Abstract
The cortical responses to auditory stimuli undergo rapid and dramatic changes during the first three years of life in normally developing (ND) children with decreases in latency and changes in amplitude in the primary peaks. However, most previous studies have focused on children above three years of age. The analysis of data during the early stages of development is challenging because the temporal pattern of the evoked responses change with age (e.g. additional peaks emerge with increasing age) and peak latency decreases with age. This study used the topography of the auditory evoked magnetic field (AEF) to identify the auditory components in ND children between 6 and 68 months (n=48). The latencies of the peaks in the AEF produced by a tone burst (ISI 2 ± 0.2 s) during sleep decreased with age, consistent with previous reports in awake children. The peak latencies of the AEFs were compared between the ND children and children with autism spectrum disorder (ASD). Previous studies indicate that the latencies of the initial components of the auditory evoked potential (AEP) as well as the AEF are delayed in children with an ASD compared to age-matched ND children greater than 4 years of age. We asked whether the AEF latencies decrease with age in children diagnosed with ASD as in ND children, but with uniformly longer latencies before about 4 years old. Contrary to this hypothesis, the peak latencies did not decrease with age in ASD group (24-62 months, n=16) unlike the age matched controls during sleep although the mean latencies were longer for ASD compared to the ND group as in previous studies. These results are consistent with previous studies indicating delays in auditory latencies and indicate a different maturational pattern in ASD relative to ND children. Longitudinal studies are needed to confirm whether the AEF latencies diverge with age starting around 3 years of age in these two groups of children.
Keywords: human auditory system, magnetoencephalography (MEG), auditory evoked magnetic field (AEF), auditory evoked potential (AEP), autism spectrum disorders
Introduction
The development of auditory responses has been studied across a broad age range from newborns to adults using both auditory evoked potentials (AEPs) measured with electroencephalography (EEG) and auditory evoked fields (AEFs) measured with magnetoencephalography (MEG) [1-10]. These studies have demonstrated a decrease in peak latency of auditory evoked responses with increasing age in normally developing (ND) children and narrowing of peak widths [1,2,6,9,11-17]. Most of these developmental studies, however, have focused on children older than three years of age or studied a small number of children across a broad age-range, limiting the ability to reliably map developmental patterns in young children. A notable exception is a set of early AEP studies [18-20] which demonstrated a pattern of decreasing latency in children 0-3 years of age measured during sleep [20]. Furthermore, the parameters vary across studies, e.g. understanding the response to changes in pitch [21-23], changing the interstimulus interval [24], or assessing the response to repeated stimuli using the paired click paradigm [25]. Therefore, a gap remains in our knowledge of the maturation of cortical responses to repeated auditory stimuli in ND children before three years of age.
During the first three years of life, AEPs and AEFs show dramatic changes in temporal waveforms and latencies [1,2,6,9,11-17]. These changes present a challenge in identifying the same components so that their developmental trend can be identified. Young children also present a methodological challenge since they may move during testing. Furthermore, children with autism spectrum disorders (ASD) may experience a low tolerance for novel surroundings. Thus, we studied auditory responses while the children slept while controlling for arousal state [26,27] by analyzing responses during sleep. Based on the previous studies we hypothesized a decrease in peak latency with increasing age in ND children.
Most of the auditory studies in children with an ASD have been carried out in children older than 4 years of age. Gage et al. [28] identified a decrease in M100 peak latency with age in 8-16 year old ND children but no change in latency with age in children with an ASD. In the same study, children with an ASD had longer M100 peak latencies than the age-matched controls independent of age. Furthermore, these group differences were most pronounced in the right hemisphere. Bruneau et al. [29] and Cantiani et al. [30] also reported delayed auditory responses in 4-8 year old and 3-7 year old children with an ASD, respectively. Other studies [31-34] using more complex auditory stimuli (language or paired stimuli), provide a mixed view of auditory processing delays with some studies reporting facilitation of the AEF.
Based on the studies focusing on simple repeated auditory tones, we asked whether the AEF latencies decrease with age in children diagnosed with ASD as in ND children, but with uniformly longer latencies before 4-5 years of age. We focused our measurements on the right hemisphere based on prior studies. Our hypotheses were evaluated by assessing the trajectory of the AEF peak latencies with age.
Methods
Participants
This study was approved by the Human Research Review Committee at the University of New Mexico Health Sciences Center and was conducted in compliance with the Declaration of Helsinki. The procedure was described to at least one parent who signed the approved consent prior to the child’s participation. Sixty-four children (48 ND, 16 ASD) between the ages of 6-68 months participated in the study. Control children were ND children, confirmed through parental report and screening with the Ages and Stages age-appropriate questionnaire [35]. Participants were recruited from the community with demographics presented in Table 1. The children diagnosed with an ASD were recruited from the Center for Development and Disability at the University of New Mexico, which specializes in early diagnosis of ASDs. These children underwent diagnostic evaluation by a multidisciplinary team consisting of a pediatrician, clinical psychologist, speech/language therapist, and a physical or occupational therapist. The evaluation included administration of the Autism Diagnostic Observation Schedule (ADOS), other developmental measures (e.g., Mullen Scale of Early Learning, Autism Behavior Checklist, and Social Responsiveness Scale), and parental interviews. The ASD diagnosis was obtained by consensus within this diagnostic team. Drs. Brian Lopez and Dina Hill were the referring clinicians. Of the 16 ASD children, 8 met criteria for Autistic Disorder based on the criteria of an ADOS Communication and Social Interaction score greater than 12 (mean = 17.7, range 14-19).
Table 1.
Demographic Information Across Groups – Mean (Standard Error)
| ND (N = 48) |
ND ≥ 24 m (N = 22) |
ASD ≥ 24 m (N = 16) |
p-value ND vs. ASD ≥ 24 m |
|
|---|---|---|---|---|
| Age (months) Range |
25.5 (2.6) 6 – 68 m |
42.6 (2.6) 26 – 68 m |
40.6 (2.5) 24 – 62 m |
p = 0.59 |
| bsq (N=36): 19.2 (2.5) 6-68 |
bsq (N=11): 38.2 (3.8) 26-68 |
bsq (N=10): 36.4 (2.4) 24-50 |
p = 0.71 | |
| nm (N=12): 44.1 (4.1) 12-62 |
nm (N=11): 47 (3.1) 31-62 |
nm (N=6): 47.4 (4.1) 39-62 |
p = 0.94 | |
| Birth Weight (grams) | 3088 (105) | 3026 (234) | 3474 (204) | p = 0.17 |
| Gestational Age at Birth (weeks) | 39.2 (0.35) | 40.0 (0.6) | 38.3 (1.6) | p = 0.34 |
| Gender (F/M) | 22/26 | 11/11 | 3/13 | p = 0.05 |
Stimuli
Participants were presented with auditory stimuli as a part of a larger study with tactile stimuli presented separately in a randomized block design. The binaural auditory stimuli (tone burst - 800 Hz, 100 ms duration (N=45) or 1000 Hz, 100 ms duration for the data collected (N = 10) using the Elekta MEG system) were presented through an artifact-free speaker located within a magnetically shielded room. The volume was 60 dB SPL measured at the location of the child’s head. All stimuli were presented with an ISI of 2 ± 0.2 s using Neurobehavioral Systems’ Presentation software. The stimuli were presented in 30 trial blocks and were intermixed with tactile stimuli until ~180 trials were obtained for each child or until the child awoke.
babySQUID MEG data collection procedure
Data collection was performed during naptime or night-time sleep with data from most children collected during night-time sleep. The children were allowed to fall asleep naturally and were transferred to the pediatric MEG system (babySQUID, Tristan Technologies, San Diego, CA [36]) located within a magnetically shielded room (MSR) after they were asleep. The children were positioned and supported with pillows, as needed, to obtain measurements over the right hemisphere.
The babySQUID MEG system consisted of 74 sensors arranged in a hemispherical design such that a child may lie down during data collection with their head resting in the reclined bowl (see sensor layout in Fig. 1A). The sensors are located close to the surface of the dewar (7-10 mm) providing measurements very close to the child’s head while the small sensor-to-sensor distance (12-14 mm) provides good spatial resolution. The layout provides coverage of the auditory area without repositioning the head (diameter of the sensor array anterior to posterior is 11.6 cm). AEFs were collected at a sampling rate of 1000 Hz.
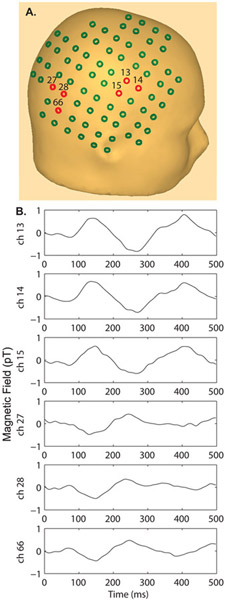
Figure 1.
A) Sensor array layout placed over the right hemisphere of an adult head. With an extent of 11 cm, good coverage of right hemisphere is obtained in children in the 6-50 month age range. Channel numbers are displayed for the waveforms shown below. B) Example single channel auditory evoked fields across 6 channels in one 9 – month old child reveal amplitude reversal (compare channels 13, 14, and 15 vs. 27, 28 and 66) representing the maxima/minima of the evoked response, consistent with a source lying midway between these groups of channels. A head surface template specific to a 9 – month old child is not available and therefore the specific sensor placement over the adult head only approximates the head position of the 9-month old child relative to the sensor array. A pediatric template MRI was used for source localization (Fig. 3) providing good approximation to the head shape. The figure demonstrates that both positive and negative maxima can be captured by the sensor array in young children when the right hemisphere is centered over the array as was done in each child.
Once the child was well positioned relative to the sensor array, a head tool device with three reflective spheres was attached to the child’s head using an elastic band. The three-dimensional positions of fiducial markers and head shape, relative to this head tool device, were collected using a stylus tool measured by the infrared NDI Medical Polaris 3-D tracking device. The tip of the stylus tool is registered relative to the head tool and fiducial points and head shape were recorded using the Polaris tracking device.
During acquisition, one investigator remained in the MSR with the child and observed the child for changes in sleep-state or movement. If the head moved during the measurements, the head position was re-measured and data collection was restarted. Parents were given the choice to remain in the room, but generally observed through the video monitor.
Whole-head Elekta VectorView MEG Data collection
Additional data were collected from 10 children (12 ND, 6 ASD) using the Elekta Neuromag 306 channel whole-head MEG system. Data were again collected while the children slept with an investigator remaining in the MSR for observation of the child. A similar binaural tone stimulus (1000 Hz 100 ms duration with a 10 ms Hanning ramp) was used with the same ISI 2 ± 0.2 s. Head position and fiducial information was obtained using the Polhemus Fastrack 3D tracking device. Head movement was compensated for using continuous head position monitoring available with the Elekta system.
Data Analysis
Only data without gross movement were processed further. Data were scored for sleep-stage (SS) using the EEG criteria of Rechtschaffen and Kales [37] and using a semi-automated program based on the automatic SS classification program for infants outlined by Estevez et al. [38]. Based on these criteria and the observation of the infants and children during data collection, we were able to distinguish between sleep-stages including: rapid eye movement (REM), sleep stage II (SSII), and slow wave sleep (SWS). The REM sleep-stage was identified based on reports of eye movements by the investigator in the room during data collection and using the theta power criterion from Estevez. SSII was identified based on presence of sleep spindles and <20% large amplitude low-frequency delta activity. SWS was determined by greater than 20% low-frequency large amplitude delta activity. Each pattern must persist for one minute to be considered a change in sleep state. We analyzed the data within each sleep stage since previous studies show that characteristics of evoked responses depend on sleep state [26,27]. In this report, we focus on data collected during SSII/SWS, since the majority of sleep was scored as SSII/SWS. After SS scoring, the raw data were filtered using a zero-phase 2-40 Hz band-pass Butterworth filter. Epochs with large amplitude noise due to movement were eliminated prior to averaging. The results of the averaging program provided a plus-minus average as well as even/odd averages. These were inspected for data quality and reproducibility. All trials were then combined into a grand average within sleep stage.
To compare latencies of individual peaks across age, we employed the following strategy. The prominent peaks in the averaged waveform were identified. The contours for the time window, which encompassed all peaks, were viewed in 10 ms intervals. Stable contour plots (consistent pattern across >30 ms), which corresponded with the peaks in the waveform, were saved. We identified 3 reliable peaks across subjects (see supplementary Figure 1) with a consistent reversal of polarity and spatial pattern across the peaks. This pattern of polarity reversals allowed us to compare peaks across individuals over the age range. Once the contour maps with the three major peaks were identified, a representative channel located at the maximum/minimum of the contours was chosen for plotting to show the pattern of development across age. Peak latencies were extracted from this maximum channel using Matlab.
Dipole modeling was performed on representative subjects across the age range to confirm that the analyzed peaks originated from the vicinity of auditory cortex. The Calibrated-Start Spatio-Temporal (CSST) algorithm [39] was used to fit an equivalent current dipole to the data, as described previously [40,41]. The approach employs a nonlinear spatio-temporal multi-dipole modeling technique. The time interval of analysis was customized to the participant to account for the changes in latency with age. Goodness of fit was determined using the reduced chi-square measure [42] with a mean value of 0.6 obtained across fits. Dipole modeling was performed on all subjects run on the Neuromag MEG system and the same peak latencies were extracted based on the auditory source originating in right auditory cortex. The three peak latencies were identified from the auditory source timecourse.
Signal-to-noise ratio (SNR) was calculated using the same maximum channel identified from the contour plots. The baseline noise from −100 to 0 ms was used to establish the noise level. The maximum amplitude in the 50-500 ms time window was used as the amplitude of the signal. SNR was then defined as the maximum signal divided by the standard deviation of the noise.
Statistical Analysis
The distribution of gender by group was tested using a chi-square test to assess proportion of male/female by group (ND vs. ASD). The Type III sum of squares results was reported from the repeated measures general linear model using SPSS 20. The within-subject repeated measure was latency for peaks 1-3 identified using the above approach. Diagnostic category (ND versus ASD) was the between-subject factor and age was the covariate in the model. Analysis of variance (ANOVA) and was used to test group effects. Linear regression was performed to determine the correlation between peak latency and age. The peak latencies were mean-centered relative to the data acquisition system (babySQUID vs. Neuromag) prior to linear regression analysis.
Results
Figure 1 shows the AEFs from a 9-month ND child. Figure 1A shows an example of the placement of the MEG sensors of the babySQUID over a standard head. Figure 1B shows the AEFs at the sensors shown in red. Note, the polarity of the waveforms in the anterior region (ch 13, 14, 15) is opposite of the polarity in the posterior region (ch 27, 28, 66).
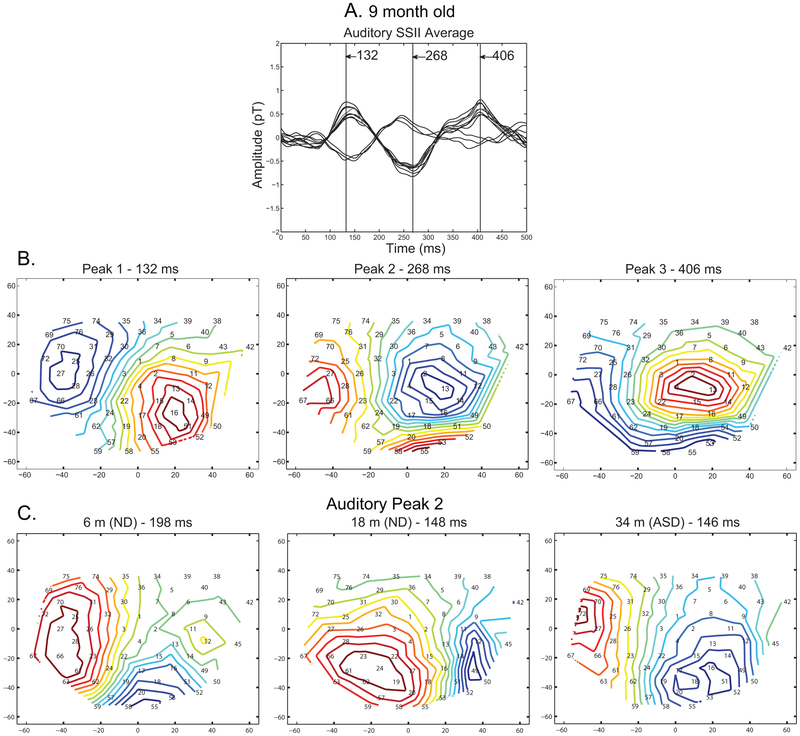
Figure 2A shows these waveforms superimposed on each other. The AEFs typically contained three peaks. In this subject, their peak latencies were 132, 268 and 406 ms after the onset of the tone burst. The temporal waveform and latency varied across the age and the two groups, but these three peaks could be identified from their spatial patterns. Figure 2B shows the spatial pattern of the AEF for the subject shown in Fig. 1 at the peak latency of the three components. For each peak, the magnetic field emerges from the scalp in the warm-color area and returns to the head in the cold-color area. Thus, the neural currents underlying peak 1, 2 and 3 are directed inferiorly, superiorly and inferiorly. These sources would give rise to a negative-positive-negative waveform at the vertex. This pattern was consistently identified across the age range. Figure 2C shows representative contour maps of peak 2 for three subjects (ND 6 m, ND 18 m and ASD 34 m).
Figure 2. Example auditory isocontours.
A) Auditory response for a 9 month-old child in channels representing the positive and negative peak associated with the source denoted by the arrow in the Peak 1 (Peak 1 - 132 ms) contour plot. The vertical lines identify the time points at which contour plots are displayed below. B) The contour plots for the 9 month-old child represent activity measured over the right hemisphere. Therefore, the nose is pointed to the left and the top of the head corresponds to the top of the plot. The first contour showed the positivity on the left side of the array, followed by a negative peak on the left side of the array, followed later by the positive pole again located on the left side of the array. Cool colors represent negative responses and warm colors represent positive responses. A negative response on the left side of the array corresponds with a downward pointing source. C) Contour maps of Peak 2 across individuals. Again, the top of the head is at the top of the array and the nose is pointed to the left. Similar dipolar activity is shown across age. The absolute position of these positive and negative poles varies across individuals due to slight changes in head position from child to child. Since the goal of the study was to characterize auditory responses, the right hemisphere was centered in the bowl; therefore, auditory cortex was consistently covered by the array across children.
There were no differences in the number of trials selected for averaging across diagnosis (ANOVA F(1,57) = 0.35, p = 0.56) and no interaction between diagnosis and MEG system (p = 0.53). The average number of trials for ND and ASD children was 65.2±6.9 and 58.3±9.4, respectively. The average SNR was statistically equivalent by group (ANOVA F(1,53) = 0.308, p = 0.58). The average SNR for the ND and ASD children was 9.7±0.85 and 10.5±1.11, respectively.
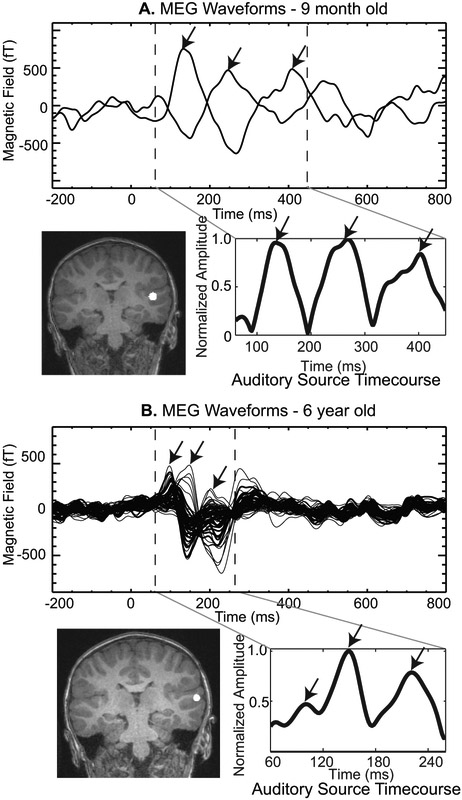
We confirmed in a subset of subjects that the neuronal activity underlying these three components was generated in the auditory cortex. Figure 3A shows the AEF waveforms from two sensors detecting magnetic fields of opposite polarity for the 9 m old child shown in Figs. 1 and 2. The dipole location providing the best fit to the data during the time segment demarcated by the two dashed lines is shown in the coronal section of the template MRI from a child of comparable age. The best fitting current dipole source (white circle in MRI) was located in auditory cortex. The timecourse of the auditory source is shown to the right of the MRI. Figure 3B shows a similar result for a second child. Again the dipole was located in auditory cortex. Source analysis was not performed in all children in this study because accurate head position information relative to the sensor array was not available for all subjects.
Figure 3. Example source analysis of the 1st three peaks.
A. The MEG waveforms for a 9 month old child are shown above with the three peaks identified by arrows and the time interval for source analysis denoted by the vertical dashed lines. Below left is the location of the auditory source projected onto a template child’s MRI. The auditory source timecourse is shown to the right with all three peaks clearly delineated in this auditory source timecourse. B. The MEG waveforms, source location and auditory source timecourse are shown for a 6 year old child also run in this same protocol. Notice the smaller time window used for the source analysis to capture the first three peaks in the older child. The same template child MRI was used to display the source location.
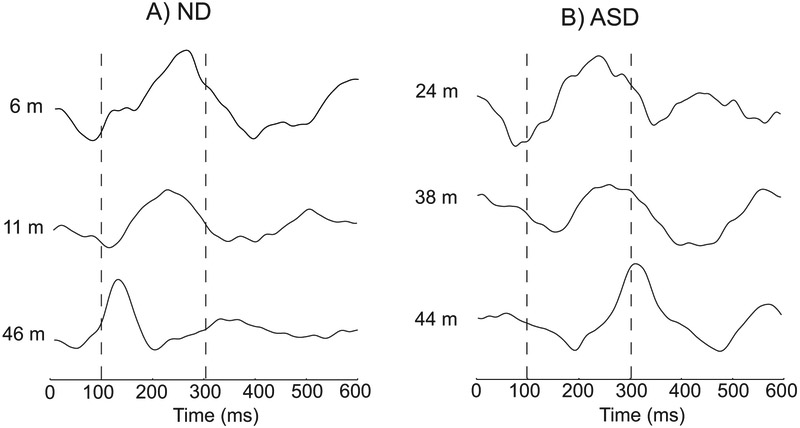
After having developed a method to identify the peaks, we analyzed the latency pattern across the age range. This analysis showed an interesting, unexpected pattern with respect to age between the ND and ASD children. Figure 4 shows an example of this difference in a subset of children. The components of the AEF waveform typically became sharper with shorter latencies with increasing age in ND children (Fig. 4A), whereas the components for the ASD children became sharper as in ND children, but their latencies did not show the same pattern of decreasing with age (Fig. 4B). This contrast can be seen clearly for peak 2 with the positive deflection in Fig. 4.
Figure 4. Example waveforms across age.
The channel with the maximal auditory response is shown across age to demonstrate the change in latency in the waveform view. One can see a change in latency with age for each of the peaks. A) Example waveforms across age in ND children. The channel which best represented the three peaks was chosen for visualization purposes. B) Example waveforms across age in ASD children. Good quality auditory responses were obtained in these children as evidenced by an equivalent SNR across diagnosis. The latencies tended to increase with age in ASD children.
Figure 5 shows the latencies with respect to age for all children studied. In the ND children (green circles), there was a clear decrease in latency with age across all three peaks. In the ASD children (blue diamonds), however, there was no clear decrease. The latencies for ASD children were quite similar to those for the ND children around the age of 24 months and diverged with increasing age. This was the case for all three components. This difference in trajectory was contrary to our initial expectation.
Figure 5. Peak latencies for all participants across age.
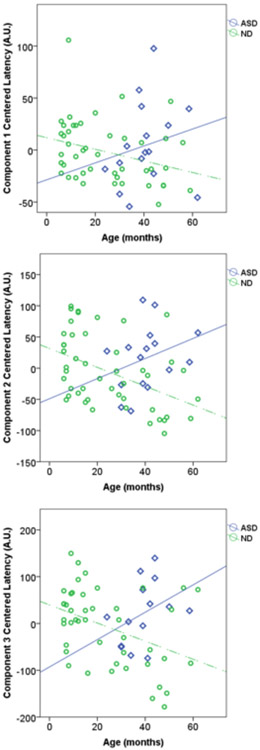
A) Component 1 plot of age versus latency for all participants. B) Component 2 plot of age versus latency for all participants. C) Component 3 plot of age versus latency for all participants. The values were mean-centered relative to the MEG system specific mean peak latency to account for a systematic delay between the MEG systems. These plots clearly show the decrease in latency across all individuals with increasing age for the ND children. At the same time the children with an ASD show a different pattern of development than the healthy control children.
We tested whether the latency decreases with age in the ND group. The negative slope of the linear regression curve was significantly different than zero for all three components (see Table 2). There was no such pattern for the ASD children (see Table 2) with the regression slopes for all three components being statistically equivalent to a zero slope. This decrease in latency in ND children shows that the evoked response latency decreases with age when measured during sleep, consistent with the same trajectory reported in earlier studies in children in the awake state. A between-subject repeated measures ANCOVA was performed for children greater than 24 months of age (age-matched to ASD group) and the interaction of age by diagnostic category was significant (F(2,29) = 5.5; p = 0.009). This is consistent with the difference in regression slopes by diagnosis seen in Fig.5. As reported in Table 2, the slope of the ASD regression curves was not statistically different from zero.
Table 2.
Regression Variables Peak Latency relative to Age
| Regression between Age and Peak Latency | ||||||
|---|---|---|---|---|---|---|
| Diagnosis | Peak | slope | R | R2 | t-statistic | p-value |
| ND (N = 46) | 1 | −0.56 | −0.31 | 0.10 | −2.13 | 0.039* |
| 2 | −1.51 | −0.45 | 0.20 | −3.39 | 0.001* | |
| 3 | −1.94 | −0.41 | 0.17 | −2.96 | 0.005* | |
| ASD (N = 15) | 1 | 0.81 | 0.20 | 0.04 | .77 | 0.45 |
| 2 | 1.61 | 0.31 | 0.10 | 1.24 | 0.24 | |
| 3 | 2.92 | 0.38 | 0.15 | 1.43 | 0.18 | |
Significant at p < 0.05
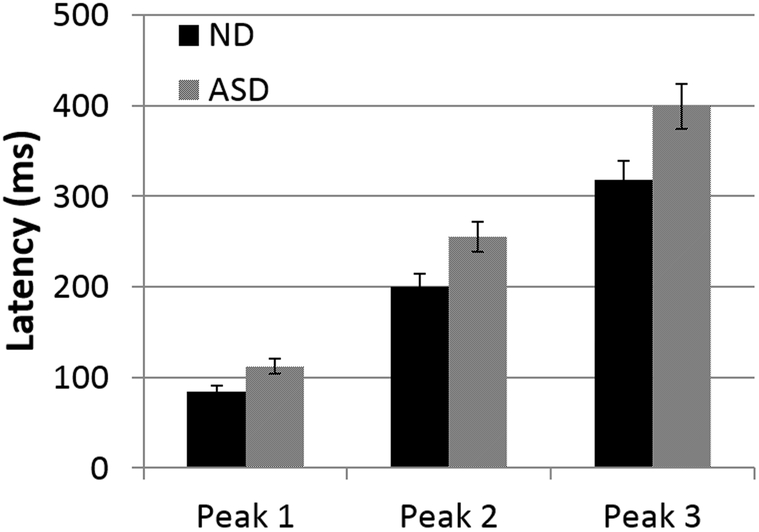
We then compared the latencies between the groups after equating for age (≥24 months, N=22 for ND and N = 16 ASD). There was no significant difference in mean age, birth weight, or gestational age between this subgroup of ND children vs. the ASD group (Table 1). Figure 6 shows the average latencies for all children in this subgroup, separately for the three peaks. A repeated measures ANOVA with peak (peaks 1, 2 and 3) as the repeated measure and diagnosis (ND ≥ 24 m versus ASD) as the between-subject factor showed that the mean latencies differed by group (F(1,27) = 11.5, p = 0.002). The peak by diagnosis interaction was also significant (F(1.4,37.8) = 5.46; p = 0.016). This is consistent with the previous studies reporting longer latencies for ASD compared to ND children in older groups. A repeated measures ANCOVA analysis with diagnosis (ND versus ASD) and gender as factors and age as a covariate with peak (peak 1, 2, and 3) as the repeated measure showed that gender did not contribute significantly to the model so it was excluded. There was also no significant interaction between diagnosis and MEG system (p = 0.95) or sleep stage (0.52).
Figure 6. Average peak latencies in children >24 months of age.
Repeated measures ANOVA confirmed a group effect of increased latency in the ASD group relative to the ND children. There was no significant peak x group interaction or group x system (babysquid vs. Neuromag) interaction.
Discussion
Decrease in auditory evoked response latency with age in ND children
The present study reports a decrease in auditory evoked peak latencies with increasing age in ND preschool aged children when measured during sleep. Few studies have focused on children in the 6-month to 4-year age range [1,2,6,9,11,15-17,22-24,43,44] with the exception of Lippe et al. [9], Paetau et al. [1] and Ohlrich et al. [20]. While Paetau and colleagues studied children from 0.3 months through to adulthood, the focus was across a wide age range with only a few preschool aged children (one 3 month old child and three children aged 3-5 years). Lippe and colleagues studied 40 children between 1 month and 5 years providing the closest comparison to the current study; although there are still limitations to direct comparisons based on differences in measurement technique (EEG versus MEG) and stimulus parameters. Unlike Lippe et al. [9], we used a longer ISI (1.2 s versus 2 s for the current study), which enhances the traditional N1 peak in young children [1]. Our results identify a peak consistent with N1 morphology, whereas Lippe et al. [9] and Yoshimura et al. [43] (ISI <1 s) did not identify a reliable N1 peak in their data. Ceponiene et al. [45], using a 700 ms interstimulus interval, and Putkinen et al. [24] using an ISI of 800 ms also reported only two peaks with a latency less than 300 ms in their youngest 4 and 2 year old participants, respectively. Our results show multiple peaks in the AEF (see Figs. 1 and 3) similar to Van Zuijen et al. [23] who studied 17 month old children with a 3.3 s ISI. Despite these differences, each study identified a consistent number of peaks across subjects when using a consistent ISI. Our results are consistent with prior studies indicating a linear decrease in latency with increasing age. Our results provide complementary results to the previous studies by showing that the same decrease in latency with age can be captured during sleep. This provides an important control for arousal state when studying children across the ASD spectrum who may not easily tolerate the laboratory environment.
However, while Wunderlich and Cone-Wesson [10] also found multiple peaks in neonates to 6 years with ~3 s ISI, they did not report a decrease in latency with age in children <6 years. However, Wunderlich and Cone-Wesson only reported group differences using 3 age groups (infant - <7 days, toddler - 13-41 months, child – 4-6 years) instead of including age as a covariate in their statistical model. Wunderlich and Cone-Wesson acknowledged considerable variability of the evoked responses within age group, which may have masked the pattern of decreased latency with age across the 13-41 month age range. An additional confound in the Wunderlich and Cone-Wesson study was that the newborn group was tested asleep whereas all other groups were tested awake.
The current results extend the linear pattern demonstrated by Wakai and colleagues [46] in neonates to 6 months of ageto a broader time window up to 5 years of age. Furthermore, our source analysis, demonstrating that the three peaks originate from the auditory cortex, are also consistent with the results of Ortiz-Mantilla et al. [22] who confirmed that both peaks identified in 6 month old children originated from auditory cortex.
Differences in peak latencies between ND and ASD children
The second major finding is that peak latencies in the age-matched groups greater than 24 months were longer for the ASD compared to the ND group. Our results for children between 24 and 68 months of age are consistent with prior ASD results showing a delay in the right hemisphere M100 response in children with ASD relative to ND children [47]. Edgar et al. [48] recently replicated the result of delayed auditory latencies in ASD children relative to ND children in a large sample. Furthermore, Bruneau et al. [29] found a delayed N100 latency in children with an ASD aged 4-8 years.
The remaining studies of ASD in children in the 3-8 year age range have focused on more complex responses such as responses to novel auditory stimuli [32,49], language stimuli [33] or changes in volume. These results were mixed with Orekhova et al.[31] reporting normal latencies in high functioning ASD individuals and other reports of faster latencies in children with an ASD [31] [32]. Brennan et al. [34] also did not report a delay in auditory latencies in response to linguistic sound stimuli suggesting that more complex stimuli may mask simple sensory processing delays. Yoshimura et al. [33] reported a lack of association between the verbal evoked P50 peak latency and language ability, but a decrease in P50 latency with increasing age in ASD children, in contrast to the results obtained in the age- and sex-matched normally developing control group. The variability in the ASD studies suggest that a systematic testing of stimulus parameters is needed to better understand how auditory processing is affected in ASD. Furthermore, the review by Kikuchi et al. [50] supports the interpretation that variability within the ASD spectrum may also explain the variability in latency results across the literature.
Different hemispheric effects have also been reported in the studies discussed above. The current study focused on right hemisphere based on Gage et al. [28,51] reporting group differences in right hemisphere, whereas left hemisphere latency change with age for ASD children was consistent with the ND rate of −4ms/year. In contrast, two ERP studies [32,52] reported abnormalities in left hemisphere in ASD children using a mismatch negativity paradigm. This focus on auditory change detection may account for the differences with the current study. Ferri and colleagues also reported shorter N1 latencies in 12 year old children with ASD with no right hemisphere abnormalities. It is important to note that based on the physiology of the auditory system, the AEP N1 component measured at Cz is a combination of left and right hemisphere responses. As mentioned by Roberts et al. [53], the contrast between MEG [28] and ERP [52] studies may be related to difficulty in separating left/right hemisphere responses in ERP studies [54]. Huang et al. [54] demonstrated that MEG is better at identifying hemispheric differences in early auditory responses.
Furthermore, a contrasting, divergent pattern of latency trajectories was observed. Although peak latency decreased with age in ND children, the slope of the linear regression curve for ASD children was not statistically different from zero. The diverging trend in the maturational pattern of auditory response latency in the two groups raises an important question. It is possible that peak latencies are similar between the ND and ASD groups around the age of 24 months. However, this cross-sectional study cannot rule out the possibility that the younger children with ASD will exhibit normal development of the AEF instead of stagnation of the AEF latencies as indicated by nonsignificant slope of the ASD regression line. Alternatively, it is possible that individuals with ASD will follow the cross-sectional developmental pattern representing little change in latency with age consistent with Gage et al. [28]. Gage and colleagues [28] reported that the latency of the M100 peak in ASD children did not change significantly with age across 8-16 years, whereas the ND M100 latencies decreased with age. However, a follow-up longitudinal study from Roberts and colleagues [55] determined that latencies in children with ASD decreased at an equivalent rate as ND children, but were delayed relative to ND children at both the initial and follow-up time points. Therefore, the nonsignificant slope in the ASD may be explained by natural variation within this spectrum disorder. This would be consistent with the finding described by Port [55]indicating that the lesser affected children with ASD are associated with reduced latency delays. Longitudinal studies are needed in this younger age range to determine the developmental pattern within individuals to better determine whether latency differences change with age and if they may indicate severity across the age spectrum.
Taken together these results further support the role of sensory deficits as a core feature of autism spectrum disorders. Recent results demonstrating both sensory and motor deficits in a rat model of autism spectrum disorder [56] provide additional support for the role of sensory processing as a core feature of the broader disorder. Understanding the role of sensory deficits in higher cognitive functioning remains an important goal in autism research [57,58].
Finally, the current study differs from previous studies since the AEFs were obtained while the children were sleeping. We collected MEG data during sleep for three primary reasons. First, collecting data during sleep allowed this study to test children across the ASD spectrum, including 8 children who met criteria for Autistic Disorder. This is in contrast to most neuroimaging studies which only recruit children with high-functioning ASD to ensure task compliance. Interestingly, a similar study [30] examining nonverbal or minimally verbal children with ASD also reported a 10 ms delay in auditory evoked potentials relative to controls in children 3-7 years of age, consistent with the current study. Second, it is now recognized that attention directly influences basic sensory responses [59,60]. Children with ASD often respond negatively to novel environments which may lead to considerable differences in attentional state between groups. Although differences in sleep have been noted in ASD, these represent a difference in the prevalence of different sleep states rather than changes of the within sleep state architecture [61]. By controlling for sleep state, the effects of attention are more carefully controlled across participants. Third, unlike EEG, MEG sensors are not attached directly to the child’s head. Therefore, with the babySQUID system movement relative to the sensor array would smear the signal spatially. Children even at 6 months of age sleep in one position for 20 minutes or longer before adjusting their position. Recent development of head-motion correction algorithms, which were used for the Neuromag data, partially alleviates this concern. There is a recognized limitation to the collection of data during sleep with regards to interpreting these results relative to findings in older children, yet extending studies to younger children and more severe forms of ASD may necessitate this approach.
Conclusions
The current study supports the hypothesis that the auditory response latencies decrease with age in normally developing (ND) preschool aged children during sleep. In contrast, the peak latencies do not decrease with age in the ASD group (<62 months) unlike the age matched controls, resulting in longer latencies in older children with ASD. Longitudinal studies are needed to determine whether individual children diagnosed with an ASD show abnormal maturational pattern in auditory responses and the consequence of sensory deficits relative to disease pathophysiology.
Supplementary Material
Acknowledgements
We thank Brian Lopez for help with recruitment. The work was funded in part by DE – FG02-08ER64581, University of New Mexico Health Sciences Center CTSC Pilot Grant, NIH P20 RR021938, NIH R21NS072729, NIH R21HD057387, NIH R01AA021771, NIH P50AA022534, and NSF 1539067. We thank the Mind Research Network for access to the magnetically shielded room for data collection.
Abbreviations
- ASD
Autism Spectrum Disorders
- ND
normally developing
- AEF
auditory evoked field
- AEP
auditory evoked potential
- ADOS
Autism Diagnostic Observation Schedule
- SWS
slow wave sleep
- SS
sleep stage
References
- 1.Paetau R, Ahonen A, Salonen O, Sams M: Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. Journal of Clinical Neurophysiology 1995;12:177–185. [DOI] [PubMed] [Google Scholar]
- 2.Ventura LM, Alvarenga Kde F, Costa Filho OA: Protocol to collect late latency auditory evoked potentials. Braz J Otorhinolaryngol 2009;75:879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KL, Nicol T, Zecker SG, Kraus N: Developmental plasticity in the human auditory brainstem. J Neurosci 2008;28:4000–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutter WJ, Maier M, Wakai RT: Development of meg sleep patterns and magnetic auditory evoked responses during early infancy. Clin Neurophysiol 2006;117:522–530. [DOI] [PubMed] [Google Scholar]
- 5.Takeshita K, Nagamine T, Thuy DH, Satow T, Matsuhashi M, Yamamoto J, Takayama M, Fujiwara N, Shibasaki H: Maturational change of parallel auditory processing in school-aged children revealed by simultaneous recording of magnetic and electric cortical responses. Clin Neurophysiol 2002;113:1470–1484. [DOI] [PubMed] [Google Scholar]
- 6.Gomes H, Dunn M, Ritter W, Kurtzberg D, Brattson A, Kreuzer JA, Vaughan HG Jr, : Spatiotemporal maturation of the central and lateral n1 components to tones. Brain Res Dev Brain Res 2001;129:147–155. [DOI] [PubMed] [Google Scholar]
- 7.Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M: Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol 2002;113:407–420. [DOI] [PubMed] [Google Scholar]
- 8.Ponton CW, Eggermont JJ, Kwong B, Don M: Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials. Clin Neurophysiol 2000;111:220–236. [DOI] [PubMed] [Google Scholar]
- 9.Lippe S, Martinez-Montes E, Arcand C, Lassonde M: Electrophysiological study of auditory development. Neuroscience 2009;164:1108–1118. [DOI] [PubMed] [Google Scholar]
- 10.Wunderlich JL, Cone-Wesson BK: Maturation of caep in infants and children: A review. Hear Res 2006;212:212–223. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Kraus N, McGee TJ, Nicol TG: Developmental changes in p1 and n1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr Clin Neurophysiol 1997;104:540–545. [DOI] [PubMed] [Google Scholar]
- 12.Tonnquist-Uhlen I, Borg E, Spens KE: Topography of auditory evoked long-latency potentials in normal children, with particular reference to the n1 component. Electroencephalogr Clin Neurophysiol 1995;95:34–41. [DOI] [PubMed] [Google Scholar]
- 13.Lauffer H, Miller C, Proschel U, Wenzel D: Simultaneous recording of brainstem and cortical acoustic evoked potentials in children: Methodical aspects and normative data. Eur J Pediatr 1993;152:682–685. [DOI] [PubMed] [Google Scholar]
- 14.Wakai RT, Lutter WJ, Chen M, Maier MM: On and off magnetic auditory evoked responses in early infancy: A possible marker of brain immaturity. Clin Neurophysiol 2007;118:1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrecht R, Suchodoletz W, Uwer R: The development of auditory evoked dipole source activity from childhood to adulthood. Clin Neurophysiol 2000;111:2268–2276. [DOI] [PubMed] [Google Scholar]
- 16.Bruneau N, Roux S, Guerin P, Barthelemy C, Lelord G: Temporal prominence of auditory evoked potentials (n1 wave) in 4–8-year-old children. Psychophysiology 1997;34:32–38. [DOI] [PubMed] [Google Scholar]
- 17.Fuchigami T, Okubo O, Fujita Y, Okuni M, Noguchi Y, Yamada T: Auditory event-related potentials and reaction time in children: Evaluation of cognitive development. Dev Med Child Neurol 1993;35:230–237. [DOI] [PubMed] [Google Scholar]
- 18.Barnet AB, Ohlrich ES, Shanks BL: Eeg evoked responses to repetitive auditory stimulation in normal and down’s syndrome infants. Dev Med Child Neurol 1971;13:321–329. [DOI] [PubMed] [Google Scholar]
- 19.Ohlrich ES, Barnet AB: Auditory evoked responses during the first year of life. Electroencephalogr Clin Neurophysiol 1972;32:161–169. [DOI] [PubMed] [Google Scholar]
- 20.Ohlrich ES, Barnet AB, Weiss IP, Shanks BL: Auditory evoked potential development in early childhood: A longitudinal study. Electroencephalogr Clin Neurophysiol 1978;44:411–423. [DOI] [PubMed] [Google Scholar]
- 21.Hamalainen JA, Ortiz-Mantilla S, Benasich AA: Source localization of event-related potentials to pitch change mapped onto age-appropriate mris at 6 months of age. Neuroimage 2011;54:1910–1918. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz-Mantilla S, Hamalainen JA, Benasich AA: Time course of erp generators to syllables in infants: A source localization study using age-appropriate brain templates. Neuroimage 2012;59:3275–3287. [DOI] [PubMed] [Google Scholar]
- 23.van Zuijen TL, Plakas A, Maassen BA, Been P, Maurits NM, Krikhaar E, van Driel J, van der Leij A: Temporal auditory processing at 17 months of age is associated with preliterate language comprehension and later word reading fluency: An erp study. Neurosci Lett 2012;528:31–35. [DOI] [PubMed] [Google Scholar]
- 24.Putkinen V, Niinikuru R, Lipsanen J, Tervaniemi M, Huotilainen M: Fast measurement of auditory event-related potential profiles in 2–3-year-olds. Dev Neuropsychol 2012;37:51–75. [DOI] [PubMed] [Google Scholar]
- 25.Hutchison AK, Hunter SK, Wagner BD, Calvin EA, Zerbe GO, Ross RG: Diminished infant p50 sensory gating predicts increased 40-month-old attention, anxiety/depression, and externalizing symptoms. Journal of attention disorders 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pihko E, Lauronen L, Wikstrom H, Taulu S, Nurminen J, Kivitie-Kallio S, Okada Y: Somatosensory evoked potentials and magnetic fields elicited by tactile stimulation of the hand during active and quiet sleep in newborns. Clinical Neurophysiology 2004;115:448–455. [DOI] [PubMed] [Google Scholar]
- 27.Lutter WJ, Wakai RT, Maier MM, Baryshnikov BV: Meg sleep pattern dependence of auditory evoked fields in young infants. Neurology & Clinical Neurophysiology 2004;2004:77. [PubMed] [Google Scholar]
- 28.Gage NM, Siegel B, Roberts TP: Cortical auditory system maturational abnormalities in children with autism disorder: An meg investigation. Brain Research Developmental Brain Research 2003;144:201–209. [DOI] [PubMed] [Google Scholar]
- 29.Bruneau N, Roux S, Adrien JL, Barthelemy C: Auditory associative cortex dysfunction in children with autism: Evidence from late auditory evoked potentials (n1 wave-t complex). Clin Neurophysiol 1999;110:1927–1934. [DOI] [PubMed] [Google Scholar]
- 30.Cantiani C, Choudhury NA, Yu YH, Shafer VL, Schwartz RG, Benasich AA: From sensory perception to lexical-semantic processing: An erp study in non-verbal children with autism. PLoS One 2016;11:e0161637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M: Sensory gating in young children with autism: Relation to age, iq, and eeg gamma oscillations. Neurosci Lett 2008;434:218–223. [DOI] [PubMed] [Google Scholar]
- 32.Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N: Hypersensitivity to acoustic change in children with autism: Electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology 2002;39:577–584. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura Y, Kikuchi M, Shitamichi K, Ueno S, Munesue T, Ono Y, Tsubokawa T, Haruta Y, Oi M, Niida Y, Remijn GB, Takahashi T, Suzuki M, Higashida H, Minabe Y: Atypical brain lateralisation in the auditory cortex and language performance in 3- to 7-year-old children with high-functioning autism spectrum disorder: A child-customised magnetoencephalography (meg) study. Molecular autism 2013;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan JR, Wagley N, Kovelman I, Bowyer SM, Richard AE, Lajiness-O’Neill R: Magnetoencephalography shows atypical sensitivity to linguistic sound sequences in autism spectrum disorder. Neuroreport 2016;27:982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Squires J, Bricker D: Ages and stages questionnaires, third edition, asq-3, ed 3rd Baltimore, MD, Brookes Publishing Co, 2009. [Google Scholar]
- 36.Okada Y, Pratt K, Atwood C, Mascarenas A, Reineman R, Nurminen J, Paulson D: Babysquid: A mobile, high-resolution multichannel magnetoencephalography system for neonatal brain assessment. Rev Sci Instrum 2006;77:24301–24309. [Google Scholar]
- 37.Rechtschaffen A, Kales (eds): A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects, Bethesda, MD, National Institutes of Health Publication, 1968, vol 204. [Google Scholar]
- 38.Estevez PA, Held CM, Holzmann CA, Perez CA, Perez JP, Heiss J, Garrido M, Peirano P: Polysomnographic pattern recognition for automated classification of sleep-waking states in infants. Med Biol Eng Comput 2002;40:105–113. [DOI] [PubMed] [Google Scholar]
- 39.Ranken D, Best E, Stephen J, Schmidt D, George J, Wood C, Huang M: Meg/eeg forward and inverse modeling using mriview; in Nowak H, Haueisen J, Gießler F, Huonker (eds): Proceedings of the 13th international conference on biomagnetism. Berlin, VDE Verlag, 2002, pp 785–787. [Google Scholar]
- 40.Stephen J, Kodituwakku P, Kodituwakku EL, Romero L, Peters AM, Sharadamma NM, Caprihan A, Coffman BA: Delays in auditory processing identified in preschool children with fasd. Alcoholism: Clinical and Experimental Research 2012;36:1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephen JM, Montano R, Donahue CH, Adair JC, Knoefel J, Qualls C, Hart B, Ranken D, Aine CJ: Somatosensory responses in normal aging, mild cognitive impairment, and alzheimer’s disease. J Neural Transm 2010;117:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Supek S, Aine CJ: Simulation studies of multiple dipole neuromagnetic source localization: Model order and limits of source resolution. IEEE Trans Biomed Eng 1993;40:529–539. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura Y, Kikuchi M, Shitamichi K, Ueno S, Remijn GB, Haruta Y, Oi M, Munesue T, Tsubokawa T, Higashida H, Minabe Y: Language performance and auditory evoked fields in 2- to 5-year-old children. Eur J Neurosci 2012;35:644–650. [DOI] [PubMed] [Google Scholar]
- 44.Plakas A, van Zuijen T, van Leeuwen T, Thomson JM, van der Leij A: Impaired non-speech auditory processing at a pre-reading age is a risk-factor for dyslexia but not a predictor: An erp study. Cortex 2013;49:1034–1045. [DOI] [PubMed] [Google Scholar]
- 45.Ceponiene R, Rinne T, Naatanen R: Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol 2002;113:870–882. [DOI] [PubMed] [Google Scholar]
- 46.Wakai RT, Lutter WJ, Chen M, Maier MM: On and off magnetic auditory evoked responses in early infancy: A possible marker of brain immaturity. Clinical Neurophysiology 2007;118:1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE, Edgar JC: Meg detection of delayed auditory evoked responses in autism spectrum disorders: Towards an imaging biomarker for autism. Autism Res 2010;3:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edgar JC, Fisk Iv CL, Berman JI, Chudnovskaya D, Liu S, Pandey J, Herrington JD, Port RG, Schultz RT, Roberts TP: Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Molecular autism 2015;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orekhova EV, Stroganova TA, Prokofiev AO, Nygren G, Gillberg C, Elam M: The right hemisphere fails to respond to temporal novelty in autism: Evidence from an erp study. Clin Neurophysiol 2009;120:520–529. [DOI] [PubMed] [Google Scholar]
- 50.Kikuchi M, Yoshimura Y, Mutou K, Minabe Y: Magnetoencephalography in the study of children with autism spectrum disorder. Psychiatry Clin Neurosci 2016;70:74–88. [DOI] [PubMed] [Google Scholar]
- 51.Gage NM, Siegel B, Callen M, Roberts TP: Cortical sound processing in children with autism disorder: An meg investigation. NeuroReport 2003;14:2047–2051. [DOI] [PubMed] [Google Scholar]
- 52.Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G: The mismatch negativity and the p3a components of the auditory event-related potentials in autistic low-functioning subjects. ClinNeurophysiol 2003;114:1671–1680. [DOI] [PubMed] [Google Scholar]
- 53.Roberts TP, Schmidt GL, Egeth M, Blaskey L, Rey MM, Edgar JC, Levy SE: Electrophysiological signatures: Magnetoencephalographic studies of the neural correlates of language impairment in autism spectrum disorders. Int J Psychophysiol 2008;68:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, Paulson KM, Weisend MP, Irwin JG, Bustillo JR, Adler LE, Miller GA, Canive JM: Predicting eeg responses using meg sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol 2003;114:835–850. [DOI] [PubMed] [Google Scholar]
- 55.Port RG, Edgar JC, Ku M, Bloy L, Murray R, Blaskey L, Levy SE, Roberts TP: Maturation of auditory neural processes in autism spectrum disorder - a longitudinal meg study. NeuroImage Clinical 2016;11:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds S, Millette A, Devine DP: Sensory and motor characterization in the postnatal valproate rat model of autism. Dev Neurosci 2012;34:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanz-Cervera P, Pastor-Cerezuela G, Fernandez-Andres MI, Tarraga-Minguez R: Sensory processing in children with autism spectrum disorder: Relationship with non-verbal iq, autism severity and attention deficit/hyperactivity disorder symptomatology. Res Dev Disabil 2015;45–46:188–201. [DOI] [PubMed] [Google Scholar]
- 58.Tomchek SD, Little LM, Dunn W: Sensory pattern contributions to developmental performance in children with autism spectrum disorder. Am J Occup Ther 2015;69:6905185040p6905185041–6905185010. [DOI] [PubMed] [Google Scholar]
- 59.Giabbiconi CM, Trujillo-Barreto NJ, Gruber T, Muller MM: Sustained spatial attention to vibration is mediated in primary somatosensory cortex. Neuroimage 2007;35:255–262. [DOI] [PubMed] [Google Scholar]
- 60.Mozolic JL, Joyner D, Hugenschmidt CE, Peiffer AM, Kraft RA, Maldjian JA, Laurienti PJ: Cross-modal deactivations during modality-specific selective attention. BMC Neurol 2008;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL: Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep 2006;29:1563–1571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.