Abstract
Objective:
To evaluate the agreement between multiple detector CT (MDCT) and laparoscopy in the preoperative categorization of peritoneal carcinomatosis, and to determine the impact of this categorization on the prediction of cytoreduction status.
Methods:
This prospective study included 80 consecutive females with primary ovarian cancer eligible for cytoreductive surgery (CRS). MDCT and diagnostic laparoscopy were performed prior to surgery for assessment of peritoneal carcinomatosis extent. Based on PCI (peritoneal cancer index) score, carcinomatosis was categorized into three groups. Categorization agreement between CT and laparoscopy was assessed and compared with the intraoperative-histopathologically proven PCI. Impact of PCI categorization on cytoreduction status was also evaluated.
Results:
The overall agreement between CT and laparoscopy in preoperative peritoneal carcinomatosis categorization was good (K =0.71-0.79) in low category group and excellent in both moderate and large group (interclass correlation coeeficient = 0.89–0.91). (p<0.01)
Optimal cytoreduction was achieved in 62/80 (77.5%) patients, PCI < 20 was detected in 48/62 (77.4%), pre-operative PCI < 20 correctly predicted optimal cytoreductive surgery (OCS) in 40/48 (83.3%) cases.
Suboptimal cytoreduction was performed in 18/80 (22.5%) patients. PCI > 20 was detected in (10/18) 55.6%, preoperative CT and laparoscopy PCI > 20 correctly predicted SCS in 8/10 (80%) cases.
The area under receiver operating characteristic curve showed that PCI cut-off <20 was the best predictor of OCS with an accuracy 85%, sensitivity 97%, specificity 40%, negative predictive value 76%, and positive predictive value 93%.
Conclusion:
Both laparoscopy and CT are equally effective in pre-operative peritoneal carcinomatosis categorization. PCI < 20 is accurate in the prediction of optimal cytoreduction. More than half of patients with suboptimal cytoreduction had PCI > 20 and interval debulking surgery can be recommended.
Advances in knowledge:
Both laparoscopy and CT are equally effective in pre-operative peritoneal carcinomatosis categorization. PCI < 20 is accurate in the prediction of optimal cytoreduction. More than half of patients with suboptimal cytoreduction had PCI > 20 and interval debulking surgery can be recommended.
Introduction
The 5-year survival rate of ovarian cancer patients is approximately 30–40% due to late presentation in an advanced stage with peritoneal cavity dissemination. Better survival was observed with cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC). Selection of patients should be done carefully to determine the cost benefit from this therapeutic approach and also treatment-related morbidity and mortality.1
Jacquet and Sugarbaker2 introduced an accurate system for quantification and distribution of peritoneal deposits at surgery, the combination of peritoneal carcinomatosis distribution at 13 abdominopelvic regions with the tumor size represents the peritoneal cancer index (PCI) score. Measurement of PC extent at surgery represents the most important indicator of prognosis in patients that will undergo CRS.
Recently different imaging techniques have been used to assess the extent and localization of peritoneal carcinomatosis. It includes ultrasound, CT, MRI, and PET.1,2 A recent meta-analysis3 have been described that CT is the best imaging modality for evaluation of peritoneal deposits due to its high per region diagnostic accuracy according to PCI.
Detailed preoperative map of carcinomatosis using PCI is mandatory to the surgeon to guide therapeutic patient management and differentiating patients who are candidates for systemic chemotherapy from those who are candidates for surgical intervention.4–7
Although staging laparotomy is considered the most accurate in determining the ability to perform complete CRS, laparotomy is a very invasive option for diagnostic aim. Diagnostic laparoscopy provides a less invasive surgical option in patients with early-stage ovarian cancer because of limited accuracy (76%) and low positive predictive value (PPV) (68%) of MDCT in predicting suboptimal cytoreduction.8 Optimal cytoreduction rate varies from 15 to 85%, survival was significantly better for patients who undergo optimal vs suboptimal cytoreduction.9 Studies attempting to identify pre-operative accuracy of MDCT and laparoscopy in the categorization of PCI and its reflection on the prediction of cytoreduction status have been limited by their retrospective design and broad inclusion criteria. The aim of this prospective study was to assess the agreement between MDCT and laparoscopy in peritoneal carcinomatosis categorization and its reflection on cytoreduction status.
Patients and methods
Patients
We conducted this prospective study between January 2015 and April 2018. It included 80 consecutive patients with primary ovarian cancer eligible for CRS. Inclusion criteria were primary ovarian cancer patients with no extraperitoneal metastatic disease at pre-operative contrast-enhanced MDCT and received pre-operative diagnostic laparoscopy. Exclusion criteria were the presence of criteria that may preclude surgery [central or multisegmental hepatic metastases, bulky lymph nodes above the renal vessels, diffuse small bowel mesentery infiltration and diffuse small bowel infiltration that could lead to short bowel syndrome after resection (remaining bowel <1.5 m)].
The local institutional review board approved the study protocol (IRB: 17100674). Written informed consent was obtained from all patients participating in the study. MDCT and laparoscopic data were compared to surgical and histopathologic findings.
CT examination
All examinations were performed using a 64-channel MDCT scanner (GE Healthcare, Waukesha, WI, USA Bright Speed 64). Patient preparation included fasting for 6 h, oral administration of 500–750 ml of diluted oral contrast 30–45 min prior to the study. All patients received 140 ml of i.v. contrast material (iopromide; Ultravist 300, Schering) with a rate of 2.3 ml s−1 via the mechanical injector. CT scanning was performed with the patient in the supine position, scanning began from the diaphragmatic dome down to the pelvic floor. Table 1 illustrated the scanning protocol parameters.
Table 1.
Scanning parameters for MDCT used in this study
| Parameter | Details |
| Scan type | Single breath-hold helical |
| Tube voltages and currents | 120 kVp, 189–200 mAs |
|
Slice thickness
Reconstruction interval Gantry rotation time Beam pitch |
1.25
mm
0.8 mm 0.33 s 1 |
| Acquisition time | 4–6 s |
| Scan acquisition | Arterial phase at 35 s; and portal venous phase at 70 s. |
MDCT, multiple detector CT.
Image interpretation
All examinations were interpreted by two radiologists with 7 and 9 years of experience in oncology imaging including CT. Radiologists reviewed all examinations using image interpretation console (Advantage Workstation 4.4, GE Healthcare, Milwaukee, WI, USA), with adjusted window width, pan, and level.
A standardized reporting template was used to calculate the PCI score. It was assessed by dividing the abdomen into 13 regions by 2 transverse planes (one at the lowest part of the costal margin level and the other at the anterior superior iliac spine level) and 2 sagittal planes (at the midclavicular line). The small intestine was assessed separately by dividing it into four different parts (upper and lower jejunum and upper and lower ileum) (Figure 1).10
Figure 1.
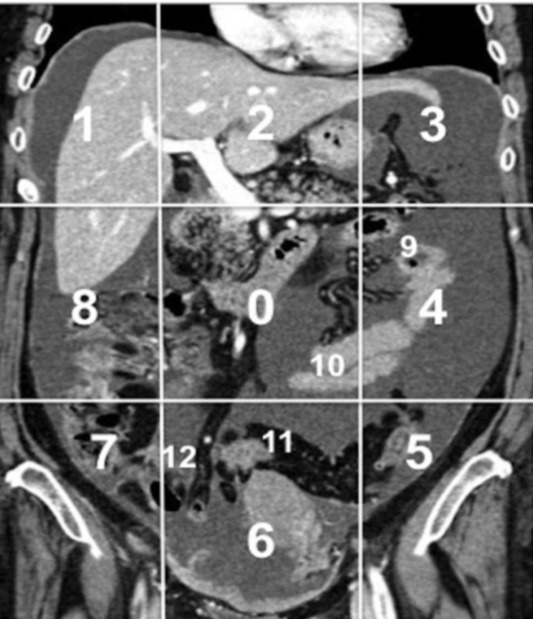
The Sugar baker's Peritoneal Cancer (PCI) describes the distribution of the 13-abdominopelvic regions. 0: central, 1: right upper, 2: epigastric, 3: left upper, 4: left flank, 5: left lower, 6: pelvis, 7: right lower, 8: right flank, 9: upper jejunum, 10: lower jejunum, 11: Upper ileum and 12: lower ileum. PCI, peritoneal cancer index.
To quantify CT PCI, the largest deposit was chosen in the assessed region, and lesion size (LS) score was assigned from 0 to 3 points. 0 if no tumor present, 1 if implants < 0.5 cm, 2 if tumor up to 5 cm, and 3 if tumor >5 cm or confluent disease or matting to pelvic structures even if there was only a small layer of tumor deposit. LS score summation in the 13-abdominopelvic regions was the PCI. A maximal score is 39 (13 × 3). PCI was categorized into three categories: low if PCI <10, moderate if 10–20, and large if >20. Receiver operating characteristic (ROC) curve was used to determine the best PCI cut-off for SCS.
Laparoscopy and laparotomy
Treatment decision was decided during a multidisciplinary tumor board at assiut university hospital, Egypt. PCI scoring was calculated by preoperative laparoscopy and intraoperative by the same surgical team. According to the standard guidelines, standard abdominal midline laparotomy with intensive surgical staging was attempted in all patients. All patients received maximal surgical effort to achieve optimum cytoreduction with no or <1 cm gross residual tumor (total abdominal hysterectomy, bilateral salpingo-oophorectomy, total omentectomy, and appendectomy), surgical excision of all deposits was attempted. Optimal cytoreduction was defined as a residual tumor of <1 cm, more than 1 cm gross residual tumor was considered suboptimal cytoreduction. All patients underwent pelvic and para-aortic lymphadenectomy if possible. Postoperative complications were described according to the Clavien–Dindo classification.11
The range of duration between CT and laparoscopy was (4–16 day), and between laparoscopy and laparotomy (7–20 day).
Statistical analysis
CT and laparoscopic results (regarding deposits presence or absence) were determined based on intraoperative and histopathological data. Positive peritoneal deposits were considered if it was present in at least one defined area on CT or laparoscopy. True positive results were considered in a region with suspected deposits by CT, laparoscopy and confirmed by histopathologically proven intraoperative deposits (gold standard), otherwise, false positive results were considered. True negative results were considered in a region if no deposits were suspected by CT, laparoscopy and no tumor was identified by surgery, respectively; otherwise, false negative results were considered.
Agreement between CT, laparoscopy, and surgery regarding peritoneal carcinomatosis categorization was assessed using κ (κ) statistic, a k-value of 0.81–1.00, excellent agreement, a k-value of 0.61–0.80, good agreement; a k-value of 0.41–0.60, moderate agreement; a k-value of 0.21–0.40, fair agreement; a k-value of <0.20 indicates poor agreement. The consistency of the diagnoses between diagnostic modalities was estimated using interclass correlation (ICC).
Accuracy of prediction of OCS was calculated based on sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each investigated parameter. Sensitivity was defined as the number of cases with intraoperative gross residual tumor <1 cm (OCS) who were correctly classified (true positives) divided by the number of cases who underwent optimal cytoreduction (true positives + false negatives).
Specificity was defined as the number of patients with intraoperative gross residual >1 cm who were correctly classified (true negatives) divided by the number of cases who underwent suboptimal cytoreduction (true negatives + false positives).
The PPV was defined as the number of true positive cases divided by the number of all positive cases (true positives + false positives). The NPV was calculated as the number of true negative cases divided by the number of all negative cases (true negatives + false negatives). ROC curve was used to calculate the best PCI cut off for OCS.
IBM SPSS Statistics v. 21 (IBM Corp., Armonk, NY) was used for data analysis. p-value ≤ 0.05. was considered as statistically significant.
Results
The clinicopathological characteristics and surgical data of all patients are presented in Table 2.
Table 2.
Patient and tumor-related characteristics and surgical outcome. †
| Characteristics N (%) | Total number = 80 |
| Median age in years (range) | 49 y; (range: 24–69 y) |
| Histology | |
| Serous | 64 (80%) |
| Mucinous | 6 (7.5%) |
| Endometrioid | 10 (12.5%) |
| FIGO stage | |
| II | 34 (42.5%) |
| IIIA | 24 (30%) |
| IIIB | 14 (18%) |
| IIIC | 8 (10%) |
| Grade | |
| Low grade | 67 (83.8%) |
| High grade | 13 (16.3%) |
| Ascites | 34 (42.5%) |
| Serum CA-125 level | |
| >500 U Ml−1 | 14 (18%) |
| <500 U Ml−1 | 66 (82.5%) |
| Surgical out come | |
| optimal CRS | 62 (77.5%) |
| suboptimal CRS | 18 (22.5%) |
| Post-operative complication | |
| Clavien–Dindo grade II | 30 (37.5%) |
| Clavien– Dindo grade III to IV | 8 (10%) |
| 90 day postoperative mortality rate | 0 |
CRS, cytoreductive surgery.
Values are given as number or percentage when appropriate.
Out of 80 ovarian cancer patients, intraoperative-histopathologic PCI confirmed low carcinomatosis in 40% (32/80) of patients, moderate carcinomatosis in 35% (28/80) and the remaining 25% (20/80) showed large tumor burden.
The overall agreement of CT in peritoneal carcinomatosis categorization was good (K = 0.70–0.80). It was excellent (ICC = 0.90–0.94) in both moderate and large group, and good (ICC = 0.63–0.79) in low group. (p < 0.02). Dis-concordance was found in (14/80) 17.5% cases. CT overestimated 10 cases (12.5%) from low to moderate group, underestimated 4 cases (5%) from moderate to low group. (Figures 2–4).
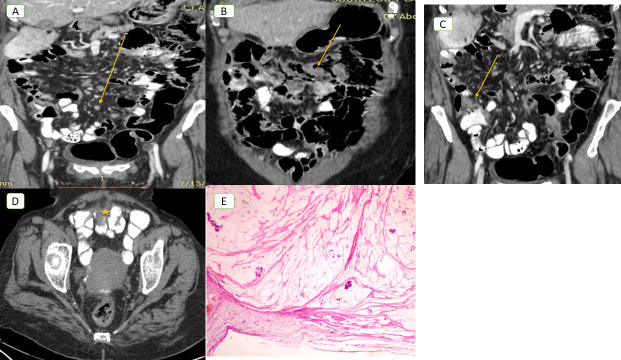
Figure 2.
A 49-year-old female with primary mucinous ovarian cancer. (A) Coronal post-contrast-enhanced CT image demonstrates multiple small soft tissue peritoneal nodules at region 3 (yellow arrow), at region 8 (star), at region 2 (white arrow), at region 1 (black arrow) and at region 11 (green arrow). (PCI CT = 11; PCI laparoscopy = 6; PCI pathologic value = 2). The CT examination falsely up staged the case to moderate tumor burden group, but laparoscopy categorized it in the low group, complete cytoreduction was done and pathology confirmed peritoneal implants only in region 8, but mesothelial hyperplasia only was detected with no tumor tissue at the remaining regions (B). PCI, peritoneal cancer index.
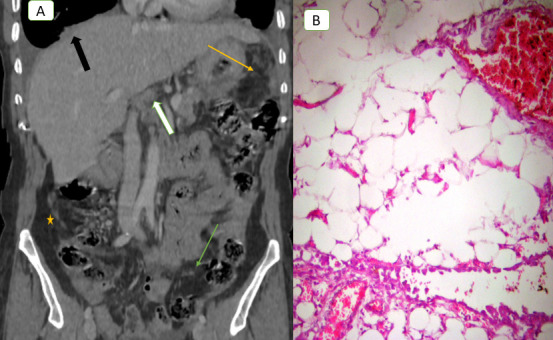
Figure 3.
A 57-year-old female with primary mucinous ovarian cancer. (A) Coronal contrast-enhanced CT scan obtained during venous phase demonstrates multiple small soft tissue peritoneal nodules at region 2 (lesser omentum nodules white arrow), calcified soft tissue deposit at liver hilum (black arrow), and soft tissue serosal implant on ascending colon (star), CT PCI = 11. Laparoscopic image (B) showed multiple small soft tissue nodules scattered in lateral peritoneal fold (missed by CT), laparoscopy and pathology PCI = 9, CT falsely upstaged the case from low to moderate group, patient underwent optimal cytoreduction. PCI, peritoneal cancer index.
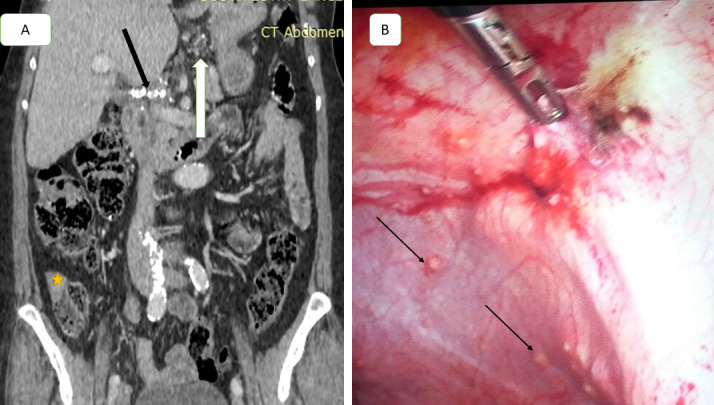
Figure 4.
A 48-year-old female with ovarian cancer, axial contrast-enhanced CT image shows multiple small soft tissue nodules scattered in the small intestinal mesentery (arrow in A), thickening and enhancement of small bowel wall over long irregular segments (arrow in B), soft tissue implant seen at the terminal ilium (arrow in C), soft tissue deposit implanted at the serosal surface of jejunum at region 11 (star in D). Frozen section biopsy with histologic confirmation (E) showed peritoneal deposits consist of dissecting pools of mucin, some of which contain strips of malignant epithelium (H&E, 200x). CT and laparoscopic (total PCI = 19), patient underwent suboptimal cytoreduction.
The overall agreement of laparoscopy in peritoneal carcinomatosis categorization was excellent (ICC = 0.81–0.95). (p < 0.04). Dis-concordance was found in (10/80) 12.5% cases. Laparoscopy overestimated four cases (5%) from moderate to large group, underestimated six cases (7.5%) from large to moderate group. Table 3 demonstrated the estimation rate of carcinomatosis category on CT and laparoscopy.
Table 3.
The estimation rate of carcinomatosis category on CT and laparoscopy. †
| Accurate estimation | Overestimation | Underestimation | |
| CT | 66/80 (82.5%) | 10/80 (12.5%) | 4/80 (5%) |
| Laparoscopy | 70/80 (87.5%) | 4/80 (5%) | 6/80 (7.5%) |
Values are given as number and percentage.
The overall agreement between CT and laparoscopy was good (K = 0.71–0.79) in low category group and excellent in both moderate and large group (ICC = 0.89–0.91). (p < 0.01)
At surgery, 62 of 80 patients (77.5%) had optimal cytoreduction. PCI < 20 was detected in 48/62 (77.4%), PCI > 20 was detected in (14/62) 22.6%. Pre-operative PCI < 20 correctly predicted OCS in 40/48 (83.3%) cases. The area under ROC curve showed that PCI cut-off < 20 was the best predictor of OCS with an accuracy 85%, sensitivity 97%, specificity 40%, NPV 76%, and PPV 93%.
Table 4 shows the accuracy of the diagnostic techniques in the prediction of OCS.
Table 4.
Accuracy of predicting optimal cytoreduction by different techniques
| Sensitivity (95% CI) | Specificity (95% CI) |
PPV (95% CI) | NPV (95% CI) | Accuracy | |
| CT | 90% (79–91%) |
39% (33–40%) |
75% (70–75%) |
70% (69–79%) |
69% |
| Laparoscopy | 89% (77–90%) |
42% (35–43%) |
76% (69–79%) |
71% (70–77%) |
71% |
| CT plus laparoscopy | 97% (89–97%) |
40% (38–49%) |
93% (89–95%) |
76% (70–78%) |
85% |
| Laparotomy | 91% (89–94%) |
83% (81–89%) |
88% (79–89%) |
90% (79–90%) |
89% |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
95% CI: 95% confidence interval.
Suboptimal cytoreduction was performed in 18/80 (22.5%) patients. PCI < 20 was detected in 8/18 (44.4%), small intestinal ± mesentery invasion (region 9–12) justified suboptimal cytoreduction, the other involved regions in each patient showed respectable deposits without residual. The most common abdominal region found to be significantly associated with suboptimal cytoreduction in PCI < 20 was the small intestinal regions (9–12 region). (p < 0.01)
Pre-operative CT and laparoscopy PCI > 20 correctly predicted SCS in 10/18 (55.6%). Multiple regions were involved with residual deposits. Region 2 in three patients due to massive involvement of the lesser curvature of the stomach, Region 1 in four patients due to massive involvement of the right diaphragmatic peritoneum with serious technical difficulties, and in three patients surgery was aborted due to significant bleeding at the attempt of resection of pelvic sidewall invasion at Region 6. However, no significant region was involved in suboptimal cytoreduction (p > 0.05).
Discussion
Pre-operative CT and laparoscopy correctly predicted OCS in 83.3% of cases with PCI < 20 with an accuracy 85%, sensitivity 97%, and PPV 93%.
CT over- and underestimation rate of peritoneal carcinomatosis in our study was 12.5%, and 5%, respectively. Laparotomy achieved optimal cytoreduction in (12/14) 85.7% of the disconcordant cases. From a clinical point of view, overestimation was diagnosed if resulted in an intraoperative PCI > 20 that would exclude patient eligibility for CRS. We believe that these disconcordance were not clinically relevant as 85.7% of patients underwent optimal CRS.
CT and surgical PCI concordance have been previously described.3,4,12 Koh et al4 reported 7% overestimation in a small cohort of 19 patients; they did not report how the discordance of CT PCI altered patient's selection for CRS. Esquivel et al12 described relevant clinical discordance in 12% of cases.
Laghi et al ’s3 meta-analysis reported variable agreement between CT and open PCI scores ranging from 0.49 [95% CI (41–56%)]12 to 0.96.13 CT underestimation rate varied from 12 to 33%.4,12–15 Our high CT agreement was in line with previously reported.1,10,16–18
Our low underestimation rate can be explained by the high prevalence of large lesion size (LS score ≥ 2 was detected in 67.5% of all involved regions). We believe that the accuracy of CT depends mainly on radiologist experience rather than the technical aspect, however, all image in our study were reconstructed at 1.25 mm slice thickness.
Laparoscopic over- and underestimation rate of peritoneal carcinomatosis in our study was 2%, and 7.5%, respectively. Laparotomy achieved optimal cytoreduction in (6/10) 60% of these disconcordant cases. Our results were in line with Campbell et al and Garruto Campanile et al.16,17 In a recent prospective multicentric trial of Fagotti et al,19 they reported (4/120) 3.3% laparoscopic underestimation rate due to the presence of adhesions (prevent the access to the peritoneal cavity, or impaired complete exploration of the all abdominal regions), or diffuse carcinomatosis. Although we had no adhesions in our cases, we think adhesions significantly affect laparoscopic accuracy. In addition, laparoscopy may be unable to evaluate the retroperitoneal space, which may prevent optimal cytoreduction in many cases.8 However, there is low complication rate after laparoscopy; some of these complications (bowel and vascular injuries) can be severe.8 The reported clinical incidence of port-site tumor recurrence after laparoscopy varies from 2 to 6%.8 In our study, we have no significant complications after laparoscopy.
Our analysis indicated general good agreement between CT and laparoscopy in categorizing peritoneal carcinomatosis, it agreed with the recently published studies,16,17 , other reports have shown conflicting results.20–23 This discrepancy could be explained by several reasons; PCI reporting on CT is prone to error due to unclear boundaries between the 13 regions, especially for the small bowel. In addition, variable morphological presentations of peritoneal deposits (nodular, plaque-like, omental cake) increase the likelihood of inaccurate estimation of tumor size. PCI calculation depends purely on the summation of lesion size, which might be liable for high rates of intra and inter radiologist variability and may produce statistically significant discordance during its estimation. Total PCI is a summation of the observations in the 13 regions; it may be misleading due to its aggregating effect. We believe that regional peritoneal carcinomatosis is more relevant than total PCI score for surgical planning.
Reportedly, there were different radiological predictors of suboptimal cytoreduction in ovarian cancer patients.24–28 Axtell et al reported that diaphragmatic and large bowel mesenteric deposits represented significant predictors.27 Dowdy et al considered diffuse peritoneal thickening as the only significant predictor of suboptimal cytoreduction.28 Suidan et al11 considered small bowel infiltration as the most important predictor. Similarly, we found that diffuse small intestinal carcinomatosis is a significant predictor of suboptimal cytoreduction in patients with PCI > 20. It is known that the extent of small bowel involvement constitutes a limiting criterion in the surgical decision because enough small intestine is needed to maintain adequate oral nutrition in the future, extensive infiltration renders effective cytoreduction impossible.13,19,29–31 Irregular thickening, distortion, and fixation of mesenteric folds (frozen mesentery) could be introduced as an exclusive criterion in the selection process for CRS.13
In the case-control study of Rivard et al,32 they demonstrated a higher risk of unresectability in the presence of two or more concerning CT imaging features (intestinal obstruction, the involvement of pelvic sidewall, hepatic hilum involvement, retroperitoneal lymph nodes, ascites, hepatic metastases, and pulmonary metastases). They concluded that no specific CT sign could exclude a patient from an attempted CRS. Chandramohan et al33 described that involvement of unfavorable sites (at the epigastric, retroperitoneal, or pelvic regions) increases surgical complexity and may require input from a different surgical speciality. In our study, preoperative CT and laparoscopy correctly predicted OCS in (40/48) 83.3% of cases with PCI < 20, the remaining eight cases underwent suboptimal cytoreduction. It was justified in all eight cases by diffuse unrespectable small intestinal ± mesentery involvement (region 9–12) by scattered soft tissue nodules, its size ranging from 1 to 2 cm. The other involved regions in each patient showed resectable deposits without residual. In 14 patients with PCI > 20, optimal cytoreduction was achieved by additional diaphragm stripping in 7 patients, and visceral resection in 7 cases (distal pancreatectomy in 3 cases, and splenectomy in 4 cases). Accordingly, we believe that the peritoneal carcinomatosis extent is not the sole factor influencing the cytoreduction status; it also depends on the involved region and the surgical effort.
In a recent review of N.R. Gómez-Hidalgo et al,34 they stated that CT-based predictive models for prediction of optimal cytoreduction were accurate, however, they have not assessed for external validity. In our opinion, valid predictive model should include clinical, radiological, laparoscopic and surgical data to identify all parameters used in the clinical practice.
Laparoscopic accuracy was explored due to difficulties in predicting SCS based on imaging alone. A laparoscopic model based on a scoring system from 0 to 12 was described by Fagotti et al.35 They reported 67% optimal cytoreduction rate with good PPV and acceptable NPV for scores of <2 and>8. However, a variable rate of unnecessary exploration was performed for scores of 2–8. Brun et al36 performed an external validation of this score and reported 69% optimal cytoreduction rate with an accuracy of 60%. Recently, Petrillo et al37 reported 80% optimal cytoreduction rate by introducing upper abdominal surgery into their procedures. In our opinion, diagnostic laparoscopy is helpful before CRS in suspected advanced cases to predict the risk of residual disease and avoid futile laparotomies. Our results confirmed that PCI < 20 by combined preoperative CT and laparoscopy had an accuracy 85% in the prediction of OCS with PPV 93% which means that only 7% (1 − PPV) of cases are at risk for unnecessary laparotomy.
The National Comprehensive Cancer Network considered cytoreductive surgery the main primary treatment for ovarian cancer, the extent, and type of surgery depends on the cancer stage.38,39 At our study, optimal cytoreduction was achieved in 77.5% of cases with 47.5% post-operative morbidity and 0.0 post-operative mortality. Reportedly, the rates of optimal cytoreduction, post-operative morbidity and post-operative mortality ranging from 67 to 86%, 11.0 to 67.0% and 0.0 to 6.7%, respectively.24 We have no FIGO Stage IV cases in the current study; we think the surgical outcome may differ in this advanced stage.
The current US SGO and ASCO guidelines recommended interval-debulking surgery for the treatment of advanced epithelial ovarian cancer.40 At south egypt cancer institute, interval-debulking surgery was recommended for non-surgical candidates (hepatic metastasis in three or more hepatic segments, severe hepatic pedicle involvement, and diffuse infiltration of the small bowel) and patients with expected high complication rates (e.g. poor performance status, severe comorbidities).
Reportedly, neoadjuvant chemotherapy had a higher rate of optimal cytoreduction, lower perioperative morbidity and better quality of life compared to primary surgery in advanced cases,41,42 however, with comparable survival rate.43 Fagotti et al44 established a laparoscopic model for identification of patients who would benefit from interval CRS, they found that predictive index value > 4 had a PPV of 100% in the prediction of SOC with a probability equal to 0 for optimal cytoreduction at laparotomy. Chéreau et al45 described that a cut-off predictive index value of ≤4 had 95% sensitivity and 82% PPV in predicting optimal cytoreduction among females undergoing interval CRS.
In the current study, 10/18 (55.6%) patients with SCS had had Stage IIIB disease and preoperative PCI > 20, based on our results, and results of previous studies,41,42 it was better to send these 10 cases to neoadjuvant chemotherapy.
This study's strength, therefore, would be its prospective design. It provides meaningful criteria by combined CT and laparoscopy to predict which patients are a suitable candidate for optimal cytoreduction. Limitation of our study includes; our results represent tertiary cancer center experience with an experienced gynecologic, oncologist and dedicated radiologists; variable results may be achieved with different surgical skills and institutional experience. We recommended further studies to confirm our data and to determine their generalizability.
Conclusions
Both laparoscopy and CT are equally effective in preoperative peritoneal carcinomatosis categorization. PCI < 20 is accurate in the prediction of optimal cytoreduction. More than half of patients with suboptimal cytoreduction had PCI > 20 and interval debulking surgery can be recommended.
Footnotes
Disclosure: The scientific guarantor of this publication is DR: Shimaa Abdalla Ahmed. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethics approval: Institutional Review Board approval was obtained. Study subjects or cohorts overlap: no cohorts have been previously reported in our study. These findings were not presented at any meetings.
Contributor Information
Shimaa Abdalla Ahmed, Email: shimaaabdalla@aun.edu.eg.
Hisham Abou-Taleb, Email: hishamaboutaleb1@yahoo.com.
Noha Ali, Email: noha_soli_2010@yahoo.com.
Dalia M. Badary, Email: hamasat82@yahoo.com.
REFERENCES
- 1. Mazzei MA , Khader L , Cirigliano A , Cioffi Squitieri N , Guerrini S , Forzoni B , et al. . Accuracy of MDCT in the preoperative definition of peritoneal cancer index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC . Abdom Imaging 2013. ; 38 : 1422 – 30 . doi: 10.1007/s00261-013-0013-9 [DOI] [PubMed] [Google Scholar]
- 2. Jacquet P , Sugarbaker PH . Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis . : Sugarbaker P. H , Peritoneal carcinomatosis: principles of management . Boston, USA: : The British Institute of Radiology. ; 1996. . . 359 – 74 . [DOI] [PubMed] [Google Scholar]
- 3. Laghi A , Bellini D , Rengo M , Accarpio F , Caruso D , Biacchi D , et al. . Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: systematic review and meta-analysis . Radiol Med 2017. ; 122 : 1 – 15 . doi: 10.1007/s11547-016-0682-x [DOI] [PubMed] [Google Scholar]
- 4. Koh J-L , Yan TD , Glenn D , Morris DL . Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis . Ann Surg Oncol 2009. ; 16 : 327 – 33 . doi: 10.1245/s10434-008-0234-2 [DOI] [PubMed] [Google Scholar]
- 5. Iafrate F , Ciolina M , Sammartino P , Baldassari P , Rengo M , Lucchesi P , et al. . Peritoneal carcinomatosis: imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging . Abdom Imaging 2012. ; 37 : 616 – 27 . doi: 10.1007/s00261-011-9804-z [DOI] [PubMed] [Google Scholar]
- 6. Qi Z , Zhang Y , Dai Q , Xia Y , Jiang Y . Peritoneal carcinomatosis in primary ovarian cancer: ultrasound detection and comparison with computed tomography . Ultrasound Med Biol 2017. ; 43 : 1811 – 9 . doi: 10.1016/j.ultrasmedbio.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 7. Spiliotis J , Halkia E , De Bree E . Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy—current perspectives . Curr Oncol 2016. ; 23 : 266 – 75 . doi: 10.3747/co.23.2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeff N . Role of laparoscopy in initial tumour staging in advanced epithelial ovarian cancer: a systematic review . Pleura and Peritoneum 2018. ; 3 : 1 – 9 . doi: 10.1515/pp-2018-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suidan RS , Ramirez PT , Sarasohn DM , Teitcher JB , Mironov S , Iyer RB , Rudy S , Suidan a , Pedro T , Ramirez b , Debra c M , Jerrold c B , et al. . A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer . Gynecol Oncol 2014. ; 134 : 455 – 61 . doi: 10.1016/j.ygyno.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajan J , Kuriakose S , Rajendran VR , Sumangaladevi D . Radiological and surgical correlation of disease burden in advanced ovarian cancer using peritoneal carcinomatosis index . Indian J Gynecol Oncolog 2018. ; 16 : 7 . doi: 10.1007/s40944-018-0175-z [DOI] [Google Scholar]
- 11. Clavien PA , Sanabria JR , Strasberg SM . Proposed classification of complications of surgery with examples of utility in cholecystectomy . Surgery 1992. ; 111 : 518 – 26 . [PubMed] [Google Scholar]
- 12. Esquivel J , Chua TC , Stojadinovic A , Melero JT , Levine EA , Gutman M , et al. . Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study . J Surg Oncol 2010. ; 102 : 565 – 70 . doi: 10.1002/jso.21601 [DOI] [PubMed] [Google Scholar]
- 13. Courcoutsakis N , Tentes AA , Astrinakis E , Zezos P , Prassopoulos P . CT-Enteroclysis in the preoperative assessment of the small-bowel involvement in patients with peritoneal carcinomatosis, candidates for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy . Abdom Imaging 2013. ; 38 : 56 – 63 . doi: 10.1007/s00261-012-9869-3 [DOI] [PubMed] [Google Scholar]
- 14. Duhr CD , Kenn W , Kickuth R , Kerscher AG , Germer C-T , Hahn D , et al. . Optimizing of preoperative computed tomography for diagnosis in patients with peritoneal carcinomatosis . World J Surg Oncol 2011. ; 9 : 171 . doi: 10.1186/1477-7819-9-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Low RN , Barone RM , Lucero J . Comparison of MRI and CT for predicting the peritoneal cancer index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures . Ann Surg Oncol 2015. ; 22 : 1708 – 15 . doi: 10.1245/s10434-014-4041-7 [DOI] [PubMed] [Google Scholar]
- 16. Campbell J , Rothman RK , Sarwani N , Pameijer CR , Wong J , Laparoscopic Cof . Correlation of laparoscopic, open, and radiographic peritoneal cancer index scores in patients with peritoneal carcinomatosis . Journal of the American College of Surgeons 2016. ; 223 ( no. 4 ): e184 : e184 . doi: 10.1016/j.jamcollsurg.2016.08.465 [DOI] [Google Scholar]
- 17. Garruto Campanile R , Soleymani Majd H , Casarin J , Morotti M , Tozzi R . A prospective study on the diagnostic pathway of patients with stage IIIC-IV ovarian cancer: can laparoscopy improve CT-scan? Gynecologic Oncology 2018. ; 149 : 237 vol. p. . doi: 10.1016/j.ygyno.2018.04.534 [DOI] [Google Scholar]
- 18. Meleis MH , El-Agwany AMS . Peritoneal carcinomatosis index in advanced ovarian malignancy either by multislice CT verus laparotomy: a comparative study . Indian J Gynecol Oncolog 2015. ; 13 . doi: 10.1007/s40944-015-0028-y [DOI] [Google Scholar]
- 19. Fagotti A , Vizzielli G , De Iaco P , Surico D , Buda A , Mandato VD , et al. . A multicentric trial (Olympia–MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer . American Journal of Obstetrics and Gynecology 2013. ; 209 : 462.e1 – 462.e11 . doi: 10.1016/j.ajog.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 20. Marmor RA , Kelly KJ , Lowy AM , Baumgartner JM . Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery . Ann Surg Oncol 2016. ; 23 : 1461 – 7 . doi: 10.1245/s10434-015-4958-5 [DOI] [PubMed] [Google Scholar]
- 21. Shivkumaran S , Mandakulutur G , Banavara K . Role of staging laparoscopy to evaluate feasibility of performing optimal cytoreductive surgery in epithelial ovarian cancers . Int J Res Med Sci 2016. ; 4 : 5099 – 102 . doi: 10.18203/2320-6012.ijrms20164062 [DOI] [Google Scholar]
- 22. Denzer U , Hoffmann S , Helmreich-Becker I , Kauczor HU , Thelen M , Kanzler S , et al. . Minilaparoscopy in the diagnosis of peritoneal tumor spread: prospective controlled comparison with computed tomography . Surg Endosc 2004. ; 18 : 1067 – 70 . doi: 10.1007/s00464-003-9139-0 [DOI] [PubMed] [Google Scholar]
- 23. El-Agwany AS . Laparoscopy and computed tomography imaging in advanced ovarian tumors: a roadmap for prediction of optimal cytoreductive surgery . Gynecol Minim Invasive Ther 2018. ; 7 : 66 – 9 . doi: 10.4103/GMIT.GMIT_1_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llueca A , Serra A , Rivadulla I , Gomez L , Escrig J . Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index . World J Surg Oncol 2018. ; 16 : 37 . doi: 10.1186/s12957-018-1339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer JI , Kennedy AW , Friedman R , Ayoub A , Zepp RC . Ovarian carcinoma: value of CT in predicting success of debulking surgery . AJR Am J Roentgenol 1995. ; 165 : 875 – 8 . doi: 10.2214/ajr.165.4.7676985 [DOI] [PubMed] [Google Scholar]
- 26. Bristow RE , Duska LR , Lambrou NC , Fishman EK , O'Neill MJ , Trimble EL , et al. . A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography . Cancer 2000. ; 89 : 1532 – 40 . doi: [DOI] [PubMed] [Google Scholar]
- 27. Axtell AE , Lee MH , Bristow RE , Dowdy SC , Cliby WA , Raman S , et al. . Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer . JCO 2007. ; 25 : 384 – 9 . doi: 10.1200/JCO.2006.07.7800 [DOI] [PubMed] [Google Scholar]
- 28. Dowdy SC , Mullany SA , Brandt KR , Huppert BJ , Cliby WA . The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma . Cancer 2004. ; 101 : 346 – 52 . doi: 10.1002/cncr.20376 [DOI] [PubMed] [Google Scholar]
- 29. Roviello F , Caruso S , Marrelli D , Pedrazzani C , Neri A , De Stefano A , et al. . Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments . Surg Oncol 2011. ; 20 : e38 – 54 . doi: 10.1016/j.suronc.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 30. Yan TD , Sim J , Morris DL . Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy . Ann Surg Oncol 2007. ; 14 : 1807 – 17 . doi: 10.1245/s10434-007-9350-7 [DOI] [PubMed] [Google Scholar]
- 31. Cotte E , Passot G , Gilly F-N , Glehen O . Selection of patients and staging of peritoneal surface malignancies . World J Gastrointest Oncol 2010. ; 2 : 31 – 5 . doi: 10.4251/wjgo.v2.i1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivard JD , Temple WJ , McConnell YJ , Sultan H , Mack LA . Preoperative computed tomography does not predict resectability in peritoneal carcinomatosis . Am J Surg 2014. ; 207 : 760 – 5 . doi: 10.1016/j.amjsurg.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 33. Chandramohan A , Thrower A , Smith SA , Shah N , Moran B . "PAUSE": a method for communicating radiological extent of peritoneal malignancy . Clin Radiol 2017. ; 72 : 972 – 80 . doi: 10.1016/j.crad.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 34. Gómez-Hidalgo NR , Martinez-Cannon BA , Nick AM , Lu KH , Sood AK , Coleman RL , et al. . Predictors of optimal cytoreduction in patients with newly diagnosed advanced-stage epithelial ovarian cancer: time to incorporate laparoscopic assessment into the standard of care . Gynecologic Oncology 2015. ; 137 : 553 – 8 . doi: 10.1016/j.ygyno.2015.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fagotti A , Ferrandina G , Fanfani F , Ercoli A , Lorusso D , Rossi M , et al. . A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study . Ann Surg Oncol 2006. ; 13 : 1156 – 61 . doi: 10.1245/ASO.2006.08.021 [DOI] [PubMed] [Google Scholar]
- 36. Brun J-L , Rouzier R , Uzan S , Daraï E . External validation of a laparoscopic-based score to evaluate resectability of advanced ovarian cancers: clues for a simplified score . Gynecol Oncol 2008. ; 110 : 354 – 9 . doi: 10.1016/j.ygyno.2008.04.042 [DOI] [PubMed] [Google Scholar]
- 37. Petrillo M , Vizzielli G , Fanfani F , Gallotta V , Cosentino F , Chiantera V , et al. . Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: proof of a concept . Gynecologic Oncology 2015. ; 139 : 5 – 9 . doi: 10.1016/j.ygyno.2015.07.095 [DOI] [PubMed] [Google Scholar]
- 38. Aletti GD , Peiretti M . Quality control in ovarian cancer surgery . Best Pract Res Clin Obstet Gynaecol 2017. ; 41 : 96 – 107 . doi: 10.1016/j.bpobgyn.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 39. Hacker NF . State of the art of surgery in advanced epithelial ovarian cancer . Annals of Oncology 2013. ; 24 ( Suppl 10 ): x27 – 32 . doi: 10.1093/annonc/mdt465 [DOI] [PubMed] [Google Scholar]
- 40. Wright AA , Bohlke K , Armstrong DK , Bookman MA , Cliby WA , Coleman RL , et al. . Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of gynecologic oncology and American Society of clinical oncology clinical practice guideline . JCO 2016. ; 34 : 3460 – 73 . doi: 10.1200/JCO.2016.68.6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang L , Zhang B , Xing G , Du J , Yang B , Yuan Q , et al. . Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: a meta-analysis of peri-operative outcome . PLoS One 2017. ; 12 : e0186725 : e0186725 . doi: 10.1371/journal.pone.0186725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fagotti A , Ferrandina G , Vizzielli G , Fanfani F , Gallotta V , Chiantera V , et al. . Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (scorpion trial): final analysis of peri-operative outcome . Eur J Cancer 2016. ; 59 : 22 – 33 . doi: 10.1016/j.ejca.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 43. Lee YJ , Chung YS , Lee J-Y , Nam EJ , Kim SW , Kim S , et al. . Impact of the time interval from completion of neoadjuvant chemotherapy to initiation of postoperative adjuvant chemotherapy on the survival of patients with advanced ovarian cancer . Gynecologic Oncology 2018. ; 148 : 62 – 7 . doi: 10.1016/j.ygyno.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 44. Fagotti A , Fanfani F , Vizzielli G , Gallotta V , Ercoli A , Paglia A , et al. . Should laparoscopy be included in the work-up of advanced ovarian cancer patients attempting interval debulking surgery? Gynecol Oncol 2010. ; 116 : 72 – 7 . doi: 10.1016/j.ygyno.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 45. Chereau E , Lavou V , BallesterM CC , Selle F , Cortez A , et al. . External validation of a laparoscopic-based score to evaluate resectability for patients with advanced ovarian cancer undergoing interval debulking surgery . Anticancer Res 2011. ; 12 : 4469 – 74 . [PubMed] [Google Scholar]