Abstract
Ecdysteroids play an essential role in controlling insect development and reproduction. Their pathway is regulated by a group of enzymes called Halloween gene proteins. The relationship between the Halloween genes and ecdysteroid synthesis has yet to be clearly understood in diamondback moth, Plutella xylostella (L.), a worldwide Lepidoptera pest attacking cruciferous crops and wild plants. In this study, complete sequences for six Halloween genes, neverland (nvd), shroud (sro), spook (spo), phantom (phm), disembodied (dib), shadow (sad), and shade (shd), were identified. Phylogenetic analysis revealed a strong conservation in insects, including Halloween genes of P. xylostella that was clustered with all other Lepidoptera species. Three Halloween genes, dib, sad, and shd were highly expressed in the adult stage, while nvd and spo were highly expressed in the egg and pupal stages, respectively. Five Halloween genes were highly expressed specifically in the prothorax, which is the major site of ecdysone production. However, shd was expressed predominantly in the fat body to convert ecdysone into 20-hydroxyecdysone. RNAi-based knockdown of sad, which is involved in the last step of ecdysone biosynthesis, significantly reduced the 20E titer and resulted in a longer developmental duration and lower pupation of fourth-instar larvae, as well as caused shorter ovarioles and fewer fully developed eggs of P. xylostella. Furthermore, after the knockdown of sad, the expression levels of Vg and VgR genes were significantly decreased by 77.1 and 53.0%. Meanwhile, the number of eggs laid after 3 days was significantly reduced in sad knockdown females. These results suggest that Halloween genes may play a critical role in the biosynthesis of ecdysteroids and be involved in the development and reproduction of P. xylostella. Our work provides a solid basis for understanding the functional importance of these genes, which will help to screening potential genes for pest management of P. xylostella.
Keywords: Plutella xylostella, Halloween genes, ecdysteroid, knockdown of sad expression, development, reproduction
Introduction
Insect molting hormones, known also as ecdysterones, are a type of steroid compounds mainly produced at the larval stage by prothoracic glands, as well as sex glands in adults (Christiaens et al., 2010; Marchal et al., 2011). 20-hydroxyecdysone (20E) is an active ecdysone that is involved in the development and reproduction of insects and other arthropods (Jia et al., 2013a; Lehmann, 2018; Ruang-Rit and Park, 2018). For example, 20E plays important roles in larval development, pupal formation and wing dimorphism (Niitsu et al., 2008; Rharrabe et al., 2009). It is also involved in a series of reproductive physiological processes, including vitellogenin (Vg) biosynthesis and transport, germ cell differentiation, oogenesis, ovarian development (Parthasarathy et al., 2010; Dana et al., 2011) as well as in the control of oocyte development by regulating the autophagy of fat body (Bryant and Raikhel, 2011).
The 20E precursor, ecdysone (E), is mainly synthesized from dietary cholesterol or other sterols in prothoracic glands of insects, which are unable to de novo synthesize these precursor cholesterols (Clark and Block, 1959; Christiaens et al., 2010; Iga and Kataoka, 2012). A group of enzymes involved in 20E synthesis have been described in Drosophila melanogaster (Niwa and Niwa, 2011) and in crustaceans (Asazuma et al., 2009; Sumiya et al., 2014). This group of enzymes called Halloween genes include Neverland (nvd), Non-molting glossy (nm-g)/shroud (sro), Spook (spo; CYP307A1), Phantom (phm: CYP306A1), Disembodied (dib: CYP302A1), Shadow (sad: CYP315A1), and Shade (shd: CYP314A1) (Luan et al., 2013). Firstly, the dietary cholesterol is converted into 7-dehydrocholesterol (7dC) under the action of 7, 8-dehydrogenase (Nvd). The malfunction of this enzyme can severely inhibit the growth and molting of D. melanogaster and Bombyx mori (Yoshiyama et al., 2006; Yoshiyama-Yanagawa et al., 2011). Subsequently, the conversion of 7dC to 2,22,25-trideoxyecdysone (ketodiol) is performed by a string of unknown reactions called the “Black Box” (Gilbert and Warren, 2005). The hypothetical enzymes of Nm-g/Sro, Spo and Spook may be involved in this complex multi-step conversion process (Namiki et al., 2005; Ono et al., 2006; Niwa and Niwa, 2011; Iga and Kataoka, 2012). Then, the ketodiol is transformed into 22, 2-dideoxidized ecdysone (ketotriol), 2-deoxidized ecdysone and ecdysone in turn with the help of Phm, Dib and Sad, respectively, (Warren et al., 2004; Iga and Kataoka, 2012). Finally, Shd catalyzes the transformation of ecdysone into active 20-hydroxyecdysone (20E) in peripheral tissues, including epidermis, midgut, fat body, and Malpighian tubule.
Previous research has identified or predicted the Halloween genes in several insects, showing high variability in number of genes among species (Rewitz et al., 2006a, b, 2007; Christiaens et al., 2010; Luan et al., 2013; Cabrera et al., 2015; Wan et al., 2015). For example, only three orthologs of the Halloween genes (spook, dib, and shd) have been found in Varroa destructor, speculating that the absence of these genes may be correlated with its ectoparasitic life (Cabrera et al., 2015). Six Halloween genes, except for sro, participate in the 20E synthesis of Bemisia tabaci (Luan et al., 2013). In Acyrthosiphon pisum, 20E biosynthesis is controlled by five Halloween genes coding for the P450 enzymes, including spo, phm, dib, sad, and shd (Christiaens et al., 2010). The functions of Halloween genes have been characterized mostly in model insects (Yoshiyama-Yanagawa et al., 2011; Ameku et al., 2017). For example, phm mutant causes low ecdysteroid titers and severe disruptions in morphogenesis of D. melanogaster and B. mori (Niwa et al., 2004; Warren et al., 2004). Suppressing nvd expression in mated female by RNAi can significantly reduce the number of germline stem cells in D. melanogaster ovary (Ameku and Niwa, 2016).
Investigation of molecular characteristics and functions of Halloween genes should be of great importance for understanding the development and reproduction of the diamondback moth, Plutella xylostella (Lepidoptera, Plutellidae), which is a cosmopolitan Lepidoptera pest attacking cruciferous crops and wild plants (Li et al., 2016). High fecundity of P. xylostella allows it to invade regions where cruciferous plants can grow, thus becoming one of the most widespread lepidopteran pests (Zalucki et al., 2012; Furlong et al., 2013). Although the Halloween genes have been predicted from the genome sequences and transcriptome data of P. xylostella (He et al., 2012; You et al., 2013; Tang et al., 2014), it is still necessary to further identify and characterize these genes in order to lay the foundation for in-depth analysis of their functions. Here, we identified the molecular characteristics and spatio-temporal variation in expression of Halloween genes, and explored the functions of sad, which is involved in last step of ecdysone production in the 20E synthesis, in the development and reproduction of P. xylostella. This study aims to further screen potential genes that can be potentially used to improve the control of P. xylostella populations.
Materials and Methods
Insect Culture
The individuals of P. xylostella used for this study were collected from a cabbage (Brassica oleracea var. capitata) field in Fuzhou (Southeast of China; 26.08°N,119.28°E) in 2004, and has since been maintained in laboratory at the Fujian Agriculture and Forestry University (You et al., 2013; Peng et al., 2015). The colony was mass reared on radish seedlings (Raphanus sativus) at 25 ± 2°C, 75 ± 5% relative humidity and L:D = 16:8 photoperiod, and insects were not exposed to any insecticide during this period.
Total RNA Isolation and cDNA Synthesis
For each of the individuals used in the following experiments, total RNA of each individual and tissue was isolated with the TRIzol® reagent (Invitrogen, United States) and RNeasy micro kit (Qiagen, Germany) following the manufacturer’s instructions, respectively. The purity of RNA samples was verified using the NanoDrop2000® spectrophotometer (Thermo, United States) based on the values of OD260/280 and OD260/230. A Reverse Transcription System (Promega, Shanghai, China) was used for synthesis of the first-strand cDNA following the manufacturer’s instructions.
Cloning and Sequencing of Halloween Genes in P. xylostella
To verify the putative Halloween genes, the homologous protein sequences of B. mori, Spodoptera litura, S. littoralis, Maduca sexta and D. melanogaster were downloaded from the NCBI genome database1, and then used as the queries to conduct TBLASTP searches for DBM genome database2 (Tang et al., 2014) based on the cutoff e-value <e–20. The candidate sequences were authenticated by normal PCR using specific primers designed by Primer Premier 5.1 software (Table 1). After the separation on the agarose gel and purification using Universal DNA Purification Kit (Tiangen, Beijing, China), the PCR products were cloned into the pGEM-T vector (Promega, Beijing, China) for sequencing. Additionally, 3′-RACE PCR was used for sad due to a failure of amplification on 3′ sequence of sad with normal PCR.
TABLE 1.
Primers used for identification and analysis of the P. xylostella Halloween genes.
| Gene |
Primer sequences (5′–3′) |
||
| Forward | Reverse | ||
| Sequence PCR | nvd | GCAGACCCACAGACAGAAGAGC | TCGACAGTAAGTTAGGAGCCGA |
| spo | GCAACCTCCCGCCAAAAACAG | CGAGAACAAAAACACGGCACAG | |
| sro | ATGGCAGTTACTCGAGTGGTGG | AGTTGGAAATCACCGGGAAAGC | |
| dib | TTCAATGAAATACCCGGACCCA | TTGGTTCAAGTGATAACCGCAC | |
| sad | TTTTATGGATGGCGAAGAATGG | CCGAAGGATGAATATGGAAGTT | |
| shd | AATTGGAGCGTCTGCCTGTC | AAACTGGTGGATAGCAAACGACAA | |
| Quantitative PCR | nvd | ATGAGACGGGAAGGTGTAATAGGGTG | ATGGTCCATTTCGGTGGGTTGC |
| spo | GTAGCGTGGAGAAAGGAGGC | CACCACTGGAAACGGAACG | |
| sro | TACGAGTGGCAGAGCACGGAAAT | TCTGGCAGGAACTGGGAGATGAC | |
| dib | TGCCAAGAAACACTATACAAAGAGG | TCTCCCGACTCCGATAGACACT | |
| sad | ATTCAGTGCGCTTTGTGATGTTA | TTGTAGTGATAATGGGTGGCTTT | |
| shd | ATGCTGGGCTGTCGGCTAGGGT | TTTGTGCCCGGAAGTGCGTCTT | |
| Vg | AACCAGGGACAAGTGAACAAC | CTCGCTGAGGCGGGGAAGGAT | |
| VgR | ATTGTGACCCCGATGGACTG | TGCAGCGGGTCTCATTCATAG | |
| ribp | CAATCAGGCCAATTTACCGC | CTGCGTTTACGCCAGTTACG | |
| RNAi | dssad | TAATACGACTCACTATAGGG ACGCATAGAGAAGTCACGGA | TAATACGACTCACTATAGGG AGCGACGATAGTAGCCCACC |
†T7 promoter sequences are underlined.
Sequence Comparison and Phylogenetic Analysis of Halloween Genes
DNAMAN 6.0 software was used to predict the open reading frame (ORF). NCBI CDD database3 was applied to identify the conserved domains. The amino acid sequences of homologous Halloween genes were aligned with Clustal W2.0 (Larkin et al., 2007). Phylogenetic tree was constructed using the method of neighbor-joining (NJ) with a bootstrap value of 1000 replicates.
Expression Profiling of Halloween Genes in P. xylostella
For stage- and sex-specific expression profiles, eggs, 1- to 3-day-old fourth-instar larvae, 1- to 3-day-old pupae, and 0-, 12-, 24-, 48-, 72-h adults were sampled (3 samples of 50–100 mg (i.e., pooled individuals) per stage and sex). For tissue-specific expression patterns, 100 two-day-old fourth-instar larvae (head, prothorax, midgut and fat body) and newly emerged adults (head, prothorax, midgut and ovary) were dissected in RNase and DNase free water (QIAGEN, Germany), and the dissected tissues were then stored in RNA Stabilization Reagent (RNAlaterTM, Qiagen, Germany) at –80°C until total RNA isolation. For each sample, total RNA isolation and cDNA synthesis followed the process as described before. qRT-PCR was conducted with GoTaq® qPCR Master Mix Kit (Promega, Madison, WI, United States). The qPCR program was as follows: 95°C for 30 s; 95°C for 5 s and 60°C for 30 s and 44 cycles. Primer sequences are presented in the Table 1. The ribosomal protein gene L32 (GenBank acc. no. AB180441) was used as the endogenous control to normalize the mRNA levels. Each sample was further divided into two subsamples or technical replicates for testing consistency.
Preparation of dsRNA
Double-stranded RNA (dsRNA) was synthesized by the MEGAscript RNAi Kit (Ambion, United States) following the manufacturer recommendations. Here, a primer pair including a T7 recognition region was designed based on the appropriate fragments of sad with Green Fluorescent Protein (GFP) being the negative control (Table 1). The PCR of cDNA from the newly emerged adults (0–24 h after emergence) was performed with the following protocol: 95°C for 3 min; 95°C for 30 s, 59°C for 30 s and 72°C for 40 s for 34 cycles, and 72°C for 10 min for extension. The products were analyzed using 1% agarose gel and purified by Gel Extraction Kit (Omega, China), and the expected fragment was cloned and sequenced to confirm its identity. All dsRNA preparations were dissolved in nuclease free water, and then stored at −80°C.
RNA Interference Experiments
To examine the effects of RNA interference on P. xylostella, two separate experiments were conducted: (1) for development, 2-day fourth-instar larvae were injected with 207 nL of dssad and, (2) for reproduction, 2-day female pupae were injected with 345 nL of dssad, using the microinjection system Nanoliter 2010 (World Precision Instruments, United States). The dsegfp was used as negative control. Three injected individuals per treatment were then collected at 6, 12, and 24 h to prepare for total RNA extraction and verify RNAi efficiency after injection. The dsRNA injection was performed with three and six replicates for larvae and pupae, respectively, at each sampling time. To better understand the effects of sad on individuals on ecdysteroid titer, larval development, Vg and Vg receptor (VgR) expression, and reproduction, three separate experiments were completed as described in the following subsections.
Effect of Sad on Ecdysteroid Titers
Ecdysteroid titers from the individuals injected with the dsRNA at 6, 12, and 24 h were measured using the Insect Ecdysteroid Enzyme-linked immunosorbent assay ELISA Kit (Shanghai Meilian Bio Technology Co., Ltd.). Three samples were used for each time. Each sample was composed of 10 individuals. The individuals in each sample as a group was weighed, and was homogenized with a corresponding volume of phosphate buffered solution (PBS) at the ratio of 1 g: 9 mL and centrifuged at 8000 rpm for 20 min. The supernatants were collected and stored at −20°C until they were determined using an ELISA according to the manufacturer’s instructions.
Effect of Sad on Larval Development
A larva injected with dsRNA was placed in a Petri dish (9 cm) covered with a filter paper. A fresh radish seedling with moist cotton ball wrapped around the stem apex was provided as food. Moist cotton ball was wrapped with preservative film to maintain humidity. Fresh radish seedling and filter paper were changed daily. Three experimental groups of 30 4th-instar individuals (for a total of 90 individuals) were used to measure the developmental duration of the fourth-instar larvae, which was recorded at every 12 h after dsRNA injection until pupation, and the pupation rate was calculated.
Effect of Sad on Expression Level of PxVg and PxVgR
The expressional profile of PxVg and PxVgR from the individuals injected with the dsRNA at 6, 12, and 24 h were measured using qRT-PCR [3 samples of 50–100 mg (i.e., pooled individuals) per time]. For each sample, RNA isolation and cDNA synthesis were completed as described before. qRT-PCR was performed with GoTaq® qPCR Master Mix Kit (Promega, United States) according to the protocols of manufacturer with the following procedure: pre-denaturing at 95°C, 30 s; then 95°C, 5 s, and 60°C, 30 s for 44 cycles. The RIBP gene was used as endogenous control. Primer sequences are presented in the Table 1. Each sample was divided into three subsamples (technical replicates) for testing consistency. The comparative Ct method (2–ΔCt) was used to calculate the transcript level.
Effect of Sad on Reproduction
To verify the effects of sad on the oogenesis and ovary development of P. xylostella, 15 newly emerged females from dsRNA injected pupae were anesthetized with CO2 and then dissected in DNase and RNase free water (Qiagen, Germany) to obtain the ovaries. Dissected ovaries were washed three times using the same reagent and photographed with stereomicroscope DMi8 (Leica, Germany).
Finally, one newly emerged female from the dsRNA injected pupae was paired with one newly emerged male in a Dixie cup for mating (4 cm in top diameter, 3 cm in bottom diameter, 3.5 cm in height). A hole was drilled into the lid of each cup to place the cotton wick soaked with 10% honey solution for nutrition, and a seam (1 cm in length) was cut on the lid to put into the parafilm sheet with the radish leaf extract for egg laying. Each of the adult pairs was moved daily into a new cup with a fresh parafilm sheet and cotton wick. The number of eggs was recorded for each day’s collection for a total of 3 days of mating. Thirty pairs of P. xylostella were used to analyze fecundity for each treatment.
Statistical Analysis
For the temporal and spatial expression of Halloween genes, comparisons were performed using one-way analysis of variance (ANOVA) with Tukey HSD multiple test. Other bioassays after dsRNA injection were analyzed using independent sample t-tests. SPSS 21.0 software (SPSS Inc., Chicago, IL, United States) was employed and the significant differences were considered at P < 0.05 and P < 0.01.
Results
Identification and Characterization of Halloween Genes
We identified the complete coding sequences of P. xylostella Halloween genes, including neverland (Px-nvd, GenBank accession no. MK962642), spook (Px-spo, MK962643), non-molting glossy/shroud (Px-sro, MK962644), disembodied (Px-dib, MK962645), shadow (Px-sad, MK962646), and shade (Px-shd, MK962647), which encoded the putative protein of Px-nvd [375 amino acids (aa)], Px-spo (539 aa), Px-sro (305 aa), Px-dib (476 aa), Px-sad (392 aa), and Px-shd (470 aa). Px-spo, Px-dib, Px-sad, and Px-shd belonged to the P450 genes superfamily. Among them, Px-dib and Px-sad had five conserved insect P450 motifs, including Helix-C (WxxxR), Helix-I (GxE/DTT/S), Helix-K (ExxR), PERF motif (PxxFxPE/DRF), and haem-binding domains (PFxxGxRxCxG/A), where ‘x’ means any amino acid (Supplementary Figures S1, S2). However, Helix-C and PERF motifs were absent in Px-spo and Px-shd, respectively. There was a common microsomal P450s character in the N-terminus of Px-spo, Px-dib, and Px-shd, consisting in numerous hydrophobic residues, followed by a proline/glycine (P/G) rich region (Supplementary Figures S1, S2). Px-sro and Px-nvd did not belong to P450s superfamily but were part of SDR (short-chain dehydrogenase reductase) superfamily and rieske superfamily domains, respectively, (Supplementary Figure S2).
Sequence Comparison and Phylogenetic Analysis of Halloween Genes
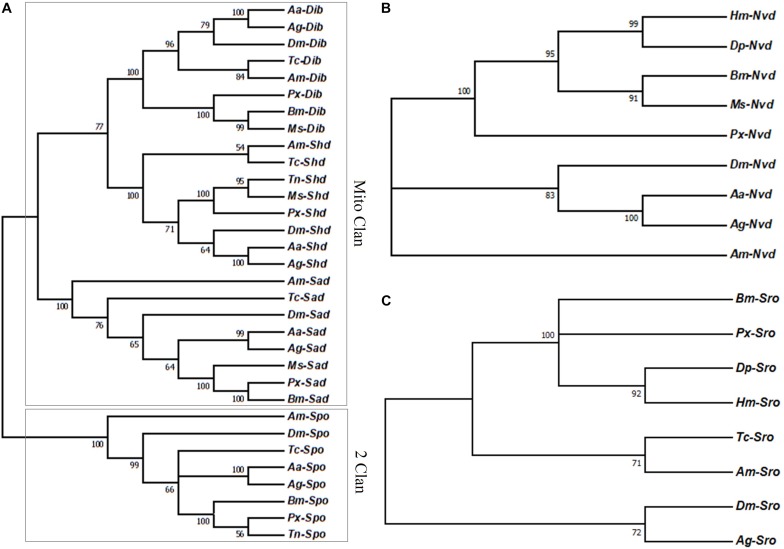
Halloween genes in P. xylostella were highly conserved (almost over 50% identical) with several homologs of Lepidoptera species. Among them, both Px-nvd and Px-sad were most similar to the homologs of B. mori with the similarity of 56.9 and 48.9% (Supplementary Table S1). However, Px-spo and Px-shd were most closely related to Trichoplusia ni (75.7 and 71.4%) (Supplementary Table S1). Px-sro and Px-dib were most similar to the homologs of Danaus plexippus (60.3%) and M. sexta (60.6%) (Supplementary Table S1). The phylogenetic tree showed Halloween genes of P. xylostella in the P450 superfamily were clustered into two specific clans with Px-dib and Px-shd sharing one branch and Px-sad another branch, thus forming the Minto Clan (Figure 1A). spo formed the second clan called 2 Clan and included Px-spo along with the other insects (Figure 1A). In addition, Px-sro and Px-nvd were both clustered with the homologs of Lepidoptera (Figures 1B,C).
FIGURE 1.
(A) Phylogenetic relationships of the Halloween genes encoding P450 enzymes, (B) Nvd, and (C) Sro between P. xylostella and the other insects. Aa, Aedes aegypti; Ag, Anopheles gambiae; Am, Apis mellifera; Bm, Bombyx mori; Dm, Drosophila melanogaster; Dp, Danaus plexippus; Hm, Heliconius Melpomene; Ms, Manduca sexta; Px, Plutella xylostella; Tc, Tribolium castaneum; Tn, Trichoplusia ni.
Expression Profile of Halloween Genes
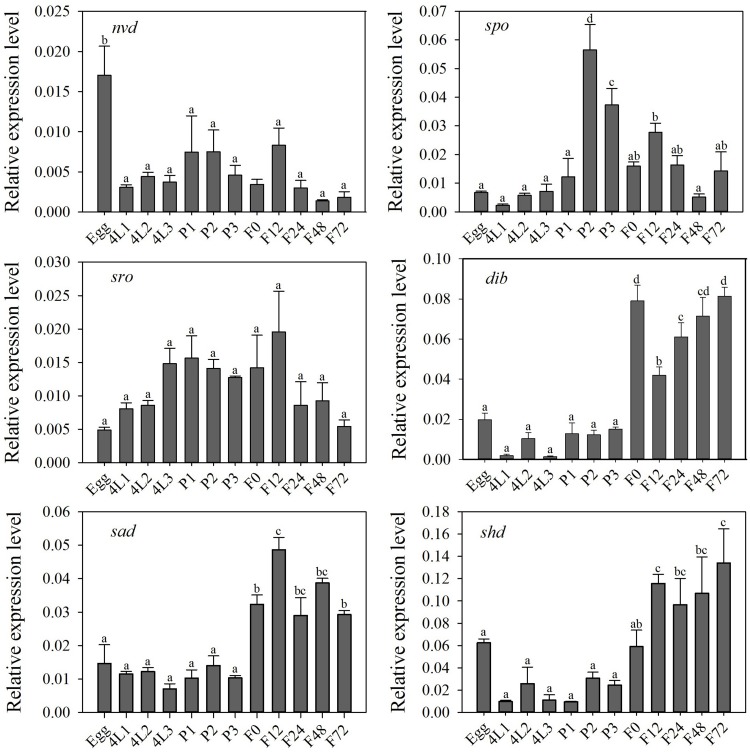
Halloween genes were expressed at each developmental stage, suggesting their importance in physiological functions of P. xylostella (Figure 2). Among these genes, the highest expression of dib, sad and shd occurred at the adult stage, and nvd was highly expressed in the eggs and spo at the pupal stage (P < 0.05) (Figure 2). There was no significant difference in relative expression level of sro among all developmental stages (P > 0.05) (Figure 2).
FIGURE 2.
Relative expression level (mean ± S.E.) of Halloween genes at different developmental stages of P. xylostella. Eggs, 4L1-3: 1 to 3 day-old 4th-instar larvae, P1-3: 1 to 3 day-old pupae, F0-72: 0 to 72 hour-old adults after eclosion. Note that the relative expression levels varied among the different genes, refer to the y-axis. Different letters above the bars indicate significant differences in different stages (P < 0.05).
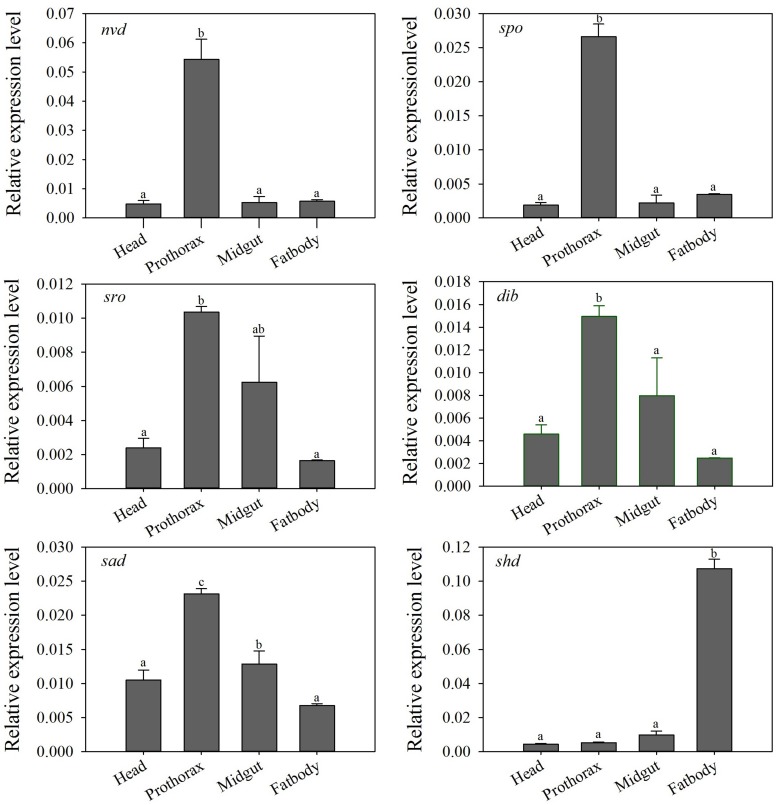
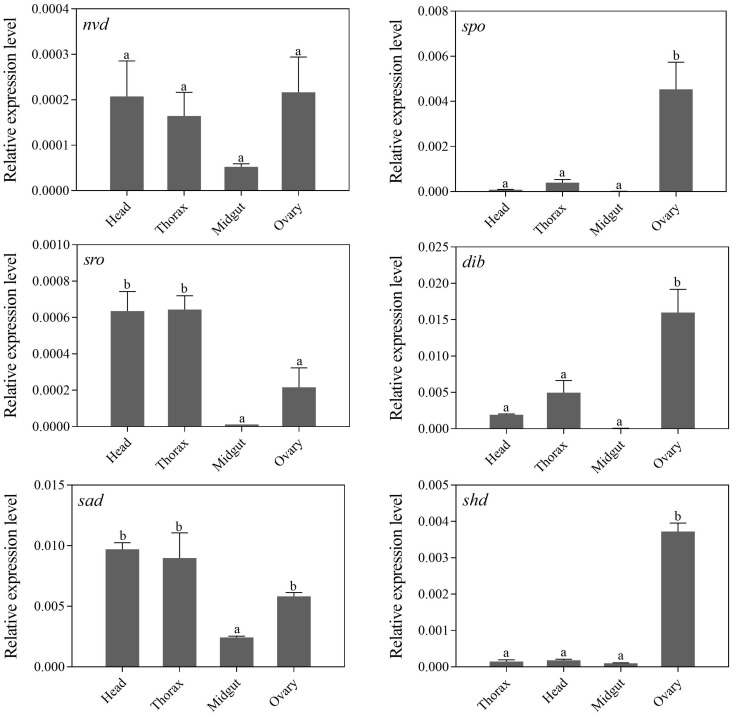
Tissue-specific expression profiles of larvae showed that the relative expression levels of the five Halloween genes, Px-nvd, Px-dib, Px-sad, Px-sro, Px-spo, were significantly higher in the prothorax than any other tissues (P < 0.05) (Figure 3). However, Px-shd, which is responsible for the transformation of ecdysone into the active 20-hydroxyecdysone, was expressed predominantly in the fat body (P < 0.05) (Figure 3). In newly emerged adults, the tissue-specific expression profiles showed that Px-spo, Px-dib and Px-shd had the highest relative expression level in the ovaries (P < 0.05), while Px-sro and Px-sad expressed predominantly in the head and thorax, with Px-sad also high in ovary (P < 0.05) (Figure 4). There was no significant difference in expressional level of Px-nvd among the different tissues (P > 0.05) (Figure 4).
FIGURE 3.
Relative expression levels (mean ± S.E.) of Halloween genes in different tissues of the P. xylostella 4th-instar larvae. Different letters above the bars indicate significant differences in different tissues (P < 0.05).
FIGURE 4.
Relative expression levels (mean ± S.E.) of Halloween genes in different tissues of newly emerged P. xylostella adults. Different letters above the bars indicate significant differences in different tissues (P < 0.05).
Effect of Sad on 20E Titers and Larval Development
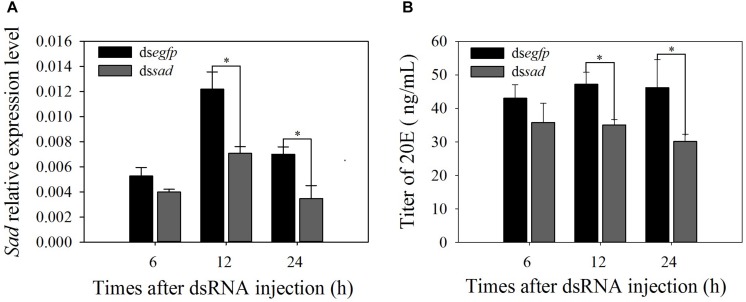
RNAi-based knockdown of sad expression was conducted to explore the functions of Halloween genes. qRT-PCR analyses showed that Px-sad transcripts were effectively suppressed after 12 and 24 h of dssad injection (decline of 41.8 and 50.0%) compared to the dsegfp injection (12 h: t = 0.174, df = 4, P = 0.026; 24 h: t = 2.899, df = 4, P = 0.044) (Figure 5A). After the knockdown of Px-sad, the 20E titers of P. xylostella were 35.1 ± 1.6 and 30.1 ± 2.2 μg/ml at 12 and 24 h, which were significantly lower than those injected with dsegfp, 47.2 ± 3.6 and 46.2 ± 8.3 μg/ml (12 h: t = 5.385, df = 4, P = 0.015; 24 h: t = 3.246, df = 4, P = 0.031) (Figure 5B).
FIGURE 5.
Relative expression levels (mean ± S.E.) of sad (A) and 20E titers (mean ± S.E.) (B) after RNAi-treated 4th-instar P. xylostella larvae. ∗Indicating significant difference between treatments (P < 0.05).
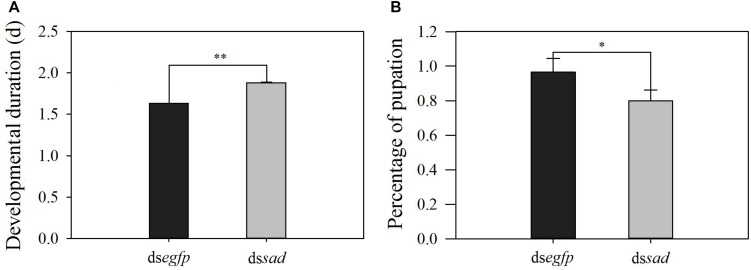
The development of the 4th-instar larvae of P. xylostella was also affected after the Px-sad knockdown. After injection with dssad, the duration from 4th-instar larva to pupa was significantly increased to 1.9 days when compared to 1.6 days for individuals injected with dsegfp (t = 4.907, df = 176, P < 0.01) (Figure 6A). The pupation rate of the dssad 4th-instar larvae was significantly reduced due to the abnormal molting (t = 3.059, df = 4, P = 0.038). The 4th-instar larvae injected with dsegfp had the pupation rate of 96.6 ± 0.08% while it was 80.0 ± 0.06% for dssad individuals (Figure 6B).
FIGURE 6.
The developmental duration (mean ± S.E.) (A) and pupation rate (mean percentage ± S.E.) (B) after RNAi-treated 4th-instar P. xylostella larvae. ∗Indicating significant difference between treatments (P < 0.05) ∗∗indicating highly significant difference between treatments (P < 0.01).
Effect of Sad on 20E Titers and Reproduction
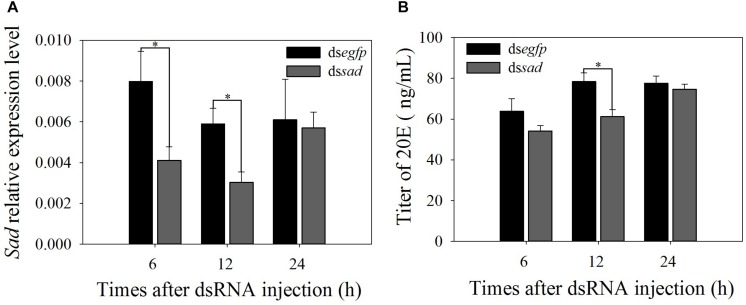
At 6 and 12 h after dssad injection, Px-sad mRNA levels in treated pupae significantly decreased by 48.5 and 48.6% when compared to dsegfp control pupae (6 h: t = 2.794, df = 10, P = 0.019; 12 h: t = 2.763, df = 10, P = 0.018) (Figure 7A). The 20E titers of sad knockdown individuals after 12 h (61.3 ± 3.4 ug/ml) were significantly lower than those injected with dsegfp (78.3 ± 4.3 ug/ml) (t = 3.123, df = 4, P = 0.035) (Figure 7B). The interference effects on sad expression (t = 0.222, df = 10, P = 0.829) and 20E titer (t = 0.095, df = 4, P = 0.929) of P. xylostella recovered by 24 h (Figures 7A,B).
FIGURE 7.
Relative expression levels (mean ± S.E.) of sad (A) and 20E titers (mean ± S.E.) (B) after RNAi-treated P. xylostella pupae. ∗Indicating significant difference between treatments (P < 0.05).
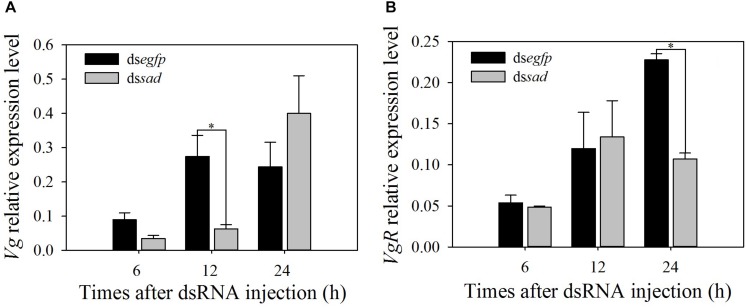
The expression levels of vitellogenin gene (Vg) and its receptor gene VgR were also measured after the sad knockdown. The Vg expression at 12 h after dssad injection significantly decreased by 77.1% when compared to the control group injected with dsegfp (t = 2.860, df = 6, P = 0.035) (Figure 8A). VgR transcripts were effectively suppressed by 53.0% after 24 h of dssad injection (Figure 8B).
FIGURE 8.
Relative expression levels (mean ± S.E.) of Vg (A) and VgR (B) after RNAi-treated P. xylostella pupae. ∗Indicating significant difference between treatments (P < 0.05).
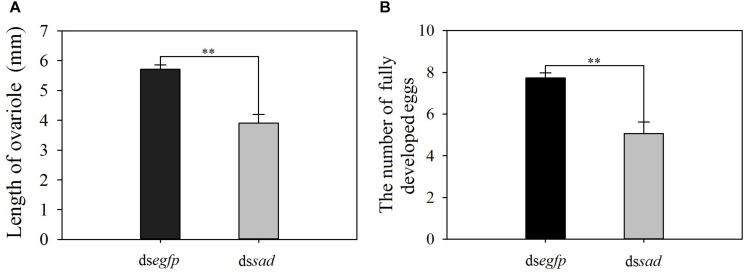
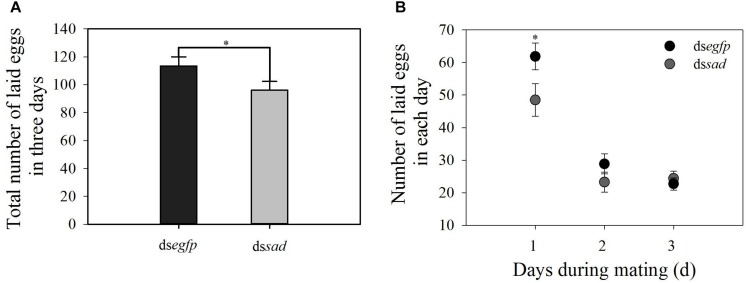
The mean ovariole length of newly emerged P. xylostella females was 3.9 ± 0.1 mm after the knockdown of sad, which was significantly shorter than that of injecting with dsegfp, 5.7 ± 0.1 mm (t = 5.582, df = 18, P < 0.01) (Figure 9A). Moreover, the average number of fully developed eggs per ovariole was significantly reduced due to RNAi-mediated knockdown of sad (5.1 ± 0.6 eggs for dssad vs. 7.7 ± 0.2 for the control) (t = 4.379, df = 28, P < 0.01) (Figure 9B). After injection with dssad, we found that the total number of eggs laid by P. xylostella females within 3 days was 96.1 ± 6.2, which was significantly lower than females injected with dsegfp, 113.4 ± 6.6 (t = 2.193, df = 53, P = 0.033) (Figure 10A). However, the results of daily oviposition showed that the difference in egg numbers of P. xylostella laid only occurred on the 1st day (t = 2.080, df = 54, P = 0.042) (Figure 10B).
FIGURE 9.
The length of ovariole (mean ± S.E.) (A) and the number of fully developed eggs (mean ± S.E.) (B) after RNAi-treated P. xylostella pupae. ∗∗Indicating highly significant difference between treatments (P < 0.01).
FIGURE 10.
(A) Total number of laid eggs in 3 days (mean ± S.E.) and (B) daily number of eggs laid (mean ± S.E.) after RNAi-treated P. xylostella pupae. ∗Indicating significant difference between treatments (P < 0.05).
Discussion
Halloween genes are involved in a series of important enzymatic steps that convert cholesterol from steroid precursors into active ecdysteroid, 20-hydroxyecdysone, which can then bind to the complex of EcR/RXR nuclear to initiate a chain of physiological processes (Tan and Palli, 2008; Fahrbach et al., 2012; Boulanger and Dura, 2015). In our study, six orthologs of the Halloween genes were identified in P. xylostella, including four P450 superfamily members spo, dib, sad, and shd, as well as the SDR and rieske superfamily members, nvd and sro. In the well-studied model species, such as B. mori, the complete 20E biosynthetic pathway involves seven conservative Halloween genes (Xia et al., 2004). However, other species, such as V. destructor (Three Halloween genes), B. tabaci (Six Halloween genes) and A. pisum (Five Halloween genes) have reduced number of the Halloween genes (Christiaens et al., 2010; Luan et al., 2013; Cabrera et al., 2015). This indicates that the number of Halloween genes involved in 20E biosynthetic pathway may be species-specific. Enya et al. (2014, 2015) found that nobo, a member of the GSTe family, was involved in cholesterol transport and/or metabolism in the prothoracic gland of D. melanogaster and B. mori. In P. xylostella genome, 22 GST genes have been identified, and five of them are GSTe subfamily genes (You et al., 2015). Whether or not any of these GSTs may be participating in 20E synthesis deserves further investigation.
Sequence alignment showed that Px-sro contained a typical SDR superfamily domain while Px-nvd had the rieske motif. These results were consistent with other studies (Niwa et al., 2010; Sandlund et al., 2018). There were completely conserved P450 motifs identified in Px-dib and Px-sad. However, Px-spo lacked the conservative domain of Helix-C. In Sogatella furcifera and S. littoralis, not all typical P450 motifs, such as Helix-C and Helix-I motifs, are conserved in spo (Iga et al., 2010; Jia et al., 2013b). Additionally, PERF motif of shd was not well conserved in P. xylostella. Sequences of shd in species, such as V. destructor, Lepeophtheirus salmonis, and Laodelphax striatellus, have five conserved cytochrome P450 domains (Jia et al., 2013a; Cabrera et al., 2015; Sandlund et al., 2018), while shd3 in A. pisum completely misses heme-binding domain (Christiaens et al., 2010). Our results suggest that conserved domain types of the family genes might be species-specific, indicating that this protein might have other functions than just being involved in 20E synthesis.
In Plutella xylostella, Halloween genes were expressed at each developmental stage, suggesting various potential physiological functions. The high expression levels of dib, sad, and shd occurred in the adult stage. This is consistent with Rewitz et al. (2006b) who report that the expression peaks for sad, shd and dib occur in the adult of M. sexta. These results speculate that 20E is involved in the reproductive functions of insect adults (Belles and Piulachs, 2015). However, the nvd was expressed the highest in the egg stage, suggesting its necessary role in embryonic development. Ameku et al. (2017) indicate that there is a significant negative impact on gametogenesis of Drosophila after knockdown of nvd expression.
The highest expression levels of five Halloween genes in the prothorax of P. xylostella further support that it was a major site for ecdysone production. These results are consistent with other insect species of Lepidoptera and Diptera (Warren et al., 2002; Niwa et al., 2004, 2005; Ono et al., 2006; Rewitz et al., 2006b). However, there was a non-negligible expression of sro, dib, and sad in other tissues, especially in the midgut, suggesting that these genes may be involved in other biological processes. For example, both dib and sad are members of cytochrome P450 gene superfamily, which may play other physiological functions in insects, such as detoxification and biocatalysis (Scott, 1999; Bernhardt, 2006). These results are similar to Zheng et al. (2017) reporting that Halloween genes encoding cytochrome P450s are expressed in prothorax, as well as in other tissues of Helicoverpa armigera.
Px-shd was expressed predominantly in the fat body of P. xylostella. The shd gene is highly expressed in midgut of M. sexta (Rewitz et al., 2006a), but in the Malpighian of S. littoralis (Iga et al., 2010), as well as expressed in other tissues (e.g., head, midgut, etc.) in S. furcifera (Jia et al., 2013b) and Schistocerca gregaria (Marchal et al., 2011). Those results may support the hypothesis of Petryk et al. (2003) suggesting that the conversion to active 20-hydroxyecdysone is catalyzed by Shd in some peripheral tissues, including epidermis, fat body, midgut and Malpighian tubule. The high expression levels of four Halloween genes (spo, dib, sad, and shd) in the ovary may suggest that they play important roles in female reproduction of P. xylostella. This is consistent with that 20E can be synthesized in the gonad of adult (Christiaens et al., 2010; Marchal et al., 2011).
In this study, we used RNAi-based knockdown of sad expression, which is involved in the last step of ecdysone biosynthesis, to further explore the functions of Halloween genes of P. xylostella. It is also necessary to note that we have focused on this gene because of its importance in a potential target for RNA-interference-based pest management as well as due to ineffective RNAi in preliminary experiments with the other five genes, a phenomenon common in Lepidoptera (Terenius et al., 2011). The fourth-instar larvae injected with dssad, showed longer developmental duration and lower pupation rate than that in the control larvae. These results are similar to those of Wan et al. (2015) for Laodelphax striatellus nymphs where sad knockdown successfully causes mortality and delays development. Injection of dssad into female pupae of P. xylostella led to a significant decrease in the expression levels of Vg and VgR gene, as well as resulted in shorter ovarioles and fewer fully developed eggs. The number of eggs laid per female was significantly reduced within 3 days. Warren et al. (2002) report that sad mutation results in embryo morphogenesis interruption and a decrease in egg production of D. melanogaster. We speculate that interference with sad expression leads to a decrease in 20E titers, thereby inhibiting Vg synthesis and transport, as well as reducing fecundity of P. xylostella. Similar results have been reported for Dermacentor variabilis (Thompson et al., 2007) and B. mori (Yuan et al., 2013).
Conclusion
This is a first report characterizing the Halloween genes in P. xylostella and confirming the important role of sad in ecdysteroids synthesis, larval development and pupation times, and reproductive functions, including ovary development, oogenesis, and egg laying. Our identified Halloween genes, neverland (nvd), shroud (sro), spook (spo), phantom (phm), disembodied (dib), shadow (sad), and shade (shd), are participating in the biosynthesis of ecdysteroids. Using RNA interference, we have examined the role of sad in the development and reproduction of P. xylostella, an important step in understanding the influence of these genes on its physiology. These findings may gradually help define potential targets for RNA-interference-based pest management. Further functional studies will be necessary to explore the responses of the other Halloween genes using more effective and suitable gene editing techniques to further investigate their related signaling pathways in P. xylostella life cycle.
Data Availability
The datasets generated for this study can be found in the GenBank, Nvd: MK962642, Spo: MK962643, Sro: MK962644, Dib: MK962645, Sad: MK962646, and Shd: MK962647.
Author Contributions
LP and M-SY designed the study. LP and LW performed the experiments. M-MZ and L-NC completed the data analysis with the help of LP and LV. LP wrote the first draft of the manuscript. M-MZ, Y-DQ, and Y-LZ wrote sections of the manuscript with collaborations of M-SY and LV. All authors have approved the manuscript and were substantially involved in revising the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by the National Natural Science Foundation of China (31772166 and 31401744), the National Natural Science Foundation of Fujian Province (2019J01666), and the Major Project of Fujian Province (2018NZ0002-1).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.01120/full#supplementary-material
References
- Ameku T., Niwa R. (2016). Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet. 12:e1006123. 10.1371/journal.pgen.1006123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameku T., Yoshinari Y., Fukuda R., Niwa R. (2017). Ovarian ecdysteroid biosynthesis and female germline stem cells. Fly. 11 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asazuma H., Nagata S., Nagasawa H. (2009). Inhibitory effect of molt-inhibiting hormone on phantom expression in the Y-organ of the kuruma prawn, Marsupenaeus japonicas. Arch. Insect Biochem. Physiol. 72 220–233. 10.1002/arch.20335 [DOI] [PubMed] [Google Scholar]
- Belles X., Piulachs M. D. (2015). Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation. Biochim. Biophys. Acta. 1849 181–186. 10.1016/j.bbagrm.2014.05.025 [DOI] [PubMed] [Google Scholar]
- Bernhardt R. (2006). Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 124 128–145. 10.1016/j.jbiotec.2006.01.026 [DOI] [PubMed] [Google Scholar]
- Boulanger A., Dura J. M. (2015). Nuclear receptors and Drosophila neuronal remodeling. Biochimi. Biophy. Acta. 1849 187–195. 10.1016/j.bbagrm.2014.05.024 [DOI] [PubMed] [Google Scholar]
- Bryant B., Raikhel A. S. (2011). Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles. PLoS One 6:e25502. 10.1371/journal.pone.0025502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera A. R., Shirk P. D., Evans J. D., Hung K., Sims J., Alborn H., et al. (2015). Three Halloween genes from the Varroa mite, Varroa destructor (Anderson & Trueman) and their expression during reproduction. Insect Mol. Biol. 24 277–292. 10.1111/imb.12155 [DOI] [PubMed] [Google Scholar]
- Christiaens O., Iga M., Velarde R. A., Rougé P., Smagghe G. (2010). Halloween genes and nuclear receptors in ecdysteroid biosynthesis and signalling in the pea aphid. Insect Mol. Biol. 19 187–200. 10.1111/j.1365-2583.2009.00957.x [DOI] [PubMed] [Google Scholar]
- Clark A. J., Block K. (1959). The absence of sterol synthesis in insects. J. Biol. Chem. 234 2578–2582. [PubMed] [Google Scholar]
- Dana G., Tamar L., Lilach G. (2011). Coordinated regulation of niche and stem cell precursors by hormonal signaling. PLoS Biol. 9:e1001202. 10.1371/journal.pbio.1001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya S., Ameku T., Igarashi F., Iga M., Kataoka H., Shinoda T., et al. (2014). A Halloween gene noppera-bo encodes a glutathione S-transferase essential for ecdysteroid biosynthesis via regulating the behaviour of cholesterol in Drosophila. Sci. Rep. 4:6586. 10.1038/srep06586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya S., Daimon T., Igarashi F., Kataoka H., Uchibori M., Sezutsu H., et al. (2015). The silkworm glutathione S-transferase gene noppera-bo is required for ecdysteroid biosynthesis and larval development. Insect Biochem. Mol. Biol. 61 1–7. 10.1016/j.ibmb.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Smagghe G., Velarde R. A. (2012). Insect nuclear receptors. Annu. Rev. Entomol. 57 83–106. 10.1146/annurev-ento-120710-100607 [DOI] [PubMed] [Google Scholar]
- Furlong M. J., Wright D. J., Dosdall L. M. (2013). Diamondback moth ecology and management: problems, progress, and prospects. Annu. Rev. Entomol. 58 517–541. 10.1146/annurev-ento-120811-153605 [DOI] [PubMed] [Google Scholar]
- Gilbert L. I., Warren J. T. (2005). A molecular genetic approach to the biosynthesis of the insect steroid molting hormone. Vitam. Horm. 73 31–57. 10.1016/s0083-6729(05)73002-8 [DOI] [PubMed] [Google Scholar]
- He W. Y., You M. S., Vasseur L., Yang G., Xie M., Cui K., et al. (2012). Developmental and insecticide-resistant insights from the de novo assembled transcriptome of the diamondback moth, Plutella xylostella. Genomics 99 169–177. 10.1016/j.ygeno.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Iga M., Kataoka H. (2012). Recent studies on insect hormone metabolic pathways mediated by cytochrome P450 enzymes. Biol. Pharm. Bull. 35 838–843. 10.1248/bpb.35.838 [DOI] [PubMed] [Google Scholar]
- Iga M., Smagghe G., Nachman R. J. (2010). Identification and expression profile of Halloween genes involved in ecdysteroid biosynthesis in Spodoptera littoralis. Peptides 31 456–467. 10.1016/j.peptides.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Jia S., Wan P. J., Zhou L. T., Mu L. L., Li G. Q. (2013a). Knockdown of a putative Halloween gene Shade reveals its role in ecdysteroidogenesis in the small brown planthopper Laodelphax striatellus. Gene 531 168–174. 10.1016/j.gene.2013.09.034 [DOI] [PubMed] [Google Scholar]
- Jia S., Wan P. J., Zhou T. L., Mu L. L., Li G. Q. (2013b). Molecular cloning and RNA interference-mediated functional characterization of a Halloween gene spook in the white-backed planthopper Sogatella furcifera. BMC Mol. Biol. 14:19. 10.1186/1471-2199-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., et al. (2007). Clustal W and clustal X version 2.0. Bioinformatics 23 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lehmann M. (2018). Endocrine and physiological regulation of neutral fat storage in Drosophila. Mol. cell Endocrinol. 461 165–177. 10.1016/j.mce.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Y., Feng X., Liu S. S., You M. S., Furlong M. J. (2016). Biology, ecology, and management of the diamondback moth in China. Annu. Rev. Entomol. 61 277–296. 10.1146/annurev-ento-010715-023622 [DOI] [PubMed] [Google Scholar]
- Luan J. B., Ghanim M., Liu S. S., Czosnek H. (2013). Silencing the ecdysone synthesis and signaling pathway genes disrupts nymphal development in the whitefly. Insect Biochem. Mol. Biol. 43 740–746. 10.1016/j.ibmb.2013.05.012 [DOI] [PubMed] [Google Scholar]
- Marchal E., Badisco L., Verlinden H., Vandersmissen T., Van Soest S., Van Wielendaele P., et al. (2011). Role of the halloween genes, spook and Phantom in ecdysteroidogenesis in the desert locust, Schistocerca gregaria. J. Insect Physiol. 57 1240–1248. 10.1016/j.jinsphys.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Namiki T., Niwa R., Sakudoh T., Itoyama K., Petryk A., Rybczynski R., et al. (2005). Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem. Bioph. Res. Co. 337 367–374. 10.1016/j.bbrc.2005.09.043 [DOI] [PubMed] [Google Scholar]
- Niitsu S., Lobbia S., Izumi S., Fujiwara H. (2008). Female-specific wing degeneration is triggered by ecdysteroid in cultures of wing discs from the bagworm moth, Eumeta variegata (Insecta: Lepidoptera, Psychidae). Cell Tissue Res. 333 169–173. 10.1007/s00441-008-0615-7 [DOI] [PubMed] [Google Scholar]
- Niwa R., Matsuda T., Yoshiyama T., Namiki T., Mita K., Fujimoto Y., et al. (2004). CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 279 35942–35949. 10.1074/jbc.m404514200 [DOI] [PubMed] [Google Scholar]
- Niwa R., Namiki T., Ito K., Shimada-Niwa Y., Kiuchi M., Kawaoka S., et al. (2010). Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid bio-synthesis pathway. Development 137 1991–1999. 10.1242/dev.045641 [DOI] [PubMed] [Google Scholar]
- Niwa R., Niwa Y. S. (2011). The fruit fly Drosophila melanogaster as a model system to study cholesterol metabolism and homeostasis. Cholesterol 2011:176802. 10.1155/2011/176802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R., Sakudoh T., Namiki T., Saida K., Fujimoto Y., Kataoka H. (2005). The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by prothoracicotropic hormone. Insect Mol. Biol. 14 563–571. 10.1111/j.1365-2583.2005.00587.x [DOI] [PubMed] [Google Scholar]
- Ono H., Rewitz K. F., Shinoda T., Itoyama K., Petryk A., Rybczynski R., et al. (2006). Spook and spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 298 555–570. 10.1016/j.ydbio.2006.07.023 [DOI] [PubMed] [Google Scholar]
- Parthasarathy R., Sun Z., Bai H., Palli S. R. (2010). Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 40 405–414. 10.1016/j.ibmb.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Zou M. M., Ren N. N., Xie M., Vasseur L., Yang Y. F., et al. (2015). Generation-based life table analysis reveals manifold effects of inbreeding on the population fitness in Plutella xylostella. Sci. Rep. 5:12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk A., Warren J. T., Marqués G., Jarcho M. P., Gilbert L. I., Kahler J., et al. (2003). Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. U.S.A. 100 13773–13778. 10.1073/pnas.2336088100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz K. F., O’Connor M. B., Gilbert L. I. (2007). Molecular evolution of the insect Halloween family of cytochrome P450s: phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. 37 741–753. 10.1016/j.ibmb.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Rewitz K. F., Rybczynski R., Warren J. T., Gilbert L. I. (2006a). Developmental expression of Manduca shade, the P450 mediating the final step in molting hormone synthesis. Mol. Cell Endocrinol. 247 166–174. 10.1016/j.mce.2005.12.053 [DOI] [PubMed] [Google Scholar]
- Rewitz K. F., Rybczynski R., Warren J. T., Gilbert L. I. (2006b). Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 36 188–199. 10.1016/j.ibmb.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Rharrabe K., Bouayad N., Sayah F. (2009). Effects of ingested 20-hydroxyecdysone on development and midgut epithelial cells of Plodia interpunctella (Lepidoptera, Pyralidae). Pestic. Biochem. Phys. 93 112–119. [Google Scholar]
- Ruang-Rit K., Park Y. (2018). Endocrine system in supernumerary molting of the flour beetle, Triboliwn freemani, under crowded conditions. Insect Biochem. Mol. Biol. 101 76–84. 10.1016/j.ibmb.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Sandlund L., Kongshaug H., Horsberg T. E., Male R., Nilsen F., Dalvin S. (2018). Identification and characterisation of the ecdysone biosynthetic genes neverland, disembodied and shade in the salmon louse Lepeophtheirus salmonis (Copepoda, Caligidae). PLoS One 13:e0191995. 10.1371/journal.pone.0191995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. G. (1999). Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 29 757–777. 10.1016/s0965-1748(99)00038-7 [DOI] [PubMed] [Google Scholar]
- Sumiya E., Ogino Y., Miyakawa H., Hiruta C., Toyota K., Miyagawa S., et al. (2014). Roles of ecdysteroids for progression of reproductive cycle in the fresh water crustacean Daphnia magna. Front. Zool. 11:60. [Google Scholar]
- Tan A., Palli S. R. (2008). Edysone receptor isoforms play distinct roles in controlling molting and metamorphosis in the red flour beetle, Tribolium castaneum. Mol. Cell Endocrinol. 291 42–49. 10.1016/j.mce.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. Q., Yu L. Y., He W. Y., Yang G., Ke F. S., Baxter S. W., et al. (2014). DBM-DB: the diamondback moth genome database. Database 2014:bat087. 10.1093/database/bat087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius O., Papanicolaou A., Garbutt J. S., Eleftherianos I., Huvenne H., Kanginakudru S., et al. (2011). RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 57 231–245. 10.1016/j.jinsphys.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Thompson D. M., Khalil S. M., Jeffers L. A., Sonenshine D. E., Mitchell R.D., Osgood C. J., et al. (2007). Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem. Mol. Biol. 37 363–374. 10.1016/j.ibmb.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Wan P. J., Jia S., Li N., Fan J. M., Li G. Q. (2015). A Halloween gene shadow is a potential target for RNA-interference-based pest management in the small brown planthopper Laodelphax striatellus. Pest Manag. Sci. 71 199–206. 10.1002/ps.3780 [DOI] [PubMed] [Google Scholar]
- Warren J. T., Petryk A., Marqúes G., Jarcho M., Parvy J. P., Dauphin-Villemant C., et al. (2002). Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 99 11043–11048. 10.1073/pnas.162375799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. T., Petryk A., Marqúes G., Parvy J. P., Shinoda T., Itoyama K., et al. (2004). Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 34 991–1010. 10.1016/j.ibmb.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Xia Q. Y., Zhou Z. Y., Lu C., Cheng D. J., Dai F. Y., Li B., et al. (2004). A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 306, 1937–1940. [DOI] [PubMed] [Google Scholar]
- Yoshiyama T., Namiki T., Mita K., Kataoka H., Niwa R. (2006). Neverland is an evolutionally conserved rieskedomain protein that is essential for ecdysone synthesis and insect growth. Development 133 2565–2574. 10.1242/dev.02428 [DOI] [PubMed] [Google Scholar]
- Yoshiyama-Yanagawa T., Enya S., Shimada-Niwa Y., Yaguchi S., Haramoto Y., Matsuya T., et al. (2011). The conserved Rieske oxygenase DAF-36/neverland is a novel cholesterol-metabolizing enzyme. J. Biol. Chem. 286 25756–25762. 10.1074/jbc.M111.244384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M. S., Yue Z., He W. Y., Yang X. H., Yang G., Xie M., et al. (2013). A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 45 220–225. 10.1038/ng.2524 [DOI] [PubMed] [Google Scholar]
- You Y. C., Xie M., Ren N. N., Cheng X. M., Li J. Y., Ma X. L., et al. (2015). Characterization and expression profiling of glutathione S-transferases in the diamondback moth, Plutella xylostella (L.). BMC Genomics 16:152. 10.1186/s12864-015-1343-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H. X., Xu X., Sima Y. H., Xu S. Q. (2013). Reproductive toxicity effects of 4-nonylphenol with known endocrine disrupting effects and induction of vitellogenin gene expression in silkworm, Bombyx mori. Chemosphere 93 263–268. 10.1016/j.chemosphere.2013.04.075 [DOI] [PubMed] [Google Scholar]
- Zalucki M. P., Shabbir A., Silva R., Adamson D., Liu S. S., Furlong M. J. (2012). Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J. Econ. Entomol. 105 1115–1129. 10.1603/ec12107 [DOI] [PubMed] [Google Scholar]
- Zheng J., Tian K., Yuan Y., Li M., Qiu X. (2017). Identification and expression patterns of Halloween genes encoding cytochrome P450s involved in ecdysteroid biosynthesis in the cotton bollworm Helicoverpa armigera. B. Entomol. Res. 107 85–95. 10.1017/S0007485316000663 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the GenBank, Nvd: MK962642, Spo: MK962643, Sro: MK962644, Dib: MK962645, Sad: MK962646, and Shd: MK962647.