Abstract
Objectives:
To investigate the clinical outcomes of fully covered self-expanding metal stent (FCSEMS) placement in patients with malignant esophageal obstruction who survived longer than 6 months.
Methods:
From January 2002 to January 2018, 88 FCSEMS were placed in 64 patients (mean age 62.9 ± 11.6 years; 58 males) with inoperable malignant esophageal obstruction with or without esophago-respiratory fistula. Only patients who survived more than 6 months with FCSEMS in place were included. Data regarding technical and clinical success, complications, reinterventions, stent patency, and patient survival were obtained from a prospectively maintained hospital database.
Results:
The technical and clinical success rates were 100 % (64/64). During follow-up, the median dysphagia score significantly improved (3.09 ± 0.68 to 1.05 ± 0.60, p < 0.001). The complication rate was 48.8 %. Multivariate analysis revealed that only longer stenting duration was associated with complications [hazard ratio = 1.220, 95 % confidence interval (CI) (1.074–2.760), p = 0.039]. The median follow-up duration was 257 days (range, 181–969). The median stent patency duration was 289 days [95% CI (209.9–368.1)]. The median survival was 254 days [95% CI (219.7–288.3)].
Conclusions:
Our data suggest that esophageal FCSEMS placement is an effective option for patients with malignant dysphagia when survival longer than 6 months is expected. The rate of complications increases with time, and SEMS development is needed to keep up with the advancement in oncological treatment.
Advances in knowledge:
Fully covered esophageal self-expandable stent placement is effective in patients surviving more than 6 months, however, the rate of complications also increases.
SEMS development is needed to cope with the advancement in oncological treatment.
Introduction
Self-expanding metal stents (SEMSs) provide rapid and effective relief of dysphagia for patients with malignant esophageal obstruction.1 Covered SEMSs are the primary type used currently with two categories available: fully covered (FC) and partially covered (PC) SEMSs.2 The latest European guideline recommends placement of either PCSEMS or FCSEMS for malignant esophageal obstruction, with no preference given to either.2 A recent randomized trial comparing PCSEMS with FCSEMS for malignant esophageal obstruction found no differences in the SEMS-related complications and recurrent obstruction rates between both types.3 However, a recognized drawback of PCSEMSs is the possibility of a difficult removal.4 Removal may be indicated in some cases, e.g. patients with severe retrosternal pain that cannot be alleviated with analgesics. In these cases, stent removal can be achieved by placing FCSEMS inside the previously placed PCSEMS (stent-in-stent technique) which should only be attempted by experienced endoscopists. Therefore, some studies recommend using FCSEMSs as the preferred option.4–7
Despite the effectiveness of SEMSs in managing dysphagia, complications do occur, particularly in patients with a better prognosis and prolonged survival.8,9 Recently, the advancement of the palliative treatment of squamous cell esophageal carcinoma led to increased survival (up to 19 months in some cases).8 The incidence of long-term esophageal SEMS-related complications varies widely, with reported values ranging from 20 to 63.5%.8,10 Most data though come from relatively small cohorts of patients with reported median stenting times shorter than 3 months.10,11 To our knowledge, only two studies have investigated SEMS-related complications in patients with esophageal malignancy who survived longer than 6 months.8,12 However, most of the SEMSs used in these two studies were PCSEMSs. To date, as far as we know, no published studies have investigated the long-term outcomes of FCSEMSs in malignant esophageal obstruction. Therefore, the purpose of our study was to investigate the clinical outcomes of FCSEMS placement among patients with malignant esophageal obstruction who survived longer than 6 months with FCSEMSs in place.
methods and materials
Patient population
This retrospective study received institutional review board approval (2018-0206), and the need to obtain written informed consent was waived. From January 2002 to January 2018, 322 FCSEMS were placed under fluoroscopic guidance in 270 patients. Patients who had FCSEMS for malignant esophageal obstruction with or without esophago-respiratory fistula (ERF) and survived longer than 6 months were included. A total of 64 patients were included in our study. The patients’ characteristics are summarized in Table 1.
Table 1.
Patients characteristics
| Characteristics | Number (n = 64) | % |
| Mean age, years (mean ± SD) | 62.9 ± 11.6 | |
| Gender (males/females) | 58/6 | 90.6/9.4 |
| Length of the strictures in cm (mean ± SD) | 5.6 ± 1.6 | |
| Dysphagia score before FCSEMS (mean ± SD) | 3.1 ± .68 | |
| Fistula before FCSEMS (yes/no) | 6/58 | 9.4 |
| Tumor origin | ||
| Esophageal | 44 | 68.8 |
| Gastric | 15 | 23.4 |
| External malignancy | 5 | 7.8 |
| Tumor location | ||
| Upper | 3 | 4.7 |
| Mid | 24 | 37.5 |
| Lower | 18 | 28.1 |
| GEJ | 19 | 29.7 |
| Tumor histology | ||
| SCC | 44 | 69.8 |
| Adenocarcinoma | 19 | 30.2 |
| Other | 1 | 1.56 |
| Indication for SEMS placement | ||
| Esophageal lesion | 54 | 84.4 |
| Fistula with respiratory tract | 6 | 9.4 |
| External compression | 4 | 6.2 |
| Eastern Cooperative Oncology Group scale score | ||
| 0 | 29 | 45.3 |
| 1 | 21 | 32.8 |
| 2 | 9 | 14 |
| 3 | 5 | 7.8 |
| Therapy before FCSEMS | ||
| Radiotherapy (yes/no) | 19/45 | |
| Chemotherapy (yes/no) | 36/28 | |
| Therapy after FCSEMS | ||
| Radiotherapy (yes/no) | 6/58 | |
| Chemotherapy (yes/no) | 21/43 | |
| TNM staging | ||
| 2 | 9 | |
| 3 | 13 | |
| 4 | 24 |
FCSEMS, fully covered self-expandable metallic stent; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma; SD, standard deviation; TNM, tumor/nodes/metastasis; Tx, unknown tumor stage.
FCSEMS and techniques of stent placement and removal
Two types of FCSEMSs were used in our study, the Niti-S single stent (Taewoong-Medical Co., Ltd., Ilsan, Korea) and the EGIS (S&G Biotech, Seongnam, Korea)5,7,13 (Figure 1). The Niti-S was developed from a single 0.2 mm thread of nitinol wire that had a tubular configuration and an inner polytetrafluoroethylene (PTFE) coating. The stent consisted of three parts: head, body, and tail. The head and tail parts were 24 mm in diameter, and the body part of the stent was 16 or 18 mm in diameter when fully expanded. The head and tail were attached to the body at a right angle to prevent migration. The EGIS was knitted from two threads of nitinol wire with a diameter of 0.154 and 0.127 mm, respectively. It had a tubular configuration with an outer PTFE coating. The flared ends had a unique "double-stepped" shoulders design to reduce migration. The outer shoulders were 24–28 mm in diameter, and the inner shoulders were 20–24 mm in diameter; the shaft was 16–20 mm in diameter when fully expanded. A drawstring made of nylon monofilament was attached to the proximal and distal inner margin of the FCSEMSs to facilitate removal.
Figure 1.
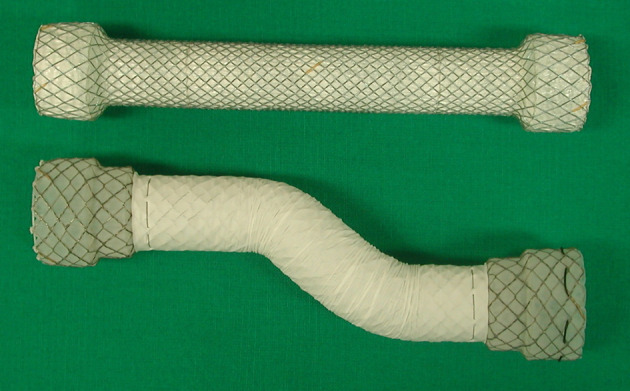
Photograph shows the Niti-s (upper), and the EGIS fully covered esophageal self-expanding metal stents.
All FCSEMSs were placed under fluoroscopic guidance without sedation. Topical anesthesia of the pharynx with lidocaine spray was performed before the procedure. The techniques used for SEMS placement and removal have been described in detail elsewhere.7,14 In summary, a 0.035-inch exchange stiff guidewire (Radiofocus Guidewire M; Terumo Corp., Tokyo, Japan) was introduced through the mouth across the obstruction into the stomach. FCSEMSs were placed using a 6 mm introducer system. If complications occurred, the FCSEMSs were removed using a retrieval set.14 The retrieval set consisted of a 13-Fr sheath, a 10-Fr dilator, a hook wire, and a 0.035-inch guide wire. Esophagography with water-soluble contrast medium was performed immediately after stent placement or removal to assess the esophageal patency or complications. When the patients showed good contrast passage right after stent placement, they were placed on a liquid diet for 1–3 days.
Follow-up
All patients underwent additional esophagography 1–3 days after FCSEMS placement to evaluate FCSEMS expansion and position. Clinical examination and esophagography were performed to assess patient condition and to evaluate FCSEMS patency and position at 4 weeks after FCSEMS placement. Thereafter, follow-up was based on monthly examinations in the outpatient clinic. Further esophagography was performed only for the patients with recurrent dysphagia, and reintervention was considered.
Study definitions
Technical success was defined as the successful placement of an FCSEMS in the proper position and good passage of contrast medium through the FCSEMS into the stomach under fluoroscopic guidance. Dysphagia was scored on a 5-point scale: 0, normal swallowing; 1, ability to swallow a semisolid diet; 2, ability to swallow a soft diet; 3, ability to swallow liquids only; and 4, complete dysphagia.7 Clinical success was defined as dysphagia score improvement by at least 1 grade within 7 days after FCSEMS placement. Recurrence of obstructive symptoms was defined as a worsening by at least one grade in the dysphagia score. Complications were defined according to the Society of Interventional Radiology Clinical Practice Guidelines.15 If the patient had a recurrence of a complication after initial management, it was considered a new complication. FCSEMS patency was defined as the period from the initial placement to the recurrence of obstructive symptoms caused by obstruction or migration. Patients were censored if no recurrent obstructive symptoms occurred during the patient’s lifetime. Overall survival was defined as the time from FCSEMS placement to death.
Statistical analysis
For categorical comparison of data, the χ 2 and Fisher’s exact tests were used, as appropriate. Dysphagia scores before and after FCSEMS placement were analyzed using the Wilcoxon signed-rank test. Time-to-event distributions were estimated using the Kaplan–Meier method and compared using the logrank test. Univariate analysis by Cox regression was used to explore the correlation between the predictor variables and the outcome variable (complications). The variables with p < 0.1 were included in the multivariate analysis by Cox regression. A two-sided p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS, v. 24.0 (IBM Corp., Armonk, NY).
Results
Technical and clinical outcomes
A total of 88 FCSEMSs were placed in 64 patients. 58 patients received Niti-S FCSEMSs, and the remaining 6 patients received EGIS FCSEMSs. FCSEMS placement was technically successful in all patients (100%), with no procedure-related complications. Clinical success was achieved in all patients (100%) after FCSEMS placement. During follow-up, the mean dysphagia score significantly improved from 3.09 ± 0.68 to 1.05 ± 0.60 (p < 0.001). In the six patients with ERF, the fistula tracts were completely sealed after FCSEMS placement.
Complications and re-interventions
Complications occurred in 31 (48.4%) of the 64 patients. Four patients had more than one complication. A total of 36 complications (mean of 1.2 complications per patient) occurred 17–345 (median, 183) days after FCSEMS placement. There were no deaths secondary to FCSEMS placement. Most complications (n = 31, 86%) were minor and successfully managed under fluoroscopic guidance by an interventional radiologist. ERF, which is a potentially life-threatening complication, occurred in five patients (14%). The ERFs were successfully managed by a second FCSEMS placement (n = 4) or a gastrostomy tube insertion (n = 1). The patients who received the EGIS FCSEMSs had four events of tumor overgrowth and one event of FCSEMS migration, and all were successfully managed by a second FCSEMS placement. One case had tumor ingrowth although the stent due to separation of the PTFE membrane. When a second SEMS was needed to treat a complication, and the first SEMS was still in place, it was placed inside the original SEMS (stent in stent). Complications and related secondary treatments are summarized in Tables 2 and 3.
Table 2.
Complications and secondary treatment
| Complication (n) | Time, range in days (median) | Secondary treatment |
| Tumor overgrowth (14) | 54–337 (170) | Second stent (14) |
| Migration (10) | 17–296 (206) | Removal and second stent (2), Second stent (3), None (5) |
| Fistula (5) | 99–308 (180) | Second stent (4), Gastrostomy (1) |
| Food impaction (4) | 65–345 (206) | Balloon (3), Removal and second stent (1) |
| Granulation tissue (2) | 183, 245 | Removal (1), Second stent (1) |
| Tumor ingrowth a (1) | 274 | None (1) |
The patient had a Niti-S FCSEMS; the PTFE membrane was separated from the stent and tumor invaded the central part of the SEMS.
Table 3.
Patients who had more than one complication and reintervention
| Patient number | Diagnosis | First complication (days) | Reintervention |
Second complication
(days) |
Reintervention | Third complication (days) | Survival (days) |
| 1 | SCC | ERF (180) | Second FCSEMS | Migration (7) | Third FCSEMS | Migration (266) a | 582 |
| 2 | SCC | Tumor overgrowth (163) | Second FCSEMS | Food impaction (212) | Balloon dilation | - | 368 |
| 3 | SCC | Tumor overgrowth (175) | Second FCSEMS | Tumor overgrowth (164) | Third FCSEMS | - | 377 |
| 4 | ACG | Granulation tissue (183) | Second FCSEMS | EFR (136) | Third FCSEMS | - | 415 |
ACG, gastric adenocarcinoma; ERF, esophago-respiratory fistula; FCSEMS, fully covered self-expanding metallic stent SCC, squamous cell carcinoma.
Patient had gastrostomy tube
Risk factors for complications
The univariate analysis showed that performance status, stent diameter, stent duration, and chemotherapy or radiotherapy before FCSEMS placement increased the risk of complications. However, on multivariate analysis, only stenting duration was associated with an increased risk of complications. Patients with a longer stenting duration were more likely to experience complications [hazard ratio (HR)=1.220, 95% confidence interval (CI) (1.074–2.760), p = 0.039]. There was no statistically significant association between complications and patient age, tumor characteristics, and chemotherapy or radiotherapy after FCSEMS placement (Table 4).
Table 4.
Univariate and multivariate analysis by Cox regression of the risk factors for complications
| Univariate | Multivariate | |||||
| Variables | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value |
| Age | 0.978 | 0.944–1.013 | 0.213 | |||
| Tumor origin (esophagus/extraesophageal) | 1.405 | 0.423–4.669 | 0.579 | |||
| Lesion across GEJ | 0.966 | 0.425–2.195 | 0.933 | |||
| Tumor location (upper/distal) | 0.882 | 0.423–1.840 | 0.737 | |||
| Histological type (adenocarcinoma/SCC) | 1.451 | 0.647–3.127 | 0.342 | |||
| TNM | 0.571 | 0.282–2.010 | 0.571 | |||
| Performance status (0/1–3) | 2.498 | 1.083–5.760 | 0.032 | 2.25 | 0.949–5.340 | 0.065 |
| Stricture length | 0.853 | 0.684–1.065 | 0.162 | |||
| FCSEMS length | 0.923 | 0.744–1.145 | 0.466 | |||
| FCSEMS diameter | 0.399 | 0.147–1.081 | 0.071 | 0.535 | .194–1.477 | 0.492 |
| Fistula (yes/no) | 0.329 | 0.044–2.427 | 0.275 | |||
| Chemotherapy before FCSEMS (yes/no) | 2.845 | 1.296–6.243 | 0.009 | 2.886 | 1.142–7.293 | 0.093 |
| Chemotherapy after FCSEMS (yes/no) | 0.596 | 0.262–1.359 | 0.219 | |||
| Radiotherapy before FCSEMS (yes/no) | 2.240 | 1.066–4.710 | 0.033 | 1.001 | 0.394–2.546 | 0.660 |
| Radiotherapy after FCSEMS (yes/no) | 0.995 | 0.300–3.355 | 0.995 | |||
| Stenting duration | 0.996 | 0.992–1.0 | 0.03 | 1.220 | 1.074–2.760 | 0.039 |
CI, confidence interval;FCSEMS, fully covered self-expanding metallic stent; GEJ, gastroesophageal junction; SCC, squamous cell cancer; TNM, tumor/nodes/metastasis.
There was no significant difference in the migration rates between lesions crossing the gastroesophageal junction (GEJ) (4 events in 19 patients, 21%) compared with lesions at other parts of the esophagus (6 events in 45 patients, 13.3%), p = 0.477. The difference in migration rate among patients who received chemotherapy (20%) and patients who did not receive chemotherapy (10.7%) was not statistically significant (p = 0.45). Also, the migration rate among patients who underwent radiotherapy before stenting (21%) was higher than the migration rate among patients who did not undergo radiotherapy before stenting (11.1%), but this difference was not statistically significant (p = 0.23). However, radiotherapy before stenting significantly increased the rate of de novo fistula development (21% vs 2.2%, p = 0.006).
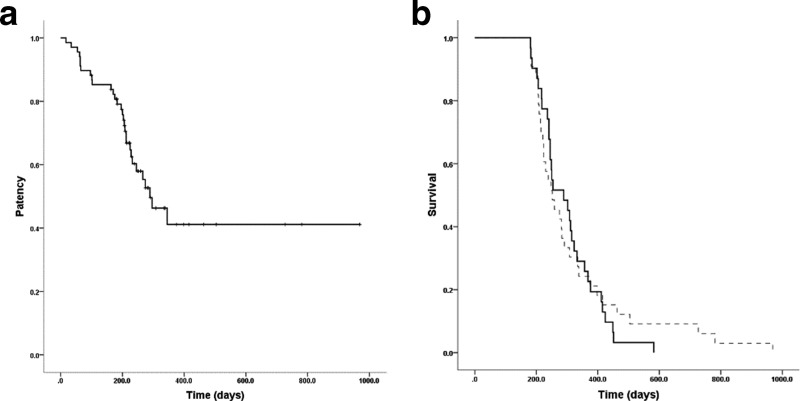
FCSEMS patency and patient survival
The median follow-up duration was 257 (range, 181–969) days. Only four patients had their FCSEMS removed during follow-up. The median stent patency was 289 days [95% CI (209.9–368.1)] (Figure 2a). The median overall survival was 254 days [95% CI (219.7–288.3)]. The median survival periods of the patients who had one complication and those with more than one complication were 247 and 391.5 days, respectively; this difference was not statistically significant (p = 0.57). The median survival periods for patients with or without complications were 289 days [95% CI (224.6–353.5)] and 252 days [95% CI (201.4–302.6)], respectively (Figure 2b). There was no significant difference in survival rate between patients with and without complications (p = 0.77).
Figure 2.
Kaplan–Meier analysis of (a) FCSEMS patency duration. (b) Overall patient survival showing no significant difference between the patients with (dotted line) or without complications. FCSEMS, fully covered self-expandingmetal stent.
Discussion
In this study, FCSEMS placement was technically successful and associated with significant dysphagia relief for all patients with malignant esophageal obstruction who survived more than 6 months after stent insertion. The complication rate was 48.4%, and most complications were minor and readily managed by re-interventions under fluoroscopic guidance. Patients who received radiotherapy before FCSEMS placement had a higher chance of developing an ERF. A longer stenting duration was the only risk factor associated with a higher complication rate.
SEMS-related complications remain a major concern, especially among patients with prolonged survival.4,16 Two studies have investigated the long-term outcomes of patients with malignant esophageal obstruction who had SEMSs in place for more than 6 months.8,12 Their complication rates were higher than our study (63.5 and 59% vs 48.4%), and one study had three deaths related to SEMS placement. In these two studies, the most commonly used SEMS were partially covered (89 and 71% of the cases). One study8 had a higher rate of tumor ingrowth or overgrowth (32 of 63 patients) compared to ours (15 of 64 patients), with a similar rate of migration (9 vs 10 patients in our study). The other study12 had a lower rate of tumor ingrowth or overgrowth, but the rate of migration was higher (36% vs 14% in our study). In our experience, FCSEMSs are associated with fewer complications, especially tumor ingrowth and overgrowth, especially when they remain in place for a longer time.
We believe that there is a cumulative increase in the rate of complications with long-term survival because there is more time for complications to occur and be observed. In our study, patients who experienced complications had a longer median survival than those who did not experience complications. Also, patients who experienced more than one complication had a longer median survival than those who had only one complication. Additionally, Cox regression analysis revealed that longer stenting duration was the only risk factor for complications on multivariate analysis [HR = 1.220, 95% CI (1.074–2.760) p = 0.039]. However, most complications were not life-threatening (86%) and were successfully managed under fluoroscopic guidance. Medeiros et al8 investigated 63 patients with malignant obstruction who had esophageal SEMSs in place for more than 6 months. In concordance with our results, they found that patients with stent-related adverse events survived longer than patients without adverse events (13.2 vs 7.9 months, p < .001). Moreover, Bick et al17 investigated the risk factors for stent-associated ERF and found that longer stenting duration increased the likelihood of SEMS-induced ERF.
Migration of the FCSEMS is a recognized drawback with reported rates of up to 60%.3,18,19 This is due to the lack of anchoring of the FCSEMS to the esophageal wall, especially when the FCSEMSs are placed across the gastroesophageal junction.2 Several modifications in the FCSEMS design have been developed to overcome this problem.20 In our experience, using FCSEMSs with the shouldered design—especially the new EGIS (S&G Biotech) with a unique "double-stepped" shoulders design—can decrease the rate of FCSMES migration.7,13 Park et al found an FCSEMS migration incidence of 12.6% among 332 patients with malignant esophageal obstruction.21 Patients with longer survival had a higher incidence of stent migration (odds ratio = 1.994, p < 0.001). In our study, FCSEMS migration was the second most frequent complication (9 of 64 patients, 14%) with one patient experiencing two migration events. In half of the cases, however, no further intervention was needed because the patients’ dysphagia improved. Medeiros et al reported similar results, as 9 of their 63 patients had SEMS migration.8 Interestingly, in their study all the cases with SEMS migration were PCSEMSs. Another factor that is thought to increase the migration rate of esophageal SEMSs is chemotherapy before or after SEMS placement.7,19 The most plausible explanation for this observation is that the chemotherapy results in shrinkage of the tumor and decreased anchoring of the SEMS to the esophageal wall. However, there remains some debate regarding the chemotherapy effect because other reports have found no relationship between chemotherapy and SEMS migration.22 In our study, we did not find an association between chemotherapy and SEMS migration. It worth mentioning that we did not find a significant increase in the migration rate with SEMSs placed across the GEJ. This may be due to the small number of patients (19 patients) which could have led to a sampling error.
ERF is a potentially life-threatening complication that occurs after esophageal SEMS placement.17 Previous studies have reported ERF rates of up to 9%; however, prolonged stenting may increase this rate to even 20% [8, 18].8,18 Medeiros et al8 reported 13 ERFs developing out of 63 patients, with 2 patients dying from pulmonary sepsis. In our study, ERF developed in 5 of 64 patients (7.8%) after a median duration of 180 days. All ERF patients were successfully managed by inserting another FCSEMS or a gastrostomy tube, and there were no fistula-related deaths. One study that investigated the risk factors of stent-associated ERFs found that ERFs occurred more frequently with lesions of the proximal and middle esophagus.17 This may be the reason that our study had fewer ERF cases than the study conducted by Medeiros et al because we had fewer patients with proximal and middle esophageal strictures (42.2 vs 66.7%). The radiotherapy effect of increasing fistula rates has been reported in several studies.7,23 In our study, patients who received radiotherapy before FCSEMS placement had a significantly higher rate of fistula development. Radiotherapy induces esophagitis, ulcerations, and ischemic damage of the esophageal wall that may cause esophageal perforations and ERFs.24 The pressure exerted by SEMSs on a damaged esophageal wall can increase the risk of necrosis and fistula development. Therefore, in patients with a history of previous radiotherapy, ERF should be anticipated and managed in a timely fashion if they do develop.
Previous reports have suggested factors that affect the occurrence of complications, including SEMS-related (e.g., length, diameter) or tumor-related (e.g. site, length and histological types) factors.20 We performed a Cox regression analysis to investigate the possible factors that affect the occurrence of complications associated with long-term esophageal stenting. Only patients with a prolonged stenting duration had an increased rate of complications. Rodriguez et al12 investigated 42 patients with malignant esophageal obstruction who survived more than 6 months. They found that the presence of tight malignant strictures (only transposable using an ultrathin gastroscope and not with a standard gastroscope) was the only factor associated with a higher rate of complications. They hypothesized that less tight strictures decreased SEMS apposition to the esophageal wall and increased the rate of migration, which was the main complication in their series. Medeiros et al8 found that poor performance status increased the rate of long-term SEMS-related complications. They hypothesized that poor performance status could be associated with malignancies that have aggressive biologic behavior, which could explain the higher rate of complications. In our opinion, however, this hypothesis is implausible because poor performance status is theoretically associated with shorter rather than longer survival. Further studies are warranted to clarify this association.
Our study has some limitations. It was a retrospective study with an inherent risk of bias. All the procedures were done by a single interventional radiologist at a tertiary referral center, which makes it difficult to generalize the outcomes, especially concerning the management of complications. Also, minor complications are difficult to assess, especially pain and gastroesophageal reflux. Additionally, the number of patients included in this study may be not have been adequate to detect some important associations that have previously reported. For example, there was no association between the migration rate and chemotherapy status or lesion location at the gastroesophageal junction. For the same reason, no difference was found between the two types of FCSEMS. However, this study gives a better understanding of the long-term outcomes associated with esophageal FCSEMS placement in a relatively large cohort of patients with malignant dysphagia.
In conclusion, esophageal FCSEMS placement is effective at relieving dysphagia in patients with advanced malignant disease who survive more than 6 months. The complication rate in our series was relatively high; however, most complications were minor and readily managed under fluoroscopic guidance with no effect on mortality. Also, the complications were lower than reported by similar studies that used PCSEMSs for most of the cases. Patients who received radiotherapy before FCSEMS placement had a higher risk of developing an ERF. Longer stenting duration was the only risk factor associated with a higher complication rate. Our data suggest that esophageal FCSEMS placement is an effective option for patients with malignant dysphagia when survival longer than 6 months is expected. The rate of complications increases with time, and SEMS development in needed to keep up with the advancement in oncological treatment.
Footnotes
Acknowledgment: This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant no. HI15C0484 to H.Y.S.). We would like to thank Prof. Richard A. Kozarek for his constructive criticism of this manuscript.
*Nader Bakheet and Jung-Hoon Park contributed equally to this work and are the co-first authors.
Contributor Information
Nader Bakheet, Email: dr.nader.gamal@gmail.com.
Jung-Hoon Park, Email: jhparkz1125@gmail.com.
Hong-Tao Hu, Email: hht19761213@gmail.com.
Sung Hwan Yoon, Email: ysungh0706@naver.com.
Kun Yung Kim, Email: kky2kkw@gmail.com.
Wang Zhe, Email: scorpiowangzhe@163.com.
Jae Yong Jeon, Email: jyjeon71@gmail.com.
Ho-Young Song, Email: hysong@amc.seoul.kr.
REFERENCES
- 1. Rustgi AK , El-Serag HB . Esophageal carcinoma . N Engl J Med 2014. ; 371 : 2499 – 509 . doi: 10.1056/NEJMra1314530 [DOI] [PubMed] [Google Scholar]
- 2. Sharma P , Kozarek R , Practice Parameters Committee of American College of Gastroenterology. . Role of esophageal stents in benign and malignant diseases . Am J Gastroenterol 2010. ; 105 : 258 – 73 . doi: 10.1038/ajg.2009.684 [DOI] [PubMed] [Google Scholar]
- 3. Didden P , Reijm AN , Erler NS , Wolters LMM , Tang TJ , Ter Borg PCJ , et al. . Fully vs. partially covered selfexpandable metal stent for palliation of malignant esophageal strictures: a randomized trial (the COPAC study) . Endoscopy 2018. ; 50 : 961 – 71 . doi: 10.1055/a-0620-8135 [DOI] [PubMed] [Google Scholar]
- 4. Vermeulen BD , Siersema PD . Esophageal stenting in clinical practice: an overview . Curr Treat Options Gastroenterol 2018. ; 16 : 260 – 73 . doi: 10.1007/s11938-018-0181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi SJ , Kim JH , Choi JW , Lim SG , Shin SJ , Lee KM , et al. . Fully covered, Retrievable self-expanding metal stents (Niti-S) in palliation of malignant dysphagia: long-term results of a prospective study . Scand J Gastroenterol 2011. ; 46 ( 7-8 ): 875 – 80 . doi: 10.3109/00365521.2011.571706 [DOI] [PubMed] [Google Scholar]
- 6. Na HK , Song H-Y , Kim JH , Park J-H , Kang MK , Lee J , et al. . How to design the optimal self-expandable oesophageal metallic stents: 22 years of experience in 645 patients with malignant strictures . Eur Radiol 2013. ; 23 : 786 – 96 . doi: 10.1007/s00330-012-2661-5 [DOI] [PubMed] [Google Scholar]
- 7. Park J-H , Song H-Y , Kim JH , Jung H-Y , Kim J-H , Kim S-B , et al. . Polytetrafluoroethylene-covered Retrievable expandable nitinol stents for malignant esophageal obstructions: factors influencing the outcome of 270 patients . AJR Am J Roentgenol 2012. ; 199 : 1380 – 6 . doi: 10.2214/AJR.10.6306 [DOI] [PubMed] [Google Scholar]
- 8. Medeiros VS , Martins BC , Lenz L , Ribeiro MSI , de Paulo GA , Lima MS , et al. . Adverse events of self-expandable esophageal metallic stents in patients with long-term survival from advanced malignant disease . Gastrointest Endosc 2017. ; 86 : 299 – 306 . doi: 10.1016/j.gie.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 9. Bedenne L , Michel P , Bouché O , Milan C , Mariette C , Conroy T , et al. . Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102 . J Clin Oncol 2007. ; 25 : 1160 – 8 . doi: 10.1200/JCO.2005.04.7118 [DOI] [PubMed] [Google Scholar]
- 10. Schoppmann SF , Langer FB , Prager G , Zacherl J . Outcome and complications of long-term self-expanding esophageal stenting . Dis Esophagus 2013. ; 26 : 154 – 8 . doi: 10.1111/j.1442-2050.2012.01337.x [DOI] [PubMed] [Google Scholar]
- 11. Homann N , Noftz MR , Klingenberg-Noftz RD , Ludwig D . Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate . Dig Dis Sci 2008. ; 53 : 334 – 40 . doi: 10.1007/s10620-007-9862-9 [DOI] [PubMed] [Google Scholar]
- 12. Rodrigues-Pinto E , Pereira P , Baron TH , Macedo G . Self-expandable metal stents are a valid option in long-term survivors of advanced esophageal cancer . Rev Esp Enferm Dig 2018. ; 110 : 500 – 4 . doi: 10.17235/reed.2018.5323/2017 [DOI] [PubMed] [Google Scholar]
- 13. Kim KY , Tsauo J , Song H-Y , Park J-H , Jun EJ , Zhou W-Z , et al. . Evaluation of a new esophageal stent for the treatment of malignant and benign esophageal strictures . Cardiovasc Intervent Radiol 2017. ; 40 : 1576 – 85 . doi: 10.1007/s00270-017-1677-2 [DOI] [PubMed] [Google Scholar]
- 14. Song H-Y , Lee DH , Seo T-S , Kim S-B , Jung H-Y , Kim J-H , et al. . Retrievable covered nitinol stents: experiences in 108 patients with malignant esophageal strictures . J Vasc Interv Radiol 2002. ; 13 : 285 – 92 . doi: 10.1016/S1051-0443(07)61722-9 [DOI] [PubMed] [Google Scholar]
- 15. Sacks D , McClenny TE , Cardella JF , Lewis CA . Society of interventional radiology clinical practice guidelines . J Vasc Interv Radiol 2003. ; 14 ( 9 Pt 2 ): S199 – S202 . doi: 10.1097/01.RVI.0000094584.83406.3e [DOI] [PubMed] [Google Scholar]
- 16. Hussain Z , Diamantopoulos A , Krokidis M , Katsanos K . Double-layered covered stent for the treatment of malignant oesophageal obstructions: systematic review and meta-analysis . World J Gastroenterol 2016. ; 22 : 7841 – 50 . doi: 10.3748/wjg.v22.i34.7841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bick BL , Song LMWK , Buttar NS , Baron TH , Nichols FC , Maldonado F , et al. . Stent-associated esophagorespiratory fistulas: incidence and risk factors . Gastrointest Endosc 2013. ; 77 : 181 – 9 . doi: 10.1016/j.gie.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 18. Vleggaar FP , Siersema PD . Expandable stents for malignant esophageal disease . Gastrointest Endosc Clin N Am 2011. ; 21 : 377 – 88 vii . doi: 10.1016/j.giec.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 19. Pellen MGC , Sabri S , Razack A , Gilani SQ , Jain PK . Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer . Dis Esophagus 2012. ; 25 : 48 – 53 . doi: 10.1111/j.1442-2050.2011.01206.x [DOI] [PubMed] [Google Scholar]
- 20. Kim KY , Tsauo J , Song HY , Kim PH , Park JH . Self-Expandable metallic stent placement for the palliation of esophageal cancer . J Korean Med Sci 2017. ; 32 : 1062 – 71 . doi: 10.3346/jkms.2017.32.7.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park J-H , Song H-Y , Shin JH , Cho YC , Kim JH , Kim SH , et al. . Migration of Retrievable expandable metallic stents inserted for malignant esophageal strictures: incidence, management, and prognostic factors in 332 patients . AJR Am J Roentgenol 2015. ; 204 : 1109 – 14 . doi: 10.2214/AJR.14.13172 [DOI] [PubMed] [Google Scholar]
- 22. Homs MYV , Hansen BE , van Blankenstein M , Haringsma J , Kuipers EJ , Siersema PD . Prior radiation and/or chemotherapy has no effect on the outcome of metal stent placement for oesophagogastric carcinoma . Eur J Gastroenterol Hepatol 2004. ; 16 : 163 – 70 . doi: 10.1097/00042737-200402000-00007 [DOI] [PubMed] [Google Scholar]
- 23. Lecleire S , Di Fiore F , Ben-Soussan E , Antonietti M , Hellot M-F , Paillot B , et al. . Prior chemoradiotherapy is associated with a higher life-threatening complication rate after palliative insertion of metal stents in patients with oesophageal cancer . Aliment Pharmacol Ther 2006. ; 23 : 1693 – 702 . doi: 10.1111/j.1365-2036.2006.02946.x [DOI] [PubMed] [Google Scholar]
- 24. Spaander MCW , Baron TH , Siersema PD , Fuccio L , Schumacher B , Escorsell Àngels , et al. . Esophageal stenting for benign and malignant disease: European Society of gastrointestinal endoscopy (ESGE) clinical guideline . Endoscopy 2016. ; 48 : 939 – 48 . doi: 10.1055/s-0042-114210 [DOI] [PubMed] [Google Scholar]