Abstract
OBJECTIVE:
To assess the biological effects of injection on the viability and metabolic activity of culture-expanded, human adipose derived mesenchymal stromal/stem cells (AMSCs).
DESIGN:
Prospective observational pilot study.
SETTING:
Academic medical center.
PARTICIPANTS:
Patient-derived clinical-grade culture expanded AMSCs.
INTERVENTIONS:
AMSCs were passed through syringes without a needle attached (control), with an 18-gauge (25.4mm) needle attached and with a 30-gauge (19mm) needle attached at a constant injection flow rate and constant cell concentrations. Each injection condition was completed in triplicate.
MAIN OUTCOME MEASURES:
Cell number and viability, proliferative capacity, metabolic activity, and acute gene expression as measured by cell counts, mitochondrial activity, and quantitative real time reverse-transcriptase polymerase chain reaction (qRT-PCR) on day 0 (immediately), day 1, and day 4 after injection.
RESULTS:
AMSC viability was not significantly affected by injection and cells proliferated normally regardless of study group. Post-injection, AMSCs robustly expressed both proliferation markers and extracellular matrix proteins. Stress-response mRNAs were markedly but transiently increased independently of needle size within the first day in culture post- injection.
CONCLUSIONS:
Human, culture expanded AMSCs maintain their viability, proliferative capacity and metabolic function following passage through needles as small as 30 gauge at constant flow rates of 4 ml/min, despite an early, non-specific stress/cytoprotective response. These initial findings suggest that culture expanded AMSCs should tolerate the injection process during most cell-based therapeutic interventions.
Keywords: cell therapy, stem cell, transplant, regenerative medicine, joint degeneration, osteoarthritis, tendinopathy
INTRODUCTION
Since the initial characterization of mesenchymal stromal/stem cells (MSCs) by Friedenstein and colleagues half a century ago1, 2, these cells have been isolated from a variety of human tissues3–8. In addition to their capacity for proliferation and multi-lineage differentiation, MSCs possess potent anti-inflammatory, immunomodulatory, anti-apoptotic and trophic capabilities9–19. Recently, MSC-based therapies have demonstrated potential benefits in the treatment of a wide range of medical conditions, including myocardial infarction, kidney injury, multiple sclerosis, type I diabetes, and spinal cord injury11, 12, 16, 20–26. Our group focuses on clinical applications of adipose-tissue derived mesenchymal stromal/stem cells (AMSCs) cultured in platelet lysate for joint regeneration and repair of skeletal tissues13–15.
Osteoarthritis (OA) is the most common adult joint disease and currently affects 27 million individuals in the United States27, 28. OA is characterized by cartilage loss, changes in subchondral bone, synovitis, and skeletal deformity29–33. The economic burden of OA is high, with over 185 billion dollars spent annually to diagnose and treat patients suffering from OA34. These costs are expected to rise with the aging population, presenting a need to develop effective conservative treatments35. MSCs have potential therapeutic value in OA because they can reduce inflammation and programmed cell death (apoptosis), and in theory are capable of restoring cartilaginous tissue damaged by OA or traumatic injury9, 10, 18, 19.
Preliminary clinical and translational studies suggest that both culture-expanded bone marrow-derived mesenchymal stromal/stem cells (BMSCs) and AMSCs may diminish OA symptoms and possibly heal tissues in some individuals. Several reports have indicated that injections of BMSCs into patients with moderate to severe knee OA may improve pain by 40– 90% within two to six months without serious adverse events36–39. In some patients, magnetic resonance imaging (MRI) also revealed evidence for meniscal or hyaline cartilage regeneration. In a more recent study, a single, variable dose, AMSC injection in patients with moderate to severe knee OA improved pain by approximately 50% without serious adverse events40. Moreover, patients receiving the highest cell dose (100 million cells) exhibited evidence of cartilage healing, consistent with the potential disease-modifying effects of AMSCs40. Although further investigation is needed to explore the relative therapeutic benefit of AMSCs vs BMSCs for OA, AMSCs do offer several advantages over BMSCs, including their relative ease of harvest, higher yield, and resistance to age-related declines in proliferative potential and cellular function11–13, 16, 40–42.
For MSCs to have therapeutic affects for OA, they must be successfully delivered into the joint with minimal effects on viability and function. However, during needle expulsion, MSCs may experience levels of fluid pressure and shear stress beyond those that they naturally encounter in the body. Prior studies have documented adverse effects of shear stresses applied to red blood cells by a rotational viscometer43. Because the approximate diameter of MSCs can range up to 20 to 30 μm it can be expected that MSCs will experience fluid shear stresses as they pass through needles commonly used for clinical care, and these stresses vary as a function of needle bore diameter via the Hagen-Poiseuille equation seen below (i.e., gauge)44. Despite the expectation that fluid shear stress may compromise cell viability, few studies have investigated the biological effects of needle gauge on human MSCs (Table 1)45–48. Furthermore, no previous study has investigated the biological effects of needle passage on human AMSCS.
Table 1:
Studies Examining the Effect of “Injection” on Mesenchymal Stem Cell (MSC) Viability or Function.
| Author (year) | Cell Source | Needle Size Tested | Flow Rate (/min) | Concentration (cells/mL) | Time Point Studied | Outcome | Results |
|---|---|---|---|---|---|---|---|
| Walker et al. (2010) | Murine and Human Bone Marrow | 20G 25.4mm 25G 25.4mm 30G 25.4mm Control: uninjected cells | 1ml, 2ml, 4ml, 8.3ml | 1 × l06 | Immediate Post-injection, 24hrc | Viabilityd Phenotypee Differentiationf | For human, 25G and 30G reduced viability 24hr post-injection at all flow rates versus control. No effects on function or terminal differentiation ability. |
| Garvican et al. (2014) | Equine Bone Marrow | 19G 50mm 21G 50mm 23G 50mm Control: uninjected cells |
15ml | 5 × l06 | Immediate Post-injection, 2hr, 4hr, 24hrj | Viabilityk Differentiationm | 21G and 23G resulted in increased apoptosis immediately, whereas 19G resulted in increased apoptosis at 2hr mark. All gauges reduced metabolic activity for first 2 hours post-injection. No change in differentiation at any time point. |
= 26s-gauge needles have inner diameter of 0.127 mm where as a standard 26-gauge needles have inner diameter of 0.260 mm.
= Live/Dead Solution (Iivitrogen, Paisley, UK), CellTiter AQ One Solution Cell Proliferation assay kit (Promega, Southampton, UK), and CaspACE Assay System (Promega, Southampton, UK)
= Propidium iodide, Thiazole orange, Fluorescein-isothiocyanate-conjugated Annexin V (BD Sciences, San Jose, CA)
= Viability was tested immediately and at 24hrs. Phenotyping was tested only immediately after injections. Terminal differentiation timeline was not explicitly mentioned.
= Antibodies for CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1 (manufacture information not reported)
= Alizarin red, Oil Red (Chemicon, Temecula, CA), and “induction medium supplied by Invitrogen” (Invitrogen, Carlsbad, CA)
= 7-amino actinomycin D and Senescence beta-Galactosidase Staining kit (Cell Signaling Technologies, Danvers, MA)
= Antibodies for CD90, CD44, CD73, CD166, CD34, CD45, and HLA-DR (BD Pharmingen, San Diego, CA)
= Alizarin red, Oil Red, and Alcian blue (manufacture information not reported)
= Viability was tested at all 4 time points. Terminal differentiation timeline was not explicitly mentioned.
= Trypan blue (Sigma-Aldrich, St. Louis, MO), Proprium iodide/Annexin V assay (Cayman Chemical, Ann Arbor, MI), alamarBlue assay(AbD Serotec, Kidlington, UK)
= no specific assay was mentioned but manuscript states the study tested cells’ tri-lineage differentiation (chondrogenic, adipogenic, and osteogenic properties).
As experimental MSC-based therapies for the treatment of joint degeneration typically require expulsion of cells through needles of different gauges, there is a compelling need for additional studies on the mechanosensitivity of cells during expulsion. Even though AMSCs have emerged as a viable stromal/stem-cell source for the treatment of OA and other joint disorders, it remains unclear whether passage through a needle will affect AMSC viability or function during injection procedures.40 Therefore, the purpose of this investigation was to examine the effects of needle expulsion (i.e. injection) on human AMSC viability and function using clinically applicable needle gauges as a function of time following needle passage. We hypothesized that human, culture expanded AMSCs subjected to injection stresses would exhibit changes in their biological phenotype (e.g., cell viability and function). Clinically, the results of this study will provide guidance for the selection of appropriate needle sizes for regenerative medicine procedures.
MATERIALS AND METHODS
General Design
Human AMSCs were subjected to needle passage at a constant rate under three conditions: (a) 6-mL plastic syringe with no needle (control), (b) 6-mL syringe attached to an 18- gauge (G), 25.4 mm stainless steel needle, and (c) 6-mL syringe attached to 30-gauge, 19 mm stainless steel needle. Cells were passed through the needles into a vial and analyzed for viability and function immediately post-injection (day 0), and also at 1 (day 1) and 4 days (day 4) following simulated injection.
Cell Isolation
Following informed consent and IRB approval at the authors′ institution, human AMSCs were isolated from lipo-aspirates obtained from a representative donor using previously described methods13, 14,49. The donor-derived AMSCs selected in this study have previously been shown to express standard MSC markers and are capable of multi-lineage differentiation13–15. In brief, donor lipo-tissue was aspirated and then digested using Type I collagenase (Worthington Biochemicals, Lakewood, NJ) for 1.5 hours at 37°C, centrifuged at 400g for 5 min, rinsed with phosphate buffered saline (Life Technologies, Grand Island, NY), and strained using 70μm cell strainers (BD Biosciences, San Jose, CA). Following this, the aspirate was treated with 154 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA for erythrocyte lysis. The remaining AMSCs were expanded in advanced minimum essential medium (Life Technologies, Grand Island, NY), supplemented with 5% (vol/vol) human platelet lysate (PLTMax; MillCreekLifeSciences, Rochester, MN), 2mM Glutamax (Life Technologies, Grand Island, NY), 2 U/mL heparin, and 1% Penn-Strep (100 U/mL penicillin, 100 μg/mL streptomycin; Cellgro, Corning, NY). All AMSCs used in experimentation were of passage 7.
Simulated Cell Injection
AMSCs were detached from the culturing flask using TrypLE Express (Life Technologies, Grand Island, NY) and counted using a hemocytometer (Thermo Fisher Scientific, Waltham, MA). A total of 1 mL of cell suspension was loaded into a 6 mL plastic syringe (BD biosciences, San Jose, CA) just prior to each injection. A standard syringe pump (Sage Instruments, Freedom, CA) was used for all AMSC injections. The device was pre -calibrated to confirm isokinetic cell expulsion at an average injection flow rate of approximately 4 mL/min (Figure 1A). Three conditions were tested: (a) 6-mL plastic syringe with no needle (control), (b) 6-mL syringe attached to an 18-gauge, 25.4 mm stainless steel needle (Covidien, Mansfield, MA), and (c) 6-mL syringe attached to 30-gauge, 19 mm stainless steel needle (Covidien, Mansfield, MA). The two needle sizes were chosen to represent a range of needle gauges commonly used in clinical practice. Once loaded, the syringe pump was used to inject the cells into a vessel, which was then divided for subsequent analysis (see below). Three injections were performed for each condition, resulting in a total of 9 trials for this investigation.
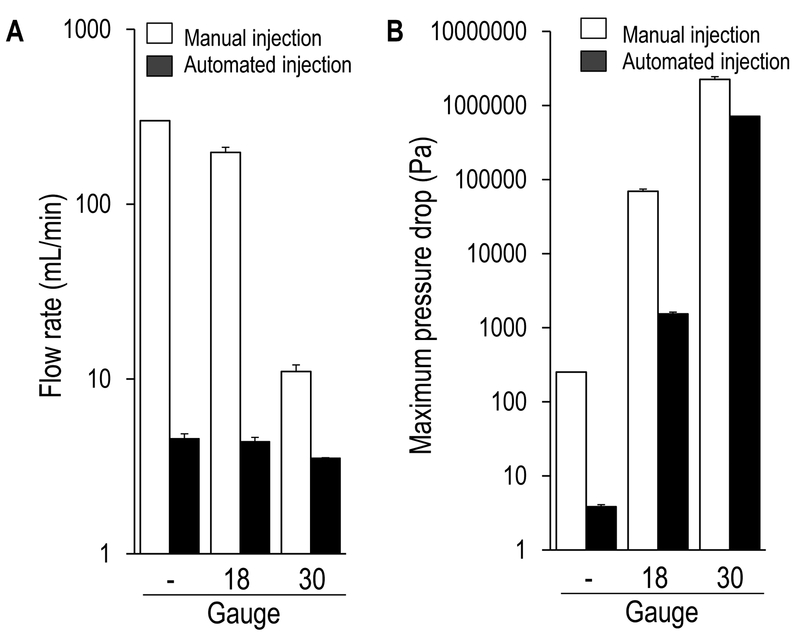
Figure 1:
Physical properties of manually operated (white bars) and syringe-pump automated (black bars) injections. (A) The flow rate (mL/min) during a manual injection varied based on injection condition, whereas the syringe pump utilized in the current investigation maintained an isokinetic flow rate regardless of injection condition. (B) The maximal pressure drop during injection increased as the needle gauge decreased, consistent with increased mechanical shear stresses experienced by cells injected through smaller needles. Pilot data generated by authors’.- = syringe only (i.e. no needle), 18 = 18 gauge, 25.4 mm needle, 30 = 30 gauge, 19 mm needle.
Assessment of AMSC Viability, Proliferation and Metabolic Activity
To assess cell viability and proliferation potential post-injection, two cell counting methods were used: Trypan Blue (day 0 post-injection, Sigma-Aldrich, St. Louis, MO) and DAPI (4′,6-diamidino-2-phenylindole) staining (days 1 and 4 post injection, Life Technologies, Grand Island, NY) paired with ImageJ analysis, an open-source NIH-supported image analysis program. All images were acquired using an inverted light microscope (Zeiss, Cambridge, MA). Trypan Blue does not penetrate the membranes of viable cells. Therefore, the presence or absence of Trypan Blue reflects cell viability immediately post-injection; cells that remain impermeable to the dye are viable.
To assess the mitrochondrial metabolic activity of injected AMSCs, MTS (3-(4,5- dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) colorimetric assays (Promega, Madison, WI) were performed at days 0, 1, and 4 post-injection. MTS assays were read using a SpectraMax Plus Plate Reader (Molecular Devices, Sunnyvale, CA) at an absorbance wave-length of 490 nm.
Gene Expression of AMSCs in Response to Injection via mRNA analysis
AMSC gene expression during the first 24 hours post-injection was utilized to assess the acute effect of mechanical stimulation/shear stress on cell activity and behavior. AMSCs were subjected to qRT-PCR to analyze mRNA expression. First, RNA was isolated using the miRNeasy Mini Kit (Qiagen, Valencia, CA) and total RNA yield was evaluated using a Nanodrop 2000 (Thermo Scientific, Inc., Waltham, MA). Reverse-transcriptase PCR was performed to obtain cDNA using SuperScript III (Invitrogen, Carlsbad, CA). Then, cDNA was analyzed by qRT-PCR (C1000 Touch Thermal Cycler, BioRad, Hercules, CA) using SYBR Green detection with specific primers to define expression levels of mRNA biomarkers for proliferation, ECM production, stress/cytoprotective responses, stem cell surface markers, and transcription factors. Primer sequences are given in Table 2. Results were normalized to GAPDH within each sample.
Table 2:
mRNA primer sequences.
| Gene | Primers, 5’−3’ | |
|---|---|---|
| Forward | Reverse | |
| EGR1 | ACCCCTCTGTCTACTATTAAGGC | TGGGACTGGTAGCTGGTATTG |
| EGR2 | ATTCTGAGGCCTCGCAAGTA | GCTTATGCCCAGTGTGGATT |
| EGR3 | GCGACCTCTACTCAGAGCC | CTTGGCCGATTGGTAATCCTG |
| JUN | AGTCCCAGGAGCGGATCAA | TTCCTTTTTCGGCACTTGGA |
| JUNB | TGGCCCAGCTCAAACAGAAG | CAGAAGGCGTGTCCCTTGAC |
| JUND | GTGAAGACCCTCAAGAGTCAGA | GACGTGGCTGAGGACTTTCT |
| FOS | GAGAATCCGAAGGGAAAGGAAT | TCCGCTTGGAGTGTATCAGTCA |
| FOSB | GCTGCAAGATCCCCTACGAAG | ACGAAGAAGTGTACGAAGGGTT |
| FOSL1 | GCCGCCCTGTACCTTGTATCT | CAGTGCCTCAGGTTCAAGCA |
| FOSL2 | CCTCGAACCTCGTCTTCACCTA | AGCAAGATTCGGAGGGACAT |
| COL1A1 | GTAACAGCGGTGAACCTGG | CCTCGCTTTCCTTCCTCTCC |
| COL3A1 | TTGAAGGAGGATGTTCCCATCT | ACAGACACATATTTGGCATGGTT |
| DCN | ATGAAGGCCACTATCATCCTCC | GTCGCGGTCATCAGGAACTT |
| MKI67 | ACGCCTGGTTACTATCAAAAGG | CAGACCCATTTACTTGTGTTGGA |
| HIST2H4 | AGCTGTCTATCGGGCTCCAG | CCTTTGCCTAAGCCTTTTCC |
| CCNB2 | CCGACGGTGTCCAGTGATTT | TGTTGTTTTGGTGGGTTGAACT |
| CD44 | CTGCCGCTTTGCAGGTGTA | CATTGTGGGCAAGGTGCTATT |
| CD90 | ATGAAGGTCCTCTACTTATCCGC | GCACTGTGACGTTCTGGGA |
| CD105 | TGCACTTGGCCTACAATTCCA | AGCTGCCCACTCAAGGATCT |
| SP1 | CAGGTGCAAACCAACAGATTA | GCTGGAGTAGGTTTGGCATAG |
| PRRX1 | CAGGCGGATGAGAACGTGG | AAAAGCATCAGGATAGTGTGTCC |
| ATF1 | CTGGAGTTTCTGCTGCTGTC | GGCAATGGCAATGTACTGTC |
Statistical Analysis
Descriptive statistics were utilized to demonstrate the effects of injection on AMSC cell counts (i.e., viability), mitochondrial activity, and qRT-PCR on day 0, day 1, and day 4 post-injection. Results were analyzed by one-way or two-way ANOVA, and unpaired Student’s t-test. Statistical significance was set at p < 0.05. Results are presented as the mean ± SD.
RESULTS
Effect of Needle Gauge on Viability, Proliferation and Metabolic Activity
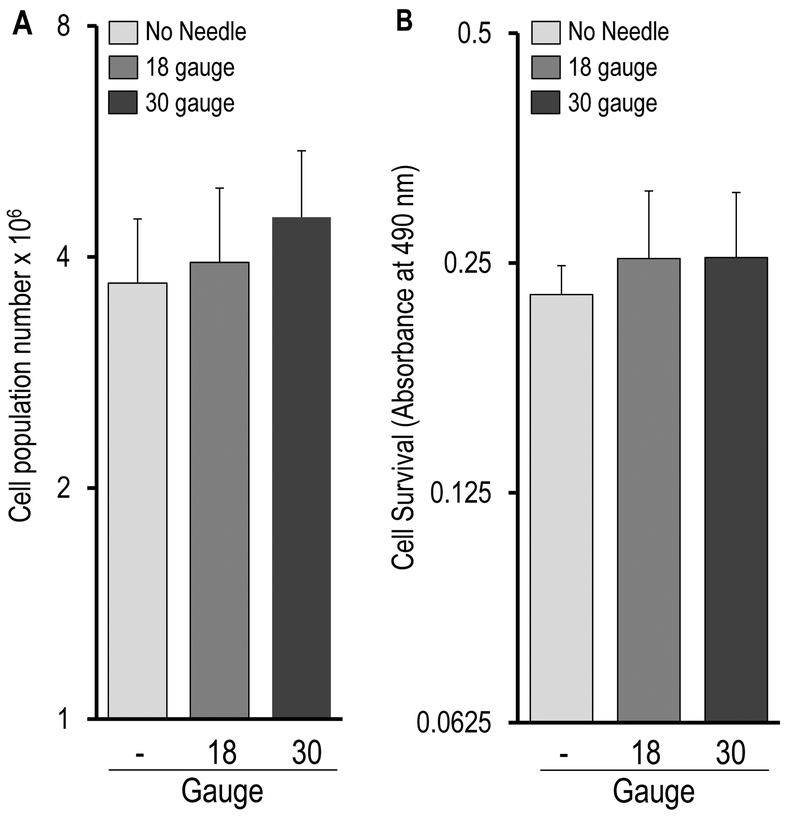
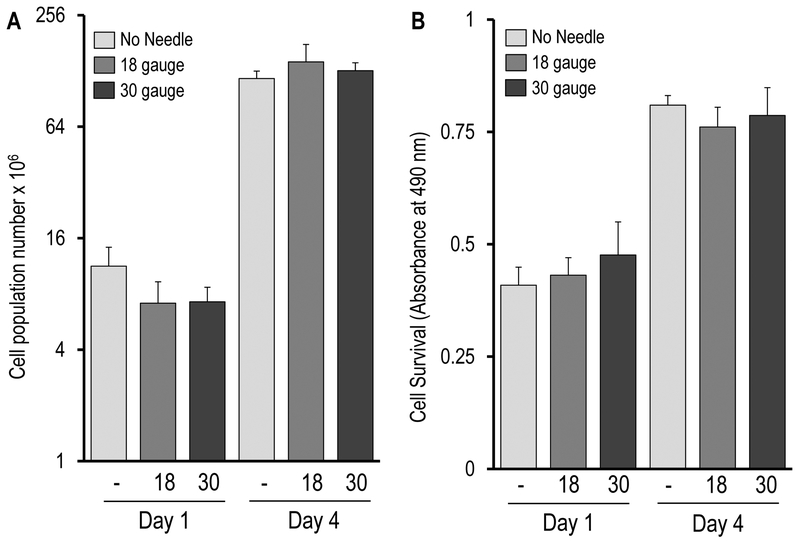
There was no statistically significant difference among the three treatment groups with respect to cell counts immediately post-injection (day 0) as determined by Trypan Blue staining (Figure 2A, syringe only = 3.70 ± 0.79 × 106 cells, 18-gauge needle = 3.94 ± 0.98 × 106 cells, and 30-gauge needle = 4.51 ± 0.99 × 106 cells), or on days 1 and 4 as determined by DAPI staining (Figure 3A, day 1: syringe only = 11.7 ± 3.00 × 106 cells, 18-gauge = 7.13 ± 2.17 × 106 cells, and 30-gauge = 7.25 ± 1.44 × 106 cells; day 4 :syringe only = 116.51 ±11.2 × 106 cells, 18-gauge = 143.18 ± 34.72 × 106 cells, and 30-gauge = 128.38 ± 13.35 ×106 cells). The increase in cell numbers between days 1 and 4 reflects approximately three to four cell divisions (i.e., about 1 cell division per day), and is consistent with previous observations showing that AMSCs exhibit exponential growth for the first four days of culture after seeding.14, 15 In summary, these Trypan Blue and DAPI results suggest that AMSC viability was not significantly affected by injection condition and that AMSCs retained their proliferative ability during the early post-injection period (i.e., up to day 4)
Figure 2:
Immediate effect (day 0) of injection on AMSC viability and function. (A) Trypan Blue stain analysis immediately post-injection. No cells stained positive for Trypan Blue, indicating that viability was not acutely effected in any of the three groups. Since none of the cells exhibited positive staining with the dye, the values directly reflect the total cell population (number × 106). (B) MTS assay results, indicating that mitochondrial metabolic activity immediately post-injection remained steady regardless of the injection condition. Values represent absorbance values measured at 490 nm. (A) & (B) collectively indicated that AMSC viability and metabolic activity were not significantly affected by simulated injection in the immediate post-injection period.
Figure 3:
AMSCs retained their proliferative capacity and metabolic function between days 1 and 4 post-injection, with no significant differences between injection groups. (A) DAPI assisted cell counts (values represent cells × 106) were similar among the three groups on days 1 and 4, and all groups demonstrated an exponential increase cell counts between days 1 and 4, reflecting maintenance of normal proliferative capacity. (B) MTS assay for mitochondrial activity revealed similar mitochondrial activity among the three treatment groups on days 1 and 4, as well a commensurate increase in mitochondrial activity between days 1 and 4. This latter finding reflects cell proliferation and corroborates the DAPI stain findings in (A).
All three treatment groups demonstrated similar mitochondrial metabolic activity at days 1 and 4 based on MTS assays (Figure 3B). As expected from actively proliferating cells, MTS absorbance increased between days 1 and 4. In summary, the MTS assays indicate that injection condition did not affect AMSC metabolic activity and corroborate the DAPI cell analysis reflecting normal AMSC proliferation in the early post-injection period.
Effect of Needle Gauge on Gene Expression
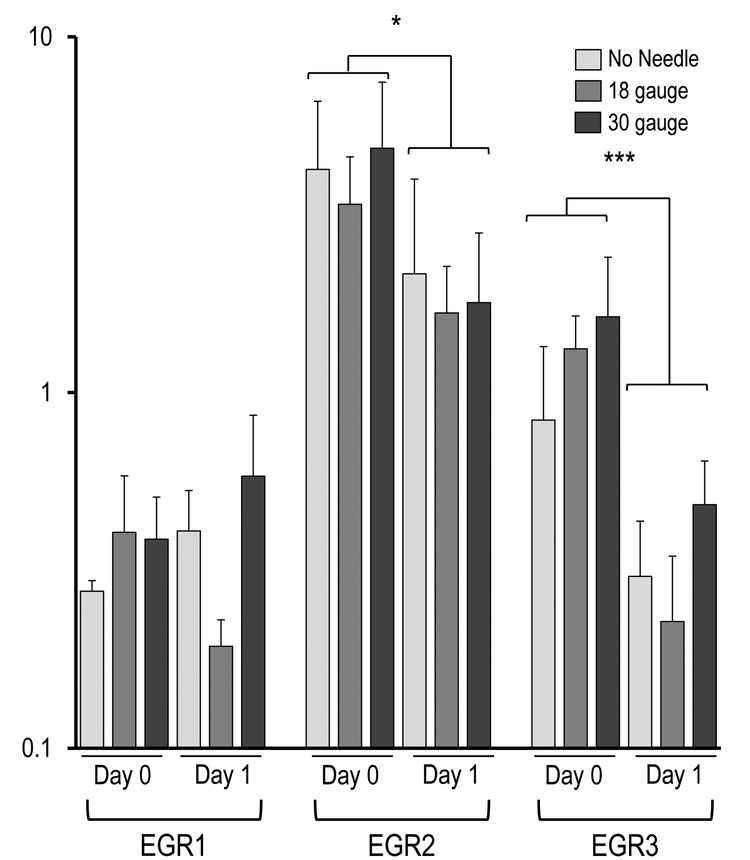
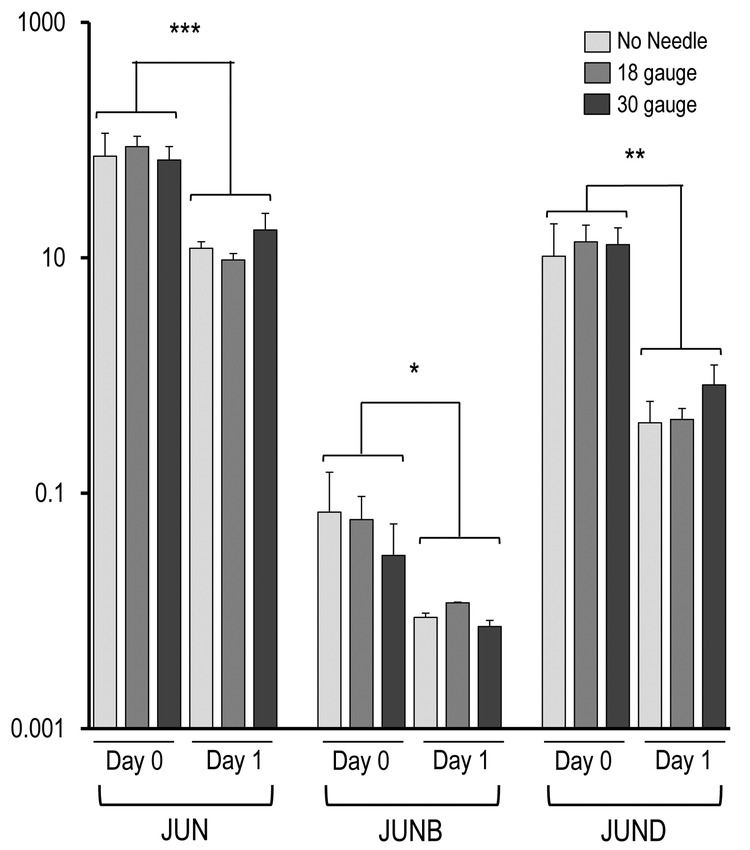
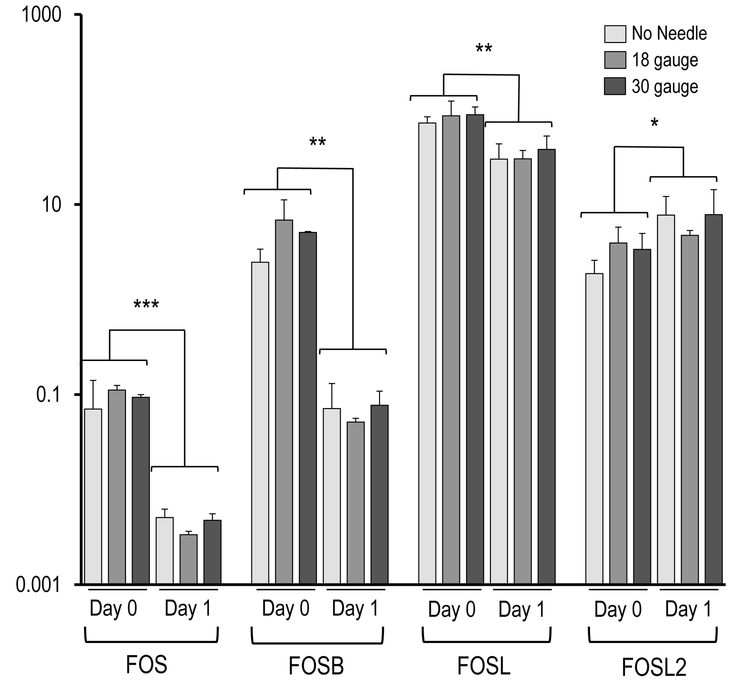
Figure 4 demonstrates the AMSC gene expression as reflected by mRNA biomarkers for the three injection groups (e.g., syringe only, 18-gauge needle, 30-gauge needle). The most prominent finding is that the AMSCs in all injection groups expressed very robust levels of genes normally associated with a stress response, cytoprotective mechanisms, and mechano-transduction, including the ‘early growth response’ transcription factors EGR2 and EGR3, as well as the seven members of the heterodimeric AP1 (FOS/JUN) gene regulatory complex (i.e., JUN, JUNB, JUND, FOS, FOSB, FOSL1, FOSL2). The robust expression of these genes observed at the time of harvest (day 0) subsided by day 1 (Figure 4A). Specifically, EGR2 (p< 0.05) and EGR3 (p < 0.001), as well as FOS (p < 0.001), FOSB (p < 0.01), FOSL1 (p < 0.01), FOSL2 (p < 0.05), JUN (p < 0.001), JUNB (p < 0.05) and JUND (p < 0.01) showed significant changes in expression during the first 24 hours, without significant differences between injection groups. These results suggest that the AMSCs mount a cytoprotective response to counteract stresses that emerged during the handling of cells and/or the injection simulation.
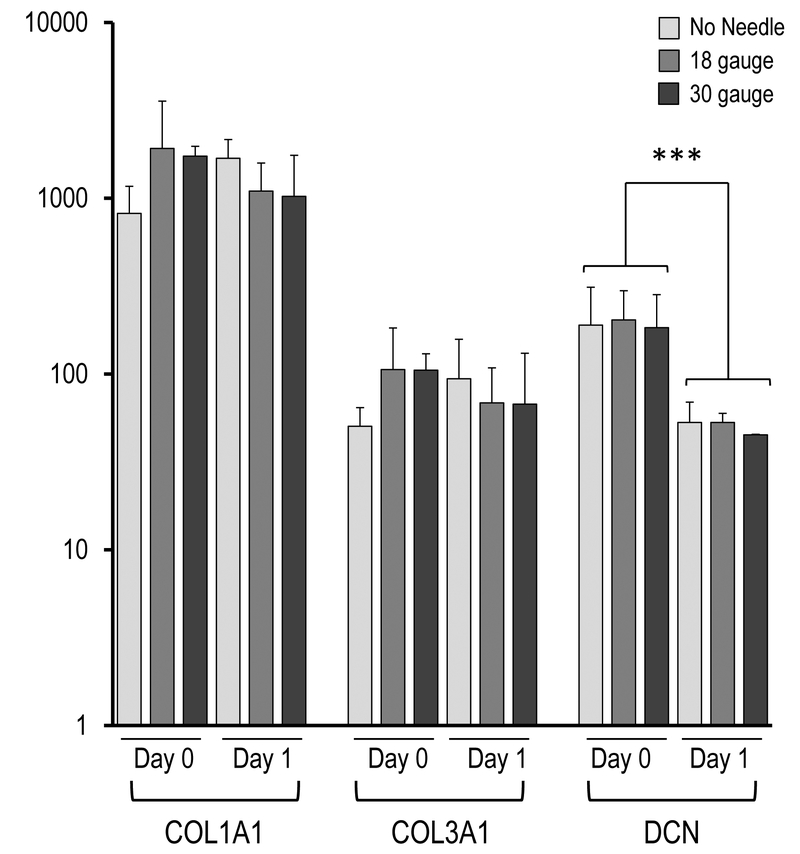
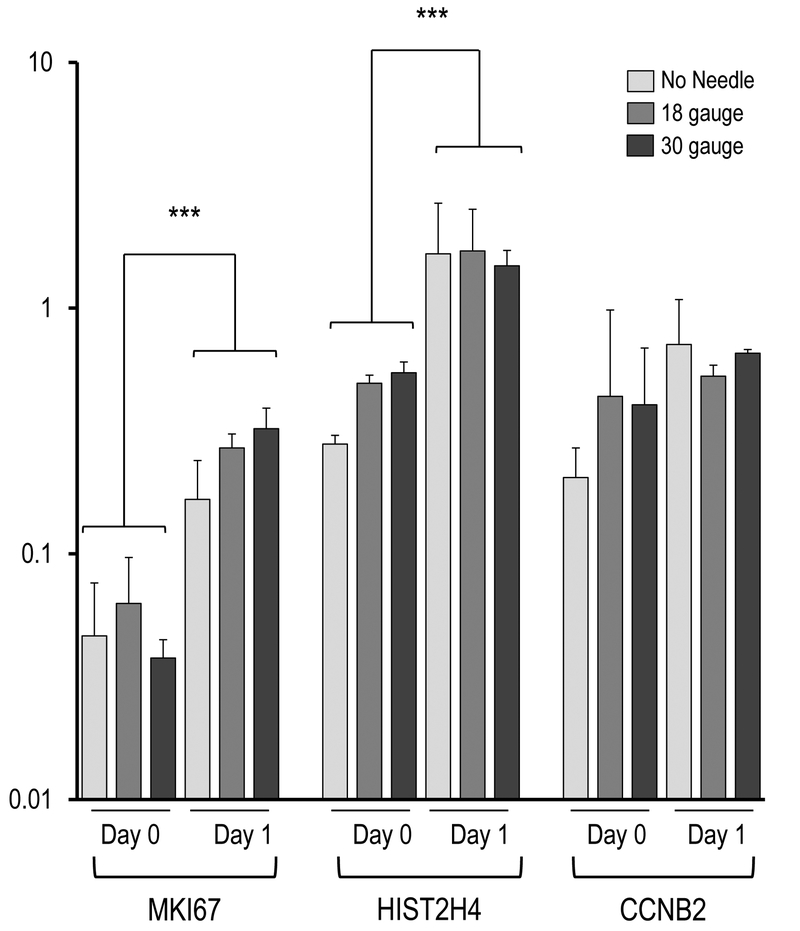
Figure 4.
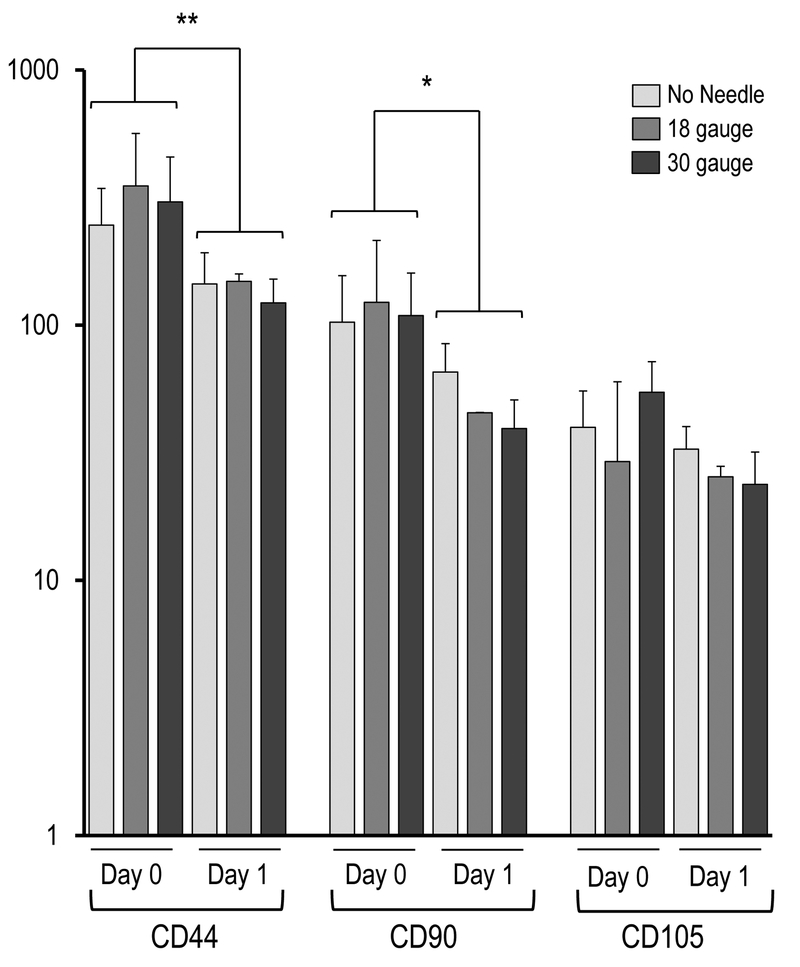
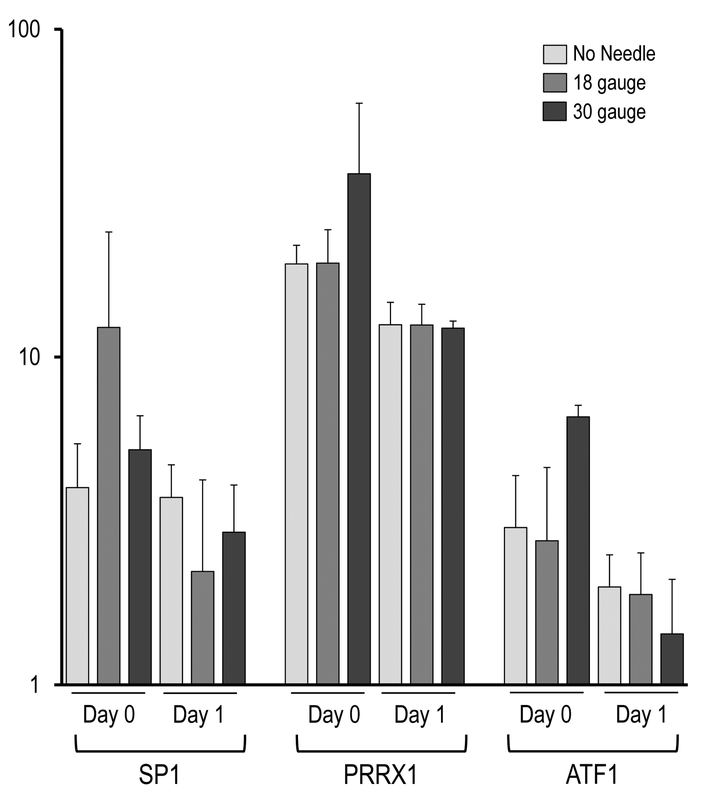
Effect of needle gauge on AMSC gene expression (as determined by mRNA analysis) following injection. Bar graphs in the panels show expression data after needle expulsion for ‘early response’ genes (A-C), extra-cellular matrix (ECM) proteins (D), proliferation markers (E), cell surface markers (F), and representative transcription factors that are ubiquitous in mesenchymal cells (G). Values represent the mean (with standard deviation) of three different AMSC injections that were sampled as biological triplicates (n=9 total samples).
Beyond the observed stress/cytoprotective gene expression response, we observed robust expression of representative extracellular matrix (ECM) markers COL1A1 and COL3A1 regardless of condition (Figure 4B), indicating that cells retain collagen anabolic activity. We note that there were modest differences in expression on Day 0 and Day 1, but these changes are not statistically significant. We also observed a decrease of the mRNA for the ECM-related non-collagenous proteoglycan DCN (p < 0.001) from day 0 to day 1. The latter result suggests that cells exhibit selective changes in the expression of ECM proteins, which reflect an altered phenotypic state.
As expected, mRNA levels for two distinct proliferation markers (HIST2H4A, p < 0.001) and MKI67 (p < 0.001) increased from day 0 to day 1 (Figure 4C). The latter findings support the conclusion that AMSCs are fully capable of resuming cell cycle progression after needle expulsion. Taken together, initiation of active proliferation between day 0 and day 1 (Figs. 3 and 4C) and selectively changes in expression of ECM proteins is broadly consistent with our previous work showing that AMSCs alter expression of ECM proteins when they stop proliferating14.
Examination of mRNA levels for three representative MSC surface markers (e.g., CD44, CD90 and CD105) revealed a decrease in their expression after one day in culture, independent of injection group. This finding is consistent with previous observations that ASMCs exhibit a general decrease in cell surface gene expression when they initiate a phase of active proliferation14. For comparison, expression of several transcription factors (e.g., SP1, PRRX1 and ATF1) that are ubiquitously expressed in MSCs was not modulated in the same manner.
In summary, the gene expression analyses indicate that AMSCs subjected to our simulated injection procedure adopt a molecular phenotype characteristic of proliferating MSCs following an initial stress/cytoprotective response.
DISCUSSION
The primary finding of the current investigation is that human, culture expanded, AMSCs maintain their viability, proliferative capacity and cellular functions following simulated injection with clinically applicable needle gauges. For decades, needle-based delivery systems have been successfully utilized for therapeutic agents such as corticosteroids and viscosupplements to treat pain and inflammation associated with degenerative and inflammatory musculoskeletal conditions. While a number of studies have investigated the emerging role of cell-based therapies to treat a variety of medical and musculoskeletal disorders, there is a paucity of experimental studies that examine the biological effects of needle-based delivery systems on the viability and cellular functions of MSCs. Importantly, to our knowledge, the current investigation is the first to examine the effect of “injection” on human, culture expanded AMSC viability or function.
Our present study complements prior investigations that examined the effects of injection on human MSCs45–48. We used AMSC suspensions of approximately 4 × 106 cells/mL and a flow rate of 4 mL/min. Two prior studies47, 48 utilized BMSCs at concentrations of 1 × 106/mL and 3 × 106/mL, flow rates of 1–8.3 mL/minute, and needle gauges ranging from 20–30 (Table 1)47, 48. A direct comparison of our work with these two previous studies is limited by methodological differences, including the source of MSCs, length and diameter of needles, flow- rates, our use of a ‘no needle’-control, and/or the longer post-injection analysis period (up to four days) of the current investigation. Mamidi et al. injected human BMSCs in suspensions of 3 × 106 cells/mL at a flow rate of 2 mL/min using needles ranging from 24- to 26-gauge47. These authors found no effect of injection on BMSC viability, morphology, surface markers, or terminal tri-lineage differentiation, but did not specify the time period(s) at which the post-injection assessments were performed47. Walker et al. examined the fate of human BMSCs following injection of 1 × 106 cells/mL at flow rates ranging from 1–8.3 mL/min through 20- to 30-gauge needles48. Unlike Walker et al., Maimidi’s group found reduced 24 hour BMSC viability at all flow rates following 25- and 30 gauge injection.48 Significant methodological differences between the two studies, as well as missing “data points” (e.g. no time course for post-injection assessment in study by Mamidi et al.) preclude identification of the likely explanation for the discrepant findings. Reassuringly, unlike the findings of Walker et al. with respect to human BMSCs, we found that human AMSC viability was unaffected by simulated injection under similar conditions. Furthermore, we observed that our passed AMSCs retained both their proliferative potential and mitochondrial metabolic activity, despite an early stress/cytoprotective response. It is possible that BMSC and AMSC preparations may differ in their inherent vulnerability to experimental handling and/or fluid shear stress during injection. Alternatively or additionally, differing methods of AMSC versus BMSC culture expansion (e.g. culture media) may select for cells with different responses to handling and/or injection related stresses. Clearly, further investigation is necessary to clarify the potential differential responses between “injected” BMSC and AMSCs.
Reduced cell viability upon expulsion from syringes is consistent with our observation that MSCs mount a cellular stress/cytoprotective response based on transiently induced expression of specific early response factors (i.e., EGR and AP1/Fos-Jun related proteins). Transit of AMSCs through syringes invokes major pressure changes that are quantitatively linked to the applied flow rate, the viscosity of the cell suspension, as well as the dimensions of needles included in our injection simulation (see equation above). For the current investigation, we selected needles with sizes near the upper and lower boundaries of clinical relevance and therefore examined the effects of both a wide (18 gauge) and narrow (30 gauge) needle. While we maintained cell suspensions and flow rates at constant and clinically realistic values, one possible limitation of our approach is that flow rates may not always be constant in clinical settings where manual administrations are typical. Cell suspensions may experience variable pressure changes during injection due to human factors (e.g., interactions between the needle gauge and forces exerted by human operator, or even manual fatigue of the operator). Alternatively, changes in resistance to flow may be imparted by the injected region. For example, injected cells suspensions may reach the volumetric limits of a closed space or encounter tissues of variable density (e.g., synovial cavity in the joint or the nucleus pulposus of a spine disk). Future studies may account for the effects of variable pressure on cell viability by performing needle expulsion trials with various levels of resistance that mimic in vivo conditions of AMSC injection.
There are several additional study limitations that warrant consideration when interpreting the results of the current investigation. First, we only examined one concentration of human, culture expanded AMSCs. Although our chosen concentration of 4 X106 cells/ml is within the range of clinical relevance, we recognize the interaction between cell concentration, viscocity, and mechanical fluid shear stress. Consequently, future studies will examine the effects of injection on more highly concentrated AMSC suspensions. Second, our longest needle was 25.4 mm. In clinical practice, longer needles are commonly used for shoulder, hip and spine injections (up to 3–4 cm). Although the effect of needle length on fluid shear stress is relatively minor when compared to the effect of needle diameter, future investigations should utilize longer needles to determine whether needle length affects AMSC viability or function following injection. Third, although our flow rate of 4 ml/min was commensurate with previously Consequently, future studies will examine the effects of high flow rate injections on AMSC viability and function.
CONCLUSION
Human, culture expanded AMSCs maintain their viability, proliferative capacity and metabolic function following passage through needles as small as 30 gauge at constant flow rates of 4 ml/min, despite an early, non-specific stress/cytoprotective response. These initial findings suggest that culture expanded AMSCs should tolerate the injection process during most cell- based therapeutic interventions. Future research should examine higher AMSC concentrations, faster injection flow rates, and longer needles, as well as identify and characterize any differences between the post-injection responses of AMSCs and BMSCs.
Acknowledgments
We thank the members of the orthopedic research laboratories and especially Matt Getzlaf and Liselotte Bulstra, for stimulating discussions and/or assistance with procedures. We appreciate the generous philanthropic support of William H. and Karen J. Eby, and the charitable foundation in their names. We also thank the Center for Regenerative Medicine of Mayo Clinic for support through career development awards (to JS and SMR) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR049069 to AvW; T32 AR56950 to EAL).
REFERENCES
- 1.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974;17:331–340. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230–247. [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 1991;9:641–650. [DOI] [PubMed] [Google Scholar]
- 4.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of cell science 2006;119:2204–2213. [DOI] [PubMed] [Google Scholar]
- 5.Kassis I, Zangi L, Rivkin R, et al. Isolation of mesenchymal stem cells from G-CSF- mobilized human peripheral blood using fibrin microbeads. Bone marrow transplantation 2006;37:967–976. [DOI] [PubMed] [Google Scholar]
- 6.Zou Z, Zhang Y, Hao L, et al. More insight into mesenchymal stem cells and their effects inside the body. Expert opinion on biological therapy 2010;10:215–230. [DOI] [PubMed] [Google Scholar]
- 7.Poznanski WJ, Waheed I, Van R. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Laboratory investigation; a journal of technical methods and pathology 1973;29:570–576. [PubMed] [Google Scholar]
- 8.Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. The Journal of clinical investigation 1976;58:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awad HA, Butler DL, Boivin GP, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue engineering 1999;5:267–277. [DOI] [PubMed] [Google Scholar]
- 10.Bai L, Caplan A, Lennon D, Miller RH. Human mesenchymal stem cells signals regulate neural stem cell fate. Neurochemical research 2007;32:353–362. [DOI] [PubMed] [Google Scholar]
- 11.Bao J, Fisher JE, Lillegard JB, et al. Serum-free medium and mesenchymal stromal cells enhance functionality and stabilize integrity of rat hepatocyte spheroids. Cell transplantation 2013;22:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen BK, Staff NP, Knight AM, et al. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting Phase I human trials. Transfusion 2015;55:1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crespo-Diaz R, Behfar A, Butler GW, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell transplantation 2011;20:797–811. [DOI] [PubMed] [Google Scholar]
- 14.Dudakovic A, Camilleri E, Riester SM, et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem 2014;115:1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudakovic A, Camilleri ET, Lewallen EA, et al. Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. Journal of cellular physiology 2015;230:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eirin A, Zhu XY, Ferguson CM, et al. Intra-renal delivery of mesenchymal stem cells attenuates myocardial injury after reversal of hypertension in porcine renovascular disease. Stem cell research & therapy 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 2007;28:3217–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell- based therapeutics. Current opinion in biotechnology 2009;20:531–536. [DOI] [PubMed] [Google Scholar]
- 20.Britten MB, Abolmaali ND, Assmus B, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 2003;108:2212–2218. [DOI] [PubMed] [Google Scholar]
- 21.Bussolati B, Hauser PV, Carvalhosa R, Camussi G. Contribution of stem cells to kidney repair. Current stem cell research & therapy 2009;4:2–8. [DOI] [PubMed] [Google Scholar]
- 22.Fellous TG, Guppy NJ, Brittan M, Alison MR. Cellular pathways to beta-cell replacement. Diabetes/metabolism research and reviews 2007;23:87–99. [DOI] [PubMed] [Google Scholar]
- 23.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009;183:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of neurology 2010;67:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation 2009;7:241–248. [PubMed] [Google Scholar]
- 26.Vassalli G, Moccetti T. Cardiac repair with allogeneic mesenchymal stem cells after myocardial infarction. Swiss medical weekly 2011;141:w13209. [DOI] [PubMed] [Google Scholar]
- 27.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis and rheumatism 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bollet AJ, Nance JL. Biochemical Findings in Normal and Osteoarthritic Articular Cartilage. II. Chondroitin Sulfate Concentration and Chain Length, Water, and Ash Content. The Journal of clinical investigation 1966;45:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brocklehurst R, Bayliss MT, Maroudas A, et al. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. The Journal of bone and joint surgery American volume 1984;66:95–106. [PubMed] [Google Scholar]
- 31.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Annals of the rheumatic diseases 1977;36:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Therapeutic advances in musculoskeletal disease 2010;2:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter DJ, Zhang W, Conaghan PG, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2011;19:557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis and rheumatism 2009;60:3546–3553. [DOI] [PubMed] [Google Scholar]
- 35.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis and rheumatism 2006;54:226–229. [DOI] [PubMed] [Google Scholar]
- 36.Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. International journal of rheumatic diseases 2011;14:211–215. [DOI] [PubMed] [Google Scholar]
- 37.Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Archives of Iranian medicine 2012;15:422–428. [PubMed] [Google Scholar]
- 38.Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation 2013;95:1535–1541. [DOI] [PubMed] [Google Scholar]
- 39.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain physician 2008;11:343–353. [PubMed] [Google Scholar]
- 40.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 2014;32:1254–1266. [DOI] [PubMed] [Google Scholar]
- 41.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 43.Leverett LB, Hellums JD, Alfrey CP, Lynch EC. Red blood cell damage by shear stress. Biophysical journal 1972;12:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge J, Guo L, Wang S, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem cell reviews 2014;10:295–303. [DOI] [PubMed] [Google Scholar]
- 45.Agashi K, Chau DY, Shakesheff KM. The effect of delivery via narrow-bore needles on mesenchymal cells. Regenerative medicine 2009;4:49–64. [DOI] [PubMed] [Google Scholar]
- 46.Garvican ER, Cree S, Bull L, Smith RK, Dudhia J. Viability of equine mesenchymal stem cells during transport and implantation. Stem cell research & therapy 2014;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mamidi MK, Singh G, Husin JM, et al. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. Journal of translational medicine 2012;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker PA, Jimenez F, Gerber MH, et al. Effect of needle diameter and flow rate on rat and human mesenchymal stromal cell characterization and viability. Tissue engineering Part C, Methods 2010;16:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. Journal of translational medicine 2013;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]