Abstract
Anatomical observations, theoretical work and lesion experiments have led to the idea that an important function of the dentate gyrus of the mammalian hippocampus is pattern separation, a neural computation that ensures new memories are encoded without interference from previously stored memories that share similar features. The dentate gyrus also exhibits a unique form of neural plasticity that results from the continuous integration of newly born excitatory granule cells, termed adult hippocampal neurogenesis. However, the manner in which adult neurogenesis contributes to dentate gyrus network activity and computations is incompletely understood. Here, we first describe the prevailing models for the role of adult neurogenesis in dentate gyrus network function and then re-evaluate these models in the light of recent findings regarding the in vivo activity of the dentate gyrus and synaptic interactions of adult born granule cells with local circuit components, as well as, inputs, and outputs of the dentate gyrus. We propose that adult neurogenesis provides flexibility for the dentate gyrus to rapidly generate a context specific, distributed representation of important sensory stimuli such as spatial cues, which ultimately gives rise to behavioral discrimination.
The dentate gyrus is one of the few areas in the mammalian brain in which new excitatory neurons are continuously generated throughout life. In addition, the dentate gyrus is characterized by largely unique neural circuitry and population activity. For instance, despite containing multiple excitatory cell types (adult-born, mature granule cells and mossy cells), its activity is dominated by inhibition, and therefore shows very low firing rates. It is therefore tempting to think that the unique properties of the dentate network could not only help to explain the reasons for adult neurogenesis in the region, but also that the study of the effects of neurogenesis on the dentate network could be instrumental in helping to better understand the distinct role for the dentate gyrus in hippocampal information processing. Here we review recent findings related to the function of the dentate gyrus in rodent spatial memory and the effects of adult-born granule cells on dentate network physiology with the goal of beginning to unify these ideas and clarifying the function of adult neurogenesis in the context of our evolving understanding of neural computations in the dentate gyrus. In doing so, we hope to answer the question of how such a small population of neurons can mediate its observed substantial effects on animal behavior.
1. The role of dentate gyrus and adult neurogenesis in spatial and contextual discrimination behaviors
The mammalian hippocampus has been shown to be a key area of the brain necessary for the encoding of spatial and episodic memories, however many questions remain unanswered regarding its computational functions as well as the mechanisms by which these are accomplished. The stimuli which comprise spatial and episodic memories often have many overlapping sensory features and consequently, making sense of the external world requires continuous discrimination of features and configurations of stimuli in order to precisely encode and recall experiences. Converging evidence across a number of species and approaches indicates that the dentate gyrus (DG) of the hippocampus is essential for contextual and spatial discrimination behaviors [1,2], suggesting that the region may play an important role in this process. While the DG has remained a relatively underexplored hippocampal sub-region with respect to patterns of in vivo activity due to its sparse neural firing, its unique anatomy has yielded theories for a distinct role in hippocampal function that mirror these behavioral findings [3]. This structure is densely packed with relatively small granule cells (GCs) that outnumber their input and output populations and differ markedly from the pyramidal neurons that make up the majority of excitatory neuronal populations elsewhere in the hippocampus and cortex. Accordingly, theoretical studies have proposed that the DG is involved in the formation of new memories protected from interference from previously stored memories by generating highly distinctive hippocampal representations of similar entorhinal inputs, through a neural computation known as pattern separation [4,5].
Adult born granule cells develop over several weeks through a highly orchestrated, multi-step neural differentiation and maturation process to reach a fully mature neuronal phenotype. Most evidence indicates that the functional properties of these neurons ultimately mirror those generated during development. However, at four to six weeks of age while still immature they display increased excitability and enhanced long-term plasticity, leading to theories about a distinct role for this developmental stage in DG function [6-9]. During this period immature granule cells (iGCs) have been shown to be behaviorally important for the encoding of new memories in the hippocampus, suggesting that they influence the function of the DG-CA3 network in a manner critical for the overall role of this region [10-12]. Moreover, recent behavioral studies using cell type-specific optogenetic or chemogenetic manipulations have suggested that iGCs transiently but distinctly contribute to encoding or retrieval stages of DG-dependent contextual and spatial discrimination behaviors in an experience dependent manner [11,13-16]. While measurement of activity within the iGC population during individual stages of discrimination tasks remains uncharted territory, these findings suggest that functionally integrated albeit synaptically immature iGCs play a distinct role in DG network dynamics compared to their mature counterparts.
Nonetheless compared with the large number of mature granule cells, it is still unknown how such a small number of iGCs constituting less than 5% of all GCs [17] exert their influence on DG circuit function. Based on anatomical, computational and behavioral studies, conceptual models for the role of adult neurogenesis in DG network function generally fall within two frameworks that describe the direct or indirect impact of iGCs on the DG network [18]. The first model, “direct encoding”, suggests that owing to their enhanced excitability as well as their functional inputs from the entorhinal cortex and outputs to the CA3, 4-6 week old iGCs preferentially encode new information during novel environmental exposure. Once mature, these GCs are thought to selectively represent the context in which they matured, providing a cellular substrate by which non-overlapping immature and mature iGCs may associate novel and familiar memory representations that occurred close together in time [12,19,20]. The second model suggests that iGCs (4-6 weeks old) modulate the activation of mature GCs (>8 weeks old) to increase the sparseness of DG representations indirectly, either by recruiting inhibitory interneurons or by competing for synaptic connections, and promote the formation of non-overlapping cell assemblies of mature GCs representing each experience [13,21-23]. While in vivo confirmation of these two models is still lacking, recent advances in functional studies in vivo as well as optimized circuit mapping technologies have revealed important functional components of the local DG network, which necessitate an update in the simplified direct vs. indirect models of iGC function. In the following sections we re-evaluate the classical concepts of iGC function in light of recent discoveries regarding the in vivo activity and network interactions of local DG circuit components, as well as inputs and outputs of the DG. We will then explore how these insights might be utilized to understand DG population encoding during behaviors in which iGCs have been demonstrated to be critical [24].
2. How do iGCs functionally interact with the local Dentate Gyrus circuitry?
The DG is composed of a densely packed granule cell layer (GCL), the overlying molecular layer, and the underlying polymorphic cell layer, the hilus. The molecular layer contains the dendrites of granule cells where they receive excitatory inputs from the entorhinal cortex and hilar mossy cells, as well as feedforward and feedback inhibition from local interneurons (Figure 1). The hilus contains granule cell axons, termed the mossy fibers, which form excitatory synapses on hilar mossy cells and inhibitory interneurons as they project to area CA3, where they subsequently synapse onto pyramidal cells and CA3 interneurons. Adult-born granule cell progenitors arise from the adult neural stem cell pool consisting of radial-glia-like precursors in the subgranular zone lining the border between GCL and hilus, which divide asymmetrically and migrate into the GCL in a series of activity-dependent steps [25]. Here they integrate into the existing local DG network where they acquire many of the long-range connections typical of mature granule cells, and synapse locally upon inhibitory interneurons as well as mossy cells. Activity patterns in the DG network in vivo are produced by an interplay between these populations of neurons, and thus the question of how iGCs interact with these circuit elements is important for our understanding of their utility in DG computations. Furthermore, recent work has begun to expand our understanding of neural representations in mature and iGCs and how they are modulated locally by interneurons and mossy cells, which is likely to illuminate the function of iGCs in the adult hippocampus.
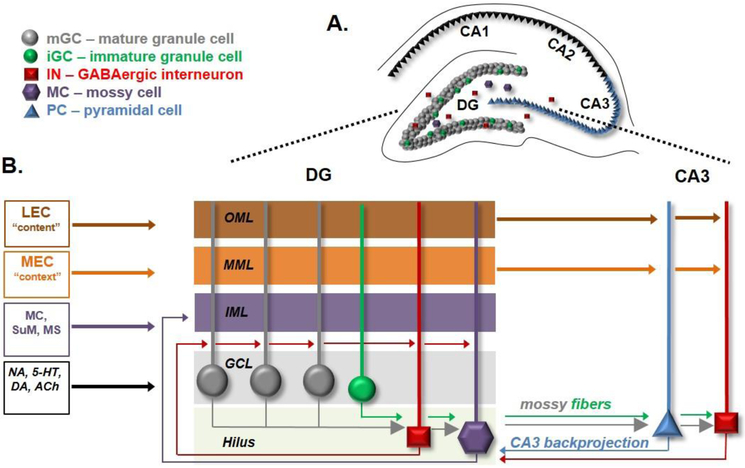
Figure 1. The extended dentate gyrus circuit.
A. The mammalian hippocampus is typically divided into areas CA1, CA2, CA3, and the dentate gyrus (DG). B. Schematic shows the laminar organization of the DG composed of the molecular layer (ML), granule cell layer (GCL) and hilus. The GCL contains the mature and adult born granule cells while the hilus contains the mossy cells and interneurons. The lateral entorhinal cortex (LEC) and the medial entorhinal cortex (MEC) innervate the outer (OML) and middle (MML) sections of the ML respectively. Mossy cells (MC), supramammillary nucleus (SuM) and medial septum innervate the inner (IML) portion of the ML. The hilus receives diverse inputs including axons of the granule cells as well as neuromodulatory inputs such noradrenergic (NA), 5-hydroxytryptamine (5-HT, serotonergic), dopaminergic (DA) and cholinergic (ACh) inputs. The outputs from the DG arise from GCs that project to the mossy cells (MC) and interneurons (IN) in the hilus and pyramidal cells (PC) and IN in the CA3. IN and MC in the hilus receive backprojections from CA3 PCs.
2.1. Sparse neural representation by dentate GCs:
Granule cells are the principal excitatory cell type in the DG and provide its only known outputs, to hippocampal area CA3. They display a number of features presumed to be critical for generating sparse and distinct neural representations of new memories without interference from previously stored memories. Strongly hyperpolarized resting membrane potentials and robust dendritic voltage attenuation results in a high activation threshold, which underlies low baseline activity levels in GCs in vivo [26,27]. Expansion of the number of units from the entorhinal cortex (EC) to the DG, in which granule cells are ~5 times more numerous than upstream EC pyramidal neurons [28], also contributes to a sparse encoding scheme as projections from individual neurons in the EC network form only a small number of synapses on each GC, yet diverge widely in the layer. Thus it is likely that a large number of inputs from distinct afferent entorhinal cortical neurons must converge in order to fire a single GC action potential. In addition, strong inhibition from local interneurons [29] and lack of direct recurrent connections between GCs [30] ensures that the mean firing rate of GCs, as well as the proportion of active cells, is low. Indeed, studies examining immediate early gene expression (IEG, such as cFos or Arc) to visualize activated neural ensembles have suggested that only a small subset of GCs (~2%), predominantly located in the outer two-thirds of the GCL suggesting a mature granule cell (mGC) identity, display behaviorally driven activation [31,32]. This is in contrast to IEG activation in ~18% and ~35% of CA3 and CA1 pyramidal neurons, respectively, in response to recent experience [33].
While IEG expression studies have been useful in mapping which cells are recruited during different behavioral experiences, to understand how different populations of neurons represent specific features of space and memories requires direct measurement of the activity of GCs in behaving animals. In the rodent hippocampus, the most distinct correlate of neural activity in all hippocampal subregions during behavior is the spatial selectivity of ‘place cells’, which collectively form a spatial map of distinct locations within the animal’s environment [34]. One feature of hippocampal place cells is that they often change their firing rate or preferred firing location in a discontinuous fashion when the environment changes, known as rate or global remapping, respectively [35]. Both rate and global remapping of spatial receptive fields are thought to support contextual discrimination behaviors through unique representations of environments and behavioral experiences with varying levels of overlap with previously stored ones. While remapping has been found in all hippocampal subregions, this feature is most consistent with the proposed pattern separation function of the DG. Similar to principal neurons in other regions of the hippocampus, GCs display spatially selective firing. However, until recently there has been contradictory physiological evidence regarding selectivity of spatial representations by GCs. While some studies have reported sparse firing GCs with single place fields that are as selective as those of CA3 place fields [36,37], other studies have reported multiple place fields in relatively active GCs that change their firing fields with subtle environmental manipulations to a greater degree than the CA3 pyramidal neurons [38]. These differences are likely because these studies utilize in vivo single-unit electrophysiological approaches, for which the yield and reliability in differentiating GCs from other cell types within the DG circuit is severely limited due to the small, densely packed nature of GCs, which make them uniquely recalcitrant to extracellular waveform clustering.
Recent studies have begun to characterize spatial representation in unambiguously identified GCs with more precise methods such as high channel density extracellular recording with spike feature analysis validated by optogenetic identification [39,40], juxtacellular recordings with morphological identification [41,42], as well as two-photon imaging of Ca2+ activity in genetically identified cell types [14,43-46]. These studies have shown that on average GCs fire very sparsely during the spatial navigation tasks in which they are typically measured. A fraction of GCs in all of these studies however were found to discharge relatively more frequently and show single distinct place fields in particular environments, comparable to place cells found in other hippocampal subregions. Despite such similarities, these studies showed an apparent discrepancy in remapping of DG firing when animals were exposed to different environments. In particular, Senzai and Buzsaki have found that in freely moving mice the same population of GCs represents two arenas, each with different visual cues on the walls but located in the same room, with small or no changes in their spatial rate maps [39]. Similarly, Hainmueller and Bartos have found that head-fixed mice exploring virtual environments with distinct visual cues have stable GC place firing [44], and do not show significant remapping between these contexts. In contrast, Danielson, et al., found that two different virtual environments with distinct textures on a circular treadmill belt and ambient multisensory stimuli (different olfactory, visual, and auditory stimuli) are represented by changes in spatial rate maps in the same mGCs (rate remapping) [14]. Furthermore, Goodsmith, et al., found that different chambers in different rooms are represented by unique populations of GCs with non-overlapping spatial rate maps (i.e. global remapping) [41]. While these divergent findings may at first appear to be in direct contrast (no remapping vs. rate remapping vs. global remapping), they could also tell us something about the types of information encoded in the DG. For instance, it is possible they reflect the distinct types and characteristics of sensory cues featured in the different ‘context’ discrimination tasks used by each study, such as global vs. local cues, or cues of different sensory modalities. Indeed, fundamental components of a context include spatial location, constellations of sensory cues as well as behavioral demands. But taken together, likely the most parsimonious explanation to reconcile these findings is that significantly different global cues lead to global remapping in GCs (non-overlapping populations of active neurons), while more slight changes in proximal cues lead to rate remapping (changes in spatial rate maps in the same neurons) or no remapping.
While position is the most distinct correlate of hippocampal neural activity, a parallel body of work suggests that other perceptual and behavioral variables, such as sensory cues [47,48] as well as goals [49,50], are encoded in addition to spatial location. Recent studies have found that GCs are strongly driven by visual [44] and tactile [40] reference cues, even though these neurons exhibit lower context specificity than neurons in CA3 or CA1. Together with a recent study that has demonstrated reward-modulated activity within the non-place coding population in the DG [51], new evidence suggests that sensory cues and goal locations strongly modulate the activity of GCs during spatial navigation tasks (Tuncdemir, Lacefield, Hen, unpublished observations). Thus, in order to understand the neural computations in the DG that organize contextual discrimination behaviors [52], it will be critical to investigate how specific components of a context are represented by individual GCs during these behaviors and to what extend individual GCs encode for similar components across different contexts . Future studies examining whether the DG primarily encodes fine-grained aspects of individual features of an environment, or whether it contains more complex representations selective for conjunctions of contextual features will provide important insights into how computations within the DG population support discrimination behaviors.
2.2. Neural representations by iGCs:
Recent studies investigating the recruitment of young versus mature GCs during encoding of contextual memories using IEG expression indicate that there is no preferential recruitment of iGCs into hippocampal memory networks [53,54], suggesting that iGCs are not the sole substrate for new memories. With the caveat that IEG induction in iGCs may not be coupled to neural activity in the same way as in mGCs [55], these studies indicate that even in the hyperplastic phase of young iGCs, the majority of neurons recruited into spatial memory networks during specific behavioral experiences will be drawn from the mGC pool [32,53,54]. Until recently, the functional contribution of iGCs to neural representations in the DG has been elusive due to the paucity of studies examining the dynamic properties of reliably identified iGCs in vivo. Danielson, et al., recently used in vivo 2-photon imaging to compare Ca2+ activity from genetically identified iGCs and mGCs in head-fixed mice running along a treadmill [14]. This work revealed that despite low overall activity levels in both populations, iGCs are more likely to be active and exhibit less selective spatial activity compared to mGCs. Among the spatially selective iGCs, sequential exposure to distinct treadmill environments (as described in the previous section) leads to similar levels of remapping of iGC firing fields compared to that of mGCs. Although reduced spatial tuning of the iGC population could in principle result from having multiple place fields[37], lower mutual information between the mouse’s position and activity of the iGC population suggests that the lower tuning specificity results from variable but not multipeaked tuning [14].
This study was the first to examine the in vivo activity of genetically identified iGCs [14] and extended previous findings showing that iGCs are more excitable however may be less suited for coding spatial information when compared to mGCs. However these findings have inspired questions about the spatial tuning of the subpopulations of young neurons and how these representations are used to guide behavior. For instance, this finding would seem to contradict a proposed role for young neurons in directly encoding novel information as their more promiscuous firing would likely decrease the network’s capacity for pattern separation, in contrast with behavioral findings. Furthermore, questions remain about the approach used in this study. First, since the activity of a mixed population of iGCs aged 6 weeks and younger was monitored, precise characterization of neural responses in iGCs on the cusp of maturity, or within their critical period during which they have been found to affect behavior, was limited. Thus more temporally precise iGC labeling, e.g. using retroviral methods, is needed to determine the changes in cellular and network dynamics of iGCs during the critical period as they transition from their early maturation phase to the later stage in which they have been hypothesized to execute computations distinct from mGCs. Second, the activity of iGCs during tasks that require memory discrimination will require further investigation, as the treadmill task did not require explicit use of information specific to each context. In particular, similar to their mature counterparts, investigating how iGCs represents non-spatial components of a context such as sensory cues and goals will likely provide a mechanistic link between in vivo activity of iGCs and their role in discrimination behaviors.
Taken together, these recent results provide new insights into the function of mature and adult born GCs through which to view the direct contributions of iGCs to the DG network function, however the question remains as to how such a small number of these cells exert an arguably outsized influence on behavior. In the following sections, we will highlight recent studies that have examined the role of iGCs within the local DG network, their impact on the CA3 outputs as well as on the inputs to the DG, which may serve to amplify their influence.
2.3. Contribution of iGCs to activity in the DG Network: monosynaptic and disynaptic effects.
Previous loss- and gain-of-function studies examining the impact of iGCs on local networks have suggested that manipulations of neurogenesis inversely correlate with the activity levels in the DG. In vivo recordings in anesthetized mice have shown that while eliminating adult neurogenesis leads to a decrease in afferent drive to the DG it also produces an increase in the amplitude of network oscillations, with an associated increase in synchronization of DG firing to these oscillatory bursts. Subsequent studies have provided evidence in support of the modulatory role of iGCs in regulating the neuronal activity of the larger population of mGCs [13,56,57] and in facilitating the recruitment of non-overlapping DG ensembles in response to distinct contexts [20]. These studies, along with the findings that chronically ablating neurogenesis results in decreased expression of vesicular GABA transporter vGAT [58] as well as increased IEG induction in mGCs [59], have led to the notion that the iGCs modulate the activity of the mGCs by recruiting feed-back inhibition via DG interneurons [22,23]. Nevertheless, whether di-synaptic inhibition of mGCs by iGCs influences the balance of excitation to inhibition within the network in a manner different than typical lateral inhibition among mGCs [29,60] remains elusive. Recent in vitro physiology studies in which iGCs were labelled at different stages of maturation with channelrhodopsin have shown that at 4 weeks of age iGCs poorly activate parvalbumin and somatostatin expressing interneurons [61,62], while recruiting increased levels of feedback inhibition onto mGCs after 6 to 8 weeks [21,61], In vivo confirmation of monosynaptic inputs from 4-6 week old iGCs onto diverse interneuron subtypes or extrasynaptic influences of young iGCs onto mGC-to-interneuron inhibitory circuits awaits further investigation, however a number of studies have highlighted the fact that the circuit interactions of iGCs with mGCs as well as DG interneurons are tightly coupled to behavioral experience. For example, ablation of neurogenesis increases IEG expression [59] and synaptic responses [63] in the DG specifically during the neurogenesis-dependent conflict condition of an active place avoidance task. In addition, optogenetic activation of iGCs decreases the size of the activated GC population only in conditions of novelty and anxiety [21]. Conversely a genetic manipulation that increases neurogenesis or a pharmacogenetic activation of iGCs results in a decrease in overall mGC activity after a chronic stress paradigm [13]. Furthermore, exposure to an enriched environment during their critical period produces a transient enhancement of innervation from DG interneurons onto iGCs [64]. Although further studies are necessary to determine whether the functional output from iGCs to inhibitory interneurons undergoes a similar activity dependent enhancement, cell-type selective connectivity mapping strategies together with task specific manipulation of iGC activity within the intact brain could shed light on this question. Taken together, these studies suggest that rather than broadly modulating sparse network coding in a uniform fashion, iGCs have a dynamic interaction with inhibitory networks such that, depending on their maturation stage and task demands, activation of iGCs shifts the excitation-inhibitory balance differentially to organize DG network activity during different behavioral contexts.
Recently, we discovered that iGCs can also influence the activity of mature GCs by direct monosynaptic inputs onto these GCs [65].These direct monosynaptic contacts which are reminiscent of the mossy fiber sprouting that both mGCs and iGCs display in response to seizures [66] are transient and disappear when iGCs reach maturity. Interestingly the impact of these monosynaptic connections is either excitatory (mediated by NMDA receptors) or inhibitory (mediated by mGluR2 receptors) depending on whether iGCs are receiving inputs rom the medial or the lateral entorhinal cortex. Together with a recent study demonstrating that blocking adult neurogenesis can reduce DG activity in response to an ambiguously cued fear conditioning task [66] , these studies reinforce the notion that the modulatory impact of iGCs on the DG may vary under different cortical inputs as engaged by distinct cognitive demands. However it is still unclear how relative contributions of direct, monosynaptic versus disynaptic pathways engaged by iGCs act to modulate mGCs.
2.4. Contribution of iGCs to DG network activity via competition with existing excitatory synapses
Another mechanism by which iGCs could impact activity levels in the DG local network is via synaptic competition between the nascent dendritic spines of newborn neurons and those of mature neurons [67]. Thus the presence of highly plastic young neurons may modulate mature granule cells by titrating away strong or inefficient connections from the entorhinal cortex onto mature neurons in the layer, decreasing their activity and increasing the sparseness of their activation and therefore enhancing their pattern separation function. Consistent with a redistribution of synapses between old and new neurons, it has been proposed that immature filopodial-like spines from young neurons invade, and may replace pre-existing afferent connections onto mature dendritic spines of mGCs [68]. By selectively manipulating the number of iGCs, recent studies lend further support to an inverse relationship between the number of iGCs and strength of synaptic inputs to the mGCs, which may occur through adjustment of the number of functional excitatory synapses on mature neurons [69,70]. Conversely, reversibly decreasing dendritic spine density of mGCs leads to an expanded population of age-matched iGCs [71], suggesting that synaptic competition dynamics are strongly coupled to network integration of iGCs. In turn, expansion of the cohort of iGCs at their critical period specifically decreases the overlap between ensembles of mGCs activated in response to temporally separated exposures to similar but not to distinct contexts, resulting in increased contextual memory precision [71]. Notably, redistribution of active synapses away from mGCs by integration of a larger number of iGCs will qualitatively have the same outcome as modulating the activity levels in the DG network via network inhibition [20,22,56], albeit on a slower temporal scale than the presumed interactions with inhibitory networks [21]. Indeed, increasing adult neurogenesis accelerates the gradual decay of long-term potentiation (LTP) in perforant path-to-DG synapses [72], while decreasing neurogenesis prolongs this type of LTP [73], indicating that neurogenic remodeling may impact the plasticity of the DG afferent circuit on a longer time scale. Moreover, environmental features and cognitive demands present during their maturation can alter iGC- dependent circuit reorganization of the DG. In addition to expanding the iGC population, environmental enrichment during their maturation changes the functional connectivity [64,74] as well as the timing of the integration of iGCs into hippocampal networks [75,76]. To dissociate the contribution of iGCs to DG network activity and computations future studies should investigate the impact of transient manipulations of iGCs activity on the mGC network over multiple temporal scales during behaviors that are dependent on neurogenesis. As such, these studies will help determine whether iGCs exert their actions through competition with excitatory synapses, disynaptic recruitment of inhibitory networks or monosynaptic activation of mGCs.
3. How do iGCs functionally interact with the CA3 network?
Mossy fibers, the axons of GCs, contact CA3 pyramidal neurons via the distinctly large and specialized mossy fiber terminals (MFTs), while filopodial extensions of these terminals selectively innervate nearby GABAergic interneurons in area CA3 [77] . Despite the sparse activation of the GCs, the large number of presynaptic release sites and active properties of the large MFT core facilitates reliable synaptic transmission from GCs to CA3 neurons [78]. Indeed, burst firing of a single GC [79] or, under some conditions, a single presynaptic MFT action potential [80] can reliably trigger spiking and cause synchronous co-activation among the downstream CA3 pyramidal neurons [39], supporting the idea that MFTs act as “detonator” synapses to these cells. On the other hand, small MFT filopodia that target CA3 interneurons far outnumber their larger counterparts [77] but have lower potency of synaptic transmission[81]. Hence, synchronous activation of a number of GCs in vivo [82], for example during a form of synchronous activity called “dentate spikes”, results in feed-forward inhibition of CA3 pyramidal neurons [83]. Taken together, findings of the target specificity and differential activity of the core and filopodia components of MFTs suggest that if iGC and mGCs fire at different frequencies they may differentially impact the CA3 network function.
Recent studies have begun expanding our understanding of the functional connectivity of iGCs with CA3 and are likely to illuminate how adult born GCs affect the CA3 network during discrimination behaviors. Optogenetic stimulation of 4 week old iGCs in vitro activates both excitatory and inhibitory synaptic responses in CA3 pyramidal neurons, with amplitudes similar to those of their mature counterparts [11,61]. Although these studies have suggested that iGCs are capable of relaying neural representations to downstream areas, the net impact of iGCs on the activity of CA3 network during their critical period may in fact be inhibitory. Structural analyses of presynaptic terminals suggest that morphological maturation of large MFT core targeting pyramidal neurons proceeds at a slower pace compared to those of small MFT filopodia contacting interneurons. For instance, 4 week old iGCs contact fewer CA3 pyramidal neuron spines, and with smaller synaptic vesicles and active zones [68,84], while they at the same time have a greater density of small MFT filopodia in the CA3 when compared to presynaptic terminals of both 6-8 week old iGCs as well as mGCs [85]. Consistent with these findings, optogenetic stimulation of 4 week old iGCs induces c-fos expression primarily in GAD67+ CA3 interneurons with an opposite pattern of activation in GAD67−, presumed pyramidal neurons upon stimulation of older iGCs [85]. By driving feed-forward inhibition in the CA3, iGCs may decrease the overlap between ensembles of CA3 activated by two similar but distinct environments associated with fear memories, resulting in increased contextual behavioral discrimination[53,86].
These studies are consistent with recent work suggesting that DG-CA3 inhibitory connections play critical roles in memory processing. Spatial and contextual discrimination learning leads to an increase in the number of MFT filopodial contacts with parvalbumin expressing CA3 interneurons with no discernible changes in the density of MFT core contacts onto CA3 pyramidal neurons [87,88]. Learning induced recruitment of feed-forward inhibition in CA3 is decreased over time in parallel to the generalization of remote contextual and spatial memories [87], and both connectivity and memory precision could be augmented by manipulating MFT cytoskeletal protein expression [87,88]. Taken together, these findings provide strong support for a relationship between learning associated changes in feed-forward inhibition of CA3 by GCs and discrimination behavior. However, determining whether MFTs arising from iGCs or mGCs differentially impact CA3 network function [12] will require cell-type specific manipulation of their activity or expression of MFT cytoskeletal factors, such as β-adducin [88] or ABLIM3 [87], and testing the resulting connectivity profiles and behavioral outcomes.
4. How do iGCs functionally interact with the inputs to the DG?
Understanding the computations involved in contextual and spatial representations by GCs requires a detailed analysis of the information encoded by its input structures. Afferent innervation to the GCs is highly segregated, with different inputs targeting distinct zones within the molecular layer of the DG [3]. The primary long-range inputs to the GCs are the glutamatergic projections from the entorhinal cortex, and the two major divisions of the entorhinal cortex synapse in largely non-overlapping regions of GC dendrites. Lateral entorhinal cortex (LEC) axons terminate in the outer one-third of the molecular layer while the medial entorhinal cortex (MEC) axons terminate in the middle one-third of the molecular layer. Glutamatergic projections from distally located mossy cells within the contra-and ipsi-lateral DG and hypothalamic supramammillary nucleus terminate in the inner one-third of the molecular layer, while neuromodulatory projections from other subcortical regions terminate in the hilus as well as the molecular layer.
The entorhinal cortex is the main interface between sensory cortical areas and the hippocampus, and its two major divisions are thought to form two functionally distinct processing streams in the DG. In particular, the MEC contains spatially modulated neurons (such as grid or border cells) and conveys movement related spatial inputs supporting hippocampus dependent spatial memory and the precision and stability of spatial firing patterns of place cells [89,90]. Recent optogenetic and imaging studies suggests that MEC neurons that project to DG contribute to context-dependent processing of fear memories [91] and navigation to a learned place [92] by drastically altering their firing rates between distinct environments or through a visual-cue-dependent sustained elevation in activity of DG specific projections, respectively. Despite the extensive body of knowledge regarding the dynamics of MEC, only a handful of studies have monitored or manipulated LEC activity. These studies have shown that neurons in the LEC show weak spatial modulation [93,94] but are more highly tuned to sensory inputs [95,96], supporting association of objects with environments [97] as well as neurogenesis dependent spatial discrimination [98]. Together, these data suggest that each of the entorhinal areas may support complementary modes of processing important for navigation, MEC for self-motion based navigation, or “path integration”, and LEC for sensory cue based navigation [99], and that these two channels of information are combined in the dendrites of individual granule cells. While the impact of MEC or LEC-specific projections on the activity of mGCs during spatial and contextual discrimination behaviors remains to be determined, cortical inputs to the mGCs can also be modulated by intrahippocampal or subcortical inputs depending on behavioral experience in order to integrate information about, e.g., behavioral state.
Located in the DG hilus region, mossy cells have extensive projections extending to the ipsilateral and contralateral DG [100]. Recent in vivo studies have shown that mossy cells fire frequently and have multiple place fields that strongly remap in different environments [39,41,43]. Precise behavioral impact of the pronounced differences in the spatial representations by mossy cells and mGCs awaits further investigation, but a number of studies have suggested that a major effect of mossy cells is to inhibit mGC network activity (Bui et al. 2018; reviewed in Scharfman 2016). In particular, a recent study demonstrated that optogenetic stimulation of mossy cell projections from contralateral DG reduces mGC firing when the stimulus precedes stimulation of MEC or LEC inputs [102]. Conversely, ascending projections from the supramammillary nucleus of the hypothalamus (SuM), long considered a key structure in the integration of cognitive and emotional aspects of goal-directed spatial learning behavior [103], potentiates mGC firing evoked by cortical inputs when temporally associated with MEC and LEC inputs [104,105]. Uniquely both mossy cells and SuM projections preferentially target the DG compared to CA3, unlike projections from the entorhinal cortex which similarly target both regions [3,104]. Since area CA3 receives the same direct cortical input from MEC and LEC as does the DG [3], modulatory control of the cortical inputs to the mGCs through selective SuM and mossy cell innervation may be critical for changing the activity patterns in the DG based upon an animal’s behavioral state.
An additional way that the cortical inputs onto the DG can alter the activity patterns in CA3 during discrimination behaviors is functional integration of iGCs into DG afferents. As such, recent work has begun to expand our understanding of differences in the long-range inputs to the iGCs compared to those of mGCs, which may help explain the effects of adult neurogenesis on behavior. Studies using the rabies virus-based monosynaptic retrograde tracing method in conjunction with retroviral or genetic labeling of iGCs [98,106] or mGCs [107], respectively, to map brain-wide monosynaptic inputs to these subtypes have revealed that 4 week old iGCs receive input from largely similar regions compared to mGCs. Despite their common input sources, recent evidence indicates that iGCs receive preferential inputs from the LEC compared to the relatively balanced inputs from LEC and MEC to mGCs [98,108]. In addition, a recent study from our group has shown that iGCs reduce the activity of mGCs in response to LEC inputs but increase the activity of mGCs in response to MEC inputs [65], providing a crucial insight into the mechanisms by which differential afferent wiring of iGCs functionally regulates the activity of mGCs. Furthermore, differential input from LEC vs. MEC populations onto iGCs may indicate a selective involvement of adult-born neurons in the encoding of sensory cues by the DG. While future studies will be necessary to elucidate whether behavioral experience differentially modulates afferent connectivity of young [64,74] and mature GCs, a unique function of iGCs may be to increase the dynamic range of cortical and/or subcortical inputs to the local mGC network in response to different behavioral stimuli. Selective recruitment of iGCs by different entorhinal inputs or other afferents might provide additional flexibility to the DG to modulate the encoding of sequences of important behavioral events in the downstream CA3 recurrent network.
5. An updated mechanistic view of adult neurogenesis in DG circuit function: focus on CA3
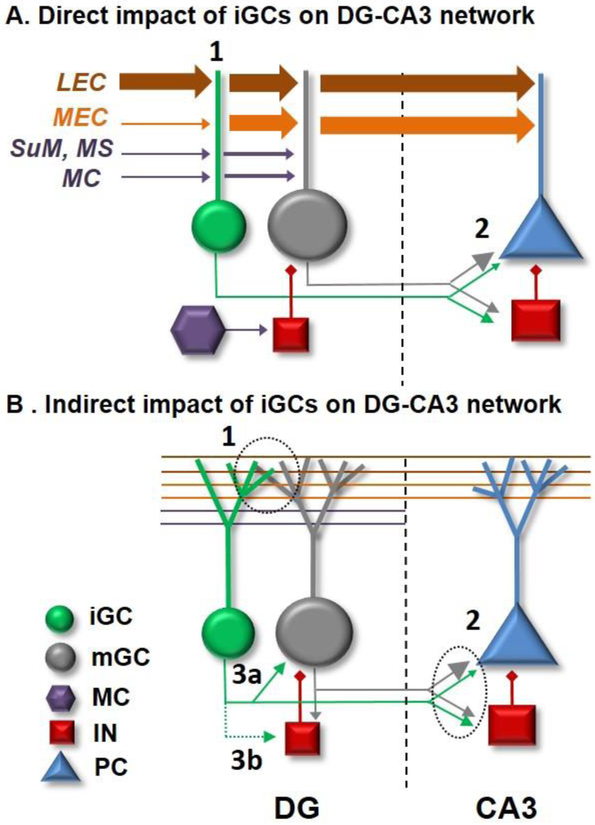
Ultimately, the behaviorally relevant effects of iGCs on the DG network are contingent upon outputs of the DG to CA3. As we have summarized in Section 2.1, the main DG outputs to CA3 include a relatively active subpopulation of GCs which are highly tuned to discrete features within an environment such as sensory cues (sensory stimuli or objects encountered during navigation) despite appearing to have lower context selectivity than mossy cells, or neurons in CA3 and CA1 [39-41,43,44]. These representations are putatively supported by the multisensory inputs from LEC and MEC, which send parallel projections to both DG and the CA3. Taking the recent studies into consideration, we propose an updated hypothetical dual circuit model by which iGCs can directly and/or indirectly alter the activity patterns in the DG-CA3 network. As a consequence of the stronger inputs from LEC than MEC onto iGCs compared to the more balanced inputs onto mGCs, iGCs may differentially gate cue-associated activity from LEC when engaged by distinct cognitive demands (Figure 2A,1). Selective modification of the inhibitory/excitatory balance in CA3 by iGCs might further increase the flexibility of the DG to alter the downstream CA3 activity (Figure 2A,2). Integration of iGCs into DG-CA3 network may also indirectly modify the activity levels by competing with the existing synaptic innervation from EC afferents onto the DG (Figure 2B,1) as well as mossy fiber innervation onto CA3 (Figure 2B,2). Alternatively, iGCs may modify the local DG network activity by monosynaptically recruiting mGCs (Figure 2B, 3a) or disynaptically affecting them via inhibitory networks (Figure 2B, 3b). Hence, due to their differential functional input and output connectivity, as well as their ability to modulate the activity levels in the DG, iGCs may provide additional processing pathways by which upstream EC inputs may change the activity patterns in the downstream CA3 recurrent network.
Figure 2. The role of adult neurogenesis in DG circuit function.
A. Adult born, immature granule cells (iGCs) directly impact the activity of DG-CA3 network. IGCs receive stronger inputs from lateral entorhinal cortex (LEC) than medial entorhinal cortex (MEC) compared to more similar strength inputs onto mGCs, while innervation from mossy cells (MC) and ascending inputs from areas such as supramammmillary nucleus (SuM) and medial septum (MS) onto iGCs increase as they mature (1). Both MC and SuM projections preferentially target the DG compared to CA3, unlike projections from LEC and MEC, which may be critical for changing the activity patterns in the downstream CA3 recurrent network. Maturation of large mossy fiber terminals of iGCs targeting pyramidal neurons proceeds at a slower pace compared to those of filopodia contacting interneurons (2), suggesting that the net impact of iGCs on the activity of CA3 network during their critical period may in fact be inhibitory. B. IGCs indirectly modify the activity of DG-CA3 network. IGCs can modulate the local DG network activity through competition with the existing excitatory synapses from afferents onto mGCs (1) or mossy fiber innervation onto CA3 (2). Alternatively, iGCs may monosynaptically activate mGCs (3a) or disynaptically recruit inhibitory interneurons (3b) to modulate the outputs of the DG to CA3.
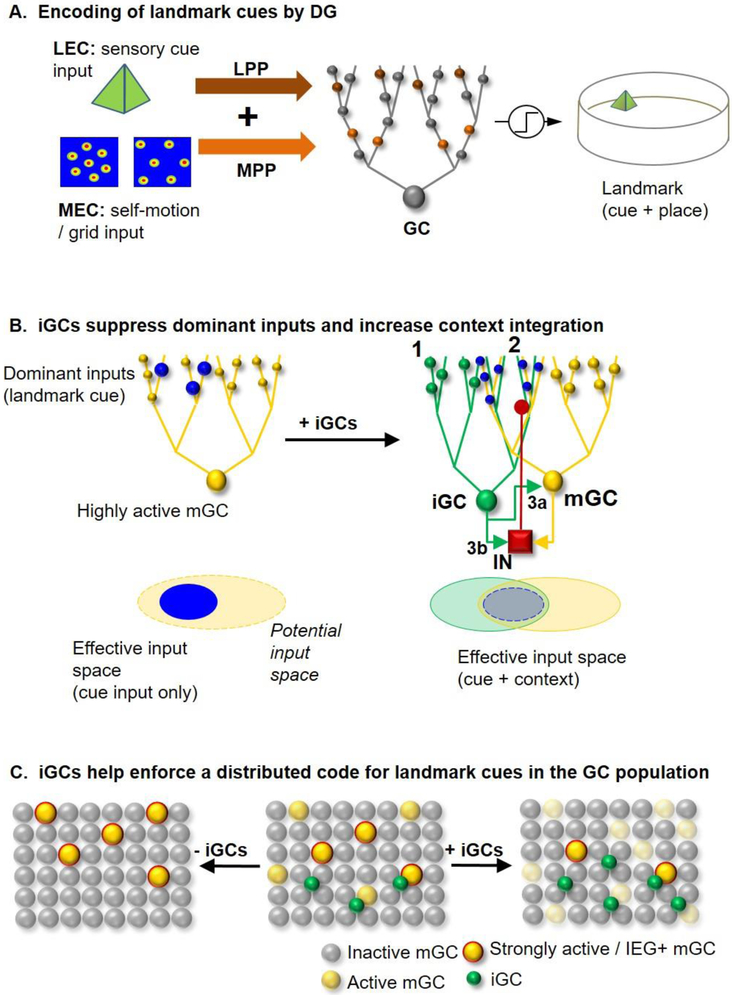
Figure 3. Model of the effects of adult neurogenesis in neural computations performed within the DG.
A. We hypothesize that the DG encodes landmarks within an environment. Sensory cue input from the lateral entorhinal cortex (LEC) and self-motion/grid cell input from the medial entorhinal cortex (MEC) travel via the lateral and medial perforant path (LPP, MPP), which synapse onto granule cell dendrites within the outer and inner molecular layer, respectively. Repeated coincident cue and spatial input from these two pathways during exploration engage plasticity to encode specific sensory cues in specific locations, i.e. landmarks. B. Adult-born, immature granule cells suppress dominant GC inputs and increase context integration. A small number of potentiated inputs, for instance ones associated with invariant landmark cues, may come to dominate GC firing which might lead to activity with respect to similar cues but in different environments, and compromise pattern separation and contextual discrimination. The integration of new neurons into this niche diffuses the dominance of these inputs and encourages the integration of additional associated, “contextual” inputs. Three mechanisms by which they might do this are: 1) Direct encoding of cue information along with other associated inputs, 2) Indirect actions on mature granule cell encoding by competing away excessively strong inputs from entorhinal cortex, or 3) Modulation of landmark-cue encoding by mature granule cells through monosynaptic excitation (a) or disynaptic inhibition (b). The next effect is to expand the input space of important cue input by integrating surrounding associated inputs (bottom). C. Immature granule cells enforce the distributed code for important landmark cues in the DG. Removal of adult-born neurons (left) leads to a code dominated by a relatively small number of highly active cells which are active regardless of context. Conditions of enhanced neurogenesis (right) lead to a more highly distributed code, where there are more weakly active cells that are more sensitive to cue-associated or contextual inputs. Note that overall this notion predicts the involvement of a larger number of neurons in encoding a cue, however most of these may fall below a threshold for IEG induction.
6. iGCs impact sparseness of representations in the DG and enforce a distributed code for landmarks
Taking these ideas together, an updated systems-level model of the effects of adult neurogenesis in neural computations performed within the DG emerges. The key feature of our model is that new neurons integrate into networks carrying unique information with relation to the DG function, particularly the representations of landmarks [48] (sensory cues at particular places that an animal encounters during navigation that it and can use to direct its behavior or discriminate one environment from another), potentially giving the new neurons an outsized influence on behavior. While the general mechanisms predicted below are generally similar to ones we and others have previously proposed [19,22,23], these mechanisms have specific implications due to this interpretation.
During spatial experience, the DG forms a sparse representation of sensory cues within an environment, promoted by inherently strong inhibition and high spiking thresholds in mGCs, which require a massive convergence of inputs in order to discharge. Based upon the fact that GC dendrites receive inputs from the MEC as well as the LEC, we propose that GC activity is preferentially recruited by sensory cues (LEC input) with a consistent relationship to space (and/or time, e.g. grid-cell inputs from MEC) thus selectively encoding sensory cues that happen at particular places, e.g. landmarks (Fig. 3A). We hypothesize that landmark representation may be important for spatial map formation in the DG and that the specificity of cell firing to sensory cues may be modulated by the spatial location of the cue. Indeed, this might underlie “pattern separation” at the level of the GC population activity, where similar cues that appear in different contexts (conjunctions of spatial and sensory information within an environment) are represented by a similar population of neurons, but where the firing of some neurons is stronger or weaker at some locations. This is also consistent with new evidence about the remapping strength GC spatial representations during context encoding [39,41,43,44].
New neurons are generated and integrated into the DG network in an activity-dependent fashion [64,71,74], which likely involves the neurons that are relatively active within the GC population. By integrating into DG networks specifically representing these “landmarks”, iGCs help to form a more distributed representation of important cues within the environment, which has a special impact on behavior. Although there may be other possible roles for iGCs in the DG network, below we examine how adult neurogenesis may influence the distributed code for landmarks in three major ways. These mechanisms are generally similar to those proposed in our earlier work; however, we will explore their implications on the computational function of the dentate relative to this idea.
First, iGCs as a population may redundantly encode information about landmarks as well as other salient contextual features (“direct encoding”, Fig. 3B, 1). The major inputs of these new neurons are largely similar to the highly tuned mGCs whose connections they invade through their activity-dependent wiring process, thus tending to recapitulate the firing selectivity of relatively active mGCs. Yet, owing to their elevated excitability and lower selectivity [6-9,14] , iGCs may potentially also integrate diverse sets of additional co-active inputs encoding features of the context distinct from mGC landmark representations. As iGCs mature, integration of other inputs to which the mature neurons may have not shown responses could help build a more context-selective representations of sensory cues within the overall population of GCs. For example, two iGCs that respond to a similar sensory cue might fire differentially when the cue is presented in different contexts by virtue of their putatively distinct unique additional inputs, further specifying their activity. This model thus predicts that thanks to the increased degree convergence of inputs unto new neurons, they may be more highly tuned for context than for individual discrete cues or positions independent of context.
Second, a modulatory role of iGCs on mGC encoding may occur as integration of new neurons into the DG network enforces a distributed representation by competing for afferent connections onto mGCs carrying landmark information (Fig. 3B, 2). This competition has the effect of selectively diffusing the influence of strong inputs that drive activity in mGCs and therefore diversifying the inputs required to fire them. Similar to the above mechanisms for integration of contextual information, the mGC whose inputs the new neuron partially replaces still fires in response to similar sensory cues, however it does so in a manner that depends on a wider variety of associated stimuli/inputs and therefore will tend to fire in response to the more restricted set of stimuli in which these inputs are co-active.
Finally, the bidirectional modulatory influence of iGCs on the DG depending on their activation by MEC or LEC inputs, may regulate the size of the mGC population recruited to represent features of a context [65] . Activation of a larger population of mGCs via monosynaptic excitation from iGCs upon MEC activation (Fig. 3B, 3a) would shift the balance in the mode of processing towards self-motion based navigation, which in effect ensures landmark representations rely less on sensory LEC input. On the other hand, the recruitment of iGCs when the network is dominated by LEC inputs may increase the sparseness of mature neuron populations activated in response to these cues by causing increased surround inhibition (Fig. 3B, 3b). In this latter case, iGC-mediated recruitment of inhibition may moderate the influence of small numbers of strong inputs carrying cue information regardless of context, therefore enhancing the requirement for summation with associated contextual information. The context-specific effect of iGCs might be further modulated by the activity of mossy cells, which pool information from multiple granule neurons and feed back onto both granule cells and interneurons, further sculpting the selectivity of important cue representations.
Together these three features enable the dentate to build representations of important sensory stimuli in an animal’s environment such as landmarks based on a more equally distributed set of inputs (Fig. 3C.), thus integrating a more varied set of associated sensory stimuli and/or spatial inputs (i.e. a “context”). This representation based on a more diffuse set of inputs may in turn lead to lower peak firing rates and enhanced context selectivity observed in the neurogenic DG. Furthermore the increased stimulus/context selectivity of this distributed code could promote the emergence of distinct population activity in area CA3 (i.e. global remapping) in response to different behavioral or spatial contexts. This could in turn affect spatial discrimination behaviors and the specificity of memory encoding and retrieval, which depend upon sequential encoding of behavioral events within the CA3 recurrent auto-associative network, based upon context-dependent landmark information encoded in the DG.
7. Conclusions and perspectives
Previous studies have tried to achieve a unifying model for iGCs through competing models of their direct and indirect impact on encoding within the neural circuit of the DG [23,24,109]. Yet, emerging evidence reveals that iGC modulation of the DG network is diverse, operate at multiple temporal scales and is engaged by a diverse set of inputs according to behavioral task demands. Due to these features, adult neurogenesis provides additional flexibility for DG to support temporally diverse events ranging from milliseconds to days, by generating a more distributed representation of important sensory stimuli in an animal’s environment given different behavioral contexts, which ultimately gives rise to behavioral discrimination. Here we focused on recent studies to highlight the essential nodes within the DG circuit along its input and output domains that could be monitored and manipulated in future studies to interrogate and interpret the contributions of adult neurogenesis to DG network activity and behavior. Of particular interest is whether well-defined principal neurons of the DG (adult born immature and mature GCs) represent behavioral experiences as complex contextual representations that include behavioral drives or as piecemeal representations of discrete and neutral sensory cues that acquire valence downstream of the hippocampus. We have also discussed a series of recent findings indicating that iGCs can modulate the activity of mGCs and CA3 via both monosynaptic and disynaptic pathways that are differentially recruited depending on environmental conditions, ultimately resulting in improved contextual discrimination. Since hippocampal dysfunction has been implicated in the cognitive discrimination impairments associated with Alzheimer’s disease and PTSD [110,111], constructing a dynamic picture of the DG, an often overlooked hippocampal region, at cellular and circuit resolutions during the formation of episodic memories may have an important clinical relevance.
Acknowledgments:
We thank Drs. Victor M. Luna and Andres D. Grosmark for helpful comments on the manuscript and figures.
Funding: This work was supported by grants from National Institutes of Health (R37 MH068542; R01 MH083862; R01 AG043688; R01 NS081203; T32 MH01574), NYSTEM (C029157) and the Hope for Depression Research Foundation (HDRF RGA-13-003) to RH.
Footnotes
Competing interests: The authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Gilbert PE, Kesner RP, Lee I, Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1, Hippocampus. 11 (2001) 626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- [2].Yassa MA, Stark CEL, Pattern separation in the hippocampus, Trends in Neurosciences. 34 (2011) 515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amaral DG, Scharfman HE, Lavenex P, The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies), Prog Brain Res. 163 (2007) 3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Treves A, Rolls ET, Computational analysis of the role of the hippocampus in memory, Hippocampus. 4 (1994) 374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- [5].Knierim JJ, Neunuebel JP, Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics, Neurobiology of Learning and Memory. 129 (2016) 38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H, A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain, Neuron. 54 (2007) 559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marín-Burgin A, Mongiat LA, Pardi MB, Schinder AF, Unique processing during a period of high excitation/inhibition balance in adult-born neurons, Science. 335 (2012) 1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schmidt-Hieber C, Jonas P, Bischofberger J, Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus, Nature. 429 (2004) 184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- [9].Snyder JS, Kee N, Wojtowicz JM, Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus, J. Neurophysiol. 85 (2001) 2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- [10].Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR, 4-to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning, Hippocampus. 22 (2012) 1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S, Optical controlling reveals time-dependent roles for adult-born dentate granule cells, Nat. Neurosci. 15 (2012) 1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S, Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion, Cell. 149 (2012) 188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R, Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus, Nature. 559 (2018) 98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A, Kheirbek MA, Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding, Neuron. 90 (2016) 101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huckleberry KA, Shue F, Copeland T, Chitwood RA, Yin W, Drew MR, Dorsal and ventral hippocampal adultborn neurons contribute to context fear memory, Neuropsychopharmacology. 43 (2018) 2487–2496. doi: 10.1038/s41386-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhuo J-M, Tseng H-A, Desai M, Bucklin ME, Mohammed AI, Robinson NT, Boyden ES, Rangel LM, Jasanoff AP, Gritton HJ, Han X, Young adult born neurons enhance hippocampal dependent performance via influences on bilateral networks, Elife. 5 (2016). doi: 10.7554/eLife.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH, A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis, J. Comp. Neurol. 518 (2010) 4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Piatti VC, Ewell LA, Leutgeb JK, Neurogenesis in the dentate gyrus: carrying the message or dictating the tone, Front Neurosci. 7 (2013) 50. doi: 10.3389/fnins.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aimone JB, Wiles J, Gage FH, Computational influence of adult neurogenesis on memory encoding, Neuron. 61 (2009) 187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK, Temporally selective contextual encoding in the dentate gyrus of the hippocampus, Nat Commun. 5 (2014) 3181. doi: 10.1038/ncomms4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Drew LJ, Kheirbek MA, Luna VM, Denny CA, Cloidt MA, Wu MV, Jain S, Scharfman HE, Hen R, Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons, Hippocampus. 26 (2016) 763–778. doi: 10.1002/hipo.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA, Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus, Hippocampus. 22 (2012) 106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sahay A, Wilson DA, Hen R, Pattern separation: a common function for new neurons in hippocampus and olfactory bulb, Neuron. 70 (2011) 582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anacker C, Hen R, Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood, Nat. Rev. Neurosci. 18 (2017) 335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pilz G-A, Bottes S, Betizeau M, Jörg DJ, Carta S, Simons BD, Helmchen F, Jessberger S, Live imaging of neurogenesis in the adult mouse hippocampus, Science. 359 (2018) 658–662. doi: 10.1126/science.aao5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Krueppel R, Remy S, Beck H, Dendritic integration in hippocampal dentate granule cells, Neuron. 71 (2011) 512–528. doi: 10.1016/j.neuron.2011.05.043. [DOI] [PubMed] [Google Scholar]
- [27].Pernía-Andrade AJ, Jonas P, Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations, Neuron. 81 (2014) 140–152. doi: 10.1016/j.neuron.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Witter MP, The perforant path: projections from the entorhinal cortex to the dentate gyrus, Prog. Brain Res. 163 (2007) 43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- [29].Espinoza C, Guzman SJ, Zhang X, Jonas P, Parvalbumin+ interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus, Nature Communications. 9 (2018). doi: 10.1038/s41467-018-06899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Claiborne BJ, Amaral DG, Cowan WM, A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus, J. Comp. Neurol 246 (1986) 435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- [31].Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA, Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience, Hippocampus. 15 (2005) 579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- [32].Deng W, Mayford M, Gage FH, Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice, Elife. 2 (2013) e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vazdarjanova A, Guzowski JF, Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles, J. Neurosci. 24 (2004) 6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burgess N, O’Keefe J, Neuronal computations underlying the firing of place cells and their role in navigation, Hippocampus. 6 (1996) 749–762. doi:. [DOI] [PubMed] [Google Scholar]
- [35].Colgin LL, Moser EI, Moser M-B, Understanding memory through hippocampal remapping, Trends Neurosci. 31 (2008) 469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- [36].Jung MW, McNaughton BL, Spatial selectivity of unit activity in the hippocampal granular layer, Hippocampus. 3 (1993) 165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- [37].Neunuebel JP, Knierim JJ, Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus, J. Neurosci. 32 (2012) 3848–3858. doi: 10.1523/JNEUROSCI.6038-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leutgeb JK, Leutgeb S, Moser M-B, Moser EI, Pattern separation in the dentate gyrus and CA3 of the hippocampus, Science. 315 (2007) 961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- [39].Senzai Y, Buzsáki G, Physiological Properties and Behavioral Correlates of Hippocampal Granule Cells and Mossy Cells, Neuron. 93 (2017) 691–704.e5. doi: 10.1016/j.neuron.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jung D, Kim S, Sariev A, Sharif F, Kim D, Royer S, Dentate granule and mossy cells exhibit distinct spatiotemporal responses to local change in a one-dimensional landscape of visual-tactile cues, Sci Rep. 9 (2019) 9545. doi: 10.1038/s41598-019-45983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].GoodSmith D, Chen X, Wang C, Kim SH, Song H, Burgalossi A, Christian KM, Knierim JJ, Spatial Representations of Granule Cells and Mossy Cells of the Dentate Gyrus, Neuron. 93 (2017) 677–690.e5. doi: 10.1016/j.neuron.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Diamantaki M, Frey M, Berens P, Preston-Ferrer P, Burgalossi A, Sparse activity of identified dentate granule cells during spatial exploration, Elife. 5 (2016). doi: 10.7554/eLife.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Danielson NB, Turi GF, Ladow M, Chavlis S, Petrantonakis PC, Poirazi P, Losonczy A, In Vivo Imaging of Dentate Gyrus Mossy Cells in Behaving Mice, Neuron. 93 (2017) 552–559.e4. doi: 10.1016/j.neuron.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hainmueller T, Bartos M, Parallel emergence of stable and dynamic memory engrams in the hippocampus, Nature. 558 (2018) 292–296. doi: 10.1038/s41586-018-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stefanini F, Kheirbek M, Kushnir L, Jennings JH, Stuber G, Hen R, Fusi S, A distributed neural code in ensembles of dentate gyrus granule cells, BioRxiv. (2018) 292953. doi: 10.1101/292953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pilz G-A, Carta S, Stäuble A, Ayaz A, Jessberger S, Helmchen F, Functional Imaging of Dentate Granule Cells in the Adult Mouse Hippocampus, J. Neurosci. 36 (2016) 7407–7414. doi: 10.1523/JNEUROSCI.3065-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wood ER, Dudchenko PA, Eichenbaum H, The global record of memory in hippocampal neuronal activity, Nature. 397 (1999) 613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- [48].Deshmukh SS, Knierim JJ, Influence of local objects on hippocampal representations: Landmark vectors and memory, Hippocampus. 23 (2013) 253–267. doi: 10.1002/hipo.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gauthier JL, Tank DW, A Dedicated Population for Reward Coding in the Hippocampus, Neuron. 99 (2018) 179–193.e7. doi: 10.1016/j.neuron.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J, The reorganization and reactivation of hippocampal maps predict spatial memory performance, Nat. Neurosci. 13 (2010) 995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sasaki T, Piatti VC, Hwaun E, Ahmadi S, Lisman JE, Leutgeb S, Leutgeb JK, Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons, Nat. Neurosci. 21 (2018) 258–269. doi: 10.1038/s41593-017-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van Dijk MT, Fenton AA, On How the Dentate Gyrus Contributes to Memory Discrimination, Neuron. 98 (2018) 832–845.e5. doi: 10.1016/j.neuron.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R, Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis, Neuron. 83 (2014) 189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stone SSD, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW, Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory, Hippocampus. 21 (2011) 1348–1362. doi: 10.1002/hipo.20845. [DOI] [PubMed] [Google Scholar]
- [55].Kuipers SD, Tiron A, Soule J, Messaoudi E, Trentani A, Bramham CR, Selective survival and maturation of adult-born dentate granule cells expressing the immediate early gene Arc/Arg3.1, PLoS ONE. 4 (2009) e4885. doi: 10.1371/journal.pone.0004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ikrar T, Guo N, He K, Besnard A, Levinson S, Hill A, Lee H-K, Hen R, Xu X, Sahay A, Adult neurogenesis modifies excitability of the dentate gyrus, Front Neural Circuits. 7 (2013) 204. doi: 10.3389/fncir.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Iyengar SS, LaFrancois JJ, Friedman D, Drew LJ, Denny CA, Burghardt NS, Wu MV, Hsieh J, Hen R, Scharfman HE, Suppression of adult neurogenesis increases the acute effects of kainic acid, Exp. Neurol. 264 (2015) 135–149. doi: 10.1016/j.expneurol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Singer BH, Gamelli AE, Fuller CL, Temme SJ, Parent JM, Murphy GG, Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice, Proc. Natl. Acad. Sci. U.S.A. 108 (2011) 5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Burghardt NS, Park EH, Hen R, Fenton AA, Adult-born hippocampal neurons promote cognitive flexibility in mice, Hippocampus. 22 (2012) 1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P, Hippocampal Somatostatin Interneurons Control the Size of Neuronal Memory Ensembles, Neuron. 89 (2016) 1074–1085. doi: 10.1016/j.neuron.2016.01.024. [DOI] [PubMed] [Google Scholar]
- [61].Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF, Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells, Neuron. 85 (2015) 116–130. doi: 10.1016/j.neuron.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Groisman AI, Yang SM, Schinder AF, Differential coupling of adult-born granule cells to parvalbumin and somatostatin interneurons, BioRxiv. (2019) 598615. doi: 10.1101/598615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA, Experience-Dependent Regulation of Dentate Gyrus Excitability by Adult-Born Granule Cells, J. Neurosci. 35 (2015) 11656–11666. doi: 10.1523/JNEUROSCI.0885-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann K-K, Schinder AF, Götz M, Berninger B, A critical period for experience-dependent remodeling of adult-born neuron connectivity, Neuron. 85 (2015) 710–717. doi: 10.1016/j.neuron.2015.01.001. [DOI] [PubMed] [Google Scholar]
- [65].Luna VM, Anacker C, Burghardt NS, Khandaker H, Andreu V, Millette A, Leary P, Ravenelle R, Jimenez JC, Mastrodonato A, Denny CA, Fenton AA, Scharfman HE, Hen R, Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus, Science. 364 (2019) 578–583. doi: 10.1126/science.aat8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hendricks WD, Chen Y, Bensen AL, Westbrook GL, Schnell E, Short-Term Depression of Sprouted Mossy Fiber Synapses from Adult-Born Granule Cells, J. Neurosci. 37 (2017) 5722–5735. doi: 10.1523/JNEUROSCI.0761-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Toni N, Schinder AF, Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus, Cold Spring Harb Perspect Biol. 8 (2015) a018903. doi: 10.1101/cshperspect.a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF, Neurons born in the adult dentate gyrus form functional synapses with target cells, Nat. Neurosci. 11 (2008) 901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Adlaf EW, Vaden RJ, Niver AJ, Manuel AF, Onyilo VC, Araujo MT, Dieni CV, Vo HT, King GD, Wadiche JI, Overstreet-Wadiche L, Adult-born neurons modify excitatory synaptic transmission to existing neurons, Elife. 6 (2017). doi: 10.7554/eLife.19886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kim WR, Park O-H, Choi S, Choi S-Y, Park SK, Lee KJ, Rhyu IJ, Kim H, Lee YK, Kim HT, Oppenheim RW, Sun W, The maintenance of specific aspects of neuronal function and behavior is dependent on programmed cell death of adult-generated neurons in the dentate gyrus, Eur. J. Neurosci. 29 (2009) 1408–1421. doi: 10.1111/j.1460-9568.2009.06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McAvoy KM, Scobie KN, Berger S, Russo C, Guo N, Decharatanachart P, Vega-Ramirez H, Miake-Lye S, Whalen M, Nelson M, Bergami M, Bartsch D, Hen R, Berninger B, Sahay A, Modulating Neuronal Competition Dynamics in the Dentate Gyrus to Rejuvenate Aging Memory Circuits, Neuron. 91 (2016) 1356–1373. doi: 10.1016/j.neuron.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Abraham WC, Logan B, Greenwood JM, Dragunow M, Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus, J. Neurosci. 22 (2002) 9626–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K, Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory, Cell. 139 (2009) 814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- [74].Vivar C, Peterson BD, van Praag H, Running rewires the neuronal network of adult-born dentate granule cells, Neuroimage. 131 (2016) 29–41. doi: 10.1016/j.neuroimage.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gonçalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, Tran T, Chang T, Gage FH, In vivo imaging of dendritic pruning in dentate granule cells, Nat. Neurosci. 19 (2016) 788–791. doi: 10.1038/nn.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Piatti VC, Davies-Sala MG, Espósito MS, Mongiat LA, Trinchero MF, Schinder AF, The timing for neuronal maturation in the adult hippocampus is modulated by local network activity, J. Neurosci. 31 (2011) 7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G, GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus, J. Neurosci. 18 (1998) 3386–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Engel D, Jonas P, Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons, Neuron. 45 (2005) 405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- [79].Henze DA, Wittner L, Buzsáki G, Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo, Nat. Neurosci. 5 (2002) 790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- [80].Vyleta NP, Borges-Merjane C, Jonas P, Plasticity-dependent, full detonation at hippocampal mossy fiber-CA3 pyramidal neuron synapses, Elife. 5 (2016). doi: 10.7554/eLife.17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lawrence JJ, McBain CJ, Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus, Trends Neurosci. 26 (2003) 631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- [82].Lee J, Yun M, Cho E, Lee JW, Lee D, Jung MW, Transient effect of mossy fiber stimulation on spatial firing of CA3 neurons, Hippocampus. 0 (n.d.). doi: 10.1002/hipo.23066. [DOI] [PubMed] [Google Scholar]
- [83].Penttonen M, Kamondi A, Sik A, Acsády L, Buzsáki G, Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts, Hippocampus. 7 (1997) 437–450. doi:. [DOI] [PubMed] [Google Scholar]
- [84].Faulkner RL, Jang M-H, Liu X-B, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming G, Song H, Cheng H-J, Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain, Proc. Natl. Acad. Sci. U.S.A. 105 (2008) 14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Restivo L, Niibori Y, Mercaldo V, Josselyn SA, Frankland PW, Development of Adult-Generated Cell Connectivity with Excitatory and Inhibitory Cell Populations in the Hippocampus, J. Neurosci. 35 (2015) 10600–10612. doi: 10.1523/JNEUROSCI.3238-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Niibori Y, Yu T-S, Epp JR, Akers KG, Josselyn SA, Frankland PW, Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region, Nat Commun. 3 (2012) 1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Guo N, Soden ME, Herber C, Kim MT, Besnard A, Lin P, Ma X, Cepko CL, Zweifel LS, Sahay A, Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization, Nat. Med. 24 (2018) 438–449. doi: 10.1038/nm.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, Sacchetti B, Caroni P, Learning-related feedforward inhibitory connectivity growth required for memory precision, Nature. 473 (2011) 514–518. doi: 10.1038/nature09946. [DOI] [PubMed] [Google Scholar]
- [89].Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI, Microstructure of a spatial map in the entorhinal cortex, Nature. 436 (2005) 801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- [90].Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, Moser EI, Conjunctive representation of position, direction, and velocity in entorhinal cortex, Science. 312 (2006) 758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- [91].Kitamura T, Sun C, Martin J, Kitch LJ, Schnitzer MJ, Tonegawa S, Entorhinal Cortical Ocean Cells Encode Specific Contexts and Drive Context-Specific Fear Memory, Neuron. 87 (2015) 1317–1331. doi: 10.1016/j.neuron.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Qin H, Fu L, Hu B, Liao X, Lu J, He W, Liang S, Zhang K, Li R, Yao J, Yan J, Chen H, Jia H, Zott B, Konnerth A, Chen X, A Visual-Cue-Dependent Memory Circuit for Place Navigation, Neuron. 99 (2018) 47–55.e4. doi: 10.1016/j.neuron.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hargreaves EL, Rao G, Lee I, Knierim JJ, Major dissociation between medial and lateral entorhinal input to dorsal hippocampus, Science. 308 (2005) 1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- [94].Yoganarasimha D, Rao G, Knierim JJ, Lateral entorhinal neurons are not spatially selective in cue-rich environments, Hippocampus. 21 (2011) 1363–1374. doi: 10.1002/hipo.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Deshmukh SS, Knierim JJ, Representation of non-spatial and spatial information in the lateral entorhinal cortex, Front Behav Neurosci. 5 (2011) 69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang C, Chen X, Lee H, Deshmukh SS, Yoganarasimha D, Savelli F, Knierim JJ, Egocentric coding of external items in the lateral entorhinal cortex, Science. 362 (2018) 945–949. doi: 10.1126/science.aau4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wilson DIG, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA, Lateral entorhinal cortex is critical for novel object-context recognition, Hippocampus. 23 (2013) 352–366. doi: 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vivar C, Potter MC, Choi J, Lee J-Y, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H, Monosynaptic inputs to new neurons in the dentate gyrus, Nat Commun. 3 (2012) 1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Knierim JJ, Neunuebel JP, Deshmukh SS, Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames, Philos. Trans. R. Soc. Lond., B, Biol. Sci. 369 (2014) 20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Scharfman HE, The enigmatic mossy cell of the dentate gyrus, Nat. Rev. Neurosci 17 (2016) 562–575. doi: 10.1038/nrn.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bui AD, Nguyen TM, Limouse C, Kim HK, Szabo GG, Felong S, Maroso M, Soltesz I, Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory, Science. 359 (2018) 787–790. doi: 10.1126/science.aan4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hsu T-T, Lee C-T, Tai M-H, Lien C-C, Differential Recruitment of Dentate Gyrus Interneuron Types by Commissural Versus Perforant Pathways, Cereb. Cortex. 26 (2016) 2715–2727. doi: 10.1093/cercor/bhv127. [DOI] [PubMed] [Google Scholar]
- [103].Pan W-X, McNaughton N, The supramammillary area: its organization, functions and relationship to the hippocampus, Prog. Neurobiol. 74(2004) 127–166. doi: 10.1016/j.pneurobio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [104].Hashimotodani Y, Karube F, Yanagawa Y, Fujiyama F, Kano M, Supramammillary Nucleus Afferents to the Dentate Gyrus Co-release Glutamate and GABA and Potentiate Granule Cell Output, Cell Rep. 25 (2018) 2704–2715.e4. doi: 10.1016/j.celrep.2018.11.016. [DOI] [PubMed] [Google Scholar]
- [105].Nakanishi K, Saito H, Abe K, The supramammillary nucleus contributes to associative EPSP-spike potentiation in the rat dentate gyrus in vivo, Eur. J. Neurosci. 13 (2001) 793–800. [DOI] [PubMed] [Google Scholar]
- [106].Deshpande A, Bergami M, Ghanem A, Conzelmann K-K, Lepier A, Götz M, Berninger B, Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb, Proc. Natl. Acad. Sci. U.S.A. 110 (2013) E1152–1161. doi: 10.1073/pnas.1218991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sun Y, Grieco SF, Holmes TC, Xu X, Local and Long-Range Circuit Connections to Hilar Mossy Cells in the Dentate Gyrus, ENeuro. 4 (2017). doi: 10.1523/ENEURO.0097-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Woods NI, Vaaga CE, Chatzi C, Adelson JD, Collie MF, Perederiy JV, Tovar KR, Westbrook GL, Preferential Targeting of Lateral Entorhinal Inputs onto Newly Integrated Granule Cells, J. Neurosci. 38 (2018) 5843–5853. doi: 10.1523/JNEUROSCI.1737-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Aimone JB, Deng W, Gage FH, Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation, Neuron. 70 (2011) 589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, Evans J, Lee M, Porsteinsson A, Lanctot KL, Rosenberg PB, Sultzer DL, Francis PT, Brodaty H, Padala PP, Onyike CU, Ortiz LA, Ancoli-Israel S, Bliwise DL, Martin JL, Vitiello MV, Yaffe K, Zee PC, Herrmann N, Sweet RA, Ballard C, Khin NA, Alfaro C, Murray PS, Schultz S, Lyketsos CG, Neuropsychiatric Syndromes Professional Interest Area of ISTAART, Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future, Alzheimers Dement. 9 (2013) 602–608. doi: 10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Parsons RG, Ressler KJ, Implications of memory modulation for post-traumatic stress and fear disorders, Nat. Neurosci. 16 (2013) 146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]