Abstract
Background
This study investigated a large number of patients to develop a predictive nomogram for survival and a web-based survival rate calculator that can dynamically predict the long-term survival of patients with primary gastric diffuse large B-cell lymphoma.
Methods
A total of 2647 patients diagnosed with primary gastric diffuse large B-cell lymphoma from 1998 to 2014 were extracted from the SEER database. We used the Lasso Cox regression model to identify independent risk factors for long-term survival and to develop a predictive nomogram for survival and a web-based survival rate calculator.
Results
The median (mean) follow-up time was 30 months (52.8 months). Cancer-specific survival rates decreased with time, while the 5-year conditional survival increased with time. Cancer-specific deaths were not constant. Cancer-specific deaths of patients within the first 2 years were high, while the risk remained relatively constant after 2 years. The independent risk factors included surgery, chemotherapy, tumor stage and age, according to the Lasso Cox regression analysis. We developed a predictive nomogram and a web-based survival rate calculator (https://linjuli1991.shinyapps.io/dynnomapp/). The calibration plot suggested that the actual value exhibited good agreement with the predicted value.
Conclusions
We found that patients with primary gastric diffuse large B-cell lymphoma had a high risk of death during the first 2 years. Additional active follow-up strategies should be provided during this period. This is the first study to develop a predictive nomogram and a web-based survival rate calculator that can provide evidence for individual treatment and follow-up.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-5993-6) contains supplementary material, which is available to authorized users.
Keywords: Primary gastric diffuse large B-cell lymphoma, Cancer-specific survival rate, Nomogram, Web survival rate calculator, Dynamically predict
Background
The National Comprehensive Cancer Network (NCCN) Guidelines use Ann Arbor staging of primary diffuse large B-cell lymphoma to guide clinical treatment and follow-up [1]. As a general staging system for non-Hodgkin’s lymphoma (NHL), the Ann Arbor staging system considers the location of lymph node dissemination as the basis for staging [2]. Other factors that may affect long-term survival, such as age and depth of tumor invasion, are not included. The Ann Arbor staging system is not considered the best staging system for primary gastric diffuse large B-cell lymphoma [3]. Therefore, this study investigated a large number of patients to develop a predictive nomogram for survival and a web-based survival rate calculator that can dynamically predict the long-term survival of primary gastric diffuse large B-cell lymphoma patients. Furthermore, we also investigated the probability of survival increasing over time based on survival times previously accumulated to guide treatment and follow-up strategies.
Methods
Patient selection
The inclusion criteria which has been reported in our previous study [4] were as follows: A case listing session was created from the Surveillance, Epidemiology, and End Results (SEER) program using SEER*Stat 8.2.1 (http://seer.cancer.gov/seerstat). A total of 2647 patients diagnosed with primary gastric diffuse large B-cell lymphoma from 1998 to 2014 were extracted from the SEER database. Patients diagnosed between January 1998 and December 2014; Ann Arbor [8] staging codes ([EOD] 10 - extent [1988–2003]; Collaborative Stage [CS] extension [2004+] stage; I and II, and stage III and IV ([ICD-O-3 topography); [pathological diagnosis of code], 16.0–16.9) ([ICD-O-3] 9680/3); the operation code (RX Summ--Surg Prim Site (1998+), 30–80); chemotherapy recode: chemotherapy code (yes, no/unknown)); and radiotherapy (radiation recode) code. The exclusion criteria were as follows: Ann Arbor staging was unknown, patients younger than 18 years old, multiple tumors (first malignant primary indicator), deaths that were not tumor-related (SEER “other cause of death” classification), patients who die from complications of chemotherapy or radiotherapy and patients who died within 30 days.
Statistical analysis
Statistical analyses were performed using SPSS software (version 22.0) for Windows (SPSS Inc., Chicago, IL), R software (version 3.4.0) (http://www.r-project.org/) and Rstudio software 1.1.383 (https://www.rstudio.com/). Cumulative survival rates were estimated using the Kaplan-Meier method and compared using the log-rank test. The cancer-specific survival hazard curve was plotted using the life table. We used the Lasso Cox regression model to identify independent risk factors for long-term survival [5]. The “glmnet” package was used to perform the Lasso Cox regression model analysis. Nomogram and calibration plots were generated using the “rms package” of R software. Discrimination was evaluated using a concordance index (C-index). A calibration plot was generated to explore the performance characteristics of the nomogram. In addition, we applied a bootstrapped resample with 1000 iterations to verify the accuracy of the nomogram. The “shiny” and “DynNom” packages were used to generate a web-based survival rate calculator, which can dynamically predict cancer-specific survival rates (https://www.shinyapps.io/). Conditional survival [6–8] estimates were calculated as the probability of survival for an additional 5 years (CS5), given that the patient had survived for 1, 2, 3, 4, or 5 years, and were calculated using the following formula: CS5 = S(X + 5)/S(X): For example, 5-year CS among patients who had survived 3 years from the date of surgery was calculated by dividing the 8-year survival rate by the 3-year survival rate. Two-sided P-values less than 0.05 were considered significant.
Results
Baseline characteristics of patients
The baseline clinical characteristics of the patients are shown in Table 1. Among the patients, 1240 (46.8%) were < 65 years, 608 (23%) were 65–74 years old, and 799 (30.2%) were > 75 years. The patients included 1539 (58.1%) men and 1108 (41.9%) women. A total of 2038 patients (77%) were white, and the other 609 patients were non-white (23%). A total of 646 (24.4%) patients had tumors located in the upper part of the stomach, 666 (25.2%) patients had tumors in the lower part of the stomach, 292 patients (11%) had tumors in the whole stomach, and for 1043 patients (39.4%) the location was unknown. The Ann Arbor stages were distributed as follows: 1167 (44.1%) cases were stage I, 582 (22%) cases were stage II, 211 (8%) cases were stage III, and 687 (26%) cases were stage IV. A total of 502 (19%) patients were treated with radiotherapy, and 2145 (81%) patients did not receive radiotherapy. A total of 2075 (78.4%) patients were treated with chemotherapy, and 572 (21.6%) patients did not receive chemotherapy or chemotherapy status was unknown. A total of 275 (10.4%) patients were treated with surgery, and 2372 (89.6%) patients did not undergo surgery.
Table 1.
Baseline characteristics of the patients
| Variable | 2647 (%) |
|---|---|
| Age | |
| < 65 | 1240 (46.8%) |
| 65–74 | 608 (23.0%) |
| > 74 | 799 (30.2%) |
| Gender | |
| Male | 1539 (58.1%) |
| Female | 1108 (41.9%) |
| Race | |
| White | 2038 (77.0%) |
| Other | 609 (23.0%) |
| Tumor location | |
| Upper third | 646(24.4%) |
| Mid and low third | 666(25.2%) |
| Overlapping stomach | 292 (11.0%) |
| Stomach (NOS) | 1043(39.4%) |
| Ann Arbor stage | |
| I | 1167 (44.1%) |
| II | 582 (22.0%) |
| III | 211 (8.0%) |
| IV | 687 (26.0%) |
| Radiation | |
| Yes | 502 (19.0%) |
| No | 2145 (81.0%) |
| Chemotherapy | |
| Yes | 2075 (78.4%) |
| No/Unknown | 572 (21.6%) |
| Surgery | |
| Yes | 275 (10.4%) |
| No | 2372 (89.6%) |
Long-term patient survival
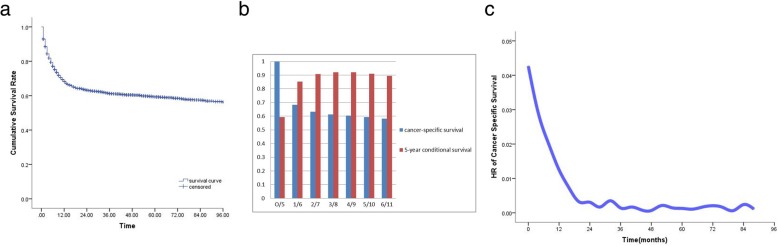
The median (mean) follow-up time was 30 months (52.8 months) for all the patients, and the cancer-specific survival rate is shown in Fig. 1a. The cancer-specific survival rates for 1, 2, 3, 4 and 5 years were 68.4, 63.2, 61.2, 60.4 and 59.3%, respectively, indicating a decreasing trend over time. The CS rates for 1, 2, 3, 4 and 5 years were 85.2, 90.8, 92.1 and 92% and 91.1%, respectively. The CS5 rate increased over time (Fig. 1b). The cancer-specific survival risk hazard is shown in Fig. 1c, which indicates that cancer-specific death of primary gastric diffuse large B cells is not constant. The rate of cancer-specific death within the first 2 years was high, while the risk remained relatively constant after 2 years. 5-year cancer specific survival rate of patients is 59.3% and 5-year overall survival rate of patients 52.4% (Additional file 1: Figure S1). 5-year cancer specific survival rate of patients who receive chemotherapy is 65.1% while no chemotherapy is 38.1%% (Additional file 2: Figure S2). 5-year cancer specific survival rate of patients who receive partial gastrectomy is 63.3% total gastrectomy is 64.9% (Additional file 3: Figure S3).
Fig. 1.
a Kaplan-Meier curve for cancer-specific survival of the patients. b 5-year conditional survival (CS5). c Cancer-specific survival hazard curve
Analysis of long-term patient survival
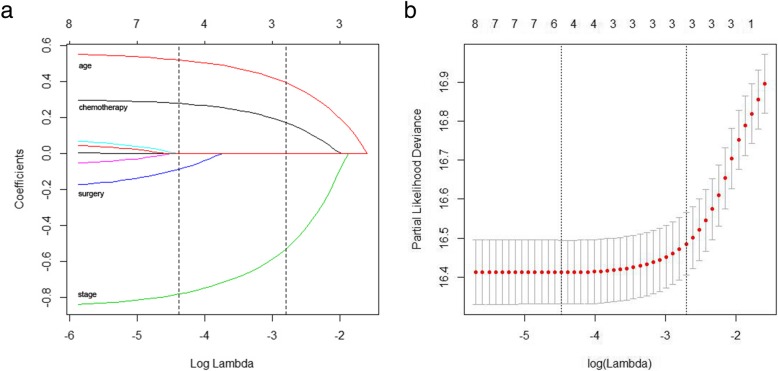
The independent risk factors affecting the long-term survival of patients were determined by Lasso Cox regression analysis. Each colored line represents a variable in the model. With increases in λ, the coefficient of each variable decreased. When the λ was optimal, the coefficients of some variables were compressed to 0; therefore, the variables that were not 0 were retained, and variable selection was performed. The blue, green, black and red solid lines that cross the dashed line in Fig. 2a indicate the independent prognostic factors, including surgery, chemotherapy, tumor stage and age, respectively. The minimum cross-validation error was 0.0114, and the maximum cross-validation error was 0.067 (Fig. 2b), which is within the range of standard error.
Fig. 2.
a Lasso regression coefficients. b Lasso cross-validation.
Predictive nomogram for cancer-specific survival
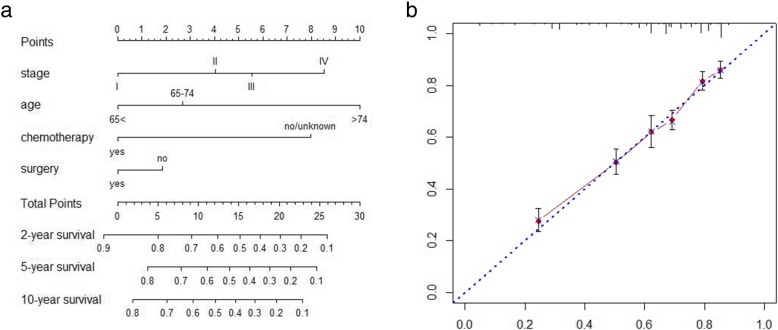
Figure 1 illustrates the predictive nomogram established for cancer-specific survival rates based on independent risk factors such as Ann Arbor stage, age, surgery and chemotherapy. The discriminative ability of the nomogram was superior to that of the Ann Arbor stages (C-index of 0.714 vs. 0.56, P < 0.01). The calibration plot (Fig. 3b) suggested that the actual value exhibited good agreement with the predicted value.
Fig. 3.
a Predictive nomogram for cancer-specific survival (C-index 0.714). b Calibration plot of 5-year cancer-specific survival
Web-based survival rate calculator
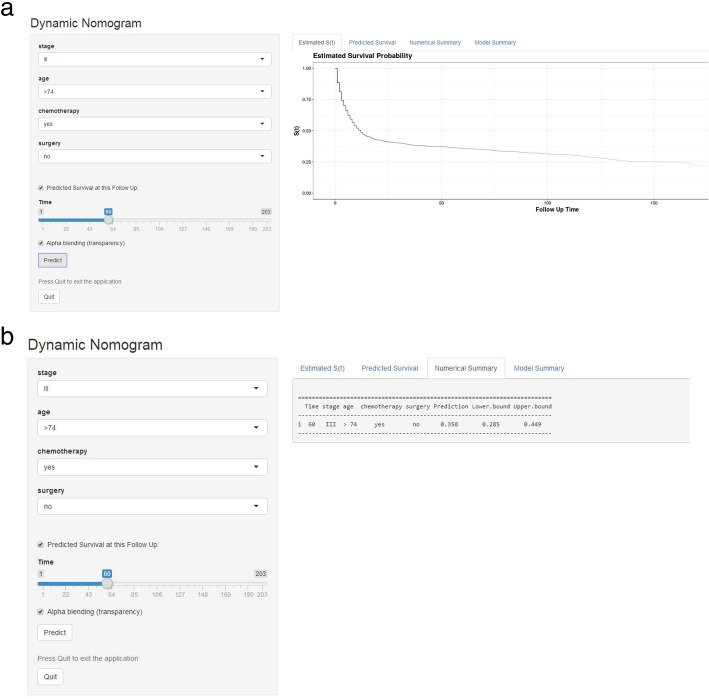
According to the above results, we established a dynamic web-based survival rate calculator (https://linjuli1991.shinyapps.io/dynnomapp/) to predict the long-term survival of patients with primary diffuse large B cells based on a nomogram (Fig. 4a). The calculator can individually predict the survival of patients according to their clinical characteristics. For example, the 5-year cancer-specific survival rate is approximately 35.8% (95% CI 28.5–44.9%) for patients with Ann Arbor stage of III, aged > 74 years, without surgery and chemotherapy (Fig. 4b).
Fig. 4.
a Patient with an Ann Arbor stage of III, age > 74 years, no surgery, who received chemotherapy according to the web survival rate calculator (95% CI 28.5–44.9%). b 95% confidence interval according to the web survival rate calculator
Discussion
Precision medicine rapidly developed in recent years. Clinicians must generate individualized treatment and follow-up strategies for patients, which requires more precise and convenient survival models. Nomograms integrate tumor stage and multiple prognostic factors into a simple and practical tool that has been widely used to predict the long-term survival of patients with malignant tumors. The accuracy is usually superior to traditional tumor staging systems [9–13]. In addition, to increase the convenience of predictive models, some scholars have used a web-based survival rate calculator [13–15] to predict the long-term survival of cancer patients. Primary gastric diffuse large B-cell lymphoma is a rare malignant tumor with an incidence of less than 5% of gastric malignant tumors [16]. A nomogram to predict survival and a web-based survival rate calculator for primary gastric diffuse large B-cell lymphoma have not been previously reported. Therefore, this study examined 2647 patients in the SEER database to analyze factors that affect long-term survival and used a conditional nomogram web-based survival rate calculator to dynamically predict the prognosis of primary gastric diffuse large B-cell lymphoma with different risk factors to determine individual treatment and follow-up strategies.
The traditional cancer-specific survival rate of tumors is an important basis for guiding treatment, follow-up and surveillance. However, the risk of cancer-specific-death is not constant and often changes over time. The risk of recurrence and death is usually the highest during the first few years after treatment, while the survival rate tends to be constant over time. Therefore, traditional survival rates exhibit a significant deficiency for dynamically evaluating the survival of tumor patients. CS [6–8, 17–19], which accounts for survival time already accumulated, provides a more “dynamic” estimate of the risk of death over time. It is an important tool for guiding long-term follow-up. In addition, the NCCN guidelines recommend that patients with diffuse large B-cell lymphoma should be followed up every 3–6 months for 5 years. This study combined cancer-specific survival rates, CS5 and hazard curves; the risk of death was high during the first 2 years after treatment and remained relatively constant after 2 years, which suggested that primary gastric diffuse large B-cell lymphoma patients should receive additional active follow-up during the first 2 years after treatment.
In addition, Lasso Cox regression analysis was used to analyze the prognostic risk factors. In contrast to the traditional stepwise Cox regression analysis, Lasso Cox regression can analyze all independent variables at the same time rather than using gradual processing. It is a new method for variable selection and shrinkage in a Cox proportional hazards model. It minimizes the log partial likelihood subject to the sum of the absolute values of the parameters being bound by a constant. Due to the nature of this constraint, it minimizes coefficients and produces some coefficients that are exactly zero. Therefore, it reduces the estimation variance while providing an interpretable final model. Simulations have indicated that Lasso regression is more accurate than stepwise selection [5, 13]. According to the Lasso Cox regression analysis, we found that age, tumor stage, chemotherapy and surgery are independent risk factors for long-term survival. Due to the overall poor functionality, low resilience and short life expectancy, the survival rate of elderly patients was relatively low. Previous studies [20, 21] have suggested that tumor stage is an important risk factor for long-term survival, and the survival rate of early-stage patients is higher than that of patients with advanced stage tumors. Chemotherapy is one of the main treatment methods according to the guidelines for lymphoma published by the Japanese Gastric Cancer Association (JGCA) and the NCCN Guideline [1, 22]. This treatment plays an important role in the prognosis of patients. Surgical indications are limited to bleeding, acute perforation, pyloric obstruction, or contraindications to chemotherapy during the course of non-surgical treatment. Studies [23, 24] have shown that combined chemotherapy and surgery could not significantly improve the long-term survival of patients. However, some studies [21, 25] have found that patients who received surgery combined with chemotherapy had significantly better prognoses than those who received chemotherapy alone. In this study, Lasso Cox regression analysis showed that surgical treatment was an independent factor for survival.
In this study, we try to incorporate factors which may have an impact on survival. Many studies [9, 10, 12, 26, 27] have suggested that nomograms may be useful tools for predicting long-term prognoses. Using a simple graphical representation, nomograms enable the incorporation of multiple relevant clinical predictors and can be applied to an individual patient’s combination of relevant clinical factors. Our predictive nomogram included age, Ann Arbor stage, chemotherapy and surgery and was superior in assessing prognoses to the Ann Arbor stage alone (C-index 0.714 vs. 0.56, P < 0.01). Although this nomogram was highly accurate, it may be difficult to use in clinical settings due to the need to perform manual calculations. Therefore, our team, for the first time, developed a web-based survival rate calculator based on prediction nomograms of primary gastric diffuse large B lymphoma. In this study, the 5-year survival rate of a patient with an Ann Arbor stage of III, aged > 74 years, without surgery who received chemotherapy was 35.8% (95% CI 28.5–44.9%). The prediction accuracy of the web-based calculator was higher than that of the nomogram according to the 95% confidence interval. The web-based calculator allows better visualization than other web-based survival rate calculators. It can dynamically predict the cancer-specific survival rate of patients at different time points and help to identify patients at high risk of cancer-specific death.
In this study, we compared overall survival and cancer specific survival of patients. 5-year CSS (59.3%) was significant higher than 5-year OS (52.4%). In order to explore the impact of the disease on long-term survival of patients and make the purpose of this study clear, we included CSS as the end point of the study. The majority within 30 days is most caused by surgery or complications. Cancer-specific survival can be better studied by excluding patients who died within 30 days.
Our study had several limitations which has been reported in our previous study [4] were as follows: A data selection bias existed because this study was analyzed in a retrospective manner. The generalizability of the results also requires further validation with external data. We try to incorporate factors which may have an impact on survival, but the SEER database was lacking of detail information of adjuvant therapy, detailed pathological types, gene expression and postoperative complications. We are unable to further analyze the impact of rituxan, non-anthracycline chemotherapy, specific dosage of radiation, different pathological types, gene types and complications on the survival of patients. And it can to be confirmed by more prospective multi-center clinical research data. However, primary gastric diffuse large B-cell lymphoma is a relatively rare disease, and studies with large numbers of patients are lacking. This study may serve as a basis for subsequent research. The web-based survival calculator can be updated and further validated externally.
Conclusion
In conclusion, our study investigated a large number of patients and analyzed clinical characteristics and prognostic factors. We found that patients with primary gastric diffuse large B-cell lymphoma had a high risk of death during the first 2 years. Active follow-up strategies should be increased during this period. This is the first study to develop a predictive nomogram and a web-based survival rate calculator that can provide evidence for individual treatment and follow-up strategies.
Additional files
Figure S1. 5-year cancer specific survival rate of patients is 59.3% and 5-year overall survival rate of patients 52.4%. (JPG 27 kb)
Figure S2. 5-year cancer specific survival rate of patients who receive chemotherapy is 65.1% while no chemotherapy is 38.1%%. (JPG 27 kb)
Figure S3. 5-year cancer specific survival rate of patients who receive partial gastrectomy is 63.3% total gastrectomy is 64.9%. (JPG 25 kb)
Acknowledgements
The authors thank all the medical staff who contributed to the maintenance of the medical record database.
Abbreviations
- CS5
5-year conditional survival
- CSS
Cancer-specific survival
- JGCA
Japanese Gastric Cancer Association
- NCCN
National Comprehensive Cancer Network
- NHL
Non-Hodgkin’s lymphoma
- OS
Overall survival
- SEER
Surveillance, Epidemiology, and End Results
Authors’ contributions
JLL, JX L, CHZ and CMH conceived of the study, analyzed the data, and drafted the manuscript; PL, JWX, JBW and JL helped revise the manuscript critically for important intellectual content; QYC and LLC helped collect data and design the study. All authors read and approved the final manuscript.
Funding
Supported by: Scientific and technological innovation joint capital projects of Fujian Province, China (No.2016Y9031). Fujian province medical innovation project (2015-CXB-16). The funding bodies were neither involved in the design of the study, nor in the collection, analysis, or interpretation of data nor in the writing of the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study was approved by the ethics committee of Fujian Union Hospital. Because patient data in the SEER database were de-identified, signed informed consent was waived for the present study.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest associated with the publication of this manuscript to declare. The authors report no relevant financial disclosures related to this current work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chang-Ming Huang and Chao-Hui Zheng contributed equally to this work and should be considered co-first authors
Contributor Information
Chang-Ming Huang, Phone: +86-591-83363366, Email: hcmlr2002@163.com.
Chao-Hui Zheng, Phone: +86-591-83363366, Email: wwkzch@163.com.
References
- 1.Zelenetz AD, Gordon LI, Wierda WG, et al. Diffuse large B-cell lymphoma version 1.2016. J Natl Compr Cancer Netw. 2016;14(2):196–231. doi: 10.6004/jnccn.2016.0023. [DOI] [PubMed] [Google Scholar]
- 2.Musshoff K. Clinical staging classification of non-Hodgkin's lymphomas (author’s transl) Strahlentherapie. 1977;153(4):218–221. [PubMed] [Google Scholar]
- 3.Ruskone-Fourmestraux A, Dragosics B, Morgner A, et al. Paris staging system for primary gastrointestinal lymphomas. Gut. 2003;52(6):912–913. doi: 10.1136/gut.52.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JL, Lin JX, Li P, et al. The impact of surgery on long-term survival of patients with primary gastric diffuse large B-cell lymphoma: a SEER population-based study. Gastroenterol Res Pract. 2019;2019:9683298. doi: 10.1155/2019/9683298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–395. doi: 10.1002/(SICI)1097-0258(19970228)16:4<385::AID-SIM380>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Ejaz A, Spolverato G, et al. Conditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the us gastric cancer collaborative. Ann Surg Oncol. 2015;22(2):557–564. doi: 10.1245/s10434-014-4116-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang SJ, Emery R, Fuller CD, et al. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer. 2007;10(3):153–158. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee JW, Ali B, Yoo HM, et al. Conditional survival analysis in Korean patients with gastric cancer undergoing curative gastrectomy. BMC Cancer. 2015;15:1005. doi: 10.1186/s12885-015-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30(31):3834–3840. doi: 10.1200/JCO.2012.41.8343. [DOI] [PubMed] [Google Scholar]
- 10.Groot Koerkamp B, Wiggers JK, Gonen M, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015;26(9):1930–1935. doi: 10.1093/annonc/mdv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 12.Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21(19):3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 13.Battersby NJ, Bouliotis G, Emmertsen KJ, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut. 2017;67(4):688–696. doi: 10.1136/gutjnl-2016-312695. [DOI] [PubMed] [Google Scholar]
- 14.Renfro LA, Grothey A, Xue Y, et al. ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer. J Natl Cancer Inst. 2014;106(12):dju333. doi: 10.1093/jnci/dju333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations to estimate survival in patients with colorectal cancer: cohort study. BMJ. 2017;357:j2497. doi: 10.1136/bmj.j2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Akwaa AM, Siddiqui N, Al-Mofleh IA. Primary gastric lymphoma. World J Gastroenterol. 2004;10(1):5–11. doi: 10.3748/wjg.v10.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikken JL, Baser RE, Gonen M, et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann Surg Oncol. 2013;20(5):1623–1630. doi: 10.1245/s10434-012-2723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harshman LC, Xie W, Bjarnason GA, et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol. 2012;13(9):927–935. doi: 10.1016/S1470-2045(12)70285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haydu LE, Scolyer RA, Lo S, et al. Conditional survival: an assessment of the prognosis of patients at time points after initial diagnosis and treatment of Locoregional melanoma metastasis. J Clin Oncol. 2017;35(15):1721–1729. doi: 10.1200/JCO.2016.71.9393. [DOI] [PubMed] [Google Scholar]
- 20.Bozer M, Eroglu A, Unal E, et al. Survival after curative resection for stage IE and IIE primary gastric lymphoma. Hepatogastroenterology. 2001;48(40):1202–1205. [PubMed] [Google Scholar]
- 21.Wang YG, Zhao LY, Liu CQ, et al. Clinical characteristics and prognostic factors of primary gastric lymphoma: a retrospective study with 165 cases. Medicine (Baltimore) 2016;95(31):e4250. doi: 10.1097/MD.0000000000004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14(2):113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 23.Aviles A, Nambo MJ, Neri N, et al. The role of surgery in primary gastric lymphoma: results of a controlled clinical trial. Ann Surg. 2004;240(1):44–50. doi: 10.1097/01.sla.0000129354.31318.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96) J Clin Oncol. 2005;23(28):7050–7059. doi: 10.1200/JCO.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Jezersek Novakovic B, Vovk M, Juznic Setina T. A single-center study of treatment outcomes and survival in patients with primary gastric lymphomas between 1990 and 2003. Ann Hematol. 2006;85(12):849–856. doi: 10.1007/s00277-006-0172-7. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Zhang Q, Hu Y, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267(3):504–513. doi: 10.1097/SLA.0000000000002116. [DOI] [PubMed] [Google Scholar]
- 27.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. 2017;35(36):4057–4065. doi: 10.1200/JCO.2016.72.0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 5-year cancer specific survival rate of patients is 59.3% and 5-year overall survival rate of patients 52.4%. (JPG 27 kb)
Figure S2. 5-year cancer specific survival rate of patients who receive chemotherapy is 65.1% while no chemotherapy is 38.1%%. (JPG 27 kb)
Figure S3. 5-year cancer specific survival rate of patients who receive partial gastrectomy is 63.3% total gastrectomy is 64.9%. (JPG 25 kb)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.