Abstract
Infertility affects 30% to 50% of women with endometriosis. Women with endometriosis are at risk of decreased ovarian reserve, both because of the pathophysiology of the disease and iatrogenic injury resulting from surgical intervention. Fertility preservation must occur at multiple levels, including careful selection of surgical candidates, avoidance of repeat procedures, and meticulous surgical technique. Fertility preservation with oocyte or ovarian tissue cryopreservation may be considered on an individual basis for women with endometriosis, particularly those at risk of bilateral ovarian injury, such as women with bilateral endometriomas.
Keywords: Endometriosis, infertility, fertility preservation, endometrioma
Introduction
Endometriosis is chronic inflammatory condition characterized by the presence of endometrial glands outside of the uterus. This estrogen-dependent disease affects up to 10% of reproductive-aged women and up to 50% of women with infertility.1-6
Infertility is a major cause of morbidity in women with endometriosis. Thirty to fifty percent of endometriosis patients face infertility, and the condition reduces fecundity from 15% to 20% per month in healthy women to 2% to 5% per month in women with endometriosis.7-9
Infertility in endometriosis has both pathologic and iatrogenic causes, and fertility preservation should occur at multiple levels. First, iatrogenic injury to the adnexa during surgery must be minimized. Surgery should be performed by expert surgeons using meticulous surgical technique to avoid follicular damage. The benefits of surgery must always be weighed against the potential for damage to the ovarian reserve, and repeat surgery with the aim of optimizing fertility should be avoided. Although controversial, ovulation suppression with oral contraceptives may represent a preventive strategy for the development of endometriomas. Furthermore, women with endometriosis should be counseled about reproductive planning, including the possibility of decreased ovarian reserve and the risks of delayed childbearing. Although little data are available to guide recommendations for use in endometriosis patients, fertility preservation with oocyte or ovarian tissue cryopreservation may be offered on an individual basis to young women at high risk of endometriosis recurrence, or those at risk of damage to the bilateral ovaries, such as women with bilateral endometriomas. In the discussion that follows, we review the mechanisms of infertility in endometriosis and consider strategies for optimizing and preserving fertility.
Pathophysiology of Endometriosis in Infertility
Multiple mechanisms contribute to infertility in endometriosis. First, the distorted pelvic anatomy seen in moderate-to-severe disease may inhibit ovum capture and fertilization.10 Second, the inflammatory environment that characterizes the peritoneal fluid in endometriosis negatively impacts conception and embryo development at multiple points. The peritoneal fluid of women with endometriosis inhibits sperm motility, likely due to increased macrophage activity and cytokines.11-13 In addition, inflammatory factors in the peritoneal fluid impair tubal motility. When incubated in the peritoneal fluid of women with endometriosis, fallopian tubes demonstrate decreased beat frequency compared with controls.9,11,14 Furthermore, inflammatory cells in the peritoneal fluid as well as free radicals in the endometrium negatively impact embryo development and viability.13,15-17
In addition to anatomic distortion and peritoneal inflammation, endometriosis also negatively impacts the endometrium. Abnormalities of the eutopic endometrium in women with endometriosis contribute to implantation failure.10 Dysregulation of the progesterone receptor resulting in progesterone resistance leads to decreased endometrial receptivity and luteal phase dysfunction.11,13,18,19 Autoantibodies to antigens in the endometrium may further disrupt endometrial receptivity and implantation.9
The ovarian reserve is also negatively affected by endometriosis. The presence of ovarian endometriomas appears to damage the ovarian reserve by exposing healthy ovarian tissue to free radicals, reducing the pool of primordial follicles, and exposing the ovary to mechanical stretch.20-22 At baseline, even prior to surgery, women with endometriomas have significantly lower anti-Müllerian hormone (AMH) levels than healthy women, and the decrease in AMH levels is greater with bilateral compared with unilateral endometriomas.23 The clinical significance of these reductions in AMH is unclear, as AMH has been shown to be a poor predictor of spontaneous fertility.24 Similarly, although women with endometriomas undergoing IVF have a higher rate of cycle cancellation, and a lower mean number of oocytes retrieved, this does not lead to a decrease in the clinical pregnancy or live birth rate.25
Another potential mechanism of infertility in endometriosis is that affected women may have impaired oocyte and embryo quality compared with healthy women.10 This hypothesis is supported by studies of donor oocytes. Whereas women with advanced endometriosis who receive embryos from healthy women have normal implantation and pregnancy rates, healthy women who receive embryos from women with endometriosis demonstrate compromised implantation rates and embryo quality.26-31 Furthermore, embryos from women with endometriosis grow more slowly and demonstrate increased rates of arrested and abnormal development.10,15,29 These data suggest that poor oocyte and embryo quality contribute to decreased implantation and pregnancy rates.10 Despite these challenges, IVF maximizes fertility for endometriosis patients, and most studies show that women with endometriosis have similar IVF outcomes to women with other causes of infertility.13,32-34
Surgical Treatment for Endometriosis and Its Effects on Ovarian Reserve
Surgical treatment has been shown in several randomized controlled trials to improve fertility outcomes in women with stage I to II endometriosis.35,36 The largest of these trials, titled Endocan, evaluated 341 patients with endometriosis who were randomly assigned to diagnostic laparoscopy or laparoscopy with excision/ablation of endometriosis.35 Their results demonstrated a significantly higher pregnancy rate in patients treated with laparoscopic excision or ablation compared with diagnostic laparoscopy (30% vs 17%).35 Two additional randomized controlled trials demonstrated improved pregnancy rates after ablation/excision of endometriosis compared with diagnostic laparoscopy (28% vs 23%37 and 24% vs 28%38); however, the results of the latter study were not statistically significant.38 Both studies had small sample sizes (20 and 38 patients, respectively) and lacked statistical power.37,38 In addition, none of the 3 studies evaluated excision and ablation separately. A 2014 Cochrane Review pooled the results of these 3 randomized trials and found a significant benefit of laparoscopic surgery for improving live birth or ongoing pregnancy rate, with a number needed to treat of 8,39 assuming that all patients at laparoscopy have endometriosis.
For patients with advanced-stage endometriosis, the available data are limited to observational studies. These observational studies do suggest a benefit in fecundity after laparoscopic treatment of endometriosis, with pregnancy rates ranging from 30% to 60% in the 1 to 3 years following surgery.10,40-43 Data regarding the impact of deep infiltration endometriosis (DIE) on fertility are mixed. Retrospective studies of DIE involving the bowel have demonstrated improved fertility after colorectal resection.44-46 Similarly, a prospective cohort study of 179 women with DIE compared immediate IVF with laparoscopy followed by IVF; results demonstrated significantly improved implantation (19% vs 32%) and pregnancy rates (24% vs 41%) after laparoscopy. A second prospective cohort study of 483 patients with DIE also reported a higher clinical pregnancy rate with a combination of IVF and laparoscopy than with surgery or IVF alone.47 Conversely, a third prospective study of rectovaginal septum excision showed no improvement in fertility outcomes when compared with expectant management.48 Although DIE itself appears to have a negative impact on fertility that can be ameliorated with surgical intervention, the risks and benefits of surgical excision must be carefully considered. Prevailing expert consensus holds that IVF, rather than surgery, should be the first-line treatment for women with DIE who primarily desire fertility.49,50
Regarding ovarian endometriomas, data support the removal of large endometriomas prior to attempting spontaneous conception; however, the management of endometriomas in the setting of IVF is controversial. A 2008 Cochrane review demonstrated that the excision of ovarian endometriomas substantially improved spontaneous conception rates (OR = 5.21, 95% CI = [2.04, 13.29]). Several randomized controlled trials address the question of whether to excise endometriomas prior to IVF. The potential benefits of endometrioma excision prior to IVF include facilitation of oocyte retrieval and prevention of spillage of endometrioma contents onto oocytes.10 These benefits must be weighed against the risk of ovarian damage, as well as the impact of delaying IVF. A recent meta-analysis of surgical treatment for endometriomas prior to IVF evaluated 33 studies, including 3 randomized controlled trials. Results demonstrated a higher rate of cycle cancellation, and a lower mean number of oocytes retrieved in the untreated endometrioma group; however, this did not lead to a decrease in the clinical pregnancy or live birth rate.25 Similarly, 2 other meta-analyses failed to demonstrate a difference in pregnancy rates between surgically treated and untreated endometriomas prior to IVF.51,52 Although there is no definitive evidence to support endometrioma excision prior to IVF, the available literature does not consider endometrioma size, and the excision of large endometriomas may be considered on an individual basis to prevent endometrioma spillage and allow for oocyte retrieval.10,53
The benefits of all surgical interventions for endometriosis must be weighed against the potential for ovarian injury.10 Damage to the ovary can result from the excision of excessive ovarian tissue or injury to the ovarian vasculature.10 A number of studies have demonstrated the harmful effects of adnexal surgery on ovarian reserve, particularly in the case of bilateral endometriomas.54-56 Studies have shown a decrease in AMH of up to 30% after excision of a unilateral endometrioma and up to 44% after excision of bilateral endometriomas.20,49
Repeated surgery for endometriosis does not appear to improve fertility outcomes and often results in damage to the ovarian reserve; therefore, patients who are unable to conceive after a single procedure should be counseled to pursue IVF rather than repeat surgery. A retrospective study of women with advanced endometriosis evaluated pregnancy rates after repeat surgery versus IVF.57 The authors demonstrated a cumulative pregnancy rate of 70% after 2 IVF cycles, compared with 24% 9 months after repeat surgery.57 Similarly, surgery for recurrent endometriomas causes a greater decline in AMH and antral follicle count than the initial surgery.58,59 The decision to pursue surgery for endometriosis patients desiring fertility must be considered carefully in the context of the potential for decreased ovarian reserve.
Minimizing the Effects of Surgery on Ovarian Reserve
Optimizing fertility for patients with endometriosis begins with reducing iatrogenic harm to the ovarian reserve. Several surgical strategies have been shown to minimize ovarian damage during laparoscopy, including meticulous surgical technique and sparing use of electrosurgery.
Several surgical approaches for the management of ovarian endometriomas have been described: cystectomy, ablation, and combinations thereof. According to a Cochrane review of 3 randomized controlled trials, when compared with ablation, excision of endometriomas results in higher rates of spontaneous conception and resolution of pain, as well as lower recurrence rates.60 Despite these favorable outcomes, there have been numerous reports of decreased ovarian reserve following cystectomy, up to 30% after excision of a unilateral endometrioma and up to 44% after excision of bilateral endometriomas.20,49 Therefore, for patients unlikely to conceive spontaneously, ablation may represent an alternative to excision that allows for better conservation of the ovarian reserve.49 Ablation can be achieved through multiple modalities, including monopolar and bipolar electrosurgery, CO2 laser, and plasma energy. Data suggest that the use of the CO2 laser and plasma energy may result in less thermal injury and improved ovarian reserve compared with ovarian cystectomy or the use of electrosurgery for ablation.61,62 Because of the potential for reduced impact on ovarian reserve, some authors have suggested approaches that combine ablation with other modalities. Donnez et al42 have described a 3-stage approach to the management of endometriomas larger than 3 cm involving (1) laparoscopic cyst drainage with biopsy to confirm the diagnosis of endometriosis, (2) treatment with 12 weeks of a gonadotropin receptor hormone (GnRH) agonist with the goal of reducing cyst diameter and mitotic activity, and (3) laparoscopic ablation of the cyst wall using a CO2 laser. Tsolakidis et al published a randomized controlled trial comparing ovarian cystectomy to the 3-stage approach for the management of endometrioma and reported a significant decrease in postoperative AMH decline with the 3-stage approach (3.9-2.9 in the cystectomy group compared with 4.5-3.99 in the 3-stage group). Recurrence rates for this method are reported to be 8% at 2 years. The clear drawback to this approach is the need for multiple laparoscopies. Obviating the need for multiple procedures, combined excision and ablative approaches have also been evaluated. The combined excision and ablative approach allows for excision of 80% to 90% of the endometrioma, followed by ablation of the remaining 10% to 20% of the cyst wall adjacent to the hilum, where bleeding and decortication are most likely to occur. A prospective study of 52 women undergoing combined excision/ablation with CO2 laser reported a pregnancy rate of 41% at 8 months and a recurrence rate of 2%.63 A randomized controlled trial compared laparoscopic cystectomy to combined excision/ablation with bipolar electrosurgery and demonstrated no difference in recurrence rate or antral follicle count; however, the study was small (n = 51) and underpowered.64 Overall, excision remains the standard of surgical management for ovarian endometriomas; however, based on available data, combined excision/ablation methods offer an alternative with the potential for decreased ovarian injury and acceptable recurrence rates.
To optimize fertility when excising an endometrioma, the plane between the endometrioma and the ovarian cortex must be carefully delineated to minimize injury to viable ovarian tissue.10 This plane is often distorted by a fibrotic capsule surrounding the endometrioma. Dilute vasopressin may be used to reduce bleeding and demarcate the correct plane; however, it must be noted that vasopressin is not Food and Drug Administration (FDA)-approved for this indication.49 In addition, care must be taken with dissection near the hilum, where bleeding is likely to occur. When hemostasis is required, electrosurgery should be used sparingly, and alternatives should be used whenever possible. Several studies, including a meta-analysis of 3 randomized controlled trials, demonstrate a smaller decline in postoperative AMH levels with the use of suturing or hemostatic sealants rather than bipolar electrosurgery to obtain hemostasis in the endometrioma cyst bed.65-67 Two randomized controlled trials evaluated the role of hemostatic sealants in ovarian cystectomy; both used a gelatin matrix and thrombin solution (FLOSEAL, Baxter).67,68 In the largest of the 2 randomized trials, 100 patients were randomized to hemostasis with bipolar electrosurgery versus a hemostatic sealant. Results demonstrated a smaller decline in postoperative AMH levels at 3 months in the hemostatic sealant compared with the bipolar group (16% vs 41%). Six percent of patients in the hemostatic sealant group required additional hemostasis with bipolar electrosurgery.67 The use of suturing or hemostatic agents should be used as an alternative to electrosurgery whenever possible. In addition, the use of adhesion barriers, including oxidized regenerated cellulose (Interceed, Gynecare), expanded polytetrafluoroethylene (Gore-Tex), sodium hyaluronate and carboxymethylcellulose (Seprafilm, Sanofi), and fibrin sheets, may reduce postoperative adhesions that distort anatomy and interfere with ovum capture.69 Excellent hemostasis must be achieved for adhesion barriers to work effectively.
Finally, surgeon experience appears to play a role in fertility outcomes after endometriosis surgery, particularly for endometrioma excision. Experienced surgeons remove less ovarian tissue along with the cyst wall at the time of ovarian cystectomy,70 and retrospective data suggest that surgeon experience may affect live birth rate after endometrioma excision.71 A retrospective study at a teaching hospital in Taiwan demonstrated a significantly higher live birth rate after IVF in patients who had undergone prior endometrioma excision by an attending physician as compared with a resident or fellow.71
The Role of Medical Therapy in Endometriosis-Related Infertility
Medical therapy has a limited role in the treatment of endometriosis-related infertility. Although it is effective for reducing pain, medical therapy does not improve fertility outcomes, and hormonal treatments inhibit ovulation.72
Data regarding adjuvant ovulation suppression are mixed; however, adjuvant hormonal therapy is a low-cost, low-risk intervention with the potential to result in substantial fertility benefits.22 A recent meta-analysis that included 965 women demonstrated a dramatic reduction in recurrent endometriomas with long-term postoperative oral contraceptive use.73 Given the profound effect of endometriomas and their treatment on ovarian reserve, adjuvant suppression should be considered in patients with ovarian endometriomas. Ovulation suppression may also be considered in adolescent women with significant dysmenorrhea, family history, or other risk factors for endometriosis.
Finally, some data support pretreatment with GnRH agonists or oral contraceptive pills (OCPs) prior to initiating IVF in women with endometriosis. A 2006 systematic review evaluated GnRH agonist pretreatment prior to IVF, including 3 randomized controlled trials of 165 women with endometriosis. Their results demonstrated a 4-fold increase in the IVF clinical pregnancy rate after 3 to 6 months of pretreatment with a GnRH agonist.74 Although live birth rate was reported in only 1 of the 3 studies, it was also increased after GnRH agonist pretreatment.74 Similarly, treatment with 6 to 8 weeks of OCPs prior to IVF increased pregnancy rates in a retrospective study.75 Despite these favorable results, more recent randomized controlled trial data have failed to show a significant benefit of GnRH pretreatment.76 If beneficial, the ideal duration of pre-IVF medical treatment and the cohort of patients most likely to benefit have not yet been established.10 In addition, the potential benefits of pretreatment must be weighed against additional costs, delays in the initiation of IVF, and the possibility of decreased response to ovarian stimulation.10
Fertility Preservation
Given that women with endometriosis are at risk of compromised ovarian reserve due to both pathologic and iatrogenic causes, consideration should be given to fertility preservation with oocyte or embryo cryopreservation. The use of oocyte cryopreservation has increased among women prior to exposure to gonadotoxic therapies, and among women desiring to delay childbearing for elective purposes;77 however, data are limited regarding fertility preservation in endometriosis patients.78
The first case report of oocyte cryopreservation for the indication of endometriosis was published in 2009.79 The patient was a 25 years old with 4 prior endometriosis surgeries, including a right oophorectomy, who presented for management of recurrent pain and dyspareunia. The antral follicle count in the remaining ovary was 3, consistent with diminished ovarian reserve. The patient was considered to be at high risk of incurring further damage to her ovarian reserve with additional surgery and was therefore counseled regarding oocyte cryopreservation prior to further treatment.79 After 3 controlled ovarian hyperstimulation (COH) cycles, a total of 21 mature oocytes were retrieved and vitrified.79 Since 2009, there has been only 1 case series of fertility preservation in endometriosis patients.80 Raad et al reported on a series of 49 patients who underwent COH for fertility preservation. Most of the included patients had deep infiltrating endometriosis and/or endometriomas. The authors noted a significantly decreased response to COH in patients with a history of a prior endometrioma surgery, suggesting that COH may be considered prior to endometrioma excision in select patients.80 Neither of these reports evaluated pregnancy outcomes after thaw and transfer of vitrified oocytes. Women considering oocyte cryopreservation should be counseled that fertility preservation does not guarantee pregnancy and be provided with age-related success rates. Studies evaluating the efficiency of oocyte cryopreservation in women undergoing elective fertility preservation demonstrate that the live birth rate per warmed vitrified oocyte varies by age, and ranges from 5% in women aged 38 years and older to 7.4% in women aged under 30 years at the time of COH.81 Therefore, to have a realistic chance of a live birth, women aged under 38 years are recommended to cryopreserve 15 to 20 oocytes, and those aged 38 to 40 years should be advised to cryopreserve 25 to 30 oocytes.82 Although data regarding pregnancy rates after oocyte cryopreservation may be cautiously extrapolated from trials evaluating IVF outcomes in women with endometriosis,13,32-34 further study is required to verify these assumptions.22 Pregnancy rates after IVF are similar for women with endometriosis compared with women with other causes of infertility; however, patients with endometriomas have significantly lower pregnancy and live birth rates.13,32-34 Women with endometriomas may be among the group most likely to benefit from fertility preservation, highlighting the need for further evaluation of oocyte cryopreservation among endometriosis patients.22
Ovarian tissue cryopreservation is an option for patients who are unable or unwilling to undergo COH, or who require oophorectomy. In 2005, Donnez et al83 described the orthotopic transplantation of 2 cortical strips from an ovary containing a 9 cm endometrioma. The patient was unable to conceive spontaneously, but became pregnant after 3 cycles of IVF.83 Although recent data from high-volume centers suggest that live birth and clinical pregnancy rates after ovarian tissue cryopreservation compare favorably with oocyte cryopreservation for women undergoing gonadotoxic treatments,84 the technology remains experimental, and the removal of healthy ovarian tissue may negatively affect ovarian reserve.10,85 If oocyte cryopreservation is to be used more widely among endometriosis patients, the technique will require further study in this patient population. Because the quality of the ovarian follicles adjacent to endometriomas may be compromised, the reproductive potential of this tissue must be further evaluated.86 In addition, some data suggest that the technology may have limited efficacy among women with decreased ovarian reserve, a common concern among endometriosis patients.85 Table 1 outlines the advantages and disadvantages to both ovarian tissue and oocyte cryopreservation.
Table 1.
Options for Fertility Preservation in Women With Endometriosis.
| Oocyte and embryo cryopreservation | Ovarian tissue cryopreservation | |
|---|---|---|
| Benefits | High success rates, particularly with embryos | Option for women who are unable or unwilling to undergo ovarian stimulation |
| Avoids a laparoscopic procedure | Option for women who require oophorectomy | |
| Avoids risk of damage to ovarian tissue | Could be performed at the time of excision surgery for at-risk patients | |
| Risks | Reproductive potential of follicles from endometriosis patients requires further study | Experimental technology |
| Need to cyropreserve large numbers of oocytes (15-20 in women aged <38 years and 25-30 in women aged ⩾38 years) | Potential for damage to viable ovarian tissue | |
| Possibility of impaired oocyte and embryo quality | Risks of laparoscopy |
It is important to note that, although AMH levels are lower in women with endometriomas at baseline,23 AMH is a poor predictor of spontaneous fertility.24 In the absence of other indications for fertility preservation, decreased AMH alone is not a compelling reason to pursue oocyte or ovarian tissue cryopreservation.
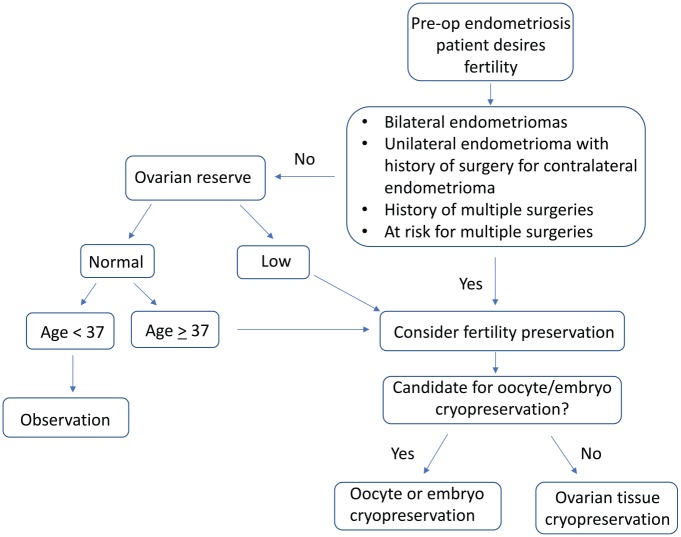
Because of the limited data regarding outcomes, use, and cost-effectiveness of fertility preservation among endometriosis patients, recommendations regarding who would most benefit from these technologies are largely speculative. Younger women, for example, may have an increased risk of endometriosis recurrence, and the quality of banked oocytes is likely to be higher than in older women.22 Women with a threat of damage to both ovaries may particularly benefit from fertility preservation; such women may include those with bilateral endometriomas, or a history of a unilateral cystectomy with a contralateral recurrence.22 Figure 1 outlines a practical approach to fertility preservation in the preoperative endometriosis patient (Figure 1). Counseling regarding fertility preservation must be individualized, taking into account age, ovarian reserve, prior and planned surgical interventions, and the role and success rates of fertility preservation technologies.10,22,86
Figure 1.
Practical approach to fertility preservation in presurgical endometriosis patients.
Conclusions
The pathogenesis of endometriosis and its surgical treatment both contribute to decreased ovarian reserve. Optimizing and preserving fertility in women with endometriosis begins with preventing iatrogenic injury. Surgery must be performed judiciously with attention to the possibility of damage to the ovarian reserve. Repeat surgeries for endometriosis do not improve fertility outcomes, and patients who do not become pregnant after the first procedure should be counseled to undergo IVF. Although data are mixed, ovarian suppression may provide a low-cost, low-risk method of preventing the development of endometriomas, which are a major threat to fertility. Fertility preservation with oocyte or ovarian tissue cryopreservation should be considered on an individual basis for women with endometriosis. More data are needed to establish outcomes for fertility preservation in endometriosis and guide recommendations regarding those most likely to benefit.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Note: Rebecca L Flyckt is now affiliated with University Hospitals Fertility Center, Cleveland, OH, USA.
Author Contributions: TF, RLF and NCL contributed to the conception and design of the paper. NCL drafted the manuscript; NCL, TF, and RLF critically revised the content of the manuscript. All three authors reviewed and approved the final manuscript.
ORCID iD: Tommaso Falcone  https://orcid.org/0000-0002-9343-9789
https://orcid.org/0000-0002-9343-9789
References
- 1. Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46. [PubMed] [Google Scholar]
- 2. Parazzini F, Vercellini P, Pelucchi C. Endometriosis: epidemiology, and etiological factors. In: Giudice LC, Evers JLH, Healy DL, eds. Endometriosis: Science and Practice. Hoboken, NJ: John Wiley;2012:19-26. doi: 10.1002/9781444398519.ch2. [DOI] [Google Scholar]
- 3. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235-258. doi: 10.1016/S0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 4. Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann NY Acad Sci. 2002;955:11-22. doi: 10.1111/j.1749-6632.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 5. Strathy JH, Molgaard CA, Coulam CB, Melton LJ. Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril. 1982;38:667-672. doi: 10.1016/s0015-0282(16)46691-4. [DOI] [PubMed] [Google Scholar]
- 6. Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Hum Reprod. 1991;6:544-549. doi: 10.1093/oxfordjournals.humrep.a137377. [DOI] [PubMed] [Google Scholar]
- 7. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil Steril. 1993;59:363-970. doi: 10.1016/S0015-0282(16)55911-1. [DOI] [PubMed] [Google Scholar]
- 8. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 9. Evans MB, Decherney AH. Fertility and endometriosis. Clin Obstet Gynecol. 2017;60:497-502. doi: 10.1097/GRF.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 10. Llarena N, Flyckt R. Strategies to preserve and optimize fertility for patients with endometriosis. J Endometr Pelvic Pain Disord. 2017;9:98-104. doi: 10.5301/jeppd.5000278. [DOI] [Google Scholar]
- 11. Holoch K, Lessey B. Endometriosis and infertility. Clin Obstet Gynecol. 2010;53:429-438. [DOI] [PubMed] [Google Scholar]
- 12. Oral E, Arici A, Olive DL, Huszar G. Peritoneal fluid from women with moderate or severe endometriosis inhibits sperm motility: the role of seminal fluid components. Fertil Steril. 1996;66:787-792. doi: 10.1016/s0015-0282(16)58637-3. [DOI] [PubMed] [Google Scholar]
- 13. Macer ML, Taylor HS. Endometriosis and infertility. a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39:535-549. doi: 10.1016/j.ogc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyons RA, Djahanbakhch O, Saridogan E, et al. Peritoneal fluid, endometriosis, and ciliary beat frequency in the human fallopian tube. Lancet. 2002;360:1221-1222. doi: 10.1016/S0140-6736(02)11247-5. [DOI] [PubMed] [Google Scholar]
- 15. Ota H, Igarashi S, Kato N, Tanaka T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril. 2000;74:313-318. doi: 10.1016/s0015-0282(00)00638-5. [DOI] [PubMed] [Google Scholar]
- 16. Ota H, Igarashi S, Sato N, Tanaka H, Tanaka T. Involvement of catalase in the endometrium of patients with endometriosis and adenomyosis. Fertil Steril. 2002;78:313-318. doi: 10.1016/s0015-0282(02)03344-7. [DOI] [PubMed] [Google Scholar]
- 17. Morcos RN, Gibbons WE, Findley WE. Effect of peritoneal fluid on in vitro cleavage of 2-cell mouse embryos: possible role in infertility associated with endometriosis. Fertil Steril. 1985;44:678-683. doi: 10.1016/s0015-0282(16)48987-9. [DOI] [PubMed] [Google Scholar]
- 18. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789-1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 19. Santamaria X, Massasa EE, Taylor HS. Migration of cells from experimental endometriosis to the uterine endometrium. Endocrinology. 2012;153:5566-5574. doi: 10.1210/en.2012-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kitajima M, Dolmans MM, Donnez O, Masuzaki H, Soares M, Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril. 2014;101:1031-1037. doi: 10.1016/j.fertnstert.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez AM, Vigano P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. 2014;20:217-230. doi: 10.1093/humupd/dmt053. [DOI] [PubMed] [Google Scholar]
- 22. Somigliana E, Vigano P, Filippi F, et al. Fertility preservation in women with endometriosis: for all, for some, for none? Hum Reprod. 2015;30:1280-1286. doi: 10.1093/humrep/dev078. [DOI] [PubMed] [Google Scholar]
- 23. Nieweglowska D, Hajdyla-Banas I, Pitynski K, et al. Age-related trends in anti-Mullerian hormone serum level in women with unilateral and bilateral ovarian endometriomas prior to surgery. Reprod Biol Endocrinol. 2015;13:128. doi: 10.1186/s12958-015-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318:1367-1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamdan M, Dunselman G, Li TC, Cheong Y. The impact of endometrioma on IVF/ICSI outcomes: a systematic review and meta-analysis. Hum Reprod Update. 2015;21:809-825. doi: 10.1093/humupd/dmv035. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Velasco JA, Arici A. Is the endometrium or oocyte/embryo affected in endometriosis? Hum Reprod. 1999;14:77-89. doi: 10.1093/humrep/14.suppl_2.77. [DOI] [PubMed] [Google Scholar]
- 27. Simon C, Gutierrez A, Vidal A, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9:725-729. doi: 10.1093/oxfordjournals.humrep.a138578. [DOI] [PubMed] [Google Scholar]
- 28. Sung L, Mukherjee T, Takeshige T, Bustillo M, Copperman AB. Endometriosis is not detrimental to embryo implantation in oocyte recipients. J Assist Reprod Genet. 1997;14:152-156. doi: 10.1007/BF02766132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pellicer A, Oliveira N, Ruiz A, Remohi J, Simon C. Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction. Hum Reprod. 1995;10:91-97. doi: 10.1093/humrep/10.suppl_2.91. [DOI] [PubMed] [Google Scholar]
- 30. Diaz I, Navarro J, Blasco L, Simon C, Pellicer A, Remohi J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril. 2000;74:31-34. doi: 10.1016/s0015-0282(00)00570-7. [DOI] [PubMed] [Google Scholar]
- 31. Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod. 2002;8:95-103. doi: 10.1093/humupd/8.1.95. [DOI] [PubMed] [Google Scholar]
- 32. Society for Assisted Reproductive Technology, American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2010 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproduction registry. Birmingham, AL: American Society for Reproductive Medicine; 2012. [Google Scholar]
- 33. Bukulmez O, Yarali H, Gurgan T. The presence and extent of endometriosis do not effect clinical pregnancy and implantation rates in patients undergoing intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2001;96:102-107. doi: 10.1016/S0301-2115(00)00379-1. [DOI] [PubMed] [Google Scholar]
- 34. Opoien HK, Fedorcsak P, Omland AK, et al. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril. 2012;97:912-918. doi: 10.1016/j.fertnstert.2012.01.112. [DOI] [PubMed] [Google Scholar]
- 35. Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. N Engl J Med. 1997;337:217-222. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- 36. Parazzini F. Ablation of lesions or no treatment in minimal–mild endometriosis in infertile women: a randomized trial Gruppo Italiano per lo Studio dell’Endometriosi. Hum Reprod. 1999;14:1332-1334. [DOI] [PubMed] [Google Scholar]
- 37. Gad MS, Badaoui MHH. Evidence-based therapy for infertility associated with early stage endometriosis. Int J Gynaecol Obstet. 2012;119:S531-S867. [Google Scholar]
- 38. Moini A, Bahar L, Ashrafinia M, Eslami B, Hosseini R, Ashrafinia N. Fertility outcome after operative laparoscopy versus no treatment in infertile women with minimal or mild endometriosis. Int J Fertil Steril. 2012;5:235-240. [PMC free article] [PubMed] [Google Scholar]
- 39. Duffy J, Arambage K, Correa F, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;3:CD011031. doi: 10.1002/14651858.CD011031.pub2. [DOI] [PubMed] [Google Scholar]
- 40. Crosignani PG, Vercellini P, Biffignandi F, Costantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711. doi: 10.1016/s0015-0282(16)58622-1. [DOI] [PubMed] [Google Scholar]
- 41. Wood C, Maher P, Hill D. Diagnosis and surgical management of endometriomas. Aust N Z J Obstet Gynaecol. 1992;32:161-163. doi: 10.1111/j.1479-828X.1992.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 42. Donnez J, Nisolle M, Gillet N, Smets M, Bassil S, Casanas-Roux F. Large ovarian endometriomas. Hum Reprod. 1996;113:641-646. doi: 10.1093/HUMREP/11.3.641. [DOI] [PubMed] [Google Scholar]
- 43. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488-1504. doi: 10.1016/0002-9378(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 44. Darai E, Carbonnel M, Dubernard G, et al. Determinant factors of fertility outcomes after laparoscopic colorectal resection for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;149:210-214. doi: 10.1016/j.ejogrb.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 45. Ferrero S, Anserini P, Abbamonte LH, Ragni N, Camerini G, Remorgida V. Fertility after bowel resection for endometriosis. Fertil Steril. 2009;92:41-46. doi: 10.1016/j.fertnstert.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 46. Darai E, Dubernard G, Coutant C, Frey C, Rouzier R, Ballester M. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023. doi: 10.1097/SLA.0b013e3181d9691d. [DOI] [PubMed] [Google Scholar]
- 47. Barri PN, Coroleu B, Tur R, Barri-Soldevila PN, Rodriguez I. Endometriosis-associated infertility: surgery and IVF, a comprehensive therapeutic approach. Reprod Biomed Online. 2010;21:179-185. doi: 10.1016/j.rbmo.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 48. Vercellini P, Pietropaolo G, De Giorgi O, Daguati R, Pasin R, Crosignani PG. Reproductive performance in infertile women with rectovaginal endometriosis: is surgery worthwhile. Am J Obstet Gynecol. 2006;195:1303-1310. doi: 10.1016/j.ajog.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 49. Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571. doi: 10.1097/AOG.0000000000002469. [DOI] [PubMed] [Google Scholar]
- 50. Abrao MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339. doi: 10.1093/humupd/dmv003. [DOI] [PubMed] [Google Scholar]
- 51. Tsoumpou I, Kyrgiou M, Gelbaya TA, Nardo LG. The effect of surgical treatment for endometrioma on in vitro fertilization outcomes: a systematic review and meta-analysis. Fertil Steril. 2009;92:75-87. doi: 10.1016/j.fertnstert.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 52. Benschop L, Farquhar C, van der Poel N, Heineman MJ. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst Rev. 2010;11:CD008571. doi: 10.1002/14651858.CD008571.pub2. [DOI] [PubMed] [Google Scholar]
- 53. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98:591-598. doi: 10.1016/j.fertnstert.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 54. Goodman LR, Goldberg JM, Flyckt RL, Gupta M, Harwalker J, Falcone T. Effect of surgery on ovarian reserve in women with endometriomas, endometriosis and controls. Am J Obstet Gynecol. 2016;215:589.e1-589.e6. doi: 10.1016/j.ajog.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 55. Seyhan A, Ata B, Uncu G. The impact of endometriosis and its treatment on ovarian reserve. Semin Reprod Med. 2015;33:422-428. doi: 10.1055/s-0035-1567820. [DOI] [PubMed] [Google Scholar]
- 56. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cysts: a prospective clinical study of 191 patients. Fertil Steril. 2009;92:1428-1435. doi: 10.1016/j.fertnstert.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 57. Pagidas K, Falcone T, Hemmings R, Miron P. Comparison of reoperation for moderate (stage III) and severe (stage IV) endometriosis-related infertility with in vitro fertilization-embryo transfer. Fertil Steril. 1996;65:791-795. doi: 10.1016/s0015-0282(16)58215-6. [DOI] [PubMed] [Google Scholar]
- 58. Muzii L, Achilli C, Lecce F, et al. Second surgery for recurrent endometriomas is more harmful to healthy ovarian tissue and ovarian reserve than first surgery. Fertil Steril. 2015;103:738-743. doi: 10.1016/j.fertnstert.2014.12.101. [DOI] [PubMed] [Google Scholar]
- 59. Ferrero S, Scala C, Racca A, et al. Second surgery for recurrent unilateral endometriomas and impact on ovarian reserve: a case-control study. Fertil Steril. 2015;103:1236-1243. doi: 10.1016/j.fertnstert.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 60. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;16:CD004992. doi: 10.1002/14651858.CD004992.pub3. [DOI] [PubMed] [Google Scholar]
- 61. Candiani M, Ottolina J, Posadzka E, et al. Assessment of ovarian reserve after cystectomy versus “one-step” laser vaporization in the treatment of ovarian endometrioma: a small randomized clinical trial. Hum Reprod. 2018;33:2205-2211. doi: 10.1093/humrep/dey305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pedroso J, Gutierrez M, Volker KW. Comparative thermal effects of J-plasma, monopolar, argon and laser electrosurgery in a porcine tissue model. J Minim Invasive Gynecol. 2014;21:S59. doi: 10.1016/j.jmig.2014.08.210. [DOI] [PubMed] [Google Scholar]
- 63. Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94:28-32. doi: 10.1016/j.fertnstert.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 64. Muzii L, Achilli C, Bergamini V, et al. Comparison between the stripping technique and the combined excisional/ablative technique for the treatment of bilateral ovarian endometriomas: a multicentre RCT. Hum Reprod. 2016;31:339-344. doi: 10.1093/humrep/dev313. [DOI] [PubMed] [Google Scholar]
- 65. Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22:363-372. doi: 10.1016/j.jmig.2014.12.168. [DOI] [PubMed] [Google Scholar]
- 66. Asgari Z, Rouholamin S, Hosseini R, Sepidarkish M, Hafizi L, Javaheri A. Comparing ovarian reserve after laparoscopic excision of endometriotic cysts and hemostasis achieved either by bipolar coagulation or suturing: a randomized clinical trial. Arch Gynecol Obstet. 2016;293:1015-1022. doi: 10.1007/s00404-015-3918-4. [DOI] [PubMed] [Google Scholar]
- 67. Song T, Lee SH, Kim WY. Additional benefit of hemostatic sealant in preservation of ovarianreserve during laparoscopic ovarian cystectomy: a multi-center, randomized controlled trial. Hum Reprod. 2014;29:1659-1665. doi: 10.1093/humrep/deu125. [DOI] [PubMed] [Google Scholar]
- 68. Sonmezer M, Taskin S, Gemici A, et al. Can ovarian damage be reduced using hemostatic matrix during laparoscopic endometrioma surgery? a prospective, randomized study. Arch Gynecol Obstet. 2013;287:1251-1257. doi: 10.1007/s00404-012-2704-9. [DOI] [PubMed] [Google Scholar]
- 69. Ahmad G, O’Flynn H, Hindocha A, Watson A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2015;2:CD000475. doi: 10.1002/14651858.CD000475.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muzii L, Marana R, Angioli R, et al. Histologic analysis of specimens from laparoscopic endometrioma excision performed by different surgeons: does the surgeon matter. Fertil Steril. 2011;95:2116-2119. doi: 10.1016/j.fertnstert.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 71. Yu HT, Huang HY, Soong YK, Lee CL, Chao A, Wang CJ. Laparoscopic ovarian cystectomy of endometriomas: surgeons’ experience may affect ovarian reserve and live-born rate in infertile patients with in vitro fertilization-intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2010;152:172-175. doi: 10.1016/j.ejogrb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 72. Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev. 2007;3:CD000155. doi: 10.1002/14651858.CD000155.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vercellini P, DE Matteis S, Somigliana E, Buggio L, Frattaruolo MP, Fedele L. Long-term adjuvant therapy for the prevention of postoperative endometrioma recurrence: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2013;92:8-16. doi: 10.1111/j.1600-0412.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 74. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006;1:CD004635. doi: 10.1002/14651858.CD004635.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dicker D, Goldman JA, Levy T, Feldberg D, Ashkenazi J. The impact of long-term gonadotropin-releasing hormone analogue treatment on preclinical abortions in patients with severe endometriosis undergoing in vitro fertilization-embryo transfer. Fertil Steril. 1992;57:597-600. doi: 10.1016/s0015-0282(16)54906-1. [DOI] [PubMed] [Google Scholar]
- 76. Rodriguez-Tarrega E, Monzo A, Quiroga R, et al. Randomized controlled trial to evaluate the usefulness of GnRH agonist versus placebo on the outcome of IVF in infertile patients with endometriosis. Hum Reprod. 2016;31:267-267. [Google Scholar]
- 77. Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients. Fertil Steril. 2013;99:1485-1495. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 78. De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302-1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Elizur SE, Chian RC, Holzer HEG, Gidoni Y, Tulandi T, Tan SL. Cryopreservation of oocytes in a young woman with severe and symptomatic endometriosis: a new indication for fertility preservation. Fertil Steril. 2009;91:293.e1-293.e3. doi: 10.1016/j.fertnstert.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 80. Raad J, Sonigo C, Tran C, Sifer C, Durnerin IC, Grynberg M. Oocyte vitrification for preserving fertility in patients with endometriosis: first observational cohort study. Eur J Obstet Gynecol Reprod Biol. 2018;220:140-141. doi: 10.1016/j.ejogrb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 81. Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016;105:459-466. doi: 10.1016/j.fertnstert.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 82. Streuli I, Benard J, Hugon-Rodin J, Chapron C, Santulli P, Pluchino N. Shedding light on the fertility preservation debate in women with endometriosis: a SWOT analysis. Eur J Obstet Gynecol Reprod Biol. 2018;229:172-178. doi: 10.1016/j.ejogrb.2018.08.577. [DOI] [PubMed] [Google Scholar]
- 83. Donnez J, Squifflet J, Dolmans MM, Martinez-Madrid B, Jadoul P, Van Langendonckt A. Orthotopic transplantation of fresh ovarian cortex: a report of two cases. Fertil Steril. 2005;84:1018.e1-1018.e3. doi: 10.1016/j.fertnstert.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 84. Diaz-Garcia C, Domingo J, Garcia-Velasco JA, et al. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril. 2018;109:478-485. doi: 10.1016/j.fertnstert.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 85. Forman EJ. Ovarian tissue cryopreservation: still experimental? Fertil Steril. 2018;109:443-444. [DOI] [PubMed] [Google Scholar]
- 86. Carrillo L, Seidman DS, Cittadini E, Meirow D. The role of fertility preservation in patients with endometriosis. J Assist Reprod Genet. 2016;33:317-323. doi: 10.1007/s10815-016-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]