Abstract
The myocardium and the cardiovascular system are often involved in patients with sarcoidosis. As therapy should be started as early as possible to avoid complications such as left ventricular dysfunction, a prompt and reliable diagnosis by means of non-invasive tests would be highly warranted. Among other techniques, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has emerged as a high sensitive tool to detect sites of inflammation before morphological changes are visible to conventional imaging techniques. We therefore aim at summarizing the most relevant findings in the literature on the use of 18F-fluorodeoxyglucose PET in the diagnostic workup of cardiac sarcoidosis and to underline future perspectives.
Introduction
The cardiovascular system is the third most frequently disease targeted site in sarcoidosis,1 being involved in 25–70% of patients according to autoptic data.2–5
In order to assess the extension of the disease, a comprehensive evaluation of integrated anatomical and metabolic information is needed. In this regard, an early diagnosis of cardiac involvement plays a pivotal role in the management of patients affected by sarcoidosis, as it is well established that high-dose steroid therapy should be started before left ventricular systolic dysfunction in patients with confirmed cardiac sarcoid and active inflammation.6–8
Cardiac magnetic resonance (CMR) has high spatial resolution and allows for a very precise evaluation of both left and right ventricular function; furthermore, it allows us to identify areas of oedema, perfusion defects and scarring areas. However, while the association of the assessment of Late Gadolinium Enhancement (LGE) demonstrated high sensitivity and specificity in the diagnosis of cardiac sarcoidosis (CS) (76–100% and 78–92%, respectively), the use of CMR in clinical practice is hampered in the presence of implantable devices or, to a lesser extent, renal function impairment.9–11
It should also be considered that the findings highlighted by LGE assessment are not able to differentiate between active and chronic lesions in the course of CS. To that end, whole body scintigraphy with 67Ga was performed in the past, showing up to 80% sensitivity in detecting CS in symptomatic patients with either AV block or ventricular tachycardia.12 However, inconsistent diagnostic performance and suboptimal radiation exposure for the patients are reported in the literature,13 and to date 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET), eventually fused with CT, has emerged as a high sensitive tool to detect sites of inflammation before morphological changes are visible to conventional imaging techniques. Among other advantages, FDG PET/CT has the ability to perform quantitative analyses and to combine functional and anatomical imaging.14
In this review, we aim to summarize the most relevant findings in the literature on the use of 18F-FDG PET in the diagnostic workup of CS and to underline future perspectives.
Patient preparation for 18F-FDG PET/CT in cardiac sarcoidosis
When performing cardiac PET with 18F-FDG, the patient’s preparation plays a pivotal role to yield an adequate diagnostic accuracy.
In fact, dietary carbohydrate intake normally triggers insulin secretion, which activates the predominantly expressed glucose transporter GLUT4 in normal myocardium and allows glucose to enter cells. On the other hand, in inflammatory cells (such as neutrophils, monocytes, and macrophages), glucose enters the cell via a glucose transporter GLUT1 and GLUT3 (which are overexpressed)15,16 and 18F-FDG is trapped by phosphorylation.17 As such, separately visualizing FDG uptake in CS lesions is possible if GLUT4 is adequately suppressed in normal myocardial cells.15,18
In this regard, several strategies for patient preparation have been developed in order to suppress physiological 18F-FDG uptake in the heart and minimize false-positive results: prolonged fasting, dietary modification, and pharmacological approaches.
While a prolonged fasting state alone has been reported to adequately reduce physiologic myocardial 18F-FDG uptake, thus increasing specificity and sensitivity of PET19–31 a more efficient patient preparation should include dietary modifications with a high-fat, low-carbohydrate, high-protein diet (HFLC). The latter has the ability to shift myocardial metabolism to fatty acid and suppress glucose utilization by normal myocardium.32
The superiority of a HFLC diet compared to fasting only has been demonstrated in some studies33,34 wherein the physiologic 18F-FDG uptake was more often adequately suppressed in the diet group than in a fast–alone group. Some other studies expanded on this important topic,35–37 also showing that the efficacy of FDG uptake suppression is highest if a 72 h pre-test HFLC diet preparation protocol is followed.38 On the contrary, adding high-fat beverage to the HFLC diet just before the 18F-FDG does not seem to have additive benefit.39
A randomized trial conducted by Demeure et al40 involved 36 volunteers and compared the effectiveness of 4 different approaches: a HFLC diet followed by a 12 h fast, HFLC diet followed by a high-fat drink 1 h prior to imaging, and a HFLC diet followed by 12 h fast and oral verapamil. In their study, the rate of suppression of cardiac glucose metabolism was higher in the group receiving HFLC diet followed by 12 h fasting (89% vs 50%). The rationale for adding Verapamil is that intracellular calcium is known to increase glucose uptake, and calcium channel antagonism has reduced myocardial 18F-FDG uptake in a mouse model.41 Unfortunately, the use of calcium-channels antagonist failed to prove beneficial to improve myocardial suppression, having no clear benefit over other preparations.40
Intravenous administration of unfractionated heparin (UFH) activates lipoprotein and hepatic lipases to increase plasma FFAs levels, causing a suppression of myocardial glucose utilization. In a Japanese CS study cohort after a fasting period of at least 6 h, 50 IU/kg of UFH were injected 15 min before application of PET tracer,22 resulting in a robust suppression of cardiac FDG uptake.
A combination of approaches to reduce physiologic myocardial 18F-FDG uptake has been also used in a few studies, showing that the injection of Heparin in addition to HFLC diet preparation outperforms a dietary preparation without injection.29,42
It should be noted that UFH injection alone seems to be less effective than an adequate dietary preparation.28,29
Although there is currently no consensus on the best protocol for suppressing cardiac FDG-uptake, current SNMMI/ASNC guidelines recommend preparation with a high-fat (>35g), low-carbohydrate (<3g) diet the day before the study and then fast for at least 4–12 h. An alternative option (especially for patients who cannot follow the dietary recommendation) is for the patient to fast for more than 18 h before the study. The additional use of heparin to dietary preparation can be considered, but its role in the suppression of myocardial glucose uptake is unclear and its impact on suppression of physiologic myocardial glucose uptake may be lower than originally thought.43 We provide a proposal for a preparatory schema in Table 1.
Table 1.
Proposal for a protocol for patient preparation before undergoing 18F-FDG PET/CT for suspected cardiac sarcoidosis
| Dietary preparation | High fat, low carbohydrates diet 72 h before the procedure |
| Fasting state | min. 6 h before the procedure |
| Intravenous heparin | 3 × 200 IU Heparin at 0, 7 and 15 min |
| 18F-FDG administration | 15 min after the last i.v. application of Heparin. Dose: 3.5 MBq/Kg (max 500 MBq) |
| Image acquisition | 60 min after FDG administration, 90 s/bed position from skull to mid tigh. Supplemental bed position over the heart (10 min, matrix 256 × 256). Low-dose CT-based AC |
| Image interpretation | Comparison with 82Rb PET/CT at rest or cardiac MR Both AC and non-AC corrected images are separately evaluated. SUVmax and TLG are also determined. |
AC, attenuation correction; 18F-FDG, 18F-fludeoxyglucose; IU, international unit; PET, positron emission tomography; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis.
18F-FDG PET in the diagnostic workup of cardiac sarcoidosis
A correct interpretation of 18F-FDG PET images requires normally a comparison with myocardial resting perfusion imaging (13N-ammonia or 82Rb PET, SPECT perfusion imaging with tracers like 201Thallium or 99mTc-tetrofosmin/sestamibi or CMR) to discriminate between active inflammation and scar tissue (Figure 1): the degree of match or mismatch between myocardial regional FDG uptake and regional perfusion allows for the identification of specific categories.43
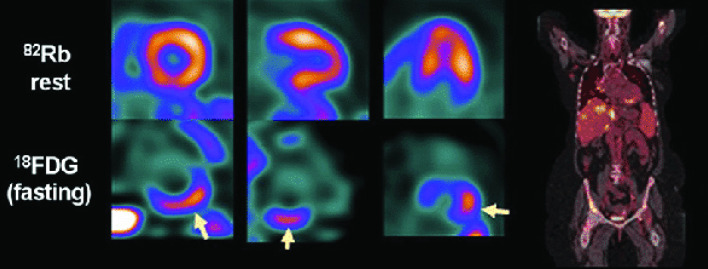
Figure 1.
PET/CT images in a patients with cardiac sarcoidosis. Perfusion imaging (above, arrow) show hypoperfusion in the basal inferior wall, corresponding with increased FDG uptake (under, arrow). Fused whole-body coronal imaging shows the systemic extent of sarcoidosis. Reprinted with permission from Springer, from Caobelli F and Bengel FM. J Nucl Cardiol 2015;22: 971–974. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Each stage of CS has different 18F-FDG-avidity and perfusion.44
Early active inflammation has abnormal18F-FDG uptake and normal perfusion.
The second stage of progression is the mismatch pattern.
The third stage is a burned-out granuloma or “scar” with decreased perfusion and lack of 18F-FDG uptake.
Many works in literature demonstrate the usefulness of 18F-FDG PET in the diagnostic assessment of CS. In a meta-analysis including 7 studies and 164 patients, Youssef et al reported 89% sensitivity and 78% specificity for CS detection with 18F-FDG PET.45 Furthermore, Manabe and colleagues showed that FDG uptake in the right ventricle in patients with high suspicion of CS was associated to a greater number of LV-involved segments, thus meeting more frequently the current clinical diagnostic criteria.46 The value in the prognostic assessment was also demonstrated by Blankstein and colleagues, wherein focal perfusion defects and FDG uptake on cardiac PET identified patients at higher risk of death or ventricular tachycardia.19
Besides a visual evaluation, also semi-quantitative parameters like standardized uptake value (SUV) may have a value in the diagnostic assessment. Although current guidelines suggest to use these parameters with caution,47 a recent work showed that SUV quantitation and localization of myocardial FDG uptake has significant associations with future cardiac events.48
These recent evidences led to the draft of an expert consensus document on the role of 18F-FDG PET in cardiac sarcoid detection and therapy monitoring. In this paper, specific scenarios were identified, in which cardiac PET can yield important information in case of suspected or known CS: (1) when abnormal screening for CS is associated with histologic evidence of extracardiac sarcoidosis; (2) in patients younger than 60 with unexplained, new onset conduction disease; (3) in patients with idiopathic sustained ventricular tachycardia and, finally, (4) in patients with proven CS in order to follow up the response to treatment.43
As of now, however, there is a lack of evidence in the literature as to whether 18F-FDG PET can be considered superior to CMR. The only published work comparing CMR and 18F-FDG PET in terms of diagnostic accuracy was published in 2008 by Ohira and colleagues. In this work, the authors showed that 18F-FDG PET has a higher sensitivity, likely due to its ability in detecting early inflammatory lesions, and a lower specificity linked to the greater confidence of the LGE assessment in the identification of areas of necrosis and fibrosis.49
It should be noted, however, that the acquisition of whole-body 18F-FDG PET images has the advantage of evaluating the extent of systemic disease beyond the myocardium. Moreover,18F-FDG PET allows us to perform a quantification of glucometabolic activity, which has been shown useful in predicting prognosis or evaluating treatment response.50
Hybrid imaging in cardiac sarcoidosis: PET/MR
The relative reduced 18F-FDG PET specificity in the diagnostic workup of CS can be compensated, in selected patients, by the comparison with CMR which, in turn, benefits from a noticeable greater sensitivity guaranteed by the evaluation of the glucose metabolism.
In fact, LGE is the expression of damage of myocardial tissue or a scar, while an increase in the uptake of FDG is a positive indicator for the presence of active inflammation.51,52
In this regard, the use of a hybrid PET/MR approach may help overcoming current reservations on the use of non-invasive imaging in the evaluation of treatment response. LGE usually persists after therapy, thus failing to differentiate between active inflammation and chronic scarring.21 Similarly, T2 weighted imaging of the oedema may be inaccurate. Conversely, the reduction of 18F-FDG uptake seems to be a more useful marker of treatment response,42 especially in the evaluation of immunosuppressive therapy, where the benefit must be carefully balanced with the potentially severe side-effects.
As such, the association of imaging modalities that can provide information on different pathways may be a definite breakthrough. Although many challenges and obstacles still need to be overcome, including costings, training staff and resources available at the time, it can be maintained that 18F-FDG PET/MR has great potential as a diagnostic standard in the future.
The association with MR assures not only attenuation correction (AC) and anatomic reference for the PET scan, as it is provided by CT, but also tissue characterization.51 Furthermore, MR has the added benefit of radiation exposure reduction, partially due to the use of MR data for AC,53 but also thanks to a reduction in the administered dose of 18F-FDG to half of standard activity,54 made possible by the increase of the acquisition time which can be doubled without affecting the overall scan time and the diagnostic accuracy.55 Moreover, further dose reduction can be pursued, taking advantage of the different PET detectors used in PET/MR, which allow higher count rate and improved image quality.56
As a consequence, by combining the two modalities no information is lost,57 and a better detection rate and an improvement of image quality and detection rate in PET/MRI in comparison to PET/CT can be achieved58,59 (Figure 2).
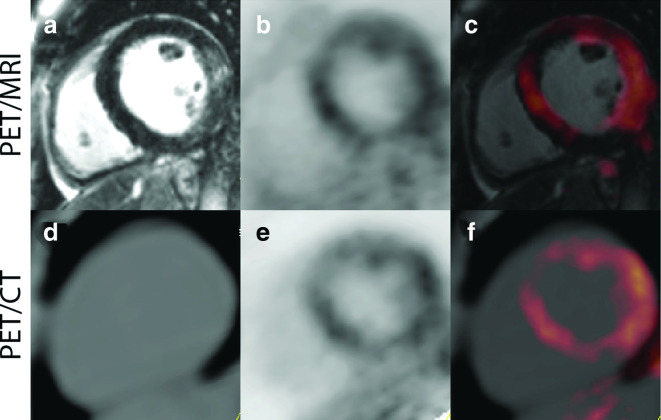
Figure 2.
Representative Images from a Patient with cardiac sarcoidosis, imaged with PET/MR (A–C) and PET/CT (D–F). There is evidence of enhanced signal seen on the delayed enhancement MR images in the lateral wall (A), and the increased 18F-FDG uptake in the septal, anterior, and lateral regions on both the PET/CT and PET/MR images (C, F). This patient had an ejection fraction of 49% with mild global hypokinesis, without wall motion abnormalities. Reprinted under the terms of the Creative Commons Attribution 4.0 International License (http://creat ivecommons.org/licenses/by/4.0/) from Wisenberg G et al. J Nucl Cardiol. 2019. doi: 10.1007/s12350-018-01578-8 [56]. No changes were made. 18F-FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography.
It was postulated that the simultaneous evaluation of 18F-FDG uptake, hyperintensities on T2 weighted imaging and LGE may benefit from an integrated PET/MR system, as the same assessments proved incongruous if standalone PET and MR studies were sequentially performed.52 This concept was investigated by Schneider et al,58 wherein a one-stop shop examination on 51 consecutive patients with a hybrid 18F-FDG PET/MR scan yielded significant improvement in diagnostic accuracy over PET and MRI alone. Similar results were reported by Wicks et al,60 wherein sensitivity of PET and MR alone for the detection of CS were 85 and 82%, respectively, and improved to 94% with hybrid PET/MR. This discrepancy is somewhat expected, as high 18F-FDG uptake and LGE represent different stages of disease.
In a recent study, the integrated role of PET/MR in CS has been investigated by classifying patients in four groups: (1) MR + PET + (co-localization of LGE and FDG uptake) classified as active CS; (2) MR-PET- (neither LGE or FDG uptake); (3) MR-PET + (no LGE but FDG uptake); and (4) MR + PET- (LGE with no FDG uptake). Simultaneous acquisition allowed accurate co-registration of PET and CMR images, thus permitting a full comparison between patterns of injury identified on LGE-CMR and regions of increased disease activity identified on FDG PET. Interestingly, the dynamic profiling of FDG uptake and its intensity appeared to be a useful tool for differentiation of different stages of the disease. In fact, a specific focal-on-diffuse pattern of glucose hypermetabolism can be observed in patients with suspected CS. While its significance is debatable, as it may represent an alternative PET pattern of failed myocardial suppression, or rather a region of early myocardial inflammation detectable with PET but not CMR,61 the fact that time activity curves of glucose consumption showed a plateau at 60 min after injection, 10 min earlier than MR + PET +, suggests a different uptake mechanism, which may be interpreted as failed suppression of physiological myocardial uptake.
18F-FDG PET and CMR provide different and complementary information on different stages of the disease. Future prospective studies should investigate the role of hybrid PET/MR as possible new clinical criteria for the diagnosis of cardiac sarcoidosis. Furthermore, studies are warranted on the role of PET/MR in the evaluation of immunosuppressive therapy, also highlighting technical issues due to the presence of device implantation artefacts, which may impair image interpretation, thus posing a challenge in following up patients with PET/MRI.
18F FDG PET/CT in the therapy response evaluation
The treatment of sarcoidosis is not specific for the disorder and has the goal of slowing the evolution of fibrosis. More aggressive treatment is required in patients in whom myocardium is involved.62 However, there are currently no clinical guidelines on the management of CS and the timing, dose and duration of immunosuppressive therapy.8 As a consequence, patients with CS undergoing a treatment need to be constantly monitored over time, in order to guide in the choice of modulating doses and duration of therapy.63
To that end, 18F-FDG PET/CT has been proposed in view of its capability to reveal the down-regulation of macrophage glucose transport, thus reflecting the effectiveness of steroids therapy.64 Unfortunately, the metabolic alterations related to prolonged corticosteroid therapy may hamper the interpretation of 18F-FDG PET/CT. In fact, elevations in serum glucose and insulin levels due to the use of steroids may adversely affect 18F-FDG uptake by normal myocytes and reduce test specificity. Hence, a reduction in myocardial 18F-FDG uptake may either reflect resolution of inflammation (normal myocytes) or progression of disease process and fibrosis.65
The evaluation of the response to the therapy is very complex and requires necessarily the performance of a baseline 18F-FDG PET/CT scan. Furthermore, all the scans at follow-up should be ideally carried out with the same procedures, in order to guarantee the correct processing of the images.43
There is scarce evidence in literature on the role of 18F-FDG PET in the evaluation of the therapy response, and the majority of papers include small patient samples or single case reports, with large differences in timing for 18F-FDG PET/CT during and at the end of corticosteoid therapy. Nevertheless, preliminary data suggest that changes in the extent of inflammatory activity in CS can be effectively traced by means of follow-up 18F-FDG PET scans,66,67 and these metabolic modifications correlate to improvements and/or disappearance of conduction abnormalities or ventricular tachycardias.21
A correlation between 18F-FDG uptake and clinical presentation was also investigated. Of note, higher FDG uptake can be seen in patients with CS presenting with ventricular tachycardia compared to those with advanced atrioventricular block or clinically silent.68,69 Furthermore, a reduction in both the intensity and extent of inflammation on a follow-up 18F-FDG PET is associated with an increase in left ventricular ejection fraction.70 The correlation between PET findings and clinical presentation has been confirmed by Shelke et al, wherein therapy responders showed a significant decrease in the extent of myocardial FDG uptake and in the number of involved LV segments. Conversely, 18F-FDG PET/CT criteria predictive for steroid resistance were reported: (1) increased SUVmax over time, (2) increased area of myocardial inflammation (increase in number of LV segments involved) and (3) baseline heterogeneous uptake.71
Besides qualitative parameters, also semi-quantitative methods have been suggested to yield a more standardized evaluation of therapy response. Ahmadian et al described a method for quantifying 18F-FDG uptake in CS, using a SUV threshold method similar to the measurement of total lesion glycolysis (TLG) in oncologic 18F-FDG PET/CT imaging, named ‘‘cardiac metabolic activity’’.50 The same authors also evaluated other semiquantitative values such as SUVmax, cardiac metabolic volume (i.e. volume of abnormal FDG) and cardiac metabolic activity (i.e. volume of abnormal FDG uptake multiplied by the mean SUV of that volume) and compared them to visual interpretation. The results of their study confirm that semiquantitative interpretation may aid in defining the severity of the inflammation, outperforming visual interpretation only,64 although patients with extensive disease do not always present with intense FDG uptake8 (Figure 3).
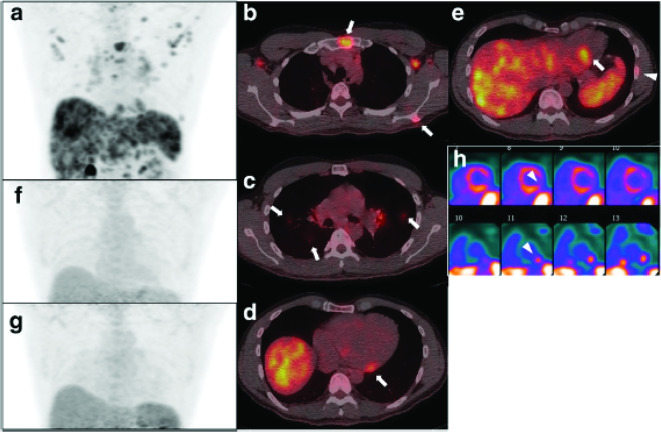
Figure 3.
Representative pre-treatment and follow-up FDG PET/CT scan (a: 3D maximum intensity projection; b, c, d, e: fused PET/CT). There is evidence of multiple organ disease involving lymph nodes (thoracic and abdominal), the lung (c, arrows), bones (e arrowhead), heart (d, e arrows), liver, and spleen. Reconstructed short axis images of the left ventricle (h perfusion images on the top and FDG images on the bottom) show increased metabolic activity in the inferolateral wall with corresponding mildly reduced perfusion (arrowheads), suggestive of active inflammation. The max SUV, metabolic volume, and total lesion glycolysis of all involved organ system and the heart are 7.5 (liver), 1860.9 ml, and 6866.9 g, and 5.7, 84.1 ml, and 294.4 g, respectively. Follow-up PET/CTs performed at 3 months (f) and 9 months (g) since the initial study show complete metabolic resolution. Reprinted under the terms of the Creative Commons Attribution 4.0 International License (http://creat ivecommons.org/licenses/by/4.0/) from EJNMMI Res (2017) 7:67 [8]. No changes were made. FDG, fluorodeoxyglucose; PET, positron emission tomography; SUVmax, maximum standardized uptake value.
The added value of semi-quantitative parameters is supported by the fact that corticosteroid therapy may have an impact on background activity, thus hampering visual interpretation. Furuya et al retrospectively analyzed 38 patients with CS in order to evaluate the effect of steroids on background 18F-FDG uptake such as that of the liver and blood pool. The SUVs obtained from the liver during steroid therapy were significantly higher than those of the pre-therapy scan, whereas the SUVs from blood pool were not altered by steroid therapy. Because 18F-FDG uptake increases in the presence of inflammation, the increase of liver uptake may also be due to an inflammatory process caused by steroid therapy. Thus, individual FDG uptake thresholds to assess the metabolic volume difference between time points before and during steroid therapy should be determined.72
The relationship between glucose metabolism and coronary perfusion in patients with CS and their modifications on immunosuppressive therapy is another matter of debate. Kruse et al analyzed 32 CS patients with the aim to study how immunosuppressive treatment would affect inflammation and myocardial blood flow performing both 18F-FDG PET/CT and 13N-ammonia PET/CT. In their study, patients responding to immune-suppressive therapy presented with decreased 18F-FDG uptake and preserved coronary circulatory function compared to baseline, whereas an increase of 18F-FDG uptake was associated with a significant worsening of the coronary circulatory function, not limited to the inflamed regions.73
Novel PET tracers in the management of cardiac sarcoidosis
Although FDG imaging displays good sensitivity and specificity in the detection of CS,45,74 non-specific myocardial uptake may be observed in up to 20% of patients despite various dietary preparations,43 potentially hampering the interpretation of the pattern of FDG uptake. Hence, PET tracers other than FDG are expected to overcome these limitations.
Somatostatin receptors
Molecular imaging of somatostatin receptor (SSTR) currently represents the major candidate to compete with FDG in PET imaging of CS. SSTR are abundant on the surface of activated inflammatory cells like macrophages, epithelioid cells and multinucleated giant cells, which are highly represented within the sarcoid granuloma26 and are normally absent in healthy heart tissues. Therefore, targeted SSTR imaging ligands do not accumulate in healthy heart tissue and any uptake pattern over blood pool activity can be considered diagnostic, providing greater reproducibility of images interpretation and increased specificity. General feasibility of SSTR targeted imaging of inflammatory cells in sarcoidosis was firstly tested by means of 111In-Pentetreotide in the late 90s.75,76 In the latest years, several studies demonstrated the great potential of 68Ga labelled DOTA-peptides in this field (Figure 4). Reiter and colleagues firstly showed the co-localization of 68Ga-DOTATOC and the T2 and contrast-enhanced CMR images in a 54-year-old patient affected by CS.77 More recently, the same group compared 68Ga-DOTATOC PET/CT and CMR imaging findings in 15 patients with biopsy-proven CS or systemic sarcoidosis with clinical suspicion of cardiac involvement. 68Ga-DOTATOC PET/CT and CMR imaging were concordant in 79.3% of all CMR positive segments, while the overall concordance of all segments was documented in 96.1%.78 Intriguingly, in three cases, 68Ga-DOTATOC PET/CT and CMR showed different results (negative PET/CT vs positive CMR). In one of these patients, increased FDG uptake was not evident owing to minor wall involvement in CMR (<25% of wall thickness) and the consequent partial volume effect, while the two remaining subjects were under immunosuppressants at the time of imaging. Both these subjects displayed no adverse cardiac events during the subsequent follow-up and the repeated CMR imaging revealed stable morphologic myocardial changes consistent with chronic post-inflammatory processes. These findings indirectly configure the potential role of 68Ga-DOTATOC PET/CT imaging in the differential diagnosis between active (PET+/CMR+) and chronic (PET-/CMR+) sarcoidotic lesions within the myocardium. This is coherent with the notion that in the chronic phase of sarcoidosis, fibrotic tissue is formed. Fibrotic tissue lacks substantial SSTR2 expression, potentially resulting in a low signal in SSTR2 PET/CT.79 On the other hand, Lapa and colleagues78 highlighted that 68Ga-DOTATOC PET/CT can detect acute inflammatory processes only in patients not receiving immunosuppressants and this finding was reproduced also by Pizzarro et al..80 Therefore, it is open to question, whether 68Ga-DOTATOC PET/CT may potentially become a useful tool to monitor disease activity under immunosuppressive therapy, especially in patients with contraindications to CMR like those with device therapy or renal impairment. Finally, the expression of SSTR in sarcoid tissue might be used in the theranostic approach. High SSTR expression might suggest the application of somatostatin treatment as an option in patients that are resistant to conventional treatment with corticosteroids. In these cases, molecular imaging of SSTR expression might be helpful to predict the effect of treatment with somatostatin.
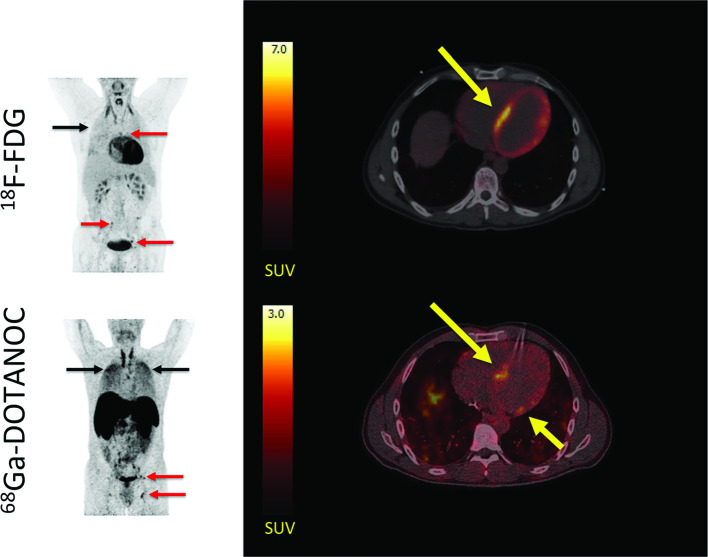
Figure 4.
Representative 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT images in a patient with cardiac sarcoidosis. Left panel: MIPs showing dilated cardiomyopathy and multiple 18F-FDG and 68Ga-DOTANOC avid lymph nodes (red arrows). In addition, there is massive and diffusely increased activity in the lung parenchyma (black arrows) representing active pulmonary sarcoidosis. Right panel: while 18F-FDG PET/CT was inconclusive due to failed suppression of tracer uptake from the myocardium (top), 68Ga-DOTANOC images showed a clearly pathological uptake in the septum (bottom). Reprinted under the terms of the Creative Commons Attribution 4.0 International License (http://creat ivecommons.org/licenses/by/4.0/) from EJNMMI Res. 2016;6(1):52 [82]. No changes were made.18F-FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography.
To date, the real diagnostic potential of SSTR PET imaging in the field of CS, remains, however, a grey area. SSTR ligands on inflammatory cells are also abundant in post-infarction myocarditis and in pericarditis81 and the exact relationship between FDG and SSTR signal in the different phases of the disease still needs to be elucidated.82
Markers of cellular proliferation
Few other PET tracers have been tested in CS in humans. Recently, Weinberg et al investigated 18F-NaF PET/CT for detection of CS in three patients.83 However, they reported that differently to FDG, 18F-NaF may not be able to effectively image active inflammation due to CS.
Conversely, 18F-fluorothymidine (FLT) has been shown to display the myocardial involvement by active sarcoid lesions in a case of a 57-year-old female with systemic sarcoidosis with cardiac involvement.84 This finding nicely agrees with the previous experimental observation that FLT accumulates in chronic granulomatous lesions with proliferative inflammation.85 Of note, FLT PET images after immunosuppressive therapy showed reduced myocardial FLT uptake. The same group recently reported the results of a retrospective study involving 20 patients with known or suspected CS who underwent both FDG and FLT PET/CT.86 This study further confirmed the feasibility of this method, but the authors failed to demonstrate a superiority of FLT imaging over FDG in the detection of sarcoidotic lesions.
Another potentially useful tracer is the imaging marker of proliferation 11C-thiothymidine (4DST). In a recent study, 4DST PET showed higher diagnostic accuracy than FDG PET compared to CMR.87
Markers of hypoxia
Molecular imaging of hypoxia represents another potential diagnostic approach. Manabe et al88 recently reported the cardiac involvement by means of 18F-FMISO PET/CT in a histologically proven systemic sarcoidosis patient. Of note, FMISO uptake was co-localized with FDG uptake pattern within the left ventricular myocardium. This finding is coherent with immune-chemistry data suggesting that the expression of hypoxia-inducible factor HIF-1a and vascular endothelial growth factor (VEGF) are expressed within granulomas in sarcoidosis89 and suggest the potential capability of this approach to distinguish hypoxic from normoxic sarcoid lesions.
Conclusion
18F-FDG PET/CT represents a powerful tool for the diagnosis and the evaluation of therapy response in patients with suspected or confirmed CS. The association with perfusion imaging allows for a precise localization of foci of inflammation within the myocardium. New tracers have been also recently developed to overcome inherent limitation of 18F-FDG, but to date no significant advantages over 18F-FDG could be demonstrated, and further pre-clinical studies are highly warranted to search for a radiopharmaceutical providing more robust evidence.
It can be foreseen that an evaluation with hybrid PET/MR, both with 18F-FDG and with other tracers will prove even more beneficial in the future, capitalizing on the feasibility of a simultaneous tissue characterization.
Footnotes
* These two authors (DG and MB) equally contributed to the present paper.
Disclosure: The authors report no disclosures relevant to the manuscript. Specifically, no financial conflict of interest is present. The authors transfer the copyright of the present paper to BJR should their work be accepted for publication. They state that the work is original and has not been submitted elsewhere. All authors participated in drafting the manuscript, which they approve in its content.
Contributor Information
Dario Genovesi, Email: dgenovesi@ftgm.it.
Matteo Bauckneht, Email: matteo.bauckneht@gmail.com.
Corinna Altini, Email: corinna.altini@hotmail.it.
Cristina Elena Popescu, Email: cristinamed@yahoo.com.
Paola Ferro, Email: paola.ferro01@gmail.com.
Lavinia Monaco, Email: laviniamonaco92@gmail.com.
Anna Borra, Email: fedefournier@libero.it.
Cristina Ferrari, Email: ferrari_cristina@inwind.it.
Federico Caobelli, Email: federico.caobelli@usb.ch.
REFERENCES
- 1. Roberts WC , McAllister HA , Ferrans VJ . Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) . Am J Med 1977. ; 63 : 86 – 108 . [DOI] [PubMed] [Google Scholar]
- 2. Johns CJ , Michele TM . The clinical management of sarcoidosis. A 50-year experience at the Johns Hopkins Hospital . Medicine 1999. ; 78 : 65 – 111 . doi: 10.1097/00005792-199903000-00001 [DOI] [PubMed] [Google Scholar]
- 3. Kandolin R , Lehtonen J , Airaksinen J , Vihinen T , Miettinen H , Ylitalo K , et al. . Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study . Circulation 2015. ; 131 : 624 – 32 . doi: 10.1161/CIRCULATIONAHA.114.011522 [DOI] [PubMed] [Google Scholar]
- 4. Matsui Y , Iwai K , Tachibana T , Fruie T , Shigematsu N , Izumi T , et al. . Clinicopathological study of fatal myocardial sarcoidosis . Ann N Y Acad Sci 1976. ; 278 ( 1 Seventh Inter ): 455 – 69 . doi: 10.1111/j.1749-6632.1976.tb47058.x [DOI] [PubMed] [Google Scholar]
- 5. Silverman KJ , Hutchins GM , Bulkley BH . Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis . Circulation 1978. ; 58 : 1204 – 11 . doi: 10.1161/01.CIR.58.6.1204 [DOI] [PubMed] [Google Scholar]
- 6. Nunes H , Freynet O , Naggara N , Soussan M , Weinman P , Diebold B , et al. . Cardiac sarcoidosis . Semin Respir Crit Care Med 2010. ; 31 : 428 – 41 . doi: 10.1055/s-0030-1262211 [DOI] [PubMed] [Google Scholar]
- 7. Cohen Aubart F , Nunes H , Mathian A , Haroche J , Hié M , Le-Thi Huong Boutin D , et al. . Cardiac sarcoidosis: diagnosis and therapeutic challenges . Rev Med Interne 2017. ; 38 : 28 – 35 . doi: 10.1016/j.revmed.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 8. Ishiyama M , Soine LA , Vesselle HJ . Semi-quantitative metabolic values on FDG PET/CT including extracardiac sites of disease as a predictor of treatment course in patients with cardiac sarcoidosis . EJNMMI Res 2017. ; 7 : 67 . doi: 10.1186/s13550-017-0315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smedema J-P , Snoep G , van Kroonenburgh MPG , van Geuns R-J , Dassen WRM , Gorgels APM , et al. . Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis . J Am Coll Cardiol 2005. ; 45 : 1683 – 90 . doi: 10.1016/j.jacc.2005.01.047 [DOI] [PubMed] [Google Scholar]
- 10. Yoshida A , Ishibashi-Ueda H , Yamada N , Kanzaki H , Hasegawa T , Takahama H , et al. . Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failure . Eur J Heart Fail 2013. ; 15 : 166 – 75 . doi: 10.1093/eurjhf/hfs206 [DOI] [PubMed] [Google Scholar]
- 11. Aggarwal NR , Snipelisky D , Young PM , Gersh BJ , Cooper LT , Chareonthaitawee P , et al. . Advances in imaging for diagnosis and management of cardiac sarcoidosis . Eur Heart J Cardiovasc Imaging 2015. ; 16 : 949 – 58 . doi: 10.1093/ehjci/jev142 [DOI] [PubMed] [Google Scholar]
- 12. Banba K , Kusano KF , Nakamura K , Morita H , Ogawa A , Ohtsuka F , et al. . Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis . Heart Rhythm 2007. ; 4 : 1292 – 9 . doi: 10.1016/j.hrthm.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 13. Okayama K , Kurata C , Tawarahara K , Wakabayashi Y , Chida K , Sato A , et al. . Diagnostic and prognostic value of myocardial scintigraphy with thallium-201 and gallium-67 in cardiac sarcoidosis . Chest 1995. ; 107 : 330 – 4 . doi: 10.1378/chest.107.2.330 [DOI] [PubMed] [Google Scholar]
- 14. Vaidyanathan S , Patel CN , Scarsbrook AF , Chowdhury FU . FDG PET/CT in infection and inflammation--current and emerging clinical applications . Clin Radiol 2015. ; 70 : 787 – 800 . doi: 10.1016/j.crad.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 15. Yamada S , Kubota K , Kubota R , Ido T , Tamahashi N . High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue . J Nucl Med 1995. ; 7 : 1301 – 6 . [PubMed] [Google Scholar]
- 16. Mochizuki T , Tsukamoto E , Kuge Y , Kanegae K , Zhao S , Hikosaka K , et al. . FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models . J Nucl Med 2001. ; 42 : 1551 – 5 . [PubMed] [Google Scholar]
- 17. Nuutila P , Koivisto VA , Knuuti J , Ruotsalainen U , Teräs M , Haaparanta M , et al. . Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo . J Clin Invest 1992. ; 89 : 1767 – 74 . doi: 10.1172/JCI115780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyagawa M , Yokoyama R , Nishiyama Y , Ogimoto A , Higaki J , Mochizuki T , et al. . Positron emission tomography-computed tomography for imaging of inflammatory cardiovascular diseases . Circ J 2014. ; 78 : 1302 – 10 . doi: 10.1253/circj.CJ-14-0250 [DOI] [PubMed] [Google Scholar]
- 19. Blankstein R , Osborne M , Naya M , Waller A , Kim CK , Murthy VL , et al. . Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis . J Am Coll Cardiol 2014. ; 63 : 329 – 36 . doi: 10.1016/j.jacc.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ambrosini V , Zompatori M , Fasano L , Nanni C , Nava S , Rubello D , et al. . 18)F-FDG PET/CT for the assessment of disease extension and activity in patients with sarcoidosis: results of a preliminary prospective study . Clin Nucl Med 2013. ; 38 : e171 – 7 . doi: 10.1097/RLU.0b013e31827a27df [DOI] [PubMed] [Google Scholar]
- 21. Yamagishi H , Shirai N , Takagi M , Yoshiyama M , Akioka K , Takeuchi K , et al. . Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET . J Nucl Med 2003. ; 44 : 1030 – 6 . [PubMed] [Google Scholar]
- 22. Ishimaru S , Tsujino I , Takei T , Tsukamoto E , Sakaue S , Kamigaki M , et al. . Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis . Eur Heart J 2005. ; 26 : 1538 – 43 . doi: 10.1093/eurheartj/ehi180 [DOI] [PubMed] [Google Scholar]
- 23. Okumura W , Iwasaki T , Toyama T , Iso T , Arai M , Oriuchi N , et al. . Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis . J Nucl Med 2004. ; 45 : 1989 – 98 . [PubMed] [Google Scholar]
- 24. Ishida Y , Yoshinaga K , Miyagawa M , Moroi M , Kondoh C , Kiso K , et al. . Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations . Ann Nucl Med 2014. ; 28 : 393 – 403 . doi: 10.1007/s12149-014-0806-0 [DOI] [PubMed] [Google Scholar]
- 25. Tahara N , Tahara A , Nitta Y , Kodama N , Mizoguchi M , Kaida H , et al. . Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis . JACC Cardiovasc Imaging 2010. ; 3 : 1219 – 28 . doi: 10.1016/j.jcmg.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 26. Langah R , Spicer K , Gebregziabher M , Gordon L . Effectiveness of prolonged fasting 18F-FDG PET-CT in the detection of cardiac sarcoidosis . J Nucl Cardiol 2009. ; 16 : 801 – 10 . doi: 10.1007/s12350-009-9110-0 [DOI] [PubMed] [Google Scholar]
- 27. Wykrzykowska J , Lehman S , Williams G , Parker JA , Palmer MR , Varkey S , et al. . Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation . J Nucl Med 2009. ; 50 : 563 – 8 . doi: 10.2967/jnumed.108.055616 [DOI] [PubMed] [Google Scholar]
- 28. Morooka M , Moroi M , Uno K , Ito K , Wu J , Nakagawa T , et al. . Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis . EJNMMI Res 2014. ; 4 : 1 – 11 . doi: 10.1186/2191-219X-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manabe O , Yoshinaga K , Ohira H , Masuda A , Sato T , Tsujino I , et al. . The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial (18)F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis . J Nucl Cardiol 2016. ; 23 : 244 – 52 . doi: 10.1007/s12350-015-0226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yokoyama R , Miyagawa M , Okayama H , Inoue T , Miki H , Ogimoto A , et al. . Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis . Int J Cardiol 2015. ; 195 : 180 – 7 . doi: 10.1016/j.ijcard.2015.05.075 [DOI] [PubMed] [Google Scholar]
- 31. Miyagawa M , Yokoyama R , Nishiyama Y , Mochizuki T , et al. . Recent advancement in diagnostic imaging around cardiac sarcoidosis . JJSOGD 2015. ; 35 : 31 – 7 [in Japanese] . doi: 10.7878/jjsogd.35.31 [DOI] [Google Scholar]
- 32. Lum DP , Wandell S , Ko J , Coel MN . Reduction of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction . Mol Imaging Biol 2002. ; 4 : 232 – 7 . doi: 10.1016/S1095-0397(01)00062-0 [DOI] [PubMed] [Google Scholar]
- 33. Coulden R , Chung P , Sonnex E , Ibrahim Q , Maguire C , Abele J , et al. . Suppression of myocardial 18F-FDG uptake with a preparatory "Atkins-style" low-carbohydrate diet . Eur Radiol 2012. ; 22 : 2221 – 8 . doi: 10.1007/s00330-012-2478-2 [DOI] [PubMed] [Google Scholar]
- 34. Harisankar CNB , Mittal BR , Agrawal KL , Abrar ML , Bhattacharya A . Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake . J Nucl Cardiol 2011. ; 18 : 926 – 36 . doi: 10.1007/s12350-011-9422-8 [DOI] [PubMed] [Google Scholar]
- 35. Williams G , Kolodny GM . Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet . AJR Am J Roentgenol 2008. ; 190 : W151 – W156 . doi: 10.2214/AJR.07.2409 [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi Y , Kumita S-ichiro , Fukushima Y , Ishihara K , Suda M , Sakurai M . Significant suppression of myocardial (18)F-fluorodeoxyglucose uptake using 24-h carbohydrate restriction and a low-carbohydrate, high-fat diet . J Cardiol 2013. ; 62 : 314 – 9 . doi: 10.1016/j.jjcc.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 37. Balink H , Hut E , Pol T , Flokstra F-J , Roef M . Suppression of 18F-FDG myocardial uptake using a Fat-Allowed, Carbohydrate-Restricted diet . J Nucl Med Technol 2011. ; 39 : 185 – 9 . doi: 10.2967/jnmt.110.076489 [DOI] [PubMed] [Google Scholar]
- 38. Lu Y , Grant C , Xie K , Sweiss NJ . Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and Very-Low-Carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis . Clin Nucl Med 2017. ; 42 : 88 – 94 . doi: 10.1097/RLU.0000000000001465 [DOI] [PubMed] [Google Scholar]
- 39. Cheng VY , Slomka PJ , Ahlen M , Thomson LEJ , Waxman AD , Berman DS . Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: a randomized controlled trial . J Nucl Cardiol 2010. ; 17 : 286 – 91 . doi: 10.1007/s12350-009-9179-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Demeure F , Hanin F-X , Bol A , Vincent M-F , Pouleur A-C , Gerber B , et al. . A randomized trial on the optimization of 18F-FDG myocardial uptake suppression: implications for vulnerable coronary plaque imaging . J Nucl Med 2014. ; 55 : 1629 – 35 . doi: 10.2967/jnumed.114.138594 [DOI] [PubMed] [Google Scholar]
- 41. Gaeta C , Fernández Y , Pavía J , Flotats A , Artigas C , Deportos J , et al. . Reduced myocardial 18F-FDG uptake after calcium channel blocker administration. Initial observation for a potential new method to improve plaque detection . Eur J Nucl Med Mol Imaging 2011. ; 38 : 2018 – 24 . doi: 10.1007/s00259-011-1873-2 [DOI] [PubMed] [Google Scholar]
- 42. Scholtens AM , Verberne HJ , Budde RPJ , Lam MGEH , et al. . Additional heparin preadministration improves cardiac glucose metabolism suppression over low-carbohydrate diet alone in 18F-FDG PET imaging . Journal of Nuclear Medicine 2016. ; 57 : 568 – 73 . doi: 10.2967/jnumed.115.166884 [DOI] [PubMed] [Google Scholar]
- 43. Chareonthaitawee P , Beanlands RS , Chen W , Dorbala S , Miller EJ , Murthy VL , et al. . Joint SNMMI-ASNC Expert Consensus Document on the Role of 18 F-FDG PET/CT in Cardiac Sarcoid Detection and Therapy Monitoring . J Nucl Med 2017. ; 58 : 1341 – 53 . doi: 10.2967/jnumed.117.196287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brett W . Sperry prognostic impact of extent, severity . and Heterogeneity of Abnormalities on 18F-FDG PET Scans for Suspected Cardiac Sarcoidosis ACC: Cardiovascular Imaging 2018. ; 11 : 336 – 45 . [DOI] [PubMed] [Google Scholar]
- 45. Youssef G , Leung E , Mylonas I , Nery P , Williams K , Wisenberg G , et al. . The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience . J Nucl Med 2012. ; 53 : 241 – 8 . doi: 10.2967/jnumed.111.090662 [DOI] [PubMed] [Google Scholar]
- 46. Manabe O , Yoshinaga K , Ohira H , Sato T , Tsujino I , Yamada A , et al. . Right ventricular (18)F-FDG uptake is an important indicator for cardiac involvement in patients with suspected cardiac sarcoidosis . Ann Nucl Med 2014. ; 28 : 656 – 63 . doi: 10.1007/s12149-014-0860-7 [DOI] [PubMed] [Google Scholar]
- 47. Jamar F , Buscombe J , Chiti A , Christian PE , Delbeke D , Donohoe KJ , et al. . EANM/SNMMI guideline for 18F-FDG use in inflammation and infection . J Nucl Med 2013. ; 54 : 647 – 58 . doi: 10.2967/jnumed.112.112524 [DOI] [PubMed] [Google Scholar]
- 48. Flores RJ , Flaherty KR , Jin Z , Bokhari S , et al. . The prognostic value of quantitating and localizing F-18 FDG uptake in cardiac sarcoidosis . J Nucl Cardiol 2018. ; 25 [Epub ahead of print] . doi: 10.1007/s12350-018-01504-y [DOI] [PubMed] [Google Scholar]
- 49. Ohira H , Birnie DH , Pena E , Bernick J , Mc Ardle B , Leung E , et al. . Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis . Eur J Nucl Med Mol Imaging 2016. ; 43 : 259 – 69 . doi: 10.1007/s00259-015-3181-8 [DOI] [PubMed] [Google Scholar]
- 50. Ahmadian A , Brogan A , Berman J , Sverdlov AL , Mercier G , Mazzini M , et al. . Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis . J Nucl Cardiol 2014. ; 21 : 925 – 39 . doi: 10.1007/s12350-014-9901-9 [DOI] [PubMed] [Google Scholar]
- 51. Ohira H , Tsujino I , Ishimaru S , Oyama N , Takei T , Tsukamoto E , et al. . Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis . Eur J Nucl Med Mol Imaging 2008. ; 35 : 933 – 41 . doi: 10.1007/s00259-007-0650-8 [DOI] [PubMed] [Google Scholar]
- 52. Sgard B , Brillet P-Y , Bouvry D , Djelbani S , Nunes H , Meune C , et al. . Evaluation of FDG PET combined with cardiac MRI for the diagnosis and therapeutic monitoring of cardiac sarcoidosis . Clinical Radiology 2019. ; 74 : 81.e9 – 81.e18 e18 . doi: 10.1016/j.crad.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 53. Flotats A , Knuuti J , Gutberlet M , Marcassa C , Bengel FM , Kaufmann PA , et al. . Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European association of nuclear medicine (EANM), the European Society of cardiac radiology (ESCR) and the European Council of nuclear cardiology (ECNC . Eur J Nucl Med Mol Imaging 2011. ; 38 : 201 – 12 . doi: 10.1007/s00259-010-1586-y [DOI] [PubMed] [Google Scholar]
- 54. Slomka PJ , Pan T , Berman DS , Germano G . Advances in SPECT and PET hardware . Prog Cardiovasc Dis 2015. ; 57 : 566 – 78 . doi: 10.1016/j.pcad.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 55. von Olshausen G , Hyafil F , Langwieser N , Laugwitz K-L , Schwaiger M , Ibrahim T . Detection of acute inflammatory myocarditis in Epstein Barr virus infection using hybrid 18F-fluoro-deoxyglucose-positron emission tomography/magnetic resonance imaging . Circulation 2014. ; 130 : 925 – 6 . doi: 10.1161/CIRCULATIONAHA.114.011000 [DOI] [PubMed] [Google Scholar]
- 56. Brendle C , Schmidt H , Oergel A , Bezrukov I , Mueller M , Schraml C , et al. . Segmentation-based attenuation correction in positron emission tomography/magnetic resonance: Erroneous tissue identification and its impact on positron emission tomography interpretation . Invest Radiol United States 2015. ; 50 : 339 – 46 . [DOI] [PubMed] [Google Scholar]
- 57. Wisenberg G , Thiessen JD , Pavlovsky W , Butler J , Wilk B , Prato FS .; in press. Same day comparison of PET/CT and PET/MR in patients with cardiac sarcoidosis . J. Nucl. Cardiol. 2019. ; 19 . doi: 10.1007/s12350-018-01578-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schneider S , Batrice A , Rischpler C , Eiber M , Ibrahim T , Nekolla SG . Utility of multimodal cardiac imaging with PET/MRI in cardiac sarcoidosis: implications for diagnosis, monitoring and treatment . Eur Heart J 2014. ; 35 : 312 . doi: 10.1093/eurheartj/eht335 [DOI] [PubMed] [Google Scholar]
- 59. Hanneman K , Kadoch M , Guo HH , Jamali M , Quon A , Iagaru A , et al. . Initial experience with simultaneous 18F-FDG PET/MRI in the evaluation of cardiac sarcoidosis and myocarditis . Clin Nucl Med 2017. ; 42 : e328 – 34 . doi: 10.1097/RLU.0000000000001669 [DOI] [PubMed] [Google Scholar]
- 60. Garrett E , Sekhri N , Barnes A , Mohiddin SA , Wicks EC , Menezes LJ , et al. . Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis . Eur Hear J - Cardiovasc Imaging 2018. ; 19 : 757 – 67 . [DOI] [PubMed] [Google Scholar]
- 61. Dweck MR , Abgral R , Trivieri MG , Robson PM , Karakatsanis N , Mani V , et al. . Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis . JACC Cardiovasc Imaging 2018. ; 11 : 94 – 107 . doi: 10.1016/j.jcmg.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Valeyre D , Prasse A , Nunes H , Uzunhan Y , Brillet P-Y , Müller-Quernheim J . Sarcoidosis . The Lancet 2014. ; 383 : 1155 – 67 . doi: 10.1016/S0140-6736(13)60680-7 [DOI] [PubMed] [Google Scholar]
- 63. Tan JL , Fong HK , Birati EY , Han Y , Sarcoidosis C . Cardiac sarcoidosis . Am J Cardiol 2019. ; 123 : 513 – 22 . doi: 10.1016/j.amjcard.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 64. Ahmadian A , Pawar S , Govender P , Berman J , Ruberg FL , Miller EJ . The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis . J Nucl Cardiol 2017. ; 24 : 413 – 24 . doi: 10.1007/s12350-016-0490-7 [DOI] [PubMed] [Google Scholar]
- 65. Skali H , Schulman AR , Dorbala S . 18F-FDG PET/CT for the assessment of myocardial sarcoidosis . Curr Cardiol Rep 2013. ; 15 : 370 . doi: 10.1007/s11886-013-0370-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sobic-Saranovic DP , Grozdic IT , Videnovic-Ivanov J , Vucinic-Mihailovic V , Artiko VM , Saranovic DZ , et al. . Responsiveness of FDG PET/CT to treatment of patients with active chronic sarcoidosis . Clin Nucl Med 2013. ; 38 : 516 – 21 . doi: 10.1097/RLU.0b013e31828731f5 [DOI] [PubMed] [Google Scholar]
- 67. Matthews R , Bench T , Meng H , Franceschi D , Relan N , Brown DL . Diagnosis and monitoring of cardiac sarcoidosis with delayed-enhanced MRI and 18F-FDG PET-CT . J Nucl Cardiol 2012. ; 19 : 807 – 10 . doi: 10.1007/s12350-012-9550-9 [DOI] [PubMed] [Google Scholar]
- 68. Mc Ardle BA , Birnie DH , Klein R , de Kemp RA , Leung E , Renaud J , et al. . Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by ¹⁸F- fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging 2013. ; 6 : 617 – 26 . doi: 10.1161/CIRCIMAGING.112.000289 [DOI] [PubMed] [Google Scholar]
- 69. Mc Ardle BA , Leung E , Ohira H , Cocker MS , deKemp RA , DaSilva J , et al. . The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis . J Nucl Cardiol 2013. ; 20 : 297 – 306 . doi: 10.1007/s12350-012-9668-9 [DOI] [PubMed] [Google Scholar]
- 70. Osborne MT , Hulten EA , Singh A , Waller AH , Bittencourt MS , Stewart GC , et al. . Reduction in ¹⁸F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis . J Nucl Cardiol 2014. ; 21 : 166 – 74 . doi: 10.1007/s12350-013-9828-6 [DOI] [PubMed] [Google Scholar]
- 71. Shelke AB , Aurangabadkar HU , Bradfield JS , Ali Z , Kumar KS , Narasimhan C . Serial FDG-PET scans help to identify steroid resistance in cardiac sarcoidosis . Int J Cardiol 2017. ; 228 : 717 – 22 . doi: 10.1016/j.ijcard.2016.11.142 [DOI] [PubMed] [Google Scholar]
- 72. Furuya S , Manabe O , Ohira H , Hirata K , Aikawa T , Naya M , et al. . Which is the proper reference tissue for measuring the change in FDG PET metabolic volume of cardiac sarcoidosis before and after steroid therapy? EJNMMI Res 2018. ; 8 : 94 . doi: 10.1186/s13550-018-0447-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kruse MJ , Kovell L , Kasper EK , Pomper MG , Moller DR , Solnes L , et al. . Myocardial Blood Flow and Inflammatory Cardiac Sarcoidosis . JACC Cardiovasc Imaging 2017. ; 10 : 157 – 67 . doi: 10.1016/j.jcmg.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 74. Kim S-J , Pak K , Kim K . Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis; a systematic review and meta-analysis . J. Nucl. Cardiol. 2019. ; 336 [Epub ahead of print]. . doi: 10.1007/s12350-018-01582-y [DOI] [PubMed] [Google Scholar]
- 75. Kwekkeboom DJ , Krenning EP , Kho GS , Breeman WAP , Van Hagen PM . Somatostatin receptor imaging in patients with sarcoidosis . European Journal of Nuclear Medicine and Molecular Imaging 1998. ; 25 : 1284 – 92 . doi: 10.1007/s002590050297 [DOI] [PubMed] [Google Scholar]
- 76. Lebtahi R , Crestani B , Belmatoug N , Daou D , Delahaye N , Dombret MC , et al. . Somatostatin receptor scintigraphy in patients with sarcoidosis: comparison with gallium-67 scintigraphy . J Nucl Med 1998. ; 39 : 44p [PubMed] [Google Scholar]
- 77. Reiter T , Werner RA , Bauer WR , Lapa C . Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography . Eur Heart J 2015. ; 36 : 2404 . doi: 10.1093/eurheartj/ehv278 [DOI] [PubMed] [Google Scholar]
- 78. Lapa C , Reiter T , Kircher M , Schirbel A , Werner RA , Pelzer T , et al. . Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI . Oncotarget 2016. ; 7 : 77807 – 14 . doi: 10.18632/oncotarget.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nobashi T , Nakamoto Y , Kubo T , Ishimori T , Handa T , Tanizawa K , et al. . The utility of PET/CT with (68)Ga-DOTATOC in sarcoidosis: comparison with (67)Ga-scintigraphy . Ann Nucl Med 2016. ; 30 : 544 – 52 . doi: 10.1007/s12149-016-1095-6 [DOI] [PubMed] [Google Scholar]
- 80. Pizarro C , Kluenker F , Dabir D , Thomas D , Gaertner FC , Essler M , et al. . Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis . ESC Heart Fail 2018. ; 5 : 249 – 61 . doi: 10.1002/ehf2.12243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lapa C , Reiter T , Li X , Werner RA , Samnick S , Jahns R , et al. . Imaging of myocardial inflammation with somatostatin receptor based PET/CT - A comparison to cardiac MRI . Int J Cardiol 2015. ; 194 : 44 – 9 . doi: 10.1016/j.ijcard.2015.05.073 [DOI] [PubMed] [Google Scholar]
- 82. Gormsen LC , Haraldsen A , Kramer S , Dias AH , Kim WY , Borghammer P . A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis . EJNMMI Res 2016. ; 6 : 52 . doi: 10.1186/s13550-016-0207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weinberg RL , Morgenstern R , DeLuca A , Chen J . Bokhari S. F-18 sodium fluoride PET/CT does not effectively image myocardial inflammation due to suspected cardiac sarcoidosis . J Nucl Cardiol 2016. ; [Epub ahead of print]. . [DOI] [PubMed] [Google Scholar]
- 84. Norikane T , Yamamoto Y , Maeda Y , Noma T , Nishiyama Y . 18F-FLT PET imaging in a patient with sarcoidosis with cardiac involvement . Clin Nucl Med 2015. ; 40 : 433 – 4 . doi: 10.1097/RLU.0000000000000653 [DOI] [PubMed] [Google Scholar]
- 85. Zhao S , Kuge Y , Kohanawa M , Takahashi T , Zhao Y , Yi M , et al. . Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: a comparison with 18F-FDG and 18F-FLT . J Nucl Med 2008. ; 49 : 135 – 41 . doi: 10.2967/jnumed.107.044578 [DOI] [PubMed] [Google Scholar]
- 86. Norikane T , Yamamoto Y , Maeda Y , Noma T , Dobashi H , Nishiyama Y . Comparative evaluation of 18 F-FLT and 18 F-FDG for detecting cardiac and extra-cardiac thoracic involvement in patients with newly diagnosed sarcoidosis . EJNMMI Res 2017. ; 7 : 69 . doi: 10.1186/s13550-017-0321-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hotta M , Minamimoto R , Kubota S , Awaya T , Hiroi Y . 11C-4DST PET/CT imaging of cardiac sarcoidosis: comparison with 18F-FDG and cardiac MRI . Clin Nucl Med 2018. ; 43 : 458 – 9 . doi: 10.1097/RLU.0000000000002059 [DOI] [PubMed] [Google Scholar]
- 88. Manabe O , Hirata K , Shozo O , Shiga T , Uchiyama Y , Kobayashi K , et al. . 18 F-fluoromisonidazole (FMISO) PET may have the potential to detect cardiac sarcoidosis . J Nucl Cardiol 2017. ; 24 : 329 – 31 . doi: 10.1007/s12350-016-0495-2 [DOI] [PubMed] [Google Scholar]
- 89. Piotrowski WJ , Kiszałkiewicz J , Pastuszak-Lewandoska D , Górski P , Antczak A , Migdalska-Sęk M , et al. . Expression of HIF-1A/VEGF/ING-4 axis in pulmonary sarcoidosis . Adv Exp Med Biol 2015. ; 866 : 61 – 9 . doi: 10.1007/5584_2015_144 [DOI] [PubMed] [Google Scholar]