Abstract
Background
The aim of this study was to review the efficacy and safety of intra-articular (IA) viscosupplementation (VS) for hip osteoarthritis (OA).
Material/Methods
We searched Medline, Clinical Trial Register Center, EMBASE, and Cochrane databases for randomized controlled trials (RCTs) comparing VS with placebo injection for hip OA. We included suitable studies, assessed the quality of studies, and extracted data on pain reduction, function improvement at different time points, and safety profiles. The comparisons of pain and function outcome were performed by meta-analysis.
Results
Five high-quality randomized controlled studies trials (RCTs) with 591 patients with hip OA were identified. Although several trials demonstrated a significant decline in pain in VS groups during follow-up compared to baseline, without severe adverse events, the pooled analysis did not show VS was superior to placebo at any time windows [7–14 days: standardized mean difference (SMD): −0.18; 95% CI, −0.47 to 0.10, p=0.21; 28–30 days: 0.02 (−0.15, 0.19), p=0.82; or at final visit: −0.14 (−0.46, 0.18), p=0.38]. Similar results were also observed in the combined data of functional results.
Conclusions
IA VS does not reduce pain or improve function significantly better than placebo in a short-term follow-up. The benefits and safety of VS should be further assessed by sufficiently-sized, methodologically sound studies with validated assessment of more clinically relevant end-points.
MeSH Keywords: Meta-Analysis; Osteoarthritis, Hip; Pain Measurement; Viscosupplementation
Background
Hip osteoarthritis (OA), with a prevalence of approximately of 1.1% for elderly men and 3.6% for elderly women, greatly affects their daily activity and quality of life by causing joint pain and stiffness [1].
The pathogenesis of hip OA is characterized by femoroacetabular degeneration, with chronic wearing of cartilage tissue during the inflammatory process [2]. The rate of response to conventional pharmacologic therapy is mostly limited, and then total hip replacements (THR) might be indicated. For large populations waiting for or unwilling to undergo surgical treatment, a more effective adjunct therapy is still needed [3].
With restoration of the protective viscoelasticity of synovial fluid, intra-articular (IA) viscosupplementation (VS) with use of hyaluronic acid (HA) solution effectively reduces friction and improves mobility in OA patients [4,5].
However, there is little published data on the efficacy of VS for hip OA, especially from large-scale randomized controlled trials (RCTs) with validated methodological quality, and the results have been conflicting. A previous meta-analysis showed a statistically significant difference in visual analogue scale (VAS) using VS in hip OA, but the conclusions were mainly based on a large proportion of poorly-designed cohort or uncontrolled studies, which only allowed for limited interpretation [6]. Another recent systematic review and meta-analysis showed little evidence of VS efficacy in pain relief at 3 months, but they failed to perform a pooled analysis, and the functional score was not reported [7].
Unanswered questions on this topic include the followings: Is VS superior to placebo in reducing hip OA pain? How fast does VS act and how long is it still effective? What are the potential adverse effects? Therefore, we performed the present meta-analysis with high-quality RCTs, comparing efficacy between VS and placebo for hip OA. Subgroup analysis at different time windows was also carried out by meta-analysis, and we summarized the safety profiles of the interventions as well.
Material and Methods
Literature search
Two authors (Lin and Liao) independently searched Medline, Clinical Trial Register Center, EMBASE, and Cochrane databases without limits up to January 26, 2019. We used: “(hyaluronan OR hyaluronic acid OR viscosupplementation) AND Hip osteoarthritis“ as terms and Boolean operators. All searches were limited to human randomized clinical trials reported in journals, with no language restrictions.
Inclusion and exclusion criteria
The studies were included in which: (a) the trials were RCTs, (b) the target population were adults with primary hip OA defined according to the American College of Rheumatology (ACR) criteria and/or radiographic changes, and (c) the trials at least performed 1 comparison of VS versus placebo control. The studies were excluded if they were: (a) not for hip OA patients, (b) clinical study design or protocol, cohort or non-controlled studies, case series, and reviews, and (c) only abstracts. Any disagreements among authors were resolved by discussion.
Quality evaluation
The methodological validity of included studies was evaluated independently by 2 authors (Liao and Lin) using a tool for assessment of risk of bias, which primarily comprised 6 specific contents, including randomization schedule, allocation concealment, double-blinding, data completeness, and selective outcome [8]. Means of κ test were used to assess disagreements, which were resolved by discussion of the 2 reviewers (Liao and Lin).
Data extraction, imputation, and analysis
Two reviewers (Zhu and Shi) seperately abstracted the useful outcome data and confirmed the precision. In each included study, the study design, number of patients, demographic data (age and sex), OA severity, inclusion criteria, intervention protocol, guidance of injections, duration of follow-up, loss to follow-up, outcome measurement, and co-interventions, were extracted and summarized. When possible, we sent emails to the corresponding author of each included article to check the appropriateness of the extracted outcome and the quality evaluation.
The pain scores of patients after treatments were the main outcome. We used endpoint data rather than change data to maximize data availability. When an article provided data on more than 1 pain scale, we referred to a previously described hierarchy of pain-related outcomes and extracted the outcome that was highest on this list [9]. Global pain took precedence over pain on walking and pain subscales on the Western Ontario and McMaster Universities arthritis index (WOMAC). If a trial provided data on both – for example, global pain scores and WOMAC pain subscores – we recorded only data on global pain scores. If the outcome data were not available in tables or text, we measured and calculated them from figures of the original article. If standard deviations (SDs) were not provided, we calculated them from standard errors or confidence intervals, as described elsewhere [10]. Moreover, WOMAC function score or Lequesne index was used for the pooled analysis of functional outcomes, and we summarized the safety profile whenever available.
Since the treatment duration and the post-treatment assessment time points varied among the trials, we grouped the time points of outcome assessments of individual trials into 3 intervals: 7 to 14 days, 28 to 30 days, and 56 to 182 days (at final visit). If more than 1 time point was reported in a window, we extracted the data nearest to the latest follow-up time within the window. This grouping was made to make maximum use of all available data and to assess the persistence of treatment effects from different time points.
The pain and function scores at different time intervals were converted to standardized mean differences (SMD) using Review Manager 5.2 software. This ensured comparability between different outcomes assessed with different instruments, with effect sizes expressed in common units (i.e., SD). A negative effect size indicates a benefit of the VS intervention. Sensitivity analysis was conducted in STATA 11.
The statistical heterogeneity was evaluated by a χ2 test on N-1 degree of freedom (p<0.1 indicated significance). The formula [(Q–df)/Q]×100% (Q: the χ2 statistic; df: degree of freedom) was used to calculate the inconsistency I2, which represented the ratio of the consistency in effect size due to variation among the included studies. A I2 value over 50% indicated significant inconsistency and we used the random-effects model to combine data from different studies; otherwise, we used the fixed-effects model [8].
We performed sensitivity analysis to test the stability of our main outcomes by omitting one single trial each time (only 5 studies were analyzed). Funnel plot analysis and meta-regression analysis could not be performed due to the insufficient number of studies included.
Results
Selection of studies
The initial literature research found 86 relevant studies, of which 58 were excluded after eliminating duplicate studies or due to inappropriateness based on titles, and a further 22 studies were excluded after reading the abstracts. In total, 80 studies were excluded. Of the remaining 6 studies, 1 reported a dichotomous outcome for pain relief and it was excluded [11]. In a study by Migliore et al., local anesthesia with mepivacaine was administrated to the placebo group [12]. Because the effects of local anesthetics only persisted for a few hours, the study was included after discussion. Overall, as presented in Figure 1, there were 5 RCTs with 591 patients included in our meta-analysis [12–16].
Figure 1.
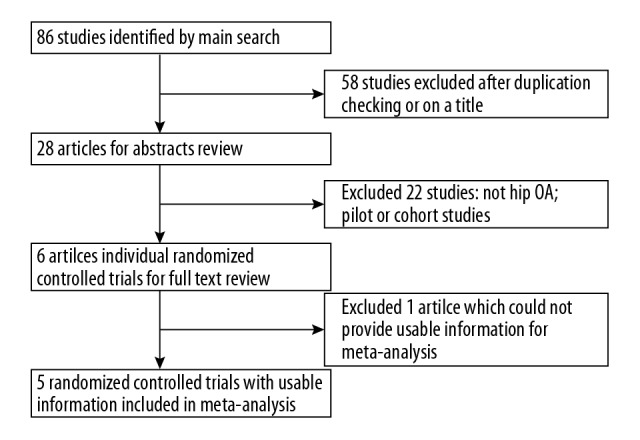
Study selection for the meta-analysis.
Information of included studies
The main data from the 5 included trials are summarized in Table 1. All the studies were designed as placebo-applied RCTs, of which 3 studies directly compared the HA to a placebo control group [12,14,16], and the other 2 were multi-armed studies, both using HA, steroid, and placebo groups [13,15]. Fluoroscopic guidance or ultrasound guidance was used to assist IA injection in all studies. Only aged adults (mean age range: 60.2–70 years) with symptomatic primary hip OA were included in all of the studies. The weighted kappa value for the consistency on inclusion between the 2 authors was 0.85 (95% CI: 0.76–0.94).
Table 1.
The overall information of the included trials.
| Author/ year/area | Sample size (VS/PLB) | Ages (mean+SD) and gender (Female/Male) | Moderate-severity OA* (%) | Inclusion criteria | Injection | Guidance | Pain outcome extracted | Functional outcome extracted | Duration (days) | Co-factors | Loss to follow-up (VS/PLB) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atchia/ 2011/ UK | 58 (19/19) | 69±8 (43/34) | 81% | 1. Aged over 50 years; 2. Primary unilateral hip OA; 3. Pain duration was more than a month or listed for elective THR |
Single injection. PLB: 3 ml NS; VS: 3ml Durolane | Ultrasound | Global pain (NRS) | WOMAC function subscale | 56 | NA | 1/0 (5.3%/ 0) |
| Brander/ 2018/ USA | 357 (182/175) | 60.3±9.4 (211/146) | 62% | 1. WOMAC score of 5 to 8 2. Age ≥35 years; 3. Symptomatic hip OA (radiographically confirmed KL grade II or III per ACR criteria) |
Single image-guided, VS: 6-mL injection of hylan G-F 20; PLB: 6 mL saline | Fuoroscopy/Ultrasound | WOMAC pain (NRS) | WOMAC function subscale | 182 | NA | 3/3 (1.7%/ 1.7%) |
| Migliore/ 2009/ Italy | 42 (22/20) | 70±8.9 | 91% | 1. Age >40 years; 2. Ambulant without assistance; 3. Hip OA by ACR; baseline VAS ≥4 cm; 4. Persistence of hip pain for at least 1 month before baseline |
Two injections. VS: 60 mg Hyalubrix; Local analgesic 4 ml 2% Carbocaine | Ultrasound | Global pain (VAS) | Lequesne | 180 | NA | 5/3 (22.7%/15%) |
| Qvistgaard/2006/Denmark | 69 (33/36) | 66±12 (65/36) | 43% | 1. Aged above 18 years; 2. Hip OA as defined by the ACR criteria and radiographic changes; 3. Stable medication for at least 3 weeks before inclusion |
Three injections with 14 days interval. PLB: 2 ml NS; VS: 3 ml Hyalgan; Steroid: (One 1 ml/40 mg depome-drone, followed by two sham injections) | Ultrasound | Pain on walking | Lequesne | 90 | 1 mL of 1% lidocaine | 5/3 (12%/8%) |
| Richette/ 2009/ France | 85 (42/43) | 60.2±11.4 (50/35) | 65.40% | 1. Aged above 30 years; 2. Hip OA as defined by the ACR criteria and radiographic changes; 3. Symptoms lasted for at least 1 month |
Single injection: PLB: 2.5 ml NS; VS: 2.5 ml Adant | Fuoroscopy | Global pain (VAS) | WOMAC function subscale | 90 | Treated with NSAIDs if necessary. | (6/4) (14.3%/9.3%) |
VS – viscosupplementation; PLB – placebo control; SD – standard deviation; UK – United Kindom; OA – osteoarthritis; THR – total hip replacement; NS – saline water; NRS – numerical rating scale (0–10); WOMAC – Western Ontario and McMaster Universities Arthritis Index; ACR – American College of Rheumatology radiographic criteria; VAS – visual analogue scale; NA – not available; NSAIDs – non-steroidal antiinflammatory drugs.
Kellgren/Lawrence III/IV and radiographic grading (Croft) III/IV are considered as “moderate-severity” OA.
Methodological quality
The methodological validity was assessed independently by 2 authors (Lin and Liao). The randomization schedule was based on randomly permuted blocks in 1 study [13], and its allocation concealment was not explicitly stated, while the other 4 studies [12,14–16] had adequate computer-generated sequences and detailed allocation concealment. Intention-to-treat (ITT) analysis was clearly applied in 4 studies [13–16], and selective reporting was not found in any of the studies. Overall, the 5 studies used adequate blinding and reported the details of loss to follow-up (Table 2). The weighted kappa value for consistency of methodological validity between the 2 authors was 0.88 (95% CI: 0.79–0.97).
Table 2.
Quality assessment of the included trials.
| Study | Randomized adequately* | Allocation concealed | Blinding** | Selective reporting | Similar co-factors# | (%) Loss to follow-up (VS/PLB)## | ITT analysis |
|---|---|---|---|---|---|---|---|
| Atchia 2011 | Adequately | Yes | Double Blinded | No | Yes | 5.3%/0 | Yes |
| Brander 2018 | Adequately | Yes | Double Blinded | No | Yes | 1.7%/1.7% | Yes |
| Migliore 2009 | Adequately | Unclear | Double Blinded | No | No | (22.7%/15%) | Unclear |
| Qvistgaard 2006 | Inadequately | Unclear | Double Blinded | No | Yes | 12%/8% | Yes |
| Richette 2009 | Adequately | Yes | Double Blinded | No | Yes | 14.3%/9.3% | Yes |
VS – viscosupplementation; PLB – placebo; ITT – itention to treat.
Randomization schedules based on randomly permuted blocks were considered not adequately according to Cochrane Handbook 5.0.2 [2008];
All the studies declared double-blind intervention, adequate methods of which were decribed explictly; outcome assessors were also considered to be blinded;
Qvistgaard’ study added lidocaine during injection equally in all groups;
Less than 25% loss-to-follow-up rate was considered acceptable.
Efficacy of measurement for pain relief after injection
Most trials demonstrated relief in pain in both the VS and placebo groups at different time intervals compared to baseline (Table 3). Specifically, VS showed a more dramatic pain reduction compared to baseline in 3 studies [12,13,16], even at the final visit (Brander et al: −34.4%, p<0.001; Migliore et al: −29.7%, p<0.01; and Qvistgaard et al., −22.4%, p<0.05). However, only in Migliore et al. did the VS group still have better pain relief than the placebo group (−29.7% versus −16.7%, p<0.05). No difference between the 2 groups was reported in the other studies. In addition, neither the VS group nor the placebo group had obvious pain alleviation compared to baseline in 2 studies [14,15].
Table 3.
Percentage changes from baseline of pain score in the included trials.
| Study | Outcomes | Treatments | No. of patients | Baseline | % Change from baseline at different time points | ||
|---|---|---|---|---|---|---|---|
| Day 7–14 | Day 28–30 | Final visit | |||||
| Atchia 2011 | NRS pain (0–10) | Durolane | 19 | 6.2 | NS | NS | NS |
| Saline | 19 | 6.3 | NS | NS | NS | ||
| Brander 2018 | WOMAC Pain score | Hylan G-F 20 | 182 | 6.42±0.06 | NA | −27.6%*** | −34.4%*** |
| Saline | 175 | 6.48±0.07 | NA | −30.1%*** | −37.3%*** | ||
| Migliore 2009 | Pain score (0–10 VAS) | Hyalubrix | 22 | 6.4±1.94 | NA | NA | −29.7%**,# |
| Mepivacaine | 20 | 6.0±1.94 | NA | NA | −16.7%** | ||
| Qvistgaard 2006 | Pain on walking (0–100 mm VAS) | Hyalgan | 33 | 49.2±24.8 | −20.3%* | −22.4%* | −22.4%* |
| Saline | 36 | 42.4±19.7 | +4.7% | −2.4% | −11.8% | ||
| Richette 2009 | Pain score (0–100 mm VAS) | Adant | 42 | 58.4±14.2 | NA | NA | −13.4% |
| Saline | 43 | 60.4±10.2 | NA | NA | −15.1% | ||
NRS – numerical rating scale (0–10); NS – not significant; WOMAC – Western Ontario and McMaster Universities Arthritis Index; NA – not available; VAS – visual analogue scale.
Achieved statistical significance (* p<0.05; ** p<0.01; *** p<0.001) compared to baseline;
achieved statistical significance (# p<0.05; ## p<0.01; ### p<0.001) compared to placebo control.
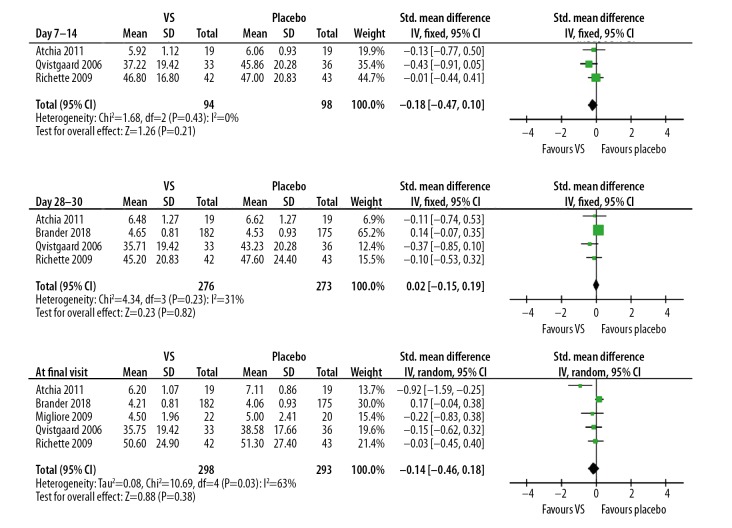
The pooled analysis confirmed the above results, showing that VS probably was not significantly more effective than placebo control during any time window [7–14 days: 3 studies, SMD (95% CI): −0.18 (−0.47, 0.10), p=0.21, I2=0%; 28–30 days: 4 studies, SMD (95% CI): 0.02 (−0.15, 0.19), p=0.82, I2=31%; at final visit: 5 studies, SMD (95% CI): −0.14 (−0.46, 0.18), p=0.38, I2=63%, Figure 2].
Figure 2.
Forest plots of pain scores at different time points.
Efficacy of measurement for function improvement after injection
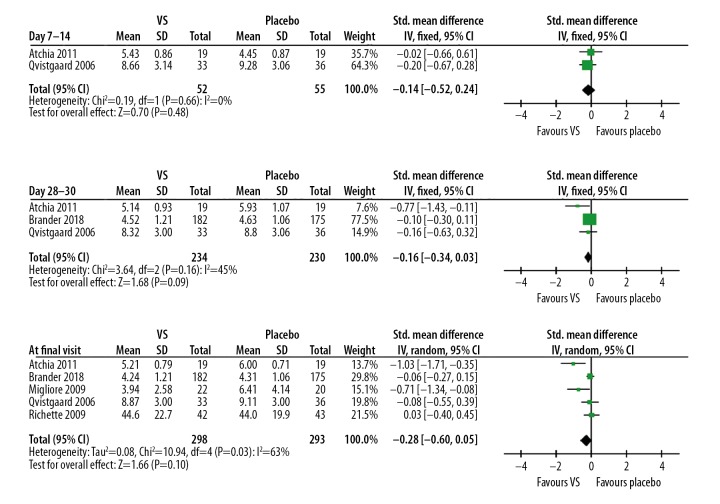
Similarly, there was significant improvement in function scores in the VS group compared to baseline in 2 studies [12,16], but not in the other studies [13–15] (Table 4). Not surprisingly, our meta-analysis shows the function scores did not differ between the VS and placebo groups at any time points [7–14 days: 2 studies, SMD (95% CI): −0.14 (−0.52, 0.24), p=0.48, I2=0%; 28–30 days: 3 studies, SMD (95% CI): −0.16 (−0.34, 0.03), p=0.09, I2=45%; at final visit: 5 studies, SMD (95% CI): −0.28 (−0.60, 0.05), p=0.10, I2=63%, Figure 3].
Table 4.
Percentage changes from baseline of function score in the included trials.
| Study | Outcomes | Treatments | No. of patients | Baseline | % Change from baseline at different time points | ||
|---|---|---|---|---|---|---|---|
| Day 7–14 | Day 28–30 | Final visit | |||||
| Atchia 2011 | WOMAC Function score | Durolane | 19 | 6 | NS | NS | NS |
| Saline | 19 | 6 | NS | NS | NS | ||
| Brander 2018 | WOMAC Function score | Hylan G-F 20 | 182 | 6.33±0.09 | NA | −28.6%*** | −33.0%*** |
| Saline | 175 | 6.44±0.08 | NA | −28.4%*** | −33.1%*** | ||
| Migliore 2009 | Lequesne index | Hyalubrix | 22 | 7.09±3.78 | NA | NA | −44.4%**,# |
| Mepivacaine | 20 | 7.75±4.15 | NA | NA | −17.2%** | ||
| Qvistgaard 2006 | Lequesne index (1–24) | Hyalgan | 33 | 10.0±4.0 | NS | NS | NS |
| Saline | 36 | 9.5±3.8 | NS | NS | NS | ||
| Richette 2009 | WOMAC Function score | Adant | 42 | 51.3±16.8 | NA | NA | −13.1% |
| Saline | 43 | 49.7±13.4 | NA | NA | −11.5% | ||
NS – not significant; WOMAC – Western Ontario and McMaster Universities Arthritis Index; NA – not available; VAS – visual analogue scale.
Achieved statistical significance (* p<0.05; ** p<0.01; *** p<0.001) compared to baseline;
achieved statistical significance (# p<0.05; ## p<0.01; ### p<0.001) compared to placebo control.
Figure 3.
Forest plots of function scores at different time points.
Heterogeneity and sensitivity analysis
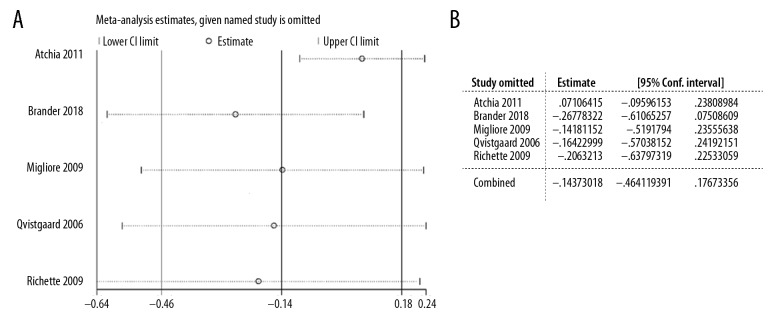
Overall homogeneity among studies was observed in comparisons of pain and functional scores (7–14 days and 28–30 days); therefore, the pooled analysis was performed with fixed-effects models (Figures 2, 3). When we detected substantial heterogeneity in comparisons of pain (I2=63%, p=0.03) and function (I2=63%, p=0.03) assessment at final visit, we used randomized-effects models to synthesize the results (Figures 2, 3). For the main outcome – pain score comparison at final visit between the 2 groups – we carried out sensitivity analysis by omitting one single study each time to evaluate the stability of the results. By doing this analysis, we were also able to clarify the sources of heterogeneity. When omitting Atchia et al., the heterogeneity (I2=63%, p=0.03) was reduced to be minimal in the comparison (I2=0%, p=0.41, data not shown), indicating that study might be the main source of the heterogeneity. However, as shown in Figure 4, we did not observe any substantial alternation of overall results by deleting any single study.
Figure 4.
Sensitivity analysis of pain scores at final visit. (A) Overall changes in standardized mean difference (SMD) and 95% confidential interval (CI) after omitting each trial. (B) Quantification results of A.
Adverse events
IA treatment with fluoroscopic or ultrasound guidance seemed to be well tolerated and safe for hip OA patients. Infections were most concerning because the risk would be greatly increased by invasive procedures, particularly for deep joints. Confirmed infection was rather rare, and only 1 case in the VS group in Atchia et al. was reported [15], and other rare adverse effects possibly related to the treatment (e.g., pruritus or hematoma at the injection area) were also only reported once in Richette et al., in 2 patients [14]. The most common adverse effects were slight or moderate flare pain during or after injection: 4 patients of 19 in the VS groups in Atchia et al. [15]; 1 of 21 in the placebo group and 3 of 101 in total in Qvistgaard et al. [13]; 2 of 43 in the placebo group and 3 of 42 in the VS group in Richette et al. [14]; and 4 of 172 in the placebo group and 12 of 182 in the VS group in Brander et al. [16]. The pain was self-restricted and balanced in all injection groups. Withdrawals related to adverse events were reported only in Brander et al. [16] (placebo: 10/172; VS: 10/182).
Discussion
VS is believed to restore the rheological properties of synovial fluid by supplementing exogenous hyaluronic acid into painful joints, thereby increasing viscosity and generating analgesic, anti-inflammatory, and chondroprotective effects. However, contrary to the promising results of knee OA, we found that VS for hip OA did not achieve significant improvement in pain or function compared to a placebo control, which is consistent with a recent systematic review [7]. However, the major limitation of this review was failure to perform a pooled analysis due to the heterogeneous design and varied outcome measurements among the included studies [7].
A meta-analysis by Lieberman et al. [6] showed that VS generated a minor but significant improvement over controls. However, the heterogeneous control groups (placebo, methylprednisolone, and other HA formulations) and the combined various time intervals (1–6 months) greatly hinder the interpretation of their results.
In our review, we exclusively targeted double-blind RCTs, 4 of which did report ITT data [13–16]. Only 1 trial did not use ITT methods, but it had low drop-out rates and satisfactory quality, and sensitivity analysis suggested it did not affect the overall results [12].
In addition, we determined which measures to pool according to a hierarchy of recommended pain evaluations, and we converted the various outcome measures to standardized mean differences and successfully pooled effect sizes with unit-less measures of treatment efficacy. The quantitative results of our review then were highly informative in assessing the magnitude of therapeutic efficacy of VS. Furthermore, we examined the therapeutic response over time by separately pooling the data for each time interval. The outputs of this analysis elucidated the mode of therapeutic response attributable to VS intervention.
Our review strictly followed the instruction of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [8]. The detailed inclusion and exclusion criteria, the explicit demonstration of study selection and methodological quality assessment, and the quantitative analysis ensured the reproducibility of the methods. Selection bias was further avoided by elimination of duplicate studies.
In the present review, 3 of 5 studies showed that VS produced a dramatic pain reduction compared to baseline [12,13,16]. Specifically, data from Brander et al. strongly suggested that VS significantly decreased pain scores compared to baseline during a 6-month follow-up after injection. These results were from a study with an adequate sample size (n=357), and the study was industry-sponsored, but they did not demonstrate the superiority of VS over placebo.
Our pooled analysis did not find differences between VS and placebo in pain or in function scores, which might be due to the small sample sizes of the included studies that failed to detect differences [17]. However, the benefits of VS in hip OA was only demonstrated in open-label cohort studies, most of which included early OA [18–21].
A placebo, even normal saline, might appear to have a pain reduction effect due to the dilution of proinflammatory cytokines. For example, a recent meta-analysis by Zhang et al. found that IA placebo is efficacious for OA, especially for self-reported pain and function outcome measurements [22].
The degree of radiographic severity of OA was reported to be strongly associated with the response to IA steroid injection of the hip, showing that more severe OA at baseline generally predicted less pain relief after IA injection [23,24]. In our meta-analysis (Table 1), 4 of 5 studies accounted for the majority of moderate-severity OA (over 50%) patients, which might have limited the response rate in the VS group [12,14–16]. Therefore, the strong placebo effects or the preponderance of moderate-severity cases might also explain the lack of statistical significance.
Our analysis has certain limitations that should be considered. (a) There was slight variability in the selection criteria of individual trials, including sex ratio and ages of patients. (b) Due to the insufficient number of trials, some relevant factors, such as the formulation of VS and the dose or number of injections, could not be analyzed. It has been reported that that higher viscosity and longer half-life result in better outcomes by increasing molecular weight of intra-articular hyaluronic acid [25], but this is controversial. In the studies included in our review, 5 products were used, in which 2 (Durolane and Hylan G-F 20) [15,16] have high molecular weights and the other 3 (Hyalubrix, Hyalgan, and Adant) have low-to-intermediate molecular weight [12–14] according to de Rezende et al. [26]. Multiple injections (2 for Hyalubrix, 3 for Hyalgan) were generally used in low-to-intermediate molecular weight products, while Durolane and Hylan G-F 20 were injected only a single time according to their individual instructions, which could have reduced the discrepancy in their sustaining period among products with different molecular weights.
However, due to the complexity and chronic nature of OA, different phenotypes, long-term observation, and appropriate comparator should be fully considered in future RCTs; otherwise, there will still be a gap between recommendations from RCTs and clinical practice [27]. We also agree that these influential factors should be further clarified by conducting subgroup analysis of a sufficient number of RCTs in future meta-analysis.
Our review shows that IA treatment with radiological guidance is well tolerated and safe for hip OA patients. Only 1 confirmed infection was reported in the VS group in Atchia et al. [15]. The most common adverse effects were moderate pain during injection or lasting for a short time after injection, which were resolved without treatment. Whether pre-operated IA VS increases the risk of prosthetic infection after THR was not studied in this review, and whether the patients waiting for THR could receive IA VS remains unclear and should be carefully considered in future research.
Conclusions
Our analysis was not able to show that IA VS reduces pain and improves function significantly better than placebo in a short-term follow-up. The benefits and safety of VS should be further assessed by sufficiently-sized, methodologically-sound studies with validated assessment of more clinically relevant end-points.
Footnotes
Source of support: The project was supported by the National Natural Science Foundation of China (81703017), by the Science and Technology Projects of Guangzhou, China (201804010080), and by the Science and Technology Projects of Huizhou, China (2018Y304)
References
- 1.Guillemin F, Rat AC, Mazieres B, et al. Prevalence of symptomatic hip and knee osteoarthritis: A two-phase population-based survey. Osteoarthritis Cartilage. 2011;19(11):1314–22. doi: 10.1016/j.joca.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Shen J, Zhao W, et al. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy NJ, Eyles JP, Hunter DJ. Hip osteoarthritis: Etiopathogenesis and implications for management. Adv Ther. 2016;33(11):1921–46. doi: 10.1007/s12325-016-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140–49. doi: 10.1016/j.semarthrit.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Legre-Boyer V. Viscosupplementation: Techniques, indications, results. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S101–8. doi: 10.1016/j.otsr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JR, Engstrom SM, Solovyova O, et al. Is intra-articular hyaluronic acid effective in treating osteoarthritis of the hip joint? J Arthroplast. 2015;30(3):507–11. doi: 10.1016/j.arth.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Leite VF, Daud Amadera JE, Buehler AM. Viscosupplementation for Hip osteoarthritis: A systematic review and meta-analysis of the efficacy on pain and disability, and the occurrence of adverse events. Arch Phys Med Rehabil. 2018;99(3):574–83.e1. doi: 10.1016/j.apmr.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Green S. Cochrane Collaboration: Cochrane handbook for systematic reviews of interventions. xxi. Chichester, England; Hoboken, NJ: Wiley-Blackwell; 2008. p. 649. [Google Scholar]
- 9.Juni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Pract Res Clin Rheumatol. 2006;20(4):721–40. doi: 10.1016/j.berh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan J, Casale FF, Thomas TL, Desai KB. Intra-articular injection for pain relief in patients awaiting hip replacement. Ann R Coll Surg Engl. 1988;70(3):156–57. [PMC free article] [PubMed] [Google Scholar]
- 12.Migliore A, Massafra U, Bizzi E, et al. Comparative, double-blind, controlled study of intra-articular hyaluronic acid (Hyalubrix) injections versus local anesthetic in osteoarthritis of the hip. Arthritis Res Ther. 2009;11(6):R183. doi: 10.1186/ar2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qvistgaard E, Christensen R, Torp-Pedersen S, Bliddal H. Intra-articular treatment of hip osteoarthritis: A randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage. 2006;14(2):163–70. doi: 10.1016/j.joca.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Richette P, Ravaud P, Conrozier T, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: A multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60(3):824–30. doi: 10.1002/art.24301. [DOI] [PubMed] [Google Scholar]
- 15.Atchia I, Kane D, Reed MR, Isaacs JD, Birrell F. Efficacy of a single ultrasound-guided injection for the treatment of hiposteoarthritis. Ann Rheum Dis. 2011;70(1):110–16. doi: 10.1136/ard.2009.127183. [DOI] [PubMed] [Google Scholar]
- 16.Brander V, Skrepnik N, Petrella RJ, et al. Evaluating the use of intra-articular injections as a treatment for painful hip osteoarthritis: A randomized, double-blind, multicenter, parallel-group study comparing a single 6-mL injection of hylan G-F 20 with saline. Osteoarthritis Cartilage. 2019;27(1):59–70. doi: 10.1016/j.joca.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Ersboll AK, Ersboll BK. Epidemiological studies based on small sample sizes – a statistician’s point of view. Acta Vet Scand Suppl. 2003;98:127–40. [PubMed] [Google Scholar]
- 18.Migliore A, Tormenta S, Massafra U, et al. Intra-articular administration of hylan G-F 20 in patients with symptomatic hip osteoarthritis: Tolerability and effectiveness in a large cohort study in clinical practice. Curr Med Res Opin. 2008;24(5):1309–16. doi: 10.1185/030079908x291930. [DOI] [PubMed] [Google Scholar]
- 19.Brocq O, Tran G, Breuil V, et al. Hip osteoarthritis: Short-term efficacy and safety of viscosupplementation by hylan G-F. 20. An open-label study in 22 patients. Joint Bone Spine. 2002;69(4):388–91. doi: 10.1016/s1297-319x(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 20.Conrozier T, Bertin P, Bailleul F, et al. Clinical response to intra-articular injections of hylan G-F 20 in symptomatic hip osteoarthritis: The OMERACT-OARSI criteria applied to the results of a pilot study. Joint Bone Spine. 2006;73(6):705–9. doi: 10.1016/j.jbspin.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Vad VB, Sakalkale D, Sculco TP, Wickiewicz TL. Role of hylan G-F 20 in treatment of osteoarthritis of the hip joint. Arch Phys Med Rehabil. 2003;84(8):1224–26. doi: 10.1016/s0003-9993(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Robertson J, Jones AC, et al. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716–23. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 23.Deshmukh AJ, Panagopoulos G, Alizadeh A, et al. Intra-articular hip injection: Does pain relief correlate with radiographic severity of osteoarthritis? Skeletal Radiol. 2011;40(11):1449–54. doi: 10.1007/s00256-011-1120-8. [DOI] [PubMed] [Google Scholar]
- 24.van Middelkoop M, Arden NK, Atchia I, et al. The OA Trial Bank: Meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage. 2016;24(7):1143–52. doi: 10.1016/j.joca.2016.01.983. [DOI] [PubMed] [Google Scholar]
- 25.Waddell DD, Bricker DC. Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis. J Manage Care Pharm. 2007;13(2):113–21. doi: 10.18553/jmcp.2007.13.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Rezende MU, de Campos GC. Viscosupplementation. Rev Bras Ortop. 2012;47(2):160–64. [Google Scholar]
- 27.Migliore A, Bizzi E, Herrero-Beaumont J, et al. The discrepancy between recommendations and clinical practice for viscosupplementation in osteoarthritis: mind the gap! Eur Rev Med Pharmacol Sci. 2015;19(7):1124–29. [PubMed] [Google Scholar]