Abstract
Neocortical neurons spontaneously fire action potentials during active network states; how are dendritic synaptic inputs integrated into the ongoing action potential output pattern of neurons? Here, the efficacy of barrages of simulated EPSPs generated at known dendritic sites on the rate and pattern of ongoing action potential firing is determined using multisite whole-cell recording techniques from rat layer 5 neocortical pyramidal neurons in vitro. Under quiescent conditions, the somatic impact of proximal (253 ± 15 μm from soma; n = 28) dendritic barrages of simulated EPSPs was 4.7-fold greater than identical barrages of EPSPs generated from distal (572 ± 13 μm from soma) sites. In contrast, barrages of proximal simulated EPSPs enhanced the rate of ongoing action potential firing, evoked by somatic simulated EPSPs, by only 1.6-fold more than distal simulated EPSPs. This relationship was apparent across a wide frequency range of action potential firing (6-22 Hz) and dendritic excitation (100-500 Hz). The efficacy of distal dendritic EPSPs was formed by the recruitment of active dendritic processes that transformed the ongoing action potential firing pattern, promoting action potential burst firing. Paired recordings (n = 42) revealed that patterns of action potential firing generated by concerted somatic and distal dendritic excitation reliably and powerfully drove postsynaptic excitation as a result of enhanced reliability of transmitter release during bursts of action potential firing. During active states, therefore, distal excitatory synaptic inputs decisively control the excitatory synaptic output of layer 5 neocortical pyramidal neurons and so powerfully influence network activity in the neocortex.
Keywords: EPSP, action potential, neocortex, dendrite, sodium channel, HCN channel
Introduction
In vivo neocortical neurons fire action potentials as a consequence of structured network activity (Arieli et al., 1996; Tsodyks et al., 1999; Sanchez-Vives and McCormick, 2000; Steriade et al., 2001; Cossart et al., 2003; Ikegaya et al., 2004). Because the action potential output of single neurons in response to natural stimuli is tightly linked to the level of cortical network activity (Arieli et al., 1996; Tsodyks et al., 1999; Anderson et al., 2000), stimulus-dependent synaptic inputs are likely to be bound into the ongoing action potential firing pattern of neurons. How are synaptic inputs generated at sites throughout the dendritic tree integrated under active action potential firing states?
Previous studies have explored the mechanisms controlling the somatic amplitude and time course of dendritically generated EPSPs under quiescent states in vitro (Stuart and Spruston, 1998; Magee, 1999; Magee and Cook, 2000; Williams and Stuart, 2000a, 2002; Berger et al., 2001). In neocortical pyramidal neurons, EPSPs generated from increasing remote dendritic sites are predicted to have a diminishing influence on action potential output (Stuart and Spruston, 1998; Williams and Stuart, 2000a, 2002; Berger et al., 2001). During ongoing action potential firing, however, these integration rules may not apply as the neuronal membrane potential is dynamically evolving, allowing interaction of synaptic potentials with classes of dendritically located voltage-activated channels (Häusser et al., 2000). Modeling studies have shown that the site-dependent variability of the somatic amplitude of dendritic EPSPs may be minimized by the interaction of EPSPs with dendritic voltage-activated channels (De Schutter and Bower, 1994; Cook and Johnston, 1997, 1999). Furthermore, the impact of dendritic excitatory input on action potential output has been shown to be amplified by the recruitment of dendritic voltage-activated channels (Williams and Stuart, 2003). Recently, two studies have examined the control of action potential output by dendritic EPSPs in neocortical layer 5 pyramidal neurons (Oviedo and Reyes, 2002; Larkum et al., 2004). Oviedo and Reyes (2002) found that the action potential output evoked by barrages of simulated EPSPs (sEPSPs) generated from proximal apical dendritic sites (<300 μm from the soma) was augmented by the recruitment of proximal apical dendritic sodium channels. In contrast, Larkum et al. (2004) demonstrated that the generation of a barrage of excitation at distal apical dendritic sites (>500 μm from the soma) decreased the threshold and augmented the rate of action potential firing generated by concerted somatic excitation. In light of these findings, the present experiments were conducted to test the hypothesis that active dendritic mechanisms act to normalize the efficacy of barrages of apical dendritic EPSPs generated at sites throughout the apical dendritic arbor, despite a uniformity of underlying synaptic conductance (Williams and Stuart, 2002). Results indicate that identical barrages of simulated EPSPs (100-500 Hz) presented from proximal or distal apical dendritic sites enhanced the mean rate of ongoing action potential firing with modest site-dependent variability, despite intense site-dependent subthreshold voltage attenuation. Distal excitatory inputs were found to transform the pattern of ongoing action potential firing by promoting the occurrence of burst discharges, the physiological significance of which was explored by paired recordings between layer 5 pyramidal neurons.
Materials and Methods
Recordings were made from layer 5 pyramidal neurons visualized in neocortical brain slices (300 μm) prepared from Wistar rats [postnatal day 24 (P24) to P32] following Institutional and UK Home Office guidelines. Slices were perfused with a solution of the following composition (in mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 3 Na pyruvic acid, and 25 glucose at 35-37°C. Triple and quadruple whole-cell recordings were made with identical current-clamp amplifiers (BVC 700; Dagan, Minneapolis, MN). Pipettes were filled with the following (in mm): 135 K-gluconate, 7 NaCl, 10 HEPES, 2 Na2-ATP, 0.3 Na2-GTP, and 2 MgCl2, pH 7.2-7.3 with KOH. Signals were filtered at 10 kHz and acquired at 20-50 kHz using Axograph (Molecular Devices, Union City, CA). Data were analyzed, and curve fitting was performed using Axograph. Numerical values are expressed as mean ± SEM, unless otherwise stated. Statistical analysis included the Kolmogorov-Smirnov and Student's t test.
Generation of simulated postsynaptic potentials. Simulated EPSPs were generated either as ideal current sources (see Figs. 1, 2, 4) or as conductance changes (see Figs. 3, 5, 6, 7). A barrage of 1000 sEPSCs (τrise = 0.2 ms; τdecay = 2 ms) (Williams and Stuart, 2002) was generated over a 2 s period with random times of occurrence. In each recording, this barrage of sEPSCs was injected at the soma to generate ongoing action potential firing that was stable from trial to trial (data not shown) (Mainen and Sejnowski, 1995; Harsch and Robinson, 2000). The frequency of action potential firing (calculated by the number of action potentials generated over a 2 s period) was varied by altering the unitary sEPSC amplitude (200-400 pA). A separate barrage of randomly generated sEPSCs was injected at apical dendritic sites (unitary amplitude, 200 pA; τrise = 0.2 ms; τdecay = 2 ms; frequency, 100-500 Hz). In each triple recording, identical barrages of sEPSPs were generated at a proximal or distal dendritic site in the absence of, or in concert with, somatic sEPSPs. In some triple recordings, the impact of dendritic excitation was not tested for each of the four frequency bands of ongoing action potential firing. Conductance injection was achieved using a real-time dynamic clamp (Harsch and Robinson, 2000), using closely spaced (<10 μm) pipettes for voltage recording and current injection at dendritic sites or by double somatic recording (Williams, 2004). At dendritic sites, each conductance EPSP (gEPSP) was simulated as an AMPA- and NMDA-type conductance (AMPA: τrise = 0.2 ms, τdecay = 2 ms, gAMPA = 4 nS, EAMPA = 0 mV; NMDA: τrise = 5 ms, τdecay = 150 ms, gNMDA = 0.4 nS, ENMDA = 0 mV) (Harsch and Robinson, 2000; Williams, 2004), whereas at somatic sites, gEPSPs were simulated solely as an AMPA conductance. In some experiments, a barrage of gIPSPs (τrise = 0.5 ms; τdecay = 5 ms; gIPSC = 4 nS; EIPSC = -70 mV; frequency, 50-500 Hz) was coinjected with gEPSPs.
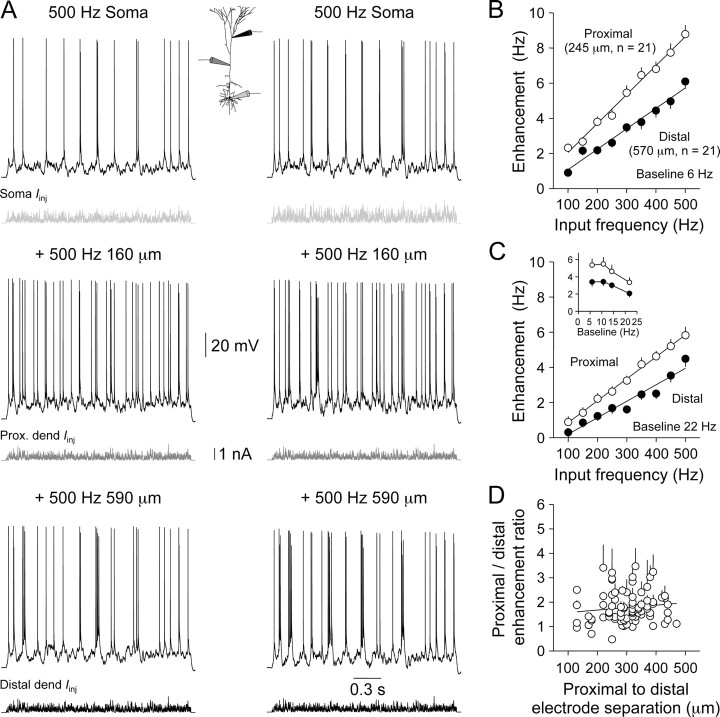
Figure 1.
Subthreshold impact of dendritic excitation. A, Somatic voltage responses evoked by dendritic excitation generated from the indicated sites. The bottom trace shows the injected current (Dend Iinj). B, The amplitude of somatic voltage responses decreased as dendritic excitation was generated from progressively remote dendritic loci (350 Hz; n = 56). The division between proximal and distal apical dendritic recording sites is illustrated; the line represents a single exponential fit. C, Pooled data showing the average somatic voltage deviation produced by dendritic excitation of increasing frequency generated at proximal or distal sites. Note the disparity between the relationship generated from proximal and distal dendritic loci. Error bars represent SEM. D, The subthreshold somatic impact of dendritic excitation does not explain the enhancement of action potential firing rate. The graph shows the ratio of the somatic voltage responses evoked by proximal and distal dendritic excitation (500 Hz) for each triple recording (filled symbols; subthreshold), plotted as a function of the distance between dendritic electrodes. In comparison, in the same neurons, the ratio of the enhancement of ongoing action potential firing rate generated by proximal and distal dendritic excitation (500 Hz) is shown (open symbols; firing). The line through each data set represents a linear regression.
Figure 2.
Enhancement of action potential firing by dendritic excitation. A, Somatic voltage records of axonal action potential firing generated by the presentation of random patterns of EPSPs delivered at the soma only (top traces; unitary EPSC amplitude: 260 pA, left trace; 360 pA, right trace), at the soma plus proximal apical dendrite (Prox. dend; middle traces), and at the soma plus distal dendrite (Distal dend; bottom traces). Iinj, Injected current. B, Enhancement of ongoing low-frequency action potential firing as a function of the frequency of dendritic excitation, when delivered at proximal (245 ± 18 μm; n = 21; open symbols) and distal (570 ± 17 μm; n = 21; filled symbols) loci. The baseline firing rate is indicated. C, In the presence of a higher frequency of ongoing action potential firing, the frequency-dependent enhancement of action potential firing rate generated by proximal or distal excitation is relatively modest (proximal: 268 ± 18 μm, n = 21; distal: 566 ± 15 μm, n = 21). The baseline firing rate is indicated. The inset shows the enhancement of firing rate produced by dendritic excitation as a function of baseline firing frequency. Note the parallel relationship for proximal and distal excitation. D, Relationship between the ratio of firing rate enhancement generated by proximal and distal excitation as a function of the physical distance between proximal and distal recording sites. For each neuron, the average ± SEM value of enhancement ratio across the frequency range of dendritic EPSP generation, for each baseline action potential firing frequency, is plotted. The fitted line is a linear regression.
Figure 4.
Blockade of distal but not proximal sodium channels disrupts rate enhancement. A, Dendritic voltage records generated by combined somatic and dendritic excitation (top trace; 500 μm from the soma) under control and after local dendritic application of TTX (1 μm). The occurrences of axonal action potentials (APs) are shown as vertical lines. Note that TTX attenuated dendritic electrogenesis and reduced action potential burst firing. B, Summary data showing the action potential firing rate evoked by concerted somatic and apical dendritic excitation under control and after local application of TTX. Note that local application of TTX at proximal dendritic sites (open symbols) failed to alter the action potential firing rate. The line has a slope of 1. C, Pooled data showing the amplitude of the first backpropagating action potential evoked by somatic excitation under control and after local application of TTX. The line has a slope of 1. The inset shows the simultaneous recording of an action potential at the soma and a proximal dendritic site (380 μm from the soma) under control and after local dendritic application of TTX. D, Cumulative probability distribution of action potential instantaneous (Inst.) firing frequency generated by proximal (pooled data from n = 8 neurons) or distal (n = 6 neurons) excitation. Note that local application of TTX at distal dendritic sites dramatically decreased the proportion of action potentials generated at high frequency. Dend, Dendritic.
Figure 3.
Distal dendritic excitation increases action potential variability. A, Modeling excitation as a conductance source does not alter rate enhancement. Dendritic voltage records (Dend Vm; 710 μm from soma) generated in response to somatic excitation alone (top trace) or by somatic plus dendritic excitation are shown. Somatically recorded action potentials (APs) are shown as vertical lines, and the current delivered by the dynamic clamp is shown below (gclamp). B, Summary data describing the percentage enhancement of action potential firing rate as a function of the frequency of dendritic excitation when modeled as a conductance source and delivered at proximal (301 ± 10 μm; n = 7; open symbols) or distal (607 ± 19 μm; n = 10; filled symbols) loci. C, The coefficient of variation of action potential firing is enhanced by distal but not proximal dendritic excitation. D, Proximal excitation does not change the pattern of action potential generation. Cumulative probability distribution of instantaneous (Inst.) firing frequency under the indicated experimental conditions. E, Distal excitation increased the proportion of action potentials generated at high instantaneous firing frequencies, an effect that is vetoed by synaptic inhibition (gGABA). Error bars represent SEM. Dend, Dendritic.
Figure 5.
Dendritic excitation is vetoed by inhibition. A, Synaptic inhibition vetoes rate enhancement. Dendritic voltage traces (Dend Vm; 640 μm from soma) generated in response to somatic plus dendritic excitation in the absence (top traces) and presence (gGABA; bottom traces) of inhibition. Somatically recorded action potentials (APs) are shown as vertical lines, and the current delivered by the dynamic clamp (gclamp) is shown below. B, Pooled data describing the progressive reduction of action potential firing rate as a function of the frequency of IPSP generation when delivered in concert with proximal (open symbols) or distal (filled symbols) excitation. C, Synaptic inhibition decreases the coefficient of variation of action potential firing when generated at distal but not proximal sites. Dend, Dendritic.
Figure 6.
Short-term dynamics of synaptic transmission. A, Paired-pulse facilitation of unitary EPSPs recorded between a pair of layer 5 pyramidal neurons. Vpost, Postsynaptic voltage; Vpre, presynaptic voltage. B, The majority of uEPSPs exhibited paired-pulse facilitation (action potentials delivered at 40 Hz; cumulative distribution of 42 paired recordings). C, The failure rate of uEPSPs is reduced by a preceding action potential [action potentials (APs) were generated at 40 Hz; cumulative distribution of 42 paired recordings]. D, Pooled data demonstrating that paired-pulse facilitation increased as a function of presynaptic action potential firing frequency (n = 15; the line represents a single exponential fit). Error bars represent SEM. E, Representative example of a train of uEPSPs evoked by a high-frequency (300 Hz) burst of four presynaptic action potentials. The bottom trace shows a train of simulated gEPSPs generated at the same frequency. F, Peak amplitude of uEPSPs (gray lines; average shown as filled symbols) generated by a high-frequency burst (4 action potentials delivered at 300 Hz). The peak amplitude of the first uEPSP of each burst has been normalized. In comparison, the amplitude of simulated gEPSPs (1 nS) delivered at the same frequency is illustrated (black lines; average shown as open symbols).
Figure 7.
Distal dendritic inputs drive powerful and reliable postsynaptic excitation. A, Averaged uEPSPs evoked by a train of presynaptic action potentials. The firing patterns were replicated from a somatodendritic recording, where action potential firing was generated by somatic excitation alone or soma plus dendritic (640 μm) excitation. Vpost, Postsynaptic voltage; Vpre, presynaptic voltage. B, Response of an ideal synapse (gEPSP) generated with identical timings. C, Ratio of the cumulative amplitude of EPSPs recorded in response to soma and soma plus dendritic excitation. Note that in the majority of recordings the ratio of uEPSPs is greater than those of gEPSPs (1 and 3 nS). The dashed lines represent ±3 SD. The inset shows the paired-pulse ratio (PP; 40 Hz) of uEPSPs that performed suboptimally (sub.; filled bar) or greater than suboptimally (open bar). D, Failure analysis of uEPSPs evoked during soma only and soma plus dendrite action potential trains. The gray boxes delineate action potential burst discharges. Error bars represent SEM.
Paired recording. Synaptic connectivity was examined between groups of three or four layer 5 pyramidal neurons, with somatic separation of <100 μm. Short-term dynamics were investigated with a paired-pulse paradigm (40-200 Hz; repeated every 5 s; interleaved with single action potential trials). The amplitude of the second unitary EPSP (uEPSP) of a pair was measured after the digital subtraction of a scaled single uEPSP. Failures of transmission were detected, using a threshold algorithm, individually inspected, and digitally averaged to ensure the absence of small-amplitude uEPSPs (Silver et al., 2003). To examine the impact of action potential trains, the absolute time of each action potential of a train evoked by somatic excitation or somatic plus distal dendritic excitation (640 μm from the soma) was measured, and replica trains of 20 μs transistor-transistor logic (TTL) pulses were produced. TTL pulses were used to gate the current injection circuit of the amplifier to produce current pulses of 5-10 nA that evoked presynaptic action potential firing with high temporal precision. Interleaved trains of presynaptic action potentials were repeated every 30 s, and averages of between 30 and 80 trials were calculated. For averaged trials, the peak amplitude of each uEPSP was measured and summed to produce the cumulative amplitude. Trains of gEPSPs, simulated as an AMPA conductance change, with the same times of occurrence were averaged (30-50 trials) and analyzed in the same manner.
Results
Subthreshold somatic impact of EPSPs
To provide a framework to explore the efficacy of dendritic EPSPs during active action potential firing states in neocortical pyramidal neurons, the subthreshold somatic impact of barrages of simulated dendritic EPSPs was defined. Identical barrages of sEPSPs (frequency, 100-500 Hz; for properties, see Materials and Methods) were generated from a proximal (253 ± 15 μm from soma; n = 28) and distal apical dendritic site (572 ± 13 μm from soma; n = 28) that in each case did not directly evoke action potential output (Fig. 1A). The somatic voltage response evoked by barrages of dendritic EPSPs was found to decrease exponential as the site of sEPSP generation was made from progressively remote dendritic sites (Fig. 1B). Pooled data demonstrated that proximal dendritic sEPSPs evoked somatic voltage responses of magnitude on average 4.7 ± 0.2 times greater than distal sEPSPs (averaged across the frequency range of dendritic sEPSP generation; n = 28 neurons) (Fig. 1C). Furthermore, when the ratio of somatic voltage responses generated by proximal and distal sEPSPs was plotted as a function of the distance between dendritic excitation sites, a steep positive linear relationship was found (slope, 0.95 per 100 μm) (Fig. 1D, filled symbols).
Encoding of dendritic EPSPs in the action potential output
To examine the integration of dendritic EPSPs during periods of action potential firing, action potential output was evoked by the somatic delivery of a random barrage of simulated EPSPs (frequency, 500 Hz) and the impact of a barrage of dendritic sEPSPs on the action potential firing rate explored (sEPSP properties identical to those used to determine the subthreshold impact) (Fig. 2). During trains of ongoing action potential firing evoked by somatic sEPSPs, dendritic sEPSPs powerfully enhanced the rate of action potential firing (Fig. 2A-C). Surprisingly, sEPSPs generated from proximal apical dendritic sites were found to be on average only 1.6 ± 0.1 times more effective than identical inputs generated from distal dendritic sites at enhancing the action potential firing rate (Fig. 2A-C). This relationship held when dendritic sEPSPs were generated across a wide range of frequencies (100-500 Hz) (Fig. 2B,C) and for a broad frequency range of ongoing action potential firing (6-22 Hz) (Fig. 2C, inset). Furthermore, when the ratio of enhancement of action potential firing rate produced by proximal and distal barrages of sEPSPs was plotted as a function of the distance between dendritic sites of excitation, a shallow linear relationship was found (slope, 0.10 per 100 μm) (Fig. 2D). This distance-dependent profile was in stark contrast with the steep relationship found for subthreshold voltage responses in the same neurons [Fig. 1D, compare firing (open symbols) and subthreshold relationship]. These data indicate that the impact of distal dendritic EPSPs during active states cannot be explained by the direct spread of excitation from site of generation to the soma and axon but suggest that active dendritic mechanisms are engaged during action potential firing states.
Active dendritic mechanisms shape synaptic integration during spike trains
Previous observations have shown that the conductance load imparted by synaptic activity acts to compartmentalize the dendritic arbor of neocortical pyramidal neurons, by increasing voltage attenuation and hampering the propagation of regenerative activity (Koch et al., 1990; Rapp et al., 1996; Williams, 2004). To inquire whether the observed relationship between dendritic synapse location and the control of ongoing action potential firing rate was sustainable under a high synaptic conductance state, EPSPs were simulated as AMPA and NMDA receptor-mediated conductance changes (for properties, see Material and Methods) (Fig. 3A). Results indicated that the spatial pattern of action potential rate enhancement was unperturbed by the conductance load imparted by gEPSPs (compare Figs. 1B, 3B). When evoked at distal, but not proximal, dendritic sites, barrages of gEPSPs led to the generation of repeated periods of large-amplitude dendritic electrogenesis that promoted action potential burst firing (Fig. 3A). Pooled data reveled that distal, but not proximal, barrages of gEPSPs increased the coefficient of variation (CV) of action potential firing (Fig. 3C) and transformed the distribution of instantaneous action potential firing frequency, increasing the proportion of action potentials generated at high (>50 Hz) frequency (Fig. 3D,E). These data directly show that identical barrages of gEPSPs generated at proximal or distal apical dendritic sites are encoded into action potential output in a qualitatively different manner.
Previous observations have indicated that single distal dendritic EPSPs, or tonic membrane depolarization, can augment the amplitude of single backpropagating (BPAPs) action potentials and so facilitate interaction of BPAPs with classes of dendritic ion channels (Magee and Johnston, 1997; Larkum et al., 1999; Williams and Stuart, 2000b; Stuart and Häusser, 2001; Waters et al., 2003). To examine whether interaction between dendritic EPSPs and BPAPs was necessary for the expression of rate enhancement, the sodium channel blocker tetrodotoxin (TTX) was locally applied at the dendritic site of sEPSP generation (Williams and Stuart, 2000b). Local application of TTX to the region of the distal site of sEPSP generation prevented the generation of distal dendritic electrogenesis and significantly decreased the enhancement of action potential firing rate generated by concerted somatic and distal dendritic excitation (somatic excitation alone: control, 11.2 ± 1.6 Hz, TTX, 11.0 ± 1.7 Hz, recovery, 11.1 ± 2.1 Hz; somatic plus distal dendritic excitation: control, 17.3 ± 1.5 Hz, TTX, 14.2 ± 1.8 Hz, recovery, 17.0 ± 1.9 Hz; p < 0.01; n = 6; 545 ± 28 μm from the soma) (Fig. 4A,B). This reduction of efficacy was accompanied by a dramatic reduction of action potential burst firing (Fig. 4A,D). In contrast, application of TTX to proximal dendritic sites failed to disturb the enhancement of ongoing action potential firing or the distribution of instantaneous firing frequency produced by proximal dendritic excitation (somatic excitation alone: control, 8.6 ± 0.5 Hz, TTX, 8.6 ± 0.5 Hz, recovery, 8.0 ± 0.6 Hz; somatic plus proximal dendritic excitation: control, 18.0 ± 1.7 Hz, TTX, 17.4 ± 1.8 Hz, recovery, 16.5 ± 1.9 Hz; n = 8; 284 ± 11 μm from the soma) (Fig. 4B,D), despite acting to powerfully attenuate the amplitude of BPAPs at proximal apical dendritic sites (control, 59.1 ± 2.4 mV; local TTX, 36.2 ± 2.8 mV; p < 0.01; n = 8) (Fig. 4C). Parallel experiments revealed that tonic distal dendritic membrane hyperpolarization significantly decreased the enhancement of action potential firing generated by distal dendritic sEPSPs (control, 5.6 ± 0.6 Hz; hyperpolarization, 1.3 ± 0.5 Hz; p < 0.001; n = 12; 558 ± 22 μm from the soma) (supplemental Fig. 1A,B, available at www.jneurosci.org as supplemental material), an effect mediated by the annihilation of periods of frank dendritic electrogenesis (supplemental Fig. 1C, available at www.jneurosci.org as supplemental material).
These findings suggest that the enhancement of action potential firing rate by dendritic EPSPs may be tightly controlled by synaptic inhibition. To test this, the impact of barrages of simulated dendritic gIPSPs delivered in concert with gEPSPs was explored (Fig. 5A). Pooled data demonstrated that the enhancement of action potential firing rate was reduced and finally abolished by synaptic inhibition in a frequency-dependent manner (50-500 Hz) (Fig. 5B). In common with dendritic excitation, synaptic inhibition operated distinctly when generated from proximal and distal dendritic sites, with distal dendritic inhibition curtailing and finally preventing the recruitment of dendritic electrogenesis, resulting in a progressive reduction of the CV of action potential discharges (Fig. 5C) and deconstructing the redistribution of instantaneous firing frequency (Fig. 3E). Notably, however, the frequency-dependent reduction of rate enhancement by gIPSPs proceeded in a parallel manner at proximal and distal dendritic sites, maintaining the relative impact of dendritic gEPSPs on the action potential firing rate (Fig. 5B).
Decoding of the action potential train
How are changes of the rate and pattern of ongoing action potential firing decoded by postsynaptic neurons of the cortical network? To address this, paired recordings were made between layer 5 pyramidal neurons, and the short-term dynamics of uEPSPs were examined (Fig. 6A). Pooled data revealed that the majority of uEPSPs exhibited paired-pulse facilitation (paired-pulse ratio at 40 Hz, 1.8 ± 0.1; n = 42) (Fig. 6B), an effect mediated, in part, by a reduction in the number of failures of transmission in response to the second action potential of a pair (Fig. 6C). Paired-pulse facilitation was found to increase exponentially with increasing frequency of action potential presentation (Fig. 6D). Furthermore, in response to a high-frequency (300 Hz) burst of four presynaptic action potentials, uEPSPs typically showed facilitation relative to the first uEPSP of the burst to yield summated responses of amplitude greater than those observed for the linear summation of simulated gEPSPs, which possessed no use-dependent properties (gEPSCs: τrise = 0.2 ms; τdecay = 2 ms; gEPSC = 1 nS; EEPSC = 0 mV; n = 10 double somatic recordings) (Fig. 6E,F). Together, the short-term dynamics of synaptic transmission suggest that action potential firing patterns generated by combined somatic and distal dendritic excitation, which exhibit repeated periods of action potential burst firing, should produce powerful postsynaptic excitation.
To directly test this hypothesis, trains of presynaptic action potentials with spike times taken from a somatodendritic recording were generated (see Materials and Methods) (Fig. 7A). A train of action potentials generated by somatic excitation alone produced a series of uEPSPs that on average showed little progressive alteration of amplitude (Fig. 7A, top traces). In contrast, a train of action potentials generated by combined somatic and distal dendritic excitation evoked uEPSPs that showed complex behavior, demonstrating intense facilitation and summation during periods of action potential burst firing (Fig. 7A, bottom traces). Across neurons, the cumulative amplitude of uEPSPs generated by the somato-distal dendritic spike pattern was 4.0 ± 0.2-fold (n = 24) greater than for the soma alone pattern. This difference was not simply a reflection of the number of uEPSPs generated in each train, because trains composed of the same number of uEPSPs generated at fixed intervals yielded a ratio of just 2.7 ± 0.1 (soma, 6.98 Hz; soma plus distal dendrite, 17.4 Hz; n = 24; data not shown). To highlight the role of short-term dynamics in this relationship, a series of simulated gEPSPs that possessed no use-dependent properties were generated with the same timing to provide a linear summation reference point (τrise = 0.2 ms; τdecay = 2 ms; gEPSC = 1 or 3 nS; EEPSC = 0 mV; n = 10 double somatic recordings) (Fig. 7B). Calculation of the cumulative amplitude ratio of trains of gEPSPs representing somatic and somato-distal dendritic excitation revealed a ratio of 3.7 ± 0.04 (gEPSC = 1 nS), a value that remained unchanged for gEPSPs simulated with a greater unitary conductance (3.7 ± 0.03; gEPSC = 3 nS) (Fig. 7C). Direct comparison revealed that the dynamics of uEPSPs ensured that the majority of investigated synaptic contacts produced a cumulative amplitude ratio that was equal to or greater than those directly observed for linear EPSP summation (Fig. 7C). In the few contacts that exhibited a ratio less (-3 SD) than simulated EPSPs, a low level of short-term plasticity was found (Fig. 7C, inset). As a final step, the fidelity of synaptic transmission was examined for each action potential of the spike trains to inquire whether distal dendritic excitation leads to an increase in the reliability of synaptic transmission. The failure rate for each action potential of the soma-only pattern of action potential firing remained at high levels throughout the spike train (n = 10 paired recordings) (Fig. 7D). In contrast, the failure rate of transmission declined during each period of burst firing evoked during action potential trains representing somatic and distal dendritic excitation (Fig. 7D). In summary, these data indicate that trains of action potentials evoked by concerted somatic and distal dendritic excitation reliably and powerfully drive postsynaptic excitation in the neocortex.
Discussion
In vivo the action potential firing pattern of neocortical pyramidal neurons appears random, exhibiting a high coefficient of variation (Softky and Koch, 1993; Shadlen and Newsome, 1998; Stevens and Zador, 1998). This observation has triggered debate concerning: (1) the mechanism(s) that neocortical neurons use to encode synaptic input into action potential output and (2) which aspect of the temporal structure of action potential output, the average rate or interspike timing, is decoded by postsynaptic neurons of the cortical network (Softky and Koch, 1993; Tovee et al., 1993; Shadlen and Newsome, 1994, 1998; Stevens and Zador, 1998; Williams and Stuart, 2000b). Here, these issues were directly investigated in layer 5 neocortical pyramidal neurons in vitro.
Control of action potential output by dendritic excitation
Identical patterns of synaptic input generated from proximal or distal apical dendritic loci enhanced the rate of ongoing action potential firing with minimal site-dependent variation. Although in the majority of trials, proximal excitation enhanced the rate of action potential firing to a greater degree than distal excitation, pooled data revealed a shallow relationship between the ratio of action potential firing rate enhancement and the physical separation between dendritic sites of excitation. In contrast, in the absence of ongoing action potential firing, the subthreshold somatic impact of dendritic excitation showed a steep distance-dependent relationship, as predicted by cable filtering coupled with the constraint of EPSP temporal summation imposed by interaction of EPSPs with dendritically located hyperpolarization-activated channels (Stuart and Spruston, 1998; Magee, 1999; Williams and Stuart, 2000a, 2002; Berger et al., 2001). During action potential firing states, therefore, the impact of dendritic excitation on action potential output is amplified in a distance-dependent manner, acting to mostly compensate for the effects of dendro-somatic EPSP voltage attenuation.
Encoding of dendritic excitation
The presented data suggest that proximal and distal dendritic EPSPs are encoded into the action potential output of neocortical pyramidal neurons in qualitatively different ways. From proximal apical dendritic sites, barrages of sEPSPs enhanced action potential firing rate without altering the CV, coaligned synaptic inhibition controlled the action potential firing rate but not variability, and local proximal dendritic application of the sodium channel blocker TTX failed to perturb the enhancement of action potential firing rate. These data indicate that proximal apical dendritic sEPSPs do not engage active dendritic mechanisms but spread in a passive manner to the axon to influence action potential initiation. Previous experiments have, however, shown that the impact of proximal dendritic sEPSPs on neuronal output is boosted by the recruitment of proximal apical dendritic sodium channels (Oviedo and Reyes, 2002). This difference may have arisen from contrasting methodology, because the barrages of proximal apical dendritic sEPSPs reported here were subthreshold for the generation of action potentials, whereas Oviedo and Reyes (2002) generated suprathreshold barrages of proximal dendritic sEPSPs. The impact of proximal dendritic excitation on action potential output may, therefore, be dependent on the level of synaptic input.
In contrast, barrages of sEPSPs generated from distal apical dendritic sites dramatically increased the CV of ongoing action potential discharges, suggesting the recruitment of additional nonlinear dendritic processes. Indeed, distal dendritic excitation generated periods of large amplitude dendritic electrogenesis that drove axonal action potential burst firing. The parallel reduction of dendritic electrogenesis, action potential firing rate, and CV by the generation of coaligned synaptic inhibition, the local distal dendritic application of TTX, and direct distal membrane potential hyperpolarization indicated that the recruitment of active dendritic processes powerfully contribute to this behavior. Previous investigations have shown that interaction between single BPAPs and appropriately timed subthreshold distal dendritic EPSPs leads to the generation of action potential burst firing in neocortical pyramidal neurons as a consequence of the regenerative recruitment of distal dendritic sodium and calcium channels (Larkum et al., 1999, 2004; Stuart and Häusser, 2001; Waters et al., 2003). The presented data are compatible with this mechanism and suggest that such interaction acts to enhance the impact of distal dendritic excitation across a fourfold range of action potential firing frequencies and across a wide frequency range of EPSP generation (100-500 Hz).
In summary, enhancement of the rate of ongoing action potential firing by proximal and distal apical dendritic excitation is proposed to occur by divergent mechanisms. Barrages of proximal dendritic EPSPs do not evoke active dendritic processes but enhance action potential firing by providing additional depolarization at the axonal site of action potential initiation. In contrast, distal dendritic EPSPs enhance action potential firing by the combination of (1) the spread of depolarization from site of generation to the axon and (2) the initiation of bursts of axonal action potentials generated by the interplay between BPAPs and distal dendritic sodium and calcium channels. This distal dendritic nonlinearity acts to boost the impact of distal excitatory inputs on action potential output, transforms the action potential firing pattern, and so flattens the distance-dependent relationship between the site of excitation and the control of the rate of ongoing action potential firing.
The transformation of the action potential firing pattern by distal dendritic excitation suggests that the highly variable discharge pattern of cortical neurons in vivo may occur as a consequence of postsynaptic integrative mechanisms and so is not reliant on temporal coherence among excitatory synaptic inputs (Stevens and Zador, 1998). Notably, a simulation study has demonstrated that active dendritic conductances lead to the production of highly variable action potential firing in response to un-correlated synaptic input (Softky and Koch, 1993). The reported findings reveal that such a dendritic nonlinearity shapes the variability of action potential firing and indicate that physiological levels of action potential variability naturally arise when excitatory input is received across the dendritic arbor of neocortical pyramidal neurons.
These results reveal that synaptic integration in cortical pyramidal neurons is a state-dependent process. Under quiescent conditions, apparent in brain slices, neocortical neurons are functionally compartmentalized; powerful dendro-somatic voltage attenuation ensures that distal dendritic excitation has a minimal direct influence at the axon (Stuart and Spruston, 1998; Berger et al., 2001; Williams and Stuart, 2002). Under these conditions, distal dendritic excitatory inputs predominantly influence neuronal output after the local dendritic integration of temporally correlated EPSPs leading to the generation of dendritic spikes that forward propagate to the axon to initiate action potential output (Williams and Stuart, 2002; Williams, 2004). Although high-frequency barrages of distal dendritic EPSPs may directly initiate axonal action potentials after voltage spread from the site of generation to the axon (Stuart et al., 1997; Zhu, 2000; Larkum and Zhu, 2002), the synaptic conductance dependent increase of dendro-somatic voltage attenuation is predicted to hamper the direct initiation of axonal action potentials (Williams, 2004). During ongoing action potential firing states, however, barrages of EPSPs generated at proximal and distal dendritic loci powerfully control the rate of action potential generation. This control is apparent across a wide range of parameters and is not perturbed by synaptic conductance and so will be sustainable during active network states in vivo (Kamondi et al., 1998; Waters and Helmchen, 2004). It should be noted, however, that the pattern of synaptic activity generated here does not reflect conditions where synaptic activity is generated at sites distributed throughout the dendritic arbor but more faithfully reflects alterations of the firing rate of presynaptic neurons that target discrete apical dendritic sites (Williams and Stuart, 2002). A dramatic distributed increase of ongoing synaptic activity is predicted to attenuate the rate enhancement generated by localized barrages of dendritic EPSPs by shunting the dendritic membrane to increase voltage attenuation and attenuate the backpropagation of action potentials (Koch et al., 1990; Rapp et al., 1996; Williams, 2004).
Decoding of dendritic excitation
To explore how action potential firing patterns were decoded by postsynaptic neurons of the cortical network, paired recordings were made between layer 5 pyramidal neurons. In response to a train of action potentials generated by somatic excitation alone, frequent failure of transmission occurred, suggesting that the rate of action potential firing is unreliably signaled. In contrast, an action potential firing pattern generated by concerted somatic and distal dendritic excitation evoked reliable synaptic transmission during periods of action potential burst firing. These data indicate, in common with other central synapses, that action potential burst firing increases the security of transmission (Lisman, 1997) and suggest that synaptic mechanisms act to reliably decode the fine structure of action potential firing patterns in layer 5 pyramidal neurons. This behavior resulted from the short-term dynamics of uEPSPs that exhibited frequency-dependent paired-pulse facilitation and demonstrate the influence of such dynamics throughout a train of action potential firing. Previous investigations of synaptic transmission between layer 5 pyramidal neurons have highlighted the presence of use-dependent depression (Thomson et al., 1993; Markram et al., 1997; Fuhrmann et al., 2002). The short-term dynamics of transmission are, however, developmentally regulated in layer 5 pyramidal neurons, switching from depression to facilitation with age (Reyes and Sakmann, 1999). Furthermore, synaptic depression is relieved after induction of long-term potentiation (Markram and Tsodyks, 1996) and regulated by neuromodulators (Tsodyks and Markram, 1997). In contrast with previous suggestions that synaptic transmission between layer 5 pyramidal neurons is tuned to encode low-frequency rate codes, as a result of synaptic depression (Fuhrmann et al., 2002), the present findings indicate that in mature cortical circuits transmission is tuned to respond to the temporal features of presynaptic spike trains, increasing gain in response to periods of high-frequency action potential firing. Together, these properties indicate that the postsynaptic saliency of pyramidal neuron output, generated by proximal (basal and proximal apical dendritic) and distal apical dendritic excitatory synaptic inputs are controlled according to a logical AND operation. Because distal apical dendritic excitatory inputs to cortical pyramidal neurons are primarily composed of corticocortical connections (Salin and Bullier, 1995; Cauller et al., 1998), which functionally convey contextual and attentional information (Gilbert, 1992; Desimone and Duncan, 1995; Hupe et al., 1998), it is tempting to speculate that activity within these pathways has a decisive influence on network activity within the neocortex and the control of subcortical structures (Brecht et al., 2004).
Footnotes
I am grateful to S. Atkinson, L. Gentet, and L. Lagnado for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Stephen R. Williams, Neurobiology Division, Medical Research Council, Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, UK. E-mail: srw@mrc-lmb.cam.ac.uk.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255894-09$15.00/0
References
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D (2000) Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat Neurosci 3: 617-621. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A (1996) Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868-1871. [DOI] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher HR (2001) High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 85: 855-868. [DOI] [PubMed] [Google Scholar]
- Brecht M, Schneider M, Sakmann B, Margrie TW (2004) Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427: 704-710. [DOI] [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW (1998) Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol 390: 297-310. [PubMed] [Google Scholar]
- Cook EP, Johnston D (1997) Active dendrites reduce location-dependent variability of synaptic input trains. J Neurophysiol 78: 2116-2128. [DOI] [PubMed] [Google Scholar]
- Cook EP, Johnston D (1999) Voltage-dependent properties of dendrites that eliminate location-dependent variability of synaptic input. J Neurophysiol 81: 535-543. [DOI] [PubMed] [Google Scholar]
- Cossart R, Aronov D, Yuste R (2003) Attractor dynamics of network UP states in the neocortex. Nature 423: 283-288. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Bower JM (1994) Simulated responses of cerebellar Purkinje cells are independent of the dendritic location of granule cell synaptic inputs. Proc Natl Acad Sci USA 91: 4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193-222. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G, Segev I, Markram H, Tsodyks M (2002) Coding of temporal information by activity-dependent synapses. J Neurophysiol 87: 140-148. [DOI] [PubMed] [Google Scholar]
- Gilbert CD (1992) Horizontal integration and cortical dynamics. Neuron 9: 1-13. [DOI] [PubMed] [Google Scholar]
- Harsch A, Robinson HP (2000) Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance. J Neurosci 20: 6181-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Spruston N, Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science 290: 739-744. [DOI] [PubMed] [Google Scholar]
- Hupe JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J (1998) Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature 394: 784-787. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R (2004) Synfire chains and cortical songs: temporal modules of cortical activity. Science 304: 559-564. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Buzsaki G (1998) Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci 18: 3919-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Douglas R, Wehmeier U (1990) Visibility of synaptically induced conductance changes: theory and simulations of anatomically characterized cortical pyramidal cells. J Neurosci 10: 1728-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ (2002) Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo J Neurosci 22: 6991-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B (1999) A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398: 338-341. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Luscher HR (2004) Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex 14: 1059-1070. [DOI] [PubMed] [Google Scholar]
- Lisman JE (1997) Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38-43. [DOI] [PubMed] [Google Scholar]
- Magee JC (1999) Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 508-514. [DOI] [PubMed] [Google Scholar]
- Magee JC, Cook EP (2000) Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci 3: 895-903. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D (1997) A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275: 209-213. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ (1995) Reliability of spike timing in neocortical neurons. Science 268: 1503-1506. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M (1996) Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382: 807-810. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B (1997) Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol (Lond) 500: 409-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo H, Reyes AD (2002) Boosting of neuronal firing evoked with asynchronous and synchronous inputs to the dendrite. Nat Neurosci 5: 261-266. [DOI] [PubMed] [Google Scholar]
- Rapp M, Yarom Y, Segev I (1996) Modeling back propagating action potential in weakly excitable dendrites of neocortical pyramidal cells. Proc Natl Acad Sci USA 93: 11985-11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Sakmann B (1999) Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci 19: 3827-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Bullier J (1995) Corticocortical connections in the visual system: structure and function. Physiol Rev 75: 107-154. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA (2000) Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027-1034. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT (1994) Noise, neural codes and cortical organization. Curr Opin Neurobiol 4: 569-579. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT (1998) The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18: 3870-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA, Lubke J, Sakmann B, Feldmeyer D (2003) High-probability uniquantal transmission at excitatory synapses in barrel cortex. Science 302: 1981-1984. [DOI] [PubMed] [Google Scholar]
- Softky WR, Koch C (1993) The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci 13: 334-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F (2001) Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969-1985. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Zador AM (1998) Input synchrony and the irregular firing of cortical neurons. Nat Neurosci 1: 210-217. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Häusser M (2001) Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci 4: 63-71. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N (1998) Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci 18: 3501-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B (1997) Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol (Lond) 505: 617-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC (1993) Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol 70: 2354-2369. [DOI] [PubMed] [Google Scholar]
- Tovee MJ, Rolls ET, Treves A, Bellis RP (1993) Information encoding and the responses of single neurons in the primate temporal visual cortex. J Neurophysiol 70: 640-654. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A (1999) Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286: 1943-1946. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H (1997) The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA 94: 719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Helmchen F (2004) Boosting of action potential backpropagation by neocortical network activity in vivo J Neurosci 24: 11127-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Larkum M, Sakmann B, Helmchen F (2003) Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo J Neurosci 23: 8558-8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR (2004) Spatial compartmentalization and functional impact of conductance in pyramidal neurons. Nat Neurosci 7: 961-967. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ (2000a) Site independence of EPSP time course is mediated by dendritic Ih in neocortical pyramidal neurons. J Neurophysiol 83: 3177-3182. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ (2000b) Backpropagation of physiological spike trains in neocortical pyramidal neurons: implications for temporal coding in dendrites. J Neurosci 20: 8238-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ (2002) Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295: 1907-1910. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ (2003) Role of dendritic synapse location in the control of action potential output. Trends Neurosci 26: 147-154. [DOI] [PubMed] [Google Scholar]
- Zhu JJ (2000) Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol (Lond) 526: 571-587. [DOI] [PMC free article] [PubMed] [Google Scholar]