Abstract
Objective
To investigate the effects of different concentrations of Fe3+ on the acute toxicity and regeneration of planarian at different temperatures.
Method
The planarians were treated with 40 mg/l, 50 mg/l, 60 mg/l, and 70 mg/l Fe3+ solution and placed in 15°C, 20°C, and 25°C, respectively, to observe the mortality and the poisoning pattern of the planarian. In addition, the planarians were cut into three parts of head, trunk, and tail, then placed in Fe3+ solution at concentrations of 10 mg/l, 15 mg/l, 20 mg/l, and 30 mg/l, and placed in 15°C, 20°C, and 25°C respectively, and the regeneration rate of the planarian was investigated.
Results
At the same temperature, in the concentration of Fe3+ from 40 mg/l to 70 mg/l, the mortality of the planarian increased with the increasing of the concentration of Fe3+; at the same concentration and different temperatures, the death speed of the planarian is the fastest at 20°C, the next at 25°C, and the lowest at 15°C, indicating that the toxic effect of Fe3+ can be accelerated at a suitable temperature of 20°C. At the same temperature, in the low concentration of Fe3+ from 10 mg/l to 30 mg/l, the regeneration rate of the planarian gradually decreased with the increasing of the concentration of Fe3+; at the same concentration and different temperature, the regeneration rate of planarian was faster at 20°C and 25°C, but the difference between 20°C and 25°C was small, and the slowest at 15°C, indicating that the low temperature significantly affects the planarian regeneration speed. The study also found the regeneration rates of the head, trunk, and tail of the planarian were different; the head regeneration was the fastest, the trunk was the second, and the tail was the slowest.
Conclusion
Fe3+ had obvious toxic effects on the survival and regeneration of planarian; the planarian is sensitive to Fe3+ and may be used to detect Fe3+ water pollution; in addition, temperature can affect the toxic effects of Fe3+ and thus affect the survival and regeneration of the planarian. Therefore, the temperature should be taken into consideration when detecting water Fe3+ pollution.
1. Introduction
In recent years, people have paid more and more attention to environmental issues, and the development of modern industry and agriculture has caused a large number of heavy metal ions to accumulate continuously in the water, causing serious harm to the water environment [1, 2].
Iron is an indispensable micronutrient for animals and plants. It is a component of chlorophyll and heme. It is also an important component of some enzymes and plays an essential role in the process of biological redox. A certain extent of iron is beneficial to the life activities of aquatic animals. However, with the development of the economy, too much excess iron is enriched in water. The excessive iron is poisonous and can cause poisoning or even death of aquatic plants and animals.
Industrial production waste liquid contains ferric ion. Iron exposure to the environment mainly occurs through mining, manufacturing units, and municipal or industrial wastewater. Iron pollution also occurs in response to corrosion of pipes and water supply from groundwater systems and from the atmosphere via rainwater [3]. When the same metals such as Fe accumulate at levels greater than the threshold level they become toxic. This induces the generation of reactive nitrogen and oxygen species (RNS; ROS), which results in the peroxidation of lipids in the plasma membrane [3]. In addition, iron packaging materials and iron products can also form waste ferric ion. These ferric ions cause water and soil pollution; in particular, the large amount of ferric ion in water causes severe water pollution and brings great harm to aquatic organisms [2, 4, 5]. The toxic effects of heavy metals on aquatic animals such as prawn and grouper have been studied intensively, and the results showed that heavy metal pollution may be further enriched between organisms through food chains [6–10], which in turn may threaten the survival of human. The accumulation of heavy metals in the human body leads to severe injury to various organs, specifically the respiratory, nervous, and reproductive systems and digestive tract [11–14]. Excess iron accumulation in tissue triggers iron-dependent oxidative stress [15]. Iron overload in the skeletal muscle not only negatively affects muscle contractility but also might impact its endocrine function, thus possibly affecting the clinical outcome of diseases, including neurodegenerative diseases [15]. Therefore, it is extremely important to effectively monitor water pollution and prevent the harmful impacts on biological health.
Planarian Dugesia japonica are flatworms (phylum Platyhelminthes) found in freshwater. They are widely distributed in China and have strong regeneration abilities. For over 200 years, planarians have been a classical model for studies on tissue regeneration [16, 17]. The body length of the planarian is generally 5-15 mm. They are soft, flat leaf-like, and bilateral symmetrical body shape. The head is triangular and the two eye-spots are used to detect the intensity of light. In addition, planarian is hermaphroditic; it can reproduce sexually or asexually. Previous studies showed that planarian is able to monitor water pollution [18, 19] and is sensitive to many pollutants [20–22]. The toxic effects of metal ions such as Cu2+ and Pb2+ and cadmium and other factors such as herbicides on planarian have been studied previously [15, 23–27]. Zhang Xiufang and Temenouga Guecheva confirmed that Cu2+ has a direct toxic effect on planarian, and planarian plays an important role in the biological monitoring of heavy metal copper ions [28, 29]. But there are few reports on the toxicity of iron ions to planarian.
In the present study, we focus on the effects of Fe3+ on the death and regeneration of Dugesia japonica under different temperatures; the results are useful for iron monitoring by planarian.
2. Materials and Methods
2.1. Experimental Materials
Planarian Dugesia ZB-1, a clonal propagation line of the Dugesia japonica, is preserved in our laboratory. FeCl3∙6H2O is produced by Tianjin Kaitong Chemical Reagent Co., Ltd.
2.2. Experimental Methods
2.2.1. Preparation of Fe3+
10 g of analytically pure FeCl3∙6H2O crystals was dissolved in 100 mL distilled water to make 100 g/l iron solution. When used, dilute it to the exact concentration.
2.2.2. Effect of Fe3+ on the Survival of Planarian
From the 100 g/l stock solution, four concentrations 40, 50, 60, and 70 mg/l of Fe3+ were made. Each concentration had 3 replicates. 10 planarians were placed in each concentration. At the same time, the four groups of planarian were cultured in 3 different constant temperature incubators: 15°C, 20°C, and 25°C. The culture medium was changed every day. The body changes of the planarian were observed at different time intervals.
2.2.3. Effects of Fe3+ on the Regeneration of Planarian
Fe3+ was prepared in 100 g/l stock solution and then diluted to 10, 15, 20, and 30 mg/l solution. Each concentration had 3 replicates. 10 planarians were cut into three sections as head, trunk and tail and then placed in these four different concentrations. The four groups of regeneration concentrations were cultured at 15°C, 20°C, and 25°C. At the same time, set a cup of the same number of planarians in tap water at each temperature as control, and change the culture solution every day. The regeneration of the planarian was observed at different time intervals and repeated three times.
2.3. Data Processing
Data was performed by Graphpad Prism5 software, and statistical analysis was performed by Excel software and SPSS software.
3. Results
3.1. Effects of High Concentration of Fe3+ on the Survival of Planarian
3.1.1. The Poisoning Form of Planarian
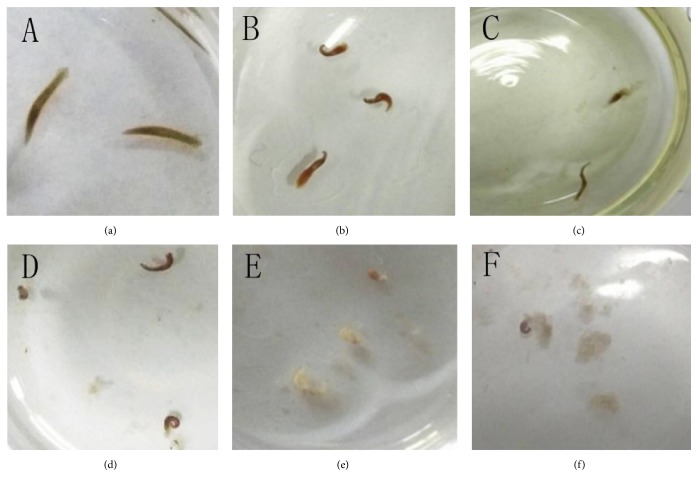
There were some differences in the toxic morphology of planarian in response to the various heavy metal ions. Planarians treated with different concentrations of Fe3+ showed that, in the early stage of treatments, the body of the planarian deformed and distorted, and the body seemed like “S” (Figure 1(b)). The effect was similar to these when the planarian was treated by copper and phenol [30]. After longer treatment the body state of the planarian in the lower concentration of Fe3+ returned to normal. However, the planarian shrank into a mass and stopped growing in the higher concentration of Fe3+. Additionally, the body of planarian was disintegrated (Figures 1(c) and 1(d)). Eventually, the whole body of the planarian was decomposed into white flocculence (Figures 1(e) and 1(f)), which indicated that long incubation time of high concentration of Fe3+ was able to make planarian die.
Figure 1.
The poisoning pattern of the planarian on Fe3+. (a) The normal form of the planarian in clear water, naturally stretched; (b) the initial poisoning form of the planarian, the body is distorted, showing an “S” shape; (c-d) the head of the planarian is more sensitive, and the head disintegrates after the body is distorted; (e-f) as time goes by, the whole body of the planarian is completely decomposed, showing a diffuse, flocculent shape.
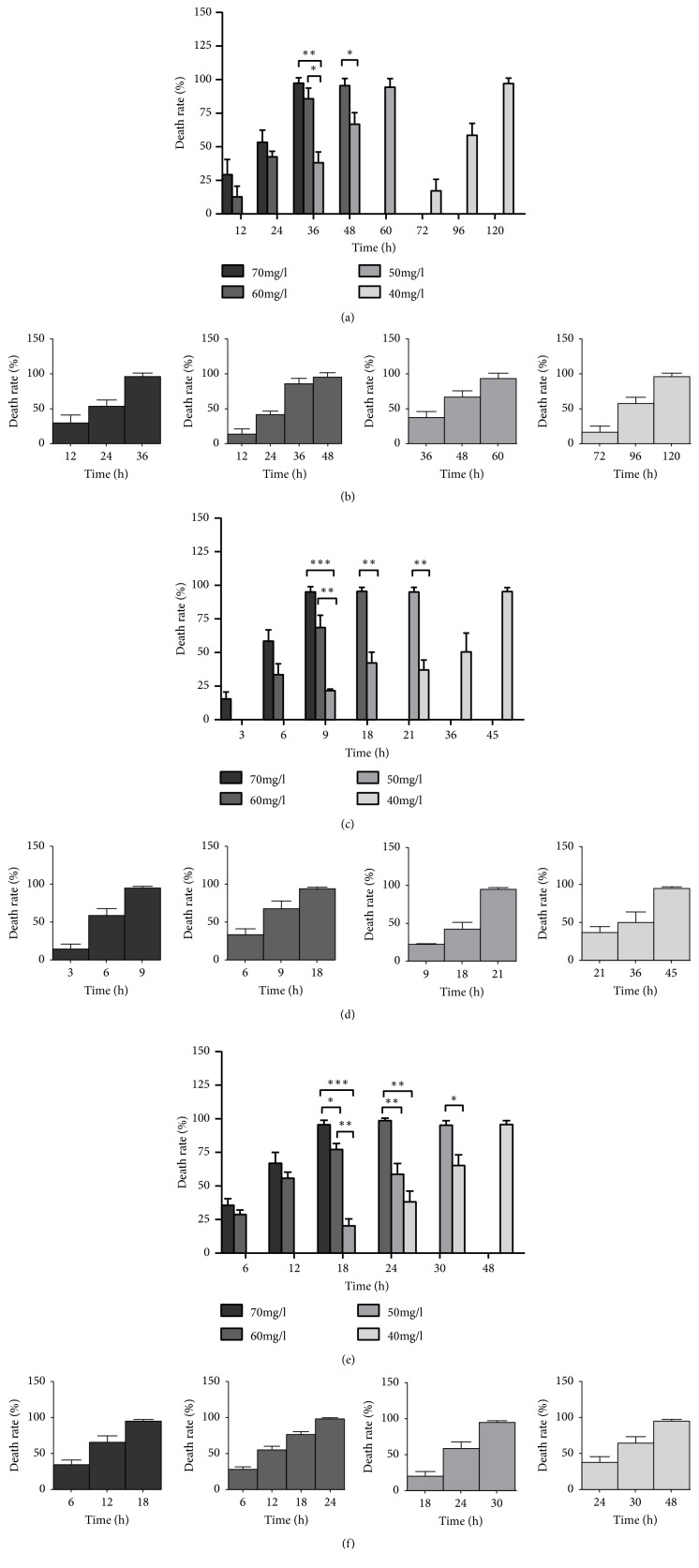
3.1.2. Mortality Changes in Different Concentrations
At the same temperature, with the increase of Fe3+ concentration, the mortality of planarian increased gradually. At 15°C, all the planarian died after treatment for 36 h in 70 mg/l, 48 h in 60 mg/l, 60 h in 50 mg/l, and 120 h in 40 mg/l (Figures 2(a) and 2(b)). At 20°C and 25°C, there were obvious regularities between different concentrations; that is, the higher the concentration, the shorter the time to reach 100% mortality. At 20°C, all the planarian died after treatment for 9 h in 70 mg/l, 18 h in 60 mg/l, 21 h in 50 mg/l, and 45 h in 40 mg/l; at 25°C, all the planarian died after treatment for 18 h in 70 mg/l, 24 h in 60 mg/l, 30 h in 50 mg/l, and 48 h in 40 mg/l (Figures 2(c)–2(f)). The results indicated that the higher the concentration is, the faster the planarian die which revealed toxic effects of Fe3+ on planarian.
Figure 2.
Effect of different temperatures and concentrations on the survival of planarian. (a-b) At 15°C, after treatment with different Fe3+ concentrations, the variation trend of the worm mortality was changed with time; (b) shows the change of mortality corresponding to each concentration; (c-d) similarly, it shows the distribution of the change in the mortality of the planarian after treatment at different concentrations of Fe3+ at 20°C; (e-f) changes in the mortality of the worms at different treatment concentrations at 25°C. Note: ∗∗∗ indicates P < 0.001, ∗∗ indicates P < 0.01 with extremely significant difference, and ∗ indicates P < 0.05 with significant difference.
3.1.3. Mortality Changes at Different Temperatures
Under the same Fe3+ concentration, there were also obvious regularities between different temperatures. Under 70 mg/l Fe3+, at 15°C, all planarian died after incubation for 36 h; at 25°C, all planarian died for 18 h, and at 20°C, all planarian died for 9 h (Figure 2). The differences were obvious. Similarly, when the concentrations were 60, 50, and 40 mg/l, from Figures 2, we could observe that the death rate was the highest at 20°C, but the lowest at 15°C, and the differences were significant. These mean that low temperature could slow down the effect of Fe3+ on the planarian toxicity, and at a suitable temperature, the toxic effects of Fe3+ on the planarian can be accelerated, which leads to the increased planarian mortality.
3.2. Effects of Nonlethal Concentrations of Fe3+ on the Regeneration of Planarian
It was found in the experiment that low concentration of Fe3+ did not cause the planarian death, and the change of poison status is not obvious. However, lower concentrations of Fe3+ can affect the regeneration process of the planarian.
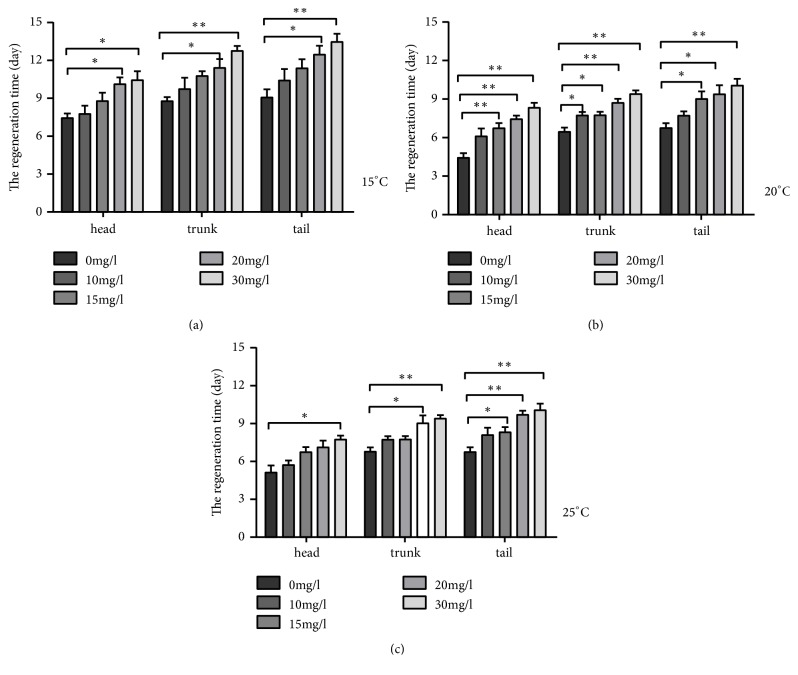
3.2.1. Changes in Regeneration Rate of Different Concentrations at the Same Temperature
At the same temperature, as the iron concentration increased, the regeneration rate also became slow. At 15°C, 30 mg/l, the time when all three parts of the planarian regenerated completely is 10-14 days, which is a larger difference than the control group, which is 7 to 8 days; at 20 mg/l, the time when all three parts of the planarian regenerated completely is 10-12 days, also having a large difference compared with the control group (Figure 3(a)). Similarly, at 20°C and 25°C, with the increase of Fe3+ concentration, the regeneration completion times were correspondingly longer, and the differences between the experiment groups and the control group were significant (Figures 3(b) and 3(c)). The higher the concentration, the more significant the difference. According to a comprehensive comparison of the significant differences in different concentrations of the three groups of temperatures in Table 3, there was a significant difference between the different experiment groups and the control group. The different regeneration rate of the planarian between the treatment concentrations of each group indicates that the lower concentration of Fe3+ also affects the physiology of the planarian. The present study was similar to that of the published results, where the higher toxic concentration, the lower planarian regenerative capacity [15, 30].
Figure 3.
Effect of Fe3+ on the regeneration of planarian at different temperatures. (a) Contrast histogram of the number of days after regeneration of the head, trunk, and tail of the planarian at 15°C, under different treatment concentrations and water treatment; (b) similarly, at 20°C, contrast histogram of the number of days after the regeneration of the planarian; (c) similarly, the comparison histogram of the number of days of the regeneration of the planarian at 25°C. Note: ∗∗∗ indicates P < 0.001, ∗∗ indicates P < 0.01 with extremely significant difference, and ∗ indicates P < 0.05 with significant difference.
Table 3.
Comparison of significant differences among the different concentrations of iron.
| Dependent Variable: Regeneration days | |||||||
|---|---|---|---|---|---|---|---|
| (I)concentration | (J) concentration | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||||
| LSD | 0mg/l | 10mg/l | -1.07∗ | .235 | .000 | -1.54 | -.61 |
| 15mg/l | -1.78∗ | .235 | .000 | -2.25 | -1.31 | ||
| 20mg/l | -2.67∗ | .235 | .000 | -3.13 | -2.20 | ||
| 30mg/l | -3.37∗ | .235 | .000 | -3.84 | -2.90 | ||
| 10mg/l | 0mg/l | 1.07∗ | .235 | .000 | .61 | 1.54 | |
| 15mg/l | -.70∗ | .235 | .004 | -1.17 | -.24 | ||
| 20mg/l | -1.59∗ | .235 | .000 | -2.06 | -1.12 | ||
| 30mg/l | -2.30∗ | .235 | .000 | -2.76 | -1.83 | ||
| 15mg/l | 0mg/l | 1.78∗ | .235 | .000 | 1.31 | 2.25 | |
| 10mg/l | .70∗ | .235 | .004 | .24 | 1.17 | ||
| 20mg/l | -.89∗ | .235 | .000 | -1.36 | -.42 | ||
| 30mg/l | -1.59∗ | .235 | .000 | -2.06 | -1.12 | ||
| 20mg/l | 0mg/l | 2.67∗ | .235 | .000 | 2.20 | 3.13 | |
| 10mg/l | 1.59∗ | .235 | .000 | 1.12 | 2.06 | ||
| 15mg/l | .89∗ | .235 | .000 | .42 | 1.36 | ||
| 30mg/l | -.70∗ | .235 | .004 | -1.17 | -.24 | ||
| 30mg/l | 0mg/l | 3.37∗ | .235 | .000 | 2.90 | 3.84 | |
| 10mg/l | 2.30∗ | .235 | .000 | 1.83 | 2.76 | ||
| 15mg/l | 1.59∗ | .235 | .000 | 1.12 | 2.06 | ||
| 20mg/l | .70∗ | .235 | .004 | .24 | 1.17 | ||
Based on observed means.
The error term is Mean Square (Error) =0.748.
∗ The mean difference is significant at the 0.05 level.
3.2.2. Changes in Regeneration Rate of Different Temperatures at the Same Concentration
At the same concentration, the results showed that the regeneration rate of the planarian was the slowest at 15°C and the fastest at 20°C (Figure 3). When the concentration was 30 mg/l, the average regeneration time of the head, trunk, and tail parts at 15°C was about 10-14 days (Figure 3(a)), while the regeneration time was not more than 9 days at 20°C (Figure 3(b)). The difference is obvious, and the same is true at the concentrations of 20 mg/l, 15 mg/l, and 10 mg/l. It showed that Fe3+ had a low toxic effect on the planarian in a low temperature, and at a suitable temperature, the regeneration rate of the planarian could be significantly suppressed. According to the results displayed in Table 1, there was a significant difference in the regeneration rate between 15°C and 20°C and between 15°C and 25°C, but there is no significant difference between 20°C and 25°C. The speeds of the planarian regeneration were not much different, and it is estimated that the suitable regeneration temperature of planarian would be between 20°C and 25°C.
Table 1.
Comparison of significant differences among the three groups at different temperatures.
| Dependent Variable: Regeneration days | |||||||
|---|---|---|---|---|---|---|---|
| (I) temperature | (J) temperature | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||||
| LSD | 15°C | 20°C | 2.56∗ | .182 | .000 | 2.19 | 2.92 |
| 25°C | 2.56∗ | .182 | .000 | 2.19 | 2.92 | ||
| 20°C | 15°C | -2.56∗ | .182 | .000 | -2.92 | -2.19 | |
| 25°C | .00 | .182 | 1.000 | -.36 | .36 | ||
| 25°C | 15°C | -2.56∗ | .182 | .000 | -2.92 | -2.19 | |
| 20°C | .00 | .182 | 1.000 | -.36 | .36 | ||
Based on observed means.
The error term is Mean Square (Error) = 0.748.
∗ The mean difference is significant at the 0.05 level.
4. Discussion
4.1. Effect of Fe3+ on the Survival of Planarian at Different Temperatures
Horvat T described that the herbicide might cause damage to the outer mucosa and other tissues of planarian and cause DNA damage [25]. Pagan OR reported that dimethyl sulfoxide (DMSO) can affect various aspects of the toxicity and behavioral effects in the planarian, revealing restricted levels of drug use and the effects on aquatic organisms [31, 32], and Guecheva Temenouga N [23] studied the effects of copper on the stressor protein response and catalase activity. In the study of Ofoegbu PU [33], the work showed that the S. mediterranea is sensitive to TBT, and TBT can influence the planarian survival, locomotion, head regeneration, and DNA damage. In the present study, iron ions are used to investigate the poison effect to planarian. Iron ions are a kind of trace elements necessary for animals and plants, and they are also a kind of water pollutants.
The study compared the effects of Fe3+ on the mortality of the planarian at different temperatures for different time periods. The mortality of the control group was 0. At the same temperature and different concentrations, with the increase of the concentration of Fe3+, the mortality of the planarian was distinct. The higher the concentration of Fe3+, the higher the mortality. At 15°C, the planarian all died in the 70 mg/l concentration of Fe3+ solution for about 36 h (Figures 2(a) and 2(b)). When the concentration was decreased, the time to reach 100% mortality is also extended, but it still caused death damage to the planarian body. Similarly, at 20°C and 25°C, the mortality rate was increased because of the increased concentration. At the same concentration and different temperatures, it can be seen from Figure 2 that, at the lowering temperature of 15°C, the death rate of the planarian is slower than other temperatures. At 20°C, the rate of death of the planarian was the fastest. This may be caused by the high activity of the related enzymes and the fastest metabolism at 20°C, which may accelerate the toxic effects of Fe3+ on the planarian. The slow metabolism at 15°C may weaken the toxic effect of Fe3+. At 25°C, the temperature-induced stress on the planarian was not obvious, and the effect of the worm's metabolism on the toxic effect was slightly smaller than that at 20°C. That is, the speed of the planarian death at the same concentration at 20°C is the fastest and at 25°C and 15°C is the slowest.
4.2. Effects of Fe3+ on the Regeneration of Planarian at Different Temperatures
According to the analysis, at the same temperature and different concentrations, with the increase of Fe3+ concentration, the regeneration rate of the head, trunk, tail, and adult of the planarian decreased, and the regeneration completion time was prolonged. At the same concentration and different temperature, the regeneration rate of the planarian at 20°C is slightly faster than that of the other temperature conditions, but it is not much different from that at 25°C (Table 1). The statistical analysis by SPSS software showed that there was a significant difference between 15°C and 20°C and between 15°C and 25°C, but there was no significant difference between 20°C and 25°C, which indicated that low temperature has a significant impact on the rate of regeneration of the planarian, and it was estimated that the optimum regeneration temperature of the planarian should be between 20°C and 25°C. Temperature can affect many biology aspects of planarian Schmidtea mediterranea [34]. As to planarian Dugesia japonica, in the previous study, the results showed that the optimal regeneration temperature of the eye point is 22°C [35, 36], and the optimal regeneration temperature of the planarian was 22°C [37], which was consistent with the present study. But there are differences about planarian Schmidtea mediterranea; it was reported that, at 19°C, the appearance of the eyes from the trunk was confirmed at five days after amputation; at 26°C and 28°C, the eyes were observed at three days after amputation[28]. However, the optimal living conditions of the planarian are 18°C-20°C; whether it is easier to regenerate for lower creatures under adversity condition, further research is needed. The regeneration rate was the slowest at 15°C, which may be because of the low activity of related enzymes in the planarian. In addition, Table 3 showed the differences between the different treatment concentrations. There were significant differences between groups which further showed that Fe3+ had strong toxic effects on the regeneration of planarian. In addition, it was found that there was decomposition of the head of the 30 mg/l Fe3+ treated planarian at 20°C and 25°C, indicating that the head of the planarian is less resistant to toxicity, so the survival rate was lower than that of the trunk and the tail parts, but the specific reasons still need further research. In this analysis, the time of regeneration of the head was the fastest. Results showed that there were significant differences in regeneration rates between different body parts (Table 2), but in natural conditions, they already showed the regeneration differences between different body parts of the planarian. So, whether Fe3+ affects the regeneration rate and how does it work still need further study.
Table 2.
Comparison of regeneration days in planarian head, trunk and tail fragments.
| Dependent Variable: Regeneration days | |||||||
|---|---|---|---|---|---|---|---|
| (I)Part | (J) Part | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||||
| LSD | Head | trunk | -1.62∗ | .182 | .000 | -1.98 | -1.26 |
| Tail | -2.20∗ | .182 | .000 | -2.56 | -1.84 | ||
| trunk | Head | 1.62∗ | .182 | .000 | 1.26 | 1.98 | |
| Tail | -.58∗ | .182 | .002 | -.94 | -.22 | ||
| Tail | Head | 2.20∗ | .182 | .000 | 1.84 | 2.56 | |
| trunk | .58∗ | .182 | .002 | .22 | .94 | ||
Based on observed means.
The error term is Mean Square (Error) =0.748.
∗ The mean difference is significant at the 0.05 level.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31550005, 31350004).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors report no conflicts of interest in this work.
Authors' Contributions
Xue Ding and Linxia Song contributed equally to this work.
References
- 1.Balistrieri L. S., Mebane C. A. Predicting the toxicity of metal mixtures. Science of the Total Environment. 2014;466-467:788–799. doi: 10.1016/j.scitotenv.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 2.Rabadjieva D., Tepavitcharova S., Todorov T., Dassenakis M., Paraskevopoulou V., Petrov M. Chemical speciation in mining affected waters: the case study of Asarel-Medet mine. Environmental Modeling & Assessment. 2009;159(1-4):353–366. doi: 10.1007/s10661-008-0634-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim J. J., Kim Y. S., Kumar V. Heavy metal toxicity: an update of chelating therapeutic strategies. Journal of Trace Elements in Medicine and Biology. 2019;54:226–231. doi: 10.1016/j.jtemb.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Mackay A. K., Taylor M. P. Floodwater metal contaminants in an australian dryland river: a baseline for assessing change downstream of a major lead-zinc-silver and copper mine. Journal of Environmental Quality. 2013;42(2):474–483. doi: 10.2134/jeq2010.0349. [DOI] [PubMed] [Google Scholar]
- 5.Geny P., Dohen E. Measures against water pollution in the iron and steel industry. Pure and Applied Chemistry. 1972;29(1-3):191–200. doi: 10.1351/pac197229010191. [DOI] [PubMed] [Google Scholar]
- 6.Bossuyt B. T., Janssen C. R. Copper toxicity to different field-collected cladoceran species: intra- and inter-species sensitivity. Environmental Pollution. 2005;136(1):145–154. doi: 10.1016/j.envpol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Heijerick D. G., Bossuyt B. T., De Schamphelaere K. A., et al. Effect of varying physicochemistry of European surface waters on the copper toxicity to the green alga pseudokirchneriella subcapitata. Ecotoxicology. 2005;14(6):661–670. doi: 10.1007/s10646-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen T., Hou H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth factor-β/Smad signaling pathway. Journal of Photochemistry and Photobiology B: Biology. 2016;162:633–640. doi: 10.1016/j.jphotobiol.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Li H., Zhang J., Wang T., et al. Elemental selenium particles at nano-size (Nano-Se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquatic Toxicology. 2008;89(4):251–256. doi: 10.1016/j.aquatox.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Langiano V. d., Martinez C. B. Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2008;147(2):222–231. doi: 10.1016/j.cbpc.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Jan A. T., Azam M., Siddiqui K., Ali A., Choi I., Haq Q. M. R. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. International Journal of Molecular Sciences. 2015;16(12):29592–29630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J. Heavy metal toxicity and the environmen. Experientia Supplementum. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huat T. J., Camats-Perna J., Newcombe E. A., Valmas N., Kitazawa M., Medeiros R. Metal toxicity links to alzheimer's disease and neuroinflammation. Journal of Molecular Biology. 2019;431(9):1843–1868. doi: 10.1016/j.jmb.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flora S. J. S., Mittal M., Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian Journal of Medical Research. 2008;128(4):501–523. [PubMed] [Google Scholar]
- 15.Halon-Golabek M., Borkowska A., Herman-Antosiewicz A., Antosiewicz J. Iron metabolism of the skeletal muscle and neurodegeneration. Frontiers in Neuroscience. 2019;13, article 165 doi: 10.3389/fnins.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris T. H., Hoyer K. K., Oviedo N. J. Innate immune system and tissue regeneration in planarians: an area ripe for exploration. Seminars in Immunology. 2014;26(4):295–302. doi: 10.1016/j.smim.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkholz T. R., Van Huizen A. V., Beane W. S. Staying in shape: planarians as a model for understanding regenerative morphology. Seminars in Cell & Developmental Biology. 2019;87:105–115. doi: 10.1016/j.semcdb.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Lau A. H., Knakievicz T., Prá D., Erdtmann B. Freshwater planarians as novel organisms for genotoxicity testing: analysis of chromosome aberrations. Environmental and Molecular Mutagenesis. 2007;48(6):475–482. doi: 10.1002/em.20307. [DOI] [PubMed] [Google Scholar]
- 19.Prá D., Lau A. H., Knakievicz T., Carneiro F. R., Erdtmann B. Environmental genotoxicity assessment of an urban stream using freshwater planarians. Mutation Research - Genetic Toxicology and Environmental Mutagenesis. 2005;585(1-2):79–85. doi: 10.1016/j.mrgentox.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Maranho L. A., Baena-Nogueras R. M., Lara-Martín P. A., DelValls T. A., Martín-Díaz M. L. Bioavailability, oxidative stress, neurotoxicity and genotoxicity of pharmaceuticals bound to marine sediments. The use of the polychaete Hediste diversicolor as bioindicator species. Environmental Research. 2014;134:353–365. doi: 10.1016/j.envres.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Ma K., Yang Y., Shi C., Chen G., Liu D. CuZnSOD and MnSOD from freshwater planarian Dugesia japonica: cDNA cloning, mRNA expression and enzyme activity in response to environmental pollutants. Aquatic Toxicology. 2019;208:12–19. doi: 10.1016/j.aquatox.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., Ma K., Yang Y., Shi C., Chen G., Liu D. Molecular cloning, characterization, expression and enzyme activity of catalase from planarian Dugesia japonica in response to environmental pollutants. Ecotoxicology and Environmental Safety. 2018;165:88–95. doi: 10.1016/j.ecoenv.2018.08.083. [DOI] [PubMed] [Google Scholar]
- 23.Guecheva T. N., Erdtmann B., Benfato M. S., Henriques J. A. P. Stress protein response and catalase activity in freshwater planarian Dugesia (Girardia) schubarti exposed to copper. Ecotoxicology and Environmental Safety. 2003;56(3):351–357. doi: 10.1016/S0147-6513(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Knakievicz T., Ferreira H. B. Evaluation of copper effects upon Girardia tigrina freshwater planarians based on a set of biomarkers. Chemosphere. 2008;71(3):419–428. doi: 10.1016/j.chemosphere.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Horvat T., Kalafatić M., Kopjar N., Kovačević G. Toxicity testing of herbicide norflurazon on an aquatic bioindicator species – the planarian Polycelis felina (Daly.) Aquatic Toxicology. 2005;73(4):342–352. doi: 10.1016/j.aquatox.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Mo Y., Zhou L., Wang Y., Wang Z., Zhao B. Induction of hsp70, hsp90, and catalase activity in planarian Dugesia japonica exposed to cadmium. Toxicology & Industrial Health. 2016;32(8):1373–1380. doi: 10.1177/0748233714561488. [DOI] [PubMed] [Google Scholar]
- 27.Plusquin M., Stevens A.-S., van Belleghem F., et al. Physiological and molecular characterisation of cadmium stress in Schmidtea mediterranea. The International Journal of Developmental Biology. 2012;56(1–3):183–191. doi: 10.1387/ijdb.113485mp. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Zhang B., Yi H., Zhao B. Mortality and antioxidant responses in the planarian (Dugesia japonica) after exposure to copper. Toxicology & Industrial Health. 2014;30(2):123–131. doi: 10.1177/0748233712452600. [DOI] [PubMed] [Google Scholar]
- 29.Guecheva T., Henriques J. A., Erdtmann B. Genotoxic effects of copper sulphate in freshwater planarian in vivo, studied with the single-cell gel test (comet assay) Mutation Research - Genetic Toxicology and Environmental Mutagenesis. 2001;497(1-2):19–27. doi: 10.1016/S1383-5718(01)00244-3. [DOI] [PubMed] [Google Scholar]
- 30.Grebe E., Schaeffer D. J. Planarians in toxicology, standardization of a rapid neurobehavioral toxicity test using phenol in a crossover study. Bulletin of Environmental Contamination and Toxicology. 1991;46(6):866–870. doi: 10.1007/BF01689731. [DOI] [PubMed] [Google Scholar]
- 31.Pagán O. R., Rowlands A. L., Urban K. R. Toxicity and behavioral effects of dimethylsulfoxide in planaria. Neuroscience Letters. 2006;407(3):274–278. doi: 10.1016/j.neulet.2006.08.073. [DOI] [PubMed] [Google Scholar]
- 32.Pagán O. R., Rowlands A. L., Fattore A. L., et al. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. European Journal of Pharmacology. 2009;615(1-3):118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ofoegbu P. U., Simão F. C., Cruz A., Mendo S., Soares A. M., Pestana J. L. Toxicity of tributyltin (TBT) to the freshwater planarian Schmidtea mediterranea. Chemosphere. 2016;148:61–67. doi: 10.1016/j.chemosphere.2015.12.131. [DOI] [PubMed] [Google Scholar]
- 34.Hammoudi N., Torre C., Ghigo E., Drancourt M. Temperature affects the biology of Schmidtea mediterranea. Scientific Reports. 2018;8(1, article 14934) doi: 10.1038/s41598-018-33355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Z., Yuwen Y., Wang Q., Chen G., Liu D. Expression analysis of Djsix-1 gene during regeneration of planarian eyespots. Molecular Biology Reports. 2011;38(6):3977–3982. doi: 10.1007/s11033-010-0515-2. [DOI] [PubMed] [Google Scholar]
- 36.Cheng G., Cheng F., Wang Q., Dong Z., Liu D. Influence of temperature on dugesia japonica eyespots regeneration. Journal of Henan Normal University. 2012;40(6):122–125. [Google Scholar]
- 37.Li X. Temperatures effect for turbellarians regeneration. Journal of Linyi Normal College. 2000;22(6):43–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.