Abstract
Small-bore pleural drainage device insertion has become a first-line therapy for the treatment of pleural effusions (PLEFF) in the intensive care unit; however, no data are available regarding the performance of resident doctors in the execution of this procedure. Our aim was to assess the prevalence of complications related to ultrasound-guided percutaneous small-bore pleural drain insertion by resident doctors. In this single-center observational study, the primary outcome was the occurrence of complications. Secondary outcomes studied were as follows: estimation of PLEFF size by ultrasound and postprocedure changes in PaO2/FiO2 ratio. In all, 87 pleural drains were inserted in 88 attempts. Of these, 16 were positioned by the senior intensivist following a failed attempt by the resident, giving a total of 71 successful placements performed by residents. In 13 cases (14.8%), difficulties were encountered in advancing the catheter over the guidewire. In 16 cases (18.4%), the drain was positioned by a senior intensivist after a failed attempt by a resident. In 8 cases (9.2%), the final chest X-ray revealed a kink in the catheter. A pneumothorax was identified in 21.8% of cases with a mean size (±SD) of just 10 mm (±6; maximum size: 20 mm). The mean size of PLEFF was 57.4 mm (±19.9), corresponding to 1148 mL (±430) according to Balik’s formula. Ultrasound-guided placement of a small-bore pleural drain by resident doctors is a safe procedure, although it is associated with a rather high incidence of irrelevant pneumothoraces.
Keywords: Lung ultrasound, pleural effusion, respiratory failure, intensive care, pleural drainage
Introduction
Critically ill patients who undergo pleural drain insertion have a 10% risk of complications, the consequences of which may be life-threatening.1-4 These adverse events seem to be directly related to the size of the catheter used (the larger the catheter, the more severe the adverse event) and to the experience level of the operator.5-7 Feasibility and periprocedural safety in this context have been analyzed and are the subject of various guidelines, underlining the importance of the training process and the use of reduced caliber drains and point-of-care ultrasound.8-10
Forty-one percent of patients admitted to intensive care unit (ICU) show various degrees of pleural effusion (PLEFF), whereas another 20% will develop it during their stay in the ICU.11-13 Consequently, a successful PLEFF drainage constitutes a fundamental component of patient care and may be required to obtain a diagnosis (even in the case of small effusions) or to increase patient oxygenation by re-expanding a collapsed lung, with subsequent benefits in ameliorated patient symptoms and fatigue.14,15 However, many doubts persist about the actual effectiveness of this procedure in the ICU setting and the timing of the procedure (early or delayed), and data from large controlled clinical trials are still lacking. Although the placement of a pleural drain is on the core curriculum of an intensivist, many are not certified in execution proficiency as no universal standards exist regarding certification.
Our residents in Anesthesia and Intensive Care Medicine undergo both theoretical and practical courses, and their competences are repeatedly tested in a simulator-based environment until they achieve adequate proficiency before dealing with real patients (under continuous expert supervision). We, therefore, hypothesized that the prevalence of complications in ultrasound-guided small-bore pleural drain insertions performed by our residents would be relatively low, residing within the bottom end of the range of complication rates reported in the literature.
The primary aim of this observational study was to assess the prevalence of complications related to ultrasound-guided percutaneous small-bore pleural drain insertion. Secondary objectives were to study the correlation between thoracic ultrasound (TUS) estimation of PLEFF and the amount of fluid drained and to analyze patient respiratory outcomes after the procedure.
Materials and Methods
Study population
This was a single-center observational retrospective study, approved by our Institutional Ethics Committee on October 5, 2017, protocol number #12489, in which the principal investigator is LV. Given its retrospective design, patient consent was waived, but we respected the European Privacy Regulation 2016/679 on General Data Protection Regulation.
All participating patients had been admitted to the ICU of the tertiary care academic hospital (Azienda Sanitaria Universitaria Integrata) of Udine, Italy; this ICU is primarily, but not exclusively, dedicated to postsurgical patients and liver transplant recipients. All patients had received a percutaneous small-bore pleural drain placed by a senior resident (with the supervision of an intensivist with at least 5 years of experience) using the Seldinger technique. Exclusion criteria were the following: chest drain already in place at the time of admission, surgical placement of a thoracic drain, loculated effusion, hemothorax, and patient age <18 years.
The decision regarding whether to place the drain was made on clinical grounds alone and was not protocol-driven. The correct placement of the catheter was verified by an anterior-posterior chest X-ray obtained by a radiologist, with 12 years of experience, blinded to the procedure. Pneumothorax was measured, using the available chest X-ray projection (anteroposterior, taken in the ICU), at the level that showed the largest distance in millimeter between the outer margin of the collapsed lung and the chest wall. Measurements were performed using electronic calipers on images displayed on a picture-archiving and communication system (SuitEstensa; Esaote, Genoa, Italy). An incorrect drain position was defined as the drain being inserted too deeply into the pleural cavity (ie, catheter tip exceeding half of the hemithorax) and/or the presence of an abnormal kink in the catheter along its path from the subcutaneous tissue into the pleural space.
Ultrasound techniques
Lung ultrasound was used to evaluate and quantify PLEFF and performed according to Balik’s instructions.16 The patient was placed in the supine position with mild torso elevation (15°). The size of the PLEFF was measured in millimeters (Sep mm) as the maximal distance at end-expiration between visceral and parietal pleura along the posterior axillary line with the probe in transverse position. The effusion volume was estimated using the formula: V (mL) = 20 × Sep (mm). We have previously described the methodology used in a step-by-step manner17 as well as the importance of correct patient positioning.18 A Philips Envisor C 1.2 (Philips, Andover, MA, USA) ultrasound device with a curvilinear 2 to 5 MHz probe was used.
Education and simulator-based training
Our residency training involves a hands-on educational program in which theoretical knowledge provided by experienced assessors through frontal lessons19 (Supplemental material 1) is combined with simulator-based practical learning using an Ultrasound Thoracentesis Model THM-30 (Simulab, Seattle, WA, USA)—this manikin is used to practice the execution of both landmark-guided and ultrasound-guided thoracentesis techniques. Residents are evaluated by calculating a score developed to assess the proficiency acquired in this procedure and which we also use to continually assess our teaching methods.20
Outcome measures and data collection
The primary outcome was the occurrence of complications (including malpositioning) in small-bore pleural drain positioning. Secondary outcome measures were PLEFF size estimation by TUS, the amount of fluid drained, and the postprocedure change in PaO2/FiO2 ratio.
A register was prepared to record the following information: demographic data, reasons for ICU admission, type of ventilation, blood gas analysis, drainage side, TUS effusion size (measured as described above), and any difficulties encountered during the procedure. Following placement of the small-bore pleural drain, the amount of fluid drained in mL and the length of ICU stay were recorded. To minimize the possibility of bias in PLEFF estimated and the volume actually drained, a lung ultrasound examination was performed in the hours following the procedure as a control.
Statistical analysis
As described in the literature, the prevalence of complications during chest tube placement stands at around 10%.1 We calculated a sample size of at least 71 patients to detect the prevalence of complications with an estimated accuracy of 86% (estimated error ± 7%) and a confidence level of 95%.
Descriptive statistical analysis was performed for the main study variables: means and standard deviations were calculated for quantitative variables, and absolute and relative frequencies were calculated for qualitative variables. The 95% confidence interval was also calculated when relevant and applicable. The prevalence of complications was calculated. Univariate t tests were performed to evaluate differences between independent variables. The differences in proportions between categorical data were evaluated using the Fisher test. The effusion estimate was calculated using Balik’s formula: V (mL) = 20 × Sep (mm).15 To analyze the correlation between 2 variables, both the Pearson correlation coefficient r and Spearman rho were calculated, as differences between the two can provide additional information. The amount of PLEFF estimated by ultrasound was compared with the actual amount drained using the Bland and Altman method. We calculated both the Spearman correlation coefficient and the concordance correlation coefficient as correlation indices of the 2 variables. To evaluate whether the size of the PLEFF or the extent of improvement in PaO2/FiO2 ratio was able to predict patient respiratory outcome following thoracic drain positioning, receiver operating characteristic (ROC) curves and the area under the respective ROC (AUC-ROC) curves were calculated, as were values for sensitivity and specificity. All tests were 2-tailed and P values <.05 were considered significant. Statistical analysis was performed using a specially designed Microsoft Excel 2010 spreadsheet (Microsoft, Redmond, WA, USA) and GraphPad Prism software version 6.01 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Study population
A total of 71 patients were enrolled and a total of 87 small-bore pleural drains were positioned during the study period. Thirteen patients (18.3%) required the insertion of 2 chest tubes, and 2 other patients (4.2%) required 3 catheters during their ICU stay. In total, 88 attempts to insert a small-bore pleural drain were made, 87 of which were successful. Of these, 16 were positioned by the senior intensivist following a failed attempt by the resident, giving a total of 71 successful placements performed by residents. The demographic characteristics of the study population are shown in Table 1. In our ICU, mean patient age was 69 ± 11.55 years and advanced cardiac and/or pulmonary disease plus end-stage liver disease were present in 57.7% of all cases. The ICU mortality rate in this study was 28%—greater than the mean value reported in a recent European multicentre cohort study (23.9%).21
Table 1.
Demographic data and patient characteristics.
| Total patients (N) | 71 |
| M/F | 41/30 (58%) |
| Mean age, y | 69.48 ± 11.55 |
| Cause of admission | |
| Postsurgical | 25 (35.2%) |
| Medical | 45 (57.7%) |
| Trauma | 1 (1.4%) |
| ICU LOS, d | 16.45 ± 16.76 |
| Exitus, n | 20 (28.2%) |
| No. of catheters per patient | No. of patients |
| 1 | 55 (77.5%) |
| 2 | 13 (18.3%) |
| 3 | 2 (4.2%) |
Abbreviations: F, female; ICU, intensive care unit; LOS, length of stay; M, male.
Primary results
Out of a total of 87 small-bore pleural drains, 51 (58.6%) were placed in the right hemithorax and 36 (41.4%) in the left. Overall, 71 (81.6%) pig-tail catheters and 16 straight catheters (18.4%) were positioned; bore size was always less than 16F (Table 2).
Table 2.
Site of insertion, catheter type, and complications after drain insertion.
| Drain insertion | ||
|---|---|---|
| Intercostal space | No. (%) | |
| 4-5 | 6 (6.9) | |
| 5-6 | 26 (29.9) | |
| 6-7 | 26 (29.9) | |
| 7-8 | 21 (24.4) | |
| 8-9 | 5 (5.7) | |
| 9-10 | 3 (3.4) | |
| Drain type | ||
| Caliber (F) | Pig-tail n = 71 (81.6%) |
Chest tube n = 16 (18.4%) |
| 8 | 30 (42.2%) | 2 (12.5%) |
| 10 | 36 (50.7%) | 2 (12.5%) |
| 12 | 1 (1.4%) | 7 (43.7%) |
| 14 | 4 (5.7%) | 5 (31.3%) |
| Complications | ||
| Event | No. (%) | 95% CI |
| Abnormal kink | 8 (9.2) | 4.1-17.3 |
| Tip beyond half of hemithorax | 28 (32.2) | 22.5-43.1 |
| PNX | 19 (21.8) | 13.7-31.9 |
| Positioning failure | 1 (1.1) | 0.03-6.1 |
| Positioning failure by resident | 17 (19) | |
Abbreviations: CI, confidence interval; PNX, pneumothorax.
In 13 cases (14.8%), difficulties were encountered by the resident during the catheter’s progression over the Seldinger guidewire: 9 of these cases (69.2%) entailed pig-tail catheters, whereas straight catheters were being used in the remaining 4 cases (30.8%). In 16 cases (18.4%), the chest tube was ultimately positioned by the senior intensivist, who took over due to difficulties being encountered by the resident. In only 1 case did the procedure fail (1.1%, 95% CI = 0.03-6.1) for both the resident and the senior intensivist: the placement of a small-bore pleural drain was deemed impossible following 3 failed attempts and the procedure was interrupted. In the failed case, a large tube was subsequently successfully placed by the thoracic surgeon.
In 8 cases (9.2%, 95% CI: 4.0%-17.3%), a successfully positioned catheter showed an abnormal kink in its course through the thoracic wall, as highlighted by chest X-ray, but in only 1 of these 8 cases were difficulties in insertion reported. Small pneumothoraces were found in 21.8% (95% CI: 13.7%-31.9%) of the study population, with a mean size of just 10 ± 6 mm (maximum size: 20 mm); no cases of hemothorax occurred.
Secondary results
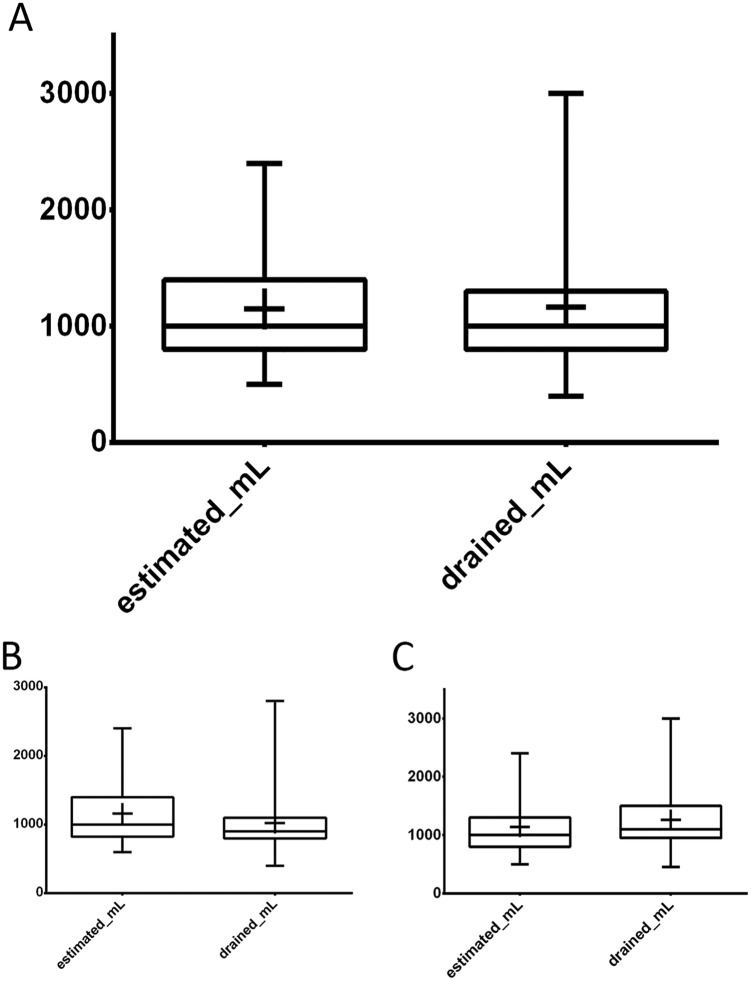
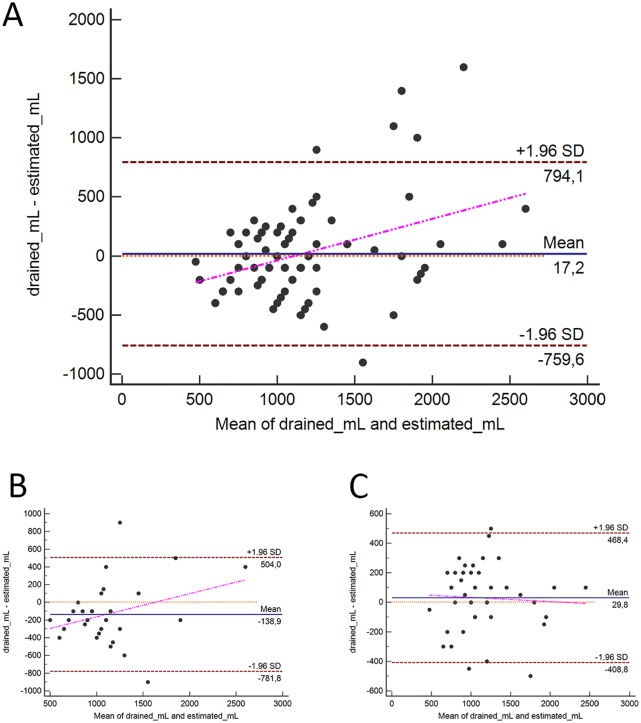
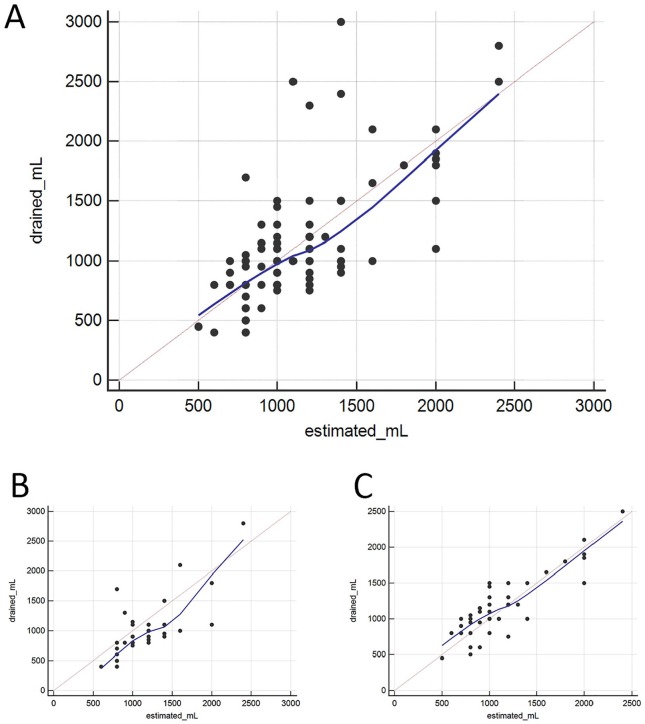
Ultrasound assessment of the PLEFF size was performed in all cases, with an average value of 57.4 ± 19.9 mm, corresponding to 1148 ± 430 estimated mL according to Balik’s formula (Table 3 and Figure 1). The amount of drained fluid following catheter placement was on average 1107 ± 487 mL. The correlation coefficients and Bland-Altman analysis used to evaluate the reliability of Balik’s equation in predicting the amount of drained effusion gave overall positive results. However, a difference was noted between the 2 sides: the correlation for the right hemithorax was significantly better than that for the left hemithorax (Table 3, Figures 2 and 3).
Table 3.
Pleural effusion measures, BA analysis, and correlation coefficient.
| Overall N = 83 |
Right hemithorax n = 47 |
Left hemithorax n = 36 |
|
|---|---|---|---|
| TUS measure, mm | 57.4 ± 19.9 | 56.3 ± 20.4 | 58.1 ± 19.5 |
| Balik’s formula estimate, mL | 1141 ± 406 | 1126 ± 422 | 1162 ± 390 |
| Drained amount, mL | 1098 ± 449 | 1155 ± 411 | 1022 ± 490 |
| BA bias, mL | 43 | −29.8 | 139 |
| 95% LoA, mL | −515 to 601 | −468 to 409 | −504 to 782 |
| CCC | 0.775 | 0.853 | 0.691 |
| Pearson ρ (precision) | 0.783 | 0.856 | 0.745 |
| Bias correction factor Cb (accuracy) | 0.989 | 0.997 | 0.927 |
Abbreviations: BA, Bland-Altman; CCC, concordance correlation coefficient; LoA, limits of agreement; TUS, thorax ultrasound.
Figure 1.
Box and whisker plot showing TUS estimate of the pleural effusion size according to Balik’s formula and the volume in mL actually drained. Colored boxes extend from the 25th to the 75th percentiles; whiskers indicate the minimum and maximum values; plus signs indicate the mean value. (A) whole data set, (B) left hemithorax, and (C) right hemithorax. TUS indicates thoracic ultrasound.
Figure 2.
Bland-Altman plots for measurement comparisons of estimated volumes (mL), according to Balik’s formula, and the volume actually drained: (A) whole data set, (B) left hemithorax, and (C): right hemithorax.
Figure 3.
Scatter diagram of TUS estimated volume and the corresponding actual drained volume for each patient: (A) whole data set, (B) left hemithorax, and (C) right hemithorax.
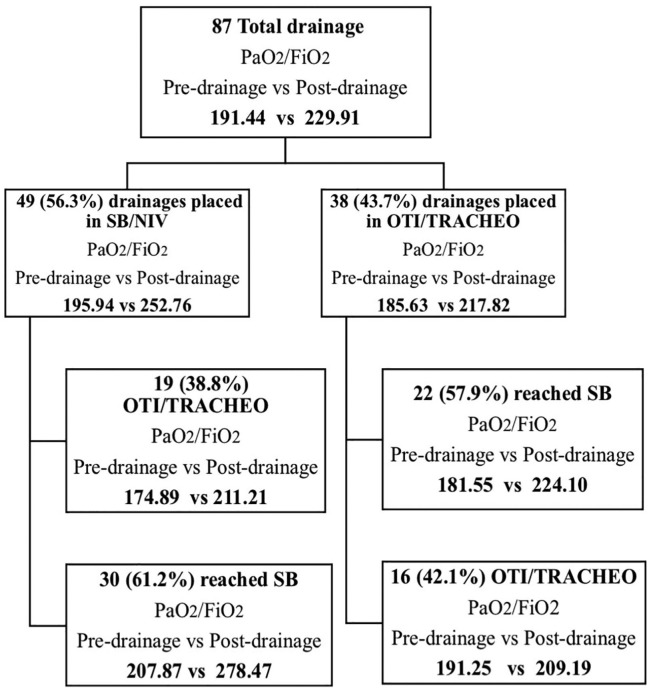
Regarding the blood-gas analysis, the overall PaO2/FiO2 ratio (expressed as mean ± SD) ranged from 191.44 ± 62.41 before pleural drain insertion to 229.91 ± 79.72 after the procedure (P < .0001). Forty-nine (56.3%) small-bore pleural drains were placed in nonintubated patients; the average PaO2/FiO2 ratio before placement was 195.94 ± 63.12 and rose to 252.76 ± 84.26 after drain insertion. Of these patients, 19 (38.8%) were subsequently intubated (orotracheal intubation) after catheter placement, whereas the remaining 30 (61.2%) maintained noninvasive ventilation. In these 30 patients (who were not intubated), the mean PaO2/FiO2 ratio before drain insertion rose from 207.87 ± 67.51 to 278.47 ± 87.11 after catheter positioning (mean of differences: +70.6, P < .0001). Considering the 19 patients who were intubated after drain insertion, the mean PaO2/FiO2 ratios before and after drainage were 174.89 ± 55.71 and 211.21 ± 73.31, respectively, revealing the limited efficacy of the procedure in these cases (mean of differences: +36.3, P = .0354; Figure 4).
Figure 4.
Study flowchart of patient outcome. NIV indicates noninvasive ventilation; OTI, orotracheal intubation; SB, spontaneous breathing; TRACHEO, tracheotomy.
Thirty-eight (43.7%) small-bore pleural drains were positioned in intubated or tracheostomized and mechanically ventilated patients, and the average PaO2/FiO2 ratio rose from 185.63 ± 61.84 to 217.82 ± 69.67. Regarding these 38 patients, 22 (57.9%) achieved spontaneous respiration in the 7 days following the procedure, whereas 16 (42.1%) remained mechanically ventilated. In the 22 patients who were upgraded to spontaneous breathing after drain insertion, the mean PaO2/FiO2 ratios before and after catheter positioning were 181.55 ± 60.62 and 224.10 ± 66.1 (P = .0095), respectively. In the 16 patients who did not achieve spontaneous respiration following chest drainage, the mean PaO2/FiO2 ratios before and after drainage were 191.25 ± 65.03 and 209.19 ± 76.64 (P = .0390), respectively (Table 4). Considering these data, it appears that the patients in whom the drainage was most effective in terms of PaO2/FiO2 ratio increase are also those who did not require intubation or were successfully weaned from mechanical ventilation (Table 4).
Table 4.
Ventilatory outcomes after pleural drainage.
| Spontaneous breathing patients n = 49 (56.3%) |
Mechanically ventilated patients n = 38 (43.7%) |
|||
|---|---|---|---|---|
| Avoided intubation n = 30 |
Subsequently intubated n = 19 |
Successful weaning n = 22 |
Failed weaning n = 16 |
|
| Mean P/F change | 70.6 ± 73.3 | 36.3 ± 69.6 | 42.5 ± 70 | 17.9 ± 31.7 |
| p | 0.11 | 0.199 | ||
| AUC-ROC | 0.623 | 0.632 | ||
| 95% CI | 0.4640-0.7816 | 0.4536-0.8106 | ||
| Cut-off P/F change | ⩾78 | ⩾36 | ||
| Sn | 79% | 81.2% | ||
| Sp | 46.7% | 54.5% | ||
Abbreviations: AUC-ROC, area under the receiver operating characteristic curves; CI, confidence interval; p, paired sample T-T; Sn, sensibility; Sp, specificity.
Finally, we also tested the hypothesis that the amount (in millimeters) of PLEFF measured by means of TUS could predict the patient’s respiratory outcome (maintenance of spontaneous breathing or weaning from mechanical ventilation); this hypothesis was based on the prediction that the greater the volume of PLEFF to drain, the greater the benefit the patient would obtain from receiving a pleural drain. However, the data collected in this study did not show any clear association (AUC-ROC = 0.611 and 0.553 in spontaneous breathing and mechanically ventilated patients, respectively). Furthermore, no correlation was found between quantity (in milliliters) of drained effusion and improvement in respiratory exchanges (Spearman ρ = 0.154, Pearson ρ = 0.181; see Supplemental materials 2-4).
Discussion
The main finding of our study was the absence of serious complications resulting from the positioning of small-bore pleural drains by resident doctors in the ICU, although a rather high number of irrelevant pneumothoraces did occur despite the use of TUS. Small-bore pleural drain insertion is becoming a first-line therapy for the treatment of benign PLEFF in the ICU; it is also used for malignant PLEFF by pulmonologists.22,23 In a recent consensus conference in the United States, experts stated that “pulmonology fellows should perform a minimum of 20 image-guided thoracostomies and 20 indwelling pleural catheter placement procedures annually to obtain standard accreditation.”24 Whereas, for a senior intensivist, the performance of at least 10 procedures per year as baseline should provide procedural supervision proficiency.25 To the best of our knowledge, no such accreditation program exists for resident doctors and intensivists in the Anesthesia and Intensive Care setting. In the University Hospital of Udine, Italy, resident doctors are trained using a phantom model before approaching real patients, and TUS is routinely used to identify the best puncture sites.19,20 It is probable that both these factors help our doctors to become quickly proficient in this procedure. Medical simulators have greatly evolved over the past 10 years, and their use means that hands-on experience with patients can be avoided before minimum skill thresholds are surpassed. Furthermore, e-learning programs with serious games have been shown to be a valid and effective tool in medical education for chest tube insertion. The use of video recording provides us with a new tool that can help make the resident’s curriculum more objective.26,27 Thus, video recording of procedures should be explored as an option in future prospective trials addressing resident doctor training in patient safety. Nevertheless, some clarifications must be made regarding our results: no serious complications occurred, such as hemothoraces or clinically relevant pneumothorax, but 21.8% of our patients developed small, clinically irrelevant pneumothoraces (in the literature, pneumothoraces have been reported to occur in 3.7%-18% of cases when ultrasound is not used).8,28 We must, however, restate that the pneumothoraces never caused any serious problems in the patients, as the mean size of pneumothorax was just 10 ± 6 mm (maximum size: 20 mm). Although the use of TUS seems to be associated with a reduced rate of pneumothorax, in our particular case, we must point out that the low experience level of the residents (the use of TUS makes the procedure longer and more technically demanding) could explain the high incidence rate of small pneumothorax. We can therefore conclude that the skill and experience level of the operator is a variable to which the safety of the maneuver is related, regardless of whether ultrasound is used or not. Moreover, we emphasize the fact that an expert radiologist was involved in the evaluation of the control chest X-rays, so the sensitivity of our study in the diagnosis of pneumothorax, especially small pneumothoraces, was probably very high. That said, the sensitivity of chest X-rays for the detection of pneumothoraces is not very high; thus, it is also possible that that incidence of pneumothoraces has been underestimated in previous studies.29,30 Regarding the rate of drain “malpositioning,” in this study, it occurred at a rate of 1.1%, concordant with literature data.31 We also report that most of the catheters considered malpositioned were placed quite caudally, beyond the fifth intercostal space; this was a result of the use of TUS, which indicated the best position regardless of anatomical landmarks.9
As a secondary outcome, we studied the reliability of PLEFF estimation using TUS data and Balik’s formula. This formula is the most widespread method used in clinical practice to estimate PLEFF but to use it correctly it is very important to consider certain variables such as patient positioning, type of ventilation (mechanical versus spontaneous ventilation), the use of positive-end expiration pressure, and the side of the chest.18,31,32 Using the Bland-Altman analysis to evaluate the reliability of Balik’s equation, we found fair overall agreement between estimated effusion volumes and the volumes drained, but with better results for the right side compared with the left hemithorax. This finding supports the results reported by Vignon et al, who suggested that the heart on the left hemithorax acts like “a stone in a water recipient.”31,32 In our study, the ultrasound-guided placement of a small-bore pleural drain proved to be both a safe and an effective procedure for improving patient respiratory gas exchange, with a mean gain of 46.1 ± 68.0 points in the PaO2/FiO2 ratio (implicating a 22% increase from preprocedural data; a relatively high value compared with other studies).14,33 However, our study did not find a robust association between the level of the PLEFF and the improvement in respiratory gas exchange after chest tube placement, corroborating the conclusions of Dres et al.34 This means that the amount of PLEFF estimated by means of ultrasound cannot be the sole parameter used in the decision-making process regarding the use of a pleural drain.35 Conversely, from our data, it appears that the patients with good outcome (ie, who avoided intubation or were successfully weaned from mechanical ventilation) were those in whom the drain was the most effective in terms of PaO2/FiO2 gain (see Table 4). The positioning of a small-bore pleural drain is thus an important procedure which can induce a positive change in the patient, but deciding which patients are the best candidates for the procedure must always be carefully evaluated. Although our data were not statistically powerful enough to draw any definitive conclusions, they do point toward some interesting statistical tendencies that are certainly worth considering when formulating a predictive index and deserve further study.
Study limitations
Some limitations of our study need to be acknowledged: first of all, this was a single-center, observational study; second, we extensively use lung ultrasound in ICU, and our resident doctors complete an annual internal course with a practical “hands-on” section in which they are required to place a small-bore pleural drain in a phantom model (note that there are no limits in access to this training); although we aimed at including all consecutive patients receiving small chest drains, some are missing from the data set due to the procedures being performed at night or over the weekend. Finally, we recognize the potential for distortion introduced by the resident doctor underreporting the complications encountered during the procedure.
Conclusions
We found ultrasound-guided placement of a small-bore pleural drain by resident doctors to be a safe procedure, although it is associated with a high rate of irrelevant pneumothoraces. The procedure improved patient respiratory gas exchanges, but did not correlate with the maintenance of spontaneous breathing or with weaning from mechanical ventilation. Estimating the amount of PLEFF by ultrasound is necessary to standardize the procedure, and, finally, resident doctor training and proficiency assessment and their supervision by senior intensivists should be formalized.
Supplemental Material
Supplemental material, 2706#Supplemental_material_1 for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental Material
Supplemental material, Suppl_4 for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental Material
Supplemental material, Supp_2_outcome_effusion_pz_VAM_-_weaning for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental Material
Supplemental material, Supp_3_outcome_effusion_pz_RS_-_IOT for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LV, GMG, FB, SD, SD and DO planned the study and drafted the manuscript. LV, GMG, FB, SD, SD, DO and RG collected images, helped develop the bibliography and drafted the manuscript. GV and TB supervised the writing of the manuscript. All the authors read and approved the final manuscript.
ORCID iDs: Luigi Vetrugno  https://orcid.org/0000-0003-3745-8368
https://orcid.org/0000-0003-3745-8368
Daniele Orso  https://orcid.org/0000-0001-7136-0343
https://orcid.org/0000-0001-7136-0343
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Ill Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goltz JP, Gorski A, Bohler J, Kickuth R, Hahn D, Ritter CO. Iatrogenic perforation of the left heart during placement of a chest drain. Diagn Interv Radiol. 2011;17:229-231. doi: 10.4261/1305-3825.DIR.3131-09.0. [DOI] [PubMed] [Google Scholar]
- 3. Bozzani A, Arici V, Bellinzona G, Pirrelli S, Forni E, Odero A. Iatrogenic pulmonary artery rupture due to chest-tube insertion. Tex Heart Inst J. 2010;37:732-733. [PMC free article] [PubMed] [Google Scholar]
- 4. Harris A, O’Driscoll BR, Turkington PM. Survey of major complications of intercostal chest drain insertion in the UK. Postgrad Med J. 2010;86:68-72. doi: 10.1136/pgmj.2009.087759. [DOI] [PubMed] [Google Scholar]
- 5. Kesieme EB, Dongo A, Ezemba N, Irekpita E, Jebbin N, Kesieme C. Tube thoracostomy: complications and its management. Pulm Med. 2012;2012:256878. doi: 10.1155/2012/256878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao M, Hughes R, Papadimos TJ, Stawicki SP. Complications of chest tubes: a focused clinical synopsis. Curr Opin Pulm Med. 2015;21:376-386. doi: 10.1097/MCP.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 7. Filosso PL, Guerrera F, Sandri A, et al. Errors and complications in chest tube placement. Thorac Surg Clin. 2017;27:57-67. doi: 10.1016/j.thorsurg.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 8. Hooper C, Maskell N. British Thoracic Society national pleural procedures audit 2010. Thorax. 2011;66:636-637. doi: 10.1136/thoraxjnl-2011-200077. [DOI] [PubMed] [Google Scholar]
- 9. Havelock T, Teoh R, Laws D, Gleeson F; BTS Pleural Disease Guideline Group Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65:ii61-ii76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 10. Brogi E, Gargani L, Bignami E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care. 2017;21:325. doi: 10.1186/s13054-017-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15:R46. doi: 10.1186/cc10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vetrugno L, Bignami E, Orso D, et al. Utility of pleural effusion drainage in the ICU: an updated systematic review and META-analysis. J Crit Care. 2019;52:22-32. doi: 10.1016/j.jcrc.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 13. Razazi K, Thille AW, Carteaux G, et al. Effects of pleural effusion drainage on oxygenation, respiratory mechanics, and hemodynamics in mechanically ventilated patients. Ann Am Thorac Soc. 2014;11:1018-1024. doi: 10.1513/AnnalsATS.201404-152OC. [DOI] [PubMed] [Google Scholar]
- 14. Cartaxo AM, Vargas FS, Salge JM, et al. Improvements in the 6-min walk test and spirometry following thoracentesis for symptomatic pleural effusions. Chest. 2011;139:1424-1429. doi: 10.1378/chest.10-1679. [DOI] [PubMed] [Google Scholar]
- 15. Talmor M, Hydo L, Gershenwald JG, Barie PS. Beneficial effects of chest tube drainage of pleural effusion in acute respiratory failure refractory to positive end-expiratory pressure ventilation. Surgery. 1998;123:137-143. [PubMed] [Google Scholar]
- 16. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32:318. doi: 10.1007/s00134-005-0024-2. [DOI] [PubMed] [Google Scholar]
- 17. Vetrugno L, Guadagnin GM, Orso D, Boero E, Bignami E, Bove T. An easier and safe affair, pleural drainage with ultrasound in critical patient: a technical note. Crit Ultrasound J. 2018;10:18. doi: 10.1186/s13089-018-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vetrugno L, Bove T. Lung ultrasound estimation of pleural effusion fluid and the importance of patient position. Ann Intensive Care. 2018;8:125. doi: 10.1186/s13613-018-0471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://www.uniud.it/it/didattica/formazione-post-laurea/scuole-di-specializzazione/area-medica/elenco-scuole-di-specializzazione-con-sede-udine/anestesia-rianimazione-e-terapia-intensiva. Accessed March, 2019.
- 20. Vetrugno L, Volpicelli G, Barbariol F, et al. Phantom model and scoring system to assess ability in ultrasound-guided chest drain positioning. Crit Ultrasound J. 2016;8:1. doi: 10.1186/s13089-016-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Capuzzo M, Volta C, Tassinati T, et al. Hospital mortality of adults admitted to Intensive Care Units in hospitals with and without Intermediate Care Units: a multicentre European cohort study. Crit Care. 2014;18:551. doi: 10.1186/s13054-014-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bintcliffe OJ, Lee GYC, Rahman NM, Maskell NA. The management of benign non-infective pleural effusions. Eur Respir Rev. 2016;25:303-316. doi: 10.1183/16000617.0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desai NR, Lee HJ. Diagnosis and management of malignant pleural effusions: state of the art in 2017. J Thorac Dis. 2017;9:S1111-S1122. doi: 10.21037/jtd.2017.07.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mullon JJ, Burkart KM, Silvestri G, et al. Interventional pulmonology fellowship accreditation standards. Chest. 2017;151:1114-1121. doi: 10.1016/j.chest.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 25. Gaba DM, Dunn WF. Procedural risks in thoracentesis: process, progress, and proficiency. Chest. 2009;135:1120-1123. doi: 10.1378/chest.09-0306. [DOI] [PubMed] [Google Scholar]
- 26. Friedrich M, Bergdolt C, Haubruck P, et al. App-based serious gaming for training of chest tube insertion: study protocol for a randomized controlled trial. Trials. 2017;18:56. doi: 10.1186/s13063-017-1799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haubruck P, Nickel F, Ober J, et al. Evaluation of app-based serious gaming as a training method in teaching chest tube insertion to medical students: randomized controlled trial. J Med Internet Res. 2018;20:e195. doi: 10.2196/jmir.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagarsheth K, Kurek S. Ultrasound detection of pneumothorax compared with chest X-ray and computed tomography scan. Am Surg. 2011;77:480-484. [PubMed] [Google Scholar]
- 29. Raja AS, Jacobus CH. How accurate is ultrasonography for excluding pneumothorax. Ann Emerg Med. 2013;61:207-208. doi: 10.1016/j.annemergmed.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 30. Corcoran JP, Psallidas I, Wrightson JM, Hallifax RJ, Rahman NM. Pleural procedural complications: prevention and management. J Thorac Dis. 2015;7:1058-1067. doi: 10.3978/j.issn.2072-1439.2015.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33:1757-1763. doi: 10.1097/01.ccm.0000171532.02639.08. [DOI] [PubMed] [Google Scholar]
- 32. Vetrugno L, Brogi E, Barbariol F, Forfori F, Bignami E. A message in the bottle. Anesthesiology. 2018;128:677. doi: 10.1097/ALN.0000000000002039. [DOI] [PubMed] [Google Scholar]
- 33. Goligher EC, Ferguson ND. Utility of draining pleural effusions in mechanically ventilated patients. Curr Opin Pulm Med. 2012;18:359-365. doi: 10.1097/MCP.0b013e32835395ef. [DOI] [PubMed] [Google Scholar]
- 34. Dres M, Roux D, Pham T, et al. Prevalence and impact on weaning of pleural effusion at the time of liberation from mechanical ventilation. Anesthesiology. 2017;126:1107-1115. doi: 10.1097/ALN.0000000000001621. [DOI] [PubMed] [Google Scholar]
- 35. Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42:1107-1117. doi: 10.1007/s00134-016-4245-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 2706#Supplemental_material_1 for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental material, Suppl_4 for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental material, Supp_2_outcome_effusion_pz_VAM_-_weaning for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Supplemental material, Supp_3_outcome_effusion_pz_RS_-_IOT for Assessment of Pleural Effusion and Small Pleural Drain Insertion by Resident Doctors in an Intensive Care Unit: An Observational Study by Luigi Vetrugno, Giovanni M Guadagnin, Federico Barbariol, Stefano D’Incà, Silvia Delrio, Daniele Orso, Rossano Girometti, Giovanni Volpicelli and Tiziana Bove in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine