Abstract
Background
Irritable bowel syndrome (IBS) is a common, chronic disorder that leads to decreased health‐related quality of life and work productivity. A previous version of this review was not able to draw firm conclusions about the effectiveness of homeopathic treatment for IBS and recommended that further high quality RCTs were conducted to explore the clinical and cost effectiveness of homeopathic treatment for IBS. Two types of homeopathic treatment were evaluated in this systematic review: 1. Clinical homeopathy where a specific remedy is prescribed for a specific condition; 2. Individualised homeopathic treatment, where a homeopathic remedy based on a person's individual symptoms is prescribed after a detailed consultation.
Objectives
To assess the effectiveness and safety of homeopathic treatment for IBS.
Search methods
For this update we searched MEDLINE, CENTRAL, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine Database (AMED), the Cochrane IBD Group Specialised Register and trials registers from inception to 31 August 2018.
Selection criteria
Randomised controlled trials (RCTs), cohort and case‐control studies that compared homeopathic treatment with placebo, other control treatments, or usual care, in adults with IBS were considered for inclusion.
Data collection and analysis
Two authors independently assessed the risk of bias and extracted data. The primary outcome was global improvement in IBS as measured by an IBS symptom severity score. Secondary outcomes included quality of life, abdominal pain, stool frequency, stool consistency, and adverse events. The overall certainty of the evidence supporting the primary and secondary outcomes was assessed using the GRADE criteria. We used the Cochrane risk of bias tool to assess risk of bias. We calculated the mean difference (MD) and 95% confidence interval (CI) for continuous outcomes and the risk ratio (RR) and 95% CI for dichotomous outcomes.
Main results
Four RCTs (307 participants) were included. Two studies compared clinical homeopathy (homeopathic remedy, asafoetida or asafoetida plus nux vomica) to placebo for IBS with constipation (IBS‐C). One study compared individualised homeopathic treatment (consultation plus remedy) to usual care for the treatment of IBS in female patients. One study was a three armed RCT comparing individualised homeopathic treatment to supportive listening or usual care. The risk of bias in three studies (the two studies assessing clinical homeopathy and the study comparing individualised homeopathic treatment to usual care) was unclear on most criteria and high for selective reporting in one of the clinical homeopathy studies. The three armed study comparing individualised homeopathic treatment to usual care and supportive listening was at low risk of bias in four of the domains and high risk of bias in two (performance bias and detection bias).
A meta‐analysis of the studies assessing clinical homeopathy, (171 participants with IBS‐C) was conducted. At short‐term follow‐up of two weeks, global improvement in symptoms was experienced by 73% (46/63) of asafoetida participants compared to 45% (30/66) of placebo participants (RR 1.61, 95% CI 1.18 to 2.18; 2 studies, very low certainty evidence). In the other clinical homeopathy study at two weeks, 68% (13/19) of those in the asafoetida plus nux vomica arm and 52% (12/23) of those in the placebo arm experienced a global improvement in symptoms (RR 1.31, 95% CI 0.80 to 2.15; very low certainty evidence). In the study comparing individualised homeopathic treatment to usual care (N = 20), the mean global improvement score (feeling unwell) at 12 weeks was 1.44 + 4.55 (n = 9) in the individualised homeopathic treatment arm compared to 1.41 + 1.97 (n=11) in the usual care arm (MD 0.03; 95% CI ‐3.16 to 3.22; very low certainty evidence).
In the study comparing individualised homeopathic treatment to usual care, the mean IBS symptom severity score at 6 months was 210.44 + 112.4 (n = 16) in the individualised homeopathic treatment arm compared to 237.3 + 110.22 (n = 60) in the usual care arm (MD ‐26.86, 95% CI ‐88.59 to 34.87; low certainty evidence). The mean quality of life score (EQ‐5D) at 6 months in homeopathy participants was 69.07 (SD 17.35) compared to 63.41 (SD 23.31) in usual care participants (MD 5.66, 95% CI ‐4.69 to 16.01; low certainty evidence).
For In the study comparing individualised homeopathic treatment to supportive listening, the mean IBS symptom severity score at 6 months was 210.44 + 112.4 (n = 16) in the individualised homeopathic treatment arm compared to 262 + 120.72 (n = 18) in the supportive listening arm (MD ‐51.56, 95% CI ‐129.94 to 26.82; very low certainty evidence). The mean quality of life score at 6 months in homeopathy participants was 69.07 (SD 17.35) compared to 63.09 (SD 24.38) in supportive listening participants (MD 5.98, 95% CI ‐8.13 to 20.09; very low certainty evidence).
None of the included studies reported on abdominal pain, stool frequency, stool consistency, or adverse events.
Authors' conclusions
The results for the outcomes assessed in this review are uncertain. Thus no firm conclusions regarding the effectiveness and safety of homeopathy for the treatment of IBS can be drawn. Further high quality, adequately powered RCTs are required to assess the efficacy and safety of clinical and individualised homeopathy for IBS compared to placebo or usual care.
Keywords: Female, Humans, Male, Constipation, Constipation/etiology, Constipation/therapy, Dietary Fiber, Dietary Fiber/therapeutic use, Homeopathy, Homeopathy/methods, Irritable Bowel Syndrome, Irritable Bowel Syndrome/therapy, Phytotherapy, Phytotherapy/methods, Quality of Life, Randomized Controlled Trials as Topic
Plain language summary
Homeopathy for treatment of irritable bowel syndrome
What is irritable bowel syndrome?
Irritable bowel syndrome (IBS) is a common chronic disorder where a person experiences the following symptoms: abdominal pain, discomfort, bloating, constipation or diarrhoea or both. It is difficult to treat because different people experience different symptoms. Some people experience constipation as the main symptom, this form of IBS is known as IBS‐C, while others experience diarrhoea as the main symptom. This form of IBS is known as IBS‐D. Others experience both constipation and diarrhoea, this form of IBS is known as IBS‐M where the M stands for mixed. Currently there is no agreement on the best form of treatment for IBS.This means that it is important to evaluate the effectiveness and safety of treatments, including homeopathic treatment, which some IBS sufferers use.
What is homeopathy?
There are different types of homeopathy. Clinical homeopathy matches a 'remedy' to a specific condition, such as IBS and everybody who has that condition would be given the same remedy. Individualised homeopathy involves a series of in‐depth consultations to assess symptoms and other issues that may affect the patient. Following an in‐depth consultation the homeopath will select the most appropriate remedy based on the persons' individual symptoms. Individualised homeopathy includes both a consultation and a remedy, whereas clinical homeopathy consists of a remedy without the in‐depth consultation.
What did the researchers investigate?
The researchers investigated whether homeopathic treatment led to the improvement of the symptoms of IBS in people with IBS.
What did the researchers find?
Four randomised controlled trials (RCTs) with 307 participants with IBS were included. Two RCTs (129 participants) compared a homeopathic remedy (asafoetida and asafoetida plus nux vomica) to a placebo remedy for the treatment of people with IBS‐C. One study (23 participants) compared individualised homeopathic treatment to usual care in female patients diagnosed with IBS. One study (94 participants) was a three armed study comparing individualised homeopathic treatment plus usual care, supportive listening plus usual care and usual care.
The four trials tested the effects of homeopathic treatment on the severity of IBS symptoms. No conclusions can be drawn from the RCT comparing individualised homeopathic treatment to usual care due to the small number of participants and the low quality of reporting in this trial. This study was carried out in 1990 and usual care for IBS may have changed since then making the results difficult to compare to current treatments.
No conclusions can be drawn from the three armed study comparing individualised homeopathic treatment plus usual care, supportive listening plus usual care and usual care due to the small number of participants in the homeopathic treatment arm (n=16).
The results of two small studies were combined (129 participants) and this suggested that there may be a possible benefit for clinical homeopathy, using the remedy asafoetida, over placebo for patients with IBS‐C at a short‐term follow‐up of two weeks. However both of the studies were carried out in the 1970s when the reporting of trials was not as comprehensive as it is now and we are very uncertain about these results and cannot suggest a possible benefit for clinical homeopathy.
Conclusions
The results for the outcomes assessed in this review are uncertain. Thus no firm conclusions regarding the effectiveness and safety of homeopathy for the treatment of IBS can be drawn. Further high quality RCTs enrolling larger numbers of patients are required to assess the effectiveness and safety of clinical and individualised homeopathy for IBS.
Summary of findings
Summary of findings for the main comparison. Homeopathy versus placebo.
| Homeopathy versus placebo for the treatment of irritable bowel syndrome | ||||||
| Patient or population: patients with irritable bowel syndrome Settings: outpatients Intervention: homeopathy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with homeopathy | |||||
| Global improvement (Asafoetida subgroup) Follow‐up: 2 weeks | 455 per 1000 | 732 per 1000 (536 to 991) | RR 1.61 (1.18 to 2.18) | 129 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | Global improvement defined as self improvement on a 3 point Likert scale |
| Global improvement (Asafoetida + nux vom subgroup) Follow‐up: 2 weeks | 522 per 1000 | 683 per 1000 (417 to 1000) | RR 1.31 (0.8 to 2.15) | 42 (1 study) | ⊕⊝⊝⊝ very low1,3,4 | Global improvement defined as self improvement on a 3 point Likert scale |
| Quality of life | Not reported | This outcome was not reported | ||||
| Abdominal pain | Not reported | This outcome was not reported | ||||
| Stool frequency | Not reported | This outcome was not reported | ||||
| Stool consistency | Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to high risk of bias 2 Downgraded one level due to sparse data (76 events) 3 Downgraded one level due to the endpoint time (2 weeks). Given the long term nature of IBS it is not clear how useful a two week outcome is for patients' and clinicians' decision making. 4 Downgraded two levels due to very sparse data ( 25 events)
Summary of findings 2. Homeopathy versus usual care.
| Homeopathy versus usual care for the treatment of irritable bowel syndrome | ||||||
| Patient or population: patients with irritable bowel syndrome Settings: outpatients Intervention: homeopathy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Homeopathy | |||||
| Global improvement (Feeling unwell) Follow‐up: 12 weeks | The mean global improvement score was 1.41 (SD = 1.97) | The mean global improvement score was 1.44 (SD = 4.55) MD 0.03 higher (3.16 lower to 3.22 higher) |
‐ | 20 (1 study) | ⊕⊝⊝⊝ very low1,2 | Global improvement defined as a self reported improvement of symptoms. Scale from: 0 to 5 Higher scores indicate greater improvement |
| Quality of life | Not reported | This outcome was not reported | ||||
| Abdominal pain | Not reported | This outcome was not reported | ||||
| Stool frequency | Not reported | This outcome was not reported | ||||
| Stool consistency | Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to high risk of bias 2 Downgraded two levels due to very sparse data (20 participants)
Summary of findings 3. Homeopathy plus usual care versus usual care.
| Homeopathy plus usual care versus usual care for the treatment of irritable bowel syndrome | ||||||
| Patient or population: patients with irritable bowel syndrome Settings: outpatients Intervention: homeopathy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Homeopathy | |||||
| Global improvement (IBS‐SSS) Follow‐up: 6 months | The mean global improvement score was 237.3 (SD = 110.27) | The mean global improvement score was 210.44 (SD = 112.4) MD 26.86 lower (88.59 lower to 34.87 higher) |
‐ | 76 (1 study) | ⊕⊕⊝⊝ low1,2 | IBS‐SSS. Scale from: 0 to 400 Lower scores indicate less severe disease |
|
Quality of life Follow‐up 6 months |
The mean quality of life score was 63.41 (SD = 23.31) | The mean quality of life score was 69.07 (SD = 17.35) MD 5.66 higher (4.69 lower to 16.01 higher) |
‐ | 76 (1 study) | ⊕⊕⊝⊝ low1,2 | EQ‐5D Higher scores indicate better quality of life |
| Abdominal pain | Not reported | This outcome was not reported | ||||
| Stool frequency | Not reported | This outcome was not reported | ||||
| Stool consistency | Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to sparse data (76 participants) 2 Downgraded one level due to high risk of bias
Summary of findings 4. Homeopathy plus usual care versus supportive listening plus usual care.
| Homeopathy plus usual care versus supportive listening plus usual care for treatment of irritable bowel syndrome | ||||||
| Patient or population: patients with irritable bowel syndrome Settings: outpatients Intervention: Homeopathy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Homeopathy | |||||
| Global improvement (IBS‐SSS) Follow‐up: 6 months | The mean global improvement score was 262 (SD = 120.72) | The mean global improvement score was 210.44 (SD = 112.4) MD 51.56 lower (129.94 lower to 26.82 higher) |
‐ | 34 (1 study) | ⊕⊝⊝⊝ very low1,2 | IBS‐SSS. Scale from: 0 to 400 Lower scores indicate less severe disease |
|
Quality of life Follow‐up: 6 months |
The mean quality of life score was 63.09 (SD = 24.38) | The mean quality of life score was 69.07 (SD = 17.35) MD 5.98 higher (8.13 lower to 20.09 higher) |
‐ | 34 (1 study) | ⊕⊝⊝⊝ very low1,2 | EQ‐5D Higher scores indicate better quality of life |
| Quality of life | Not reported | This outcome was not reported | ||||
| Abdominal pain | Not reported | This outcome was not reported | ||||
| Stool frequency | Not reported | This outcome was not reported | ||||
| Stool consistency | Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded two levels due to very sparse data (34 participants) 2 Downgraded one level due to high risk of bias
Background
Description of the condition
Irritable bowel syndrome (IBS) is a common, chronic disorder that affects 10 to 22% of the population in the UK (Williams 2007). The economic costs of IBS in primary care in the UK are estimated to be over GBP 200 million per year (Akehurst 2002). It is difficult to treat because no single cause has been identified. Treatment is directed at controlling symptoms, using pharmacological and non‐pharmacological approaches (Ruepert 2011; Spiller 2007; Zijdenbos 2009).
IBS is characterised by recurrent symptoms (i.e. abdominal pain or discomfort, bloating, constipation, or diarrhoea) that indicate a dysfunctional gastrointestinal tract rather than an organic change or specific diagnosis. It has an uncertain prognosis for recovery (Mearin 2006). Such patients have a plethora of non‐colonic symptoms such as back pain, urinary frequency, and chronic fatigue which can lead to the patient being referred to the wrong specialty and having inappropriate investigations and even surgery. This can lower quality of life (Agrawal 2006; Longstreth 2007). In addition, sleep disturbance and depressed mood are common in IBS patients.
Diagnosis of IBS can be made using the Rome IV criteria (Drossman 2016), although this is largely a research tool used to allow common reporting standards of symptoms in trials and other research populations. In clinical practice the diagnosis of IBS is largely based on symptoms and should be positive rather than by exclusion, although the presence of alarm symptoms (e.g. blood in stool, weight loss or family history) should prompt further investigations (Spiller 2007). IBS can be characterised into the following subtypes: IBS with constipation, IBS with diarrhoea, IBS with mixed bowel habits and unspecified.
Usual care for IBS commonly includes advice on lifestyle, including diet and stress reduction, possibly combined with medication. There are a number of different medications used to help treat IBS: antispasmodic medicines, which help to reduce abdominal pain and cramping; laxatives, which help to treat the symptoms of constipation; anti‐motility medicines, which help to treat the symptoms of diarrhoea, and neuropathic modulators such as tricyclic antidepressants, which were originally designed to treat depression, but also help to reduce the feeling of abdominal pain and cramping. Alternative treatments such as hypnotherapy, psychotherapy and acupuncture have been tried and have a place in selected patients (Agrawal 2006,McPherson 2012). However these treatments have limited availability and are expensive and labour intensive. Despite much research into both psychological and pharmacological treatments for irritable bowel syndrome no consensus exists on its optimal treatment (Ruepert 2011; Zijdenbos 2009).
Description of the intervention
Homeopathy is a popular, albeit controversial form of complementary and alternative medicine. A UK survey has shown that 1.9% of the population consulted a homeopath in the 12 months prior to the survey and 8.6% had bought an over‐the‐counter homeopathic remedy (Thomas 2001). Homeopathy is based on treating patients with remedies prepared from substances that have been highly diluted and succussed (shaken). It was first developed by Samuel Hahnemann in the eighteenth century in Germany and works on the principle of “like cures like” whereby a substance that would cause symptoms in a healthy person cures those same symptoms in illness.
Homeopathic treatment varies among different practitioners and four main types can be identified (Linde 1997):
Individualised (or classical) homeopathy, the type most commonly practised in the UK, involves a consultation followed by the prescription of a homeopathic medicine individualised to the patient;
Clinical homeopathy, where the same homeopathic medicine is used for a group of patients all presenting with the same clinical condition (e.g. lycopodium for IBS, arnica for bruising);
Complex homeopathy, where a number of different homeopathic medicines are given either in a fixed combination or concurrently; and
Isopathy, where the homeopathic medicine is based on the substance which has led to the problem (e.g. grass pollen for hay fever).
Homeopathic medicines when prescribed by trained professionals are generally regarded as safe (Dantas 2000).
How the intervention might work
Homeopathy is based on the ‘law of similars’ i.e. a substance which causes symptoms in a healthy individual can be used to treat similar symptoms in a diseased person (Vithoulkas 1980).There is significant debate regarding the scientific basis for homeopathy amongst healthcare practitioners, scientists, politicians and policy makers and the mechanism by which homeopathic remedies may work is not completely understood.
The manufacture of homeopathic medicines involves serial dilution alternating with violent agitation (i.e. ‘succussion’). The combination of these two processes is referred to as ‘potentisation’ or ‘sequential kinetic activation’ (Gariboldi 2009). Many homeopathic medicines are diluted beyond Avogadro’s number and therefore fall under the classification of ultra‐high dilutions (UHDs). Avogadro's number is the number of molecules in a mole of a substance, approximately 6.0225 × 1023, which means that a sample diluted beyond 1024 would have reached a stage where it is very unlikely that there is even a single molecule of the original substance present. The biological efficacy of UHDs may be dependent on sequential kinetic activation (Gariboldi 2009), but the mechanism by which sequential kinetic activation enables a UHD to be biologically active is unknown. A common theory is that it involves stable water structures, created by interactions between molecules of the biological material and the water it is dissolved in, allowing the water to retain information about the biological material (Montagnier 2009).
Why it is important to do this review
This review is an update of a previously published Cochrane review on homeopathy for irritable bowel syndrome (Peckham 2012). The original review marked a small step forward in establishing whether or not homeopathy is an effective treatment for IBS; however due to the small number of studies, age of the studies and methodological limitations the original review provided only limited information. This updated review is timely not only because of the passage of time but also given the continued lack of effective treatments for IBS and the sustained interest in homeopathy as a potential treatment option. Lower gastrointestinal tract disorders account for one in 20 of all general practice consultations in the UK (Thompson 2000). In addition, gastroenterology problems are the fourth most common referral to National Health Service (NHS) homeopathic hospitals (Spence 2005) and one of the eight most common conditions treated by NHS homeopaths in general practice (Mathie 2006). The frequency with which people with IBS consult homeopaths may be some indication of the value which they place on the homeopathic approach. Homeopathic treatment may offer a treatment strategy for patients with IBS, but at present it is not clear if it offers any benefit.
Objectives
The objective of this systematic review is to assess the effectiveness and safety of homeopathic treatment for IBS.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing homeopathic treatment with placebo or active comparators were considered for inclusion regardless of blinding method, publication status and language of publication. Quasi randomised studies were also considered for inclusion, where allocation was achieved by 'quasi‐random' methods such as alternation between treatment arms, year of birth, month entered into study. Cohort and case‐control studies were also considered for inclusion.
Types of participants
All trials of patients with a diagnosis of IBS were eligible for inclusion in this review regardless of age, gender, race, educational status or duration of IBS. Trials which included IBS patients in whom 10% or more had unstable psychiatric disorders, ulcerative colitis, Crohn's disease, bowel cancer and pregnant and breastfeeding women were excluded from this review.
Types of interventions
Trials were included if one of the groups in the trial received any type of homeopathic treatment involving the delivery of a homeopathic remedy (either by a homeopath following a consultation or studies where a homeopathic remedy was delivered without a consultation) and the other received placebo, an active comparator treatment, or no treatment.
Types of outcome measures
All trials that included any one of the following outcome measures were included in the review.
Primary outcomes
The primary outcome was global improvement of symptoms (patient‐reported or clinician‐evaluated or both) as measured by a global IBS symptom score (e.g. IBS Severity Scoring System (IBS‐SSS), Adequate Relief Measure, GI Symptom Rating Scale, Functional Bowel Disorder Severity Index or IBS Symptom Questionnaire).
Secondary outcomes
Secondary outcomes included:
Quality of life as measured by validated quality of life measure e.g. EQ5D, SF36, IBS Quality of Life Measure, IBS Quality of Life Questionnaire, Functional Digestive Disorder Quality of Life Questionnaire, IBS Health Related Quality of Life Questionnaire;
Abdominal pain, discomfort and distension;
Stool frequency, bowel transit time;
Stool consistency; and
Adverse events.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched from inception to 31 August 2018:
The Cochrane Central Register of Controlled Trials (CENTRAL) on the Cochrane Library, Ovid MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and the Allied and Complementary Medicine Database (AMED). The Cochrane IBD/FBD Group Specialised Register, Clinical trials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) http://www.who.int/ictrp/en/ were also searched.
Searching other resources
1. Reference searching
The reference lists for all identified studies were inspected for additional studies.
2. Personal contact
The first author of each included study was contacted for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Two authors (EJP and SB) independently reviewed the titles and abstracts of the studies identified by the literature search. Included studies were assessed against the predefined inclusion criteria.
Data extraction and management
Two authors (EP and SB) independently extracted data from the included studies. Authors were contacted to clarify any unclear data. EP who is an author of one of the included studies was not involved in the data extraction or assessment of the risk of bias for the study that she was involved in the conduct of, GT extracted the data for this study.
Assessment of risk of bias in included studies
Two authors (SB and GT) independently assessed the methodological quality of included randomised trials using the Cochrane risk of bias tool (Higgins 2011). The following items were assessed:
sequence generation (i.e. was allocation sequence adequately generated?);
allocation sequence concealment (i.e. was allocation adequately concealed?);
blinding (i.e. was knowledge of the allocated interventions adequately prevented during the study?);
incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
other potential sources of bias (i.e. was the study apparently free of other problems that could lead to a high risk of bias e.g. baseline imbalances, evidence of carry‐over in cross‐over trials, comparability of groups in cluster trials).
It was intended that, based on these criteria the studies would be subdivided into three categories:
1. Low risk of bias i.e. all quality criteria met;
2. Medium risk of bias i.e. one or more of the quality criteria partly met; and
3. High risk of bias i.e. one or more of the quality criteria not met.
It was intended that the quality of quasi‐randomised trials, non‐randomised trials, cohort and case control studies would be assessed using a quality instrument designed for assessing the quality of non‐randomised studies (Downs 1998).
Using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (Atkins 2004, Schünemann 2011), we will assess the overall quality of the evidence supporting the following outcomes: global improvement of symptoms, quality of life, improvement in abdominal pain, stool frequency, stool consistency and adverse events. The summary of the evidence will be presented in a 'Summary of findings’ table, which will provide key information about the best estimate of the magnitude of the effect for each relevant comparison, and the rating of the overall confidence in effect estimates for each outcome. The GRADEpro Guideline Development Tool will be used to create the 'Summary of findings’ table.
Measures of treatment effect
Review Manager (RevMan 5.3.5) was used to analyse the data. For continuous outcomes the mean difference (MD) with 95% confidence interval (95% CI) was calculated. For each dichotomous outcome the risk ratio (RR) with 95% CI was calculated.
Unit of analysis issues
We did not anticipate any unit of analysis issues arising from cluster randomisation. In the case of multiple intervention groups each intervention group was analysed separately against the control group and the sample size for the control group was divided proportionately across each intervention group. We noted that if the results were reported at multiple time points in the studies, each outcome would be analysed at pre‐defined periods of follow‐up in separate meta‐analyses. Time points would be grouped as follows: less than three months, three months to one year, longer than one year. These time points were chosen as representing time frames in which a difference in the likelihood of responding could be expected.
Dealing with missing data
We intended to analyse data using the intention to treat (ITT) principle and sensitivity analyses were to be undertaken as appropriate (e.g. ITT versus available case, and study quality). However, data were analysed on an available case basis as the included studies did not provide enough detail to allow for an ITT analysis.
Assessment of heterogeneity
Statistical heterogeneity was assessed using the Chi² test and the I² statistic. If heterogeneity existed between studies (I² ≥ 50%) for the primary outcome, reasons for the heterogeneity would be explored. Clinical heterogeneity would be assessed through the description of the setting and homeopathic approach used in each study.
Assessment of reporting biases
In the protocol we noted that if more than 10 studies were identified for inclusion in this review, funnel plots would be used to assess publication biases.
Data synthesis
Data from individual trials were combined by meta‐analysis if the interventions, outcomes and patient groups were sufficiently similar (determined by consensus). For continuous data the MD with 95% CI was calculated where the same scales have been used. Where studies were deemed sufficiently similar but different scales have been used the standardised mean difference (SMD) would be used to combine data. For dichotomous outcomes the pooled RR and 95% CI were calculated.
In the protocol we specified that data would not be pooled for meta‐analysis if a high degree of heterogeneity (I² > 75%) was detected. A fixed‐effect model would be used to pool data in the absence of heterogeneity. An I² ≥ 50% is considered to represent moderate heterogeneity and in such cases (I² 50 to 75%) a random‐effects model would be used for pooling the data.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned between studies that prospectively identified IBS patients using ROME III criteria versus studies that did not use ROME III criteria to prospectively identify IBS patients. In the protocol we also noted that if data were reported separately for the different forms of IBS then a subgroup analysis comparing the different forms would be carried out. A subgroup analysis was also planned for quasi and true randomisation, different comparators (e.g. no treatment, usual care, placebo, or other active treatment) and different homeopathy interventions (e.g. individualised or clinical homeopathy).
Sensitivity analysis
In the protocol we noted that if a sufficient number of trials were identified a sensitivity analysis would be carried out by study quality to determine if the results of the primary analysis change according to which trials are incorporated into the analysis.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
Figure 1 shows details of the search and selection process. From citations initially identified, full text sources were examined (after removal of duplicates and assessment of abstract), studies were excluded for various reasons (listed in the excluded studies table) and four studies plus two secondary publications from two included studies were included in the review (Owen 1990; Peckham 2014; Rahlfs 1976; Rahlfs 1979). Two studies were included in quantitative synthesis (Rahlfs 1976; Rahlfs 1979). No cohort or case‐control studies were identified.
1.
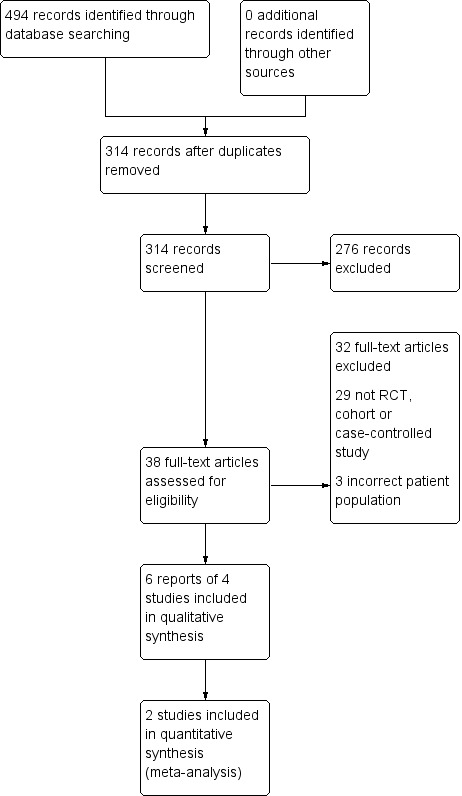
Study flow diagram.
Included studies
Four studies with a total of 307 participants were included (Owen 1990; Peckham 2014; Rahlfs 1976; Rahlfs 1979). See Characteristics of included studies. Owen 1990 and Peckham 2014 were conducted in the UK and published in English. Rahlfs 1976 and Rahlfs 1979 were conducted in the former Federal Republic of Germany and published in German and were translated from German into English. Rahlfs 1976, was a three arm trial comparing asafoetida against asafoetida + nux vomica, against placebo, whereas Rahlfs 1979 compared asafoetida versus placebo (the participants in the two trials are independent). The authors noted that Rahlfs 1976 failed to recruit its target number of participants, hence the (simplified) trial being re‐run. There were 23 participants in Owen 1990, 72 participants in Rahlfs 1976, 119 participants in Rahlfs 1979 and 94 participants in Peckham 2014. All included studies were published as full articles.
Owen 1990 compared individualised homeopathic treatment, which involved a homeopathic consultation and an individualised homeopathic remedy, to usual care which consisted of high doses of dicyclomine hydrochloride, faecal bulking agents and diet sheets asking the patient to take a high fibre diet. This study differs from other pragmatic trials of individualised homeopathic treatment, where the more common approach has been to compare individualised homeopathic treatment plus usual care to usual care alone. In Owen 1990 participants were asked to rate how unwell they felt before and after treatment, exact details of how this was scored are not given. Although Owen 1990 did not include a global measurement of IBS as one of the outcomes, we considered the rating of how unwell patients felt to provide a global measurement of the patients' health. The other outcome measures in Owen 1990 involved the patients choosing their own top four worst symptoms and grading these on a visual analogue scale, it was not specified that these symptoms had to be related to IBS, and details of the symptoms patients chose were not reported are not given, hence this outcome measure was not included in this review.
Peckham 2014 was a three armed trial that compared individualised homeopathic treatment plus usual care to supportive listening plus usual care to usual care alone. Unequal randomisation was used in Peckham 2014 with 16 participants allocated to individualised homeopathic treatment, 18 to supportive listening and 60 to usual care. Individualised homeopathic treatment involved a homeopathic consultation and an individualised homeopathic remedy. Supportive listening aimed to control for the time and attention given to the patient in the individualised homeopathic treatment arm and consisted of the same number of sessions of the same duration as the homeopathic consultation. In both the individualised homeopathic treatment and supportive listening arms patients were offered five one hour sessions with a homeopath or counsellor respectively. The primary outcome measure in Peckham 2014 was change in IBS‐SSS at 6 months.
None of the included studies reported on adverse events as an outcome.
Excluded studies
The Characteristics of excluded studies table, describes the characteristics of the 32 excluded studies along with the reason for their exclusion.
Ongoing studies
No ongoing studies were identified.
Risk of bias in included studies
The risk of bias in the included studies for each domain are discussed below. See results of the risk of bias analysis are summarized in Figure 2.
2.
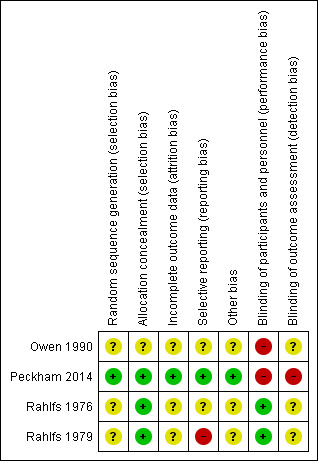
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Allocation
Owen 1990, Rahlfs 1976, Rahlfs 1979 and Peckham 2014 were described as RCTs. Owen 1990 reported that the participants were stratified and randomised into one of two treatment groups. However, no details were given about the stratification or how randomisation sequence was generated. Rahlfs 1976 reported that a chance code was used for randomisation, although what this entailed and how it was implemented was not described. Rahlfs 1979 did not report any information regarding the method of generation of the randomisation code. Peckham 2014 reported that the random sequence was generated by shuffling of sealed opaque envelopes containing the allocation and was reported as being of low risk of bias for allocation concealment. Rahlfs 1976 and Rahlfs 1979 provided medication in sequentially numbered drug containers and were rated as low risk for allocation concealment. Owen 1990 did not describe the procedure used for allocation concealment and was rated as unclear for this item.
Blinding
Participants and physicians were not blinded to treatment allocation in the Owen 1990 and Peckham 2014 studies as it was not possible to design a study where participants were not aware of their receiving an individualised homeopathic consultation, supportive listening or usual care. Owen 1990 did not report whether other key study personnel were blinded, or whether outcome assessment was carried out blind. In Peckham 2014 outcomes were participant reported and due to participants being aware of their allocation outcomes were at high risk of bias. In Rahlfs 1976 and Rahlfs 1979 the study participants and the doctors who recruited the participants were blinded to allocation by the use of an identical placebo. In Rahlfs 1979, the participant blinding was well described. Rahlfs 1976 and Rahlfs 1979 did not report whether other key study personnel were blinded, or if outcome assessment was carried out blind.
Incomplete outcome data
The number of patient withdrawals was reported for Owen 1990, Rahlfs 1976, Rahlfs 1979 and Peckham 2014. Although Owen 1990 reported the number of withdrawals and the arm from which the patients withdrew, the reasons for withdrawal were not reported. In Peckham 2014 the reasons for withdrawal were reported and a comparison of baseline data was made between those who had missing data and those who did not, this indicated that there was a relationship between age, employment status and missing data, hence employment status and age were included in the ANCOVA model for the primary outcome. Rahlfs 1976 did not report which arms that patients withdrew from and therefore it was not clear whether there may be attrition bias in this trial. Rahlfs 1979 reported the number of withdrawals from each treatment group and the reasons for withdrawal. Whilst dropouts appear to be comparable in terms of number and reason for withdrawal across both arms of this study (Rahlfs 1979), it should be remembered that any dropout threatens group comparability at baseline as random allocation seeks to distribute both known and unknown characteristics across groups, and dropouts may differ for unknown characteristics that cannot be measured.
Selective reporting
Due to insufficient reporting in Owen 1990 and Rahlfs 1976 both studies were rated as unclear for the item on selective reporting. Rahlfs 1979 was deemed to be at a high risk of bias due to selective reporting because of evidence of selective choice of data for an outcome. Some participants were excluded from the outcome analyses for not meeting the inclusion criteria while other participants who did not meet the inclusion criteria in terms of age were included in the analyses. Peckham 2014 was deemed to be at low risk of bias for selective reporting due to all the outcomes being reported in the protocol paper being presented in the main paper.
Other potential sources of bias
Due to the low quality of reporting in Owen 1990, Rahlfs 1976 and Rahlfs 1979, the potential for other sources of bias in these studies could not be assessed. No other potential sources of bias were identified in Peckham 2014, however it cannot be certain that there were no other sources of bias so this has been marked as unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Clinical homeopathic remedy versus placebo remedy
Rahlfs 1976 and Rahlfs 1979 assessed global improvement in IBS at two weeks as an outcome measure. For this outcome patients were asked to measure their improvement on a three‐point scale (Rahlfs 1976) and a four‐point scale (Rahlfs 1979). For the Rahlfs 1976 study participants were asked to rate whether they were not or negligibly improved, more than half improved or free of symptoms. Participants in the Rahlfs 1979 study were asked to rate whether they were worse, not or negligibly improved, more than half improved or free of symptoms. For the purposes of this review, we dichotomised these scales into two categories: those who had improved (more than half improved or free of symptoms) versus those who had not improved (those who were worse, or not or negligibly improved).
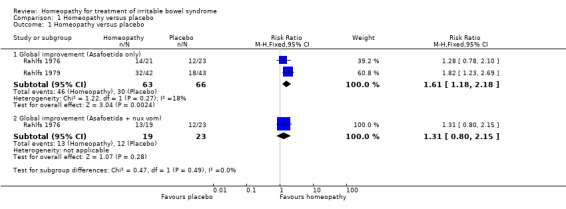
At short term follow up of two weeks, a pooled analysis (129 participants) indicated that there may be a benefit of the homeopathic treatment asafoetida over placebo. At short‐term follow up of two weeks, the RR for global improvement in symptoms for asafoetida versus placebo was 1.61 (95% CI 1.18 to 2.18; very low certainty evidence). Little heterogeneity was detected for this comparison (I² = 18%). For the study that compared homeopathic remedy (asafoetida plus nux vomica) to placebo the RR was 1.31 (95% CI 0.80 to 2.15; very low certainty evidence).
Homeopathic treatment versus usual care
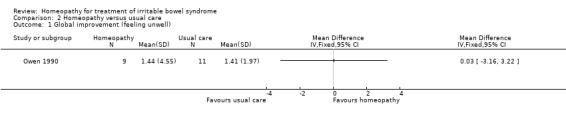
In Owen 1990 participants (N = 20) were asked to rate how unwell they felt before and after treatment. The effect of individualised homeopathic treatment was uncertain. The mean global improvement score (i.e. how unwell the participants felt ‐ a lower score indicates feeling more unwell) in the individualised homeopathic treatment arm was 1.44 (SD 4.55) compared to 1.41 (SD 1.97) in the usual care arm (MD 0.03, 95% CI ‐3.16 to 3.22; very low certainty evidence).
Homeopathic treatment plus usual care versus usual care
In Peckham 2014 participants were asked to complete the IBS‐SSS at baseline and at 6 months. The effect of individualised homeopathic treatment at 6 months was uncertain. In the individualised homeopathic treatment plus usual care arm the mean IBS‐SSS score was 210.44 (SD 112.4) compared to 237.30 (SD 110.22) in the usual care arm (MD ‐26.86, 95% CI ‐88.59 to 34.87; low certainty evidence). A lower score indicates less severity of symptoms.
Homeopathic treatment plus usual care versus supportive listening plus usual care
In Peckham 2014 participants were asked to complete the IBS‐SSS at baseline and at 6 months. The effect of homeopathic treatment compared to supportive listening was uncertain. At six months the mean IBS‐SSS score in the homeopathic treatment plus usual care arm was 210.44 (SD 112.4) compared to 262.00 (SD 120.72) in the supportive listening plus usual care arm (MD ‐51.56, 95% CI ‐129.94 to 26.82; very low certainty evidence). A lower score indicates less severity of symptoms.
Secondary outcome measures
The secondary outcomes quality of life, abdominal pain, stool frequency, stool consistency and adverse events were not reported on in Rahlfs 1976, Rahlfs 1979 or Owen 1990. Quality of life using the EQ‐5D was reported on in Peckham 2014. The effect of individualised homeopathic treatment on quality of life was uncertain. In the homeopathic treatment arm the mean EQ‐5D visual analogue score (VAS) at six months was 69.07 (SD 17.35) compared to 63.41 (SD 23.31) in the usual care arm (MD 5.66, 95% CI ‐4.69 to 16.01; low certainty evidence). In the homeopathic treatment arm the mean EQ‐5D score at six months was 69.07 (SD 17.35) compared to 63.09 (SD 24.38) in the supportive listening arm (MD 5.98, 95% CI ‐8.13 to 20.09; very low certainty evidence). A lower score indicates a worse quality of life.
Pooling of results
Outcome data from the Owen 1990 study was not pooled with the data from Rahlfs 1976 and Rahlfs 1979 because of heterogeneity between the studies. The three studies investigated two different types of homeopathy. For the same reason outcome data from Peckham 2014 was not pooled with outcome data from Rahlfs 1976 and Rahlfs 1979. Owen 1990 and Peckham 2014 investigated the effectiveness of individualised (classical) homeopathic treatment, whilst Rahlfs 1976 and Rahlfs 1979 investigated clinical homeopathy. The type of IBS investigated was also potentially different. In the Owen 1990 and Peckham 2014 studies participants were diagnosed with IBS and no further information on type was given, whilst the participants in Rahlfs 1976 and Rahlfs 1979 had constipation‐predominant IBS. In addition, the studies measured outcomes at different time points. Peckham 2014 measured outcomes at 26 weeks and Owen 1990 measured outcomes at 12 weeks, whilst Rahlfs 1976 and Rahlfs 1979 measured outcomes at 2 weeks. The primary outcome for the Owen 1990 study was not a global improvement measure and was not comparable with the other three studies. Although it may be tempting to combine studies in a meta‐analysis when it is likely to yield a statistically significant result, it is important not to combine studies where there is significant clinical heterogeneity, because these results would not be meaningful due to the large degree of differences between the studies. For these reasons the outcomes from Owen 1990, Peckham 2014, Rahlfs 1976 and Rahlfs 1979 were not combined.
Discussion
Summary of main results
Two RCTs compared a clinical homeopathic remedy (asafoetida and asafoetida plus nux vomica) with placebo for treating IBS‐C (Rahlfs 1976; Rahlfs 1979). In a meta‐analysis of these studies, the homeopathic remedy found that there may be a benefit of the remedy over placebo for improvement in global IBS symptoms at a short‐term follow‐up of two weeks. However, this result should be interpreted with caution due to the low quality of the reporting in these studies, a high or unknown risk of bias associated with the trials in this pooled analysis, short‐term follow‐up, and sparse data.
Two RCTs (Owen 1990; Peckham 2014) compared individualised homeopathic treatment with usual care. In Owen 1990 individualised homeopathic treatment was compared to usual care (dicyclomine hydrochloride, faecal bulking agents, and diet sheets advising a high fibre diet). No conclusions can be drawn from this study due to the small number of participants, the low quality of reporting in this trial and a high risk of bias. Although Peckham 2014 has a low risk of bias and the quality of the reporting is good it is difficult to draw conclusions from this study due to the small number of participants.
Overall completeness and applicability of evidence
Rahlfs 1976 and Rahlfs 1979 assessed the effectiveness of clinical homeopathy for the treatment of constipation‐predominant IBS. Therefore this review does not provide information on the effectiveness of clinical homeopathy for the treatment of IBS in general, or diarrhoea‐predominant, or mixed typology IBS. Both Rahlfs 1976 and Rahlfs 1979 reported outcomes at two weeks. Given the long term nature of IBS it is not clear how useful a two‐week outcome is for patients', clinicians' and policy makers' decision making. As people live with IBS for years, an evaluation of impact at two weeks fails to take into account possible rebound effects or longer term benefits or adverse events that would be important for patients and practitioners to know about when they consider the potential benefits and harms associated with this intervention.
Two studies that assessed the effectiveness of individualised homeopathic treatment were identified in this review (Owen 1990; Peckham 2014). The number of participants in both these studies were small (N = 23 and N = 94 respectively). Owen 1990 was conducted over 25 years ago. It is likely that there have been changes in usual care for IBS since this time, therefore Owen 1990 may not provide a full picture of the effectiveness of individualised homeopathic treatment compared to usual care. Peckham 2014 was conducted more recently and is likely to compare individualised homeopathic treatment to current usual care however given the small size of this study and the fact that it is underpowered makes it difficult to draw any firm conclusions from the study.
Quality of the evidence
The results from the pooled analysis indicate a possible benefit for homeopathic treatment using clinical homeopathy (non‐individualised homeopathic remedies) over placebo for constipation‐predominant IBS. However, this result needs to be interpreted with caution. The two studies included in the pooled analysis (Rahlfs 1976 and Rahlfs 1979) were carried out in the 1970s before the introduction of the CONSORT statement (Begg 1996), and the quality of reporting in these studies does not meet currently expected standards (Schultz 2010). The low quality of the reporting means that it is not possible to determine whether or not these studies were carried out in a rigorous manner and thus how likely it is that these results are a true reflection of the treatment effect. Both studies were determined to have an unknown risk of bias for most assessed items and Rahlfs 1979 was at a high risk of reporting bias. The quality of the evidence supporting the primary outcome (i.e. global improvement) was very low due to the low quality of reporting in the included studies, high or unknown risk of bias, sparse data and short‐term follow‐up.
Participants in the Rahlfs 1976 and Rahlfs 1979 studies were recruited through general practice as having suspected IBS. It is not clear whether diseases such as Crohn's disease or ulcerative colitis were ruled out in these participants and it is possible that some participants had diseases such as Crohn's or ulcerative colitis rather than IBS.
The quality of the reporting in the Owen 1990 study was low, and this study does not meet the current expected standards (Schultz 2010). No conclusions can be drawn from this study due to the small number of participants and risk of bias. Owen 1990 was rated as high risk of bias for blinding of participants and personnel. The study was rated as unknown risk of bias for the other assessed items. The exact details of the medication prescribed in the usual care arm, in terms of dosage and frequency were not reported.
The quality of the reporting in the Peckham 2014 was good and combined with the published protocol it meets the current expected standards (Schultz 2010). Peckham 2014 was rated as being at low risk of bias for selection bias, attrition bias and reporting bias and at high risk of bias for performance bias and detection bias. However the small number of participants in this study (n = 16 homeopathic treatment, n = 18 supportive listening and n = 60 usual care) and the fact that it was underpowered mean no firm conclusions can be drawn from this study. The quality of the evidence supporting the primary outcome (i.e. global improvement) ranged from very low to low due to sparse data and high risk of bias.
Potential biases in the review process
To avoid potential biases in the review process data extraction was carried out independently by two assessors. In addition, efforts were made to identify all studies that were potentially eligible for this review (see Search methods for identification of studies). However, It is possible that not all potentially eligible studies were identified. This could be because potentially eligible studies have been carried out and then have not been published, or that studies have been published but not in places where they could be accessed, possibly because they were published in little known non‐indexed journals or they could have been published in places where they should have been found, but were not found. One of the review authors (EJP) was a trialist for an included study (Peckham 2014). For this study, the assessment of risk of bias and data extraction was performed by other authors. Cohort and case‐control studies were considered for inclusion but none were identified by the literature search. In retrospect the inclusion of case‐control studies was not appropriate given that the main reason for including case‐control studies in a review is when an event is very rare and thus it is unlikely that any RCTs have been carried out (Reeves 2011).
Agreements and disagreements with other studies or reviews
No other systematic reviews of homeopathic treatment for IBS were identified. However non‐condition specific systematic reviews of homeopathic treatment that included the Rahlfs 1976 and Rahlfs 1979 studies have been published (Linde 1997; Shang 2005). Neither of these systematic reviews carried out any analyses on homeopathy for the treatment of IBS or specifically commented on homeopathy for IBS.
Authors' conclusions
Implications for practice.
The results for the outcomes assessed in this review are uncertain. Thus no firm conclusions regarding the effectiveness and safety of homeopathy for the treatment of IBS can be drawn.
In this review of homeopathic treatment for IBS, two of the included studies used clinical (non‐individualised) homeopathic remedies to treat patients with constipation‐predominant IBS (Rahlfs 1976; Rahlfs 1979). A meta‐analysis of these two studies found a possible benefit favouring the homeopathic remedy over placebo. However, these results should be interpreted with caution due to the low quality of reporting in these studies, a high or unknown risk of bias and sparse data. Thus it is not possible to be certain whether or not the trials were able to distinguish between true treatment effects, chance or bias. Furthermore, the low quality of reporting practice means that it is difficult to assess whether the results would be replicated in everyday practice, that is, whether the results are externally valid or generalisable. We are therefore very uncertain about these results and cannot suggest a possible benefit for clinical homeopathy.
It is of note that Rahlfs 1976 and Rahlfs 1979 reported outcomes at two weeks. Given the long term nature of IBS, it is not clear how useful a two‐week outcome is for decision making. It is essential that trials have a follow‐up period that is clinically meaningful. As people live with IBS for years, an evaluation of impact at two weeks fails to take into account any possible rebound effects, or longer term benefits or adverse events that would be important for patients and practitioners to know about when they consider the potential benefits and harms associated with this intervention.
The results from Owen 1990 are uncertain and no conclusions can be drawn from this study. Owen 1990 compared individualised homeopathic treatment and usual care consisting of dicyclomine hydrochloride and faecal bulking agents. The results from Peckham 2014 are uncertain and no conclusions can be drawn from this study. Peckham 2014 compared individualised homeopathic treatment plus usual care, supportive listening plus usual care and usual care. Individualised homeopathy is the most common form of homeopathy practised in the UK. However due to the poor quality of reporting in Owen 1990 study and the small number of participants in both Owen 1990 and Peckham 2014, no conclusions can be made regarding the usefulness of individualised homeopathic treatment for the treatment of IBS.
None of the included studies reported on adverse events therefore no conclusions can be drawn on the safety of homeopathic treatment for IBS.
Implications for research.
Further high quality, adequately powered RCTs are required to assess the efficacy and safety of clinical and individualised homeopathy for IBS compared to placebo or usual care.
Rahlfs 1976 and Rahlfs 1979 evaluated clinical homeopathy involving pre‐specified homeopathic remedies for the treatment of IBS‐C and were therefore designed to assess the effectiveness of non‐individualised homeopathic remedies. However due to the high risk of reporting bias in one of these studies and unclear reporting in both of these studies it is recommended that these trials are repeated using current reporting guidelines (Schultz 2010), to determine whether or not there is any benefit associated with homeopathy for IBS. Future high quality studies should enrol larger numbers of patients and assess longer term efficacy and safety outcomes.
Owen 1990 assessed the effectiveness of individualised homeopathic treatment compared to usual care and Peckham 2014 assessed the effectiveness of individualised homeopathic treatment plus usual care to usual care. Due to the low quality reporting in Owen 1990 and the likelihood that usual care for IBS has changed since this study was conducted, and the fact that Peckham 2014 was underpowered to detect a significant difference, it is recommended that the effectiveness and safety of individualised homeopathic treatment be evaluated in a well‐designed, adequately powered trial.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2019 | Amended | Minor typographical error in plain language summary corrected. There is no impact on the data, review findings, or interpretation. |
History
Protocol first published: Issue 3, 2012 Review first published: Issue 11, 2013
| Date | Event | Description |
|---|---|---|
| 31 August 2018 | New citation required but conclusions have not changed | Updated review, no new conclusions |
| 31 August 2018 | New search has been performed | New search and one new study added |
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search Strategies
Medline
1. Colonic disease*.mp
2. irritable bowel syndrome/
3. colonic diseases, functional/
4. irritable bowel/
5. irritable colon/
6. spastic colon/
7. functional bowel disease*.mp.
8. functional colonic disease*.mp.
9. or/1‐8
10. homeopathy/
11. homeopath*.mp.
12. homoeopath*.mp.
13. alternative medicine*.mp.
14. or/10‐13
15. 9 and 14
EMBASE
1. Colonic disease*.mp
2. irritable bowel syndrome/
3. colonic diseases, functional/
4. irritable bowel/
5. irritable colon/
6. spastic colon/
7. functional bowel disease*.mp.
8. functional colonic disease*.mp.
9. or/1‐8
10. homeopathy/
11. homeopath*.mp.
12. homoeopath*.mp.
13. alternative medicine*.mp.
14. or/10‐13
15. 9 and 14
CENTRAL
#1 MeSH: [Irritable bowel syndrome] explode all trees
#2 Colonic disease*
#3 irritable bowel syndrome
#4 colonic diseases, functional
#5 irritable bowel
#6 irritable colon
#7 spastic colon
#8 functional bowel disease*
#9 functional colonic disease*
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 homeopathy
#12 homeopath*
#13 homoeopath*
#14 alternative medicine*
#15 #11 or #12 or #13 or #14
#16 #10 and #15
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
1. (TI homeopathy or AB homeopathy) OR (TI homeopath* or AB homeopath*) OR (TI homoeopath* or AB homoeopath*) OR (TI alternative medicine* of AB alternative medicine*)
2. (TI Irritable bowel syndrome or AB Irritable bowel syndrome) OR (TI Colonic disease* or AB Colonic disease*) OR (TI irritable colon or AB irritable colon) OR (TI spastic colon or AB spastic colon) OR (TI functional bowel disease* or AB functional bowel disease*) OR (TI functional colonic disease* or AB functional colonic disease*)
Allied and Complementary Medicine Database (AMED)
1. Colonic disease*.mp
2. irritable bowel syndrome/
3. colonic diseases, functional/
4. irritable bowel/
5. irritable colon/
6. spastic colon/
7. functional bowel disease*.mp.
8. functional colonic disease*.mp.
9. or/1‐8
10. homeopathy/
11. homeopath*.mp.
12. homoeopath*.mp.
13. alternative medicine*.mp.
14. or/10‐13
15. 9 and 14
Clinical trials. Gov
1. Homeopathy and Irritable bowel syndrome
IBD specialized register
1. Irritable bowel syndrome and homeopath
2. Colonic diseases and homeopath
3. Functional bowel and homeopath
Data and analyses
Comparison 1. Homeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Homeopathy versus placebo | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Global improvement (Asafoetida only) | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.18, 2.18] |
| 1.2 Global improvement (Asafoetida + nux vom) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.15] |
1.1. Analysis.
Comparison 1 Homeopathy versus placebo, Outcome 1 Homeopathy versus placebo.
Comparison 2. Homeopathy versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global improvement (feeling unwell) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Homeopathy versus usual care, Outcome 1 Global improvement (feeling unwell).
Comparison 3. Homeopathy plus usual care versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global improvement (IBS‐SSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
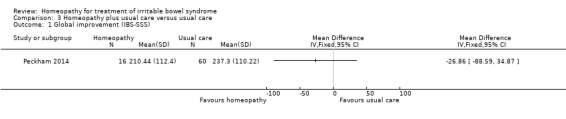
Comparison 3 Homeopathy plus usual care versus usual care, Outcome 1 Global improvement (IBS‐SSS).
3.2. Analysis.
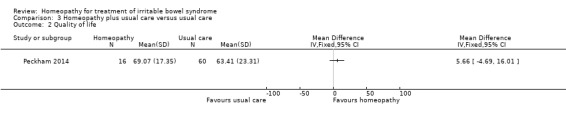
Comparison 3 Homeopathy plus usual care versus usual care, Outcome 2 Quality of life.
Comparison 4. Homeopathy plus usual care versus supportive listening plus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global improvement (IBS‐SSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
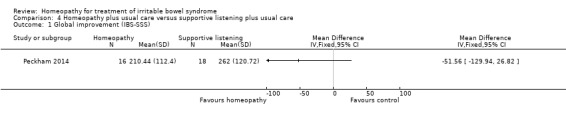
Comparison 4 Homeopathy plus usual care versus supportive listening plus usual care, Outcome 1 Global improvement (IBS‐SSS).
4.2. Analysis.
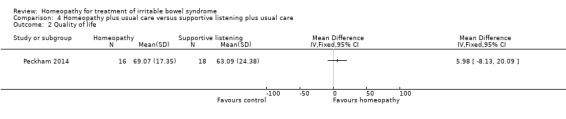
Comparison 4 Homeopathy plus usual care versus supportive listening plus usual care, Outcome 2 Quality of life.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Owen 1990.
| Methods | RCT, unblinded, parallel study, 12 weeks duration | |
| Participants | Setting; county hospital, UK Number of participants; 23 patients were allocated into one of the treatment groups, 20 patients included in analysis Recruitment methods; female patients attending the out‐patient department at a county hospital in whom a diagnosis of IBS was made Diagnosis of IBS; clinical diagnosis by a consultant gastroenterologist and consultant gynaecologist Age range of patients; 20‐69 years Gender (of treated patients); 100% female Duration of symptoms > 3 months |
|
| Interventions | 1. Individualised homeopathic treatment 2. High doses of Dicyclomine hydrocholoride (exact dose not stated), faecal bulking agents and diet sheets advising a high fibre diet |
|
| Outcomes | Patients were asked to grade: their four worst symptoms on a visual analogue scale, dysmenorrhoea, dyspareunia, and feeling unwell at baseline, 2, 6 and 12 weeks | |
| Notes | Detailed information is given on the homeopathic treatment the participants received in terms of; remedy chosen, potency and dosage, whilst no information is given on the strength and dosage of the dicyclomine hydrocholoride and faecal bulking agents prescribed in the usual care arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although it is stated that this is a randomised trial no details were given as to how randomisation was achieved |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition, whilst possible reasons for attrition were discussed for one patient, the reasons for the other two patients leaving the study were not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information is provided to be able to judge whether the study is at risk from selective reporting |
| Other bias | Unclear risk | Due to the low quality of the reporting in this study it is unclear whether the study is at risk from any other forms of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and doctors were not blinded to allocation, however it is not stated whether other key study personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not reported whether or not the outcome assessment was carried out blind |
Peckham 2014.
| Methods | Three arm, parallel group non‐blinded randomised controlled trial 26 weeks in duration | |
| Participants | Setting: Hospital outpatient, UK Number of participants; 94 patients were allocated into one of the treatment groups in a 4:1:1 ratio, 60 patients were allocated to usual care, 16 were offered homeopathic treatment plus usual care and 18 were offered supportive listening plus usual care Recruitment methods; GP database recruitment, consultant gastroenterologist in secondary care Diagnosis of IBS; diagnosed according to the Rome III criteria, potentially eligible participants were asked to complete a questionnaire which included the Rome III criteria for IBS. Participants had to score a minimum of 100 on the IBS‐SSS to be eligible to take part in the trial Mean age range of participants; 49 years Gender; 83% female Duration of symptoms > 3 months |
|
| Interventions | 1. Individualised homeopathic treatment plus usual care 2. Supportive listening plus usual care 3. Usual care |
|
| Outcomes | IBS‐SSS, EQ‐5D, HADS | |
| Notes | This study employed a Trials Within Cohorts design which recruited a cohort of people with IBS. From this cohort people were randomly selected to receive the offer of a treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by shuffling of sealed opaque envelopes containing the allocation (protocol paper) |
| Allocation concealment (selection bias) | Low risk | Questionnaires from participants consenting and meeting the eligibility criteria are taken one at a time, at the same time a sealed opaque envelope containing the allocation is taken from the top of the shuffled pack and opened and the allocation noted. This is carried out by an independent administrator at the University of Sheffield, in the presence of another independent administrator. (protocol paper). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of patients who dropped out of the usual care and supportive listening arms was similar and the reasons for dropping out were the same in both groups. 9/60 (15%) participants in the usual care arm did not return the follow‐up questionnaire All 16 participants in the homeopathy arm were included in the analysis 3/18 (17%) participants in the supportive listening arm did not return the questionnaire |
| Selective reporting (reporting bias) | Low risk | Outcome data presented for those outcome measures stated in the protocol. |
| Other bias | Low risk | The quality of reporting in this study was good and did not indicate that there were likely to be other forms of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the nature of the interventions in this study nor the study design allows for the masking of the therapists or the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were patient‐reported outcomes (i.e. the patient was the outcome assessor). As the patients were aware of the group allocation, this domain was judged as high risk of bias. |
Rahlfs 1976.
| Methods | RCT, double blind, parallel study, 2 weeks duration | |
| Participants | Setting; general practice, Germany Number of participants; 71 patients treated (number of patients randomised not clearly stated), 63 patients included in analysis Recruitment methods; patients presenting in general practice with suspected IBS Diagnosis of IBS; Clinical diagnosis plus completion of detailed questionnaire Mean age (of treated patients); 43.8 years Gender (of treated patients); 50.8% female Duration of symptoms > 14 days |
|
| Interventions | 1. 0.1% asafoetida alcohol solution, 6 x 5 drops daily 2. 0.1% asafoetida alcohol solution + 0.01% nux vomica alcohol solution, 6 x 5 drops daily 3. placebo, 45% alcohol solution, 6 x 5 drops daily |
|
| Outcomes | Self assessment on a 3 point scale; no or negligible improvement, more than half improved, free of symptoms measured on day 8 and day 15 of the study Time to recovery assessed by the patient reporting the day they felt considerable improvement Freiburg Personality Inventory |
|
| Notes | Analysed participant data were fairly well described, but a lot of pre‐randomisation and pre‐analysis data were missing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A chance code was used for the randomisation, the exact nature of which was not reported Therefore the risk of bias cannot be determined |
| Allocation concealment (selection bias) | Low risk | Medication was provided in sequentially numbered drug containers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition, some reasons for attrition are given, details of allocation are not always given |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information was provided to be able to judge whether the study was at risk from selective reporting |
| Other bias | Unclear risk | Insufficient information was provided to assess whether the study was at risk from any other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and recruiting doctors were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether outcome assessment was carried out blind |
Rahlfs 1979.
| Methods | RCT, double blind, parallel study, 2 weeks duration | |
| Participants | Setting; general practice, Germany Number of participants; 119 patients treated (number of participants randomised not clearly stated), 89 patients included in analysis Recruitment methods; patients presenting in general practice with suspected IBS Diagnosis of IBS; Clinical diagnosis plus completion of detailed questionnaire Mean age (of patients included in analysis, ages of those not included not stated); 42.5 years Gender (of those included in analysis, gender of those not included not stated); 68.5% female Duration of symptoms > 14 days |
|
| Interventions | 1. 0.1% asafoetida alcohol solution, 6 x 5 drops daily 2. placebo, 45% alcohol solution, 6 x 5 drops daily |
|
| Outcomes | Self assessment on a 4 point scale; worsening of symptoms, no or negligible improvement, more than half improved, free of symptoms, measured on day 8 and day 15 of the study Time to recovery assessed by the patient reporting the day they felt considerable improvement |
|
| Notes | Analysed participant data were fairly well described, but a lot of pre‐randomisation and pre‐analysis data were missing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although it was reported that this was a randomised trial no details were given as to how randomisation was achieved |
| Allocation concealment (selection bias) | Low risk | Medication was provided in sequentially numbered drug containers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data, reasons for missing data, and how incomplete outcome data were addressed was not clearly described |
| Selective reporting (reporting bias) | High risk | The inclusion and exclusion criteria were applied in a variable manner, some people that were subsequently found not to meet the exclusion and inclusion criteria were removed from the analysis However people who did not meet the inclusion criteria for age, being too old were still included in the analysis This leaves the study at risk of bias due to selective reporting |
| Other bias | Unclear risk | Due to the low quality of the reporting it was unclear whether the study was at risk from any other forms of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and doctors were blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether outcome assessment was carried out blind to treatment allocation |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aleem 2000 | Discussion piece and not a randomised controlled trial, cohort or case‐control study |
| Anonymous 2005 | An initial reading of this Italian article revealed it to be discussing a meta analysis by Shang 2005 Therefore a full translation was not conducted |
| Anonymous 2009 | Discussion piece and not a randomised controlled trial, cohort or case‐control study |
| Bauer 2014 | Case studies on the treatment of IBS and not a randomised controlled trial, cohort or case‐control study |
| Bhagat 2010 | Case report (n = 1) of homeopathic treatment for IBS |
| Bhattacharjee 2010 | The article was a discussion on the different homeopathic remedies used for the treatment of IBS |
| Chen 2015 | A discussion on the use of complementary therapies for the treatment of functional gastrointestinal disorders |
| Cherniack 2013 | Not on IBS |
| Chimthanawala 2004 | Case report (n = 2) of homeopathic treatment for IBS |
| Diamond 2005 | A discussion on the use of complementary therapies for the treatment of gastroenterological problems |
| Elio 2014 | Report on a series of patients attending a clinic. Not specific to IBS and not an RCT or case series |
| Feldhaus 2000 | This was a discussion piece on the treatment of IBS |
| Gamble 2007 | Discussion of a potentially new way of assessing and treating IBS, from a homeopathic perspective, using two cases as an example |
| Gebhardt 1988 | Discussion on homeopathic treatment for IBS, not a randomised controlled trial, cohort or case‐control study |
| Gray 1998 | This study was a case series of 25 patients with no comparator group |
| Greeson 2008 | Non‐randomised observational study of outcomes for patients attending a integrative medical centre where homeopathy was only one of the treatments offered |
| Grundmann 2014 | Review of treatments for IBS does not include homeopathy |
| Innes 2000 | This study was a case series (n = 20) with no comparator group |
| Jagose 2004 | Case report (n = 1) of homeopathic treatment for IBS |
| Jones 1996 | A discussion of the homeopathic treatment of IBS, illustrated by three cases |
| Jones 1997 | Discussion piece on homeopathic treatment of IBS |
| Jones 1999 | Case report study of a woman with IBS treated with homeopathy |
| Krishendu 2010 | A discussion of the different homeopathic remedies used for the treatment of IBS |
| Lobo 2000 | Case report (n = 1) on homeopathic treatment of IBS |
| Master 2008 | Discussion piece on homeopathy for IBS |
| Mohan 2006 | Case report (n = 2) of IBS treated with homeopathy |
| Pawar 2015 | A discussion on homeopathic remedies |
| Pinto 1999 | A selection of case reports on homeopathic treatment for a variety of conditions |
| Pohl 2013 | An overview of treatments for irritable bowel syndrome which does not include homeopathy |
| Slade 2003 | Case report (n = 1) of homeopathic treatment of ulcerative colitis |
| Turner 2008 | Discussion of homeopathic treatment of IBS, illustrated by eight case histories |
| White 1999 | Discussion of homeopathic treatment for IBS, not a randomised controlled trial, cohort or case‐control study |
Contributions of authors
EJP initiated, designed the study and drafted the protocol. EP, SB and GT, extracted the data and conducted the quality assessment. AA and KC arbitrated. KC provided advice on meta analysis. KC, SB, GT and AA all commented on the review.
Sources of support
Internal sources
University of Leeds, UK.
Homeopathy Research Institute, UK.
ScHARR, UK.
External sources
No sources of support supplied
Declarations of interest
EJP has contributed to the design and management of one of the included RCTs. EJP is a homeopath.
GT: None known.
SB: None known.
KC: None known.
AA: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Owen 1990 {published data only}
- Lecoyte T, Owen D, Shepherd H, Letchworth A, Mullee M. An investigation into the homeopathic treatment of patients with irritable bowel syndrome. Proceedings of the 48th LMHI Congress. Vienna, Austria, 1993.
- Owen D. An investigation into the homoeopathic treatment of patients with irritable bowel syndrome. Congress of the Faculty of Homoeopathy. Windermere, 1990.
Peckham 2014 {published data only (unpublished sought but not used)}
- Peckham E, Raw J, Relton C. Exploring the effectiveness of homeopathic treatment for irritable bowel syndrome. Homeopathy 2014;103(1):90‐1. [DOI] [PubMed] [Google Scholar]
- Peckham EJ, Relton C, Raw J, Walters C, Thomas K, Smith C, et al. Interim results of a randomised controlled trial of homeopathic treatment for irritable bowel syndrome. Homeopathy 2014;103(3):172‐7. [DOI] [PubMed] [Google Scholar]
Rahlfs 1976 {published data only}
- Rahlfs VW, Mossinger P. Treatment of irritable colon. A multicenter placebo‐controlled double‐blind study in general practice [Ein multizentrischer plazebo‐kontrollierter doppelblindversuch in der allgemeinen praxis]. Arzneimittel Forschung 1976;26(12):2230‐4. [PubMed] [Google Scholar]
Rahlfs 1979 {published data only}
- Rahlfs VW, Mossinger P. Asa foetida in the treatment of the irritable colon; a double blind trial [Asa foetida bei colon irritabile]. Deutsche Medizinische Wochenschrift 1979;104(4):140‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aleem 2000 {published data only}
- Aleem SM. Colo‐rectal disorders. National Journal of Homeopathy 2000;2(4):259‐61. [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Homeopathy and placebo [Omeopatia e placebo]. Medico e Bambino 2005;24(7):415. [Google Scholar]
Anonymous 2009 {published data only}
- Anonymous. Homoeopathy associated with improvements in health. Australian Journal of Pharmacy 2009;90:75. [Google Scholar]
Bauer 2014 {published data only}
- Bauer BA, Litin JC, Bundrick JB. Clinical pearls in complementary and integrative medicine (cim). Disease‐a‐month 2014;60(7):323‐331. [DOI] [PubMed] [Google Scholar]
Bhagat 2010 {published data only}
- Bhagat J. Case study and management of irritable bowel syndrome. Homoeopathic Heritage International 2010;35(1):18‐21. [Google Scholar]
Bhattacharjee 2010 {published data only}
- Bhattacharjee J. Irritable bowel syndrome ‐ a menace in disguise. Homoeopathic Heritage International 2010;35(1):34‐9. [Google Scholar]
Chen 2015 {published data only}
- Chen JDZ, Yin J, Takahashi T, Hou X. Complementary and Alternative Therapies for Functional Gastrointestinal Diseases. Evidence‐based Complementary and Alternative Medicine 2015;138645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherniack 2013 {published data only}
- Cherniack EP. Use of complementary and alternative medicine to treat constipation in the elderly. Geriatrics and Gerontology International 2013;13(3):533‐538. [DOI] [PubMed] [Google Scholar]
Chimthanawala 2004 {published data only}
- Chimthanawala D. Irritable bowel syndrome ‐ a few cases. National Journal of Homoeopathy 2004;6(2):102‐4. [Google Scholar]
Diamond 2005 {published data only}
- Diamond JA, Diamond JW. Common functional bowel problems. Advance for Nurse Practitioners 2005;13(5):31‐34,72. [PubMed] [Google Scholar]
Elio 2014 {published data only}
- Elio R, Marco P, Paola B, Marialessandra P, Mariella DS, Monia P, et al. Homoeopathy in the public health system: Outcome data from the homoeopathic clinic of the Campo di Marte Hospital, Lucca, Italy (1998‐2010). European Journal of Integrative Medicine 2014;6(1):39‐47. [Google Scholar]
Feldhaus 2000 {published data only}
- Feldhaus HW. Comprehensive therapy recommendation for irritable bowel syndrome [Ganzheitliche therapieempfehlung bei reizdarm: Behandlungsvorschlag aus unserem preisratsel]. Arztezeitschrift fur Naturheilverfahren 2000;4(6):336‐7. [Google Scholar]
Gamble 2007 {published data only}
- Gamble J. Case insights series. A study on irritable bowel syndrome. Similia: Journal of the Australian Homoeopathic Association 2007;19(1):19‐21. [Google Scholar]
Gebhardt 1988 {published data only}
- Gebhardt KH. Homeopathy for catarrh of the mucous membrane of the air routes, intestines and urinary tract [Homootherapie der schleimhautkatarrhe der luftwege, speisewege und harnwege]. Erfahrungsheilkunde 1988;37(4):202‐4. [Google Scholar]
Gray 1998 {published data only}
- Gray J. How I treat irritable bowel disease: a survey of 25 patients. British Homoeopathic Journal 1998;87(4):195‐202. [Google Scholar]
Greeson 2008 {published data only}
- Greeson JM, Rosenzweig S, Halbert SC, Cantor IS, Keener MT, Brainard GC. Integrative medicine research at an academic medical centre: Patient characteristics and health‐related quality‐of‐life outcomes. Journal of Alternative and Complementary Medicine 2008;14(6):763‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grundmann 2014 {published data only}
- Grundmann O, Yoon SL. Complementary and alternative medicines in irritable bowel syndrome: an integrative view. World Journal of Gastroenterology 2014;20(2):346‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Innes 2000 {published data only}
- Innes MA, Greenfield SM, Hunton M. Using case studies for prescribing research ‐ an example from homoeopathic prescribing. Journal of Clinical Pharmacy and Therapeutics 2000;25(6):399‐409. [DOI] [PubMed] [Google Scholar]
Jagose 2004 {published data only}
- Jagose A. The efficacy of homoeopathy in IBS. National Journal of Homoeopathy 2004;6(2):106‐7. [Google Scholar]
Jones 1996 {published data only}
- Jones A. Homoeopathic case studies: irritable bowel syndrome. Positive Health 1996;16:30. [Google Scholar]
Jones 1997 {published data only}
- Jones A. Irritable bowel syndrome. Homoeopathy 1997;47(5):101‐2. [Google Scholar]
Jones 1999 {published data only}
- Jones A. Homeopathic case studies: Nux Vomica for bowel symptoms. Positive Health 1999;37:25. [Google Scholar]
Krishendu 2010 {published data only}
- Krishendu. Irritable bowel syndrome ‐ a homeopathic perspective. Homoeopathic Heritage International 2010;35(1):26‐30. [Google Scholar]
Lobo 2000 {published data only}
- Lobo A. IBS: a constitutional approach. National Journal of Homoeopathy 2000;2(4):262a‐d. [Google Scholar]
Master 2008 {published data only}
- Master FJ. From the editor's desk. Homoeopatic approaches to irritable bowel syndrome. Homoeopathic Heritage International 2008;33(10):4‐5. [Google Scholar]
Mohan 2006 {published data only}
- Mohan G, Kishore KC, Ratna SA. Irritable bowel syndrome: "A challenge in medical practice". National Journal of Homoeopathy 2006;8(2):131‐3. [Google Scholar]
Pawar 2015 {published data only}
- Pawar D. Management of irritable bowel syndrome with Ranunculacae family. National Journal of Homoeopathy 2015;17(7):12‐13. [Google Scholar]
Pinto 1999 {published data only}
- Pinto G. Case studies ‐ Marylebone health centre action research enquiry. Homoeopath 1999;75:36‐41. [Google Scholar]
Pohl 2013 {published data only}
- Pohl D, Heinrich H, Misselwitz B. Irritable bowel syndrome ‐ Diagnostics and therapy [Reizdarmsyndrom ‐ Diagnostik und Therapie]. Gastroenterologe 2013;8(5):417‐24. [Google Scholar]
Slade 2003 {published data only}
- Slade N. Homeopatic casebook. Homeopathy and colitis. Positive Health 2003;86:25‐8. [Google Scholar]
Turner 2008 {published data only}
- Turner R. Homeopathic approaches to irritable bowel syndrome (IBS). Positive Health 2008;146:18‐21. [Google Scholar]
White 1999 {published data only}
- White A. Irritable bowel syndrome. Complementary Medicine Bulletin 1999;1(6):1‐2. [Google Scholar]
Additional references
Agrawal 2006
- Agrawal A, Whorwell PJ. Irritable bowel syndrome: diagnosis and management. BMJ 2006;332(7536):280‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akehurst 2002
- Akehurst RL, Brazier JE, Mathers N, O'Keefe C, Kaltenthaler E, Morgan A, et al. Health‐related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics 2002;20(7):455‐62. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting randomized controlled trials The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Dantas 2000
- Dantas F, Rampes H. Do homeopathic medicines provoke adverse effects? A systematic review. British Homoeopathic Journal 2000;89(Supplement 1):S35‐8. [DOI] [PubMed] [Google Scholar]
Downs 1998
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology and Community Health 1998;52(6):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drossman 2016
- Drossman DA, Hasler WL. Rome IV‐functional GI disorders: disorders of gut‐brain interaction. Gastroenterology 2016;150(6):1257–1261. [DOI] [PubMed] [Google Scholar]
Gariboldi 2009
- Gariboldi S, Palazzo M, Zanobbio L, Dusio GF, Mauro V, Solimene U, et al. Low dose oral administration of cytokines for treatment of allergic asthma. Pulmonary Pharmacology and Therapeutics 2009;22(6):497‐510. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Linde 1997
- Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges L, et al. Are the clinical effects of homeopathy placebo effects? A meta‐analysis of placebo controlled trials. Lancet 1997;350(9081):834‐43. [DOI] [PubMed] [Google Scholar]
Longstreth 2007
- Longstreth GF. Avoiding unnecessary surgery in irritable bowel syndrome. Gut 2007;56(5):608‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathie 2006
- Mathie RT, Robinson TW. Outcomes from homeopathic prescribing in medical practice: A prospective, research‐targeted, pilot study. Homeopathy 2006;95(4):199‐205. [DOI] [PubMed] [Google Scholar]
McPherson 2012
- MacPherson H, Tilbrook H, Bland JM, Bloor K, Brabyn S, Cox H, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterology 2012;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mearin 2006
- Mearin F, Badía X, Balboa A, Benavent J, Caballero AM, Domínguez‐Muñoz E, et al. Predictive factors of irritable bowel syndrome improvement: 1‐year prospective evaluation of 400 patients. Alimentary Pharmacology and Therapeutics 2006;23(6):815‐26. [DOI] [PubMed] [Google Scholar]
Montagnier 2009
- Montagnier L, Aïssa J, Ferris S, Montagnier JL, Lavallée C. Electromagnetic signals are produced by aqueous nanostructures derived from bacterial DNA sequences. Interdisciplinary Sciences, Computational Life Sciences 2009;1(2):81‐90. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Ruepert 2011
- Ruepert L, Quartero AO, Wit NJ, Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD003460.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2011. Vol. 5.1.0, The Cochrane Collaboration, 2011. [Google Scholar]
Shang 2005
- Shang AJ, Huwiler‐Muntene MD, Narety C, Juni P, Dorig S, Sterne JA, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo‐controlled trials of homoeopathy and allopathy. Lancet 2005;366(9487):726‐32. [DOI] [PubMed] [Google Scholar]
Spence 2005
- Spence DS, Thompson EA, Barron SJ. Homeopathic treatment for chronic disease: a 6 year, university‐hospital outpatient observational study. Journal of Alternative and Complementary Medicine 2005;11(5):793‐8. [DOI] [PubMed] [Google Scholar]
Spiller 2007
- Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56(12):1770‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2001
- Thomas KJ, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England: a population based survey. Complementary Therapies in Medicine 2001;9(1):2‐11. [DOI] [PubMed] [Google Scholar]
Thompson 2000
- Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46(1):78‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vithoulkas 1980
- Vithoulkas G. The Science of Homeopathy. New York: Grove Press, 1980. [Google Scholar]
Williams 2007
- Williams JG, Roberts SE, Ali MF, Cheung WY, Cohen DR, Demery G, et al. Gastroenterology services in the UK. The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut 2007;56 Suppl 1:1‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zijdenbos 2009
- Zijdenbos IL, Wit NJ, Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006442.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Peckham 2012
- Peckham EJ, Nelson EA, Greenhalgh J, Cooper K, Roberts ER, Agrawal A. Homeopathy for treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009710] [DOI] [PubMed] [Google Scholar]


