.1007/s00441-013-1710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida S., Green N., Coon H., Triche T., Mims S., Burg M. High NaCl induces stable changes in phenotype and karyotype of renal cells in culture. Am J Physiol. 1987;253:C230–C242. doi: 10.1152/ajpcell.1987.253.2.C230. [DOI] [PubMed] [Google Scholar]
- 20.Batchu S.N., Hughson A., Wadosky K.M., Morrell C.N., Fowell D.J., Korshunov V.A. Role of Axl in T-lymphocyte survival in salt-dependent hypertension. Arterioscler Thromb Vasc Biol. 2016;36:1638–1646. doi: 10.1161/ATVBAHA.116.307848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batchu S.N., Dugbartey G.J., Wadosky K.M., Mickelsen D.M., Ko K.A., Wood R.W., Zhao Y., Yang X., Fowell D.J., Korshunov V.A. Innate immune cells are regulated by Axl in hypertensive kidney. Am J Pathol. 2018;188:1794–1806. doi: 10.1016/j.ajpath.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batchu S.N., Xia J., Ko K.A., Doyley M.M., Abe J., Morrell C.N., Korshunov V.A. Axl modulates immune activation of smooth muscle cells in vein graft remodeling. Am J Physiol Heart Circ Physiol. 2015;309:H1048–H1058. doi: 10.1152/ajpheart.00495.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varghese J.J., Schmale I.L., Mickelsen D., Hansen M.E., Newlands S.D., Benoit D.S.W., Korshunov V.A., Ovitt C.E. Localized delivery of amifostine enhances salivary gland radioprotection. J Dent Res. 2018 doi: 10.1177/0022034518767408. 22034518767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breyer M.D., Bottinger E., Brosius F.C., 3rd, Coffman T.M., Harris R.C., Heilig C.W., Sharma K. Amdcc: mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 25.Harms J.F., Budgeon L.R., Christensen N.D., Welch D.R. Maintaining GFP tissue fluorescence through bone decalcification and long-term storage. Biotechniques. 2002;33:1197–1200. doi: 10.2144/02336bm02. [DOI] [PubMed] [Google Scholar]
- 26.Gerloff J., Korshunov V.A. Immune modulation of vascular resident cells by Axl orchestrates carotid intima-media thickening. Am J Pathol. 2012;180:2134–2143. doi: 10.1016/j.ajpath.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura T., Nojiri T., Hosoda H., Ishikane S., Shintani Y., Inoue M., Miyazato M., Okumura M., Kangawa K. Protective effects of C-type natriuretic peptide on cisplatin-induced nephrotoxicity in mice. Cancer Chemother Pharmacol. 2015;75:1057–1063. doi: 10.1007/s00280-015-2734-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee J., Williams P.G. Changes of sodium and urea concentrations in the renal papillary interstitial fluid on dehydration of rats. J Physiol. 1971;218:195–204. doi: 10.1113/jphysiol.1971.sp009610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis K.L., Newton-Cheh C., Wang T.J., Frampton C.M., Doughty R.N., Whalley G.A., Ellis C.J., Skelton L., Davis N., Yandle T.G., Troughton R.W., Richards A.M., Cameron V.A. Association of genetic variation in the natriuretic peptide system with cardiovascular outcomes. J Mol Cell Cardiol. 2011;50:695–701. doi: 10.1016/j.yjmcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Nelson C.P., Hamby S.E., Saleheen D., Hopewell J.C., Zeng L., Assimes T.L. Genetically determined height and coronary artery disease. N Engl J Med. 2015;372:1608–1618. doi: 10.1056/NEJMoa1404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakao K., Kuwahara K., Nishikimi T., Nakagawa Y., Kinoshita H., Minami T., Kuwabara Y., Yamada C., Yamada Y., Tokudome T., Nagai-Okatani C., Minamino N., Nakao Y.M., Yasuno S., Ueshima K., Sone M., Kimura T., Kangawa K., Nakao K. Endothelium-derived c-type natriuretic peptide contributes to blood pressure regulation by maintaining endothelial integrity. Hypertension. 2017;69:286–296. doi: 10.1161/HYPERTENSIONAHA.116.08219. [DOI] [PubMed] [Google Scholar]
- 32.Caniffi C., Cerniello F.M., Gobetto M.N., Sueiro M.L., Costa M.A., Arranz C. Vascular tone regulation induced by C-type natriuretic peptide: differences in endothelium-dependent and -independent mechanisms involved in normotensive and spontaneously hypertensive rats. PLoS One. 2016;11:e0167817. doi: 10.1371/journal.pone.0167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Consortium for Blood Pressure Genome-Wide Association Studies. Ehret G.B., Munroe P.B., Rice K.M., Bochud M., Johnson A.D. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiranec K., Chen W., Werner F., Nikolaev V.O., Naruke T., Koch F., Werner A., Eder-Negrin P., Dieguez-Hurtado R., Adams R.H., Baba H.A., Schmidt H., Schuh K., Skryabin B.V., Movahedi K., Schweda F., Kuhn M. Endothelial C-type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation. 2018;138:494–508. doi: 10.1161/CIRCULATIONAHA.117.033383. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Nielsen B., Schmidt-Nielsen B. On the function of the mammalian renal papilla and the peristalsis of the surrounding pelvis. Acta Physiol (Oxf) 2011;202:379–385. doi: 10.1111/j.1748-1716.2011.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bargman J., Leonard S.L., McNeely E., Robertson C., Jamison R.L. Examination of transepithelial exchange of water and solute in the rat renal pelvis. J Clin Invest. 1984;74:1860–1870. doi: 10.1172/JCI111605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gambaryan S., Subramanian H., Rukoyatkina N., Herterich S., Walter U. Soluble guanylyl cyclase is the only enzyme responsible for cyclic guanosine monophosphate synthesis in human platelets. Thromb Haemost. 2013;109:973–975. doi: 10.1160/TH12-12-0916. [DOI] [PubMed] [Google Scholar]
- 38.Jin X., Zhang Y., Li X., Zhang J., Xu D. C-type natriuretic peptide ameliorates ischemia/reperfusion-induced acute kidney injury by inhibiting apoptosis and oxidative stress in rats. Life Sci. 2014;117:40–45. doi: 10.1016/j.lfs.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Jung D.C., Kim S.H., Jung S.I., Hwang S.I., Kim S.H. Renal papillary necrosis: review and comparison of findings at multi-detector row CT and intravenous urography. Radiographics. 2006;26:1827–1836. doi: 10.1148/rg.266065039. [DOI] [PubMed] [Google Scholar]
- 40.Kolaja G.J., Packwood W.H., Bell R.R., Ratke C.C., Stout C.L. Renal papillary necrosis and urinary protein alterations induced in Fischer-344 rats by D-ormaplatin. Toxicol Pathol. 1994;22:29–38. doi: 10.1177/019262339402200105. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos N.A., Carvalho Rodrigues M.A., Martins N.M., dos Santos A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–1250. doi: 10.1007/s00204-012-0821-7. [DOI] [PubMed] [Google Scholar]
- 42.Kim C.S., Choi J.S., Park J.W., Bae E.H., Ma S.K., Lee J., Kim S.W. Altered regulation of nitric oxide and natriuretic peptide system in cisplatin-induced nephropathy. Regul Pept. 2012;174:65–70. doi: 10.1016/j.regpep.2011.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.