Abstract
Background
The role of KIF18A in tumorigenesis and tumor development has been well studied in several cancers, but not in prostate cancer. In this study, we investigated the potential prognostic utility of KIF18A and its role in prostate cancer progression.
Material/Methods
We collected prostate cancer and paracancerous tissue samples from the same patient. Immunohistochemical staining was performed to investigate the KIF18A expression levels in the clinical sample. The Cancer Genome Atlas (TCGA) database was analyzed via a bioinformatics approach to gain insight into the relationship between KIF18A expression and prognosis. We examined the effect of KIF18A knockdown on PC-3 cell proliferation via colony formation and MTT assays. Flow cytometry was used to assess the effect of KIF18A knockdown on PC-3 cell apoptosis. Transwell invasion assay was performed to assess whether KIF18A affects the invasion ability of PC-3 cells.
Results
The KIF18A protein level was higher in PCa tissue than in paracancerous tissue. The In addition, upregulated KIF18A suggested a poor tumor stage and prognosis for prostate cancer patients. Our in vitro experiments demonstrated that KIF18A knockdown in PC-3 cells significantly inhibited proliferation and metastasis.
Conclusions
High KIF18A expression in prostate cancer patients predicts a poor prognosis. KIF18A knockdown inhibits prostate cell proliferation and metastasis. Therefore, this study confirms the usefulness of KIF18A as an oncological prognostic indicator and a potential therapeutic target for prostate cancer.
MeSH Keywords: Cell Proliferation, Lymphatic Metastasis, Prognosis, Prostatic Neoplasms, Survival Analysis
Background
As the most common and non-cutaneous malignant disease in men in the United States, prostate cancer (PCa) is one of the leading causes of death in older men. In 2018, it was estimated that approximately 164 690 men would be newly diagnosed with prostate cancer and that it would lead to 29 430 deaths in the United States [1]. Although most prostate cancer cases show indolent tumor behavior, it remains a significant public health problem. It is the cancer with the second highest mortality in males. Patients with localized prostate cancer can undergo radical prostatectomy, which leads to good clinical outcomes, but some patients experience clinical relapse and die from the malignancy [2,3]. The first evidence of prostate cancer relapse is an increase in the level of prostate-specific antigen. Prostate-specific antigen (PSA) is a protein secreted primarily by prostatic epithelial cells; it is one of the most effective serum biomarkers available and has been used in clinical practice for many years [4–6]. Moreover, researchers have demonstrated that serum ALP level is an independent predictor of bone metastasis of prostate cancer [7]. However, its use can also lead to many problems, such as overdiagnosis and overtreatment, due to its lack of specificity and poor ability to reflect tumor aggressiveness [4]. Therefore, new prognostic factors are needed for detecting biomedical recurrence and predicting overall survival to establish accurate prognoses in prostate cancer patients after radical prostatectomy.
A growing number of kinesin family members are thought to be involved in the development and progression of tumors. Kinesin functions in the meiotic phase of cells, regulating cell division and cell cycle. The kinesin superfamily has been reported to be closely associated with invasion, metastasis, and poor prognosis a variety of cancers. It was further demonstrated that the ATP-binding domain of kinesin is essential for resistance to docetaxel. Ganguly et al. reported that MCAK plays a key role in microtubule detachment and is responsible for the resistance to paclitaxel [8,9]. Several compounds that inhibit 2 mitotic kinesins (KIF11 and CENPE) have entered Phase I and II clinical trials, either as monotherapies or in combination with other drugs [10]. Among the kinesin family members, researchers have suggested that high expression of KIF4A [11] and KIF22 [12] is associated with poor prognosis of prostate cancer. As a member of the kinesin-8 family, KIF18A is a multifunctional protein that uses adenosine triphosphate (ATP) hydrolysis to generate force and movement along microtubules [13,14]. It is involved in a variety of cellular functions, including cell division, movement, microtubule dynamics, and organelle transport. Scientists speculate that KIF18A may affect cell differentiation and even cancer development [15–18]. It is noteworthy that several recent reports have suggested that KIF18A is also involved in tumor behavior and could be useful as a potential biomarker in breast, colorectal, and hepatocellular cancers [19–25]. However, there are no studies on the relevance of KIF18A expression and its clinicopathological significance in prostate cancer, and the correlation between KIF18A and prostate cancer remains elusive.
In this study, we investigated the expression of KIF18A in prostate cancer tissues and its impact on the prognosis of patients with prostate cancer. In order to gain a deeper understanding of the mechanism of action of KIF18A in the development of prostate cancer, we systematically explored the roles of KIF18A in prostate cancer cells.
Material and Methods
Patients and specimens
After obtaining authorization from the Ethics Review Committee of our institution (approval 2018YJ008), prostate cancer patients (n=85) who underwent laparoscopic radical prostatectomy (LRP) at Tianjin Union Medical Center were recruited for the study. All of the patients were diagnosed with primary prostate cancer based on pathology, and none of them had received androgen deprivation treatment, radiation therapy, or chemotherapy before the surgery. We collected prostate cancer and paracancerous tissue (the term “paracancerous tissue” is defined herein as “normal adjacent prostate tissue”) samples from each patient. Fresh samples were collected just after surgery and fixed in 10% formalin before being embedded in paraffin wax. The following clinical and pathological parameters were recorded: age, preoperative serum PSA, pathological stage, Gleason score, seminal vesicle invasion status, and lymph node status.
Antibodies
The antibodies used for immunohistochemistry (IHC) and Western blot included antibodies to KIF18A (Cat. No. 19245, Proteintech, Manchester, UK); PCNA (ab92552. Abcam, Cambridge, MA, USA); Ki67 (ab16667. Abcam, Cambridge, MA, USA); caspase-3 (ab13847. Abcam, Cambridge, MA, USA); Bcl-2 (ab32124. Abcam, Cambridge, MA, USA); MMP-9 (ab76003. Abcam, Cambridge, MA, USA), and GAPDH (ab181602. Abcam, Cambridge, MA, USA).
Bioinformatics analysis
Gene Expression Profiling Interactive Analysis (GEPIA) [26] was used to perform the survival analysis. Overall survival and disease-free survival rates obtained from the TCGA public database were used to investigate the connection between the KIF18A mRNA level and patient survival time. The analysis included 477 patients (243 high-KIF18A patients and 234 low-KIF18A patients).
Immunohistochemical staining
The IHC assay to measure KIF18A expression was performed on 4-μm sections of the formalin-fixed, paraffin-embedded samples. After baking at 75°C for 40 min, deparaffinization of sections was performed in xylene followed by rehydration using an ethanol concentration gradient following standard procedures. The slices were immersed in antigen activation liquid (pH 9.0) and heated to 95°C in a microwave oven for 20 min. The endogenous peroxidase activity and nonspecific immunoglobulin binding were blocked via individual incubation in 5% hydrogen peroxide and 10% normal goat serum for 15 min. Subsequently, the sections were incubated with polyclonal rabbit anti-KIF18A antibody for 12 h at 4°C. After the sample was returned to room temperature, we added the secondary antibody and incubate the sample for 30 min. After DAB staining, the slices were counterstained with Meyer hematoxylin. Next, xylene was used for dehydration and then removed. To analyze the results, the staining intensities (0: negative/weak staining, 1: moderate staining, 2: strong staining) and distribution areas (0: less than 5% positive staining; 1: 5% to 50% positive staining; 2: more than 50% positive staining) were recorded. The expression levels (low expression: 0–2; high expression: 3–4) were represented by the sum of the above 2 parameters. Uropathologists confirmed that the normal prostate tissue acinus was small and round, the basal cells were flat and in a continuous line at the base of the gland, and the nucleus was arranged neatly. Two independent uropathologists blinded to the clinical data evaluated the scores, and the means of the scores were calculated and used as the final immunostaining score.
Cell culture
All the conventional prostate cancer cell lines used in this study – PC-3, VCap, C4-2, LNCaP, and RWPE-1 – were obtained from ATCC (Manassas, VA, USA). The characteristics of the above cell lines were as follow. The PC-3 cell line is isolated from tissue of patients with castration-resistant prostate cancer, which is derived from the patient’s bone metastases. The PC-3 cell line is a highly malignant cancer cell with a fast growth rate and strong heterogeneous tumor-forming ability. The PC-3 cell line does not express AR and PSA and is not sensitive to androgen stimulation. The VCap cell line was originally drawn from spinal metastases in a prostate cancer patient who was not affected by hormones. The VCap cell line is sensitive to androgen. The LNCaP cell line is a prostate cancer cell line obtained from a patient’s living tissue specimen, and it was isolated from a lymph node metastasis in a hormone-dependent prostate cancer patient. The LNCaP cell line simultaneously expresses AR and PSA and is sensitive to androgen stimulation. In the C42 cell line, the researchers mixed LNCaP cells with human bone stromal cell line MS and implanted them in the skin of nude mice to form a xenogenic implant tumor and then castrated the mice. Twelve weeks after the castration treatment, the researchers removed the tumor for primary culture to obtain the castration-resistant C42 cell line [27]. The RWPE-1 cell line is derived from human normal prostate epithelial cells. All the cell lines were cultured in RPMI 1640 medium (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco, Waltham, MA USA) at 37°C with 5% CO2.
Colony formation assays
Cells in logarithmic growth phase were digested with trypsin and homogenized into individual cells. Culture dishes were inoculated at the appropriate cell density and then incubated for 7 days in a 37°C, 5% CO2 incubator. The cells were cultured for 5–7 days according to a conventional cell culture method, and then the colonies were stained. The staining solution was washed away with PBS, and the colonies were counted and imaged.
Quantitative RT-PCR (qRT-PCR)
Total cellular RNAs were extracted by TRIzol reagent (Invitrogen, Thermo Fisher Scientific, MA, USA) according to the manufacturer’s instructions. cDNA from RNAs was generated using a TaqMan RNA reverse transcription kit (Applied Biosystems) following the manufacturer’s protocol. The KIF18A mRNA reverse primers were: F: 5′-TGCTGGGAAGACCCACACTAT-3′ and R: 5′-GCTGGTGTAAAGTAAGTCCATGA-3′. TaqMan Universal Master Mix II was used to determine the expression of KIF18A mRNA in qRT-PCR.
In vitro transfection
PC-3 cells were seeded in 6-well plates and then we added preparing plasmid (ORIGENE, KIF18A human shRNA plasmid, TL303697, HuSH shRNA RFP Cloning Vector, TR30014, USA) mixed with Transfection Reagent (Roche, 6365787001, Switzerland) and incubated for 48 h at 37°C, then Western blot analysis was performed to verify the efficiency of KIF18A silencing.
Western blot
We prepared protein samples according to conventional methods. Electrophoresis was performed using a conventional method. After the end of the electrophoresis, the proteins were transferred to a PVDF membrane. Then, the PVDF membrane was incubated with dried skimmed milk powder for 1 h. After washing the milk from the membrane with TBST, the membrane was incubated with primary antibody. After incubation with the secondary antibody solution for 1 h, TBST was used to remove the excess secondary antibody, and the blot was visualized (Tanon Science & Technology Co., Shanghai, China).
MTT assays
We dissolved 0.5 g of MTT in 100 ml of PBS, and the solution was sterilized by filtration through a 0.22-μL filter and stored at 4°C in the dark. After transfection for 48 h (as described above), cells were seeded in 96-well plates (100 μL/well, approximately 2×103 cells/well). The cells were cultured for several days. MTT solution and DMSO were added to each well. A microplate reader was used to measure the optical density of each well at a wavelength of 570 nm. Value-added active fold lines were drawn using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
Flow cytometry
Culture growth was terminated when the total number of cells reached 1×106. The cells were digested with trypsin, centrifuged at 1000 rpm for 3 min, and the supernatant was discarded. The cells were fixed with 70% ice-cold alcohol and allowed to stand at 4°C overnight. The cells were washed twice with ice-cold PBS, centrifuged, stained with a fluorescent solution (PE Annexin V Apoptosis, Cat No. 559763, BD Pharmingen™), and then gently mixed. After incubation for 30 min at room temperature, the cells were analyzed and mapped using a flow cytometer (Cytomics FC 500 MCL, Beckman Coulter, Inc. USA).
Transwell invasion assay
Matrigel was pre-heated to 4°C until it was liquid, then add the liquefied Matrigel to the Transwell chamber (Costar, Corning, Inc. NY, USA) for 8 h. The cell concentration was adjusted to 2×108/L with serum-free medium. We added 500 μL of fetal bovine serum-containing medium to the lower Transwell chamber, and 100 μL of the cell suspension was added to the upper Transwell chamber. Cells were incubated for at 37°C with 5% CO2. After 24–36 h, cells passing through the filter were stained and counted.
Statistical analysis
Statistical analysis was conducted with SPSS 22.0 software (SPSS, IBM Corporation, Armonk, NY, USA). Differences between categorical variables were evaluated using the chi-square test. The analyses of overall and disease-free survival were performed on the GEPIA website [26]. The Cox proportional hazard model was applied for multivariate analysis of the independent prognostic factors. Two-side values of P<0.05 were considered significant.
Results
KIF18A expression is upregulated in PCa
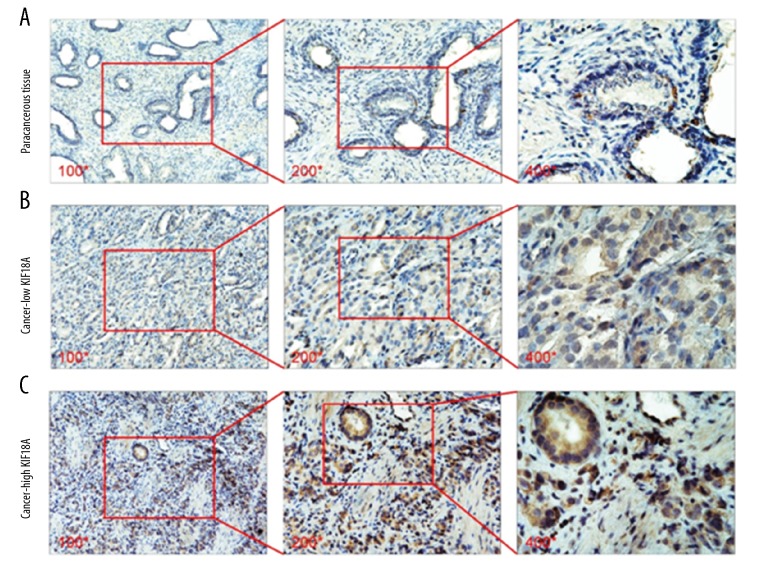
IHC was performed to measure the KIF18A protein levels in 85 pairs of PCa and paracancerous tissue samples. As shown in Figure 1, the KIF18A protein expression in PCa tissue was higher than that in paracancerous tissue (Figure 1A, 1C). KIF18A was mainly expressed in the cytoplasm, with some slight nuclear expression in the prostate tumor cells (Figure 1C). Overall, we observed that the KIF18A level was elevated in 25.9% (22/85) of the paracancerous tissue samples and 70.6% (60/85) of the prostate cancer tissue samples. Table 1 shows the statistical analysis of the above IHC experiments.
Figure 1.
Immunostaining to evaluate KIF18A expression in PCa and paracancerous tissue samples. (A) Immunostaining showed that KIF18A expression was absent or weak in paracancerous tissue. The pictures were taken by an optical microscope. The magnification of the pictures from left to right is 100 times, 200 times, and 400 times. (B) Immunostaining showed the low KIF18A expression in the cytoplasm and nucleus of PCa cells. The magnification of the pictures from left to right is 100 times, 200 times, and 400 times. (C) Immunostaining showed the high KIF18A expression in the cytoplasm and nucleus of PCa cells. The magnification of the pictures from left to right is 100 times, 200 times, and 400 times.
Table 1.
Expression of KIF18A in prostate cancer (PCa) and non-cancerous prostate tissues.
| Groups | KIF18A expression | P value# | ||
|---|---|---|---|---|
| n | High expression | % | ||
| Non-cancerous | 85 | 22 | 25.88% | <0.001* |
| PCa | 85 | 60 | 70.59% | |
P value was analyzed by chi-square test;
indicates P<0.05 with statistical significance.
The relationship between the KIF18A expression level and PCa clinicopathological features
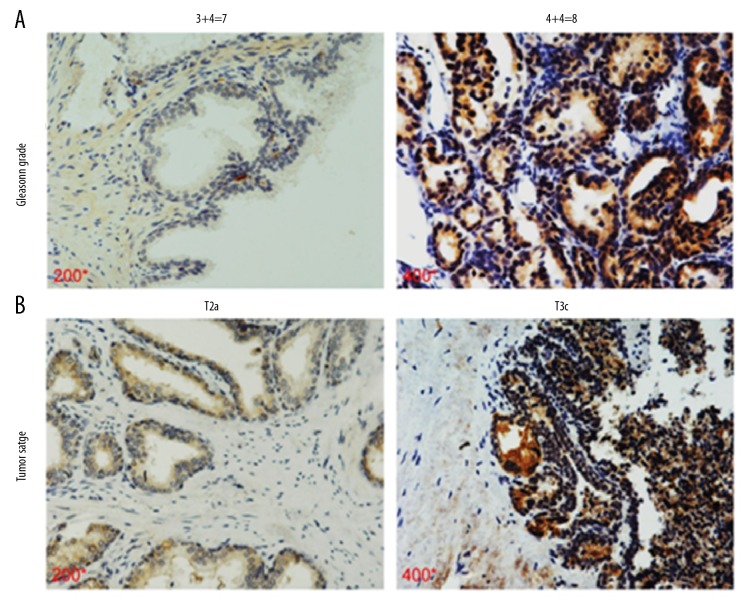
The immunohistochemical results showed that the KIF18A expression varied between low and high in the prostate cancer tissue samples (Figure 1B, 1C). We classified the PCa tissue specimens according to the KIF18A expression level based to the immunohistochemical scoring method described in the previous section. Our statistical results show that high KIF18A expression was correlated with tumor T stage (p=0.02), Gleason score (p=0.03), and lymph node metastasis status (p=0.03) (Table 2). Conversely, no significant differences were observed concerning age (p=0.80), preoperative PSA level (p=0.65), or seminal vesicle invasion (p=0.74). The IHC results also showed that PCa patients with higher Gleason grade (4+4=8) had higher levels of KIF18A expression than those with lower Gleason grade (3+4=7) (Figure 2A). Similarly, PCa patients with T3c stage had higher levels of KIF18A expression than PCa patients with T2a (Figure 2B). Based on these results, it appears that prostate cancer patients with high KIF18A expression are more likely have poor tumor stage and a higher chance for lymph node metastasis to occur.
Table 2.
Clinicopathologic variables and KIF18A expression in 85 prostate cancer patients.
| Variables | All n=85 | KIF18A | P value# | |
|---|---|---|---|---|
| Low n=25 | High n=60 | |||
| Age | ||||
| <65 | 46 | 13 | 33 | 0.8 |
| ≥65 | 39 | 12 | 27 | |
| Perioperative PSA | ||||
| >10 | 41 | 13 | 28 | 0.65 |
| ≤10 | 44 | 12 | 32 | |
| Tumor stage | ||||
| T2 | 35 | 15 | 20 | 0.02* |
| T3/T4 | 50 | 10 | 40 | |
| Gleason grade | ||||
| ≤7 | 46 | 18 | 28 | 0.03* |
| >7 | 39 | 7 | 32 | |
| Lymph node metastasis | ||||
| No | 48 | 15 | 33 | 0.03* |
| Yes | 37 | 10 | 27 | |
| Seminal vesicle metastasis | ||||
| No | 25 | 8 | 17 | 0.74 |
| Yes | 60 | 17 | 43 | |
P value was analyzed by chi-square test;
indicates P<0.05 with statistical significance.
Figure 2.
The association between KIF18A and Gleason grade and prostate cancer malignancy. (A) Immunostaining showed the KIF18A expression in PCa specimens of different Gleason grade patients. The magnification of the pictures from left to right is 200 times and 400 times. KIF18A is expressed simultaneously in the cytoplasm and nucleus. (B) Immunostaining showed that KIF18A expression in PCa specimens of different tumor stage patients. The magnification of the pictures from left to right is 200 times and 400 times. KIF18A is expressed simultaneously in the cytoplasm and nucleus.
KIF18A is a poor prognostic indicator after radical prostatectomy
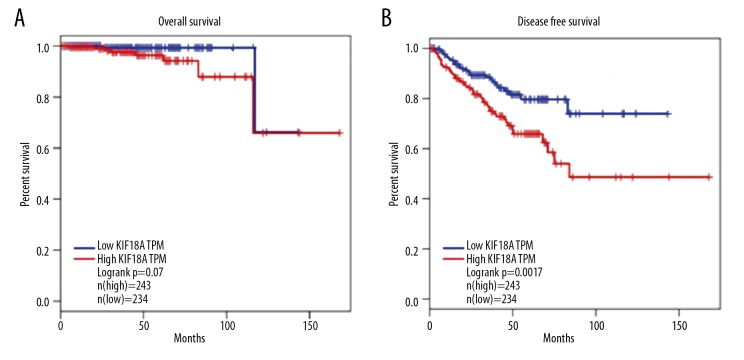
Since disease-free and overall survival are the most crucial parameters for PCa patients after radical prostatectomy [28], the Kaplan-Meier curve method was used to analyze the relationship between KIF18A expression level and overall survival and to analyze the relationship between KIF18A expression level and disease-free survival. All of the relevant data were obtained from the TCGA database. The median disease-free and overall survival times were 18 and 30 months, respectively. KIF18A mRNA expression was classified as low (n=234) or high (n=243) based on the median KIF18A mRNA expression level as the cut-off point. As shown in Figure 2, we found no significant difference in overall survival between the 2 groups (Figure 3A) (P=0.07 in the TCGA data set); however, it is worth noting that the disease-free survival time (P=0.0017, in TCGA dataset) was significantly different between the high- and low-KIF18A expression groups (Figure 3B).
Figure 3.
The association between KIF18A expression at the mRNA level and patient survival. (A) Kaplan-Meier survival analysis of overall survival associated with KIF18A expression in PCa. (B) Kaplan-Meier survival analysis of disease-free survival associated with KIF18A expression in PCa.
To further explore the prognostic value of KIF18A in PCa patients, univariate and multivariate Cox proportional hazard regression analyses were performed on the TCGA dataset to validate the clinical prognostic impact of KIF18A level (Table 3). A univariate analysis showed a significant association between disease-free survival and several factors, including KIF18A level (P=0.003), Gleason score (P<0.001), and pT stage (P<0.001). Moreover, multivariate Cox analysis showed that the Gleason score (P<0.001) is significantly associated with disease-free survival.
Table 3.
Prognostic value of KIF18A for the disease-free survival via Cox proportional hazards model.
| Covariant | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Exp(B) | 95% CI | P Value# | Exp(B) | 95% CI | P Value# | |
| KIF18A | 1.92 | 1.25–2.95 | 0.003* | 1.03 | 0.65–1.63 | 0.91 |
| Age | 1.03 | 1.00–1.06 | 0.10 | 1.00 | 0.98–1.04 | 0.67 |
| Gleason | 2.20 | 1.76–2.72 | <0.001* | 1.97 | 1.54–2.51 | <0.001* |
| pT stage | 2.62 | 1.73–3.95 | <0.001* | 1.58 | 0.98–2.56 | 0.06 |
Exp(B) indicates the exponent of B, it represents the hazard ratio; 95% CI indicates 95% confidence interval.
P value was analyzed by chi-square test;
indicates P<0.05 with statistical significance.
KIF18A expression is upregulated in PCa cells
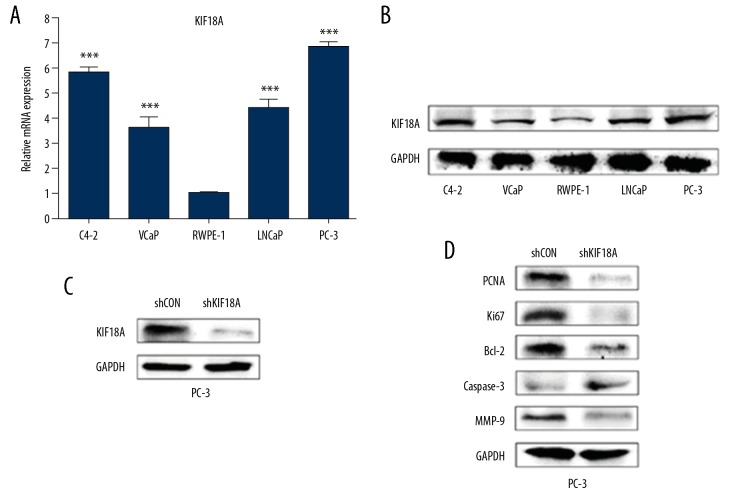
We used the C4-2, VCaP, LNCaP, and PC-3 human prostate cancer cell lines and the human normal prostate cell line RWPE-1 for our experiments. Firstly, qRT-PCR and Western blotting were performed to measure the KIF18A expression levels of mRNA and protein in these cell lines (Figure 4A, 4B). The results indicated that the expression level of KIF18A in both mRNA and protein were higher in the prostate cancer cell lines than the normal cells, which was consistent with the results from the tissue samples. We transfected PC-3 cells with the shKIF18A plasmid to obtain a PC-3shKIF18A cell line with stable KIF18A knockdown (Figure 4C).
Figure 4.
KIF18A expression is upregulated in PCa cells. (A) KIF18A mRNA expression levels in PC-3, VCap, C4-2, LNCaP, and RWPE-1 cells as measured by qRT-PCR. (B) Western blotting to detect the KIF18A protein expression levels in each of the above cell lines. (C) Western blot analysis of the effects of KIF18A knockdown after transfection of PC-3 cells with the shKIF18A plasmid. (D) Western blotting was used to measure the expression levels of corresponding protein in these 2 cell lines. GAPDH was used as an endogenous reference gene.
KIF18A has been shown to be involved in the processes of proliferation, apoptosis, and invasion in cancer cells, so we evaluated the expression levels of the corresponding proteins in different cell lines. Then, we observed the expression difference of PCNA and Ki67 in the PC-3shKIF18A cells and the control group, showing that cell proliferation was inhibited (Figure 4D). Furthermore, the Bcl2 expression level in the PC-3shKIF18A cells was lower than that in the control cells, while their caspase-3 expression level was higher than that of the control cells (Figure 4D). These results indicate that KIF18A knockdown effectively promoted apoptosis in PC-3 cells. In addition, the expression level of MMP-9 in PC-3shKIF18A cells was significantly lower than that in PC-3 cells (Figure 4D). This result indicates that knockdown of KIF18A inhibits invasion of PC-3 cells.
KIF18A knockdown inhibits PC-3 cell proliferation and metastasis
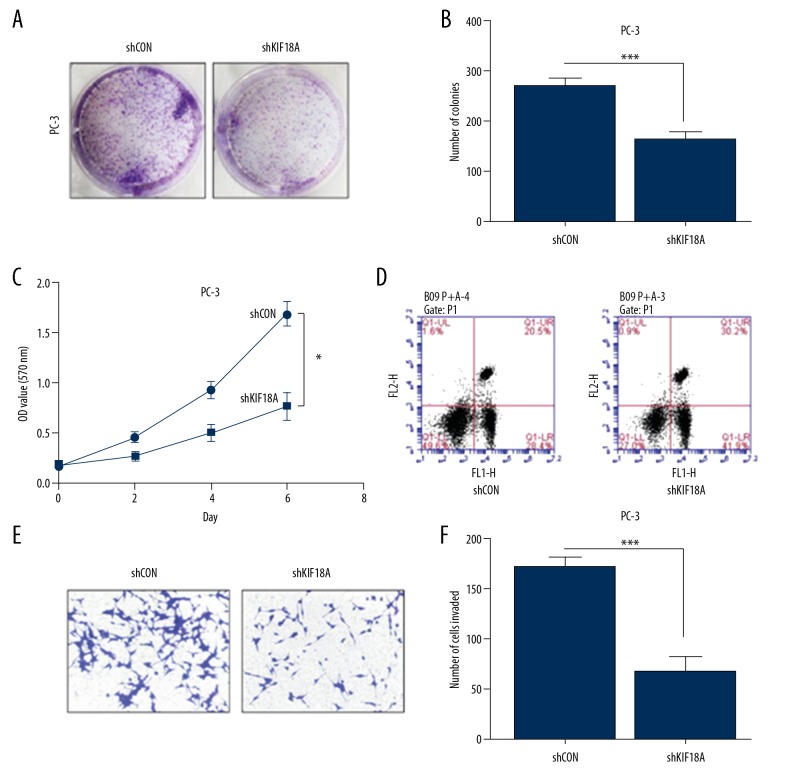
We further assessed the effect of KIF18A knockdown in prostate cancer cell lines. Colony formation assays showed a difference between the numbers of PC-3 and PC-3shKIF18A colonies (Figure 5A). The statistical analysis result suggested that knockdown KIF18A in PC-3 significantly decreased the number of colonies compared to the control cells (Figure 5B). MTT assays were used to observed the growth-inhibitory effect of knockdown KIF18A in PC-3 prostate cancer cells. As shown in the figure, the growth ability was inhibited in the PC-3shKIF18A cell line but not in the control group (Figure 5C). In summary, KIF18A knockdown significantly inhibited the proliferation of PC-3 prostate cancer cells. Moreover, flow cytometry results indicated that the number of apoptotic PC-3shKIF18A cells was significantly higher than the number of apoptotic PC-3 control cells (Figure 5D). Apart from this, after knocking down KIF18A, the invasive ability of PC-3 was significantly reduced (Figure 5E). The number of PC-3shKIF18A cells invading was significantly lower than the number of PC-3 cells invading (Figure 5F). Therefore, we propose that KIF18A knockdown promotes apoptosis in the PC-3 prostate cancer cell line.
Figure 5.
Knockdown of KIF18A suppresses the proliferation and metastasis of PC-3 cells. (A) A colony formation assay to measure the growth of PC-3 cells (left) and PC-3 cells after KIF18A knockdown (right). Cell colonies were stained with crystal violet staining solution. (B) The number of cell colonies in each group in Figure 5A. Drafting and statistical analysis using GraphPad Prism 5 software. (*** P<0.001) (C) An MTT assay was used to monitor the growth of PC-3 cells after KIF18A knockdown. The absorbance values were measured at a wavelength of 570 nm. Comparison of the difference between the 2 sets of data on the sixth day. (* P<0.05). (D) Flow cytometry was used to detect apoptosis in PC-3 cells after KIF18A knockdown. The UL region indicates cell necrosis, the LL region indicates cell survival, the LR region indicates early cell apoptosis, and the UR region indicates late cell apoptosis. LR region+UR region indicates total apoptosis. (E) Transwell invasion assays showed the invasive ability of the prostate cancer cells after knockdown of KIF18A. The invading cells were stained purple by Giemsa staining. (F) The number of invaded cells in each group in Figure 5E was counted. Drafting and statistical analysis using GraphPad Prism 5 software. (*** P<0.001).
Discussion
PCa is a heterogeneous and multifocal malignancy with an unpredictable prognosis [29–32]; therefore, it is necessary to find appropriate PCa diagnostic markers and therapeutic targets. Although the discovery of PSA and its use in diagnostics have supported early prostate cancer diagnosis [33], its ability to predict prognosis is still widely questioned by experts. Therefore, identifying an indicator that can efficiently, accurately, and feasibly predict the prognosis of prostate cancer is very important for clinical diagnosis [34–38].
Because KIF18A can affect cell mitosis, microtubule dynamics, and organelle transport, it is likely to be associated with tumor proliferation. Furthermore, scientists have concluded that KIF18A may affect the occurrence and development of tumors. Zhang et al. [20] reported that ectopic KIF overexpression affects mitosis, enhances cell multinucleation, and promotes cancer cell proliferation. These observations suggest that KIF18A can promote breast cancer cell proliferation. Kasahara et al. [19] and Nagahara et al. [21] demonstrated a relationship between KIF18A and prognosis in breast and colorectal cancer. Nagahara et al. [21] showed that KIF18A can be used as an independent predictor of overall survival after cancer surgery. These observations are consistent with our findings in prostate cancer and the clinical indicators in patients. Luo et al. [24] reported differences in KIF18A expression between cancerous and paracancerous tissues based on in vivo and in vitro experiments, and their conclusions are consistent with those of this study.
Through searches of the recent literature, we found that the present study is the first to explore the relationship between KIF18A and prostate cancer. In this study, we confirmed by immunohistochemistry that the KIF18A protein expression level was higher in prostate cancer tissue than in paracancerous tissue. The correlation between the KIF18A level and PCa clinical features was analyzed based on clinical patients. The results indicated that the KIF18A level is associated with tumor T stage (p=0.02), Gleason score (p=0.03), and lymph node metastasis status (p=0.03) in prostate cancer patients. SPSS statistical software and the Kaplan-Meier method were used to analyze overall and disease-free survival. We demonstrated that PCa patients with high KIF18A expression have a worse prognosis. In subsequent in vitro experiments, we demonstrated that KIF18A expression was significantly higher in prostate cancer cells than in normal prostate cells. Moreover, when KIF18A was knocked down in PC-3 prostate cancer cells, their proliferative capacity was significantly inhibited, while apoptosis was significantly increased. Various experimental data indicate that KIF18 is a key protein in the maintenance of prostate cancer cell proliferation. In addition, when KIF18A was knocked down, the invasive ability of PC-3 prostate cancer cells was suppressed. We conclude that KIF18A is a potential target for prostate cancer treatment.
The small sample size of this study limits the broader application of conclusions in the clinic. The mechanisms underlying the roles of KIF18A in PCa have not been clearly elucidated, and further in vivo experiments could lead to the identification of its carcinogenic mechanisms and reinforce the reliability of our conclusions. We will continue to thoroughly investigate the underlying mechanism of the role of KIF18A in prostate cancer development and progression. We plan to expand the clinical sample size and thoroughly follow up our results.
Conclusions
KIF18A expression is upregulated in PCa. High KIF18A expression in prostate cancer patients predicts a poor prognosis. KIF18A knockdown inhibits prostate cell proliferation and metastasis. Therefore, this study confirms the usefulness of KIF18A as an oncological prognostic indicator and a therapeutic target in clinical practice.
Footnotes
Source of support: This work was supported by the Tianjin Union Medical Center Research Project Fund (2018YJ008)
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Union Medical Center of Tianjin. The clinical trial registry number is 2018YJ008.
Data availability
The data used or analyzed during the present study are available from the corresponding author on reasonable request.
Conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 3.Roder MA, Brasso K, Christensen IJ, et al. Survival after radical prostatectomy for clinically localised prostate cancer: A population-based study. BJU Int. 2014;113(4):541–47. doi: 10.1111/bju.12065. [DOI] [PubMed] [Google Scholar]
- 4.Diamandis EP. Prostate-specific antigen: Its usefulness in clinical medicine. Trends Endocrinol Metab. 1998;9(8):310–16. doi: 10.1016/s1043-2760(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 5.Fenton JJ, Weyrich MS, Durbin S, et al. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(18):1914–31. doi: 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- 6.Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: A review of current evidence. JAMA. 2014;311(11):1143–49. doi: 10.1001/jama.2014.2085. [DOI] [PubMed] [Google Scholar]
- 7.Koo KC, Park SU, Kim KH, et al. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015;3(1):10–15. doi: 10.1016/j.prnil.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen T, Yang L, Zhang Z, et al. KIF20A affects the prognosis of bladder cancer by promoting the proliferation and metastasis of bladder cancer cells. Dis Markers. 2019;2019 doi: 10.1155/2019/4863182. 4863182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Gong H, Huang K. Oncogenic role of kinesin proteins and targeting kinesin therapy. Cancer Sci. 2013;104(6):651–56. doi: 10.1111/cas.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12(8):527–39. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- 11.Gao H, Chen X, Cai Q, et al. Increased KIF4A expression is a potential prognostic factor in prostate cancer. Oncol Lett. 2018;15(5):7941–47. doi: 10.3892/ol.2018.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Xie H, Zhu S, et al. High Expression of KIF22/kinesin-like DNA binding protein (Kid) as a poor prognostic factor in prostate cancer patients. Med Sci Monit. 2018;24:8190–97. doi: 10.12659/MSM.912643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 2001;98(13):7004–11. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407(6800):41–47. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- 15.Weaver LN, Ems-McClung SC, Stout JR, et al. Kif18A uses a microtubule binding site in the tail for plus-end localization and spindle length regulation. Curr Biol. 2011;21(17):1500–6. doi: 10.1016/j.cub.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y, English CA, Ohi R. The kinesin-8 Kif18A dampens microtubule plus-end dynamics. Curr Biol. 2010;20(4):374–80. doi: 10.1016/j.cub.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 17.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 18.Mayr MI, Storch M, Howard J, Mayer TU. A non-motor microtubule binding site is essential for the high processivity and mitotic function of kinesin-8 Kif18A. PLoS One. 2011;6(11):e27471. doi: 10.1371/journal.pone.0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasahara M, Nagahara M, Nakagawa T, et al. Clinicopathological relevance of kinesin family member 18A expression in invasive breast cancer. Oncol Lett. 2016;12(3):1909–14. doi: 10.3892/ol.2016.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Zhu C, Chen H, et al. Kif18A is involved in human breast carcinogenesis. Carcinogenesis. 2010;31(9):1676–84. doi: 10.1093/carcin/bgq134. [DOI] [PubMed] [Google Scholar]
- 21.Nagahara M, Nishida N, Iwatsuki M, et al. Kinesin 18A expression: Clinical relevance to colorectal cancer progression. Int J Cancer. 2011;129(11):2543–52. doi: 10.1002/ijc.25916. [DOI] [PubMed] [Google Scholar]
- 22.Rucksaken R, Khoontawad J, Roytrakul S, et al. Proteomic analysis to identify plasma orosomucoid 2 and kinesin 18A as potential biomarkers of cholangiocarcinoma. Cancer Biomark. 2012;12(2):81–95. doi: 10.3233/CBM-130296. [DOI] [PubMed] [Google Scholar]
- 23.Tooker BC, Newman LS, Bowler RP, et al. Proteomic detection of cancer in asbestosis patients using SELDI-TOF discovered serum protein biomarkers. Biomarkers. 2011;16(2):181–91. doi: 10.3109/1354750X.2010.543289. [DOI] [PubMed] [Google Scholar]
- 24.Luo W, Liao M, Liao Y, et al. The role of kinesin KIF18A in the invasion and metastasis of hepatocellular carcinoma. World J Surg Oncol. 2018;16(1):36. doi: 10.1186/s12957-018-1342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan LD, Xu YY, Yu Y, et al. Serum HER2 level measured by dot blot: A valid and inexpensive assay for monitoring breast cancer progression. PLoS One. 2011;6(4):e18764. doi: 10.1371/journal.pone.0018764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Z, Li C, Kang B, et al. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu HC, Hsieh JT, Gleave ME, et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Int J Cancer. 1994;57(3):406–12. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 28.Abd Elmageed ZY, Yang Y, Thomas R, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–97. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filella X, Alcover J, Molina R. Active surveillance in prostate cancer: The need to standardize. Tumour Biol. 2011;32(5):839–43. doi: 10.1007/s13277-011-0193-2. [DOI] [PubMed] [Google Scholar]
- 30.Morrison GJ, Goldkorn A. Development and application of liquid biopsies in metastatic prostate cancer. Curr Oncol Rep. 2018;20(4):35. doi: 10.1007/s11912-018-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: A review. JAMA. 2017;317(24):2532–42. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 32.Carlsson J. Potential for clinical radionuclide-based imaging and therapy of common cancers expressing EGFR-family receptors. Tumour Biol. 2012;33(3):653–59. doi: 10.1007/s13277-011-0307-x. [DOI] [PubMed] [Google Scholar]
- 33.Tabayoyong W, Abouassaly R. Prostate cancer screening and the associated controversy. Surg Clin North Am. 2015;95(5):1023–39. doi: 10.1016/j.suc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Khorasani M, Teimoori-Toolabi L, Farivar TN, et al. Aberrant expression of miR-141 and nuclear receptor small heterodimer partner in clinical samples of prostate cancer. Cancer Biomark. 2018;22(1):19–28. doi: 10.3233/CBM-170696. [DOI] [PubMed] [Google Scholar]
- 35.Dai Y, Li D, Chen X, et al. Circular RNA myosin light chain kinase (MYLK) promotes prostate cancer progression through modulating Mir-29a expression. Med Sci Monit. 2018;24:3462–71. doi: 10.12659/MSM.908009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Tang H, Huang J, An H. Upregulation of CXCR7 is associated with poor prognosis of prostate cancer. Med Sci Monit. 2018;24:5185–91. doi: 10.12659/MSM.906180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cozar JM, Robles-Fernandez I, Martinez-Gonzalez LJ, et al. Genetic markers a landscape in prostate cancer. Mutat Res. 2018;775:1–10. doi: 10.1016/j.mrrev.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Panigrahi GK, Ramteke A, Birks D, et al. Exosomal microRNA profiling to identify hypoxia-related biomarkers in prostate cancer. Oncotarget. 2018;9(17):13894–910. doi: 10.18632/oncotarget.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]