Abstract
The persistent isolation of Pseudomonas aeruginosa in the airways of non-cystic fibrosis bronchiectasis (NCFB) patients is associated with a worsening of the symptoms, increase of exacerbations, poor quality of life and functional impairment. The objective of this study was the analysis of the eradication rate of P. aeruginosa in the sputum of patients with NCFB treated with inhaled colistin and the effects of the treatment in the exacerbations. This was a prospective, cohort, study of 67 NCFB patients treated with inhaled colistin at the Hospital of A Coruña (Spain). We recorded dyspnoea, exacerbations, lung function and sputum cultures of P. aeruginosa in the patients. The mean age of the patients was 67.25 ± 14.6 years (59.7% male). The percentages of eradication of P. aeruginosa in sputum at 3, 6, 9 and 12 months were 61.2%, 50.7%, 43.3% and 40.3%, respectively. We observed a significant decrease in exacerbations after 1 year of colistin treatment (1.98 ± 3.62) versus the previous year (3.40 ± 4.21, p < 0.001). We conclude that treatment with inhaled colistin in patients with NCFB and P. aeruginosa in sputum can achieve high rates of eradication even in patients with several previous positive cultures, as well as a significant decrease of exacerbations and hospital admissions.
Keywords: Bronchiectasis, Pseudomonas aeruginosa, inhaled antibiotics, colistin, eradication
Introduction
In recent years, there has been an increase in the prevalence of non-cystic fibrosis bronchiectasis (NCFB)1 and in the economic burden caused by its management.2 Chronic bronchial infection (CBI) is frequently associated with NCFB and characteristically the dominant pathogens are Pseudomonas aeruginosa and Haemophilus influenzae. 3,4 Infection with P. aeruginosa is associated with worsening of symptoms,5 poorer health-related quality of life,6 increased exacerbations and accelerated decline in forced respiratory volume in the first second (FEV1).7,8 Also, it is one of the predictive factors of worse prognosis in the available severity indexes.7,9
Eradication of P. aeruginosa in CBI is challenging due to its ability to growth in biofilms, the fast appearance of resistant strains, and the difficulty of reaching adequate concentrations of antibiotics in the bronchial space with conventional treatments in some cases.10–14 However, successful eradication is usually high in young (68–93%) and adult (79%) cystic fibrosis (CF) patients using a variety of antipseudomonal regimens, with an average time of reappearance of 8–18 months.15–19
There are few studies of the possible role of inhaled antibiotics in the treatment of NCFB, and they are highly heterogeneous.13,15,20–23 Two studies with tobramycin were prospective trials with a placebo control group, but with a reduced number of patients.13,23 The few studies with inhaled colistin did not exceed 30 patients each and in only one of them systemic antibiotics had been used previously.15,21,22
Despite the limited data available, the clinical guidelines recommend the use of inhaled antibiotics for the treatment of early infection by P. aeruginosa. 24,25 In contrast to CF, for which the prescription of simultaneous systemic and inhaled antibiotics is recommended,6,26 for NCFB inhaled antibiotic (tobramycin or colistin for 3–12 months) is recommended only after failure of a previous eradicating attempt with oral ciprofloxacin and/or an intravenous (IV) cycle with two antipseudomonal antibiotics.24,25
The primary aim of this study was to evaluate the efficacy of an antibiotic eradication protocol for P. aeruginosa infection consisting of the administration of systemic antibiotics followed by inhaled colistin for 12 months in a cohort of patients with NCFB. The secondary aim was to determine whether the use of inhaled colistin is associated with decreased exacerbations with respect to the year prior to the initiation of treatment.
Material and methods
This 12-month prospective study was conducted between 1 January 2010 and 31 August 2015 at the bronchiectasis outpatient clinic of the University Hospital in A Coruña (Spain). The research protocol described here adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Review Board of the Hospital Universitario A Coruña. All patients were informed orally and in writing of the procedures, potential risks and implications of accepting this study.
This study included consecutive NCFB patients aged ≥18 years if they had (1) a diagnosis based on clinical presentation and high-resolution computed tomography (HRCT), (2) ≥1 cultures of P. aeruginosa but had not previously had eradication therapy, (3) treatment with inhaled colistin and follow-up for at least 1 year from the start of the inhaled antibiotic, and (4) no modifications in the base treatment for respiratory disease during the study period including previous year.
The exclusion criteria were (1) patients with CF or traction bronchiectasis due to pulmonary fibrosis, (2) indication of inhaled colistin as lung transplant prophylaxis, (3) infection with other Gram-negative non-fermenter bacteria, (4) having started treatment with regular nebulized antibiotics without prior IV or oral antipseudomonal antibiotics, (5) patients that achieved successful eradication of P. aeruginosa with only systemic antibiotic treatment, and (6) lack of compliance with follow-up protocol.
Patients underwent standard clinical follow-up procedures and all of them were informed about the collection of data from their medical history, and gave informed consent.
Study design
In the first assessment (inclusion visit), all patients underwent the same diagnostic protocol as suggested by the 2010 British Thoracic Society and Spanish guidelines.24,25 We routinely attempt to diagnose the aetiology of bronchiectasis following established guidelines.24
We collected data on demographics, exposure factors, familiar genetic diseases, co-morbidities, long-term treatments, respiratory symptoms (cough, sputum, dyspnoea using the Medical Research Council scale), physical exploration, microbiological findings in sputum cultures in previous year, HRCT, laboratory determinations including immunoglobulins (IgG, IgA, IgM, IgE), alfa-1 antitrypsin, autoimmunity, sweat test for chloride concentration (genetic testing in case of suspected CF) and number of exacerbations in previous year (oral cycles of antibiotics, emergency visits or hospitalizations). Clinical, radiological and treatment histories were obtained from the electronic health records and from the interview to the patient. Spirometry was performed in all patients, including forced vital capacity (FVC) and FEV1.27
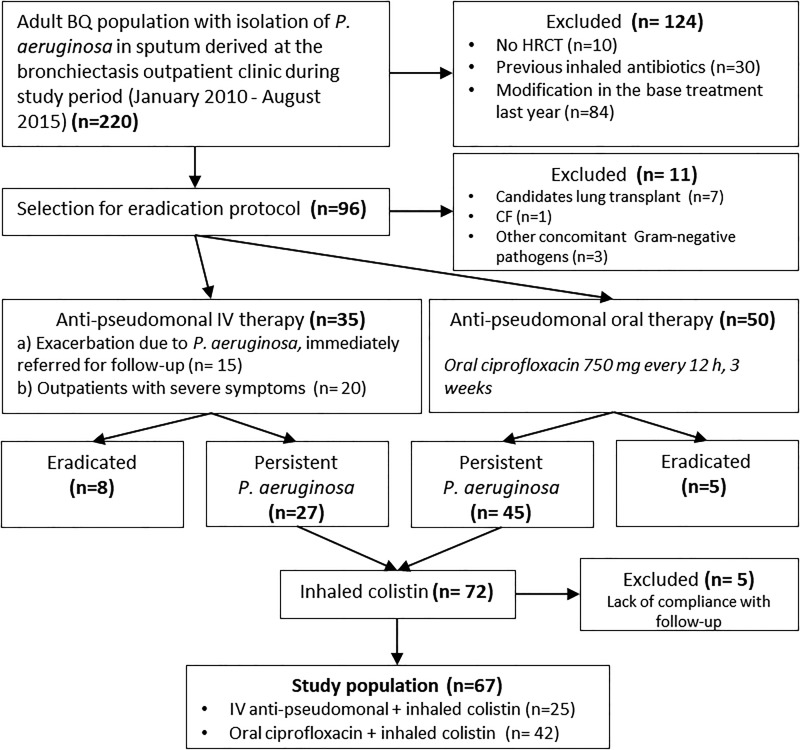
All patients with positive culture for P. aeruginosa received an eradication protocol which included oral ciprofloxacin 750 mg twice daily for 3 weeks (mild-moderate symptoms in outpatients) or IV tobramycin 5 mg/kg plus beta-lactam antipseudomonal antibiotics for 2 weeks (outpatients with severe symptoms, with purulent bronchorrhea, or patients admitted for exacerbation due to P. aeruginosa that were immediately referred for follow-up). Variations from the protocol occurred mainly because of drug allergy, intolerance or insusceptibility, and in this case two alternative IV antipseudomonal antibiotics were used at the clinician’s discretion. In case of persistence of P. aeruginosa in the sputum, inhaled colistin (Promixin 1 MIU twice daily) was administered with the I-neb® Adaptive Aerosol Delivery (AAD) device (Philips Respironics, Chichester, UK) during a 12-month period (Figure 1). Subjects were instructed to self-administer the treatment at home. A short-acting bronchodilator was administered before nebulized colistin as needed in case of cough and/or wheezing in order to reduce adverse effects.
Figure 1.
Chart showing participants in the study. BQ: bronchiectasis; HRCT: high-resolution computed tomography; CF: cystic fibrosis; IV: intravenous; OR: oral.
Monitoring of the patients followed standard clinical practice in visits every 3 months. Subjects were questioned concerning adherence to the treatment, changes in symptoms such as cough, dyspnoea, sputum production and wheezing. Blood tests (serum chemistry, haematology) and spirometry were performed in each visit. Samples of sputum were cultured monthly during the first 3 months and every 2 months thereafter.
All bacteriological analyses were performed on spontaneous sputum samples obtained before colistin administration and tested the presence of P. aeruginosa and other typical respiratory pathogens. The Murray–Washington criteria for sputum quality were used in all cases, with all samples having less than 10 squamous cells and more than 25 leukocytes per low-power microscope field. All samples were cultured in standard blood agar and Mc-Conkey and Saboraud media. The microorganisms isolated were identified by standardized or automated means, and the antibiogram was made.
Eradication of P. aeruginosa was defined as a negative culture on all sputum samples taken at the moment of evaluation (3, 6, 9 and 12 months since the beginning of treatment). Inhaled colistin was stopped only in the case of negative cultures of P. aeruginosa for at least 12 months. CBI was defined by the isolation of P. aeruginosa in sputum culture on three or more occasions, at least 1 month apart. Patients who were unable to provide sputum samples due to the absence of a productive cough were classified as not having chronic infection for the purposes of analysis. In case of CBI, inhaled colistin treatment was continued indefinitely.
The number of episodes of exacerbations was recorded for 1 year pre- and post-eradication protocol. An exacerbation of bronchiectasis was defined as a clinical deterioration for which oral or IV antibiotics were prescribed in the presence of at least one (and usually more than one) of the following symptoms: increasing cough, increasing sputum volume, worsening sputum purulence, worsening dyspnoea, increased fatigue/malaise, fever and haemoptysis. Exacerbations were classified as ambulatory management, emergency visits or hospital admission. Exacerbations were recorded from patient histories and verified using administrative databases. Previous exacerbations were defined with the same criteria as for the prospectively recorded exacerbations during colistin treatment.
Statistical analysis
We initially performed a descriptive analysis of the variables included in the study. The qualitative variables are presented as absolute values and percentages. For quantitative variables, the results are expressed as means and standard deviations, maximum and minimum values and quartiles.
Univariate comparisons between patients with or without eradication in the 3-, 6-, 9- and 12-month periods were tested with Pearson’s χ 2 or Fisher’s exact test for categorical variables. We used the Wilcoxon test to compare continuous variables after analysing the normality assumption with the Kolmogorov–Smirnov test. Variables selected for comparison between groups (eradication vs. non-eradication at different times) were age, gender (female vs. male), smoking status (never smoked vs. ex/active smoker), first positive sputum cultures of P. aeruginosa, proportion of patients with concomitant azithromycin treatment, systemic antibiotics used for eradication (oral ciprofloxacin vs. IV antipseudomonal antibiotics), P. aeruginosa morphotype mucoid, mean FVC post-bronchodilatation (% predicted) and FEV1 post-bronchodilatation (% predicted). A logistic regression analysis was performed to determine the factors related to the eradication of P. aeruginosa. Age and gender were included as covariates in all models, as well as all variables with p < 0.10 in univariate analysis (concomitant treatment with azithromycin, P. aeruginosa mucoid) and also included variables that showed significant influence in literature in eradication in CF (first positive sputum for P. aeruginosa and systemic antibiotics). A forward stepwise strategy was used. Statistical analysis was performed using the SPSS software package version 20.0 (SPSS Inc., USA). A p value <0.05 was considered statistically significant for all analyses.
Results
Ninety-six patients diagnosed with NCFB and presenting P. aeruginosa in sputum were selected for the eradication protocol. Eleven patients were excluded due to the following reasons: seven patients had indication related to infection prophylaxis for lung transplantation, one was diagnosed with CF during the follow-up, three had other concomitant Gram-negative microorganism different from P. aeruginosa when treatment started. Of the remaining 85 patients, 13 achieved successful eradication with only systemic antibiotic treatment, and 5 failed to comply with first follow-up visit. The data correspond to 67 patients with a mean age of 67.25 ± 14.6 years (range 16–87 years), predominantly males (59.7%). Patient baseline characteristics are shown in Table 1. Twenty-five patients received 2 weeks of IV antipseudomonal antibiotics and the remaining 42 patients received oral ciprofloxacin.
Table 1.
Patient characteristics at the beginning of treatment (n = 67).
| Age (years), mean ± SD (range) | 67.25 ± 14.6 (16–87) |
| Sex, % males | 59.7 |
| Tobacco use, n (%) | |
| Ex-smokers | 26 (39) |
| Smokers | 4 (6) |
| Cause of bronchiectasis, n (%) | |
| COPD | 26 (38.8) |
| Post-infective | 10 (14.9) |
| Unknown | 9 (13.4) |
| Asthma | 8 (11.9) |
| Immune defect | 5 (7.5) |
| Deficit of alfa-1 antitrypsin | 4 (6.0) |
| Rheumatoid arthritis | 2 (3.0) |
| Ciliary dyskinesia | 2 (3.0) |
| Yellow nail syndrome | 1 (1.5) |
| Coinfection with other pathogens, n (%) | |
| Staphylococcus aureus | 2 (3.0) |
| Haemophilus influenzae | 6 (10.0) |
| Streptococcus pneumoniae | 4 (6.0) |
| Pseudomonas aeruginosa infection, n (%) | |
| First-ever isolation | 19 (28.3) |
| Previously isolated | 48 (71.7) |
| Inhaled corticosteroids, n (%) | 55 (87) |
| Bronchodilators, n (%) | 60 (95) |
| Macrolides, n (%) | 46 (69) |
| Dyspnoea MRC, n (%) | |
| Grade 0 | 6 (9) |
| Grade 1 | 15 (22.4) |
| Grade 2 | 29 (43.3) |
| Grade 3 | 15 (22.4) |
| Grade 4 | 2 (3) |
| Dyspnoea MRC, media ± SD | 1.88 ± 0.96 |
| Cystic BQ (HRCT), n (%) | 15 (23) |
| Mean FVC post-bronchodilatation, L ± SD | 2.64 ± 0.88 |
| Mean FVC post-bronchodilatation, % predicted ± SD | 79.62 ± 20.50 |
| Mean FEV1 post-bronchodilatation, L ± SD | 1.44 ± 0.67 |
| Mean FEV1 post-bronchodilatation, % predicted ± SD | 57.49 ± 24.74 |
| FEV1/FVC, ratio ± SD | 54.59 ± 14.66 |
| Mean exacerbations in the previous year, mean ± SD | 3.40 ± 4.21 |
| 0 | 10 (14.9) |
| 1 | 10 (14.9) |
| 2 | 14 (20.9) |
| ≥3 | 33 (49) |
n = number of patients; SD: standard deviation; COPD = chronic obstructive pulmonary disease; MRC = dyspnoea according to the Medical Research Council scale; BQ = bronchiectasis; HRCT = high-resolution computed tomography; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second.
Table 2 shows the number of positive P. aeruginosa sputum cultures per patient prior to the administration of inhaled colistin. Nineteen patients (28.3%) started treatment after two sputum cultures positive for P. aeruginosa (one isolate prior to the systemic antibiotic regimen and persistence in control sputum), and in 48 patients (72%) P. aeruginosa had been isolated on three or more occasions.
Table 2.
Number of positive sputum for P. aeruginosa (separated at least 1 month) previous to the initiation of the treatment with systemic antibiotics followed by inhaled colistin.a
| Number | Frequency | Percentage |
|---|---|---|
| 2 | 19 | 28.3 |
| 3 | 13 | 19.4 |
| 4 | 13 | 19.4 |
| 5 | 8 | 12.0 |
| 6 | 2 | 3.0 |
| 7 | 1 | 1.5 |
| 8 | 1 | 1.5 |
| 9 | 2 | 3.0 |
| ≥10 | 8 | 12.0 |
a All patients had at least two positive sputum previous to the start of the treatment with inhaled colistin because of the two previous attempts at eradication with antibiotics.
The eradication of P. aeruginosa after 3 months was observed in 41 patients (61.2%), of whom 34 patients (50.7%) continued with negative cultures after 6 months, 29 patients (43.3%) after 9 months and 27 patients (40.3%) after 1 year. There were eight patients (11.9%) presenting positive cultures at month 3 of the treatment who were negative at month 6. Of these, seven patients (10.7%) remained negative after 9 and 12 months.
We did not observe significant differences in the eradication of P. aeruginosa in the short or long term depending on the number of previous positive sputum cultures (2 or ≥3) (Table 3). Differences in other variables between patients with and without eradication at 3, 6, 9 and 12 months are shown in Table 4. In the logistic regression model, the only variable associated to the eradication rate was the presence of the mucoid P. aeruginosa (p < 0.001).
Table 3.
Eradication of P. aeruginosa after 3, 6, 9 and 12 months as a function of number of previous positive sputum.
| Eradication | p Valuea | ||||
|---|---|---|---|---|---|
| Yes | No | ||||
| n | % | n | % | ||
| Eradication at month 3 | 0.785 | ||||
| 2 sputum, n = 19 | 11 | 58 | 8 | 42 | |
| ≥3 sputum, n = 48 | 30 | 62 | 18 | 38 | |
| Eradication at month 6 | 0.531 | ||||
| 2 sputum, n = 19 | 10 | 53 | 9 | 47 | |
| ≥3 sputum, n = 48 | 24 | 50 | 24 | 50 | |
| Eradication at month 9 | 0.438 | ||||
| 2 sputum, n = 19 | 9 | 47 | 10 | 53 | |
| ≥3 sputum, n = 48 | 20 | 42 | 28 | 58 | |
| Eradication at month 12 | 0.319 | ||||
| 2 sputum, n = 19 | 9 | 47 | 10 | 53 | |
| ≥3 sputum, n = 48 | 18 | 38 | 30 | 62 | |
a χ 2 test.
Table 4.
Characteristics of patients with NCFB with and without P. aeruginosa eradication in the 3, 6, 9 and 12 months follow-up.a,b
| Eradication | 3 months | 6 months | 9 months | 12 months | ||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 41) | No (n = 26) | Yes (n = 34) | No (n = 33) | Yes (n = 29) | No (n = 38) | Yes (n = 27) | No (n = 40) | |
| Age (years) | 65.9 ± 14.8 | 69.3 ± 14.0 | 64.5 ± 15.6 | 70.1 ± 13.0 | 63.7 ± 16.3 | 69.9 ± 12.6 | 64.4 ± 16.4 | 69.1 ± 13.1 |
| Sex, males, n (%) | 24 (58.5) | 16 (61.5) | 19 (55.9) | 21 (63.6) | 16 (55.2) | 24 (63.2) | 15 (55.6) | 25 (62.5) |
| Ex/active smokers, n (%) | 19 (46.3) | 11 (42.3) | 15 (44.1) | 15 (45.5) | 12 (41.4) | 18 (47.4) | 11 (40.7) | 19 (47.5) |
| First P. aeruginosa culture, n (%) | 11 (26.8) | 8 (30.7) | 10 (52.6) | 9 (47.4) | 9 (47.4) | 10 (52.6) | 9 (47.4) | 10 (52.6) |
| Concomitant azithromycin, n (%) | 27 (65.9) | 19 (73.1) | 23 (67.6) | 23 (69.7) | 18 (62.1) | 28 (73.7)c | 16 (59.3) | 30 (75) |
| Oral ciprofloxacin | 26 (63.4) | 16 (61.5) | 22 (64.7) | 20 (60.6) | 19 (65.5) | 23 (60.5) | 17 (63.0) | 25 (62.5) |
| P. aeruginosa mucoid | 18 (43.9) | 22 (84.6)d | 14 (41.2) | 26 (78.8)c | 10 (65.5) | 30 (78.9)d | 8 (70.4) | 32 (80)d |
| Mean FVC post-BD, % predicted | 80.4 ± 21.5 | 78.3 ± 19.2 | 80.2 ± 23.1 | 79.1 ± 17.9 | 80.3 ± 20.8 | 79.1 ± 20.4 | 80.1 ± 21.2 | 79.3 ± 20.2 |
| Mean FEV1 post-BD, % predicted | 58.3 ± 26.3 | 56.1 ± 22.5 | 57.5 ± 27.1 | 57.4 ± 22.7 | 57.6 ± 26.7 | 57.3 ± 23.4 | 56.7 ± 27.4 | 58.1 ± 23.1 |
NCFB: non-cystic fibrosis bronchiectasis; FVC post-BD: forced vital capacity post-bronchodilatation (% predicted); FEV1 post-BD: forced expiratory volume in 1 second post-bronchodilatation (% predicted).
a Data are presented as mean ± standard deviation (X ± SD).
b p Values: comparisons between groups were tested using the Pearson’s χ 2 (categorical variables) or Wilcoxon test (non-normally distributed continuous variables after analysing the normality with Kolmogorov–Smirnov test).
c p < 0.1.
d p < 0.001.
We observed a decrease in the number of exacerbations after administration of inhaled colistin from (mean ± SD) 3.40 ± 4.21 in the year before to 1.98 ± 3.62 the following year (p < 0.001). When the type of exacerbations (oral antibiotic cycles, emergency visits or hospital admissions) were analysed independently, a significant reduction in both hospital admissions (p < 0.001) and number of antibiotic cycles (p = 0.018) was also observed, but not of emergency visits (p = 0.526) (Table 5). Most patients (70%) had two or more exacerbations in the previous year. Of the 19 patients who had the first hospital admission in the previous year, 4 patients (21%) had at least one admission the following year.
Table 5.
Number of exacerbations before and during treatment with inhaled colistin.
| n | Mean ± SD | Difference | p Valuea | |
|---|---|---|---|---|
| Hospital admissions | <0.001 | |||
| Previous year | 75 | 1.14 ± 1.56 | 0.712 | |
| During treatment | 28 | 0.42 ± 1.33 | ||
| Emergency room visits | 0.526 | |||
| Previous year | 18 | 0.28 ± 0.89 | −0.077 | |
| During treatment | 23 | 0.35 ± 1.36 | ||
| Cycles of antibiotics | 0.018 | |||
| Previous year | 128 | 1.94 ± 2.80 | 0.758 | |
| During treatment | 78 | 1.18 ± 1.73 | ||
| Total exacerbations | <0.001 | |||
| Previous year | 214 | 3.40 ± 4.21 | 1.381 | |
| During treatment | 129 | 1.98 ± 3.62 |
n = number of patients; SD = standard deviation.
a Test of Wilcoxon.
Mild adverse effects (cough and/or wheezing) were reported by five (7.5%) patients during the first month of treatment but did not result in discontinuation of therapy.
Discussion
This study shows that P. aeruginosa may be eradicated in a high percentage of NCFB patients with a treatment based on systemic antibiotics followed by inhaled colistin even when previously they had presented several positive cultures. In addition, our study suggests that this therapy may be effective in reducing exacerbations. In our study, we observed eradication rates of P. aeruginosa in NCFB patients of 61.2, 50.7, 43.3 and 40.3% after 3, 6, 9 and 12 months of treatment, respectively.
There are only three published studies that analyse the use of inhaled colistin for P. aeruginosa eradication in NCFB patients. Two studies including 18 and 19 patients found that eradication success was lower than ours (21.4% and 16%, respectively) after an average 41 and 21.2 months of treatment.21,22 The possible reasons for these differences with our study are that in these cases the patients had not received systemic antibiotics previous to inhaled colistin, a different colistin regimen and type of nebulizer, or no treatment with macrolides. In our study, 69% of the patients maintained a simultaneous treatment with macrolides.
A prospective study with 25 patients receiving inhaled colistin plus systemic antibiotics for 3 months showed an eradication rate of 43% at 14.3 months, very similar to ours at 12 months.15 Although the value of systemic antibiotics in P. aeruginosa eradication therapy in NCFB patients is still under investigation, the results of White et al. and our study coincide in presenting higher eradication rates than studies that use inhaled antibiotic only. The use of oral/IV antibiotics may increase the chances of eradication because inhaled antibiotics may not reach areas of the lung due to obstruction of the airways by mucus.
The largest study to date with inhaled colistin included 144 patients (73 treated with colistin, 71 with placebo) and was carried out over 6 months with a dose of 1 MIU every 12 hours.12 Although a significant decrease in the load of P. aeruginosa was reported, the efficacy of eradication was not specified.
Two studies using only inhaled tobramycin have shown eradication rates of 35% and 22.2% at 6 and 12 weeks, respectively.13,20 The only randomized, placebo-controlled study using IV ceftazidime plus tobramycin during 14 days followed by inhaled tobramycin for 3 months reported an eradication rate of 54.5% after 15 months, in line with our results.23 The success of the eradication therapy in this last case can be due to having started after a first isolate of P. aeruginosa from sputum in contrast with typically after the second or more isolates. However, in our study, we did not obtain significant differences in the eradication rate as a function of the number of previous positive sputum with P. aeruginosa, suggesting that eradication should be attempted both when P. aeruginosa isolation is recent as if there is long-term chronic infection.
Currently the optimal treatment regimen and duration has not been determined. The studies of White and Orriols reached eradication rates similar to ours after 3 months but in the first case the treatment also included oral ciprofloxacin and in the second the treatment was started after first isolation and did not include patients with mucoid P. aeruginosa. 15,23 Compared with non-mucoid P. aeruginosa, the mucoid morphotype is associated with a lower percentage of success in eradication treatment.28,29 In our study, up to 72% of the patients had more than three P. aeruginosa-positive sputum isolations and in 59.7% of these the morphotypes were mucoid. Our results differ from some authors that suggest that the timing of the administration of antimicrobial therapy may be critical, as there may be a window of opportunity after which antibiotic eradication treatment is no longer successful. In our study, 10% of the patients reached eradication after the third month. Our current practice is to maintain inhaled antibiotic for 1 year after the last positive isolation.
Sodium colistimethate is usually used at a dose of 2 million IU every 12 hours dissolved in 4 ml of the most isotonic solution possible, but with the use of the I-neb nebulizer (Philips Respironics®) the dose can be reduced to 1 million IU every 12 hours. The medication is released only during patient inhalation and not continuously as in all other nebulizers.
Comparison of exacerbations between pre- and post-treatment with inhaled colistin found a significant reduction in the number of exacerbations during the year with treatment. We observed significantly reduced hospital admissions and antibiotic cycles in the patients treated, but not of visits to the emergency room (ER). This reflects the fact that exacerbations that require a visit to the ER are usually severe and result in hospital admission. There are a few small studies that have demonstrated a significant reduction in exacerbations in line with our study.11,14,22 Besides, in one of the most important clinical trials of aerosolized antibiotics for patients with NCFB, a clear trend of increase of time to first exacerbation was demonstrated when patients with good adherence to treatment were selected.12 Our results are consistent with a recent study that showed that an important risk factor predicting benefit from inhaled antibiotics is a history of frequent pulmonary exacerbations.30 In our case, 70% of the patients had >2 exacerbations in the previous year, while 40% of the patients in the clinical trial had no exacerbations in the previous year. A strength of our study is that the number of exacerbations is quite high in a large number of patients; in contrast, several recent trials have failed due to a low exacerbation rate in the patients.
The main limitations of our study were that it was performed in a single centre with no placebo-controlled group. However, our methods and management by a single researcher assured low variability in data acquisition and homogeneous management of patients and application of clinical protocols before and after the intervention. Other possible limitation is that, although we verified the collection of the drug at the Pharmacy and also evaluated self-reported adherence in a structured interview in each visit, we did not perform an analysis of the stored adherence data in the I-neb nebulizer system. However, all patients attended all follow-up visits. Finally, another possible limitation of the study was that the patient selection was not homogenous with regard to timing of P. aeruginosa infection. However, this fact has allowed us to observe that eradication is possible even in patients with several previous isolations.
We conclude that a high rate of long-term eradication of P. aeruginosa in NCFB patients can be achieved with administration of systemic antibiotics followed by inhaled colistin, and we recommend this approach based on our experience with patients in real clinical conditions. Pseudomonas eradication should be attempted even when Pseudomonas is present in several samples of sputum. In line with previous studies of different aerosolized antibiotics for NCFB,11,12,14,22 our study demonstrated a notable clinical impact of inhaled colistin, with a significant reduction of exacerbations and an ensuing significant decrease in hospital admissions.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Marina Blanco-Aparicio  https://orcid.org/0000-0002-5012-1746
https://orcid.org/0000-0002-5012-1746
References
- 1. Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest 2012; 142: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joish VN, Spilsbury-Cantalupo M, Operschall E, et al. Economic burden of non-cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Appl Health Econ Health Policy 2013; 11: 299–304. [DOI] [PubMed] [Google Scholar]
- 3. Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013; 187: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King PT, Holdsworth SR, Freezer NJ, et al. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006; 100: 2183–2189. [DOI] [PubMed] [Google Scholar]
- 5. Ho PL, Chan KN, Ip MS, et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest 1998; 114: 1594–1598. [DOI] [PubMed] [Google Scholar]
- 6. Canton R, Cobos N, de Gracia J, et al. Antimicrobial therapy for pulmonary pathogenic colonisation and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect 2005; 11: 690–703. [DOI] [PubMed] [Google Scholar]
- 7. Martinez-Garcia MA, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 8. Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 9. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orriols R, Roig J, Ferrer J, et al. Inhaled antibiotic therapy in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection by Pseudomonas aeruginosa . Respir Med 1999; 93: 476–480. [DOI] [PubMed] [Google Scholar]
- 11. Drobnic ME, Sune P, Montoro JB, et al. Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa . Ann Pharmacother 2005; 39: 39–44. [DOI] [PubMed] [Google Scholar]
- 12. Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014; 189: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barker AF, Couch L, Fiel SB, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med 2000; 162: 481–485. [DOI] [PubMed] [Google Scholar]
- 14. Murray MP, Govan JR, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011; 183: 491–499. [DOI] [PubMed] [Google Scholar]
- 15. White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 356–360. [DOI] [PubMed] [Google Scholar]
- 16. Littlewood JM, Miller MG, Ghoneim AT, et al. Nebulised colomycin for early pseudomonas colonisation in cystic fibrosis. Lancet 1985; 1: 865. [DOI] [PubMed] [Google Scholar]
- 17. Taccetti G, Bianchini E, Cariani L, et al. Early antibiotic treatment for Pseudomonas aeruginosa eradication in patients with cystic fibrosis: a randomised multicentre study comparing two different protocols. Thorax 2012; 67: 853–859. [DOI] [PubMed] [Google Scholar]
- 18. Ratjen F, Munck A, Kho P, et al. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 2010; 65: 286–291. [DOI] [PubMed] [Google Scholar]
- 19. Proesmans M, Vermeulen F, Boulanger L, et al. Comparison of two treatment regimens for eradication of Pseudomonas aeruginosa infection in children with cystic fibrosis. J Cyst Fibros 2013; 12: 29–34. [DOI] [PubMed] [Google Scholar]
- 20. Scheinberg P, Shore E. A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis. Chest 2005; 127: 1420–1426. [DOI] [PubMed] [Google Scholar]
- 21. Steinfort DP, Steinfort C. Effect of long-term nebulized colistin on lung function and quality of life in patients with chronic bronchial sepsis. Intern Med J 2007; 37: 495–498. [DOI] [PubMed] [Google Scholar]
- 22. Dhar R, Anwar GA, Bourke SC, et al. Efficacy of nebulised colomycin in patients with non-cystic fibrosis bronchiectasis colonised with Pseudomonas aeruginosa . Thorax 2010; 65: 553. [DOI] [PubMed] [Google Scholar]
- 23. Orriols R, Hernando R, Ferrer A, et al. Eradication therapy against Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respiration 2015; 90: 299–305. [DOI] [PubMed] [Google Scholar]
- 24. Vendrell M, de Gracia J, Olveira C, et al. Diagnóstico y tratamiento de las bronquiectasias. Normativa SEPAR. Arch Bronconeumol 2008; 44: 629–640. [DOI] [PubMed] [Google Scholar]
- 25. Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65(Suppl 1): i1–58. [DOI] [PubMed] [Google Scholar]
- 26. Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 2017; 4: CD004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchís Aldas J, Casan Ciará P, Castillo Gómez J, et al. Normativa para la práctica de la espirometría forzada. Arch Bronconeumol 1989; 25: 132–142. [Google Scholar]
- 28. Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol 1997; 23: 330–335. [DOI] [PubMed] [Google Scholar]
- 29. Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003; 167: 841–849. [DOI] [PubMed] [Google Scholar]
- 30. Nadig TR, Flume PA. Aerosolized antibiotics for patients with bronchiectasis. Am J Respir Crit Care Med 2016; 193: 808–810. [DOI] [PubMed] [Google Scholar]