Abstract
Studies disagree about whether racial and ethnic groups have lower or higher human papillomavirus (HPV) vaccination uptake, an important issue given large disparities in some HPV cancers. We sought to characterize and explain racial and ethnic differences in HPV vaccination. We systematically searched PubMed, CINAHL, Embase, and Web of Science to identify US studies through mid-2017 reporting associations of race and ethnicity with HPV vaccination. We identified 118 studies (n = 3,095,486) published in English that reported HPV vaccine initiation or follow-through in the US from which we could calculate effect sizes. We used random effects meta-analysis to synthesize effect sizes for comparisons of Whites or non-Hispanics to Blacks, Hispanics, Asians, or all minority groups combined. Studies showed no racial or ethnic differences in HPV vaccine initiation overall. However, when restricting to studies using provider-verified vaccination data, minorities were 6.1% [3.3%–8.8%] more likely than Whites to initiate HPV vaccination. Advantages were larger for Hispanics, males, and younger samples (age < 18). In contrast, minorities were 8.6% [5.6%, 11.7%], less likely than Whites to follow-through with the full HPV vaccine series, a disparity present across all participant and study characteristics. More recent studies found larger advantages for racial and ethnic minorities in HPV vaccine initiation and smaller disparities in follow-through. In summary, high-quality studies found racial and ethnic minorities are more likely to initiate but less likely to follow-through with HPV vaccination, a clear finding that self-report studies obscure. Higher HPV vaccine initiation among minorities suggests potential reductions in HPV cancer disparities.
Keywords: HPV vaccines, Minority health, Health care disparities, Metaanalysis, Systematic review
1. Introduction
Every year, over 43,000 new cases of HPV-related cancers are diagnosed among American men and women (Van Dyne et al., 2018). However, large racial and ethnic disparities exist in some HPV cancers. For example, non-Hispanic Black and Hispanic women have higher incidence of cervical cancer (9.5 and 9.7 per 100,000, respectively), compared to non-Hispanic White women in the US (7.0 per 100,000) (Bakitas, 2007). A major cause in cervical cancer disparities is lower use of screening among minority women (Fedewa et al., 2015), with three- year Pap testing rates much lower for Hispanic (51%) and Black (53%) compared to non-Hispanic White women (78%) (Rauscher et al., 2008). In addition to barriers in healthcare access (Del Carmen and Avila-Wallace, 2013), the disparity is sometimes attributed to women misbelieving they have received screening when they have not (Rauscher et al., 2008).
Routine HPV vaccination of adolescents can greatly reduce the number of HPV precancers and cancers in the US (Chesson et al., 2013). However, less than half (43%) of all 13- to 17-year-olds have completed the 3-dose HPV vaccine series (Walker et al., 2017). The Centers for Disease Control and Prevention (CDC) estimates that increasing HPV vaccination from current levels to 80% would prevent 53,000 future cervical cancers in the US among girls who now are 12 years old or younger over the course of their lifetime (Centers for Disease Control and Prevention, 2013).
HPV vaccination may offer racial and ethnic minority groups even greater potential for cancer reduction given their higher incidence of some HPV cancers. However, some studies suggest racial and ethnic disparities in HPV vaccine uptake (Chao et al., 2010; Wei et al., 2013), even as other studies suggest differences favor minorities (Clarke et al.,2016; Reiter et al., 2013) or show no difference (Brewer et al., 2011; Hirth et al., 2014). We sought to comprehensively characterize differences between racial and ethnic minorities and Whites in HPV vaccine initiation and follow-through. We also sought to identify study characteristics that explain the direction and magnitude of these differences.
2. Methods
2.1. Search strategy
We systematically searched four databases (Pubmed, CINAHL, Embase and Web of Science) to identify studies published between January 2006 (the year that the U.S. licensed the first HPV vaccine) and July 2017. Searches used combinations of terms related to HPV vaccine, uptake, and race and ethnicity as follows: (HPV vaccin* OR papillomavirus vaccin* OR papilloma virus vaccin*) AND (accept* OR initiat* OR uptake OR complet* OR compliance OR comply) AND (race* OR racial OR ethnic*), with small variations as required by the database interfaces. The full search strategy is available online (Supplementary file 1). We also searched the reference lists of included articles but identified no additional relevant studies. We completed the literature search in September 2017.
2.2. Study selection
Two reviewers (JS and WC; DZ and TM) independently examined titles and abstracts and, for relevant articles, conducted full-text re views. Eligible studies were those that used data from a US sample to report HPV vaccination rates in males, females, or both, with at least one comparison between a racial or ethnic minority and White study participants. We limited our review to studies with US samples due to the distinctive demography and health care system of the country. We included studies if they 1) were written in English; 2) were published in a peer-reviewed journal; 3) reported data from a primary study (not a review, editorial, or commentary); 4) reported quantitative data; 5) measured HPV vaccination (initiation or follow-through) as the outcome variable; 6) included White and at least one of these other groups: Black, Hispanic, Asian; and 7) reported HPV vaccination data in a way that allowed us to extract the difference between White and at least one of these minority groups. We applied eligibility criteria hierarchically in the order above. We resolved questions and disagreements through discussions with the senior author (NB).
2.3. Data extraction
Reviewers extracted data on study characteristics using a standardized coding form; two investigators (JS and WC) verified the data. When studies had missing information, we requested it from the corresponding authors. We extracted year of data collection; for studies in which data were collected over multiple years, we used the final year of data collection. We coded race and ethnicity of study participants, the percentage who were Asian, Black, White, and Hispanic; too few participants in the studies were from other categories to allow us to code them. We coded studies as gathering HPV vaccination data by self-report (i.e., from individuals or their parents) or through provider-verification (i.e., from medical records, immunization registries, or insurance claims.)
An important data source is the National Immunization Survey—Teen (NIS—Teen), an annual, nationally-representative survey of households to assesses adolescent vaccination by parent or guardian report, with vaccination confirmed via provider records for a large subset of participants. We obtained provider-verified HPV vaccine initiation and follow-through by sex and race or ethnicity through publicly available NIS-Teen data (Centers for Disease Control and Prevention, 2018). On request, the CDC provided us with HPV vaccine follow-through data by sex and race for 2015 and 2016.
2.4. Effect sizes
Two reviewers independently extracted unadjusted and, if available, adjusted effect sizes describing the association between race and ethnicity with HPV vaccine initiation (individuals with at least one dose, out of all eligible individuals) and follow-through (individuals with all recommended doses, among individuals with at least one dose). The referent group was generally “Non-Hispanic White.”, although for some studies that separated race and ethnicity, the referent was either “White” or “non-Hispanic”. Reviewers attempted to extract effect sizes separately for males and females as well overall.
For unadjusted effect sizes, reviewers extracted a risk difference, the percentage point difference between non-Hispanic Whites and a minority racial or ethnic group in HPV vaccination. Negative values for risk difference indicate disparities such that the minority group had lower vaccination coverage than Whites, and positive values indicate higher vaccination among minority groups. For adjusted effect sizes, reviewers extracted the adjusted odds ratio (aOR) where reported. ORs less than one indicate disparities, and greater than one indicate reverse disparities. When studies presented more than one adjusted estimate, we used the most fully-adjusted estimate available. When multiple studies reported on the same data source, we include one unadjusted and one adjusted estimate for each reported population, retaining the study with the most years of data and most granular data (i.e., stratifications by race, sex, and age).
2.5. Statistical analysis
First, we examined racial and ethnic differences in HPV vaccination by performing a series of random effects meta-analyses. We examined the difference between Whites and minorities (Hispanic, Black, and Asian combined and separately) for each HPV vaccination measure (initiation and follow-through). We then stratified the analyses by source of vaccination data (self-report vs. provider-verified). Self-report studies include reporting of vaccination by an individual or by their parent or guardian. Provider-verified studies include reporting by medical records, immunization registry, or insurance claims. For vaccine initiation, the number of studies was sufficient to further stratify by age (studies that restricted to only individuals under age 18 vs. those that included ages 18+).
Next, we examined whether study characteristics predicted the difference in HPV vaccination between minority groups and Whites. For characteristics with sufficient data in each group, we conducted bivariate meta-regression. Analyses involving sex as a correlate used sex-stratified vaccination data; otherwise analyses used the overall data. We report the I2 statistic as a measure of heterogeneity of effect size between studies (0% indicates no heterogeneity, 100% indicates substantial heterogeneity). Finally, we examined potential publication bias by visually inspecting a funnel plot and conducting a quantitative test as described by Egger and colleagues (Sterne et al., 2008). Analyses used Stata 15 (College Station, Tx). Statistical significance tests were two-tailed and had a critical alpha of 0.05.
3. Results
3.1. Characteristics of included studies
Of 8105 studies identified, 118 studies met eligibility criteria (Fig. 1). Included studies reported vaccination data for 3,095,486 adolescents and young adults. Of these, 102 studies reported on adjusted or unadjusted initiation of HPV vaccination and 44 studies reported vaccine follow-through (Supplemental Table 1).
Figure 1.
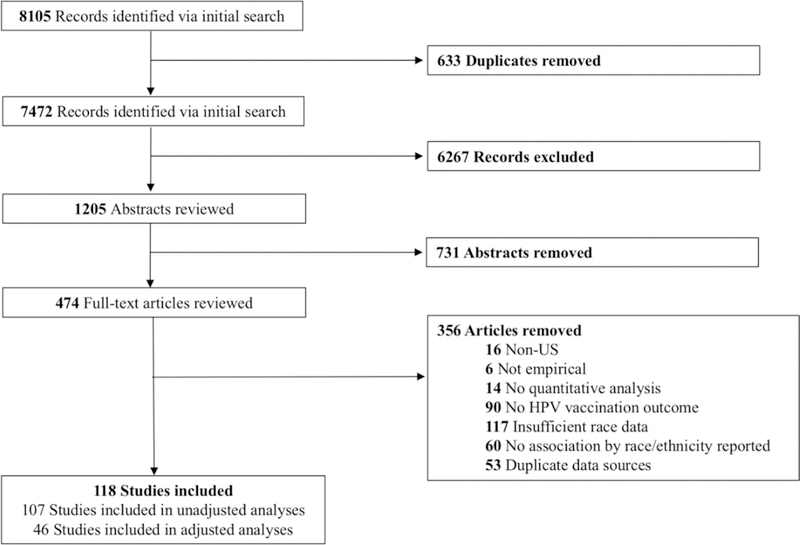
Flow diagram
Overall, 41% of studies included a national sample, and 51% used probability sampling to identify participants (Table 1). A third of studies did not report a response rate (32%) and, among those that did, rates varied widely (range: 5% to 95%) (Wilson et al., 2017; Gorbach et al., 2017). Studies generally reported on vaccination among females (69%) or both males and females (19%). About half of studies (53%) reported on 11- to 12-year-olds, the recommended age for HPV vaccine initiation, 64% reported on 13- to 17-year-olds, and 71% of studies on 18- to 26-year-olds. Vaccination was most often self-reported (58%), with a large minority of studies using more reliable methods, including clinic vaccination records (33%), insurance claims (5%) or data from an immunization registry (3%).
Table 1.
Study characteristics (k = 118)
| Characteristic | % |
|---|---|
| Publication Year | |
| 2009–2011 | 28 |
| 2012–2014 | 40 |
| 2015–2017 | 32 |
| Sampling of Clusters | |
| Single-site/Multi-site | 37 |
| Statewide/Multi-state | 21 |
| National | 41 |
| Sampling of Patients | |
| Probability | 51 |
| Convenience | 49 |
| Response Rate | |
| <50% | 14 |
| 50–75% | 19 |
| >75% | 8 |
| Census (100% included) | 26 |
| Not Reported | 32 |
| Sex | |
| Female Only | 69 |
| Male Only | 13 |
| Both | 19 |
| Ages Included1 (Years) | |
| 9–10 | 23 |
| 11–12 | 53 |
| 13–17 | 64 |
| 18–26 | 71 |
| >26 | 9 |
| Source of Race and Ethnicity Data | |
| Self-report | 75 |
| Administrative Data | 25 |
| Race- White | |
| <30% | 25 |
| 30–50% | 13 |
| 51–75% | 46 |
| >75% | 16 |
| Race- Black | |
| <10% | 40 |
| 10–20% | 26 |
| >20 | 30 |
| Not reported | 3 |
| Race- Asian | |
| <5% | 25 |
| 5–9% | 16 |
| >9% | 7 |
| Not reported | 44 |
| Hispanic Ethnicity | |
| <15% | 36 |
| 15–30% | 33 |
| >30% | 18 |
| Not reported | 13 |
| Hispanic Ethnicity Reporting | |
| Part of Race Variable | 92 |
| Separate Variable | 8 |
| Missingness of Race and Ethnicity | |
| Not reported | 86 |
| Reported, <10% | 10 |
| Reported, >10% | 4 |
| Handling of Missingness for Race and Ethnicity (k=39) | |
| Imputed | 11 |
| Excluded from analysis | 13 |
| Included in "Other" | 7 |
| Included as own category | 3 |
| Not reported | 67 |
| Source of HPV Vaccination Data | |
| Participant-report | 36 |
| Parent-report | 22 |
| Insurance Claims Data | 5 |
| Clinic vaccination record | 33 |
| Immunization registry | 3 |
| National Immunization Survey—Teen Data | |
| No | 90 |
| Yes | 10 |
| Participant Sample Size | |
| <500 | 20 |
| 500–1,999 | 30 |
| 2,000–10,000 | 19 |
| >10,000 | 30 |
| Study Design | |
| Cross-sectional | 92 |
| Longitudinal | 8 |
Studies may include more than one age category- percentages do not add to 100.
Studies did not typically report race and ethnicity as separate constructs, with the majority of studies (92%) reporting on Hispanic ethnicity within a mutually exclusive “race” category. In the majority of studies, race and ethnicity were self-reported by participants (75%), with the remaining studies using existing administrative records. Few studies discussed how they handled missing data on race and ethnicity (33%), and fewer reported the extent of missingness (14%). For studies that quantified missingness, it was generally under 10% of all observations; however missingness was as high as 50% in one large study (Chao et al., 2010). Larger studies typically handled missing data through imputation- inferring race and ethnicity through statistical methods or by participant last name. Smaller studies generally excluded those with incomplete data entirely or included those with missing data within “other” or their own category.
3.2. Meta-analyses of HPV vaccine initiation
No racial and ethnic differences were present in HPV vaccine initiation, 0.0% [95% confidence interval ‒1.8%, 1.8%], across 94 studies reporting unadjusted findings (sFigure 1). However, results differed markedly for self-reported and provider-verified HPV vaccination (Fig. 2a). Self-reported HPV vaccine initiation showed an overall disparity among racial and ethnic minorities (risk difference [RD] ‒ 3.6% [ ‒ 5.5%, ‒1.7%]) while provider-verified vaccination data found the opposite, with minorities 6.0% [3.6%, 8.5%] more likely to initiate HPV vaccine.
Figure 2.
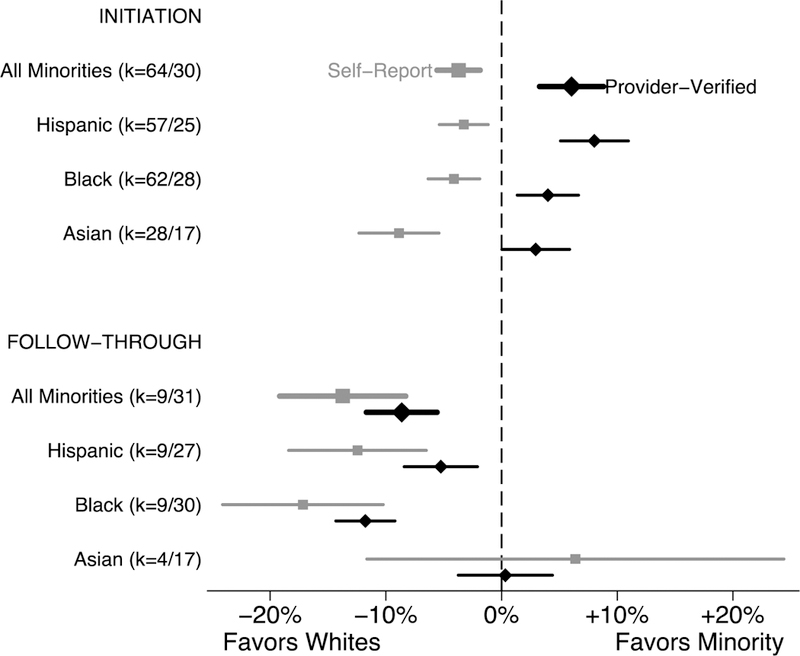
Difference in HPV Vaccination between Minority and White, by Vaccination Information Source
In studies of only patients under age 18 (Fig. 3), minorities were 7.4% [3.7%, 11.1%] more likely to initiate HPV vaccination when using provider-verified vaccination data, while self-reported data showed no difference (RD: 0.8% [‒2.4%, 4.1%]). Provider-verified initiation was higher for each minority group, with the largest advantage for Hispanic teens (8.1% [4.6%, 11.7%]). For studies including patients ages 18 and older, provider-verified initiation was higher for minorities overall (RD: 4.6% [0.1%, 9.3%]) and for Hispanics (RD: 5.7 [0.6%, 10.9%]), but Blacks and Asians did not differ from Whites. However, self-reported initiation was lower for each racial and ethnic minority as well as overall as compared to whites (RD: −5.8% [‒8.2%, ‒3.4%]).
Figure 3.
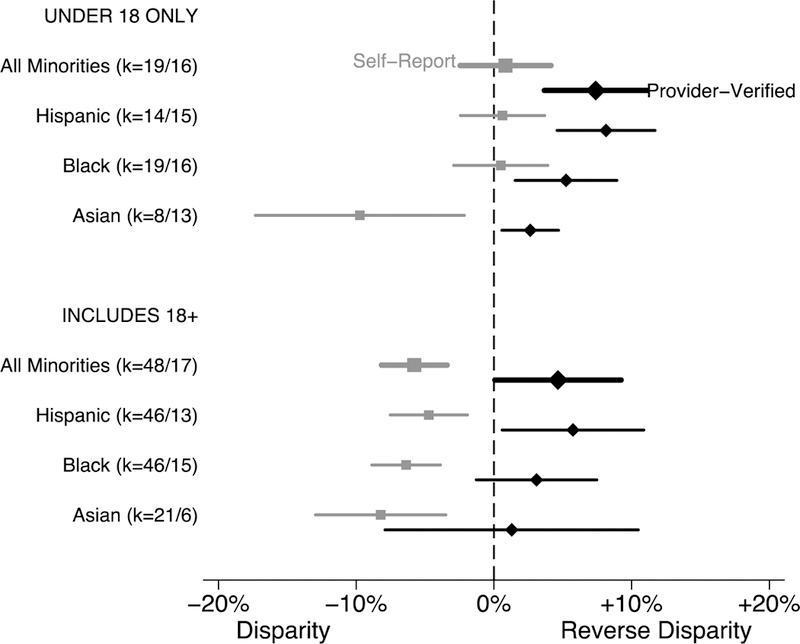
Difference in HPV Vaccine Initiation Between Minority and White, by Age and Vaccination Information Source
The largest predictor of minority-White differences in HPV vaccine initiation was source of vaccination data, in bivariate meta-regressions (Table 2). Provider-verified initiation was 9.6% higher among minorities than Whites, as compared to the same difference for self-report. Other indicators of data source were statistically significant in bivariate meta-regressions, including use of data from NIS—Teen and use of administrative, rather than self-report, data for race and ethnicity. Additionally, later year of data collection was associated with increasing difference in favor of minorities (i.e., disparities getting smaller), and racial and ethnic differences in HPV vaccine initiation among males were 8.7% larger (favoring minorities) than for females (p = 0.17), in bivariate meta-regressions. Significant disparities were seen in studies that included older individuals while studies restricting to target vaccination ages (under 17) showed significant differences in favor of minorities. In a multivariable meta-regression, vaccination data source remained statistically significant, as did age and sex, but year of data collection was no longer a significant predictor of racial differences in vaccination (Supplemental Table 2).
Table 2.
Correlates of Effect Size (Difference in HPV Vaccine Initiation between Minority and White)
| HPV VACCINE INITIATION | n | k | Effect Size | I2 | Difference | P | |
| All studies | 1,751,281 | 94 | 0.0% [-1.8%,1.8%] | 98 | - | - | |
| Correlate | |||||||
| Publication Year | 2009–2010 | 557,222 | 25 | −3.0% [−6.4%,0.3%] | 92 | ref | - |
| 2012–2014 | 248,137 | 40 | −0.3% [−2.9%,2.4%] | 97 | 2.8% | 0.20 | |
| 2015–2017 | 945,922 | 29 | 2.9% [−0.1%,6.0%] | 97 | 6.0% | 0.01 | |
| Sampling of Clusters | National | 1,430,561 | 60 | −0.2% [−2.5%,2.1%] | 98 | ref | - |
| Regional/Single Site | 320,720 | 34 | 0.1% [−2.7%,2.9%] | 98 | 0.3% | 0.88 | |
| Sampling of Individuals | Probability | 1,369,523 | 43 | 1.0% [−1.8%,3.8%] | 98 | ref | - |
| Convenience | 381,758 | 51 | −0.8% [−3.1%, 1.5%] | 98 | −1.8% | 0.34 | |
| Sex | Female | 922,842 | 80 | −1.6% [−3.5%,0.4%] | 97 | ref | - |
| Male | 828,439 | 26 | 7.1% [3.9%,10.3%] | 99 | 8.7% | <.001 | |
| Age Included (years) | Under 18 only | 1,201,965 | 35 | 4.3% [1.7%,6.9%] | 98 | ref | - |
| Includes 18+ | 549,316 | 65 | −2.5% [−4.6%, −0.4%] | 97 | −6.8% | <.001 | |
| Source of Race and Ethnicity Data | Self-Report | 448,820 | 79 | −1.3% [−3.2%,0.6%] | 97 | ref | - |
| Administrative | 1,302,461 | 15 | 5.5% [1.5%,9.5%] | 99 | 6.8% | 0.003 | |
| Source of Vaccination Data | Self-report | 264,260 | 64 | −3.6% [−5.5%, −1.7%] | 95 | ref | - |
| Provider-verified | 1,487,021 | 30 | 6.0% [3.6%,8.5%] | 99 | 9.6% | <.001 | |
| NIS—Teen Data | No | 1,604,353 | 85 | −1.1% [−2.9%,0.6%] | 98 | ref | - |
| Yes | 146,928 | 9 | 7.9% [3.3%, 12.5%] | 98 | 9.0% | <.001 | |
| HPV VACCINE FOLLOW-THROUGH | |||||||
| All studies | 355,044 | 41 | −9.6% [−12.1%, −7.1%] | 96 | - | - | |
| Correlate | |||||||
| Publication Year | 2009–2010 | 169,326 | 12 | −15.1% [−18.9%, −11.2%] | 92 | ref | - |
| 2012–2014 | 42,228 | 12 | −6.6% [−10.8%, −2.5%] | 90 | 8.4% | 0.004 | |
| 2015–2017 | 143,490 | 17 | −6.5% [−10.0%, −3.0%] | 90 | 8.6% | 0.001 | |
| Sampling of Clusters | National | 294,758 | 28 | −10.5% [−13.6%, −7.5%] | 97 | ref | - |
| Regional/Single Site | 60,286 | 13 | −7.7% [−12.1%, −3.3%] | 93 | 2.8% | 0.30 | |
| Sampling of Individuals | Probability | 291,971 | 26 | −9.7% [−13.0%, −6.5%] | 97 | ref | - |
| Convenience | 63,073 | 15 | −9.4% [−13.5%, −5.4%] | 94 | 0.3% | 0.91 | |
| Sex | Female | 259,774 | 39 | −9.4% [−11.9%, −6.8%] | 96 | ref | - |
| Male | 95,270 | 10 | −5.7% [−10.9%, −0.6%] | 87 | 3.6% | 0.22 | |
| Age Included (years) | Under 18 only | 163,432 | 16 | −8.0% [−11.8%, −4.2%] | 95 | ref | - |
| Includes 18+ | 191,612 | 27 | −10.9% [−14.0%, −7.7%] | 95 | −2.9% | 0.25 | |
| Source of Race and Ethnicity Data | Self-Report | 71,130 | 23 | −10.0% [−13.4%, −6.5%] | 93 | ref | - |
| Administrative | 283,914 | 18 | −9.2% [−13.0%, −5.5%] | 98 | 0.8% | 0.77 | |
| Source of Vaccination Data | Self-report | 7,817 | 9 | −13.7% [−19.3%, −8.1%] | 74 | ref | - |
| Provider-verified | 347,227 | 32 | −8.6% [−11.4%, −5.9%] | 97 | 5.1% | 0.11 | |
| NIS—Teen Data | No | 299,188 | 33 | −10.6% [−13.4%, −7.7%] | 96 | ref | - |
| Yes | 55,856 | 8 | −6.3% [−11.5%, −1.2%] | 96 | 4.2% | 0.16 | |
Note. Effect size is the difference in HPV vaccine initiation between minority and White. Negative numbers indicate a disparity, minorities had lower HPV vaccination coverage than Whites; positive numbers indicate a reverse disparity. NIS—Teen = National Immunization Survey—Teen.
Forty-six studies provided comparisons of racial or ethnic differences in HPV vaccine initiation after controlling for characteristics such as socioeconomic status and healthcare access (Supplemental Fig. 2). Using provider-verified data, Hispanic individuals remained more likely than Whites to initiate HPV vaccine initiation (aOR): 1.29 [1.04, 1.59]. Differences in initiation between Black and White individuals were not present. We could not examine adjusted Asian-White differences in provider-verified initiation because too few studies reported these findings.
3.3. Meta-analysis of HPV vaccine follow-through
HPV vaccine follow-through showed a large disparity across 42 studies reporting unadjusted data. Minorities were 9.4% [11.9%, 7.0%] less likely to follow-through with HPV vaccination than Whites (Fig. 2b, Supplemental Fig. 3). Disparities were generally larger when vaccination data were self-reported, although this difference was not statistically significant. Follow-through disparities were largest among Blacks (Provider-verified RD: ‒11.8% [‒14.3%, ‒9.2%]) followed by His-panics (Provider-verified RD: ‒5.2% [‒8.2%, ‒2.1%]) when compared to Whites (Fig. 2b). No differences in HPV vaccine follow- through were present when comparing Whites and Asians. Year of data collection was the only study characteristic predictive of minority-White differences in HPV vaccine follow-through in bivariate meta-regressions. Disparities shrank over time (Table 2) but remained statistically significant in all years, even in multivariate analyses (Supplemental Table 2). The disparity in follow-through was smaller among males than females. In studies that reported adjusted estimates, HPV vaccine follow-through remain higher among Hispanics (aOR: 0.83 [0.73, 0.95]) and Blacks (aOR: 0.62 [0.56, 0.70]) relative to Whites (Supplemental Fig. 1).
3.4. Publication bias
Visual inspection of funnel plots for HPV vaccine initiation unadjusted effect sizes (Supplemental Fig. 4) showed slight asymmetry, suggesting a lower than predicted number of small studies showing higher vaccination rates among minorities. Stratifying studies by source of HPV vaccination data suggested this asymmetry is likely due to differences in study quality rather than publication bias, with smaller studies generally more likely to rely on self-reported vaccination. Quantitative analysis using Egger’s test of bias suggested found no asymmetry for HPV vaccine initiation (p = 0.08) or follow-through (p = 0.35). Too few studies with adjusted effect sizes were available to support examination of publication bias.
4. Discussion
In the presence of large racial and ethnic disparities in HPV cancers, it is particularly important to understand corresponding differences in HPV vaccination. In our meta-analyses of data for over three million people in the US, high-quality data showed strong evidence of higher HPV vaccine initiation among racial and ethnic minority adolescents. In contrast, Black and Hispanic patients were less likely than Whites to follow-through with the full vaccine series after initiating. Finally, we found that self-reported HPV vaccine initiation yielded biased estimates of minority-White differences, suggesting inequity that does not exist. Our findings offer a surprising resolution to the longstanding disagreement over whether minorities have higher or lower HPV vaccination coverage than Whites.
The driver of these vaccination patterns remains unclear but are likely a result of systematic differences in care by race and ethnicity. Studies assessing perceptions of HPV vaccine have not generally found racial or ethnic difference in vaccine acceptability (Brewer and Fazekas, 2007), making it unlikely that differences are related to patient preferences. Therefore, we consider the role of both system-level and provider-level differences. On a system level, location of care differs by race and ethnicity with minorities more likely to receive care from safety-net providers (Henry et al., 2016) and health departments (Bekemeier et al., 2014). As provider recommendation is the strongest predictor of vaccine initiation (Gilkey et al., 2016), higher initiation rates may be a result of more frequent recommendations in these settings (Aragones et al., 2013). Safety-net providers are also generally associated with lower continuity of care (Doescher et al., 2001), potentially resulting in lower follow-through among minority patients. Additionally, the differences may be a result of provider-level factors, including implicit bias and communication around the vaccine. Previous studies have shown that more efficient, directive physician communication around HPV vaccination is associated with higher uptake (Sturm et al., 2017; Brewer et al., 2017). The tendency of providers to be more directive with minority patients (Cooper and Roter, 2003; Johnson et al., 2004) may result in higher uptake. However, the lack of informed discussion may result in worse patient recall of vaccines received (and associated (O’Leary et al., 2018; Otanez and Torr, 2017) under-reporting of vaccination) with the consequence of lower follow-through among minority individuals (Hirth et al., 2016; Attanasio and Mcalpine, 2014). Differential misreporting of HPV vaccination among minorities and other underserved populations (Vu et al., 2019) suggests that researchers should take care in interpretation when using self-report HPV vaccination, particularly when focusing on health disparities, as current approaches may lead to biased estimates both of HPV vaccination overall and among key ethnic and racial groups.
We additionally found that the unadjusted magnitude of initiation disparities declined over time, from no difference in the early years of the vaccine, to a difference in favor of minorities in later years. This shift appeared to be a result of changes in the composition of study populations (increasingly incorporating males and focusing on younger ages) and changes in study methods (more studies using provider-verified data). A similarly sized reduction over time in disparities for HPV vaccine follow-though occurred (~9%). However, declining disparities in follow-through are not explained by changing study populations and merit further study.
The finding of higher HPV vaccine initiation among minorities is both promising and unique, as racial and ethnic minorities are generally less likely to receive adolescent preventive services (Flores and Tomany-Korman, 2008). Vaccine initiation likely confers the main benefit, with potentially smaller additional benefit from additional doses (Hariri et al., 2018; Kreimer et al., 2015). Dosing schedules for younger adolescents have dropped from three to two doses (Meites et al., 2016), and ongoing studies are even considering whether one dose of HPV vaccine confers full protection (Sampson et al., 2018). Therefore, even in the presence of lower follow-through, racial and ethnic disparities in HPV cancers may be reduced in the future (Burger et al., 2016).
4.1. Limitations
The quality of race data was low in many studies, suggesting some tentativeness for conclusions based on our findings. Few studies reported the missingness of race in their samples, and no studies assessed potential effects of missingness on their findings. Studies that reported missingness in race data typically excluded these individuals or imputed race from other available data such as last name (Walker et al., 2017; Chao et al., 2010). For primary studies with incomplete data on race and ethnicity, authors should report the extent of any missingness as well as the analytic approach for the missing data and potential implications on findings.
Data were insufficient for examination of disparities other than between White and Black, Hispanic and Asian participants. Native Americans have disproportionately high rates of HPV-associated cancer (Siegel et al., 2018), making HPV vaccination in this group particularly important. Similarly, studies generally categorized Asian and Hispanic samples as single groups despite the presence of culturally distinct subgroups. For example, among Hispanics, vaccination rates are higher among those born in the US and who speak English as a primary language (Gerend et al., 2013). Consideration of minority groups beyond Black, Hispanic, and Asian as well as subpopulations within these broad racial and ethnic groups is important but requires high quality information on race and intentional oversampling of these groups.
Included studies represent a variety of settings within the US which may reflect varied state policies, health care resources, and demographic makeup. While we found higher HPV vaccination among minorities overall, we are unable to explore heterogeneity within the US, especially among small areas such as counties and census tracts. Additionally, racial and ethnic disparities may look very different outside the US (Fisher et al., 2014; Sadry et al., 2013), suggesting an important area for future research.
Included studies defined follow-through as completion of three HPV vaccine doses among individuals with at least one dose. However, in late 2016, new ACIP guidelines reduced the total number of recommended HPV vaccine doses from three to two for individuals who begin the series before the age of 15 (Meites et al., 2016). While the schedule change will likely result in an increase in follow-through overall, it is unclear whether this change will affect racial and ethnic disparities in vaccine follow-through.
Finally, we note that while unadjusted estimates are the most informative for understanding the direction and extent of differences in patterns of care, adjusted estimates can help to provide insight into underlying causes of racial and ethnic differences. However, we found choice of covariates varied considerably across studies, including different combinations of socioeconomic characteristics, healthcare utilization measures, provider recommendation, and knowledge of HPV vaccine. Each model, therefore, presented a fundamentally different estimate of the residual differences, making a pooled estimate difficult to interpret. Future studies should clearly identify proposed mechanisms for observed racial and ethnic differences and provide strong theoretical justification for covariate selection (VanderWeele and Robinson, 2014).
5. Conclusion
HPV vaccine is an extraordinary breakthrough in cancer prevention. Ensuring all groups have the potential to benefit from this advancement is crucial. In high quality studies, minorities had the same or higher HPV vaccine initiation rates than Whites but were less likely to follow- through with all recommended doses. Given low HPV vaccination coverage overall, programs should consider interventions to increase initiation for all groups. A focus on minority groups should exist in the context of overall improvement in HPV vaccination, as no group has yet reached the nation’s target for HPV vaccination. Importantly, higher HPV vaccine initiation among minorities has the potential to decrease in racial and ethnic disparities in HPV cancers in the future. A better understanding of the system-, patient-, and provider-level factors leading to higher uptake among generally underserved groups may help to inform interventions to increase utilization of other preventive services for minority adolescents.
Supplementary Material
Acknowledgements
We thank Teri Malo, Parth Shah and Dongyu Zhang for their contributions in the early stages of this work. We also thank Yael Symes and Tsion Ghedamu for their assistance with data extraction. Finally, we thank Tanja Walker and Shannon Stokley for providing additional data from the National Immunization Survey—Teen for this analysis.
Authors’ time (Spencer and Calo) was partially supported by the National Cancer Institute’s training grant R25CA11633 and T32CA11633. Funders played no role in (a) study design; (b) the collection, analysis, and interpretation of data; (c) the writing of the report; or (d) the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Dr. Brewer has received commercial research grants from Merck and Pfizer and served as a paid advisory board member for Merck. He is chair of the National HPV Vaccination Roundtable which is funded by CDC and hosted by the American Cancer Society.
Footnotes
Disclosures
The other authors have no financial disclosures or potential conflicts of interest to report.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2019.03.037.
References
- Aragones A, Bruno D, Gany F, 2013. Attitudes surrounding implementation of the HPV vaccine for males among primary care providers serving large minority populations. J. Health Care Poor Underserved 24 (2), 768–776. 10.1353/hpu.2013.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio L, Mcalpine D, 2014. Accuracy of parental reports of children’s HPV vaccine status: implications for estimates of disparities, 2009–2010. Public Health Rep. 129 (3), 237–244. 10.1177/003335491412900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakitas MA, 2007. Background Noise: The Experience of Chemotherapy-induced Peripheral Neuropathy. 56 10.1097/01.NNR.0000289503.22414.79. [DOI] [PubMed] [Google Scholar]
- Bekemeier B, Grembowski D, Yang Y, Herting JR, 2014. Are local public health department services related to racial disparities in mortality? SAGE Open 4 (1). 10.1177/2158244014527989.2158244014527989-. [DOI] [Google Scholar]
- Brewer NT, Fazekas KI, 2007. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev. Med. (Baltim) 45 (2–3), 107–114. 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Gottlieb SL, Reiter PL, et al. , 2011. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex. Transm. Dis. 38 (3), 197–204. 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C, 2017. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 139 (1), e20161764. 10.1542/peds.2016-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger EA, Lee K, Saraiya M, et al. , 2016. Racial and ethnic disparities in human papillomavirus-associated cancer burden with first-generation and second-generation human papillomavirus vaccines. Cancer 122 (13), 2057–2066. 10.1002/cncr.30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2013. Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013 - United States. MMWR Morb. Mortal. Wkly Rep. 62 (29), 591–595 (doi:mm6229a4 [pii]). [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. VaxView. National Immunization Survey—Teen. https://www.cdc.gov/vaccines/vaxview/index.html, Accessed date: 26 November 2017.
- Chao C, Velicer C, Slezak JM, Jacobsen SJ, 2010. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am. J. Epidemiol. 171 (3), 357–367. 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson HW, Flagg EW, Koutsky L, et al. , 2013. Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States. Vaccine 10.1016/j.vaccine.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MA, Coutinho F, Phelan-Emrick DF, Wilbur MA, Chou B, Joshu CE, 2016. Predictors of human papillomavirus vaccination in a large clinical population of males aged 11 to 26 years in Maryland, 2012–2013. Cancer Epidemiol. Biomark. Prev. 25 (2), 351–358. 10.1158/1055-9965.EPI-15-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper L, Roter D, 2003. Patient-provider communication. The effect of race and ethnicity on process and outcomes of healthcare. In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare, pp. 552–593. https://www.ncbi.nlm.nih.gov/books/NBK220354/:, Accessed date 4 June 2018. [Google Scholar]
- Del Carmen MG, Avila-Wallace M, 2013. Effect of health care disparities on screening. In: Clinical Obstetrics and Gynecology. 56 pp. 65–75. 10.1097/GRF.0b013e31827af75a. [DOI] [PubMed] [Google Scholar]
- Doescher MP, Saver BG, Fiscella K, Franks P, 2001. Racial/ethnic inequities in continuity and site of care: location, location, location. Health Serv. Res. 36 (6 Pt 2), 78–89. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1383608&tool=pmcentrez&rendertype=abstract (Accessed April 27, 2018). [PMC free article] [PubMed] [Google Scholar]
- Fedewa SA, Sauer AG, Siegel RL, Jemal A, 2015. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol. Biomark. Prev. 24 (4), 637–652. 10.1158/1055-9965.EPI-15-0134. [DOI] [PubMed] [Google Scholar]
- Fisher H, Audrey S, Mytton JA, Hickman M, Trotter C, 2014. Examining inequalities in the uptake of the school-based HPV vaccination programme in England: a retrospective cohort study. J. Public Health (United Kingdom) 36 (1), 36–45. 10.1093/pubmed/fdt042. [DOI] [PubMed] [Google Scholar]
- Flores G, Tomany-Korman SC, 2008. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics 121 (2), e286–e298. 10.1542/peds.2007-1243. [DOI] [PubMed] [Google Scholar]
- Gerend MA, Zapata C, Reyes E, 2013. Predictors of human papillomavirus vaccination among daughters of low-income Latina mothers: the role of acculturation. J. Adolesc. Health 53 (5), 623–629. 10.1016/j.jadohealth.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT, 2016. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine 34 (9), 1187–1192. 10.1016/j.vaccine.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach P, Cook R, Gratzer B, et al. , 2017. Human Papillomomavirus vaccination among young men who have sex with men and transgender women in 2 US cities, 2012–2014. Sex. Transm. Dis. 44 (7), 436–441. 10.1097/OLQ.0000000000000626.Human. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri S, Schuler MS, Naleway AL, et al. , 2018. Human papillomavirus vaccine effectiveness against incident genital warts among female health-plan enrollees, United States. Am. J. Epidemiol. 187 (2), 298–305. 10.1093/aje/kwx253. [DOI] [PubMed] [Google Scholar]
- Henry KA, Stroup AM, Warner EL, Kepka D, 2016. Geographic factors and human papillomavirus (HPV) vaccination initiation among adolescent girls in the United States. Cancer Epidemiol. Biomark. Prev. 25 (2), 309–317. 10.1158/1055-9965.EPI-15-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth JM, Rahman M, Smithc JS, Berenson AB, 2014. Regional variations in HPV vaccination among 9–17 year old adolescent females from the BRFSS, 2008–2010. Hum. Vaccin. Immunother. 10 (12), 3475–3483. 10.4161/21645515.2014.980202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth J, Kuo YF, Laz TH, et al. , 2016. Concordance of adolescent human papillomavirus vaccination parental report with provider report in the National Immunization Survey-Teen (2008–2013). Vaccine 34 (37), 4415–4421. 10.1016/j.vaccine.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Roter D, Powe NR, Cooper LA, 2004. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am. J. Public Health 94 (12), 2084–2090. 10.2105/AJPH.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. , 2015. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 16 (7), 775–786. 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites E, Kempe A, Markowitz LE, 2016. Use of a 2-dose schedule for human papillomavirus vaccination — updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly Rep. 65 (49), 1405–1408. 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- O’Leary ST, Lockhart S, Barnard J, et al. , 2018. Exploring facilitators and barriers to initiation and completion of the human papillomavirus (HPV) vaccine series among parents of girls in a safety net system. Int. J. Environ. Res. Public Health 15 (2), 185 10.3390/ijerph15020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otanez S, Torr BM, December 2017. Ethnic and racial disparities in HPV vaccination attitudes. J. Immigr. Minor. Health 1–7. 10.1007/s10903-017-0685-2. [DOI] [PubMed] [Google Scholar]
- Rauscher GH, Johnson TP, Young IC, Walk JA, 2008. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol. Biomark. Prev. 17 (4), 748–757. 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Reiter PL, McRee AL, Pepper JK, Gilkey MB, Galbraith KV, Brewer NT, 2013. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am. J. Public Health 103 (8), 1419–1427. 10.2105/AJPH.2012.301189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadry SA, De Souza LR, Yudin MH, 2013. The impact of ethnicity on awareness and knowledge of and attitudes towards the human papillomavirus and vaccine among adult women. J. Obstet. Gynaecol. Can. 35 (11), 995–1003. 10.1016/S1701-2163(15)30787-8. [DOI] [PubMed] [Google Scholar]
- Sampson JN, Hildesheim A, Herrero R, Gonzalez P, Kreimer AR, Gail MH, 2018. Design and statistical considerations for studies evaluating the efficacy of a single dose of the human papillomavirus (HPV) vaccine. Contemp. Clin. Trials 68, 35–44. 10.1016/j.cct.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2018. Cancer statistics, 2018. CA Cancer J. Clin. 68 (1), 7–30. 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Smith GD, 2008. Investigating and dealing with publication and other biases. Syst Rev Heal Care Meta-Analysis Context Second Ed 323 (7304), 189–208. 10.1002/9780470693926.ch11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm L, Donahue K, Kasting M, Kulkarni A, Brewer NT, Zimet GD, 2017. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J. Adolesc. Health 61 (2), 246–251. 10.1016/j.jadohealth.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB, 2018. Trends in human papillomavirus-associated cancers — United States, 1999–2015. MMWR Morb. Mortal. Wkly Rep. 67 (33), 918–924. 10.15585/mmwr.mm6733a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Robinson WR, 2014. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology 25 (4), 473–484. 10.1097/EDE.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu M, Luu M, Haardörfer R, Berg CJ, Escoffery C, Bednarczyk RA, 2019. A multilevel analysis of factors influencing the inaccuracy of parental reports of adolescent HPV vaccination status. Vaccine. https://doi.org/10.1016ZJ.VACCINE.2018.12.032. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TY, Elam-Evans LD, Singleton JA, et al. , 2017. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb. Mortal. Wkly Rep. 66 (33), 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Moore PC, Green AL, 2013. Geographic variability in human papillomavirus vaccination among U.S. young women. Am. J. Prev. Med. 44 (2), 154–157. 10.1016/j.amepre.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KL, Smith ML, Rosen BL, Pulczinski JC, Ory MG, 2017. HPV vaccination status and mandate support for school-aged adolescents among college females: a descriptive study. J. Sch. Nurs. 33 (3), 232–245. 10.1177/1059840516659764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


