Abstract
The source of polycystic ovarian syndrome (PCOS) is much debated and is likely to be multifactorial. There is an apparent familial inheritance with first-degree relatives of sufferers more likely to be affected. Twin studies have suggested a genetic cause but candidate genes are yet to be verified. Genes affecting insulin resistance, steroid hormone production, and inflammatory cytokine responses have all been implicated. Current thinking supports the theory that exposure to environmental factors in utero predisposes a female foetus to hyperandrogenism, insulin resistance, and polycystic ovaries in adult life. Which environmental factors have an impact on the foetus and the mechanisms of exposure are still to be confirmed. Animal studies have shown a clear correlation between hyperexposure of the foetus to androgens in utero and future development of a PCOS pattern of symptoms. Placental aromatases should neutralise androgens from the maternal circulation and prevent them reaching the foetal circulation. Our hypothesis is that the high maternal anti-Mullerian hormone (AMH) levels in PCOS block the placental aromatase and allow passage of testosterone through the placenta. This maternal testosterone acts on the foetal ovaries and ‘programmes’ them to recruit more preantral follicles and so produce higher AMH levels when they become functional at around 36 weeks of gestation. The high AMH concentrations in PCOS also seem to increase luteinizing hormone release and inhibit follicle stimulating hormone action on aromatase, so adding to the hyperandrogenic environment of adult PCOS.
Keywords: PCOS, AMH, androgens, placenta, ovary
Introduction
Polycystic ovarian syndrome (PCOS) affects 8%–18%1,2 of women of reproductive age. It is a syndrome with varied presentation and clinical history. While many sufferers are diagnosed in adolescence when investigated for symptoms such as persistent acne, hirsutism, oligomenorrhoea, or amenorrhoea,3–5 other women may be ignorant of their diagnosis until such time as they seek assistance with conception. For example, March et al2 showed as many as 70% of women identified as having PCOS in their study were previously undiagnosed.
Women with PCOS may experience symptoms such as hirsutism, acne, and fertility difficulties. They also have increased risks of metabolic syndrome6 and insulin resistance leading to cardiovascular disease and type 2 diabetes.5,7 Psychological impacts of the syndrome include increased rates of depression, eating disorders, and anxiety.8–12
The burden of this syndrome on health services is significant, involving fertility treatments, management of metabolic conditions and cardiovascular complications, obesity-related concerns, and associated psychological elements.
Obesity has been linked with PCOS ever since the first description by Stein and Leventhal in 1935. Obesity and increasing body weight can exacerbate all the symptoms and signs of the syndrome,13 and as obesity becomes more common, the diagnosis of PCOS based on clinical symptoms has also increased. It is well documented that weight loss of even 5%–10% can improve symptoms and restore ovulatory menstrual cycles in affected women.13 It is also important to acknowledge the population of lean women with PCOS who are also hyperandrogenic and anovulatory and may be the most insulin resistant, irrespective of body mass index (BMI).14
The criteria for diagnosis have been revised several times and there is no universally accepted version. The most widely used are the Rotterdam criteria, which specify that for diagnosis of PCOS a woman must have 2 out of the following 3 criteria; oligomenorrhoea or amenorrhoea, biochemical or clinical evidence of hyperandrogenism (specifically hirsutism and acne), and typical ultrasound characteristics of a polycystic ovary.
The other most commonly utilised diagnostic classification is the American National Institutes of Health (NIH) system which defines PCOS as a combination of hyperandrogenism and ovulatory dysfunction.15
Diagnosis of this syndrome is important for research as correct identification of subjects is vital for accuracy and the different sets of diagnostic criteria can complicate this. It is also important to identify women with the diagnosis so that their metabolic and cardiovascular health can be monitored and optimised given the associated risks. There is no doubt that the better we can understand the aetiology of this syndrome, the more likely we are to be able to effectively treat or prevent it in the future.
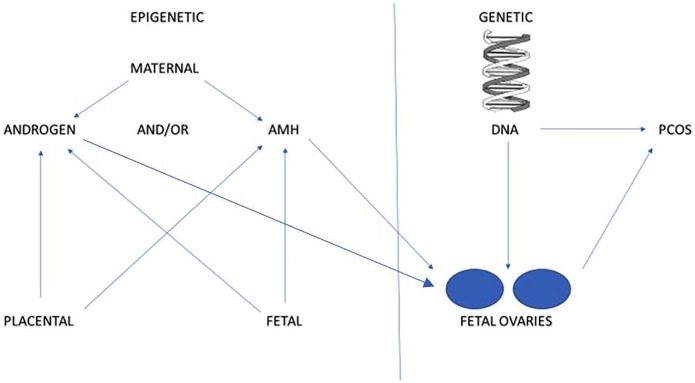
Differences in diagnostic criteria are one of the factors hampering the search for the aetiology of this syndrome. Theories regarding pathogenesis exist supporting the possibility of genetic inheritance and for epigenetic alterations related to hormone exposure. There may well be interactions between genetic factors and environmental factors and research is ongoing into these areas (Figure 1).
Figure 1.
Potential sources of PCOS.
AMH indicates anti-Mullerian hormone; PCOS, polycystic ovarian syndrome.
Genetics and Inheritance
There is clear evidence that first-degree female relatives of women with PCOS are more likely to have the syndrome themselves16 and that there are seemingly familial patterns of inheritance of the syndrome.17 The search for candidate genes or polymorphisms is ongoing and yet to elucidate a clear answer. The genes that have been nominated as candidates tend to fall into four different categories; those related to insulin resistance, those related to androgen biosynthesis and actions, those responsible for inflammatory cytokine responses, and others.18
Although some small studies have suggested an autosomal dominant inheritance pattern,19,20 family studies and twin studies have shown confounding patterns of inheritance and these patterns do not fit a Mendelian inheritance pattern.21 One twin study comparing monozygotic and dizygotic twins showed a concordance of diagnosis of 63% for the monozygotic and 67% for the dizygotic pairs (no significant difference).22 A larger Dutch study showed higher correlation between PCOS diagnoses in monozygotic twin pairs compared with dizygotic and singleton sisters. However, the diagnostic criteria were based on a threshold from a model and relied on self-reported characteristics rather than medical diagnoses.23
More recently, genome-wide association studies (GWAS) have found 16 different genetic loci observed in groups with self-reported PCOS and that fit the NIH diagnostic criteria.24 The largest meta-analysis to date, looking at more than 113 000 women, compared results between the diagnostic groups – Rotterdam, NIH, and self-reported PCOS. This study identified 14 genetic loci independently associated with risk for PCOS which applied to all diagnostic groups and remained after adjustment for age and BMI. There were two possible candidate genes discovered which were both endocrine-related.25
Interestingly, there is a link shown between genetic predisposition to high BMI and PCOS and evidence that insulin resistance is an independent risk factor. Genetic variants have also been associated with PCOS and separately with depression which may help explain the high incidence of depression within the PCOS population. The genetic loci related to PCOS have links to neuroendocrine, metabolic, and reproductive pathways and links with the genetic associations of menopause, metabolic disorders, depression, and male-pattern balding.25
Literature regarding the search for candidate genes or genetic loci related to PCOS is inconclusive and evidence supporting the relationship between loci/genes and the clinical picture is still far from conclusive.26
Epigenetic Theories
Epigenetic changes could explain the difference illustrated in the twin studies combined with the genetic loci identified above. If external factors alter the expression of these genes then this could contribute to PCOS pathogenesis and symptom development. One plausible theory is that hyperexposure to androgens in utero programmes the expression of foetal genes, which then destine the foetus to develop polycystic ovaries, hyperandrogenism, insulin resistance, and hormonal profiles in keeping with the syndrome in adult life.26–29
The Barker hypothesis was first described in 1990 when a study showed a link between birthweight and cardiovascular disease risk as an adult.30,31 The hypothesis was that the prenatal environment altered gene expression and ‘programmed’ future health. With regard to PCOS, the theory is that exposure to excess androgens in utero could have epigenetic consequences and effectively programme a female foetus to develop PCOS later in life.26,31 These effects are thought to be the direct consequence of the actions of androgens on the expression of genes controlling ovarian steroid production, folliculogenesis, gonadotropin-releasing hormone (GnRH) pulsatility, and insulin resistance.32
The theory of prenatal androgen exposure resulting in metabolic changes, insulin resistance, and a PCOS has mostly been developed in response to various animal studies where pregnant mothers were exposed to increased steroid hormones.31,33 These studies have shown consistent results across various animal species including sheep,33 monkeys,27,31 mice,34 and rats.35
The studies on rats were first published in the 1970s and showed disordered follicle recruitment and ovulatory patterns in rats that had been exposed to testosterone prenatally.36 Other studies have demonstrated similar effects of androgen exposure on rodents.34,35 Although these studies help validate the hypothesis of foetal androgen exposure being the trigger for ‘programming’ offspring towards a PCOS, rodents have different gonadal development patterns and ovulatory cycles which limits the utility of these results with regard to human application.32
Padmanabhan and Veiga-Lopez33 suggest that sheep are an excellent choice of research subject because their organ development follows a more rapid but similarly sequenced trajectory. The foetus of a sheep starts the process of gonadal differentiation at 30 days (humans 45–62 days) and has antral follicles present in the foetal ovary by day 135 (humans day 230). A meta-analysis of the results of different sheep studies was performed and showed that the timing of testosterone treatment was important in influencing the consequences – If testosterone was given too early in the gestation period, virilisation of the genitalia occurred.33
Ovarian biopsies have been performed in androgen-treated sheep and were found to have lower levels of primordial follicles and higher numbers of primary follicles suggesting increased recruitment.37 Ultrasound demonstrated higher numbers of antral follicles, persistence of follicles, and increased follicular recruitment.38,39 Insulin resistance was also seen in sheep as early as 5 weeks after birth continuing into adulthood.33,40 In summary, the sheep that had been prenatally exposed to testosterone demonstrated all the metabolic changes required to fit a human diagnosis of PCOS.
Regarding fertility, it was noted that male sheep, when mixed with prenatally androgen-exposed sheep and the control offspring, preferentially chose to mate with the control group.41
Abbott et al31 studied rhesus monkeys and injected pregnant mothers with testosterone at one of two stages during their pregnancies. When followed up to puberty and beyond, the offspring of the injected monkeys demonstrated irregular menstruation, high serum luteinizing hormone (LH) levels, insulin resistance, and polycystic ovaries.
Clearly there will never be human studies replicating the animal research that exposes pregnant mothers to exogenous steroid hormones. Assessing the endogenous hormone levels in the foetal circulation or environment is challenging. Studies have investigated androgen levels in amniotic fluid and have illustrated raised amniotic testosterone in the second trimester of foetuses with PCOS mothers42 suggesting increased production of androgen. Cord blood samples have also been tested but study results have been varied.43,44 It may be possible to quantify in utero androgen exposure by using foetal sebum levels as a surrogate marker45 but this needs further research.
The difficulty in linking maternal testosterone or androgen levels to foetal programming towards a PCOS picture is the placenta. As Franks32 asks, where does the excess androgen originate and what is the mechanism of it reaching and affecting the foetus? Normally, it would be expected that maternal testosterone could not cross the placenta as sex hormone–binding globulin should bind testosterone and render it inactive and the placenta has high levels of aromatase enzymes that convert androgens to oestrogens. This mechanism has long been attributed to the protection of the foetus from a hyperandrogenic state. However, in the placental tissue of women with PCOS, reduced p450 aromatase and increased androgen-producing enzyme activity have been shown.46 These changes in the placenta of a mother with PCOS could facilitate hyperexposure of the foetus to maternal androgens.
It is possible that the ovary is genetically predisposed to hypersecrete androgens both during foetal development and from puberty.27,47 However, as described above, no candidate genes have been validated despite extensive investigation.
Anti-Mullerian Hormone
A more recent development in this search for clarification of the aetiology of PCOS is the involvement of anti-Mullerian hormone (AMH).
AMH has previously been known as the hormone responsible for foetal sex differentiation. More recently, AMH has been found to be secreted by the granulosa cells of the ovarian follicles.48 Polycystic ovaries have a larger number of follicles and granulosa cell mass29 and also a higher AMH secretion per granulosa cell49 leading to high AMH concentrations in women with PCOS.
There is a correlation between the severity of symptoms of PCOS, specifically oligomenorrhoea, amenorrhoea, and polycystic ovarian morphology50 and increased serum levels of AMH. This lends credence to the possibility that AMH is not only a marker of the syndrome but potentially a contributor to the pathogenesis.51 AMH prevents follicular recruitment and has been shown to be as much as 18 times higher in anovulatory women with PCOS compared with normo-ovulatory controls.52
AMH inhibits the actions of follicle stimulating hormone (FSH) and blocks aromatase activity leading to a reduction in estradiol levels and a prevention of multifollicular development, promoting monofollicular ovulation at normal physiological levels.52 The higher levels associated with PCOS exaggerate this response leading to anovulatory cycles.
Tata et al53 showed that these high AMH levels persisted and increased throughout pregnancy in women with PCOS compared with weight-matched, non-PCOS controls. This led to the hypothesis that AMH levels were implicated in the prenatal foetal ovarian ‘programming’ that results in PCOS later in life.
Animal studies have been designed to test this hypothesis. Pregnant mice injected with AMH demonstrated increased testosterone production, reduced placental metabolism of testosterone to estradiol and offspring with a predisposition to PCOS characteristics after puberty. The explanation suggested was that AMH stimulated GnRH neurons leading to increased LH pulsatility.53 Certainly, very high LH levels are more often associated with normal weight PCOS women compared with obese women and the highest AMH concentrations were found in normal weight PCOS subjects.53
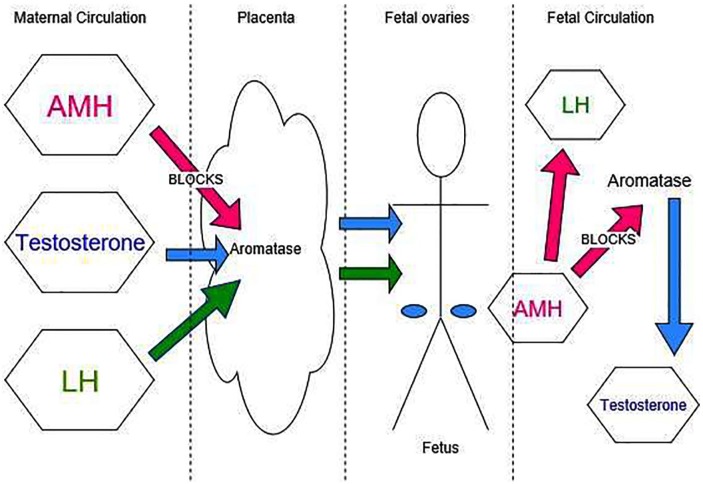
Given this information, it may be possible that, in women with high AMH concentrations, the placental aromatases are blocked, allowing passage of maternal testosterone across the placenta. Alternatively, the high LH levels produced in response to the increased AMH could cross the placenta. Exposure to one or both of these hormones could be responsible for the epigenetic alteration in development of the foetal ovary. If the ovaries then began to produce foetal AMH which in turn blocked aromatase activity, this could further increase the androgen exposure of the foetus to foetal testosterone.
Research into the tissue of foetuses from 6 weeks gestation and into infancy suggest that AMH is first expressed in male foetuses from 8.5 weeks gestation.54 The same study did not find AMH in female foetuses until 36 weeks gestation. This supports the theory that maternal AMH is not present in the foetal circulation (if it crossed the placenta this would likely happen from an earlier gestation and hence result in differentiation of gonads to testes rather than ovaries).
The early developing foetal ovary may be susceptible to epigenetic alterations in gene expression as a consequence of exposure to testosterone. It is possible that maternal testosterone encourages a response in the ovarian granulosa cells and in a positive feedback cycle, increases androgen production. Granulosa cell sensitivity to AMH would then continue throughout the reproductive life span of this offspring.
In summary, the aetiology of PCOS is still not well understood. The theory that circulating hormones coming from maternal or placental sources have an epigenetic impact and a ‘programming’ effect is plausible but difficult to prove.
The hypothesis posed by the authors is that high circulating maternal AMH levels reduce the activity of aromatase P450 in the placenta. Reduced aromatase activity combined with high maternal circulating testosterone levels allows increased placental passage and hyperexposes the foetus to androgens. The foetus in turn produces increased androgens and LH. This then has an epigenetic effect on the developing ovaries as corroborated by animal studies, predisposing them to intrauterine growth restriction, and the metabolic dysfunction, insulin resistance, and oligo-ovulatory menstrual cycles of PCOS (Figure 2).
Figure 2.
Suggested mechanisms of foetal hormone exposure.
AMH indicates anti-Mullerian hormone; LH, luteinizing hormone.
Better understanding of the mechanism by which the foetus is exposed to high androgen levels will allow research into potential treatments to reduce the risk of PCOS.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Idea conceived by RH, drafted by CR and refined by both.
ORCID iD: Roy Homburg  https://orcid.org/0000-0003-3863-2831
https://orcid.org/0000-0003-3863-2831
References
- 1. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685-697. [DOI] [PubMed] [Google Scholar]
- 2. March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551. [DOI] [PubMed] [Google Scholar]
- 3. Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among south Asians and Caucasians: is there a difference. Clin Endocrinol. 2002;57:343-350. [DOI] [PubMed] [Google Scholar]
- 4. Azziz R, Sanchez L, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89: 453-462. [DOI] [PubMed] [Google Scholar]
- 5. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Hum Reprod. 2012;27:14-24. [DOI] [PubMed] [Google Scholar]
- 6. Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95:2038-2049. [DOI] [PubMed] [Google Scholar]
- 7. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type II diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347-363. [DOI] [PubMed] [Google Scholar]
- 8. Merikangas KR, Spence A, Kupfer DJ. Linkage studies of bipolar disorder: methodologic and analytic issues. Arch Gen Psychiatry. 1989;46:1137-1141. [DOI] [PubMed] [Google Scholar]
- 9. Klipstein KG, Goldberg JF. Screening for bipolar disorder in women with polycystic ovary syndrome: a pilot study. J Affect Disord. 2006;91:205-209. [DOI] [PubMed] [Google Scholar]
- 10. Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril. 2007;87:1369-1376. [DOI] [PubMed] [Google Scholar]
- 11. Rassi A, Veras AB, dos Reis M, et al. Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Compr Psychiatry. 2010;51:599-602. [DOI] [PubMed] [Google Scholar]
- 12. Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2012;97:225-230. [DOI] [PubMed] [Google Scholar]
- 13. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol. 2007;67:735-742. [DOI] [PubMed] [Google Scholar]
- 15. Zawadzki JK, Dunaif A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. Boston, MA: Blackwell Scientific Publications; 1992. [Google Scholar]
- 16. Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first degree relatives of patients with PCOS. Fertil Steril. 2001;75:53-58. [DOI] [PubMed] [Google Scholar]
- 17. Azziz ed R. The Polycystic Ovary Syndrome: Current Concepts on Pathogenesis and Clinical Care. New York, NY: Springer; 2007:1-15, 29-42. [Google Scholar]
- 18. Deligeoroglou E, Kouskouti C, Christopoulos P. The role of genes in the polycystic ovary syndrome: predisposition and mechanisms. Gynecol Endocrinol. 2009;25:603-609. [DOI] [PubMed] [Google Scholar]
- 19. Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84:38-43. [DOI] [PubMed] [Google Scholar]
- 20. Carey AH, Chan KL, Short F, White D, Williamson R, Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol. 1993;38:653-658. [DOI] [PubMed] [Google Scholar]
- 21. Lunde O, Magnus P, Sandvik L, Hoglo S. Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Invest. 1989;28:23-30. [DOI] [PubMed] [Google Scholar]
- 22. Jahanfar S, Eden J, Warren P, Seppala M, Nguyen T. A twin study of polycystic ovary syndrome. Fertil Steril. 1995;63:478-486. [PubMed] [Google Scholar]
- 23. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91: 2100-2104. [DOI] [PubMed] [Google Scholar]
- 24. Day FR, Hinds DA, Tung JY, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovarian syndrome. Nat Commun. 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Day FR, Karaderi T, Jones MR, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnostic criteria. PLoS Genet. 2018;14:e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Filippou P, Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23:421-432. [DOI] [PubMed] [Google Scholar]
- 27. Abbott DH, Dumesic DA, Franks S. Developmental origins of polycystic ovary syndrome – a hypothesis. J Endocrinol. 2002;174:1-5. [DOI] [PubMed] [Google Scholar]
- 28. Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278-285; discussion 286. [DOI] [PubMed] [Google Scholar]
- 29. Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017-1021. [DOI] [PubMed] [Google Scholar]
- 30. Barker DJ. The fetal and infant origins of adult disease. Br Med J. 1990;301:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz JE, Nestler JE, Dewailly D, eds. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. Totowa, NJ: Humana Press; 2006: 259-272. [Google Scholar]
- 32. Franks S. Animal models and the developmental origins of polycystic ovary syndrome: increasing evidence for the role of androgens in programming reproductive and metabolic dysfunction. Endocrinology. 2012;153:2536-2538. [DOI] [PubMed] [Google Scholar]
- 33. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101:7129-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fels E, Bosch LR. Effect of prenatal administration of testosterone on ovarian function in rats. Am J Obstet Gynecol. 1971;111:964-969. [DOI] [PubMed] [Google Scholar]
- 36. Parker CR, Jr, Mahesh VB. Interrelationship between excessive levels of circulating androgens in blood and ovulatory failure. J Reprod Med. 1976;17:75-90. [PubMed] [Google Scholar]
- 37. Forsdike RA, Hardy K, Bull L, et al. Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol. 2007;192:421-428. [DOI] [PubMed] [Google Scholar]
- 38. Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve and ovarian morphology in sheep. Biol Reprod. 2009;80:726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally androgenised lambs. Mol Cell Endocrinol. 2001;185:51-59. [DOI] [PubMed] [Google Scholar]
- 40. Recabarren SE, Padmanabhan V, Codner E, et al. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289:E801-E806. [DOI] [PubMed] [Google Scholar]
- 41. Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007;67:459-467. [DOI] [PubMed] [Google Scholar]
- 42. Palomba S, Marotta R, Di Cello A, et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol. 2012;77:898-904. [DOI] [PubMed] [Google Scholar]
- 43. Barry JA, Kay AR, Navaratnarajah R, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30:444-446. [DOI] [PubMed] [Google Scholar]
- 44. Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95:2180-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Homburg R, Gudi A, Shah AM, Layton A. A novel method to demonstrate that pregnant women with polycystic ovary syndrome hyper-expose their fetus to androgens as a possible stepping stone for the developmental theory of PCOS. Reprod Biol Endocrinol. 2017;15:61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maliqueo M, Lara HE, Sanchez F, Echiburu B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166:151-155. [DOI] [PubMed] [Google Scholar]
- 47. Cole B, Hensinger K, Maciel Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006;91:3654-3661. [DOI] [PubMed] [Google Scholar]
- 48. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92: 240-245. [DOI] [PubMed] [Google Scholar]
- 49. Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456-4461. [DOI] [PubMed] [Google Scholar]
- 50. Homburg R, Ray A, Bhide P, et al. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod. 2013;28:1077-1083. [DOI] [PubMed] [Google Scholar]
- 51. Homburg R, Crawford G. The role of AMH in anovulation associated with PCOS: a hypothesis. Hum Reprod. 2014;29:1117-1121. [DOI] [PubMed] [Google Scholar]
- 52. Pellatt L, Rice S, Mason HD. Anti-Mullerian hormone and polycystic ovary syndrome: a mountain too high. Reproduction. 2010;139:825-833. [DOI] [PubMed] [Google Scholar]
- 53. Tata B, Mimouni N, Barbotin A, et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24:834-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rajpert-De Meyts E, Jørgensen N, Græm N, et al. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of sertoli and granulosa cells. J Clin Endocrinol Metab 1999;84:3836-3844. [DOI] [PubMed] [Google Scholar]