Abstract
Synthetic biology combines engineering and biology to produce artificial systems with programmable features. Specifically, engineered microenvironments have advanced immensely over the past few decades, owing in part to the merging of materials with biological mimetic structures. In this review, we adapt a traditional definition of community ecology to describe “cellular ecology”, or the study of the distribution of cell populations and interactions within their microenvironment. We discuss two exemplar hydrogel platforms: (1) self-assembling peptide (SAP) hydrogels and (2) Poly(ethylene) glycol (PEG) hydrogels and describe future opportunities for merging smart material design and synthetic biology within the scope of multicellular platforms.
Keywords: self-assembly, biomimetic, cellular ecology, multicellular
Introduction
Synthetic biology is a growing and ever-changing field that utilizes biological components to engineer synthetic or artificial systems. Over the past few decades, researchers have continually revised the definition of synthetic biology to broaden the scope as new technologies develop.1 In general, the field of synthetic biology involves the creation of artificial biological systems or the redesign of existing natural biological systems to achieve a functional output.2 Some intriguing and very practical tools have emerged from synthetic biology: some examples include programming E. coll to express recombinant proteins,3 generating induced pluripotent stem cells via genetic manipulation,4 and repurposing the CRISPR/Cas9 machinery for targeted genome editing.5 Many tools in synthetic biology focus on reprogramming the internal environment of the cell, while little attention has been given to the extracellular matrix. We believe the scope of synthetic biology should be broadened to include engineered cellular microenvironments. In fact, others have already suggested that synthetic biology should have its own niche in tissue engineering,6 especially as synthetic materials are now being combined with biologically-derived moieties to control cellular behavior. Finally, we propose that synthetic biology and materials research both have a vital role to play in recreating fundamental aspects of cellular ecology within in vitro cell culture models.
For many types of research, it is no longer enough to use a generic hydrogel scaffold, such as PEG-RGD or collagen, to model specific cellular environments. These scaffolds contain an incomplete picture of the spatiotemporal chemical and mechanical information found in native tissue. Researchers have recognized for some time that material design, cellular microenvironment, tissue dynamics, and architecture all impart meaningful information in cellular platforms. Whereas there are many approaches to engineering cellular environments (e.g. organoid culture, cellular reprogramming, etc.), this paper will discuss how synthetic materials can be used to mimic the extracellular microenvironment. We will examine various hydrogel scaffolds, or 3D constructs containing crosslinked polymers, that prove useful in modeling cellular environments. Whereas other scaffolds exist (e.g. metallic, ceramic, etc.), we will focus on hydrogel constructs for the duration of this paper. We hope, with this paper, the reader will gain an appreciation on the multifaceted nature of engineered cells and their environments, agree with the need for materials to be modified to approximate native tissue environment, and consider a role for materials research in synthetic biology.
Cellular ecology: understanding cellular interactions in their tissue-specific environments
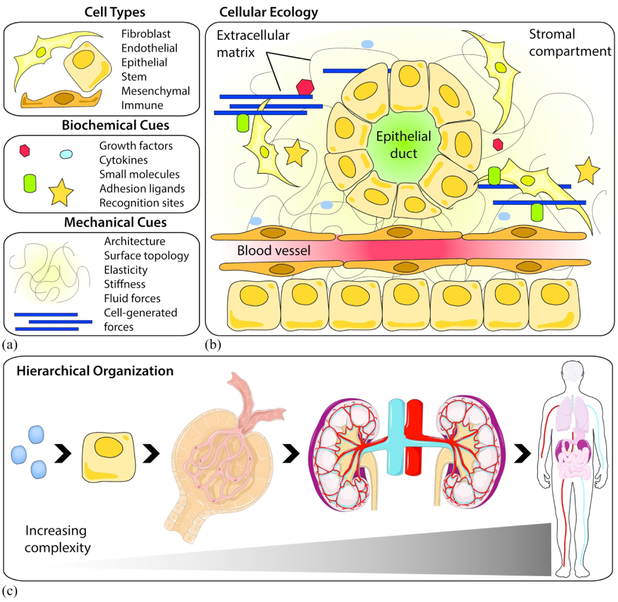
The human body contains approximately 37 trillion cells, made up at least 200 distinguishable cell types.7 All of these cell types reside in tissues with specialized modi operandi that contributes to the overall functionality of the body. Consider for a moment a “cellular ecology”, where a population of cells reside in any given organ. These cells interact with their neighboring cells and the extracellular environment [Fig. 1]. Like the wide range of ecosystems that make up the Earth’s biosphere, the organs that make up an organism are interconnected and diverse. This interaction and diversity cannot be ignored when designing new tissue and cell culture models for regenerative medicine, organogenesis, tissue engineering, and multicellular systems. Cellular ecology encompasses every level of tissue hierarchy, from the single cell that senses its microenvironment, to the spatiotemporal patterning of tissues in an organism [Fig. 1(c)]. Indeed, cellular populations from each organ experience strikingly different biochemical and biophysical cues: the bone and the brain, for example, are defined by a set of completely different material properties, ECM proteins, cell types, and architectures that drive morphogenesis and function. Even the blood vessels that perfuse nutrients through these tissues have completely different architectures and structural requirements. Cellular morphology and function are partially driven by tissue dynamics. Embryonic lung cells experience cyclic strain (stretch) via coordinated contractions,8-10 adult kidney cells sense changes in local fluid flow,11 and cancer cells respond to a stiffening extracellular matrix.12,13 Tissue dynamics and remodeling also play a role in determining large-scale architectural features, which translates to functional outputs. For instance, the force generated by cardiomyocytes to pump blood to the rest of the body or the loading required for bone to support the body’s weight changes throughout an organism’s lifetime. Deviations from coordinated tissue forces generated during development can lead to severe impairments (e.g. lung hypoplasia in congenital diaphragmatic hernia).14 Given the complexity of the cellular environment, we turn to how multidisciplinary research can fulfill unmet needs in engineered cellular platforms.
Figure 1: Components of cellular ecology and its hierarchical organization.
(a) The cellular microenvironment is composed of different cell types, biochemical cues, and mechanicals cues. Each of these components can be arranged in numerous combinations that (b) organize into tissues with unique architectures, structures, and functions. (c) Cellular ecology encompasses all levels of an organism, from single molecules to whole organs. An example of tissue hierarchy is shown as follows: biochemical molecule → epithelial cell → glomerulus → kidneys → human. Some art elements from Servier Medical Art that are registered under a CC BY license.
Synthetic bioactive material design
The extracellular matrix contains a myriad of signaling cues, including biochemical cues from growth factors, cytokines, and adhesion ligands, and mechanical cues from ECM architecture, cell-driven ECM forces, and surface topology. The most commonly used method to recreate the ECM is to fabricate synthetic or natural hydrogels. However, there is mounting evidence that synthetic materials by themselves are not enough to recapitulate cell-matrix interactions because they lack the intrinsic signaling cues of natural materials. And, although natural materials are bioactive, their material properties cannot be easily controlled, nor can their multifunctional signaling cues be decoupled. The batch-to-batch variability of natural material properties alone makes it difficult to reproduce experimental conditions faithfully. Thus, there has been a drive to improve synthetic materials so that they better capture the multifunctional bioactivity found in native ECM. One way to make synthetic materials more appealing to biologists is to design them to be “modular” by utilizing “Click” chemistry. Biorthogonal chemical group click pairs can exist in a sea of functionality found in living systems, without perturbing natural biological processes, and find its complementary click pair with high fidelity and impressive kinetics. This ‘lock and key’ method allows users to leverage click modifications to piece together functionalized “building block” units like LEGO bricks, and build more complex hybrid systems that can be tailored to mimic a specific tissue or cellular function. In this sense, the focus on biomaterials design has evolved from using a “bulk” material to a more modular “building block” design.
Although there are numerous strategies and novel materials platforms available, we discuss two exemplar hydrogel platforms that—taken together—involve many of the chemical modifications, characteristics, and biomedical applications currently accessible in the field. The platforms that we have identified with good applicability in synthetic biology include: (1) those that employ self-assembling peptides (SAPs) and (2) those that contain poly(ethylene) glycol (PEG). The use of engineered proteins that self-assemble into a soft, bioactive hydrogel was inspired by the ability for amino acid sequences to assemble into a 3D structure and elicit specific signaling cues. This class of hydrogel is created from amphiphilic or alternating hydrophilic and hydrophobic sequences. SAP hydrogels have the advantage of behaving like natural ECM proteins through their assembly (fibrillarization) and disassembly (proteolytic degradation). The second category of synthetic hydrogel fabrication are those that are made from PEG, a hydrophilic polymer. PEG is a unique biomaterial: it has a well-defined chemical structure, it can participate in a wide range of chemistries, and it can be tailored to almost any biological platform. One of the most useful properties of PEG is its ability to crosslink with other polymers, proteins, and molecules via a class of high yielding “click” chemistries. PEG has the added advantage of easily forming hydrogels with a wide range of stiffnesses independent of ligand density.
In this section on bioactive materials, we will review the different molecular building blocks available, show how they can be modified to elicit specific signaling cues, and place them in the context of in vitro cell culture models.
Self-assembling peptide hydrogels
Self-assembling peptides (SAPs) are relatively short (~5-30) amino acid sequences - often repeating - that have the capacity to fold into deterministic superstructures. SAPs can be designed based on their secondary structures (β-sheets or α-helix) or chemistry (hydrogen bonding, hydrophobicity and/or electrostatic interaction), which can then be used to fabricate self-assembled hydrogels under a specific pH.15 SAP hydrogels are mechanically soft, sometimes shear thinning, and typically fibrillar in structure. The benefit to using SAPs to other biomaterials is that their architecture lends itself to mimic native ECM proteins. They can degrade via proteolysis and their amino acid sequences can be designed via computational modeling approaches. The current limitations of SAPs are associated with the difficulty of creating them via solid phase peptide synthesis.16 As the chain length increases during synthesis, the peptide chains become vulnerable to aggregation; this leads to a mixed mode of sequences during coupling and deprotection steps and can severely limit overall yield. However, as more sequences are identified and synthesized into functional SAPs, the library of commercially available SAPs continually expands for researchers. Additionally, peptides are typically functionalized to SAP hydrogels to enhance their bioactivity. When an amino acid sequence on a native protein is identified as a motif, it can be synthesized into a peptide and tethered to a SAP or incorporated into the main chain. There are two main considerations when designing SAP hydrogels: their biochemical activity and their mechanical properties.
Biochemical Signaling
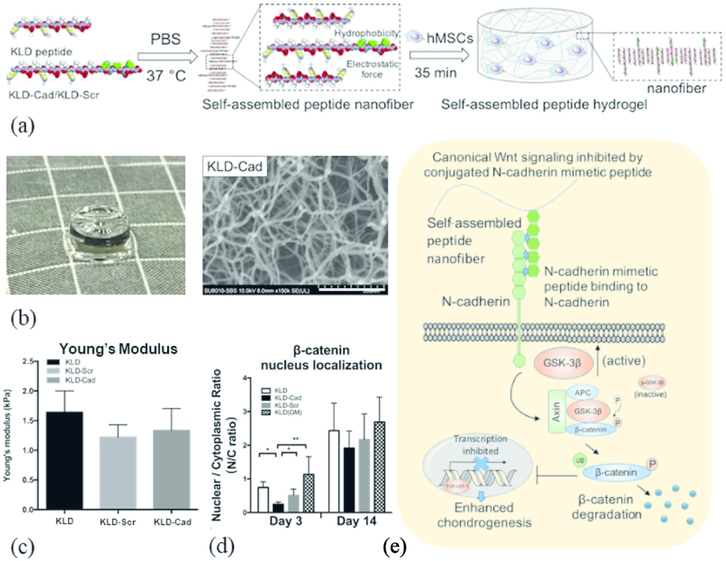
SAPs of all different types of sequences can be designed to physically support cells in 3D culture. For example, Tsukamoto et al. reported a peptide consisting of 13 amino acids (SPG-178; RLDLRLALRLDLR) that formed a stable hydrogel (SPG-178-Gel) at physiological pH.17 They showed that seeding dental pulp stem cells within the SPG-178-Gel scaffold supported osteogenic induction (with the use of induction media). However, SAP scaffolds must be functionalized if they are to actively participate in cellular signaling. The bone marrow, for example, has unique ECM composition that is not found anywhere else, so a SAP that has a bone-marrow peptide mimic can better control cellular phenotype. In one system Tsutsumi et al. synthesized a SAP (E1Y9; Ac-EYEYKYEYKY-NH2) and tethered different bioactive peptides related to osteogenic growth and adhesion to the C-terminus: ALK (ALKRQGRTLYGF) from an osteogenic growth peptide, DGR (DGRDSVAYG) from a cell adhesion motif of osteopontin, or PRG (PRGDSGYRGDS) from a cell adhesion motif of type IV collagen.18 The resulting hydrogels supported adhesion, growth, and differentiation of the pre-osteoblast cell line MC3T3-E1, while the E1Y9-ALK showed the most promise for osteogenic induction. Interestingly, the E1Y9-ALK was also the only hydrogel to contain a net positive charge and form mixed fibers/particles, suggesting a possible perturbation in its self-assembly. In a second system, Li et al. functionalized KLD (Ac-KLDLKLDLKLDL) with an N-cadherin mimetic peptide (HAVDIGG) to induce chondrogenesis in mesenchymal stem cells [Fig 2(a-b)]. By using an N-cadherin mimetic, the hydrogel suppressed canonical Wnt/β-catenin signaling in MSCs, which is known to differentially modulate chondrogenesis and osteogenesis [Fig 2(e)].19 In this hydrogel, self-assembly potential appeared to remain unperturbed (via SEM and Young’s modulus) [Fig 2(c)], while the targeting peptide induced gene expression changes in MSCs [Fig 2(d)], In a third system, Liu et al. incorporated one of three peptides—SKPPGTSS (bone marrow homing motif), FHRRIKA (heparin-binding motif) or PRGDSGYRGDS— to the C-terminus of RADA16-I (AcN-RADARADARADARADA-CONH2) to induce adipogenesis in human adipose stem cells.20 This group found that the motifs interrupted the β-sheet conformation, but alleviated the problem by adding a “certain amount of RADA16” to the functionalized peptide solution. It is important to keep in mind that when designing SAP hydrogels, the functionalized peptides should not only target specific signaling cues, but also be investigated for possible perturbations in self-assembly. SAP hydrogels that incorporate multiple bioactive peptides in a combinatorial fashion might serve to better mimic the ECM of the native tissue. We believe this is an important next step for improving biochemical signaling in SAPs.
Figure 2: Biochemical signaling cues incorporated into 3D hydrogels for culture.
(a) Schematic of functionalized SAP into nanofibullar structures, stabilized by hydrophobic and electrostatic interactions, to deliver biochemical cues to encapsulated hMSCs. (b) Picture (grid = 5 mm) and SEM image (scale bar = 300 μm) of KLD hydrogel functionalized with N-cadherin mimetic peptide (KLD-Cad). (c) Time sweep rheology studies show comparable mechanical properties across functionalized KLD hydrogels but (d) differential intracellular signaling dependent on functional moiety. (e) Schematic of the KLD-Cad hydrogel binding to hMSC transmembrane N-cadherin proteins, which promotes GSK-3β facilitated ubiquitin-mediated degradation of cytoplasmic β-catenin and the expression of pro-chondrogenic factors in hMSCs. Modified from Ref. 19 with permission from Elsevier.
Another current obstacle in SAP hydrogels is their biostability. Indeed, SAPs tend to degrade via proteolysis, which can be a double-edged sword: degradation allows cells to migrate through and remodel the hydrogel, but it is a challenge if the SAPs degrade too rapidly and lose their structural support. One way to circumvent this is to change the chirality from L-amino acids to D-amino acids. Chen et al. found that by changing the L-amino acids in RADA16 to D-amino acids, they could decrease proteolytic activity and thereby increase the biostability of the RADA16 hydrogel.21 A cursory examination of cell compatibility with the D-RADA16 hydrogel showed no detectable side effects, despite concerns over cytotoxicity (e.g. bioaccumulation) of D-amino acids.22 Others have found that substituting D- for L-enantiomeric counterparts appears to produce virtually identical hydrogels with no significant changes in biocompatibility or self-assembly potential.23 This method may prove to be a viable option for increasing SAP biostability in other cellular platforms.
Mechanical Signaling
SAP hydrogels are notorious for their weak mechanical properties. SAPs are generally assembled to maximize electrostatic interactions, hydrogen bonding, and hydrophobic clustering, but these bonds are relatively weak and still leave SAP hydrogels too soft for many applications. The mechanical weakness of a SAP hydrogel is somewhat alleviated by increasing the concentration of peptide, but increasing its concentration is only partially effective. A case in point: by independently changing the concentration of KFE-8 (FKFEFKFE) and KFE-8 functionalized with RGD peptide (KFE-8-RGD), Hogrebe et al. was able to control both the stiffness and adhesiveness of the hydrogel; however, this was achieved with nominal changes in stiffness (0.5 kPa to 3 kPa).24 In an earlier paper, the same group had increased the concentration of KFE-8 up to a stiffness of 10 kPa and seeded MSCs within.25 Although the MSCs in the softer gels successfully differentiated into adipocytes, they found that most cells in the 10 kPa gels were “round and appeared dark in color” with mixed morphologies. After 26 days in culture, a live/dead assay showed that the MSCs in the stiffer 10 kPa gel were apoptotic, reminiscent of endochondral ossification, but not quite at the point of osteogenesis. The mechanical strength of SAPs seem fall in the ideal range for neurogenesis,26-28 adipogenesis,20 and other soft tissue differentiation. For this reason and others, SAPs alone have been generally applied in the context of soft tissue models,29 hemostasis,30-32 and drug delivery.33,34
To increase mechanical strength, SAP hydrogels can be crosslinked. Pugliese et al. used sulfo-SMCC, an amine-to-sulfhydryl cross-linker with an N-hydroxysuccinimide (NHS) ester and maleimide reactive group.35 They found that by adding sulfo-SMCC to a peptide called CK (Ac-CGGLKLKLKLKLKLKGGC-CONH2), they could increase its mechanical strength 100-fold. Unfortunately, they found that the beneficial effects from increasing stiffness was somewhat negated by the depletion of free amines from the crosslinking reaction. The group postulated that their crosslinked SAP could improve neural stem cell differentiation by additional peptide functionalization. Crosslinking SAP hydrogels may be a viable alternative to altering its mechanical properties than increasing peptide concentration. However, methods are needed to prevent the crosslinking from interfering with biocompatibility.
For SAP hydrogels to truly be effective in stiffer tissues, they must somehow be able to overcome their inherent mechanical weakness. One way to increase the mechanical strength of a SAP hydrogel is to reinforce it with stiffer materials to create a composite. In bone tissue engineering, not only should the material stiffness match, but the tissue architecture should match as well. Bone tissue has both porous (hydroxyapatite) and fibrillar (collagen) components. The bone marrow niche is relatively soft (~200-20,000 Pa),36 which means that fibrillar SAP hydrogels can be used to house bone marrow-derived cells, while a stiffer, porous material can then be used as structural support. Zhang et al. may have recognized this and designed a nano-hydroxyapatite/chitosan/SAP (nHA/CTS/SAP) hydrogel that could retain the porous structure and mechanical strength of the nHA/CTS hydrogel, while also promoting cellular adhesion and growth from the SAP.37 They found that the SAP/nHA/CTS composite hydrogel significantly outperformed the nHA/CTS hydrogel in compressive tests (Young’s modulus ≫3 MPa). They hypothesized that incorporation of the SAP led to a decrease in the average pore diameter, which subsequently increased the interaction of nHA and chitosan and boosted its mechanical strength. More importantly, the synergy between the nHA/CTS and SAP provided exceptional mechanical properties and bioactivity, of which neither biomaterial could have produced on its own. Composites for bone tissue engineering has been used in similar systems.38,39 Whereas physically mixing materials can work to build a specific architecture, structured approaches of discrete layering have shown promise. Wu et al. designed a scaffold composed of peripheral blood MSCs (PBMSC) seeded within a SAP hydrogel that was sandwiched between two poly(lactic-co-glycolic acid) (PLGA) membranes.40 The PBMSC/SAP/PLGA hydrogel was implanted into a rat model of calvarial bony defect and designed such that the PLGA membranes could seal the bone defect and provide mechanical support for the SAP, which was suitable for PBMSC survival and osteogenesis. Other systems may benefit from SAP-material composites. Tendons, for example, contain elongated chondrocytes surrounded by dense bundles of collagen, which are formed from a complex interplay between tensile/compressive mechanics and FGF signaling.41 The addition of SAP-encapsulated cells to an aligned scaffold with tendon-specific peptides may prove to be a highly effective platform for tenogenesis. With the current tools at hand, there are many ways to incorporate mechanical reinforcing elements with SAP hydrogels.
Future Directions
SAP hydrogels hold promise for recapitulating biochemical and mechanical cues found in soft tissue. However, there are still many ways in which SAPs are used to improve cellular platforms. Currently, SAP hydrogels are confined to mostly monoculture models, including those that are used to direct stem cell differentiation. However, there is a critical need to include more than one cell type in these models to better mimic the “cellular ecology” of native tissue. Thus, SAP hydrogels should be incorporated in multicellular tissue platforms. Additionally, while we find that SAP hydrogels generally lack the ability to mimic the complex geometries of native tissue, they can provide useful mechanical (soft/moderately stiff) and biochemical (adhesion peptides) information for many cell niches. For tissues like the bone, SAP hydrogels are already used to support cell growth while stronger (less bioactive) materials are used to provide structural support. Other tissues that require a soft niche housed within a strong material may benefit from the addition of SAPs. Finally, the specific peptide sequences that are incorporated into SAP backbones can be used in conjunction with programmable components in synthetic biology. Thus, we encourage the community to continue to find ways to incorporate SAPs into multicellular platforms, multi-material platforms, and programmable cells in synthetic biology.
PEG-based hydrogel design
PEG comes in a variety of molecular weights (200-10000 g/mol), geometries (branched, star, comb), and chemical modifications (acrylate, divinyl sulfate). Because PEG is resistant to protein adsorption and contains no bioactive domains, it is generally considered biologically inert. Moreover, PEG can be functionalized with peptides to elicit specific cellular responses, which makes it an excellent candidate for designing tissue-specific hydrogel systems. Unlike the fibrillar SAP hydrogels, PEG-based hydrogels can have a porous architecture, which influences the way cells migrate through the hydrogel.42 PEG can also have a wide range of stiffness (~100-100,000 Pa) and can be tuned independently of ligand density.43 This is advantageous for decoupling stiffness and matrix adhesiveness for any system. The following sections will review the current status of the biochemical and mechanical properties of PEG hydrogels and their suggested functionality to increase their use in synthetic biology applications.
Biochemical signaling
PEG hydrogels decorated with RGD peptides promote cellular adhesion, but they do not capture the complexity of the ECM microenvironment, nor are they tissue-specific. Despite this, a recent study found that the vast majority (roughly 89%) of hydrogels and tissue scaffolds developed over the past five decades used the RGD peptide sequence to functionalize the material.44 Granted, RGD peptides are short, well characterized, and can be “clicked” onto many kinds of materials with relative ease. However, this finding is somewhat alarming, as the RDG motif does not represent the true diversity of native ECM. On the other hand, this study also reveals a wide-open field awaiting exploration: PEG hydrogels can be used to represent any ECM by conjugating them with tissue-specific peptides. Peptide sequences have been established from bone,45 integrin recognition sites,44 and more generally the matrisome.46 Recently, Jansen et al. identified 20 bone marrow-specific peptides using proteomic-based bioinformatics and coupled them to an 8-arm PEG via a Michael-type addition reaction.47 The resulting bone marrow hydrogel was able to maintain MSC stemness while significantly increasing bone differentiation capacity (via induction media) compared to the traditional PEG-RGD hydrogel. This method of identifying and incorporating a cocktail of bioactive peptides could be employed in other PEG hydrogels to give cells a “buffet” of signaling cues. PEG is so versatile that different strategies can be used to achieve a similar goal using completely different starting materials. For example, Anjum et al., created a chondroitin sulfate-PEG (CS-PEG) hydrogel that was grafted with MMP-Lys-peptide via FXIIIa-specific enzymatic crosslinking.48 The CS-PEG hybrid hydrogels were bound with slow releasing bone morphogenetic protein 2 (BMP-2), which improved osteogenic differentiation of encapsulated bone marrow mesenchymal stem cells. Like SAPs, PEG hydrogels have the potential to mimic any aspect of the ECM using tissue-specific peptides using straight-forward (photo)chemistry. But unlike SAPs, the addition of peptides to PEG is much less likely to disrupt the architectural properties of the hydrogel. Furthermore, PEG alone is resistant to degradation, which means it can be crosslinked with other materials (hyaluronic acid, chitosan, degradable peptides, etc.) to control cellular remodeling and employ spatiotemporal patterning.
Mechanical signaling
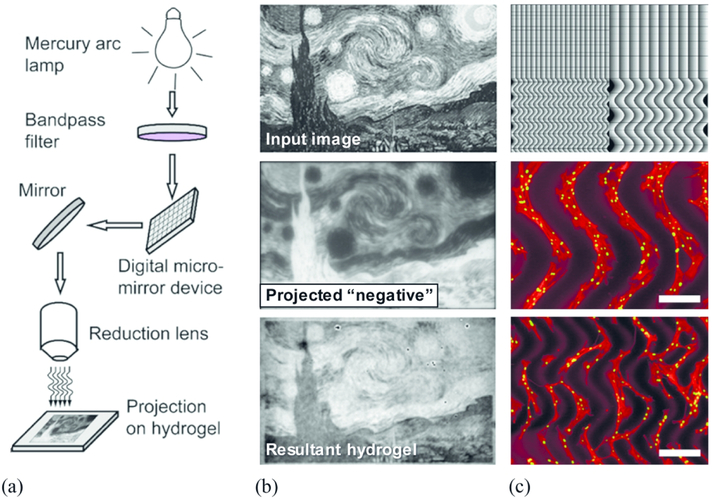
Mechanotransduction in PEG hydrogels has been widely explored, especially in the context of matrix stiffness,49,50 MMP-degradable systems,51-55 and spatial patterning.56-60 For a comprehensive review on mechanical tuning in PEG hydrogels, the reader is directed to these papers.61-63 To create photoresponsive PEG hydrogels with tunable mechanics, there are many PEG modifications that are available to choose a chemistry that best fits user needs.64 To summarize, PEG with a terminal amine can utilize efficient amide coupling reagents, like HATU, to generate stable amide linkages to carboxylic acid ends of a photoresponsive moiety. PEG with terminal acrylates can utilize the α,β unsaturated Michael acceptor to conjugate the photoresponsive moiety of choice via Michael addition (under mildly basic conditions) or radical polymerization as the radiative approach for spatiotemporal patterning. PEG with terminal azides or alkynes can use highly efficient and fast strain promoted azide-alkyne click (SPAAC) to generate a stable triazole linkage to incorporate photoresponsive moieties. A benefit to SPAAC is that the reaction can be performed under physiological conditions in under two hours of mixing. By using a combination of mechanical stiffness, protease-cleavable sites, and spatially patterned proteins or stiffness gradients, PEG hydrogels are becoming increasingly valuable for decoupling each of the cellular processes that are influenced by matrix mechanics. To spatially pattern stiffness gradients, Wu et al. developed a photoresponsive PEG hydrogel with spatiotemporal control over the mechanical properties using a fluorescent protein crosslinker, dronpa145N.65 Illuminating the hydrogel with violet (405 nm) light caused dronpa145N to monomerize, while illuminating it with green (505 nm) light brought it back to its tetrameric state. The difference in crosslinking could be seen with changes in the stiffness, which could be modulated between ~300 to 2000 Pa. The benefit to this method is two-fold: the 505 nm light is relatively non-toxic to cells, and the crosslinking is fully reversible. Another technique developed by Norris and colleagues makes use of maskless photolithography to pattern complex micron-scale stiffness gradients, as demonstrated by their beautiful recreation of Van Gogh's “Starry Night”.66 Briefly, a combination of photodegradable PDG–PEG4k-DA and nondegradable PEG4k-DA was polymerized and placed under a multiphoton microscope [Fig 3(a)]. An image file was loaded to the system, and the hydrogel was exposed to varying light intensity, resulting in a negative of the projected image [Fig 3(b)]. Different patterns and stiffness gradients were tested on hMSCs, and the authors found that the cells preferred the most degraded portions of the hydrogel. This is interesting and unexpected, because cells respond to durotaxis by migrating towards stiffer material. The hMSCs also “crossed over” from one degraded portion to the next when the gap was decreased from 268 μm to 134 μm [Fig 3(c)]. One important note about this platform: it is currently limited to 2D projections due to the set-up of the multiphoton microscope. However, if this kind of mechanical control could be implemented in 3D systems, then the opportunities to investigate the tissue microenvironment would be exciting. The ability to provide cells with unique biochemical cues and the diversity of methods in which PEG can be manipulated for spatiotemporal control over stiffness and protein patterning make it especially important for uses in dynamic co-culture models.
Figure 3: Maskless lithography techniques can spatially pattern mechanical gradients on a photodegradable hydrogel.
(a) In one method, a digital micro-mirror device (DMD) projects an image onto a hydrogel sample to propagate photodegradation-based patterning. This adaptable method allows users to alter topological material stiffness on the hydrogel. (b) The input grayscale image of The Starry Night was loaded into a SF-100 XPRESS photolithography system to project the “negative” pattern, granting the resultant degraded hydrogel. The inherent contrast can be directly visualized, as degraded regions appear darker due to differences in molar absorptivities between degraded and undegraded photolabile compound in the hydrogel. (c) Pixel projected image (negative) containing linear and curved gradient was projected to the surface of gel. Seeded hMSCs on the surface of patterned gel gathered in the degraded region and followed the pattern gradient. Scale bars = 268 μm (middle) and 134 μm (bottom). Reproduced from Ref. 66 with permission from American Chemical Society.
Future directions
PEG-based hydrogels are continuing to be at the forefront of many innovative platforms, especially those that modify hydrogel properties using external stimuli, such as light, pH, and temperature. Regarding synthetic biology, we see parallels between photoresponsive PEG hydrogels that are modified (degrade, crosslink, etc.) in response to light exposure, and optogenetics, a branch of synthetic biology that involves controlling cells that are genetically modified to respond to light inputs. We believe that PEG-based hydrogels have distinct characteristics that give them applicability in synthetic biology, especially in the field of synthetic tissues.
Leveraging dynamic spatiotemporal platforms to define cellular ecology
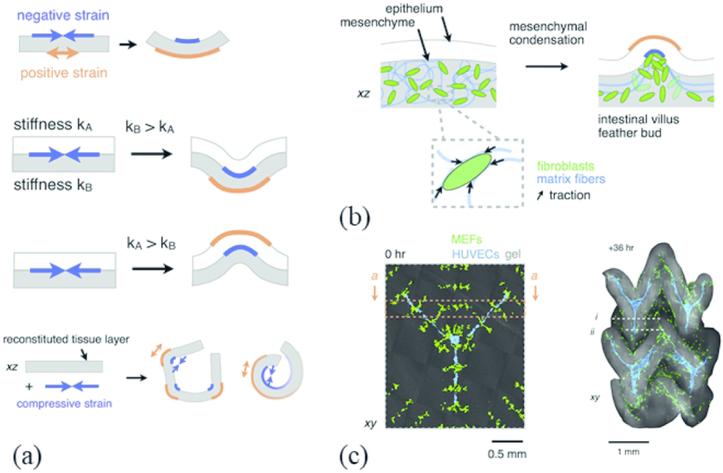
We have so far explored properties of SAPs and PEG hydrogels that mimic different aspects of the native ECM, including mechanical strength, material microstructure, biochemical cues, and spatial patterning. To date, most of the work in cellular biomaterials has focused on understanding, tuning, and developing strategies to create materials with these defined properties. Now we turn our attention to architecture and the way it drives tissue morphology. During morphogenesis, dividing cells grow, differentiate, and begin to fold into superstructures that eventually form organs, glands, and other tissues. These processes are mediated, in part, by local mechanical stresses between adjoining cells and the ECM. Fibroblasts are contractile by nature and are involved in numerous remodeling events in the body, including those in wound healing.67 Hughes and colleagues hypothesized that during morphogenesis, mesenchymal fibroblasts contract and form condensates, which leads to villi formation.68 They developed a finite element model to predict tissue curvature and autonomous tissue folding. The thought was that differences of strain at tissue interfaces results in bending, which can lead to invagination or evagination in tissue morphogenesis [Fig 4(a)]. Fibroblasts/MSCs contract to form condensates, which is thought to cause collagen alignment and “budding” [Fig 4(b)] and the initial formation of a villus. The finite element model was experimentally validated by spatially patterning mesenchymal condensates in collagen-Matrigel gel and observing deterministic folding of the hydrogel in a distinct pattern [Fig 4(c)]. The complex geometries created during development are difficult to replicate in 3D tissue models. However, it is well-known that cells actively participate in tissue contraction, compression, and folding. Taken together, Hughes and colleagues demonstrated that mechanically responsive hydrogels can be used to investigate morphogenetic processes. Furthermore, these studies also illustrate how cells can be “actuators,” which when combined with controlled cellular placement (architecture) and eventual deliberate materials design can generate 3D cellular scaffolds with complex architectural features and cell positioning. It isn’t difficult to envision synthetic biology helping the tissue engineering field by encoding synthetic light-activated contractile circuits to rearrange material architecture at a macro scale for biological utility.
Figure 4: Cell forces can induce topological folding in hydrogels.
(a) Differences in strain at tissue or material interfaces can result in folding as an engineering approach to direct tissue morphogenesis ex vivo. (b) 3D cell position patterning techniques can spatially pattern cell-ECM traction forces generated by contracting populations of mesenchymal cells termed “condensates.” Formation of these condensates at the gel-gel interface generates enough mechanical strain to induce curvature and folding of model gel “tissues.” (c) Confocal micrograph and confocal projection of a hydrogel with a cell-generated folded architecture. Condensing fibroblasts (green) drives folding trajectories and ECM compaction, and the resulting tissue topography influences endothelial cell (blue) lumenization and migration behavior biased along incipient folds. Reproduced from Ref. 68 with permission from Elsevier.
The ability to guide cells into precise spatial patterns represents an ongoing challenge in tissue engineering. There have been efforts made to mirror various in vivo tissue geometries by spatially patterning cells in hydrogels, especially in the form of 3D bioprinting.69-71 For example, Ma and coworkers encapsulated three cell types in different prepolymer solutions and used layer-by-layer UV photolithography to bioprint a triculture biomimetic liver lobule.72 The cells remained spatially organized in their hexagonal units for up to 10 days in culture with moderately good viability. This 3D bioprinting technique combines computer-aided-design (CAD) with rapid UV photopolymerization, making it possible to fabricate numerous user-defined, custom-made geometries.73 However, current bioprinted tissue models do not capture the dynamic process of tissue folding or the types of mechanical stress (compressive, tensile, etc.) found in developing organs. We postulate that combining current bioprinting techniques with cell contraction-based hydrogel folding may yield new ways to investigate morphogenesis of different tissues.
Another type of spatial patterning is accomplished by dynamically altering the geometry of the hydrogel post-polymerization. One type of dynamic shape-morphing hydrogel are self-folding hydrogels. Self-folding hydrogels have been around for more than a decade and have seen many iterations of similar shapes, materials, properties, and applications.74-76 As a recent example, Naficy et al. developed a shape morphing hydrogel using poly(NIPAM), and poly(HEMA) monomers to print patterns that could undergo shape transitions in different environmental conditions.77 These patterns spontaneously folded into cubes, pyramids (not shown), and hinges in a temperature-dependent manner (20-60 °C range) [Fig 5(c)]. In particular, the poly(NIPAM) and poly(HEMA) transitioned from a flat shape to a cube when fully swollen below 32 °C and return to a flat shape above 32 °C. These temperature ranges are strikingly close to a physiological temperature (37 °C). Despite their ability to create complex, dynamic geometric shapes, self-folding hydrogels have seen little, if any, translation into 3D cell culture models. In fact, publications related to self-folding hydrogels that are cell friendly are sparse, and the ones that exist have just barely begun to scratch the surface.78-80 Similar dynamic hydrogel platforms have been developed recently, including a temperature-responsive “buckling” hydrogel that folds into a geometry a priori [Fig 5(b)],81 an alginate/polyacrylamide hydrogel that can be physically reshaped into any geometry and then “fixed” in place using a multivalent ionic solution [Fig. 5(a)],82 and a self-folding “origami” hydrogel.79 A more thorough review on shape-morphing hydrogels can be found here.83 Many of these hydrogel folding patterns can be determined a priori through computer modeling and can be adapted to almost any 3D structure or topological feature. We see the potential for shape-morphing hydrogel systems to be incorporated in co-culture models to investigate architectural features, such as acini, ducts, alveoli, and lumen. Additionally, these hydrogels have the potential to participate in real-time folding events based on cellular cues (e.g. pH, enzyme secretion, density, etc). We propose that if these methods could be adapted to cell-friendly hydrogel systems, then they will give biomedical researchers a powerful tool for studying physiological and pathological events related to architectural changes and cell-cell communication.
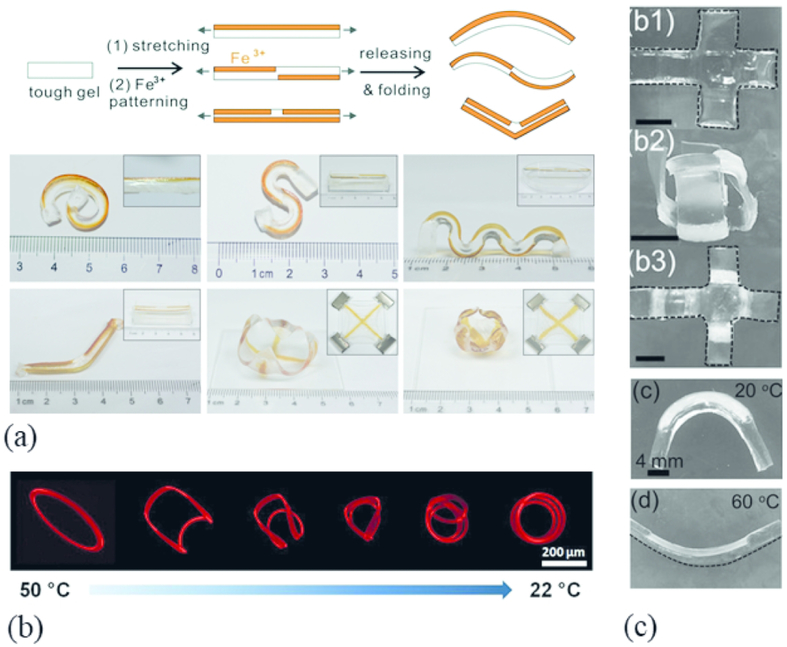
Figure 5: Asymmetric patterning of mechanically heterogeneous structures results in stiffness mismatch-induced hydrogel folding/unfolding.
(a) Various 3D folding patterns of Ca-alginate PAAm hydrogels via localized Fe3+ ion solution exposure to encode anisotropy into a hydrogel. Adapted from Ref. 82 with permission from the Royal Society of Chemistry. (b) Thermal actuation of a circular photo-patterned PNIPAM hydrogel ring. Between heating to 50° C and cooling to 22° C, the hydrogel de-swells, adopting a nearly planar shape, and swells into triple coiled state, respectively. Adapted from Ref. 81 with permission from Elsevier (c) Shape deformation of poly(NIPAM) and the poly(HEMA) induced with temperature changes. The hydrogel swollen state and adopts a folded 3D box conformation at room temperature and unfolds into the planar conformation upon heating to 60° C. Adapted from Ref. 77 with permission from of John Wiley & Sons.
Conclusions
The merging of synthetic biology with materials science creates a unique working space for synthetic tissue engineering, an area that has the potential to create novel cell-based platforms. Hitherto, we adapt the definition of conventional ecology to describe cellular ecology as the study of the distribution of cell populations, the interaction among cell types, and the interactions between cells and their microenvironment. The overarching goal is to engineer better cellular platforms for tissue engineering, regenerative medicine, and morphogenesis. The major components of cellular ecology include biochemical and mechanical cues, tissue architecture, cell types, and dynamic forces, all of which should be considered when designing next-generation synthetic hydrogels. While SAP hydrogels and PEG hydrogels have unique characteristics that can be used to probe various aspects of the cellular microenvironment, many other useable materials exist. Notwithstanding, many of the hydrogel platforms described in this review incorporate important design functionalities related to cellular ecology that can be adapted for synthetic biology applications. We leave the reader with a recent marvel in synthetic tissue engineering and optogenetics: a miniature, artificial ray fish constructed with an elastomeric body and a gold exoskeleton—powered by cardiomyocytes that contract in response to light.84
Acknowledgements
The authors would like to thank Dr. Rahul Chib for thoughtful discussions and comments. This work was supported in part by grants from the National Institutes of Health (R01HL133163, R21ES027962, T32GM008550 to A.M.D.), the National Science Foundation (1537256) the Burroughs Wellcome Fund (1017521), and the March of Dimes Basil O’Connor Award (5-FY16-33 to J.P.G).
Abbreviations:
- ECM
Extracellular Matrix
- MSC
Mesenchymal Stem Cell
- PEG
Poly(ethylene) glycol
- CS
Chondroitin Sulfate
- SAP
Self-Assembling Peptide
References
- 1.Benner SA & Sismour AM Synthetic biology. Nat. Rev. Genet 6, 533–543 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts M. a. J., Cranenburgh RM, Stevens MP & Oyston PCF Synthetic biology: biology by design. Microbiol. Read. Engl 159, 1219–1220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosano GL & Ceccarelli EA Recombinant protein expression in Escherichia coli: advances and challenges. Front. Microbiol 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 318, 1917–1920 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Hsu PD, Lander ES & Zhang F Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sia SK, Gillette BM & Yang GJ Synthetic tissue biology: Tissue engineering meets synthetic biology. Birth Defects Res. Part C Embryo Today Rev 81, 354–361 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Bianconi E et al. An estimation of the number of cells in the human body. Ann. Hum. Biol 40, 463–471 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Bokka KK et al. Morphogenetic Implications of Peristalsis-Driven Fluid Flow in the Embryonic Lung. PLOS ONE 10, e0132015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George UZ, Bokka KK, Warburton D & Lubkin SR Quantifying stretch and secretion in the embryonic lung: Implications for morphogenesis. Mech. Dev 138, 356–363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HY et al. Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Dev. Cell 34, 719–726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A The Primary cilium calcium channels and their role in flow sensing. Pflüg. Arch. - Eur. J. Physiol 467, 157–165 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Boghaert E et al. Host epithelial geometry regulates breast cancer cell invasiveness. Proc. Natl. Acad. Sci 109, 19632–19637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ameis D, Khoshgoo N & Keijzer R Abnormal lung development in congenital diaphragmatic hernia. Semin. Pediatr. Surg 26, 123–128 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Zhang W et al. Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Prog. Polym. Sci 80, 94–124 (2018). [Google Scholar]
- 16.Coin I, Beyermann M & Bienert M Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc 2, 3247 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto J et al. Efficacy of a Self-Assembling Peptide Hydrogel, SPG-178-Gel, for Bone Regeneration and Three-Dimensional Osteogenic Induction of Dental Pulp Stem Cells. Tissue Eng. Part A 23, 1394–1402 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi H, Kawamura M & Mihara H Osteoblastic differentiation on hydrogels fabricated from Ca 2+-responsive self-assembling peptides functionalized with bioactive peptides. Bioorg. Med. Chem 26, 3126–3132 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Li R et al. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 145, 33–43 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Liu X et al. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 9, 6798–6805 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Chen S et al. Designer D-form self-assembling peptide scaffolds promote the proliferation and migration of rat bone marrow-derived mesenchymal stem cells. Int. J. Mol. Med 40, 679–688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J et al. D -Amino Acids Modulate the Cellular Response of Enzymatic-Instructed Supramolecular Nanofibers of Small Peptides. Biomacromolecules 15, 3559–3568 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Du X, Wang J, Yamagata N & Xu B Enzyme-instructed self-assembly of peptides containing phosphoserine to form supramolecular hydrogels as potential soft biomaterials. Front. Chem. Sci. Eng 11, 509–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogrebe NJ et al. Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel. Acta Biomater. 70, 110–119 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Hogrebe NJ & Gooch KJ Direct influence of culture dimensionality on human mesenchymal stem cell differentiation at various matrix stiffnesses using a fibrous self-assembling peptide hydrogel: Effect of Culture Dimensionality on HMSC Differentiation. J. Biomed. Mater. Res. A 104, 2356–2368 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Tavakol S et al. Erratum to: Mechano-Transduction Signals Derived from Self-Assembling Peptide Nanofibers Containing Long Motif of Laminin Influence Neurogenesis in In-Vitro and In-Vivo. Mol. Neurobiol 54, 2497–2497 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Tavakol S et al. Self-Assembling Peptide Nanofiber Containing Long Motif of Laminin Induces Neural Differentiation, Tubulin Polymerization, and Neurogenesis: In Vitro, Ex Vivo, and In Vivo Studies. Mol. Neurobiol 53, 5288–5299 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Lu C et al. Bioactive Self-Assembling Peptide Hydrogels Functionalized with Brain-Derived Neurotrophic Factor and Nerve Growth Factor Mimicking Peptides Synergistically Promote Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng 4, 2994–3005 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Maude S, Ingham E & Aggeli A Biomimetic self-assembling peptides as scaffolds for soft tissue engineering. Nanomed. 8, 823–847 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Cheng T-Y et al. Self-assembling functionalized nanopeptides for immediate hemostasis and accelerative liver tissue regeneration. Nanoscale 5, 2734 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Saini A, Serrano K, Koss K & Unsworth LD Evaluation of the hemocompatibility and rapid hemostasis of (RADA) 4 peptide-based hydrogels. Acta Biomater. 31, 71–79 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Yang S et al. Novel hemostatic biomolecules based on elastin-like polypeptides and the self-assembling peptide RADA-16. BMC Biotechnol. 18, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altunbas A, Lee SJ, Rajasekaran SA, Schneider JP & Pochan DJ Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 32, 5906–5914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelain F, Unsworth LD & Zhang S Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J. Controlled Release 145, 231–239 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Pugliese R, Marchini A, Saracino GAA, Zuckermann RN & Gelain F Cross-linked self-assembling peptide scaffolds. Nano Res. 11, 586–602 (2018). [Google Scholar]
- 36.Jansen LE, Birch NP, Schiffman JD, Crosby AJ & Peyton SR Mechanics of intact bone marrow. J. Mech. Behav. Biomed. Mater 50, 299–307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z et al. Self-assembling peptide and nHA/CTS composite scaffolds promote bone regeneration through increasing seed cell adhesion. Mater. Sci. Eng. C 93, 445–454 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Hou T et al. A composite demineralized bone matrix – Self assembling peptide scaffold for enhancing cell and growth factor activity in bone marrow. Biomaterials 35, 5689–5699 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Li K et al. Biomimetic Ultralight, Highly Porous, Shape-Adjustable, and Biocompatible 3D Graphene Minerals via Incorporation of Self-Assembled Peptide Nanosheets. Adv. Funct. Mater 28, 1801056 (2018). [Google Scholar]
- 40.Wu G et al. Osteogenesis of peripheral blood mesenchymal stem cells in self assembling peptide nanofiber for healing critical size calvarial bony defect. Sci. Rep 5, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiele NR, Marturano JE & Kuo CK Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr. Opin. Biotechnol 24, 834–840 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Annabi N et al. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev 16, 371–383 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyton SR, Raub CB, Keschrumrus VP & Putnam AJ The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27, 4881–4893 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Huettner N, Dargaville TR & Forget A Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 36, 372–383 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Visser R, Rico-Llanos GA, Pulkkinen H & Becerra J Peptides for bone tissue engineering. J. Controlled Release 244, 122–135 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Naba A et al. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteomics 11, M111.014647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansen L, McCarthy T, Lee M & Peyton S A synthetic, three-dimensional bone marrow hydrogel. (2018). doi: 10.1101/275842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anjum F et al. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials 87, 104–117 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Lv H et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehmann MS, Luna JI, Maverakis E & Kloxin AM Tuning microenvironment modulus and biochemical composition promotes human mesenchymal stem cell tenogenic differentiation: HUMAN MESENCHYMAL STEM CELL TENOGENIC DIFFERENTIATION. J. Biomed. Mater. Res. A 104, 1162–1174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blache U et al. Dual Role of Mesenchymal Stem Cells Allows for Microvascularized Bone Tissue-Like Environments in PEG Hydrogels. Adv. Healthc. Mater 5, 489–498 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Mahadevaiah S, Robinson KG, Kharkar PM, Kiick KL & Akins RE Decreasing matrix modulus of PEG hydrogels induces a vascular phenotype in human cord blood stem cells. Biomaterials 62, 24–34 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters EB, Christoforou N, Leong KW, Truskey GA & West JL Poly(Ethylene Glycol) Hydrogel Scaffolds Containing Cell-Adhesive and Protease-Sensitive Peptides Support Microvessel Formation by Endothelial Progenitor Cells. Cell. Mol. Bioeng 9, 38–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mabry KM, Lawrence RL & Anseth KS Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 49, 47–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh SP, Schwartz MP, Lee JY, Fairbanks BD & Anseth KS A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration. Biomater. Sci 2, 1024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soman P et al. Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials 33, 7064–7070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sunyer R, Jin AJ, Nossal R & Sackett DL Fabrication of Hydrogels with Steep Stiffness Gradients for Studying Cell Mechanical Response. PLoS ONE 7, e46107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang C et al. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci 113, E4439–E4445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hahn MS, Miller JS & West JL Three-Dimensional Biochemical and Biomechanical Patterning of Hydrogels for Guiding Cell Behavior. Adv. Mater 18, 2679–2684 (2006). [Google Scholar]
- 60.Nemir S, Hayenga HN & West JL PEGDA hydrogels with patterned elasticity: Novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng 105, 636–644 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Ma Y et al. 3D Spatiotemporal Mechanical Microenvironment: A Hydrogel-Based Platform for Guiding Stem Cell Fate. Adv. Mater 1705911 (2018). doi: 10.1002/adma.201705911 [DOI] [PubMed] [Google Scholar]
- 62.Kloxin AM, Kloxin CJ, Bowman CN & Anseth KS Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv. Mater 22, 3484–3494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kloxin AM, Tibbitt MW, Kasko AM, Fairbairn JA & Anseth KS Tunable Hydrogels for External Manipulation of Cellular Microenvironments through Controlled Photodegradation. Adv. Mater 22, 61–66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sletten EM & Bertozzi CR Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed 48, 6974–6998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu X et al. Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res. 11, 5556–5565 (2018). [Google Scholar]
- 66.Norris SCP, Tseng P & Kasko AM Direct Gradient Photolithography of Photodegradable Hydrogels with Patterned Stiffness Control with Submicrometer Resolution. ACS Biomater. Sci. Eng 2, 1309–1318 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Desmouliere A, Darby IA, Laverdet B & Bonté F Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol 301 (2014). doi: 10.2147/CCID.S50046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hughes AJ et al. Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Dev. Cell 44, 165–178.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merceron TK et al. A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication 7, 035003 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Skardal A et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 25, 24–34 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Kolesky DB et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater 26, 3124–3130 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Ma X et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci 113, 2206–2211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soman P, Chung PH, Zhang AP & Chen S Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels: 3D Microstructures in Cell-Laden Hydrogels. Biotechnol. Bioeng 110, 3038–3047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Randall CL, Gultepe E & Gracias DH Self-folding devices and materials for biomedical applications. Trends Biotechnol. 30, 138–146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan J, He H, Hansford DJ & Lee LJ Self-Folding of Three-Dimensional Hydrogel Microstructures. J. Phys. Chem. B 109, 23134–23137 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Yoon C et al. Functional stimuli responsive hydrogel devices by self-folding. Smart Mater. Struct 23, 094008 (2014). [Google Scholar]
- 77.Naficy S, Gately R, Gorkin R, Xin H & Spinks GM 4D Printing of Reversible Shape Morphing Hydrogel Structures. Macromol. Mater. Eng 302, 1600212 (2017). [Google Scholar]
- 78.Kwag HR et al. A Self-Folding Hydrogel In Vitro Model for Ductal Carcinoma. Tissue Eng. Part C Methods 22, 398–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuribayashi-Shigetomi K, Onoe H & Takeuchi S Cell Origami: Self-Folding of Three-Dimensional Cell-Laden Microstructures Driven by Cell Traction Force. PLoS ONE 7, e51085 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bassik N, Stern GM, Jamal M & Gracias DH Patterning Thin Film Mechanical Properties to Drive Assembly of Complex 3D Structures. Adv. Mater 20, 4760–4764 (2008). [Google Scholar]
- 81.Bae J, Na J-H, Santangelo CD & Hayward RC Edge-defined metric buckling of temperature-responsive hydrogel ribbons and rings. Polymer 55, 5908–5914 (2014). [Google Scholar]
- 82.Li T et al. “Freezing”, morphing, and folding of stretchy tough hydrogels. J. Mater. Chem. B 5, 5726–5732 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Jeon S-J, Hauser AW & Hayward RC Shape-Morphing Materials from Stimuli-Responsive Hydrogel Hybrids. Acc. Chem. Res 50, 161–169 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Park S-J et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 353, 158–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]