Abstract
In the developing CNS, GABAA responses switch from early excitation to late mature inhibition. The developmental factors that induce the polarity switch remain to be unraveled. Here, we bring the first experimental evidence in vivo in the retina that chronic activation of GABAA receptors is necessary for the switch to occur and for the chloride extrusion mechanism (through the K+/Cl- cotransporter KCC2) to develop. Using a turtle model and calcium imaging, we investigated how chronic blockade of GABAA receptors with bicuculline during the period of the GABAergic polarity switch (from 1 week before hatching until 4 weeks after hatching) influences developmental changes in the patterns of spontaneously generated electrical activity in the retinal ganglion cell (RGC) layer. During that period, spontaneous activity normally switches from propagating waves to stationary patches of coactive cells, until correlated activity completely disappears. These changes in activity patterns coincide with the switch of GABAA responses from excitation to inhibition. When GABAA receptors are chronically blocked, GABAA responses remain excitatory and spontaneous waves keep propagating across the RGC layer. Concomitantly, the developmental upregulation of KCC2 is inhibited on dendritic processes in the inner plexiform layer, suggesting that the intracellular concentration of chloride remains higher, as in younger cells. This study presents the first demonstration in vivo that GABA autoregulates its developmental polarity switch, emphasizing the importance of GABAergic activity in controlling activity patterns in the maturing retina.
Keywords: retinal waves, retinal development, GABA, KCC2, calcium imaging, spontaneous activity
Introduction
GABA is the main inhibitory neurotransmitter in the mature CNS. However, during early development, GABA type-A neurotransmission depolarizes neurons, causing transient increases in intracellular calcium (Ben-Ari, 2002). This early phenomenon is believed to underlie GABA-mediated trophic effects involved in the control of cell survival, neurite outgrowth, and synapse formation (Owens and Kriegstein, 2002), and it is ubiquitous (Ben-Ari, 2002), including the retina (Fischer et al., 1998; Sernagor et al., 2003a; Zheng et al., 2004).
Activation of GABAA receptors increases Cl- conductance. The polarity of GABAA neurotransmission is therefore determined by the equilibrium potential for Cl- (ECl). The intracellular concentration of Cl- decreases with development because of the upregulation of a Cl- extrusion mechanism through the K+/Cl- transporter KCC2 (Payne et al., 2003), resulting in ECl shifting toward more hyperpolarized levels (Rivera et al., 1999). Developmental KCC2 upregulation in the retina (Vu et al., 2000) coincides with the switch in the polarity of GABAA responses and the disappearance of propagating waves of activity (Sernagor et al., 2003a).
What controls the developmental shift in GABAA polarity remains a major question. In the retina, visual experience influences the switch (Sernagor et al., 2003a). Whether GABAA neurotransmission per se plays a trophic role in controlling the switch remains controversial, at least based on observations from two studies on rodent hippocampal cultures. One study shows that chronic blockade of GABAA neurotransmission prevents the switch and inhibits the developmental increase in KCC2 expression (Ganguly et al., 2001), whereas the other study reports that the KCC2 developmental increase is not dependent on GABAA neurotransmission (Ludwig et al., 2003). The discrepancy between these two studies is unclear and may be attributable to differences in experimental conditions. The next step is clearly to determine whether GABA regulates its own switch in vivo, and this is what we present here, using a developing turtle retina model.
Developing turtle retinal ganglion cells (RGCs) start bursting spontaneously at embryonic stage 22 (S22), 3 weeks before hatching (Sernagor and Grzywacz, 1995, 1999; Grzywacz and Sernagor, 2000). Neighboring RGCs are coactive, resulting in propagating waves (Sernagor et al., 2001, 2003a). This spontaneous activity provides us with a good approach to observe the contribution of different neurotransmitters in an intact circuit. Waves slow down at S25, 1 week before hatching (S26). This is the time at which GABAA neurotransmission becomes involved in the circuitry that controls wave propagation and GABAA responses are depolarizing (Sernagor et al., 2003a). GABAA responses then gradually switch polarity, coinciding with the transformation of propagating waves into patches of coactivated RGCs. These local correlations disappear at 1 month after hatching, when GABAA neurotransmission switches to mature inhibition. In the present study, we found that when retinas are chronically exposed to bicuculline from S24 until 1 month after hatching, GABA remains excitatory and spontaneous waves keep propagating.
Parts of this work have been published previously in abstract form (Sernagor et al., 2003b).
Materials and Methods
We used the turtle species Pseudemys scripta elegans. Embryonic ages were determined according to specific staging criteria (Yntema, 1968). Gestation lasts ∼60 d at 29°C. S22 and S25, respectively, correspond approximately to 3 and 1 week before hatching, and S26 corresponds to the hatching process.
Surgical procedure, dye labeling, and drug application. Embryos were kept at 29°C in a dark incubator. Newly hatched turtles were immediately transferred to water tanks kept at 28°C in 12 h light/dark cycles.
The detailed surgical procedure has been described previously (Sernagor and Grzywacz, 1995).
RGC retrograde loading with Calcium Green dextran was also described previously (Sernagor et al., 2000; Sernagor et al., 2003a).
After isolation, retinas were mounted, with the RGC layer facing up, onto gray blotting paper (Millipore, Bedford, MA) and transferred to the experimental chamber onto the stage of an upright microscope [Olympus (Tokyo, Japan) AX70]. The chamber was continuously perfused (2-5 ml/min) with oxygenated Ringer's solution (containing 4.9 mm KCl instead of 2.9 mm, to increase background spontaneous activity) kept at 26-28°C.
Bicuculline (Tocris Cookson, Ballwin, MO) was bath applied through the perfusate.
GABA was applied with a micropipette in single puffs of 50 μl of a 5 mm solution to avoid receptor desensitization (Sernagor et al., 2003a). All puffs were delivered from the same point in the recording chamber, near the perfusion inflow, and GABA diffused toward the retina placed in the center of the chamber, ∼1.2 cm from where the puff was delivered (Sernagor et al., 2003a).
Analysis of calcium transients. The imaging techniques and analysis of the calcium transients were similar to those used in our previous studies (Sernagor et al., 2000, 2003a). Fluorescence changes were detected using a Video Rate Intensified CCD camera (Princeton Scientific Instruments, Monmouth Junction, NJ), recorded continuously (25 frames/s) onto videotape, and simultaneously viewed with MetaMorph imaging software (Universal Imaging, Downingtown, PA).
To calculate cellular recruitment during waves, we include all RGCs that participate in the production of one wave of activity. When the activity is patchy, later in development, we calculate cellular recruitment by sampling spontaneous activity occurring during consecutive 5 s bins (Sernagor et al., 2003a).
To calculate relative-onset plots, a cell that was activated early in a wave is arbitrarily chosen as the reference cell. Then, for every other active cell, we plot the difference in onset time for that cell and the reference cell as a function of the distance between them. When there is propagation, relative-onset plots exhibit clear oblique lines, indicating increasing delays with distance from the reference cell. When the activity is patchy, relative-onset plots exhibit clusters of horizontal lines, with each line representing one patch of neighboring coactive RGCs. When local correlations disappear with development, relative-onset plots exhibit no specific pattern.
Bicuculline-Elvax implants. Our protocol for preparing and implanting Elvax (DuPont, Billerica, MA) blocks into the eye and for determining the appropriate drug concentration in the plastic was described previously (Sernagor and Grzywacz, 1996). Spontaneous spikes in the optic nerve were recorded with an extracellular glass suction electrode placed on the cut end of the nerve. Spontaneous spikes were continuously acquired during 10-15 min on a personal computer and sorted off-line by threshold detection using Spike3 acquisition software (Cambridge Electronic Design, Cambridge, UK). The threshold was determined visually, ensuring that acquisition included only spike waveforms and no baseline noise. Although recording from intact isolated eyes is not possible in mammals because of relatively poor retinal oxygenation, it is feasible in reptiles because of their low metabolic requirements.
Immunocytochemical localization of KCC2. The protocol for immunocytochemical labeling of KCC2 was described previously (Sernagor et al., 2003a). The only difference with our previous work is that we used the same sections to detect KCC2 and cell somata rather than alternate sections. We mounted the sections in Vectashield antifading mountant containing 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) (revealing cell somata by labeling DNA). This enabled us to determine in a more direct way to what extent KCC2 was present in various retinal layers. All sections were labeled on the same day.
Sections were viewed at 40× magnification using a Leica (Nussloch, Germany) DMRA fluorescence microscope. Images were captured at the same exposure (1 s) and the same gain with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) using MetaMorph. KCC2 labeling bandwidth [thickness of labeling within the inner plexiform layer (IPL)] and labeling intensity (gray levels) were measured in MetaMorph by drawing rectangles (length, KCC2 bandwidth in IPL; width, 25 pixels) perpendicular to the IPL. The entire IPL thickness was determined by measuring the distance between the RGC layer and the inner nuclear layer, delimited by DAPI staining. The percentage occupancy of KCC2 in the IPL is calculated by normalizing the raw KCC2 bandwidth measurements to the mean IPL thickness. The average labeling intensity (within the rectangles) was normalized (in percentage) to the peak value obtained at posthatching day 28 (PH28).
Statistics. We used standard statistical tests to quantify our observations, including t tests to detect changes after chronic treatments. Values are expressed as mean ± SE. (When n has two values separated by a comma, the first value indicates the total number of measurements, and the second value indicates the number of retinas.)
Results
Developmental changes in spontaneous activity patterns and GABAA activity
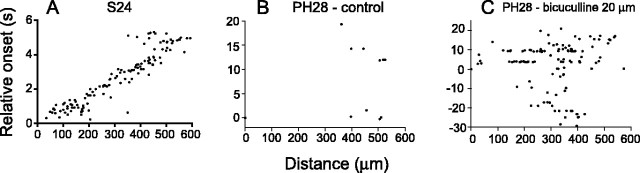
During normal development, correlated spontaneous activity recorded from turtle RGCs disappears within the first month after hatching (Sernagor et al., 2003a). Figure 1 illustrates how the activity patterns change from S24 (Fig. 1A) to PH28 (Fig. 1B). The activity is represented with relative-onset plots, as described in Materials and Methods (Sernagor et al., 2003a). Each dot represents one active cell over the sampling period (6 s for S24 and 20 s for PH28). Although the plot from S24 clearly reveals the presence of a propagating wave, barely any spontaneous activity can be recorded anymore at PH28, and virtually no local correlations are detectable between neighboring RGCs. A typical propagating wave (S24) and sparse spontaneous activity (PH28) are illustrated in supplemental movies 1 and 2, respectively (available at www.jneurosci.org as supplemental material). Figure 2D summarizes activity levels, quantified by cellular recruitment during spontaneous episodes of activity (see Materials and Methods) in different experimental conditions. The left column in the graph illustrates that only 6.4 ± 0.7% of the cells (n = 162, 4) are recruited when there is spontaneous activity at PH28.
Figure 1.
Developmental changes in spontaneous activity patterns. A, Spontaneous activity in an S24 retina visualized using a relative-onset plot (see Materials and Methods). The plot reveals clear propagation between neighboring RGCs. B, Spontaneous activity at PH28. There is barely any remaining correlated activity. C, Same retina as in B, in the presence of bicuculline. Spontaneous activity increases but shows little correlation between neighbors and no propagation.
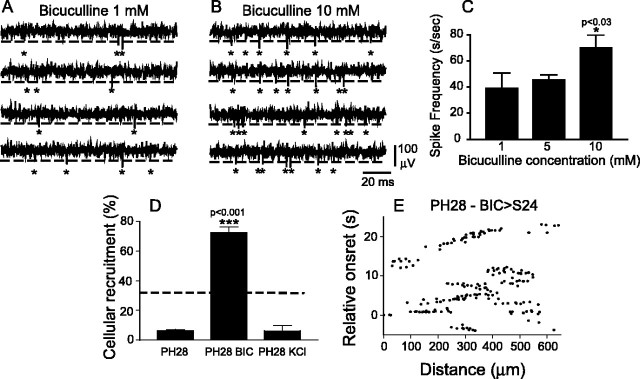
Figure 2.
Chronic blockade of GABAA receptors prevents the disappearance of correlated spontaneous activity in RGCs. A, Extracellular spikes recorded from the optic nerve in a PH5 eye that had been exposed to a 1 mm bicuculline-Elvax implant for 5 d. Spikes are acquired by threshold (dashed line) detection. Each spike detected is indicated by an asterisk. B, Same as A, except that this particular eye was exposed to 10 mm bicuculline. There are more spontaneous spikes than in A. C, Spontaneous firing recorded from optic nerves after 5 d of intraocular exposure to different doses of bicuculline. D, Cellular recruitment at PH28 in control, bicuculline (BIC)-treated, and KCl-treated retinas during spontaneous activity. The dashed line indicates cellular recruitment at hatching during normal development (Sernagor et al., 2003a). E, Spontaneous activity (at PH28) after bicuculline treatment from S24 (indicated as BIC > S24). The oblique lines indicate clear wave propagation, as in embryonic retinas (Fig. 1 A). There is no bicuculline in the bath during recordings. Error bars represent SEM.
GABAA responses, which are depolarizing at early stages, switch to inhibition during the first month after hatching, and this is what appears to cause the disappearance of correlated spontaneous activity (Sernagor et al., 2003a). Similar observations were reported in the rabbit retina, in which stage III waves are also under GABAA control and become fully propagating waves during GABAA receptor blockade (Syed et al., 2004). By PH28, when GABA puffs are delivered near the retina (see Materials and Methods), they fail to elicit depolarization (Sernagor et al., 2003a). In four PH28 retinas, GABA puffs never triggered calcium transients in any RGC. To confirm that GABAA responses are inhibitory at PH28 d, the addition of the GABAA receptor antagonist bicuculline (20 μm) raises the overall excitability of the network, with a 34.2 ± 2.3% increase in cellular recruitment during spontaneous activity (n = 4). Figure 1C shows that when GABAA receptors are blocked with bicuculline (same retina as that in Fig. 1B), there is a marked increase in the number of cells expressing spontaneous calcium transients. However, as opposed to the effect of similar drug concentrations during late gestation (Sernagor et al., 2003a), bicuculline fails to elicit activity propagation between neighbors at PH28. Instead, it remains restricted to random individual cells, except for occasional synchronization between neighbors, as illustrated by horizontal clusters [for more examples, see Materials and Methods and the study by Sernagor et al. (2003a)].
The effect of chronic blockade of GABAergic activity on spontaneous activity patterns
To investigate whether GABA regulates its own switch, retinas were chronically exposed to bicuculline-soaked intraocular implants of the slow-release polymer Elvax from S24, just before GABAA neurotransmission becomes involved in wave generation (Sernagor et al., 2003a). The optimal concentration of bicuculline in Elvax was determined by measuring the spontaneous firing frequency recorded from the optic nerve from freshly excised eyes after 5 d of exposure to implants (Sernagor and Grzywacz, 1996) containing increasing concentrations of bicuculline [1 mm (n = 2 eyes), 5 mm (n = 8), and 10 mm (n = 6)]. We implanted the eyes at PH0 and recorded the activity at PH5, to ensure that bicuculline has a clear excitatory effect on spontaneous activity (GABAA responses are already switching to inhibition at that stage) (Sernagor et al., 2003a). Figure 2 illustrates sequences of extracellular spike recordings from the optic nerve, showing that spontaneous activity is stronger in the presence of 10 mm bicuculline in the eye (Fig. 2B) than in the presence of 1 mm of the drug (Fig. 2A). All subsequent experiments were performed with 10 mm bicuculline implants, because this concentration produced a significant increase in neural activity (compared with 5 mm; p < 0.03; two-tailed t test) (Fig. 2C). At PH28, after ∼5 weeks of chronic exposure to bicuculline, spontaneous activity was significantly stronger than in age-matched controls. Cellular recruitment increased to 72.4 ± 3.7% (Fig. 2D, middle column) (n = 23, 4), similar to recruitment during waves at S24 (Sernagor et al., 2003a). Activity did not occur at random as in age-matched controls in the acute presence of bicuculline (as in Fig. 1C). Instead, spontaneous activity exhibited wave patterns, such as those at embryonic stages during normal development (such as in Fig. 1A). Indeed, we found that robust waves propagated every 2-3 min in four of five retinas (Fig. 2E) (there is no bicuculline in the bath; see an example in supplemental movie 3, available at www.jneurosci.org as supplemental material). The relative-onset plot in Figure 2E clearly reveals propagation patterns, characterized by oblique lines in the plot (see Materials and Methods). Wave speed (calculated between pairs of cells) (Sernagor et al., 2003a) in the chronic retinas was 96.8 ± 9.0 μm/s (n = 20, 4), significantly slower than at S24 (226.4 μm/s) (Sernagor et al., 2003a) but faster than at S25 (32.5 μm/s) (Sernagor et al., 2003a). The wave spatial extent was 125.8 ± 2.8 μm (n = 20, 4), similar to S24 (Sernagor et al., 2003a). After acute bath application of bicuculline (20 μm), the activity increased even further, with wave propagation speed increasing to 134.5 ± 17.6 μm/s (n = 20, 5) and wave spatial extent increasing to 132.4 ± 1.5 μm (n = 20, 5; p < 0.05; two-tailed t test compared with control chronic values). This suggests that, in our hands, chronic exposure to bicuculline did not cause GABA to remain “purely” excitatory as at S25 during normal development (Sernagor et al., 2003a). Nevertheless, GABA puffs into the recording chamber elicited large, slowly propagating, and prolonged calcium signals in all RGCs and in all retinas, suggesting that GABAA responses did not fully switch to inhibition either, as they normally do by 1 month after hatching.
To verify whether this bicuculline-induced effect could be attributable to a nonspecific increase in neural activity, we exposed retinas chronically to KCl (2 m)-soaked Elvax implants (which increased spontaneous firing in optic nerves by 114.3%; n = 4) from S24 until PH28. Considering that drug release from Elvax is stable during the 2-3 months after an initial peak (first couple of days) and amounts to ∼0.3% per day, we estimate that retinas were constantly exposed to ∼6 mm KCl. We found no significant difference with age-matched controls. Cellular recruitment was only 6.25 ± 3.5% (n = 89, 6) (Fig. 2D, right column) in these K+-treated retinas, confirming the need for specific GABAA receptor blockade to influence the switch. Inspection of the retinas after exposure to KCl did not reveal damage caused by the strong chronic depolarization. The various retinal layers appeared completely normal (data not shown).
Chronic GABAA blockade inhibits the developmental upregulation of KCC2
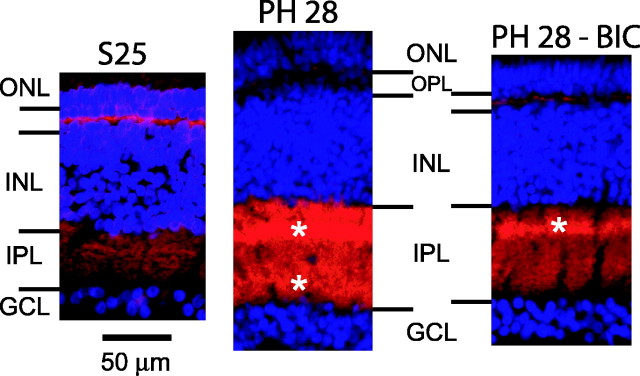
The expression of KCC2 in the IPL is low in embryonic retinas, and it increases to adult levels during the first month after hatching (Sernagor et al., 2003a). The left panel of Figure 3 shows KCC2 distribution in a retinal cross section at S25 (see Materials and Methods). Confirming our previous observations (Sernagor et al., 2003a), the expression of KCC2 is weak in the IPL, with the inner part of the IPL near the RGC layer showing even less staining than the outer part. The expression of the transporter significantly increases with development. The middle panel of Figure 3 reveals that both the intensity of the labeling and the percentage occupancy of the IPL have significantly increased by PH28. In addition, close inspection of the retinas also unveils two clear sub-bands of stronger labeling within the IPL (white asterisks), presumably corresponding to the ON (in the inner part of the IPL) and OFF (in the outer part of the IPL) laminas of RGC dendritic trees (Nelson et al., 1978). After chronic treatment with bicuculline, the expression of the transporter is much weaker than in age-matched controls (Fig. 3, compare right and middle panels), although it is higher than at S25. Interestingly, it is the inner part of the IPL, in which the ON lamina of the dendritic tree is located, that is most affected. A stronger band of KCC2 expression still remains in the outer part of the IPL (Fig. 3, white asterisks), but it is absent from the inner part of the IPL. On average, the percentage occupancy of KCC2 within the entire IPL decreases by 12.2% (compared with age-matched controls), whereas the intensity of the labeling decreases by 15.3% (n = 19, 2 for control; n = 29, 3 for bicuculline-treated retinas; six sections were used in each retina) (p < 0.001; two-tailed t test for both).
Figure 3.
Chronic blockade of GABAA receptors with bicuculline inhibits the developmental increase in KCC2 expression. Left, Cross section of an S25 retina. KCC2 is immunolabeled in red, and the somata are immunolabeled in blue (DAPI). Middle, Cross section of a retina at PH28. White asterisks indicate areas of stronger KCC2 expression within the IPL. Right, Cross section of a bicuculline-treated retina at PH28. KCC2 expression is much weaker, especially in the inner part of the IPL, reminiscent of embryonic stages. BIC, Bicuculline; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; OPL, outer plexiform layer.
Discussion
We showed previously that visual experience is necessary for GABA to switch polarity in the developing turtle retina (Sernagor et al., 2003a). However, the shift in polarity starts ∼1 week before animals become exposed to light, suggesting that factors other than light are important as well. Inspired both by the knowledge that GABA-expressing cells are present long before hatching (Nguyen and Grzywacz, 2000) and by the fact that chronic GABAA activity autoregulates the polarity switch in hippocampal cultures (Ganguly et al., 2001), we investigated whether chronic GABAA receptor blockade influences the polarity switch in vivo in the developing turtle retina and found that it is indeed necessary.
This study is the first attempt to investigate this potential trophic role of GABA using an in vivo experimental model, and it consolidates in vitro observations from rat hippocampus (Ganguly et al., 2001). In vivo models offer the advantage of allowing the manipulation of GABAergic neurotransmission and observing the consequences of these manipulations on network activity in an intact system. Changes in GABA expression affect activity correlation between neighboring RGCs and therefore are bound to influence the formation of connections in the visual system at both retinal (Wong, 1999; Sernagor et al., 2001) and extraretinal (Shatz, 1996; Crair, 1999; Grubb et al., 2003; McLaughlin et al., 2003) levels.
Our results support the idea that spontaneous neural activity during development is important not only for the maturation of excitatory circuitry but also for the development of inhibitory synaptic connections (Gaïarsa, 2004). Interestingly, the maturation of GABAergic synapses in the hippocampus occurs while the entire network expresses high, patterned neural activity (Gubellini et al., 2001; Gaïarsa, 2004), and it coincides with the period during which GABAA responses switch from excitation to inhibition (Ben-Ari et al., 1989), suggesting that high levels of GABAergic activity during that period may control the polarity switch.
In many systems, such as the spinal cord, chronic application of antagonists may result in homeostatic changes in neural networks (O'Donovan and Chub, 1997). This raises the possibility that spontaneous activity in the retina returns to baseline levels sometime after exposure to bicuculline. However, we have no indication that this is the case, because activity levels remain higher than normal even after 5 d of constant exposure to bicuculline released from Elvax (Fig. 2). Moreover, the homeostatic changes reported in the spinal cord (at relatively late developmental stages, such as in our experiments) were observed after glutamatergic rather than GABAergic blockade (O'Donovan and Chub, 1997).
Despite the controversy arising from the two in vitro studies on rodent hippocampal cultures (Ganguly et al., 2001; Ludwig et al., 2003), our observations suggest that the developmental increase in the expression of KCC2 in the IPL is GABA dependent, and they confirm that GABA controls chloride homeostasis in the developing retina, thereby acting as a self-limiting factor of its developmental polarity switch. Interestingly, after chronic GABAA receptor blockade, the expression of KCC2 decreases relatively more in the inner part of the IPL, in which RGC dendrites processing ON responses form a distinct lamina. In the developing ferret retina, when GABA switches to inhibition, it inhibits ON RGCs more strongly than OFF cells (Fischer et al., 1998), suggesting that, during normal development, extrusion of chloride by KCC2 from the inner dendritic plexus may become more pronounced than in the OFF layer. This would imply that waves occur only when ON RGCs have no inhibitory GABAergic inputs on their dendrites and that once GABAergic inhibition is removed, as in the present study, propagating waves resume.
In our hands, the expression level of KCC2 is lower at S25 than at PH28 after chronic exposure to bicuculline, indicating that the chronic treatment did not fully suppress the developmental upregulation of the transporter. Nevertheless, it is lower than in age-matched controls and appears to be sufficient to cause GABAA receptor activation to exert a depolarizing effect, suggesting that the distribution of chloride ions resembles that found in immature cells. Direct measurements of chloride reversal potential will clarify this point. Surprisingly, after chronic exposure to bicuculline, waves are somewhat faster than at S25, and this is despite the fact that KCC2 expression is higher than at late embryonic stages. Moreover, in the presence of acute bicuculline, waves accelerate even more, approaching the propagation speeds we reported at S24 during normal development (Sernagor et al., 2003a,b). This discrepancy may be attributable to developmental changes in the neural network underlying wave generation and propagation. For example, glutamate connections, which are known to control wave speed (Sernagor et al., 2000, 2003a,b), might become reinforced with retinal maturation.
A major question that remains to be answered is what are the precise and crucial steps occurring between the GABA-triggered calcium transients and the final increase in KCC2 expression, leading to the reversal of the polarity switch in GABAA responses. The answer must lie in some processes specifically triggered by GABA rather than by glutamate-evoked calcium transients, but what these factors are remains unknown.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (Swindon, UK) (E.S.). We thank C. Slater for lending the equipment for analyzing KCC2 immunofluorescence and S. J. Eglen for critical reading of this manuscript.
Correspondence should be addressed to Evelyne Sernagor at the above address. E-mail: Evelyne.Sernagor@ncl.ac.uk.
Copyright © 2005 Society for Neuroscience 0270-6474/05/254801-05$15.00/0
References
- Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3: 728-739. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaïarsa J-L (1989) Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol (Lond) 416: 303-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC (1999) Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol 9: 88-93. [DOI] [PubMed] [Google Scholar]
- Fischer KF, Lukasiewicz PD, Wong ROL (1998) Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci 18: 3767-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaïarsa J-L (2004) Plasticity of GABAergic synapses in the neonatal rat hippocampus. J Cell Mol Med 8: 31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M (2001) GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 18: 521-532. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID (2003) Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron 40: 1161-1172. [DOI] [PubMed] [Google Scholar]
- Grzywacz NM, Sernagor E (2000) Spontaneous activity in developing turtle retinal ganglion cells: statistical analysis. Vis Neurosci 17: 229-241. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaïarsa J-L (2001) Activity- and age-dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur J Neurosci 14: 1937-1946. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Li H, Saarma M, Kaila K, Rivera C (2003) Developmental upregulation of KCC2 in the absence of GABAergic and glutamatergic transmission. Eur J Neurosci 18: 3199-3206. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD (2003) Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40: 1147-1160. [DOI] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Kolb H (1978) Intracellular staining reveals different levels of stratification for on-centre and off-centre ganglion cells in the cat retina. J Neurophysiol 41: 472-483. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Grzywacz NM (2000) Colocalization of choline acetyltransferase and gamma-aminobutyric acid in the developing and adult turtle retinas. J Comp Neurol 420: 527-538. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Chub N (1997) Population behavior and self-organization in the genesis of spontaneous rhythmic activity by developing spinal networks. Semin Cell Dev Biol 8: 21-28. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3: 715-727. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipo J, Kaila K (2003) Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26: 199-206. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397: 251-255. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM (1995) Emergence of complex receptive field properties of ganglion cells in the developing turtle retina. J Neurophysiol 73: 1355-1364. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM (1996) Influence of spontaneous activity and visual experience on developing retinal receptive-fields. Curr Biol 6: 1503-1508. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM (1999) Spontaneous activity in developing turtle retinal ganglion cells: pharmacological studies. J Neurosci 19: 3874-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, O'Donovan MJ (2000) Differential effects of acetylcholine and glutamate blockade on the spatiotemporal dynamics of retinal waves. J Neurosci 20: RC56(1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, Wong ROL (2001) Development of retinal ganglion cell structure and function. Prog Retin Eye Res 20: 139-174. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Young C, Eglen SJ (2003a) Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. J Neurosci 23: 7621-7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Leitch EA, Young C, Mehta V (2003b) Developmental changes in GABAA receptor-driven activity mediate the maturation of retinal spontaneous activity patterns. Soc Neurosci Abstr 29: 655.4. [Google Scholar]
- Shatz CJ (1996) Emergence of order in visual system development. Proc Natl Acad Sci USA 93: 602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Seunghoon L, Zheng J, Zhou ZJ (2004) Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol (Lond) 560: 533-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TQ, Payne JA, Copenhagen DR (2000) Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J Neurosci 20: 1414-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ROL (1999) Retinal waves and visual system development. Annu Rev Neurosci 22: 29-47. [DOI] [PubMed] [Google Scholar]
- Yntema CL (1968) A series of stages in the embryonic development of Chelydra serpentina J Morphol 125: 219-251. [DOI] [PubMed] [Google Scholar]
- Zheng J-I, Seunghoon L, Zhou ZJ (2004) A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron 44: 851-864. [DOI] [PubMed] [Google Scholar]