Abstract
Introduction:
Lead is a multiple organ toxicant and an oxidative-stress inducer. The effect of Costus afer on metal- induced male reprotoxicity has not been previously carried out, hence this study. The present study investigates the protective effect of Costus afer aqueous leave extract on lead- induced reproductive damages in male albino Wistar rats.
Methods:
Adult male albino Wistar rats were weighed and separated into five groups of five rats each. Groups 1 & 2 served as normal and toxic controls receiving deionized and leaded (CH3COO)2Pb.3H2O and water respectively. Groups 3, 4 and 5 were given 750, 1500 and 2250mg/kg of Costus afer orally, respectively while receiving Pb2+ water ad libitum for 28 days.
Results:
The reproductive and antioxidant parameters obtained from the result served as scientific evidence in the study. The result showed non-significant changes in the absolute and relative weights of epididymis and testes in the Pb Group versus the control. Significant increases were recorded in the sperm analysis, blood lead (7.9±1.02; 1.1±0.01) level (BLL), luteinizing hormone (LH) (8.5±1.4:5.5±0.4), and a decrease in follicle stimulating hormone (FSH) (4.5±2.6:6.5±1.65), with non-significant changes in testosterone (TET) (1.3±0.00:1.6±0.2) in the Pb group compared to the control.
Conclusion:
The treatment with Costus afer exhibited dose-dependent significant changes in testicular oxidative stress, hormonal, sperm analysis and histopathological changes induced by lead. Aqueous leaves extract of Costus afer may be protective against lead induced testicular damage.
Keywords: sperm analysis, lead acetate, Costus afer, reproductive damages
INTRODUCTION
Plants represent a major part of the therapeutic ingredients in almost all systems of medical science. Herbal therapy in Africa is an age-long practice. Men’s continuous reliance on herbs for therapeutic and nutritional benefits cannot be overemphasized. Herbs and herbal products, or herbal supplements are all forms of plant materials used as complementary or alternative medicines throughout the world. Costus afer (bush cane, ginger lily) is a widespread tropical plant commonly found in shabby forest and riverbanks of West Africa (Iwu, 1993).
The study carried out by previous researchers (Morán-Martínez et al., 2013), reported the role of lead in male reproductive toxicity and its implication in infertility. Led buildup in the testis is known to have anti-spermatogenic effects, as per reported by Fahim et al. (2013). According to Anjum et al. (2017), the testis of lead-treated rats revealed remarkable degeneration and atrophied seminiferous tubules, with absence of regular differentiated stages of germ cells to mature spermatozoa. Given the high cost, scarcity and wide range of adverse effects of chelators, such as the classical antidotes of lead poisoning, continuous search for widely available ‘’natural antidotes’’ that will ameliorate or reverse the deleterious effects of lead in developing nations has been the research focus in our laboratory. The present study seeks to examine the efficacy of Costus afer in mitigating lead-induced oxidative stress and injury in the male reproductive system of male albino Wistar rats.
MATERIALS AND METHODS
Plant identification
The plant was identified and authenticated by Mr O.Ozioko A.O, International Center for Ethno Medicine and Drug Development (INTERCEDD), University of Nigeria Nsukka, Nigeria and the voucher Number is INTERCEDD/033.
Sample processing and extraction
The leaves were washed with clean water, shade-dried in a well-ventilated place for 24hrs. Two-hundred and fifty grams of the leaves were weighed and macerated into 500ml of deionized water, placed in a closed container and allowed to stand for 48hrs, under constant stirring. After 48hrs, the mixture was strained, the marc pressed, and the liquid filtered and stored in a refrigerator at 4ºC. The solution was discarded every three days and a fresh sample prepared, and the process was repeated until the end of the study.
Preparation of 2500-ppm leaded-water
A 50g of lead acetate (CH3COO)2Pb·3H2O were dissolved in 12ml of 1N HCl and made up to 20L with deionized water. Ten grams of glucose was added to improve the taste according to Sadeghi et al. (2013).
Animal Husbandry
Twenty male albino Wistar rats (Rattus norvegicus) weighing between 90-180g were purchased from the Department of Experimental Pharmacology & Toxicology - Animal house Abuja campus, Faculty of Pharmaceutical Sciences, University of Port-Harcourt, Rivers State. The rats were kept in polypropylene cages and maintained under the standard conditions prescribed by the committee for the purpose of controlling and supervising animal experiments (CPCSEA). The Institutional Animal Ethics Committee under the following approval number approved the experimental protocol: UPH/PHARM/2017/033. They were weighed and classified into five groups of five animals each, and allowed to acclimatize for two weeks. They were housed in a standard cage and maintained in standard laboratory condition at a temperature of 25±2ºC, with relative humidity of 55-64% and light and dark conditions (12/12h). They were fed with Top Feeds (Flour Mills Lagos, Nigeria.) and leaded acetate (CH3COO)2Pb·3H2O solution, except for the normal controls, that received deionized water ad libitum. Animal ethics and proper handling methods were strictly adhered to.
Design
Five groups of five male albino Wistar rats were used in the experiment. Each group was treated as follows for four weeks. Group 1, which served as the normal controls, received deionized water; Group 2 (toxic control) received lead acetate solution ad libitum. While Group 3 received lead acetate solution plus 750mg/kg bw. Costus afer. Group 4 received lead acetate solution plus 1500mg/kg b.w. Costus afer and Group 5 received lead acetate solution plus 2250mg/kg bw. Costus afer. The dose of the Costus afer extract used was based on previous studies (Ezejiofor et al., 2014), while the Sadeghi et al. method (2013) was adopted for the administration of the lead acetate solution. The body weights were monitored weekly, while the fluid and feed intake of the rats in all the groups were monitored daily for 28 days.
Necropsy
On the 28th day, the rats were fasted overnight, weighed, and slaughtered under ether anesthesia on the 29th day. The blood samples were collected by cardiac puncture and kept at a temperature of 4˚C for 6 hours. The blood samples were then centrifuged at 3000 rpm for 15 minutes and stored properly for further analysis. The testis and epididymis were harvested, absolute and relative weights were measured. The blood sample was spun at 3000rpm for 10min using a centrifuge. The left testes and epididymis were stored in 10% formaldehyde and processed for histological examination, whereas the right testes and epididymis were homogenized in ice-cold 0.1M Tris HCl buffer (pH 7.4) to produce 10% homogenate. The homogenate was centrifuged at 5000g at 4oC for 15 minutes. The supernatant was collected and used in an antioxidant assay.
Hormonal analyses
Plasma testosterone TET, luteinizing hormone (LH) and follicle stimulating hormone (FSH) assays were performed using a commercial microplate enzyme immunoassay kit, following the manufacturer’s instructions (Monobind Inc., USA). The testosterone AccuBind™ Microplate EIA Test System has a sensitivity of 0.0576 ng/ml and with a negligible cross reactivity with other androgen derivatives like androstenedione, 5α-dihydrotestosterone, and methyltestosterone.
Sperm Analysis
Spermatozoa were obtained from the epididymis by the method described previously (El-Desoky et al., 2013). The seminal fluid was collected by macerating the epididymis in phosphate buffered saline (PBS), centrifuged at 12,000 rpm for 5 minutes and incubated at 37ºC. The supernatants were assayed for sperm quality and characteristics - as described by Cheesbrough (2006). Sperm motility was assessed by the method described by Zemjanis (1970). The motility of epididymal sperm was microscopically evaluated within 2-4 minutes of their isolation from the caudal epididymis and the data was expressed as percentages. Epididymal sperm count was obtained by mincing the caudal epididymis in distilled water and filtering through a nylon mesh. The spermatozoa were counted using the hemocytometer with the improved Neubauer (Deep 1/10m, LABART, Germany) chamber, as per described by Pant and Srivastava (Pant & Srivastava, 2003). A total of 400 spermatozoa from each rat were examined for morphological traits.
Determination of Daily sperm production and testicular sperm number - TSN
Daily sperm production was determined using the frozen left testes from control and treated rats according to Joyce et al. (1993). Briefly, the testis was homogenized for 3 minutes in 25ml of physiological saline containing 0.05% (v/v) Triton X-100. Sample aliquots of 5.5µl were then placed on the hemocytometer and counted twice at 100 X magnification under the microscope to determine the average number of spermatids per sample. These values were used to obtain the total number of spermatids per testis and this number was then divided by the testes’ weights to count spermatids per gram of testes. Developing spermatids spent 4.61 days in rats. Thus 4.61 to obtain the daily sperm production (Joyce et al., 1993) divided the values for the number of spermatids per testis.
Determination of morphological abnormalities and percentage viability
The sperm suspension was placed on a glass slide, and smeared out with another slide. This was stained with Wells and Awa’s stain (0.2 g of eosin and 0.6 g of fast green dissolved in distilled water and ethanol in the 2:1 ratio) for morphological examination; and 1% eosin and 5% nigrosine in 3% sodium citrate dehydrate solution for live/dead ratio - according to the method described by Wells & Awa (1970).
Biochemical analysis
Testicular/Epididymal superoxide dismutase SOD assay
We estimated the SOD using the inhibition of superoxide-dependent reduction of tetrazolium dye, methyl thiazolyl tetrazolium (MTT) to its formazan (Madesh & Balasubramanian, 1998).
Testicular/epididymal Malondialdehyde (MDA) Determination
Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) (Ohkawa et al., 1979; Balasubramanian et al., 1988). The MDA level was calculated and expressed as nmol of MDA/g of wet tissue using the molar extinction coefficient of the chromophore (1.56×10-5/m/cm).
Testicular/Epididymal Reduced Glutathione (GSH) l assay
We estimated the GSH based on a reduced glutathione reaction with 5-5ditiobis-2-nitrobenzoic acid (DTNB). Testicular GSH was spectrophotometrically determined using Ellman’s reagent 5- 5-dithiobis (2-nitrobenzoic acid) (DTNB) as a chromogen at 412 nm (Sedlak & Lindsay, 1968).
Testicular/ Epididymal Catalase Activity assay
Catalase activity in homogenates were determined according to Clairborne (1995) with slight modifications. The specific CAT activity was calculated using the molar extinction coefficient of H2O2 at 240 nm, 43.59 l mol cm. One unit of catalase activity equals the amount of protein that converts 1 mmol H2O2 min. Activity was expressed as Units mg.
Testicular/Epididymal Glutathione-S-Transferase (GST) activity assay
The glutathione-S-transferase (GST) activity was determined according to Habig et al. (1974).
Testicular/Epididymal Glutathione Peroxidase Activity assay
The GSH-Px activity was assessed according to the methods described by Rotruck et al. (1973).
Histopathology
Formalin fixed tissues (testes and epididymis) were dehydrated through ascending grades of alcohol, cleared in three changes of xylene, and were embedded in paraffin. Serial sections, each of 4µm thickness, were cut and stained with H and E as per standard protocols (Bancroft & Gamble, 2002). Stained sections were morphologically evaluated, and the slides were used for comparison.
Statistical analysis
The data was analyzed using the one-way ANOVA - statistical package for social sciences (SPSS) version 12.0. The differences between mean values were tested using Duncan's multiple comparison tests and the significance level was set at p<0.05.
RESULTS
Body weight and organ weight
The weights of the animals at the experiment onset ranged from 90g in the control animals to 180 g in the Pb acetate + 2250mg/kg CA Group (Table 1). Lead acetate alone and in combination with different doses of the C. afer CA (750 -2250mg/kg) did not cause any significant increase in the body weight of the rats, both in control and treated Groups up to 28 days of observation (Table 1). At the end of the experiment, the percent body weight gain was high among controls (37.23%), low in the Pb-acetate group (19.87%) and even lower in decreased dosing, when compared with the controls. However, the weight increases for all groups were not significantly different after 28 days of dosing with C. afer. Furthermore, both the absolute and relative weights of the testes and epididymis did not change at the end of the experiment (Table 1).
Table 1.
Effect of the aqueous leave extract of Costus afer (CA) on the body weight, absolute and relative weights of organs
| Treatment Groups | Final Body weight | Organs | Absolute weight (g) | Relative weight (%) |
|---|---|---|---|---|
| (weight gain/% weight gain) | ||||
| Water | 99.9±7.77 | Epididymis | 0.27±0.01 | 0.27 |
| (27.10±7.45/37.23) | Testes | 1.20±0.06 | 1.20 | |
| Pb Alone | 114.52±11.3 | Epididymis | 0.60±0.00 | 0.52 |
| (18.98±6.43/19.87) | Testes | 1.33±0.05 | 1.20 | |
| Pb+750mg/kg CA | 125.7±10.2 | Epididymis | 0.13±0.00 | 0.10 |
| (21.04±6.65/22.46) | Testes | 1.40±0.06 | 1.10 | |
| Pb+1500mg/kg CA | 130±9.34 | Epididymis | 0.17±0.01 | 0.13 |
| (20.8±6.23/19.05) | Testes | 1.47±0.02 | 1.10 | |
| Pb+2250 mg/kg CA | 196.3±11.6 | Epididymis | 0.11±0.00 | 0.08 |
| (28.98±12.39/17.22) | Testes | 1.57±0.04 | 0.80 |
Data are presented as the mean±SD (n=5). There was no significant difference among the treatment groups and control values (p>0.05).
Epididymal and testicular Sperm characteristics
The effect of C. afer on the sperm characteristics (volume, pH, viability, morphology and epididymal sperm number) of the lead-exposed male rats are shown on Table 2. The volume, sperm motility and epididymal sperm count in the lead acetate Group alone decreased significantly (p<0.05), while the percent abnormal, sluggishness and dead sperm cells increased significantly (p<0.05) when compared with the Control Group. The sperm motility, volume, percent viability and epididymal sperm number of the C. afer Group were not significantly different from the control values as seen in Table 2.
Table 2.
Effect of C. afer extract on the Sperm Characteristics of Lead exposed male rats
| Treatment/parameter | Vol (ml) | pH | Viability (%) | Normal Morph (%) | Abnormal (%) | Motility (%) | Sluggish (%) | Dead (%) | ESN ml (*106) |
|---|---|---|---|---|---|---|---|---|---|
| Water | |||||||||
| Mean±SD | 0.12±0.07 | 8.0 ±0.0 | 71.7±10.4 | 65.0 ±13.2 | 26.6±10.4 | 63.3±23.6 | 5.33±2.9 | 25±18.0 | 3.9±2.0 |
| (Max-Min) | 0.2-0.05 | 8.0-8.0 | 80-60 | 75-50 | 35-15 | 90-45 | 10-5 | 40-5 | 5.0-1.0 |
| Pb alone | |||||||||
| Mean±SD | 0.08±0.03 | 8.0±0.0 | 65.0±5.0 | 60.0±5.0* | 40.0±5.4* | 45.0±5.0* | 15.0±5.0* | 43.3±5.9* | 3.0±1.0 |
| Max-Min | 0.2-0.1 | 8.0-8.0 | 65-55 | 65-55 | 45-35 | 50-40 | 20-10 | 50-40 | 4.0-2.0 |
| Pb+750mg/kg CA | |||||||||
| Mean±SD | 0.13±0.06 | 8.0±0.0 | 65.0±13.2 | 63.±7.64** | 35.0±13.2** | 58.3±2.9** | 11.67±2.9* | 30±0 | 4.2±1.4** |
| Max-Min | 0.2-0.1 | 8.0-8.0 | 75-50 | 70-55 | 50-25 | 60-55 | 15-10 | 30-30 | 5.0-2.5 |
| Pb+1500mg/kg CA | |||||||||
| Mean±SD | 0.13±0.06 | 8.0±0.0 | 68.3±7.63** | 63.3±10.4** | 36.7±10.4** | 61.7±16.7** | 11.67±5.8** | 23.3±11.5** | 4.3±7.6** |
| Max-Min | 0.2-0.1 | 8.0-8.0 | 75-60 | 75-55 | 45-25 | 80-50 | 15-5 | 30-10 | 2.0-5.0 |
| Pb+2250mg/kgCA | |||||||||
| Mean±SD | 0.17±0.0 | 8.0±0.0 | 78.3±10.4** | 71.67±12.6** | 35.0±8.67** | 78.3±2.89** | 11.67±2.9** | 13.3±2.8** | 4.6±1.0** |
| Max-Min | 0.1-0.05 | 8.0-8.0 | 90-70 | 70-55 | 45-30 | 80-75 | 15-10 | 15-10 | 6.0-4.0 |
Data expressed as mean±S.D.
Values differ significantly from control (p<0.05).
Values differ significantly from Pb alone (p<0.05).
CA = C. afer
Hormonal parameters
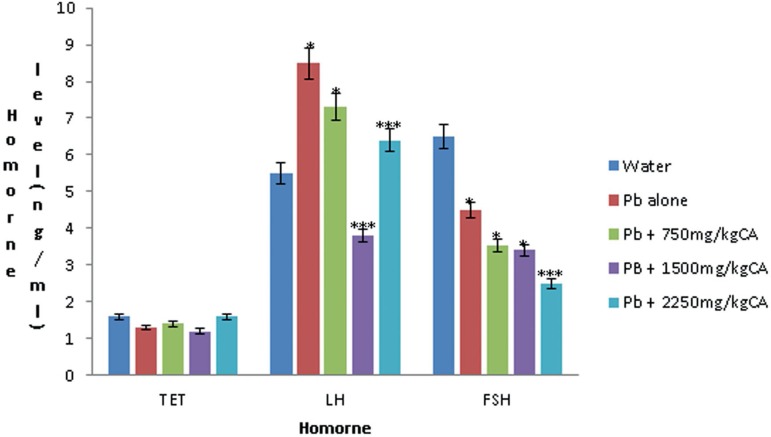
The effect of C. afer on the plasma testosterone (TET), Luteinizing hormone (LH) and Follicle stimulating hormone (FSH) levels on the lead-exposed male rats is shown in Figure 1. There was no significant difference in the TET level in lead acetate only (1.3±0.00) and lead acetate plus C. afer-treated Groups (1.4±0.1, 1.2±0.06, 1.6±0.1). Whereas a significant difference (p<0.05) was seen in the LH and FSH levels in lead acetate only (LH-8.5±1.4: FSH-4.5±2.6) and in the lead acetate plus C. afer treated groups (LH-7.3±0.5, 3.8±0.6, 6.4±0.5 and FSH-3.53±0.15, 3.2±1.1, 2.5±0.17 compared with the normal control animals (LH- 5.5±0.4 and FSH-6.5±1.65).
Figure 1.
Effect of C. afer extract on the hormonal (ng/ml) parameters of lead-exposed rats. n=5, *(significantly different from the water); ***(significantly different from the lead alone).
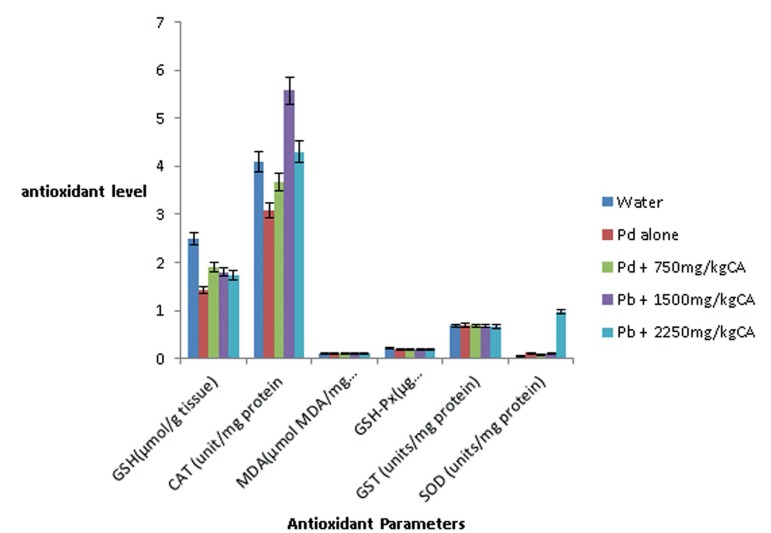
Testicular MDA and GSH level, GSH-Px and GST activities
Figure 2 shows the changes in MDA and GSH levels, as well as GST and GSH-Px activities in the testes. There were no significant changes in the level of testicular MDA, GST and GSH-Px activity in the testes of Pb acetate alone treated rats compared to those in the Control Group (0.11± 0.01mol MDA mg versus 0.11± 0.01mol MDA mg-1, 0.69±0.03 versus 0.68±0.03 and 0.22±0.04 versus 0.18±0.03g residual GSH remaining min mg respectively). There were no significant changes in the testicular MDA level, GST and GSH-Px activities in the lead acetate + C. afer treated groups compared to control animals (p>0.05).
Figure 2.
Effect of aqueous leave extract of C. afer on the testicular anti-oxidant parameters in lead-exposed rats
Changes in testicular antioxidant enzymes (CAT and SOD) activities
There were 25% and 10% decrease in testicular CAT activities in the Pb acetate alone and 750mg/kg CA treated rats, respectively, when compared with the Control Group (Figure 2); whereas there were 40% and 7.6% increases in lead acetate plus 1500mg/kg CA and lead acetate plus 2250mg/kg CA treated groups, respectively. The difference between the CAT activity in the lead acetate plus 1500mg/kg CA treated and control animals was statistically significant (p<0.05). The testicular CAT activity increased 1.36 fold (4.1±0.46 versus 5.57±0.51unit mg) in the Pb acetate plus 1500mg/kg CA rats, compared to Pb acetate only treated rats (Figure 2). There was no significant change in the testicular SOD both in the Pb acetate only and C. afer extract treated animals (Figure 2).
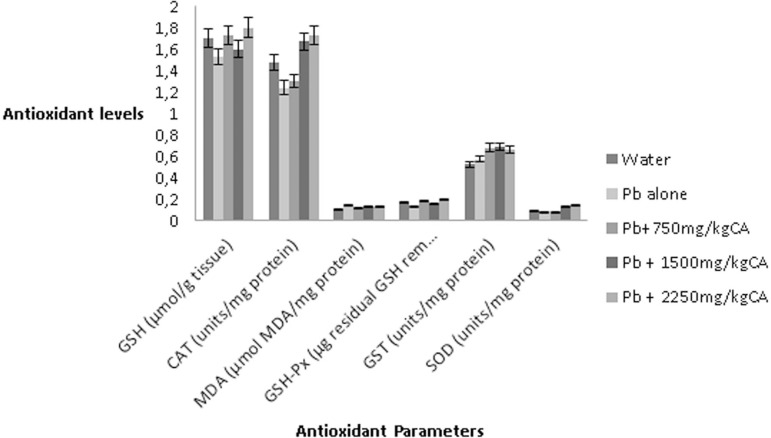
Epididymal MDA and GSH level, GSH-Px and GST enzyme activities
The effect of C. afer on the MDA and GSH levels, as well as GST and GSH-Px activities in epididymis are summarized in Figure 3 on lead exposed male rats. There was no significant change in the level of epididymal MDA, GST and GSH-Px activity in the epididymis of Pb acetate only treated rats compared to control values. There were also no statistical significant changes in epididymal MDA level, GST and GSH-Px activity in lead acetate plus C. afer-treated rats compared to controls (p>0.05).
Figure 3.
Effect of aqueous leave extract of C. afer on the epididymal anti-oxidant parameters in lead-exposed rats
Changes in epididymal antioxidant enzymes (CAT and SOD) activities
The epididymal CAT and SOD showed no changes in both the Pb acetate only and lead acetate plus C. afer-treated rats (Figure 3) when compared with the Control Group.
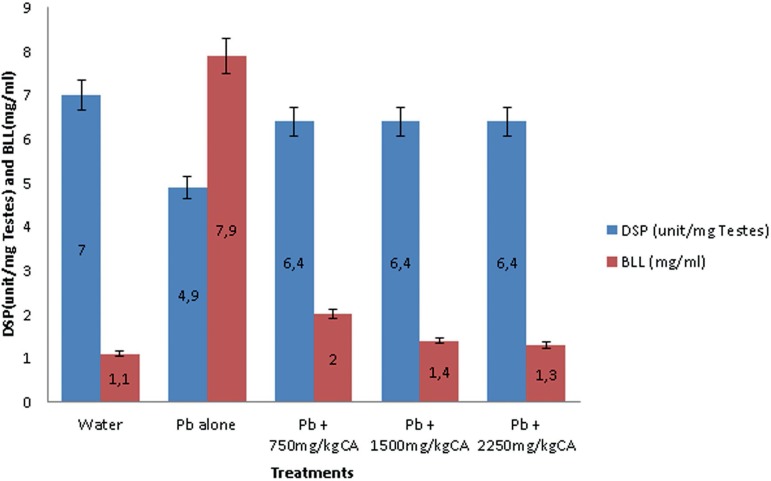
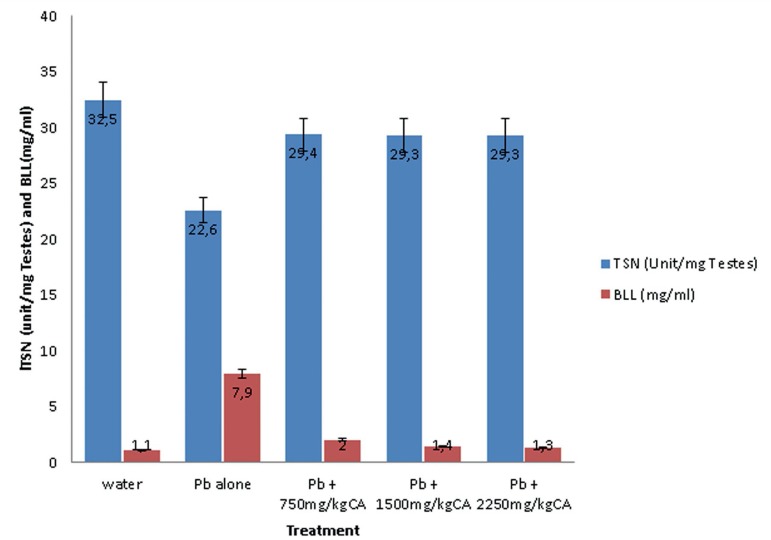
Daily Sperm Production (DSP), Testicular Sperm Number (TSN) and Blood Lead Level (BLL).
The effects of C. afer on daily sperm production (DSP) and Blood Lead Level (BLL) and testicular sperm number (TSN) and Blood Lead Level (BLL) in lead acetate treated male albino rats are shown in Figure 4 and 5, respectively. The DSP decreased and BLL increased in Pb acetate animals compared with the control. There was significant increase in DSP and a decrease in BLL following treatment with the C. afer CA extract.
Figure 4.
Effects of C. afer on Daily Sperm Production (DSP) and Blood Lead Level (BLL) in lead-exposed rats. Data is expressed as mean±S.D.; n=5
Figure 5.
Effects of C. afer on testicular sperm number (TSN) and Blood Lead Level (BLL) in lead exposed rats. Data is expressed as mean±S.D.; n=5
A similar trend was seen in Figure 5, with a decrease in Testicular Sperm Number TSN and an increase in BLL in the Pb acetate animals compared with the control value. In the C. afer treated animals, the reverse was the case with an increase in TSN and a decrease in BLL, respectively.
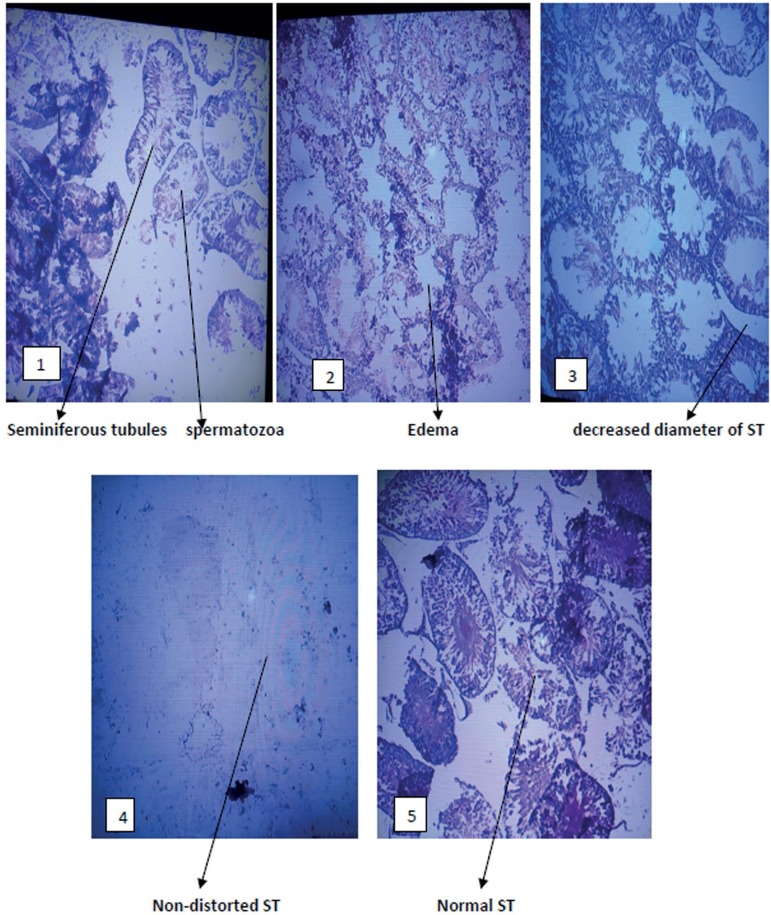
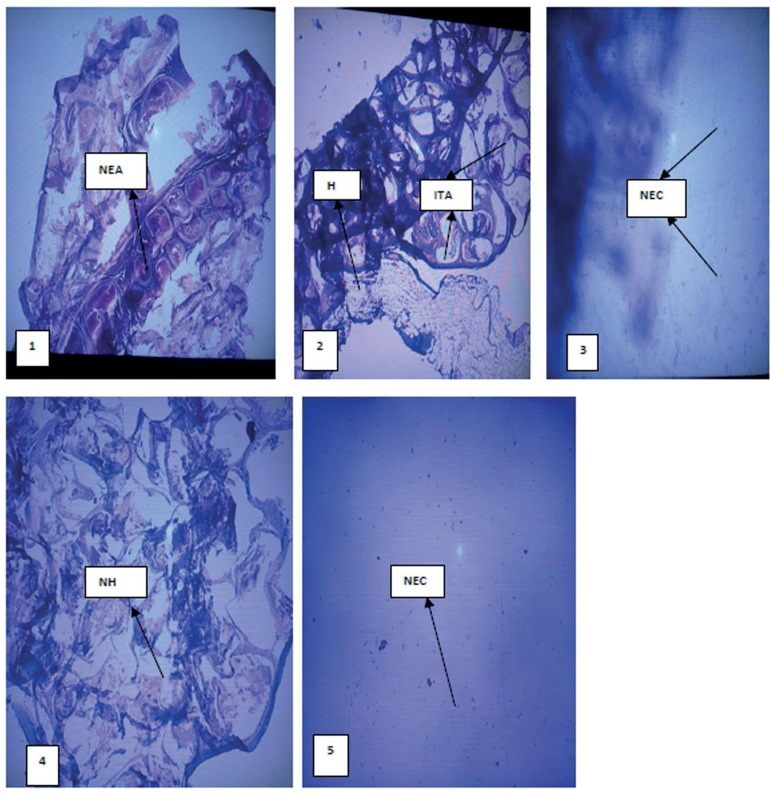
Histological examination of the testis
Figures 6 and 7 show the histopathology of the testes treated with lead acetate and C. afer. There were no histopathological changes in the testes and epididymis of rats treated with C. afer when compared with lead acetate only treated group. Edema, hydrocele and inflamed tunica albuginea were observed in the lead acetate only treated group. Such effect was alleviated by C. afer
Figure 6.
Photomicrograph of the testes: 1 (H2O), 2 (Pb alone), 3(Pb+750mg/kg CA), 4 (Pb+1500mg/kg CA) and 5 (Pb+2250mg/kg CA). All panels were stained with hematoxylin & eosin. Magnification x100. ST (Seminiferous tubules). CA=Costus afer
Figure 7.
Photomicrograph of the epididymis: 1 (H2O), 2 (Pb alone), 3(Pb+750mg/kg CA), 4 (Pb+1500mg/kg CA) and 5 (Pb+2250mg/kg CA). All panels were stained with hematoxylin & eosin, magnification x100. NEC (normal epididymal cell), NEA (normal epididymal architecture), ITA (inflamed tunica albuginea), H (hydrocele)
DISCUSSION
The testicular toxicity of Pb is mediated by oxidative damage and generation of reactive oxygen species (ROS) (Morán-Martínez et al., 2013; Fahim et al., 2013). The pathological role of ROS in infertility has been studied but not well established due to the various possible sources associated with excess production of ROS, including abnormal spermatozoa (Venkatesh et al., 2009). Oxidants seem to interfere with normal sperm function via peroxidation of unsaturated fatty acids in the sperm plasma membrane, which results in sperm dysfunction (Barros et al., 2003). Mammalian spermatozoa are coated with a membrane rich in polyunsaturated fatty acids (PUFA) which are very susceptible to oxidative damage by free radicals or ROS. The lipid peroxidation (LPO) mechanism damages the sperm cell membrane and is thought to be the main feature of the ROS-induced sperm damage leading to loss of motility, abnormal morphology and reduced capacity for sperm oocyte penetration and infertility (Storey, 1997). The body depends on strong antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase/reductase for its proper function. Storey (1997) reported that glutathione peroxidase/reductase enzymes play a central role in the defense against oxidative damage in human sperm. Seminal plasma and spermatozoa have abundance of antioxidant enzymes, namely glutathione peroxidase, glutathione reductase, superoxide dismutase (Yeung et al., 1998), and some of these antioxidant enzymes are made by the epididymis during storage (Potts et al., 2000). A decrease in the levels of reduced glutathione (GSH) during sperm production disrupts the membrane integrity of spermatozoa because of increased oxidative stress. GSH peroxidase, a selenium-containing antioxidant enzyme with GSH as the electron donor, removes peroxyl radicals from various peroxides including H2O2. GSH reductase then regenerates reduced GSH from oxidized GSH (GSSG). A selenium-associated polypeptide, presumably GSH peroxidase, has been demonstrated in rat sperm mitochondria; it plays a significant role in this peroxyl scavenging mechanism and, ultimately, in maintaining sperm motility. Although no significant changes were seen in the epididymal antioxidant parameters, treatment with C. afer significantly increased the levels of testicular GSH, CAT and SOD when compared with the Pb-acetate-only group. These antioxidant properties of C. afer may be responsible for it protective effect against the Pb-acetate-induced testicular toxicity (Dorostghoal et al., 2014; Sharma & Garu, 2011; Ansar et al., 2016). Pb can cross the blood-testis barrier, build up in the testis, and damage germinal cells at various levels of differentiation, shifting the oxidant/antioxidant profile towards the oxidant side as manifested by the marked exhaustion of the enzymatic antioxidants together with the buildup of lipid peroxidation products in the testicular tissue homogenate.
Administration of C. afer in the Pb-acetate-treated rats seemed to significantly restore seminal volume, pH, viability, morphology and ESN. There are reports of certain reductions in testis volume, seminiferous tubules diameter and germinal epithelium height increase from early weeks to 60 days of age, nearly by the onset of puberty, but it decreases afterward, so it seems that Pb has transient effects and testicular parameters become gradually better until 120 days of age. A plausible mechanism in Pb-toxicity is the loss of tissue homeostasis via an imbalance between pro and anti-oxidative factors (El-Masry et al., 2016). In a dose-dependent fashion, Pb tends to enter the tight junctions of the inter-Sertoli barrier, damage the epithelium, with a decrease in its height due to germ cell loss, thus enlarging the tubular lumen. Costus afer may hold a promise in the management of Pb-induced testicular toxicity as evidenced by it restorative effect on the various seminal parameters.
The major function of the testes is spermatogenesis and hormone production (Brennan & Capel, 2004), hence the testicular toxicity of Pb ultimately causing reduction in male sex hormones (Chowdhury, 2009). Besides the production of spermatozoa, testes are involved in the production of hormones that are required for various functions in the body, including maintenance of secondary sexual functions, and feedback on the hypothalamus and the pituitary to control the secretion of the gonadotropins. TET, LH and FSH are important hormonal components of male sexual development and fertility. A significant decline in TET or an increase in LH and FSH has been shown to adversely affect sexual maturity and fertility in male animals (Mann & Lutwak-Mann, 1981). In the present study, there was a significant increase in LH and FSH and a decrease in TET, found in the toxic control, which differs significantly from the normal controls, confirming the previous findings of Mann and Lukwat-Mann (Mann & Lutwak-Mann, 1981). It could be inferred that C. afer confers a protective effect by bringing to near normal the level of plasma TET, LH and FSH in the treated groups.
The blood lead level (BLL) was inversely related to the sperm production (DSP) and testicular sperm number (TSN). C. afer administration significantly increased both the DSP and TSN with accompanying reductions in BLL. Several studies in many rat strains and rodents indicate fairly consistently that blood lead (Pb) concentration > 30-40 µg/dl during at least 30 days of administration was associated with spermatogenesis impairment and reduced concentration of circulating androgens (Pandya et al., 2012; Assi et al., 2016). In the present study, there were increases in BLL coupled with daily decreases in sperm production (DSP), testicular sperm number (TSN) and reduced fertility indices (sperm concentration, percentage viability, individual motility and general motility) and increased percentage abnormality following lead acetate administration. These effects were either significantly reversed or brought to near normal control levels after treatment with C. afer. Histological analysis of the testis and epididymis showed marked distortions in the Pb-acetate-treated group compared with controls and C. afer-treated groups.
CONCLUSION
The present study implicated Pb as a reprotoxicant, affecting both the histological, biochemical and sperm analyses of the exposed rats, in general causing overall reproductive damage which was ameliorated by Costus afer. Taken together, aqueous leaf extract of C. afer may hold promise in alleviating Pb-induced male reproductive damage.
REFERENCES
- Anjum MR, Madhu P, Reddy KP, Reddy PS. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol Ind Health. 2017;33:265–276. doi: 10.1177/0748233716637543. [DOI] [PubMed] [Google Scholar]
- Ansar S, Hamed S, Al Ghosoon HT, Al Saedan RA, lM Iqba. The protective effect of rutin against renal toxicity induced by lead acetate. Toxin Rev. 2016;35:58–62. doi: 10.3109/15569543.2016.1155623. [DOI] [Google Scholar]
- Assi MA, Hezmee MN, Abba Y, Yusof MS, Haron AW, Rajion MA, Al-Zuhairy MA. Prophylactic effect of Nigella sativa against lead acetate induced changes in spermiogram, reproductive hormones and gonadal histology of rats. Vet World. 2016;9:1305–1311. doi: 10.14202/vetworld.2016.1305-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian KA, Manohar M, Mathan VI. An unidentified inhibitor of lipid peroxidation in intestinal mucosa. Biochim Biophys Acta. 1988;962:51–58. doi: 10.1016/0005-2760(88)90094-X. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 5th ed. New York: Churchill Livingstone; 2002. [Google Scholar]
- Barros ME, Schor N, Boim MA. Effects of an aqueous extract of phyllanthus niruri on calcium oxalate crystallization in vitro. Urol Res. 2003;30:374–379. doi: 10.1007/s00240-002-0285-y. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: Molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Cheesbrough M, editor. Examination of Semen. New York: Cambridge University Press; 2006. Medical Laboratory Manual for tropical countries Volume 2; pp. 130–132. [Google Scholar]
- Chowdhury AR. Recent advances in heavy metals induced effect on male reproductive function - A retrospective. Al Ameen J Med Sci. 2009;2:37–42. [Google Scholar]
- Clairborne A. Handbook of Methods for Oxygen Radical Research. In: Greewald AR, editor. Catalase activity. Boca Raton, FL; CRC Press: 1995. pp. 237–242. [Google Scholar]
- Dorostghoal M, Seyyednejad SM, Jabari A. Protective effects of Fumaria parviflora L. on lead-induced testicular toxicity in male rats. Andrologia. 2014;46:437–446. doi: 10.1111/and.12100. [DOI] [PubMed] [Google Scholar]
- El-Desoky GE, Bashandy SA, Alhazza IM, Al-Othman ZA, Aboul-Soud MA, Yusuf K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS One. 2013;8:e59177. doi: 10.1371/journal.pone.0059177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Masry S, Ali HAS, El-Sheikh NM, Awad SM. Dose-Dependent Effect of Coriander (Coriandrum Sativum L.) and Fennel (Foeniculum Vulgare M.) on Lead Nephrotoxicity in Rats. Int J Res Stud Biosc. 2016;4:36–45. [Google Scholar]
- Ezejiofor AN, Orish CN, Orisakwe OE. Costus afer ker gawl leaves against gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2014;8:310–313. [PubMed] [Google Scholar]
- Fahim MA, Tariq S, Adeghate E. Vitamin E modifies the ultrastructure of testis and epididymis in mice exposed to lead intoxication. Ann Anat. 2013;195:272–277. doi: 10.1016/j.aanat.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Iwu MM, editor. Handbook of African Medicinal Plants. Boca Raton: CRC Press; 1993. pp. 214–215. [Google Scholar]
- Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testes size and sperm production in the mouse. J Androl. 1993;14:448–455. doi: 10.1002/j.1939-4640.1993.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Ind J Biochem Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- Mann T, Lutwak-Mann C, editors. Male Reproductive Function and Semen: Themes and Trends in Physiology, Biochemistry and Investigative Andrology. Berlin: Springer Verlag; 1981. [Google Scholar]
- Morán-Martínez J, Carranza-Rosales P, Morales-Vallarta M, A Heredia-Rojas J, Bassol-Mayagoitia S, Denys Betancourt-Martínez N, M Cerda-Flores R. Chronic environmental exposure to lead affects semen quality in a Mexican men population. Iran J Reprod Med. 2013;4:267–274. [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pandya C, Pillai P, Nampoothiri LP, Bhatt N, Gupta S, Gupta S. Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Pant N, Srivastava SP. Testicular and spermatotoxic effects of quinalphos in rats. J Appl Toxicol. 2003;23:271–274. doi: 10.1002/jat.919. [DOI] [PubMed] [Google Scholar]
- Potts RJ, Notarianni LJ, Jefferies TM. Seminal plasma reduces exogenous oxidative damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mut Res. 2000;447:249–256. doi: 10.1016/S0027-5107(99)00215-8. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Sadeghi A, Ebrahimzadeh Bideskan A, Alipour F, Fazel A, Haghir H. The Effect of Ascorbic Acid and Garlic Administration on Lead-Induced Neural Damage in Rat Offspring's Hippocampus. Iran J Basic Med Sci. 2013;16:157–164. [PMC free article] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Sharma R, Garu U. Effects of lead toxicity on developing testes in Swiss mice. Univers J Environ Res Technol. 2011;1:390–398. [Google Scholar]
- Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997;3:203–213. doi: 10.1093/molehr/3.3.203. [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Singh G, Gupta NP, Kumar R, Deecaraman M, Dada R. Correlation of sperm morphology and oxidative stress in infertile men. Iran J Reprod Med. 2009;7:29–34. [Google Scholar]
- Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci. 1970;53:227–232. doi: 10.3168/jds.S0022-0302(70)86184-7. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG, De Geyter M, De Geyter C, Rolf C, Kamischke A, Nieschlag E. Studies on the origin of redox enzymes in seminal. Mol Hum Reprod. 1998;4:835–839. doi: 10.1093/molehr/4.9.835. [DOI] [PubMed] [Google Scholar]
- Zemjanis R. Collection and evaluation of semen. In: Zemjanis R, editor. Diagnostic and Therapeutic Technique in Animal Reproduction. 2nd ed. Baltimore: MD: The William and Wilkins Company, Waverly Press; 1970. pp. 139–153. [Google Scholar]