Abstract
Beta-hydroxy-beta-methylbutyrate (HMB) has been used extensively as a dietary supplement for athletes and physically active people. HMB is a leucine metabolite, which is one of three branched chain amino acids. HMB plays multiple roles in the human body of which most important ones include protein metabolism, insulin activity and skeletal muscle hypertrophy. The ergogenic effects of HMB supplementation are related to the enhancement of sarcolemma integrity, inhibition of protein degradation (ubiquitin pathway), decreased cell apoptosis, increased protein synthesis (mTOR pathway), stimulation of the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis and enhancement of muscle stem cells proliferation and differentiation. HMB supplementation has been carried out with various groups of athletes. In endurance and martial arts athletes, HMB supplementation revealed positive effects on specific aerobic capacity variables. Positive results were also disclosed in resistance trained athletes, where changes in strength, body fat and muscle mass as well as anaerobic performance and power output were observed. The purpose of this review was to present the main mechanisms of HMB action, especially related to muscle protein synthesis and degradation, and ergogenic effects on different types of sports and physical activities.
Key words: HMB, ergogenic aids, supplementation, athletes
Introduction
Beta-hydroxy-beta-methylbutyrate (HMB) is a natural metabolite of leucine, one of the essential amino acids (Van Koevering and Nissen, 1992). First, during reversible transamination, leucine is converted to alpha-ketoisocaproic acid (alpha-KIC) which, at the same concentration level as leucine, appears to be able to inhibit the breakdown of muscle proteins (Chua et al., 1979). Depending on whether alpha- KIC is in the mitochondria or cytoplasm, it is converted to isovaleryl-coenzyme A (isovaleryl-CoA) by KIC dehydrogenase or HMB by KIC dioxygenase, respectively (Sabourin and Bieber, 1983). Conversion to isovaleryl- CoA inside liver mitochondria occurs with 95% probability, while only 5% of leucine is converted to HMB in the cytosol (Scheme 1) (Van Koevering and Nissen, 1992). Next, the isovaleryl-CoA is subsequently metabolized by various transformations to acetyl-CoA and other compounds (Kohlmeier, 2015). Metabolism of HMB in the cytosol involves joining a coenzyme A molecule (HMB-CoA), followed by the conversion of the resulting compound into beta-hydroxy-beta-methylglutaryl coenzyme A (HMG-CoA), which is the primary metabolite of HMB in the body (Scheme 1). With the involvement of reductase, HMG-CoA is transformed into mevalonic acid, which is a precursor for the synthesis of cholesterol (Rudney, 1957). The amount of HMB excreted in urine is dose-dependent. For low doses of this compound (1 g/day), the amount of HMB excreted is 14%, while for high doses (3 g/day), it is as much as 29% (Vukovich et al., 2001).
Scheme 1.
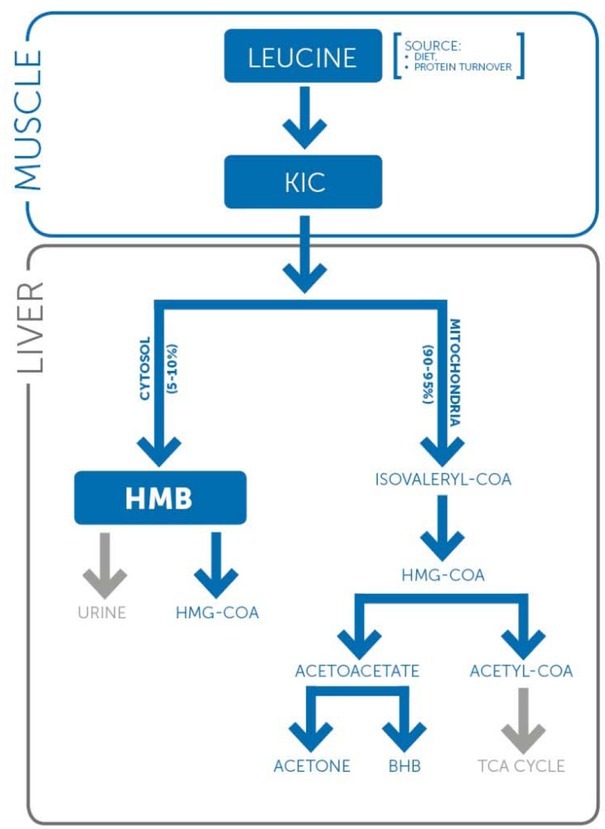
The primary metabolite of HMB in the body
The purpose of this paper was to present a comprehensive review of HMB ergogenic mechanisms which include the mTOR dependent protein synthesis, the GH and IGF-1 synthesis, satellite cell proliferation, ubiquitin-dependent protein proteolysis and cholesterol biosynthesis. The secondary purpose was to present the effects of HMB supplementation on physical fitness and sports performance.
The possible mechanisms of HMB action
The available literature shows that the mechanism of action of HMB in the body is based primarily on its ability to increase the integrity of the myocyte cell membrane, i.e. sarcolemma, and reduce the efficiency of intracellular proteolytic pathways (Asadi et al., 2017; Nissen and Abumrad, 1997; Wilson et al., 2008). It is a consequence of the antagonistic effect on the protein degradation pathway by coupling them to a specific protein, i.e. ubiquitin (Smith et al., 2005). Recent research also mentions two additional mechanisms through which HMB can have a positive effect on muscle protein anabolism (Eley et al., 2007; Gerlinger-Romero et al., 2011). These are the activation of the mTOR kinase pathway (Eley et al., 2007) as well as the increase in the level of transcription of the insulin-like growth factor-1 (IGF-1) gene (Gerlinger- Romero et al., 2011). Besides, in vivo studies point to the fact that HMB improves the proliferation of muscle stem cells in fast twitch fibers (Asadi et al., 2017). Enhanced satellite cell proliferation should increase muscle hypertrophic changes and functional changes (Alway et al., 2013).
Several possible mechanisms of HMB action on the human body are presented in Scheme 2.
Scheme 2.
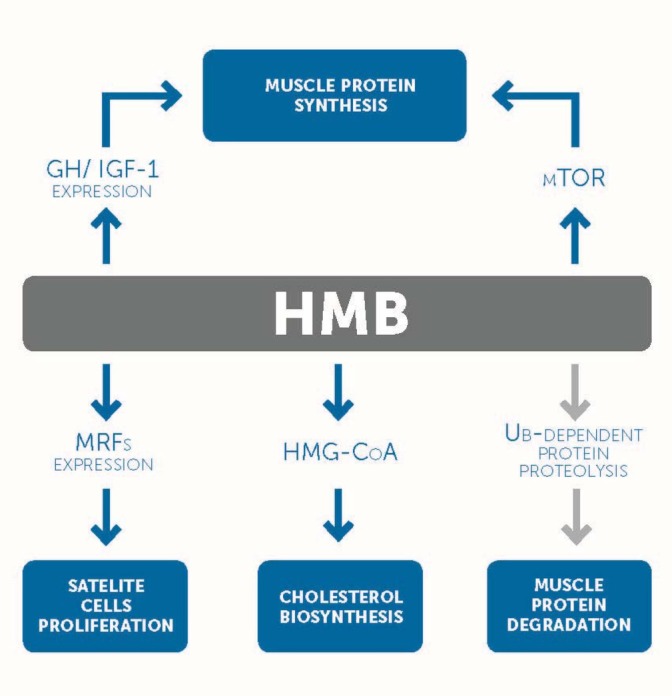
Possible mechanisms of HMB action in the human body
The effect of HMB on the mTOR kinase pathway
The fundamental function of mTOR kinase is to regulate the processes of cell transcription, translation, proliferation and growth i.e. protein synthesis (Asadi et al., 2017; Hay and Sonenberg, 2004). It seems that mTOR kinase plays a major role in the adaptation of muscle fiber synthesis as a result of intense physical effort (Hornberger, 2011), as well as hormonal and nutritional stimulation. It is known that HMB stimulates protein synthesis in skeletal muscles (Nissen and Abumrad, 1997; Smith et al., 2005). Relevant results were presented by Pimentel et al. (2011). Those researchers administered 320 mg/kg body weight of HMB to rats. After supplementation they observed significant muscle hypertrophy in the rodents; mTOR kinase expression increased by 429%. Also Baxter et al. (2005) confirm that HMB supplementation decreased muscle protein degradation while adding rapamycin, an mTOR specific inhibitor, reduced this effect significantly. Kornasio et al. (2009) in their experiment showed that HMB, apart from stimulating the mTOR kinase pathway (PI3K/Akt), additionally increased the expression of IGF-1. However, it remains unanswered whether the stimulation of mTOR by HMB took place indirectly or directly. In another study, it was observed that the activation of mTOR kinase followed myotube hypetrophy in response to IGF-1 (Arazi et al., 2018). Therefore, in future research, it is essential to determine whether HMB stimulates mTOR directly or as a result of increased expression of IGF-1 (Bodine et al., 2001). The effect of HMB on the stimulation of growth hormone / insulin-like growth factor-1 (GH / IGF-1) synthesis
In last years, researchers revealed that HMB could stimulate the GH/IGF-1 synthesis. (Arazi et al., 2015; Gerlinger- Romero et al., 2011). In the study of Garlinger-Romero et al. (2011), rats were administered 320 mg/kg body weight of HMB for 4 weeks. After this time, they observed an increase in mRNA-GH expression and GH levels of 65 and 20%, respectively. After the supplementation period, the liver mRNA of IGF-1 increased by approximately 30%, and the concentration in the serum of IGF-1 by almost 20%. In that study, however, there was no increase in IGF-1 levels in muscle cells, although in human myoblasts there was a 2-fold increase in IGF-1, additionally dependent on the dose of HMB (Kornasio et al., 2009). IGF-1 has a strong anabolic effect on skeletal muscle and is responsible for the hypertrophy of muscle fibres (Fiorotto et al., 2003). Unfortunately in the study of Portal et al. (2011), the effect of HMB on IGF-1 changes in male and female volleyball players compared to the placebo group was not confirmed. The lack of heterogeneity of the results seems to prove the need for further research to verify this hypothesis. HMB improves the proliferation of muscle stem cells
The effect of HMB on satellite cells proliferation and differentiation, at least in cell cultures, is similar, yet greater compared to the effect induced by IGF-1 (Arazi et al., 2015; Holeček et al., 2017). Furthermore, it also influences the expression of IGF-1 mRNA (Gerlinger-Romero et al., 2011). HMB also acts directly on the phosphorylation of serine-threonine kinase that is responsible for the phosphorylation of several proteins involved in the regulation of the fundamental cellular processes, including their proliferation (Arazi et al., 2015). In an in vitro study in human myoblast satellite cells, an increase in the expression of the MyoD protein, one of the markers indicating proliferation of muscle cells, was observed (Kornasio et al., 2009). The same study also noted an increase in the number of cells following the rise of the others myogenic regulatory factors (MRFs, myogenin and MEF2) that are responsible for the differentiation of satellite cells, thus suggesting a direct effect of HMB on myoblast proliferation and differentiation (Kornasio et al., 2009). A similar trend, i.e. an increase in the number of differentiated satellite cells (MyoD, myogenin myonuclei) was also found by Alway et al. (2013). In that study, an ergogenic effect of HMB on the proliferation of satellite cells of fast twitch muscle fibres was observed.
Ergogenic effects of HMB supplementation in athletes
For more than a decade, athletes have been regularly using HMB supplementation, expecting to improve athletic performance, especially muscle growth and body fat oxidation, decrease muscle tissue degradation, and improve aerobic fitness (Asadi et al., 2017; Arazi et al., 2015; Silva et al., 2017; Wilson et al., 2014). Apart from that, some authors still indicate that HMB requires more research confirming its effectiveness as an ergogenic aid (Kreider et al., 1999; Thomson et al., 2009; Vulcan et al., 2012). The basic form of HMB which has been used in research was the calcium salt HMB-Ca (Jówko et al., 2001; Kreider et al., 1999; Nissen et al., 1996). Recently, a new more effective form free acid HMB-FA formula has been developed and intensively studied (Asadi et al., 2017; Silva et al., 2017; Teixeira et al., 2019).
Influence of HMB supplementation combined with resistance training on strength variables and body composition
Among the many elements of strength training, high volume of work and overall training loads are the main factors influencing post-exercise increases in the secretion of anabolic hormones which stimulate muscle protein synthesis (Asadi et al., 2017; Wilk et al., 2018). Resistance trained athletes use supplements such as whey protein, amino acids or HMB to increase muscle mass and finally increase strength and power reducing body fat (Asadi et al., 2017; Bloomstrand et al., 2006; Panton et al., 2000; Wilk et al., 2018; Woolf, 2001). Since HMB increases beta-oxidation of the fatty acids, it is also used as a supplement to reduce body fat (Asadi et al., 2017). The first study in which the effectiveness of HMB-Ca (salt form) supplementation on muscular strength and body composition was evaluated, was published by Nissen et al. in 1996. In this work, the authors investigated the effects of two doses of HMB (1.5 and 3.0 g/per day) during a 3-week resistance training program in untrained male participants. The HMB supplemented group versus the placebo group were compared in a 1-RM testing protocol. Total strength increased by 13% (1.5 g HMB/d) and 18.4% (3.0 g HMB/d), respectively, in subjects ingesting HMB. Additionally, a positive trend in fat-free mass (FFM) was observed. Subjects who consumed 1.5 g HMB/d increased their FFM by 1.2 kg, while those that ingested 3.0 g HMB/d increased their FFM by 4 kg. Improvements in strength variables in all considered lifts, as well as in total strength values during the intake of 3 g HMB/d were also observed by Jówko et al. (2001) in a 3-week study with untrained participants. Unfortunately, research by Nissen et al. (1996) did not confirm these ergogenic effects of HMB on muscular strength in trained athletes, with the exception of the bench press exercise where significant improvements were registered. Additionally, a positive trend was observed in FFM in the group ingesting HMB (1.4 kg; 2%) compared to the placebo group (0.85 kg; 1.35%). Another study, which confirms the effectiveness of 3 g supplementation of HMB per day during 12 weeks in 20 trained male subjects was performed by Wilson et al. (2014). Those authors registered strength increases in the squat and bench press in the supplemented compared to the placebo group. Changes in body mass (+1.8 kg), FFM (+5.3 kg), BF (-3.4 kg), and quadriceps thickness (+4.8 mm), were also observed in subjects supplemented with HMB. Similarly, Asadi et al. (2017) in their six-week study with HMB-FA supplementation demonstrated its efficacy on strength and power performance. Panton et al. (2000) evaluated the influence of 4-week supplementation with 3 g of HMB per day during resistance training. After the supplementation period, increases in 1-RM strength occurred only in the HMB-group in upper-body exercises. No differences in lower body strength between the placebo and the HMB group were observed. Those authors also registered favorable changes in fat free mass (FFM) (+1.4 kg placebo vs. +2 kg HMB group) and body fat (BF) (-0.9% placebo vs. -1.5% HMB group). Thomson et al. (2009) in their 9-week study observed increases in leg extension strength and decreases in biceps curl and bench press results. They also observed decreases in overall skinfold thickness and a positive trend in BF content. Similar results were reported by Ransone et al. (2003) who confirmed that HMB supplementation decreased BF and increased FFM in professional soccer players.
However, there are also studies with athletes which reported no ergogenic effects of HMB supplementation during resistance training (Gallagher et al., 2000; Kreider et al., 1999, 2000; Slater et al., 2001). A 4-week study conducted by Kreider et al. (1999) on 40 trained males supplemented with HMB with doses of 3 to 6 g per day resulted in non-significant differences in 1-RM variables (overall gains in 1RM strength, when bench press and leg press values were combined). Similarly, they did not observe differences in body composition. In another 4 week study with 28 male football players, Kreider et al. (2000) also reported no significant influence of 3 g/d of HMB supplementation on body composition. Moreover, no significant differences were observed between groups in 1-RM tests, in the bench press, squat, power clean, and total lifting volume for all three lifts combined between the supplemented and placebo groups (Kreider et al., 2000). On the other hand, Teixeira et al. (2019) investigated changes in strength comparing HMB-Ca vs. HMB-FA supplementation in 40 males. After 8-week supplementation, there were no positive effects of both HMB-Ca and HMB-FA formula on strength measures in the 1RM protocol (1RM bench press (kg); 1RM back squat (kg)), nor muscle thickness of the vastus lateralis and rectus femoris. Additionally, the same authors, in another 8-week study with resistance training, reported no positive effect of HMB-Ca and HMB-FA on FFM and BF (Teixeira et al., 2018). Slater et al. (2001) performed a similar study with 22 trained males (water polo and Australian rowing Olympians). After 6 weeks of supplementation, they also showed no positive influence of 3 g of daily HMB-Ca and HMB-FA supplementation, on strength in the 3RM, bench press and leg press. Furthermore, no changes in body composition were observed (Slater et al., 2001). Gallagher et al. (2000) supplemented 37 male subjects with 3 and 6 g/d of HMB over 8 weeks. No differences between groups in the 1-RM test and body composition were observed. However, a positive trend in FFM (+1.9 kg) was noted in the group supplemented with HMB.
The aforementioned results indicate that in athletes the effects of HMB supplementation on strength variables and body composition are not equivocal (Assadi et al., 2017; Kreider et al., 2010; Nissen et al., 1996). This can be explained by several factors. Studies performed on trained athletes mostly resulted in no influence on strength, body composition and anaerobic power output, during resistance training protocols lasting from 3 to 9 weeks (Kreider, 1999; Slater et al., 2001). Positive results in trained individuals were yet observed in research in which the HMB supplementation period lasted 12 weeks or longer (Wilson et al., 2014). Another reason that may explain such discrepancies in literature is for the use of different training protocols. In the first study of Kreider et al. (1996), subjects maintained their usual training routine and recorded all training sessions on their training log sheets during the supplementation period, without any additional control during the workouts. Similarly, Thomson et al. (2009) did not control the quality of training sessions and based their results on self-reported training logs (84% of accuracy and similarity between participants in compliance of training). On the other hand, in Wilson et al. (2008) used a non-linear progression with tapered training volume. These may generate differences in load volume during the experiment between particular groups of subjects. Differences in diet control may have also influenced the final results (Thompson et al., 2009), especially considering that not all studies controlled the diet during the experiment. From the studies presented in this review, only four of them took diet into account (Jówko et al., 2001; Kreider et al., 2000; Slater et al., 2001; Thomson et al., 2009), while in three studies pre- and post-experimental dietary recalls were used, without diet control during the study (Gallagher et al., 2000a; Kreider et al., 1999; Teixeira et al., 2019). The lack of strict dietary control and dietary co-intervention may mask the effect of HMB supplementation or induce a synergistic effect of several variables (i.e. improvement in protein turnover, management of vitamin and mineral deficits).
Influence of HMB supplementation on anaerobic performance and power output
Besides the influence on strength and power, some researchers have investigated the effectiveness of HMB supplementation on anaerobic power (i.e. Wingate test) and countermovement jump (CMJ) performance (Kreider et al., 2000; Wilson et al., 2014). The study conducted by Wilson and et al. (2014) noticed an improvement in Wingate test results and CMJ performance, while Kreider et al. (2000) did not observe any enhancement in a 12 x 6 s sprint test with 30-s rest intervals between sprints on a computerized cycle ergometer.
The effects of HMB supplementation on inhibition of protein degradation during endurance and resistance training
Regardless of the type of training, working muscles are exposed to proteolysis (Maciejewska et al., 2017; Nissen et al., 1996; Smith et al., 2004). Different conditions, such as aerobic and anaerobic training, injury, immobilization, and fasting, may increase muscle protein degradation (Michalczyk et al., 2019; Reid, 2005; Thompson and Scordilis, 1994; Whitehouse et al., 2003). Various studies have analysed interactions between proteolytic pathways and HMB (Nissen et al., 1996; Smith et al., 2004). HMB supplementation decreases TNF-α and INF-γ, inflammatory markers, leading to the inhibition of proteolytic processes (Hoffman et al., 2016). Smith et al. (2005) showed a positive effect of HMB supplementation on the reduction of muscle protein degradation in in vitro culture of mouse myotubes infected with cancer cells, which was treated with a proteolysis inducing agent, increasing the activity of the ubiquitin-proteasome proteolytic pathway. It could be ruled out that HMB impacts this pathway indirectly, increasing the activation of IGF-1 production and finally increases muscle steam cell proliferation (Arazi et al., 2015; Eley et al., 2008; Sacheck et al., 2004). However, in a recent study, Asadi et al. (2017) confirmed that HMB-FA supplementation altered resting hormonal concentrations, and those results appear to be the first to indicate that HMB-FA supplementation for six weeks can promote post-training gains in GH and IGF-1. Those authors concluded that six weeks of HMB- FA supplementations induced significant increases in anabolic hormones with reduction of catabolic hormones and remarkable improvements in strength and power performance (Asadi et al., 2017). In a previous study, Willoughby et al. (2003) suggested that if muscle degradation following training was not intense and the activity of the ubiquitin pathway decreased, thus the effectiveness of HMB supplementation was not significant. Those results suggest that HMB supplementation to inhibit muscle mass degradation is beneficial for individuals who are recreationally physically active. However, in professional athletes during preparatory periods, when training loads are very high and different training methods are used, HMB-FA supplementation could be beneficial to protect from muscle damage (Asadi et al., 2017). Knitter et al. (2000) evaluated the influence of HMB supplementation on exercise induced muscle damage after prolonged distance runs. The study was conducted on 8 male and 8 female athletes, running a weekly distance of 48 km or more for 6 weeks. Participants were randomly assigned either to the experimental (3 g/d HMB) or the placebo group. Before and after the intervention, participants performed a 20 km cross-country running test. In the experimental group, the levels of creatine kinase (CK) and lactate dehydrogenase (LDH), markers of muscle tissue damage, were lower compared to the control group. The results indicated that HMB supplementation reduced exercise-induced muscle damage (Arazi et al., 2015; Knitter et al., 2000).
The rate of absorption and bioavailability of various HMB chemical forms
At present, two different forms of HMB are available, monohydrated calcium salt Ca (HMB)2∙H2O and free beta-hydroxy-beta-methylbutyric acid (HMB-FA). Athletes and scientists most often use the HMB-Ca form (Kreider et al., 1999; Nissen et al., 1996; Smith et al., 2004). Vulkovich et al. (2001) indicated that the concentration of HMB in the bloodstream is dose-dependent and that taking 1 g of HMB-Ca would result in the peak concentration of pure HMB in the bloodstream after 2 hours. When administering 3 g of HMB-Ca, the maximum concentration would be observed already after one hour, being at the same time three times higher than when administering 1 g. According to Fuller et al. (2011), using HMB-FA as a dietary supplement (an amount equivalent to HMB-Ca) results in a 2-fold higher HMB concentration in blood serum, which is absorbed four times faster than HMB-Ca. In addition, analysis of the area under the curve, within 180 minutes of HMB administration, demonstrated significantly better HMB-FA absorption when compared to HMB-Ca. The plasma HMB half-life, when administered in the form of HMB-FA, is 3.5 h, while form HMB-Ca it is 2.5 h. It seems that the rate of decreased peak HMB-FA concentration in the serum is 25% faster, which indicates faster HMB uptake and degradation of the compound. At the same time, it should be clearly stated that the rate of urinary excretion of this compound does not differ significantly between the analyzed forms of HMB.
Optimal doses, time and period of HMB supplementation
Based on the scientific data, it has been observed that the effect of HMB is dose-dependent and doses to be considered range between 1and 3 g per day (Fuller et al., 2011; Gallagher et al., 2000a; Willson et al., 2013). In scientific research the most commonly used doses of HMB include 1.5 g/d, 3 g/d or exceptionally 6 g/d (Zanchi et al., 2011). Supplementation with doses of HMB-Ca equal to 6 g/d or lower than 3g/d does not seem to be more effective in increasing lean body mass (LBM) and strength of the subjects compared to the 3 g/d dose (Gallagher et al., 2000a). There are also suggestions that the intake of HMB should be adjusted to 38 mg/kg of LBM (Gallagher et al., 2000a). Based on the research by Nissen et al. (1996), it can be seen that HMB-Ca most effectively inhibits protein degradation in the first two weeks of its use, while a significant reduction in serum CK activity occurs only in the third week after commencing the supplementation. It seems that 2 weeks of HMB-Ca supplementation is the minimum period that is effective in reducing muscle damage. Nissen et al. (1996) indicate that after the first 2 weeks of supplementation, a new training stimulus should be introduced, which may increase the level of muscle cell damage, and thus use the protective effect of HMB to a greater extent.
As for supplement timing, the training goal should be taken into account, and various HMB action patterns should be considered. The most frequently used supplementation protocol includes the administration of 1 g of HMB-Ca 3 time a day with meals (breakfast, lunch and dinner). It is believed that this is the most optimal model when the athlete wants to minimize the damage to the trained muscle groups. For athletes to reduce the time of post exercise recovery, it is recommended to consume 3 g/60 min/HMB-Ca or 3 g/30 min/HMB-FA before exercise (Fuller et al., 2011; Wilson et al., 2013).
The safety of HMB supplementation
Beta-hydroxy-beta-methylbutyric acid has been widely studied for over two decades, not only on cell lines, but also on animals and, above all, pleiotropically on humans (Gerlinger-Romero et al., 2011; Nissen and Abumrad, 1997; Nissen et al., 2000; Smith et al., 2005). Various studies carried out on rat models (Baxter et al., 2005) and their results normalized for human equivalent dosing indicate the safety of using HMB-Ca for an 81 kg adult male of 50 g HMB-Ca per day, for three months, with no visible side effects. At a dose of 6 g per month, no effect on the cholesterol fractions, blood variables, glycemic index, nor liver and kidney function were observed (Gallagher et al., 2000b). Similarly, during a one year HMB-Ca supplementation study (2-3 g/d), which was combined with various amino acids and carried out in elderly subjects, no adverse effects were observed (Baier et al., 2009). In conclusion, HMB in the doses described in the literature seems to be completely safe, and may improve various health markers (Arazi et al., 2015).
Conclusion
HMB is among the supplements, which can be recommended for all sport disciplines regardless of sex and age. HMB supplementation reduces post exercise muscle damage, and thus accelerates recovery. It also allows for increases in lean body mass, improved strength and aerobic capacity. For competitive athletes, the most effective dose of HMB supplementation is 3 g/daily for 3 to 4 weeks. The legitimacy of research with higher doses (up to 6 g) should be discussed. It seems that HMB supplementation with recommended doses is safe, well-tolerated and does not induce any health problems, even if consumed continuously over a period of one year.
References
- Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol. 2013;48(9):973–984. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Arazi H, Rohani H, Ghiasi A, Davaran M. The effect of HMB supplementation on cardiovascular risk factors after four weeks of resistance training in amateur athletes. Int. Cardiovasc. Res. J. 2015;9:89–93. [Google Scholar]
- Asadi A, Arazi H, Suzuki K. Effects of β-Hydroxy-β-methylbutyrate-free Acid Supplementation on Strength, Power and Hormonal Adaptations Following Resistance Training. Nutrients. 2017;9(12) doi: 10.3390/nu9121316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier S, Johannsen D, Abumrad N, Rathmacher JA, Nissen S, Flakoll P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. JPEN. J Parenter Enteral Nutr. 2009;33(1):71–82. doi: 10.1177/0148607108322403. [DOI] [PubMed] [Google Scholar]
- Baxter JH, Carlos JL, Thurmond J, Rehani RN, Bultman J, Frost D. Dietary toxicity of calcium beta-hydroxy-beta-methyl butyrate (CaHMB) Food Chem Toxicol. 2005;43(12):1731–1741. doi: 10.1016/j.fct.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Chua B, Siehl DL, Morgan HE. Effect of leucine and metabolites of branched chain amino acids on protein turnover in heart. J Biol Chem. 1979;254(17):8358–8362. [PubMed] [Google Scholar]
- Durkalec-Michalski K, Jeszka J, Podgórski T. The Effect of a 12-Week Beta-hydroxy-beta-methylbutyrate (HMB) Supplementation on Highly-Trained Combat Sports Athletes: A Randomised, Double-Blind, Placebo-Controlled Crossover Study. Nutrients. 2017;9(7) doi: 10.3390/nu9070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 2007;293(4):E923–E931. doi: 10.1152/ajpendo.00314.2007. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295(6):E1409–E1416. doi: 10.1152/ajpendo.90530.2008. [DOI] [PubMed] [Google Scholar]
- Fiorotto ML, Schwartz RJ, Delaughter MC. Persistent IGF-I overexpression in skeletal muscle transiently enhances DNA accretion and growth. FASEB J. 2003;17(1):59–60. doi: 10.1096/fj.02-0289fje. [DOI] [PubMed] [Google Scholar]
- Fuller JC, Sharp RL, Angus HF, Baier SM., Rathmacher JA. Free acid gel form of β-hydroxy-β-methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br J Nutr. 2011;105(3):367–372. doi: 10.1017/S0007114510003582. [DOI] [PubMed] [Google Scholar]
- Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta-methylbutyrate ingestion, Part I: effects on strength and fat free mass. Med Sci Sports Exerc. 2000a;32(12):2109–2115. doi: 10.1097/00005768-200012000-00022. [DOI] [PubMed] [Google Scholar]
- Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta-methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Med Sci Sports Exerc. 2000b;32(12):2116–2119. doi: 10.1097/00005768-200012000-00023. [DOI] [PubMed] [Google Scholar]
- Gerlinger-Romero F, Guimarães-Ferreira L, Giannocco G, Nunes MT. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Horm IGF Res. 2011;21(2):57–62. doi: 10.1016/j.ghir.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hoffman JR, Gepner Y, Stout JR, Hoffman MW, Ben-Dov D, Funk S, Daimont I, Jajtner AR, Townsend JR, Church DD, Shelef I, Rosen P, Avital G, Chen Y, Frankel H, Ostfeld I. β-Hydroxy-β-methylbutyrate attenuates cytokine response during sustained military training. Nutr Res. 2016;36(6):553–63. doi: 10.1016/j.nutres.2016.02.006. doi. [DOI] [PubMed] [Google Scholar]
- Holeček M.. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle. 2017;8:529–541. doi: 10.1002/jcsm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43(9):1267–1276. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jówko E, Ostaszewski P, Jank M, Sacharuk J, Zieniewicz A, Wilczak J, Nissen S. Creatine and beta-hydroxy-beta-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program. Nutrition. 2001;17(7-8):558–566. doi: 10.1016/s0899-9007(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Knitter AE, Panton L, Rathmacher JA, Petersen A, Shar R. Effects of β-hydroxy-β-methylbutyrate on muscle damage after a prolonged run. J Appl Physiol. 2000;89(4):1340–1344. doi: 10.1152/jappl.2000.89.4.1340. [DOI] [PubMed] [Google Scholar]
- Kohlmeier M. Nutrient Metabolism: Structures, Functions, and Genes. Academic Press; 2015. [Google Scholar]
- Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2009;1793(5):755–763. doi: 10.1016/j.bbamcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Kreider RB, Ferreira MP, Greenwood M, Wilson M, Grindstaff P, Plisk S, Amalda AL. Effects of Calcium β-HMB Supplementation During Training on Markers of Catabolism, Body Composition, Strength and Sprint Performance. J Exerc Physiol. 2000;3(4):48–59. [Google Scholar]
- Kreider RB, Ferreira M, Wilson M, Almada AL. Effects of calcium beta-hydroxy-beta-methylbutyrate (HMB) supplementation during resistance-training on markers of catabolism, body composition and strength. Int J Sports Med. 1999;20(8):503–509. doi: 10.1055/s-1999-8835. [DOI] [PubMed] [Google Scholar]
- Maciejewska D, Michalczyk M, Czerwińska-Rogowska M, Banaszczak M, Ryterska K, Jakubczyk K, Piotrwski J, Hołowko J, Drozd A, Wysokińki P, Ficek K, Wilk K, Lubkowska A, Cięszczyk P, Bertrand J, Stachowska E. Seeking Optimal Nutrition for Healthy Body Mass Reduction among Former Athletes. J Hum Kinet. 2017;60:63–75. doi: 10.1515/hukin-2017-0090. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk MM, Chycki J, Zajac A, Maszczyk A, Zydek G, Langfort J. Anaerobic Performance after a Low-Carbohydrate Diet (LCD) Followed by 7 Days of Carbohydrate Loading in Male Basketball Players. Nutrients. 2019;11(4):E778. doi: 10.3390/nu11040778. pii. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB) J Nutr Biochem. 1997;8(6):300–311. [Google Scholar]
- Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130(8):1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC, Abumrad N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol. 1996;81(5):2095–2104. doi: 10.1152/jappl.1996.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Panton LB, Rathmacher JA, Baier S, Nissen S. Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (hmb) during resistance training. Nutrition. 2000;16(9):734–739. doi: 10.1016/s0899-9007(00)00376-2. [DOI] [PubMed] [Google Scholar]
- Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Santos RV. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the m TOR pathway. Nutr Metab. 2011;8:11. doi: 10.1186/1743-7075-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portal S, Zadik Z, Rabinowitz J, Pilz-Burstein R, Adler-Portal D, Meckel Y, Nemet D. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double-blind, placebo-controlled study. European J Appl Physiol. 2011;111(9):2261–2269. doi: 10.1007/s00421-011-1855-x. [DOI] [PubMed] [Google Scholar]
- Ransone J, Neighbors K, Lefavi R, Chromiak J. The effect of beta-hydroxy beta-methylbutyrate on muscular strength and body composition in collegiate football players. J Strength Cond Res. 2003;17(1):34–9. doi: 10.1519/1533-4287(2003)017<0034:teohmo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reid MB. Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1423–R1431. doi: 10.1152/ajpregu.00545.2004. [DOI] [PubMed] [Google Scholar]
- Rudney H. The biosynthesis of beta-hydroxy-beta-methylglutaric acid. J Biol Chem. 1957;227(1):363–377. [PubMed] [Google Scholar]
- Sabourin PJ, Bieber LL. Formation of beta-hydroxyisovalerate by an alpha-ketoisocaproate oxygenase in human liver. Metabolism. 1983;32(2):160–164. doi: 10.1016/0026-0495(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287(4):E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- Silva VR, Belozo FL, Micheletti TO, Conrado M, Stout JR, Pimentel GD, Gonzalez AM. β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: a systematic review. Nutr Res. 2017;45:1–9. doi: 10.1016/j.nutres.2017.07.008. doi. Epub 2017 Jul 26. Review. [DOI] [PubMed] [Google Scholar]
- Slater G, Rathmacher JA, Lee H, Hahn AG, Logan P, Jenkins D, Vukovich M. β-Hydroxy-β-Methylbutyrate (HMB) Supplementation Does Not Affect Changes in Strength or Body Composition during Resistance Training in Trained Men. Int J Sport Nutr Exerc Metab. 2001;11(3):384–396. doi: 10.1123/ijsnem.11.3.384. [DOI] [PubMed] [Google Scholar]
- Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005;65(1):277–283. [PubMed] [Google Scholar]
- Smith HJ, Wyke SM, Tisdale MJ. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Cancer Res. 2004;64(23):8731–8735. doi: 10.1158/0008-5472.CAN-04-1760. [DOI] [PubMed] [Google Scholar]
- Teixeira FJ, Matias CN, Monteiro CP, Valamatos MJ, Reis JF, Batista A, Phillips SM. No effect of HMB or α-HICA supplementation on training-induced changes in body composition. Eur J Sport Sci. 2018:1–9. doi: 10.1080/17461391.2018.1552723. [DOI] [PubMed] [Google Scholar]
- Teixeira FJ, Matias CN, Monteiro CP, Valamatos MJ, Reis JF, Tavares F, Phillips SM. Leucine Metabolites Do Not Enhance Training-induced Performance or Muscle Thickness. Med Sci Sports Exerc. 2019;51(1):56–64. doi: 10.1249/MSS.0000000000001754. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Scordilis SP. Ubiquitin changes in human biceps muscle following exercise-induced damage. Biochem Biophys Res Commun. 1994;204(3):1193–1198. doi: 10.1006/bbrc.1994.2589. [DOI] [PubMed] [Google Scholar]
- Thomson JS, Watson PE, Rowlands DS. Effects of nine weeks of β-Hydroxy-β-methylbutyrate supplementation on strength and body composition in resistance trained men. J Strength Cond Res. 2009;23(3):827–835. doi: 10.1519/JSC.0b013e3181a00d47. [DOI] [PubMed] [Google Scholar]
- Van Koevering M, Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol. 1992;262(1 Pt 1):E27–E31. doi: 10.1152/ajpendo.1992.262.1.E27. [DOI] [PubMed] [Google Scholar]
- Vukovich MD, Dreifort GD. Effect of beta-hydroxy beta-methylbutyrate on the onset of blood lactate accumulation and V(O)(2) peak in endurance-trained cyclists. J Strength Cond. 2001;15(4):491–497. [PubMed] [Google Scholar]
- Vukovich MD, Slater G, Macchi MB, Turner MJ, Fallon K, Boston T, Rathmacher J. Beta-hydroxy-beta-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J Nutr Biochem. 2001;12(11):631–639. doi: 10.1016/s0955-2863(01)00182-6. [DOI] [PubMed] [Google Scholar]
- Whitehouse AS, Khal J, Tisdale MJ. Induction of protein catabolism in myotubes by 15(S)-hydroxyeicosatetraenoic acid through increased expression of the ubiquitin-proteasome pathway. Br J Cancer. 2003;89(4):737–745. doi: 10.1038/sj.bjc.6601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby DS, Taylor M, Taylor L. Glucocorticoid receptor and ubiquitin expression after repeated eccentric exercise. Med Sci Sports and Exer. 2003;35(12):2023–2031. doi: 10.1249/01.MSS.0000099100.83796.77. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr Metab. 2008;5:1. doi: 10.1186/1743-7075-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Antonio J. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB) J Int Spc Sports Nutr. 2013;10(1):6. doi: 10.1186/1550-2783-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Stout JR, Duncan N, Lowery RP, Wilson SMC, Baier SM, Rathmacher J. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: a randomized, double-blind, placebo-controlled study. Eur J App Phisiol. 2014;114(6):1217–1227. doi: 10.1007/s00421-014-2854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk M, Michalczyk M, Gołaś A, Krzysztofik M, Maszczyk A, Zając A. Endocrine responses following exhaustive strength exercise with and without the use of proteinand protein-carbohydrate supplements. Biol Sport. 2018;35(4):399–405. doi: 10.5114/biolsport.2018.75754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Lancha AH. HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids. 2011;40(4):1015–1025. doi: 10.1007/s00726-010-0678-0. [DOI] [PubMed] [Google Scholar]


