Abstract
Glioblastoma multiforme represents one of the deadliest brain tumor types, manifested by a high rate of recurrence and poor prognosis. The presence of glioma stem cells (GSCs) can repopulate the tumor posttreatment and resist therapeutics. A better understanding of GSC biology is essential for developing more effective interventions. We established a CD133 promoter-driven dual reporter, expressing green fluorescent protein (GFP) and firefly luciferase (CD133-LG), capable for in vitro and in vivo imaging of CD133+ GSCs. We first demonstrated the reporter enabled in vitro analyses of GSCs. DBTRG-05MG (Denver Brain Tumor Research Group 05) carrying CD133-LG (DBTRG-05MG-CD133-LG) system reported increased GFP/luciferase activities in neurospheres. Additionally, we identified and isolated CD133+/GFP+ cells with increased tumorigenic properties, stemness markers, Notch1, β-catenin, and Bruton’s tyrosine kinase (Btk). Furthermore, prolonged temozolomide (TMZ) treatment enriched GSCs (reflected by increased percentage of CD133+ cells). Subsequently, Btk inhibitor, ibrutinib, suppressed GSC generation and stemness markers. Finally, we demonstrated real-time evaluation of anti-GSC function of ibrutinib in vivo with TMZ-enriched GSCs. Tumorigenesis was noninvasively monitored by bioluminescence imaging and mice that received ibrutinib showed a significantly lower tumor burden, indicating ibrutinib as a potential GSC inhibitor. In conclusion, we established a dual optical imaging system which enables the identification of CD133+ GSCs and screening for anti-GSC drugs.
Keywords: glioma stem cells (GSCs), CD133, dual optical imaging system, temozolomide resistance, Btk inhibitor
Introduction
Glioma represents the most common type of brain cancer and glioblastoma multiforme (GBM) as the most prevalent and malignant subtypes of glioma.1,2 Even with aggressive interventions such as surgery, radiation, chemotherapy, and targeted therapeutics, the median survival for patients with GBM is estimated to be 12 to 15 months.3,4 Accumulating evidence has indicated that the presence of glioma stem cells (GSCs) represents as one of the key attributes to the malignant phenotypes of GBM. Glioma stem cells are reported to be responsible for repopulating GBM postaggressive treatments. For instance, GSCs express high level of stem cell markers with the properties of enhanced self-renewal ability and increased expression of multiple-drug resistant genes, enabling GSCs to repopulate and resist treatments.5,6,7 As how GSCs arise remains unclear; however, experimental evidence has suggested that chemotherapeutic agents such as temozolomide (TMZ) may actually select for and enrich CD133 + GSCs.8,9 These findings suggest the current treatment regimens may provide short-term benefits but increase the risk of disease recurrence via the generation of GSCs. Based on these premises, it is essential to gain more understanding into the molecular mechanism(s) responsible for the emergence of GSCs. So that it may accelerate the development of improved therapeutics and patient survival. However, the identification and isolation of GSCs still remain a challenging tasks both experimentally and clinically. More importantly, a real-time tracking system of the generation and development of GSCs is lacking. Therefore, it is our aim to develop a cellular imaging system that can be used to identify and isolate GSCs.
CD133 (also known as prominin 1), a glycoprotein, which has been well-recognized as a general stem cell marker and GSCs. A wealth of literature supports CD133 as a marker for GSCs as well as a marker for clinical samples with enhanced metastatic ability and drug resistance.10,11 Studies showed that CD133+ GSCs are more resistant to multiple chemotherapeutic agents12 and express higher levels of breast cancer resistance protein (drug transporter), methylguanine methyltransferase (DNA repair), and genes that inhibit apoptosis such as FLIP, Bcl-2, and Bcl-x.13 An increased expression of CD133 in GSCs was associated with a significantly increased resistance against cisplatin, via the decreased expression of miR-29a (a tumor suppressor), suggesting the increased CD133 expression could also promote drug resistance via noncoding RNA route.14 In our previous report, we showed that CD133+ GBM cells were associated with an increased level of Bruton’s tyrosine kinase (Btk) and increased the malignant properties of GBM cells such as increased resistance against chemotherapeutic agent, TMZ, and increased the generation of GSCs.15 Based on these findings, increased CD133 expression and Btk in GBM cells may serve as a valuable biomarker when screening for anti-GSC agents.
Here, we constructed a dual reporter system, GFP (fluorescence) and firefly luciferase (bioluminescence), driven by CD133 promoter (termed CD133-LG) making it selective for GSCs. We demonstrated that this dual imaging system could be used to identify and isolate CD133+ GSCs by fluorescence-activated cell sorting (FACS). In vitro assays showed GBM cell line, DBTRG-05MG (Denver Brain Tumor Research Group 05) cells expressing CD133-LG, robustly reported GFP/luciferase activities in conditions which induces CD133 expression such as neurospheres generated under serum-deprived conditions and prolonged TMZ exposure. Finally, we demonstrated its application for screening anti-GSC agent, ibrutinib, using mouse xenograft model via noninvasive bioluminescence imaging technique.
Materials and Methods
Construction of CD133 Promoter-Driven LG Reporter
The lentiviral plasmid containing dual reporter, firefly luciferase and enhanced green fluorescence protein (LG), pFULG, was a kind gift from Dr S. S. Gambhir (Molecular Imaging Program at Stanford [MIPS]), Stanford, California.16 The CD133 P5 (nucleotide position: −98/+10, 122 base pair) promoter region sequence was also a generous gift from Dr S. Tanaka.17 In brief, CD133 P5 promoter was designed to contain PacI and Xbal restriction sites first; the primer sequences used to create restriction sites were PacI 5′-TTAATTAACAGTGTCTCCCCAGA-3′ and Xbal 5′-TCTAGAAGCAACTTCTACCAGC-3′; PacI-CD133 P5-Xbal was then subcloned into pGEM-T Easy vector (Promega, Madison, WI) as the pGEMT-CD133 p5 plasmid. Subsequently, CD133 P5 was subcloned into pFULG vector using PacI and Xbal restriction enzymes. The final reconstructed vector with CD133 P5 promoter and dual reporter, LG, was named as pCD133-LG.
Cell Culture and Infection
Human malignant glioma cell line DBTRG-05MG (ATCC CRL-2020, Manassas, VA) was obtained from American Type Culture Collection (ATCC). Characteristically, DBTRG-05MG cell line was established from the tumor tissue of patient with GBM who had been treated with local brain irradiation and multidrug chemotherapy. Cells were maintained in RPMI (Roswell Park Memorial Institute) 1640 + 2 mmol/L glutamine + 1% HT + 1 mmol/L sodium pyruvate + 10% fetal bovine serum as recommended by the ATCC. The DBTRG-05MG cells were infected with viral particles containing CD133-LG vector, according to the protocol described previously.16 The resultant cells were termed DBTRG-05MG-CD133-LG cells. Ibrutinib (Ib, PCI-32765) and TMZ (SelleckChem, Taiwan) were dissolved in dimethyl sulfoxide for in vitro assays. The DBTRG-05MG-CD133-LG cells were treated with 8 µmol/L of Ib for 48 hours and harvested for further analyses. As for the experiment of prolonged TMZ treatment, DBTRG-05MG-CD133-LG cells were cultured in RPMI medium containing (50 µmol/L TMZ) and survived cells were harvested, passaged, and continued culture in RPMI medium containing (50 µmol/L TMZ) for 4 weeks (This TMZ concentration was selected based on a previous study which showed that this TMZ concentration [clinically relevant dose] increased tumorigenicity of GBM cells in vitro).9 After 4 weeks, these cells were tested their sensitivity against TMZ compared with their parental counterparts.
Fluorescence-Activated Cell Sorting Analysis and Neurosphere Generation
Fluorescence-activated cell sorting was performed to identify and isolate CD133+/GFP+ cell population in DBTRG-05MG-CD133-LG cells. CD133/1 (AC133) antibodies conjugated to APC (Miltenyi Biotec, Auburn, California) was used to identify CD133+ cells. Cells were isolated using magnetic microbeads (Coulter-Immunotech Co, Brea, California), according to our previously established protocols and conditions.15 Approximately >95% of isolated DBTRG-05MG-CD133-LG cells were found viable and cultured for further analyses. The DBTRG-05MG-CD133-LG neurospheres were generated under serum-deprived conditions, according to the established protocol with slight modifications.18 The neurosphere culture medium is RPMI containing 20 ng/mL of epidermal growth factor, basic fibroblast growth factor (both from R&D Systems Minneapolis, MN), leukemia inhibitory factor (Chemicon, Merck KGaA, Darmstadt, Germany), and B27 (1:50; Life Technologies, Carlsbad, California) as a stem cell-permissive medium.19 Sorted parental and/or TMZ-treated DBTRG-05MG-CD133-LG cells (105 cells) were seeded in Corning ultra-low attachment plates with serum-free stem cell medium (Nutristem, Biological Industries, Israel) at 37°C in a 5% CO2 incubator for at least 3 to 7 days. Neurospheres (>50 µm in diameter) were observed under a microscope and harvested for further experiments.
Cell Viability Test
The DBTRG-05MG-CD133 cells were seeded in RPMI culture medium in 96-well plates (5000 cells/well). Cells were subsequently treated with different concentrations of TMZ. After 48 hours, cells were washed in phosphate buffered saline (PBS) 3 times and fixed with 10% trichloroacetic acid, washed with ddH2O, followed by an incubation in 0.4% Sulforhodamine B (SRB) (w/v) in 1% acetic acid at room temperature. The unbound SRB dye was washed off using 1% acetic acid, air-dried, and 10 mmol/L trizma base was added. Finally, the absorbance was measured using a microplate reader at a wavelength of 570 nm.
Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Western Blotting
Total protein lysates of DBTRG-05MG-CD133-LG cells and/or their corresponding neurospheres were obtained using a protein extraction kit (Panomics, Fremont, California). Protein samples (20 μg/lane) were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane using BioRad Protean system (BioRad, Taiwan). Blots were then first rinsed with PBS and blocked with 5% skim milk in PBST (PBS plus Tween) and incubated with primary antibodies in cold overnight. All primary antibodies were purchased from Cell Signaling Technology unless otherwise specified: Bruton’s tyrosine kinase (1:1000), β-catenin (1:800), glial fibrillary acidic protein (GFAP; 1:1000), nestin (1:1000), Notch1 (1:600), and β-actin (1:10 000). Peroxidase-conjugated secondary antibodies were subsequently added, and signals were developed using ECL detection kit ECL Substrate Kit (High Sensitivity) (ab133406, abcam, Japan). The immunoblots were imaged and analyzed using UVP BioDoc-It system (Upland, California).
Mouse Xenograft GSC Model and Noninvasive Bioluminescence Imaging
The animal experiments were approved by the animal experiment committee and issued affidavit of approval of animal use protocol Taipei Medical University (Approval # LAC-2018-0223). The GSC xenograft models were established in this study. The DBTRG-05MG-CD133-LG neurospheres (50 000 cells) were orthotopically injected into nonobese diabetic/severe combined immunodeficiency mice (6-8 weeks old) following a previously established protocol.20 Originally, 5 mice in each group were injected with DBTRG-05MG-CD133-LG cells. One week postinjection, bioluminescence was used to determine the success and approximately equal starting bioluminescence intensity in each group. Three mice with approximately equal starting bioluminescence were selected in each group for experiments. To minimize the usage of laboratory animals, we abided the principles of the 3Rs (Replacement, Reduction, and Refinement) of animal welfare, required by our institution. The tumor inhibitory effect of Ib (intraperitoneal injection, 6 mg/kg, 5 times per week) and tumorigenesis over time was monitored using bioluminescence imaging technology (IVIS 200 system, Caliper, Taiwan) on a weekly basis. The tumor burden was quantified using Living Imaging software (Caliper, Taiwan). Ibrutinib was given intraperitoneally (6 mg/kg, 5 times/week). Mice were humanely killed after experiments, and tumor samples were harvested for further analyses.
Results
Establishment of CD133 Promoter-Driven Optical Imaging System
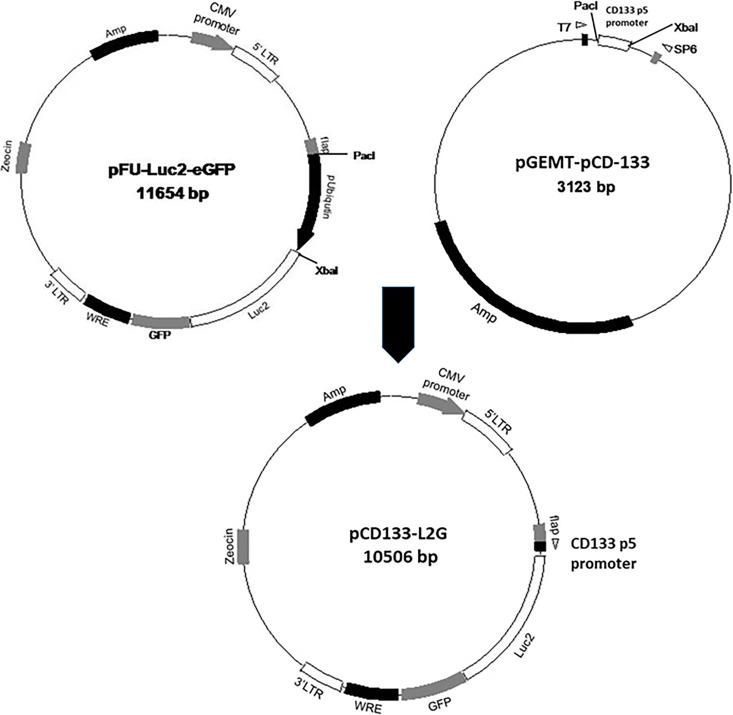
CD133-positive and/or high expressing glioma cells have been generally recognized of having stem-like properties such as enhanced self-renewal ability, increased resistance against therapeutics, and propensity for metastasis.21,22 Based on this premise, it was our intention to create an optical imaging platform for real-time in vivo tracking of GSCs. First, functional CD133 promoter region (CD133 P5)17 was subcloned into pGEMT vector through PacI and Xbal sites (right vector, Figure 1). CD133 P5 promoter region was subsequently inserted into pFULG vector (left vector, Figure 1) also by PacI and Xbal sites, yielding the final CD133-LG vector (the bottom vector, Figure 1).
Figure 1.
General scheme for generating CD133 promoter-driven dual optical imaging reporter. First, functional CD133 P5 promoter sequence was subcloned into pGEMT plasmid, resulting pGMET-CD133 P5 plasmid. The CD133 p5 promoter sequence from pGEMT was then subcloned into the pFULG plasmid which contains LG fusion gene, producing the final CD133-LG plasmid.
Generation and Characterization of CD133-LG Expressing GBM and GSC Cells
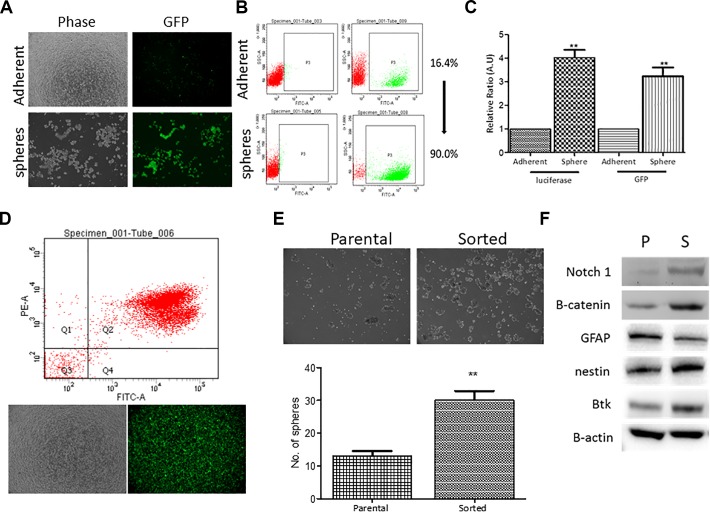
Next, we generated stable CD133-LG expressing human GBM cell line, DBTRG-CD133-LG by lentiviral infection. First, we analyzed DBTRG-05MG-CD133-LG cells microscopically. In the parental (adherent) cells, we observed some GFP-positive cells, suggesting CD133+ cell subpopulation (adherent, Figure 2A). We also cultured DBTRG-05MG-CD133-LG cells under serum-deprived conditions to generate neurospheres. The DBTRG-05MG-CD133-LG spheres were almost all GFP-positive (sphere, Figure 2A). Using flow cytometric analysis, we found that a significantly higher percentage of GFP+ cell population in the sphere group (approximately 90.0%) comparing to their parental counterparts (approximately 16.4%, Figure 2B). In addition, DBTRG-05MG-CD133-LG spheres showed a significantly higher luciferase activity and enhanced GFP intensity as compared to their adherent counterparts (Figure 2). Subsequently, we intended to demonstrate the application for cell sorting where DBTRG-05MG-CD133-LG cells were sorted using both CD133 and GFP in order to obtain a more homogenous cell population. After FACS, the resultant cells were almost all GFP positive under fluorescent microscopic examination (Figure 2D). The sorted cells were compared with the parental counterparts for their sphere-forming ability. The sorted DBTRG-05MG-CD133-LG cells exhibited an enhanced sphere-forming ability (Figure 2E). In support, the sorted cells were found to express increased level of stemness markers, Notch1, β-catenin, and nestin, as well as oncogenic marker Btk, and decreased level of GFAP (Figure 2F).
Figure 2.
Characterization of CD133-LG expressing DBTRG-05MG cells. A, Lentiviral-infected DBTRG cells. Immunofluorescent microscopic images showed that increased GFP signal in the neurospheres generated from DBTRG-05MG-CD133-LG as compared to their adherent parental counterparts. B, Flow cytometric analysis. Neurospheres generated from DBTRG-05-CD133-LG cells were approximately 90% positive CD133 and GFP while only 16.4% in the adherent counterparts. C, Dual reporter assay. As indicated, neurospheres showed significantly increased level of luciferase activity and GFP fluorescence than their adherent counterparts. D, Application for fluorescence-activated cell sorting (FACS). DBTRG-05MG-CD133-LG cells were subjected to FACS. The sorted cells were both CD133 and GFP positive (estimated 95.4%). E, Comparative neurosphere-forming ability assay. Sorted DBTRG-05MG-CD133-LG cells showed a significantly higher ability to generate neurospheres as compared to their parental counterparts. F, Comparative Western blot analysis of parental and sorted DBTRG-05MG-CD133-LG cells. Stemness markers, notch1, β-catenin, nestin, and Bruton’s tyrosine kinase (Btk) were markedly increased while differentiation marker glial fibrillary acidic protein (GFAP) was decreased in the sorted cells when compared with their parental counterparts. **P ≤ .01.
Temozolomide Treatment Enriched CD133+ GBM Cells
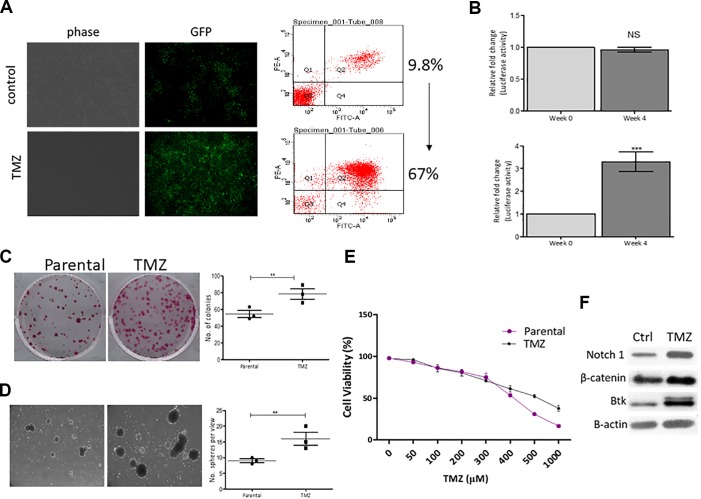
A recent study reported that the clinical dosing of TMZ actually promoted tumorigenic properties of GBM in vitro, suggesting TMZ treatment may lead to the selection of TMZ-resistant GBM cells.9 We intended to take this study further by determining whether prolonged treatment of TMZ led to the enrichment of CD133+ glioma stem-like cells using our reporter system. We exposed DBTRG-05MG-CD133-LG cells (not sorted) under a prolonged exposure of TMZ (50 µmol/L for 4 weeks) and compared these cells with nontreated counterparts using both fluorescent microscopy and flow cytometry. We observed approximately 9.8% of cells showing GFP signal in the control cells while 67% in the TMZ-treated cells (Figure 3A). In accordance, the relative luciferase activity was found to be increased in the TMZ-treated group after 4-week exposure (Figure 3B). In terms of tumorigenic properties, we observed the TMZ-treated group exhibited a significantly enhanced colony-forming ability (Figure 3C) and neurosphere-forming ability (Figure 3D) when compared with the control counterparts. More importantly, TMZ-treated cells showed an increased resistance against TMZ as compared to their parental counterparts (Figure 3E). The comparative Western blots of parental and TMZ-treated DBTRG-05MG-CD133-LG cells showed that TMZ-treated cells contained a prominently higher level of stemness markers including notch1, β-catenin, and Btk (Figure 3F).
Figure 3.
Temozolomide (TMZ) treatment enriches CD133+ glioblastoma multiforme (GBM) cell population with glioma stem cell (GSC) properties. A, Representative fluorescence micrographs (left panels) depict that DBTRG-05MG-CD133-LG post 4-week exposure of TMZ (50 µmol/L) contained an increased CD133+ cell population. Representative flow cytometric analysis shows that TMZ exposure led to a substantial increase in green fluorescent protein (GFP)/CD133+ DBTRG-05MG-CD133-LG cells, from approximately 9.0% to 67%. B, Luciferase assay showed that after 4-week low-dose TMZ exposure, the luciferase activity driven by CD133 promoter in the DBTRG-05MG-CD133-LG was significantly increased as compared to the parental cells. ***P < .001. Comparative colony (C) and neurosphere (D) forming assays. E, Cell viability assay shows that TMZ-treated DBTRG-05MG-CD133-LG became more TMZ resistant (half maximal inhibitory concentration (IC50) value increased from approximately 400-500 µmol/L). E, Western blots depicts the prominently increased expression of stemness markers notch1, β-catenin, and Bruton’s tyrosine kinase (Btk) in the TMZ-treated cells.
Ibrutinib Treatment Suppressed GBM Tumorigenesis and GSC Formation
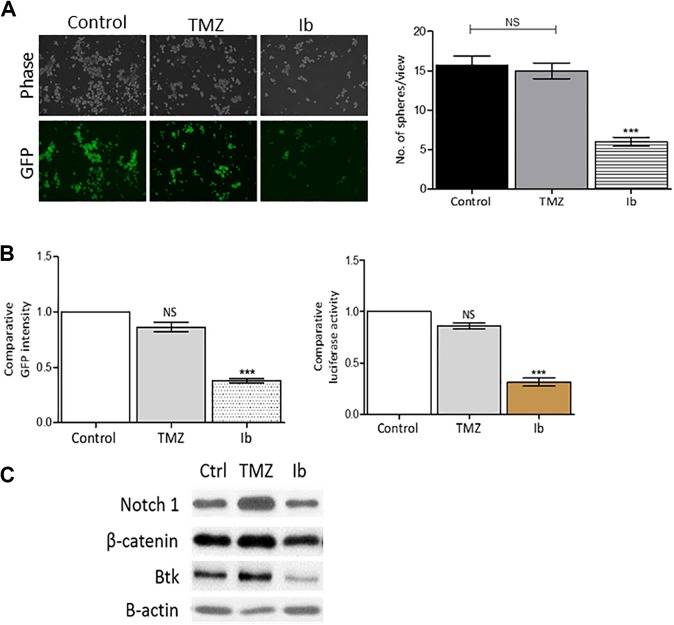
Our previous study and others demonstrated the antineoplastic effect of Ib on GBM cells in vitro and in vivo.23,24 Here, we intended to demonstrate the anti-GSC application using our CD133-LG system. First, Ib treatment significantly suppressed the sphere-forming ability in the DBTRG-05MG-CD133-LG cells as compared to TMZ (Figure 4A). Both GFP fluorescence (left panel, Figure 4B) and luciferase activity (right panel, Figure 4B) were analyzed. Ibrutinib treatment significantly reduced both GFP fluorescence and luciferase activity in DBTRG-05MG-CD133-LG cells, while no significant reduction in both reporter activities in TMZ-treated DBTRG-05MG-CD133-LG cells. In support, Western blots of the DBTRG-05MG-CD133-LG cells showed that a significantly decreased expression of Notch1, β-catenin, and Btk after Ib treatment (5 µmol/L, 48 hours) but no significant decrease in their expression when treated with TMZ (500 µmol/L, 48 hours) as depicted in Figure 4C.
Figure 4.
In vitro anti-glioma stem cell (GSC) drug screening application. A, Representative micrographs of neurosphere-forming assay. Ib treatment significantly reduced the number of GFP+ neurospheres generated from DBTRG-05MG-CD133-LG cells as compared to control and temozolomide (TMZ) groups. B, Reporter assays. Left panel, comparative GFP+ intensity readouts among control, Ib-, and TMZ-treated DBTRG-05MG-CD133-LG neurospheres. ***P < .001. Right panel, comparative luciferase activity readouts among control, Ib-, and TMZ-treated neurospheres. ***P < .001. C, Western blots showing Ib treatment prominently reduced the stemness markers notch1, β-catenin, and Bruton’s tyrosine kinase (Btk). Ib indicates ibrutinib; GFP, green fluorescent protein; NS, no significance.
Noninvasive Optical Imaging of Ib-Mediated Suppression of GSCs
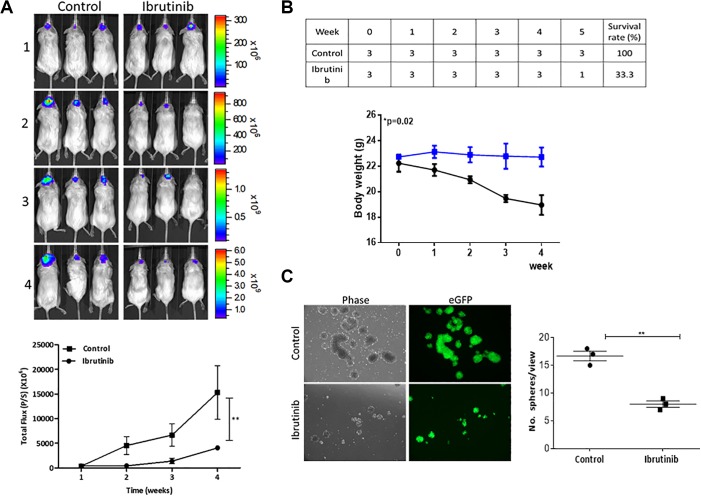
Finally, we tested our CD133-LG system using xenograft mouse model by intracranial injecting DBTRG-05MG-CD133-LG neurospheres, CD133 promoter-driven LG dual reporter showed its effectiveness in vivo. Mice treated with Ib apparently showed a significantly lower bioluminescent signal as compared to their control counterparts (Figure 5A). In addition, the bioluminescence could be semiquantitatively measured to reflect the tumor growth over time as demonstrated in the photon flux versus time curve (Figure 5B). More importantly, tumor samples harvested showed that Ib-treated group exhibited a significantly reduced ability to generate CD133+ neurospheres when cultured under serum-deprived conditions when compared to the samples from the control group (Figure 5C).
Figure 5.
Preclinical evaluation of anti-glioma stem cell (GSC) function of ibrutinib using noninvasive bioluminescence imaging. A, Representative bioluminescence images of mice bearing DBTRG-05MG-CD133-LG neurospheres with and without the treatment of ibrutinib (over the course of 5-week experiment [Ib; intraperitoneal, IP, injection; 6 mg/kg; 5 times/week]) over time. N = 3 per group. Lower panel, Average body weight over time curve shows the treatment of Ib did not significantly affect the body weight of the animals. Lower panel represents the semiquantitative analysis of tumor burden over time. Y-axis, total photo flux; X-axis, time (treatment time). B, Survival rate and body weight monitoring over time. C, Comparative neurosphere-forming ability assay. Tumor samples were harvested from both control and Ib-treated mice. Ib-treated cells formed a significantly lower number of neurospheres, corresponding with a lower percentage of GFP+ cell population. *P ≤ .05; **P ≤ .01. Ib indicates ibrutinib; GFP, green fluorescent protein.
Discussion
Glioblastoma multiforme is one of the most malignant cancer types and despite advancement in therapeutics development, GBM remains a challenging tasks in the clinics. One of the major factors for GBM’s resilience against treatments is the existence and/or generation of GSCs. A wealth of studies have demonstrated that GSCs are a subpopulation of glioma cells with the enhanced abilities to resist therapeutics, increased epithelial-mesenchymal transition/metastatic potential, and enhanced self-repopulation. All of these properties contribute toward the poor prognosis of patients with GBM. However, the identification and isolation of GSCs remain challenging.
In this study, we utilized CD133 as one of the most accepted markers for GSCs.10,25 Here, we constructed a platform where CD133 promoter drives the expression of dual optical reporters, GFP and firefly luciferase (termed CD133-LG). The DBTRG-05MG-LG neurospheres generated under serum-deprived culture conditions (surrogate of GSCs) showed a significantly increased GFP and luciferase activities. In agreement, an increased CD133 expression in GBM cells is associated with increased proliferation25 and resistance against chemotherapeutic agents.12 We also demonstrated that our system could be used to identify and isolate CD133+ GBM cells using flow cytometry; the isolated CD133+ GBM cells showed an increased GSC properties such as increased ability to generate neurospheres and resist TMZ treatment. In addition, our dual reporter is a lentiviral plasmid thus it has an advantage to better infect nondividing cells such as stem cells as opposed to other viral vectors which primarily target dividing cells.
Currently, there are different theories as how GSCs arise. For instance, evidence suggests that angiocrine factor such as nitric oxide secreted from the glioma microenvironment promotes the conversion of differentiated glioma cells to GSCs via activating stemness pathway such as Oct4.26 In addition, it has been shown that CD133 expression is associated with the mesenchymal and neural subtypes of GBM cells.27 In agreement, we showed that FASC-sorted DBTRG-05MG-CD133-LG cells express a significantly higher level of stemness marker notch1, β-catenin, and nestin. Another theory entails that GSCs are generated and/or enriched by therapeutic regimens such as chemotherapies. For instance, it has been shown that clinical dosage of TMZ promotes the proliferation of GBM cells in vitro.9 The results from this study expanded this notion further and showed that prolonged relatively low dose of TMZ led to the enrichment of CD133+ GBM cells and promoted the generation of GSCs; this phenomenon was captured and reported by the increased GFP/luciferase activities and the ability to form neurospheres by our DBTRG-05MG-CD133-LG system. Further supports came from the observations where these TMZ-trained DBTRG-05MG-CD133-LG cells became more resistant against TMZ and expressed an increased expression of stemness markers such as notch1, β-catenin, and Btk (a previous GBM stemness marker identified by our group).15 Together these findings provide supports toward the notion that conventional chemotherapeutic agents such as TMZ may provide short-term benefits but increase the potential for the enrichment/generation of TMZ-resistant GSCs. Interestingly, CD133− GBM cells have also been shown to possess GSC abilities. A previous study indicated that clinical samples of CD133− GBM cells were characterized by a lower proliferation index, whereas GFAP staining was similar as compared to their CD133+ counterparts.19 The same study revealed 117 genes were differentially expressed by CD133+ and CD133− subtypes and that CD133+ cancer stem cell maintain only a subset of primary GBM cells. The remainder population is generated from previously unknown CD133− GBM cells also with stem cell-like properties but distinct gene signatures and growth characteristics in vitro. Our CD133-LG system may be used in the future for distinguishing and analyzing the differential characteristics between CD133+ and CD133− GSCs. For instance, one could compare the self-renewal ability between these 2 subpopulations of GSCs (by limiting dilution assay). These findings may shed important lights into the biogenesis of GSCs and potential targets for GSC inhibitor development.
To this end, our previous report provided preclinical evidence for the usage of Ib, a Btk inhibitor for the suppression of GSCs. In this study, we showed that using DBTRG-05MG-CD133-LG cells as a GSC model, Ib treatment significantly reduced the generation of TMZ-resistant CD133+ cell population and suppressed tumorigenic properties. These observations further supported our dual imaging reporter system in the application for anti-GSC drug screening. Recently, Btk-mediated signaling has gained attention in GBM tumorigenesis where an elevated expression and activity of this signaling cascade is identified in GBM cells.28,29 Interestingly, a new form of Btk (p65Btk) has been identified, and p65Btk is restricted to gemistocytic cells and associated with a poor prognosis. Thus, Btk inhibitors may be of potential for combating the generation of GSCs, and our CD133-LG system may be utilized for the rapid screening of anti-GSC drugs. It is noteworthy that since CD133-LG system allowed us to identify CD133+ GSC population without the usage of anti-CD133 antibody, the real-time and long-term tracking of GSCs were made possible. In addition, the reporting of CD133+ GSCs by our system was more consistent compared to a larger variation using antibody-based FACS. Both features offered by our CD133-LG system should provide a more rapid and accurate way of identifying GSCs. Finally, DBTRG-05MG-CD133-LG neurospheres (as the surrogate for GSCs) were intracranially injected to establish a GSC mouse model for in vivo evaluation of Ib as an anti-GSC agent. In agreement, mice that received Ib treatment showed a significantly lower bioluminescent signal as compared to the vehicle control. Tumor samples harvested from Ib-treated mice also demonstrated a significantly reduced ability to generate neurospheres under serum-deprived culture conditions and with lower percentage of GFP+ cells. Based on our observations, Ib appeared to be of potential as an anti-GSC agent using our CD133-LG dual imaging system. The results from our previous15 and current studies have provided strong preclinical evidence for using Ib to target and suppress the generation of TMZ-resistant GSCs. In fact, there are increasing number of clinical trials testing the efficacy of Ib as an adjuvant therapeutic agent for patients with GBM. For instance, one undergoing trial at Case Comprehensive Cancer Center combines Ib with radiation and TMZ in patients with newly diagnosed glioblastoma (ClinicalTrials.gov Identifier: NCT03535350).
In summary, in this study, we have established a dual reporter optical imaging system driven by CD133 promoter. We first demonstrated its applications in identifying and isolating CD133+ GSCs using FACS; DBTRG-05MG-CD133-LG cells were subsequently used to generate neurospheres accompanied by the increased activities of both reporters (GFP and firefly luciferase) and stemness markers notch1, β-catenin, nestin, and Btk. Subsequently, using our system, we were able to evaluate the anti-GSC functions of Ib both in vitro and in vivo.
Supplemental Material
Supplementary_Figure_1_Characterization_of_U87MG for The Development and Applications of a Dual Optical Imaging System for Studying Glioma Stem Cells by Po-An Tai, Yen-Lin Liu, Ya-Ting Wen, Chien-Min Lin, Thanh-Tuan Huynh, Michael Hsiao, Alexander T. H. Wu and Li Wei in Molecular Imaging
Footnotes
Authors’ Note: Po-An Tai and Yen-Lin Liu contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partial supported by the following grants. TCRD-TPE-107-43 and TCRD-TPE-108-46 to Po-An Tai and Li Wei; 103TMU-TMUH-06 to Yen-Lin Liu and Alexander TH Wu; 108-wf-phd-03 to Ya-Ting Wen; MOST 104-0210-01-09-02 and MOST 105-0210-01-13-01 to Michael Hsiao.
ORCID iD: Alexander T. H. Wu  https://orcid.org/0000-0002-0178-6530
https://orcid.org/0000-0002-0178-6530
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Li B, Huang MZ, Wang XQ, et al. TMEM140 is associated with the prognosis of glioma by promoting cell viability and invasion. J Hematol Oncol. 2015;8:89 doi:10.1186/s13045-015-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3. Mostafa H, Pala A, Hogel J, et al. Immune phenotypes predict survival in patients with glioblastoma multiforme. J Hematol Oncol. 2016;9(1):77 doi:10.1186/s13045-016-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi:10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auffinger B, Spencer D, Pytel P, Ahmed AU, Lesniak MS. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev Neurother. 2015;15(7):741–752. doi:10.1586/14737175.2015.1051968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spencer DA, Auffinger BM, Murphy JP, et al. Hitting a moving target: glioma stem cells demand new approaches in glioblastoma therapy. Curr Cancer Drug Targets. 2017;17(3):236–254. doi:10.2174/1568009616666161215161924. [DOI] [PubMed] [Google Scholar]
- 7. Uribe D, Torres A, Rocha JD, et al. Multidrug resistance in glioblastoma stem-like cells: Role of the hypoxic microenvironment and adenosine signaling. Mol Aspects Med. 2017;55:140–151. doi:10.1016/j.mam.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 8. Lee G, Auffinger B, Guo D, et al. Dedifferentiation of glioma cells to glioma stem-like cells by therapeutic stress-induced HIF signaling in the recurrent GBM model. Mol Cancer Ther. 2016;15(12):3064–3076. doi:10.1158/1535-7163.Mct-15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. William D, Walther M, Schneider B, Linnebacher M, Classen CF. Temozolomide-induced increase of tumorigenicity can be diminished by targeting of mitochondria in in vitro models of patient individual glioblastoma. PLoS One. 2018;13(1):e0191511 doi:10.1371/journal.pone.0191511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bian EB, Li J, Zhao B. CD133-positive stem cells. J Neuro. 2014;120:1011–1012. doi:10.3171/2013.10.Jns132261. [DOI] [PubMed] [Google Scholar]
- 11. Ludwig K, Kornblum HI. Molecular markers in glioma. J Neurol. 2017;134(3):505–512. doi:10.1007/s11060-017-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eramo A, Ricci-Vitiani L, Zeuner A, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13(7):1238–1241. doi:10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 13. Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67 doi:10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang L, Li N, Yan Z, Li C, Zhao Z. MiR-29a-mediated CD133 expression contributes to cisplatin resistance in CD133(+) glioblastoma stem cells. J Mol Neurosci. 2018;66(3):369–377. doi:10.1007/s12031-018-1177-0. [DOI] [PubMed] [Google Scholar]
- 15. Wei L, Su YK, Lin CM, et al. Preclinical investigation of ibrutinib, a Bruton’s kinase tyrosine (Btk) inhibitor, in suppressing glioma tumorigenesis and stem cell phenotypes. Oncotarget. 2016;7(43):69961–69975. doi:10.18632/oncotarget.11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H, Patel MR, Prescher JA, et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107(42):18115–18120. doi:10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabu K, Kimura T, Sasai K, et al. Analysis of an alternative human CD133 promoter reveals the implication of Ras/ERK pathway in tumor stem-like hallmarks. Mol Cancer. 2010;9:39 doi:10.1186/1476-4598-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hitomi M, Deleyrolle LP, Mulkearns Hubert EE, et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015;11(7):1031–1042. doi:10.1016/j.celrep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–4015. doi:10.1158/0008-5472.can-06-4180. [DOI] [PubMed] [Google Scholar]
- 20. Ozawa T, James CD. Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. J Vis Exp. 2010;10 doi:10.3791/1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217. doi:10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park DM, Rich JN. Biology of glioma cancer stem cells. Mol Cells. 2009;28(1):7–12. doi:10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 23. Choy W, Nagasawa DT, Trang A, Thill K, Spasic M, Yang I. CD133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme. Neurosurg Clin N Am. 2012;23(3):391–405. doi:10.1016/j.nec.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 24. Wan F, Zhang S, Xie R, et al. The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol. 2010;20(5):877–889. doi:10.1111/j.1750-3639.2010.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown DV, Filiz G, Daniel PM, et al. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intra-tumor heterogeneity. PLoS One. 2017;12(2):e0172791 doi:10.1371/journal.pone.0172791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JK, Jeon HM, Jeon HY, et al. Conversion of glioma cells to glioma stem-like cells by angiocrine factors. Biochemi Biophys Res Commun. 2018;496(4):1013–1018. doi:10.1016/j.bbrc.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 27. Zarkoob H, Taube JH, Singh SK, Mani SA, Kohandel M. Investigating the link between molecular subtypes of glioblastoma, epithelial-mesenchymal transition, and CD133 cell surface protein. PLoS One. 2013;8(5):e64169 doi:10.1371/journal.pone.0064169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Liu X, Hong Y, et al. Ibrutinib, a Bruton’s tyrosine kinase inhibitor, exhibits antitumoral activity and induces autophagy in glioblastoma. J Exp Clin Cancer Res. 2017;36(1):96 doi:10.1186/s13046-017-0549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yue C, Niu M, Shan QQ, et al. High expression of Bruton’s tyrosine kinase (BTK) is required for EGFR-induced NF-κB activation and predicts poor prognosis in human glioma. J Exp Clin Cancer Res. 2017;36:132 doi:10.1186/s13046-017-0600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figure_1_Characterization_of_U87MG for The Development and Applications of a Dual Optical Imaging System for Studying Glioma Stem Cells by Po-An Tai, Yen-Lin Liu, Ya-Ting Wen, Chien-Min Lin, Thanh-Tuan Huynh, Michael Hsiao, Alexander T. H. Wu and Li Wei in Molecular Imaging