Abstract
In late pregnant rats, the hypothalamic-pituitary-adrenal (HPA) axis is hyporesponsive to psychogenic stressors. Here, we investigated attenuated HPA responses to an immune challenge and a role for endogenous opioids. ACTH and corticosterone were assayed in blood samples from virgin and 21 d pregnant rats before and after endotoxin [lipopolysaccharide (LPS); 1 μg/kg, i.v.], interleukin-1β (IL-1β; 500 ng/kg, i.v.), or vehicle. In virgins, plasma ACTH concentrations increased 1 h after LPS and 15 min after IL-1β, as did corticosterone, with no responses in pregnant rats. In situ hybridization revealed increased corticotrophin releasing hormone (CRH) mRNA expression in the dorsomedial parvocellular paraventricular nucleus (pPVN) and increased anterior pituitary pro-opiomelanocortin mRNA expression 4 h after IL-1β in virgins; these responses were absent in pregnant rats. In contrast, immunocytochemistry showed that Fos expression was similarly increased in the nucleus tractus solitarius (NTS) A2 region in virgin and pregnant rats 90 min and 4 h after IL-1β. Naloxone pretreatment (5 mg/kg, i.v.) restored ACTH and pPVN CRH mRNA responses after IL-1β in pregnant rats but reduced the CRH mRNA response in virgins without affecting ACTH. Proenkephalin-A and μ-opioid receptor mRNA expression in the NTS was significantly increased in the pregnant rats, indicating upregulated brainstem opioid mechanisms. IL-1β increased noradrenaline release in the PVN of virgin, but not pregnant, rats. However, naloxone infused directly into the PVN increased noradrenaline release after IL-1β in pregnant rats. Thus, the HPA axis responses to immune signals are suppressed in pregnancy at the level of pPVN CRH neurons through an opioid mechanism, possibly acting by preterminal autoinhibition of NTS projections to the pPVN.
Keywords: ACTH, CRH mRNA, IL-1β, μ-opioid receptor mRNA, noradrenaline, nucleus tractus solitarius, proenkephalin-A mRNA
Introduction
Responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis to psychogenic stressors is strongly attenuated during late pregnancy (Neumann et al., 1998; Brunton et al., 2000; Johnstone et al., 2000), but HPA axis responses in pregnancy to immune challenge, a physical stressor, have not been reported. In non-pregnant rats, HPA axis activation by immune challenge reflects the bidirectional relationship between the immune and stress axes (Besedovsky and Del Rey, 1992; Turnbull and Rivier, 1995; Mulla and Buckingham, 1999), with a vital role of the HPA axis in modulating immune responses (Turnbull and Rivier, 1995). Systemic administration of endotoxin [lipopolysaccharide (LPS)] increases corticotrophin releasing hormone (CRH) release (Givalois et al., 1995), CRH mRNA expression in the hypothalamic paraventricular nucleus (PVN) (Kakucska et al., 1993), and adrenocorticotrophic hormone (ACTH) and corticosterone secretion (Turnbull et al., 1998). LPS stimulates cytokine production by macrophages, hence increasing circulating cytokine levels (Andersson et al., 1992), including interleukin-1β (IL-1β), which can mediate HPA responses to endotoxin (Besedovsky and Del Rey, 1992). Accordingly, systemic administration of IL-1β potently activates the HPA axis (Besedovsky and Del Rey, 1992; Ericsson et al., 1994). The key role of PVN CRH neurons in this response (Berkenbosch et al., 1987; Sapolsky et al., 1987; Rivest and Rivier, 1991) involves their activation by direct noradrenergic projections from the brainstem A1 and A2 neurons in the nucleus tractus solitarius (NTS) and ventrolateral medulla (VLM) (Ericsson et al., 1994; Buller et al., 2001b), with the A2 neurons of primary importance (Ericsson et al., 1994; Buller et al., 2001b). Systemic IL-1β stimulates these neurons (Ericsson et al., 1994) by first acting on IL-1 type I receptors on the endothelium of brain blood vessels to activate local production of prostaglandins (Ericsson et al., 1995; Rivest et al., 2000), which can then excite the NTS neurons via EP3 and EP4 receptors (Zhang and Rivest, 1999).
The attenuation of HPA axis responses to psychogenic stressors in pregnancy involves inhibition by a central endogenous opioid mechanism that emerges in pregnancy (Douglas et al., 1998). Possible sources of endogenous opioid that could regulate PVN CRH neurons include noradrenergic NTS neurons, which coexpress proenkephalin-A (pENK-A) mRNA (Ceccatelli et al., 1989). μ-Opioid receptors mediate presynaptic inhibition of noradrenaline release in the hypothalamus (Onaka et al., 1995); thus, enkephalins may autoinhibit NTS neurons.
Here, we tested whether HPA axis responses to immune challenge are attenuated in late pregnancy and whether NTS neurons respond to IL-1β in pregnant rats. We also investigated whether differences in noradrenaline release in the PVN could account for attenuated HPA axis responses and investigated a role for endogenous opioids. Finally, we established whether pENK-A and μ-opioid receptor mRNA expression in NTS neurons is upregulated in pregnancy.
Materials and Methods
Animals
Female Sprague Dawley rats (initial body weight, 260-290 g; Bantin and Kingman, Hull, UK) were maintained under standard conditions of temperature (20-22°C), humidity, and lighting (12 h light/dark cycle; lights on at 7:00 A.M.) with ad libitum access to food and water. Rats were initially housed in groups of five to six and then caged individually after surgery. Pregnant rats were obtained by mating overnight with a sexually experienced male; pregnancy was confirmed by finding a vaginal plug of semen on the cage floor the following morning (day 1 of pregnancy; day 22 of pregnancy is the expected day of parturition). Virgin rats were used as controls. All procedures were performed in accordance with current United Kingdom Home Office regulations.
Surgery: jugular vein cannulation
Five days before the experiment, virgin and day 16 pregnant rats were fitted with a SILASTIC jugular vein cannula (wall, 0.25 mm; internal diameter, 0.5 mm; filled with sterile heparinized 0.9% saline; 50 U/ml) under inhalational anesthesia (2-3% halothane in 600 ml/min each of oxygen and nitrous oxide) for subsequent blood sampling and drug administration.
Blood sampling
On the morning of the experiment (between 7:30 and 9:00 A.M.), jugular cannulas were connected to polyvinylchloride (PVC) extension tubing (wall, 1 mm; internal diameter, 0.5 mm) filled with heparinized saline (50 U/ml 0.9% saline) and attached to a 1 ml syringe for remote sampling. Rats were then left undisturbed for 90 min. Blood samples for radioimmunoassay (RIA) of ACTH (0.5 ml) or ACTH and corticosterone (0.6 ml) were collected into 1 ml syringes containing 20 μl of 5% EDTA chilled on ice and centrifuged to separate plasma, which was frozen and stored at -20°C until assay. Each blood sample was replaced immediately with an equivalent volume of sterile 0.9% saline. Pregnancy status was checked postmortem, and only pregnant rats with at least six viable fetuses were included in the study (actual range, 9-18 pups). The timings of blood samples for the different experiments are given below.
Experiment 1: ACTH responses to LPS
After two basal blood samples (0.5 ml for ACTH) taken 15 min apart, rats were given either 1 μg/kg LPS endotoxin (Serotype 055:B5; 2 μg/ml, i.v.; Sigma, Poole, Dorset, UK) or vehicle (0.9% saline, i.v.). Additional blood samples were taken 60, 120, 180, and 240 min after treatment. These time points were deemed to be the most appropriate after reviewing the literature (Turnbull et al., 1998).
Experiment 2: HPA axis and NTS responses to IL-1β
After two basal blood samples taken 30 min apart, rats were given either 500 ng/kg human recombinant (rh) IL-1β (1 μg/ml, i.v.; R&D Systems, Oxon, UK) or vehicle [0.2% bovine serum albumin (BSA) in PBS]. Additional blood samples were withdrawn 15, 30, 60, 90, and 120 min after treatment. Rats were killed by conscious decapitation 4 h after treatment (optimal time to measure PVN CRH mRNA expression after stimulation) (Harbuz and Lightman, 1989). Trunk blood was collected into tubes containing chilled 5% EDTA (0.2 ml/100 g body weight), and plasma was separated and stored as above. The forebrains, brainstems, and pituitary glands were rapidly removed, frozen on dry ice, and stored at -70°C for CRH mRNA in situ hybridization (ISH), Fos immunocytochemistry, and POMC mRNA ISH (to assess corticotroph exposure to secretagogue), respectively.
To evaluate Fos expression in the NTS at 90 min after treatment (the time for peak neuronal Fos expression after stimulation) (Sharp et al., 1991), additional groups of pregnant and virgin rats were fitted with a jugular cannula as before. Five days later (on day 21 of pregnancy), the rats were given either 500 ng/kg rhIL-1β (1 μg/ml, i.v.) or vehicle (0.2% BSA in PBS) and killed 90 min later by conscious decapitation. Brainstems were rapidly removed, frozen on dry ice, and stored at -70°C for Fos immunocytochemistry.
Experiment 3: effects of naloxone on ACTH responses to IL-1β
Pregnant (day 21) and virgin rats were implanted with a jugular vein cannula as above. Immediately after the first basal blood sample was taken (0.5 ml for ACTH RIA), rats were given 5 mg/kg naloxone (10 mg/ml) or 0.9% saline intravenously. After a second blood sample 15 min later, all rats were given 500 ng/kg rhIL-1β (1 μg/ml, i.v.). Additional blood samples (0.5 ml for ACTH RIA) were taken 15, 30, 60, and 90 min after IL-1β injection.
Experiment 4: effects of naloxone on PVN CRH mRNA responses to IL-1β
Pregnant (day 21) and virgin rats were used and were implanted with a jugular vein cannula. The rats were given, as above, naloxone or vehicle followed by IL-1β intravenously and killed by decapitation 4 h later. Brains were rapidly removed, frozen on dry ice, and stored at -70°C as before for CRH mRNA ISH. These rats were blood sampled as above for other measurements (not reported here).
Experiment 5: pregnancy and pENK-A mRNA and μ-opioid receptor mRNA expression
Virgin, midpregnant (day 10), late-pregnant (day 21), parturient (90 min after the birth of the first pup), and lactating (days 7-10) rats were killed by conscious decapitation. Brainstems were rapidly removed, frozen on dry ice, and stored at -70°C until processing for pENK-A and μ-opioid receptor mRNA ISH. Brains were also collected from untreated virgin and pregnant (d21) rats. Because enkephalin is coexpressed with CRH in the parvocellular PVN (pPVN) (Hokfelt et al., 1983) and is also expressed in the perifornical region (Khachaturian et al., 1983) (a region that may influence PVN neuron responses), sections cut through the pPVN and perifornical region were also processed for pENK-A mRNA ISH.
Experiment 6: effects of naloxone on IL-1β-evoked noradrenaline release in the PVN in virgin and pregnant rats
Surgery: implantation of microdialysis probe. Virgin and pregnant (day 18/19 of pregnancy) rats were anesthetized with halothane (as above), and a U-shaped microdialysis probe (tip length, 1.4 mm; dialysis surface, 2.4 mm2) (Landgraf and Ludwig, 1991) was implanted into the right PVN [coordinates: caudal, 1.7 mm; lateral, 1.6 mm; 7.4 mm deep from the brain surface; according to Paxinos and Watson (1996) at an angle of 10° to the vertical to avoid puncturing the sagittal sinus]. Probes were secured in place with two jeweler's screws fixed to the skull and dental acrylic. Probes were flushed with sterile artificial CSF (aCSF) (in mm: 138 NaCl, 3.36 KCl, 9.52 NaHCO3, 0.49 Na2HPO4, 2.16 urea, 0.49 NaH2PO4, 1.26 CaCl2, 1.18 MgCl2, pH 7.2). Lengths of fluorinated ethylene propylene (FEP) tubing (CMA Microdialysis, Solma, Sweden) were connected to the probe inlet and outlet. Rats were also fitted with a jugular vein cannula (as before) for administration of IL-1β. After surgery, rats were housed in commercially available purpose-designed Perspex bowls (CMA Microdialysis) with food and water available ad libitum. A wire tether extending from a swiveling lever-arm suspended over the bowl was clipped to a collar placed around the rat's neck, permitting free movement of the animal without entangling/disconnection of the microdialysis tubing.
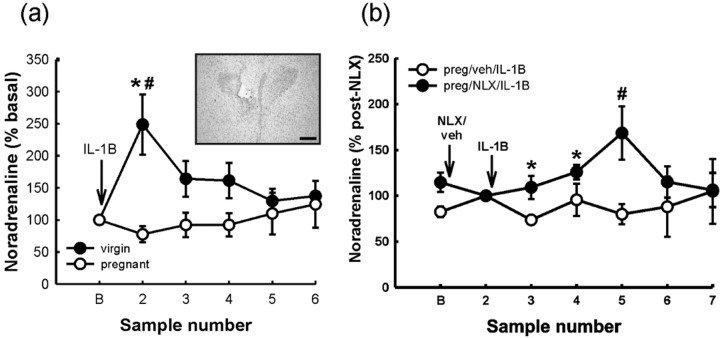
Microdialysis. Between 8:00 and 8:30 A.M., on the morning of the experiment (2-3 d after surgery; i.e., day 20/21 of pregnancy), intravenous cannulas were connected to PVC extension tubing attached to a 1 ml syringe filled with heparinized saline. A length of FEP tubing connected to a 1 ml Hamilton syringe prefilled with aCSF was attached to the inlet port of the probe. Hamilton syringes (1 ml) were secured in a microinfusion pump. Probes were flushed with aCSF (flow rate, 1.5 μl/min) for 90 min before the experiment commenced. After flushing, microdialysis samples were collected at 15 min intervals. Three dialysate samples were collected under basal conditions and then probes were either perfused with aCSF or with aCSF containing naloxone (1 mg/ml) for the remainder of the experiment. An additional three dialysate samples were collected and then all rats were administered IL-1β (500 ng/kg, i.v.). Five additional dialysate samples were collected after IL-1β administration. In each case, dialysates were collected in chilled microdialysis vials (covered in aluminum foil to minimize exposure to light) containing 2 μl of 10% acetic acid (to prevent oxidation of monoamines) and immediately after collection were frozen on dry ice and stored at -70°C until analysis. At the end of the experiment, rats were killed, and the brains were removed, frozen on dry ice, and stored at -70°C until histological processing. Coronal brain cryostat sections (15 μm) were cut through the hypothalamus, thaw-mounted onto glass microscope slides, and stained with Toluidine blue to verify microdialysis probe location. Only data from rats in which probes were correctly placed within the PVN [with reference to plates 25-26 according to Paxinos and Watson (1996)] are included in the results (see Fig. 7).
Figure 7.
The effect of IL-1β and naloxone pretreatment on noradrenaline release in the PVN of virgin and pregnant (day 21) rats. a, Six basal 15 min dialysates were collected, and the within-rat mean of these values was taken as 100% (B) and then rats were administered IL-1β (500 ng/kg, i.v.; 1 μg/ml; rh). An additional five dialysates (15 min each) were collected, and values were calculated as percentage of mean basal for each rat. Values are group means ± SEM. Group numbers are as follows: virgin, n = 8; pregnant, n = 7 rats. Two-way ANOVA for repeated measures was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus basal values in the same group; #p < 0.001 versus other group at the same time point. Inset, Representative photomicrograph of a Toluidine blue-stained coronal section (15 μm) cut through the hypothalamus after postmortem removal of the microdialysis probe. The probe tip is located in the dorsomedial parvocellular region of the PVN. Scale bar, 250 μm. b, Three basal dialysates were collected and then rats were treated with either naloxone (NLX; 1 mg/ml retrodialyzed directly into PVN) or vehicle (aCSF). Three additional dialysates were collected [the mean of these 3 samples was taken as 100% for each rat (sample 2), and the 3 preceding basal samples were normalized to sample 2 (B)] and then all rats were administered IL-1β (500 ng/kg, i.v.; rh). Five additional dialysates were collected at 15 min intervals [these values were normalized to the mean of the 3 postnaloxone or vehicle values for each rat (sample 2)]. Values are group means ± SEM. Group numbers are as follows: pregnant/vehicle, n = 7; pregnant/naloxone, n = 6 rats. Two-way ANOVA for repeated measures was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.05 and #p < 0.001 versus other group at the same time point.
HPLC. Noradrenaline contents in the dialysates were quantified at The Babraham Institute (Cambridge, UK) using reverse-phase HPLC with electrochemical detection as described previously (Kendrick et al., 1996). The detection sensitivity for a 10 μl sample injection was 5-25 pg/ml.
Radioimmunoassays. Plasma ACTH and corticosterone concentrations were determined using commercially available radioimmunoassay kits (Immunodiagnostic Systems, Tyne and Wear, UK). ACTH was measured in unextracted plasma samples using a two-site immunoradiometric assay (Hodgkinson et al., 1984). Corticosterone was measured in unextracted plasma (diluted 1:10 in assay buffer) using a double antibody radioimmunoassay with 125I-corticosterone as the tracer (Keith et al., 1978). For the ACTH and corticosterone assays, sensitivity was 1 pg/ml and 0.4 ng/ml, respectively, and the intra-assay variation was <11 and <6%. All samples from any one experiment were assayed together.
In situ hybridization. Tissue was sectioned coronally on a cryostat at 15 μm and thaw-mounted onto gelatinized slides. Sections were fixed with 4% (w/v) paraformaldehyde in 0.1 m PBS, washed in PBS, acetylated in triethanolamine/acetic anhydride solution, dehydrated in an ascending ethanol series (70-100%), delipidated in chloroform, and partially rehydrated in 95% (v/v) ethanol. All sections from a particular experiment were processed in the same hybridization reaction, and in each case, synthetic oligonucleotide probes were used (MWG-Biotech, Ebersberg, Germany). To detect CRH mRNA expression in the PVN, a 42-mer probe (5′-CCT GTT GCT GTG AGC TTG CTG AGC TAA CTG CTC TGC CCT GCC-3′), complementary to bases 496-537, which encode amino acids 22-35 of the rat CRH peptide (Jingami et al., 1985), was used. The POMC probe was a 48-mer oligonucleotide (5′-GGT CAT GAA GCC GCC GTA GCG CTT GTC CTT GGG CGG GTT GCC CCA GCG-3′) complementary to rat POMC mRNA (Nakanishi et al., 1979). The pENK-A probe was a 39-mer probe (5′-TGC ATC CTT CTT CAT GAA ACC GCC ATA CCT CTT GGC AAG-3′), complementary to bases 721-759 of pENK-A mRNA (Rosen et al., 1984), and the μ-opioid receptor probe was 39-mer (5′-TTC AGA CCG CAT GGA TCG GAC TGG TTG CCA TCA ACG TGG-3′) and complementary to bases 282-320 of rat μ-opioid receptor mRNA (Chen et al., 1993).
Probes were 3′-labeled with 35S-dATP using terminal deoxynucleotidyl transferase and purified using spin columns (QIAquick nucleotide removal kit; Qiagen, Crawley, West Sussex, UK). Sections were hybridized with radiolabeled probe (105 cpm labeled probe/section) in hybridization buffer [1% BSA (w/v), 5% dextran sulfate (w/v), 15 mm diothiothreitol, 2 mm EDTA, 1% ficoll (w/v), 50% formamide (v/v), 0.1 mg/ml PolyA, 1% polyvinylpyrrolidone (w/v), 0.2 mg/ml salmon testes DNA, 1.2 m sodium chloride, 2.5% sodium pyrophosphate (w/v), 20 mm Tris, pH 7.6, 0.1 mg/ml yeast tRNA, 0.1 mg/ml yeast total RNA] overnight at 37°C in humidified chambers. Sections were rinsed three times in 1×SSC at room temperature, washed four times for 15 min each in 1× SSC at 45-63°C (depending on hybridized probe melting temperature), and twice for 30 min each at room temperature. Sections were air-dried and exposed to autoradiographic film (Hyperfilm-β-max; Amersham Biosciences, Buckinghamshire, UK) at room temperature, with 14C polymer strip standards to check optimal exposure (Amersham Biosciences). Exposure times were 21 and 14 d for CRH and POMC mRNA, respectively. Film was then developed (Kodak D-19; Sigma) and fixed (Hypam rapid fixer; Ilford, Cheshire, UK). Slides were dipped in liquid autoradiographic emulsion (Kodak type NTB-3; Anachem, Bedfordshire, UK) and exposed (at 4°C) for 12 weeks for CRH mRNA and 21 d for pENK-A mRNA and μ-opioid receptor mRNA. Slides were developed and fixed as above and counterstained with hematoxylin and eosin. The film autoradiograms were quantified using a computerized image analysis system (objective magnification, 2.5×; NIH Image version 1.62). Grain area was measured over a minimum of six PVN profiles (in three consecutive sections) and six anterior pituitary sections from each rat for quantification of CRH and POMC mRNA expression, respectively. The area of each profile was measured to calculate grain area/PVN or anterior pituitary (square millimeter/square millimeter). Background measurements were made over areas adjacent to the region of interest and subtracted. Measurements over a 14C polymer strip were used to confirm that tissue measurements were on the dynamic part of the radioactivity/silver grain density curve.
The number of CRH mRNA-expressing cells in the PVN was counted in emulsion-dipped sections. A positive cell was defined as one with more overlying silver grains than the mean over 10 cells lateral to the PVN (background) plus three SDs. Data are presented as the number of positive cells per square millimeter PVN profile. For quantitative analysis of pENK-A and μ-opioid receptor mRNAs in the NTS, silver grain area was measured in >150-200 cells in the NTS per rat. Background measurements were made over the equivalent of 20 cells in neuropil adjacent to the NTS and subtracted. pENK-A mRNA expression in the pPVN was determined by counting the number of positively expressing cells (emulsion-dipped sections) and by measuring grain density over the pPVN (film autoradiographs). Both were measured in a minimum of six PVN profiles per rat.
For all ISH measurements, average values for each rat were used to calculate group means ± SEM.
Fos immunocytochemistry. Coronal brainstem cryostat sections (15 μm) cut through the A2 cell region of the NTS (at the level of the area postrema) were mounted on gelatinized slides. Sections were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.3, for 30 min at room temperature, followed by three 5 min washes in 0.1 m PB, pH 7.4. Endogenous peroxidases were deactivated with 1.5% hydrogen peroxide in 0.1 m PB for 15 min and then sections were washed in PB containing 0.3% Triton X-100 (PB-T). Sections were incubated in normal sheep serum (NSS) for 60 min before incubation with rabbit anti-Fos (Ab-2) antibody (Calbiochem, La Jolla, CA) (diluted 1:1000 in PB-T containing 1% NSS and 0.02% sodium azide; 750 μl/slide) at 4°C for 48 h. Sections were washed in PB-T (three times for 15 min each), incubated in excess (750 μl/slide) goat anti-rabbit peroxidase complex (Vector Laboratories, Burlingame, CA), and diluted 1:500 in PB-T containing 1% NSS for 24 h at 4°C. Next, sections were washed three times for 15 min each in 0.1 m PB, followed by a 10 min rinse in acetate buffer (0.33 m, pH 6.0). Sections were incubated in acetate buffer (0.33 m, pH 6.0) containing 2.5% nickel ammonium sulfate and 0.5% diaminobenzidine for 7-10 min until Fosimmunoreactive nuclei were visualized. The reaction was terminated with a 5 min wash in 0.165 m acetate buffer. Fos-positive cells were manually counted bilaterally in 8-10 NTS sections per rat (VLM A1 neurons are not activated by IL-1β at the dose used) (Ericsson et al., 1994). The area of the NTS profiles was measured using an AppleMac computer (Apple Computers, Cupertino, CA) and NIH Image (version 1.62) software.
Statistical analysis. Two-way repeated-measures (RM) ANOVA, followed by Student-Newman-Keuls multiple-comparison tests were used to analyze ACTH, corticosterone, and noradrenaline data. CRH mRNA, POMC mRNA, and Fos data were analyzed using a two-way ANOVA. pENK-A and μ-opioid receptor mRNA data were analyzed using a one-way ANOVA or Student's t test. Values shown are group means ± SEM. p < 0.05 was considered statistically significant.
Results
Experiment 1: ACTH responses to LPS
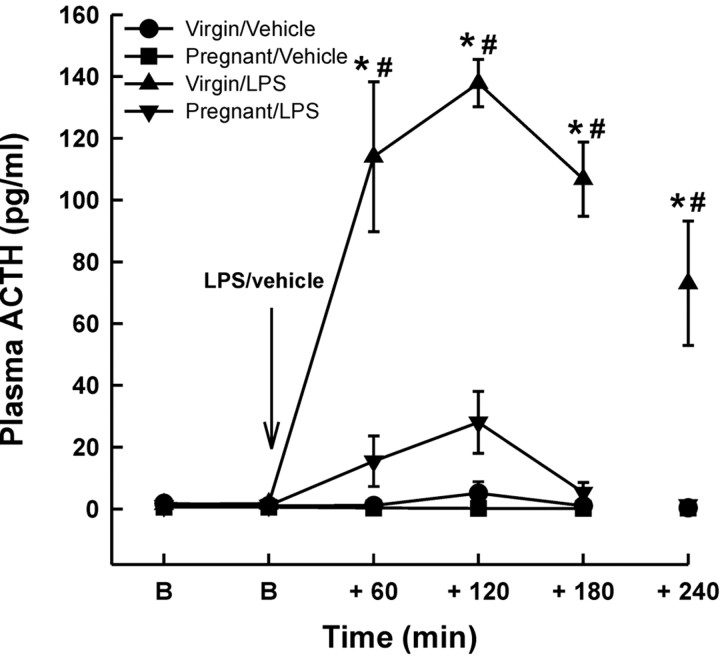
Basal plasma ACTH concentrations (1.53 ± 0.19 pg/ml in the virgin rats, n = 22 vs 0.87 ± 0.21 pg/ml in the pregnant rats, n = 17) were not significantly different between any of the groups (Fig. 1) (two-way RM ANOVA). Intravenous LPS significantly increased plasma ACTH concentration in the virgin group (p < 0.001; two-way RM ANOVA) within 60 min of the injection, which peaked at 120 min, and remained significantly elevated 240 min after LPS administration. However, LPS evoked only a small, nonsignificant increase in plasma ACTH concentration in the pregnant group (Fig. 1). Vehicle injection had no significant effect on plasma ACTH in either the virgin or the pregnant rats (Fig. 1).
Figure 1.
The effect of intravenous LPS on plasma ACTH concentrations in virgin and pregnant (day 21) rats. Two basal blood samples (B) were collected 30 min apart before intravenous injection of either LPS (1 μg/kg) or vehicle (0.5 ml/kg; 0.9% saline). Additional blood samples were withdrawn as shown. Values are group means ± SEM. Group numbers are as follows: virgin/vehicle, n = 10; pregnant/vehicle, n = 7; virgin/LPS, n = 11; pregnant/LPS, n = 10. Two-way ANOVA for repeated measures was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus basal values in the same group; #p < 0.001 versus all other groups at the same time point.
Experiment 2: HPA axis and NTS responses to intravenous IL-1β
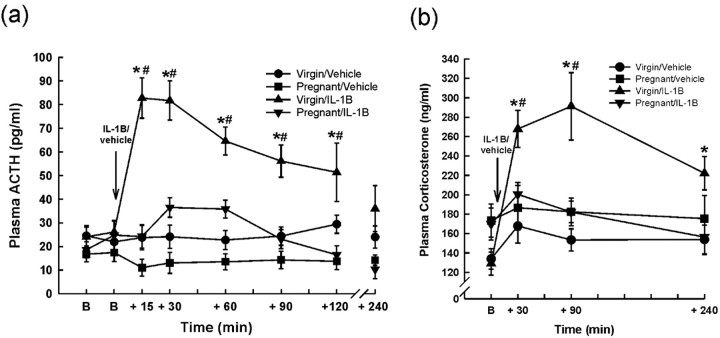
To circumvent the possibility that changes in endogenous cytokine generation may underlie the attenuated response to LPS in pregnancy, we measured HPA responses to IL-1β in subsequent experiments. Basal plasma ACTH concentrations were not significantly different between any of the groups (Fig. 2a) (p > 0.05; two-way RM ANOVA). Intravenous IL-1β rapidly increased ACTH secretion within 15 min in the virgin group (Fig. 2a) (p < 0.001; two-way RM ANOVA), which remained significantly elevated for at least 120 min after IL-1β, returning to baseline levels within 240 min. In contrast, in the pregnant group, IL-1β induced only a nonsignificant increase (1.7-fold; p = 0.093 and p = 0.084; two-way RM ANOVA vs basal and pregnant vehicle-treated groups, respectively) in plasma ACTH concentration 30 min after the injection (Fig. 2a). Plasma ACTH concentrations in the pregnant group were significantly less than those in the virgin group at all time points measured after IL-1β (p < 0.001; two-way RM ANOVA). Vehicle injection did not affect plasma ACTH concentrations in either the virgin group or the pregnant group (Fig. 2a).
Figure 2.
Effects of intravenous IL-1β on plasma ACTH and corticosterone concentrations in virgin and pregnant (day 21) rats. Basal blood samples (B) were collected for ACTH and corticosterone RIA before intravenous injection of either rhIL-1β (500 ng/kg) or vehicle (0.5 ml/kg 0.2% BSA in PBS). Additional blood samples were withdrawn for ACTH (a) and corticosterone (b) RIA as shown. Trunk blood was collected at 240 min. Values are group means ± SEM. Group numbers are as follows: virgin/vehicle, n = 9; pregnant/vehicle, n = 6; virgin/IL-1β, n = 8; pregnant/IL-1β, n = 7. Two-way ANOVA for repeated measures was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus basal values in the same group; #p < 0.005 versus all other groups at the same time point.
Basal plasma corticosterone concentration was not significantly different between groups (Fig. 2b) (p > 0.05; two-way RM ANOVA). After IL-1β administration, plasma corticosterone concentration increased significantly within 30 min in the virgin group and was still significantly greater than basal at 240 min (p < 0.001; two-way RM ANOVA). Intravenous IL-1β had no significant effect on plasma corticosterone concentration in the pregnant group (Fig. 2b). Vehicle injection had no significant effect on plasma corticosterone concentration in either group.
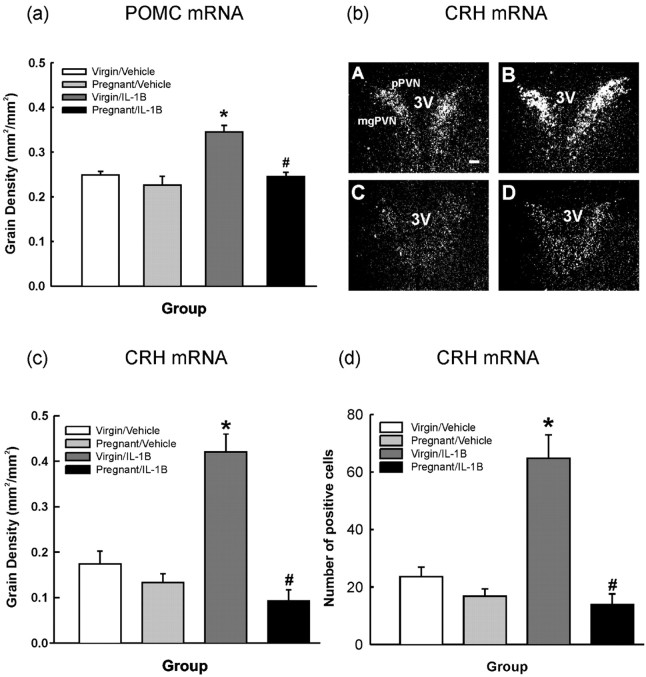
POMC mRNA expression in the anterior pituitary was similar in the vehicle-treated virgin and pregnant rats. IL-1β treatment significantly increased POMC mRNA expression in virgin rats (Fig. 3a) (p < 0.001; two-way ANOVA) but had no such effect in pregnant rats (Fig. 3a).
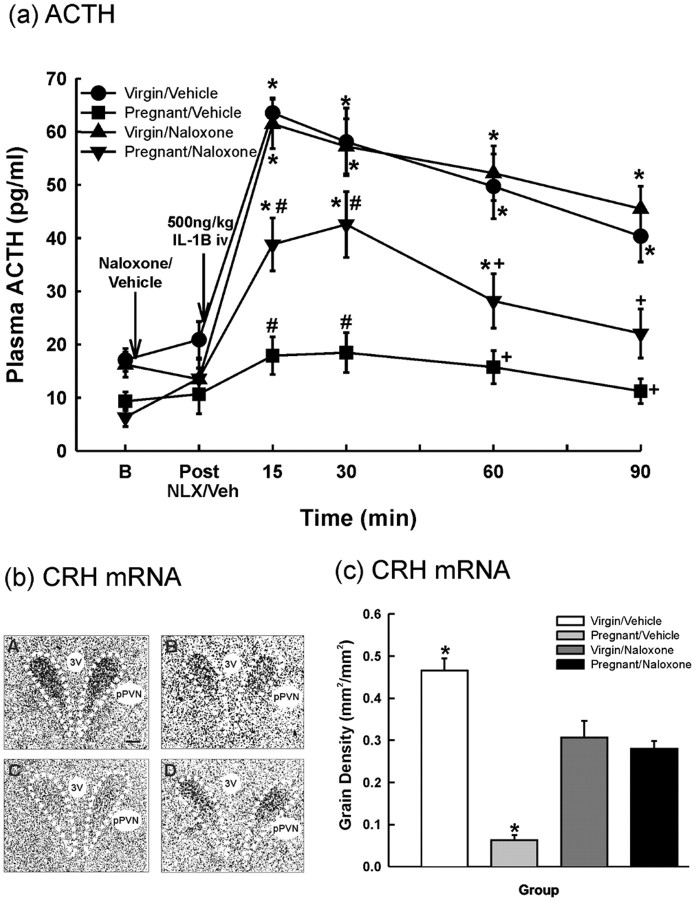
Figure 3.
Effects of intravenous IL-1β on anterior pituitary POMC mRNA and parvocellular PVN CRH mRNA expression in virgin and pregnant (day 21) rats. In situ hybridization for POMC mRNA in the anterior pituitary (a) and CRH mRNA (b-d) in the dorsomedial parvocellular PVN in 15 μm coronal sections from rats killed 4 h after intravenous injection of either rhIL-1β (IL-1B; 500 ng/kg) or 0.2% BSA in PBS (0.5 ml/kg). a, POMC mRNA: grain area measurements were made from film autoradiographs over the anterior pituitary. b, Representative dark-field autoradiographs of coronal PVN sections hybridized with an 35S-labeled oligonucleotide probe complementary to CRH mRNA from each group: A, virgin/vehicle; B, virgin/IL-1β; C, pregnant/vehicle; D, pregnant/IL-1β. 3V, Third ventricle; mgPVN, magnocellular subdivision of PVN. Scale bar, 100 μm. c, CRH mRNA: measurements of grain density were made from film autoradiographs over the pPVN. d, Number of pPVN cells expressing CRH mRNA. Values are group means ± SEM. Group numbers are as follows: virgin/vehicle, n = 10; pregnant/vehicle, n = 11; virgin/IL-1β, n = 8; pregnant/IL-1β, n = 7. Two-way ANOVA was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus control groups; #p < 0.001 versus virgin/IL-1β group.
Four hours after IL-1β treatment, CRH mRNA expression (measured as grain density and number of positive cells) in the dorsomedial pPVN was significantly increased in the virgin group (Fig. 3) (p < 0.001; two-way ANOVA), but there was no significant change in CRH mRNA expression in the pregnant group (Fig. 3). CRH mRNA expression in the PVN was not significantly different between the virgin and pregnant control rats (Fig. 3) (p = 0.233; two-way ANOVA).
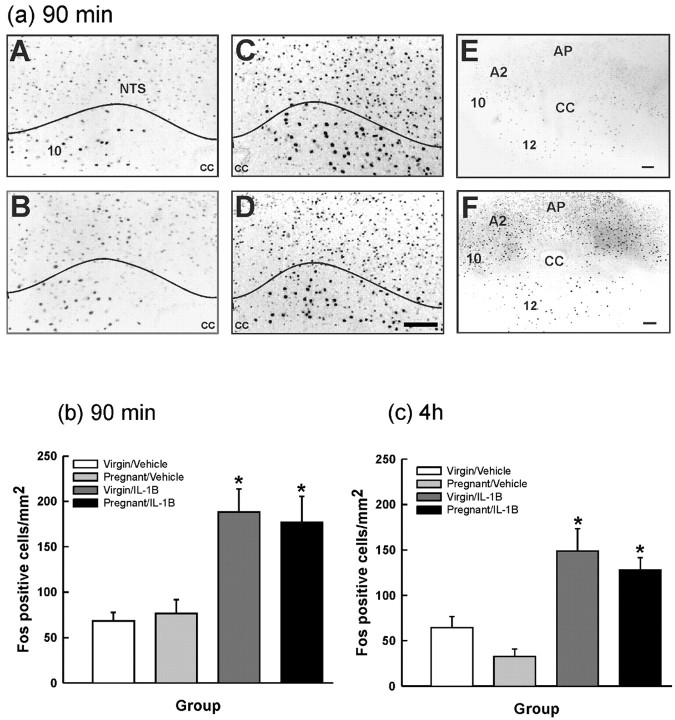
IL-1β significantly increased Fos expression in the A2 cell region of the NTS of both virgin and pregnant rats killed 90 min (Fig. 4a,b) or 240 min (Fig. 4c) after treatment; however, there were no significant differences in the number of Fos-positive neurons between the virgin and pregnant IL-1β-treated groups. There were no significant differences in the number of Fos-immunopositive NTS neurons between the virgin and pregnant groups after vehicle (Fig. 4).
Figure 4.
Effect of intravenous IL-1β on Fos expression in the A2 cell region of the NTS in virgin and pregnant (day 21) rats. Rats were given either rhIL-1β (500 ng/kg, i.v.) or vehicle (0.5 ml/kg, i.v.; 0.2% BSA in PBS) and killed after 90 min (a, b) or 4 h (c) (same rats as in Fig. 2). Cryostat 15 μm coronal brainstem sections were processed for Fos immunocytochemistry. a, Photomicrographs of representative sections showing neuronal nuclei immunoreactive for Fos (at 90 min) in the NTS from each group: A, virgin/vehicle; B, pregnant/vehicle; C, virgin/IL-1β; D, pregnant/IL-1β. Low-magnification images are shown for pregnant/vehicle (E) and pregnant/IL-1β (F). NTS, A2 cell region of the NTS; CC, central canal; 10, dorsal motor nucleus of vagus; 12 hypoglossal nucleus. Scale bar, 100 μm. b, c, Fos-positive cells were counted in the A2 cell region of the NTS at the level of the area postrema, ∼13.5-14.2 mm caudal to bregma according to the study by Paxinos and Watson (1996), and the area of the NTS profile was measured using NIH Image 1.62 software. The data are expressed as the number of Fos-positive cells per square millimeter. Values are group means ± SEM. b, Ninety minutes. Group numbers are as follows: virgin/vehicle, n = 6; pregnant/vehicle, n = 7; virgin/IL-1β, n = 6; pregnant/IL-1β, n = 8. c, Four hours. Group numbers are as follows: virgin/vehicle, n = 10; pregnant/vehicle, n = 11; virgin/IL-1β, n = 7; pregnant/IL-1β, n = 7. Two-way ANOVA was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus vehicle groups.
Experiment 3: effects of naloxone on ACTH responses to IL-1β
Basal plasma ACTH concentrations were not significantly different between any of the groups, and neither naloxone nor vehicle injection significantly affected basal plasma ACTH levels in any of the groups (Fig. 5a) (two-way RM ANOVA). IL-1β rapidly increased plasma ACTH concentration in the vehicle-treated virgin group, which peaked within 15 min (63.5 ± 2.8 pg/ml); however, there was no significant change in the vehicle-treated pregnant group (17.9 ± 3.5 pg/ml). Pretreatment with naloxone had no significant effect on the ACTH secretory response to systemic IL-1β in the virgin group (Fig. 5a). In contrast, in the pregnant group given naloxone, there was a significant ACTH response to IL-1β (p < 0.001 vs basal levels; p < 0.002 vs pregnant vehicle-treated group at 15 min; two-way RM ANOVA), although ACTH concentrations were significantly less than those in the virgin groups (Fig. 5a) at all sample times after IL-1β.
Figure 5.
The effect of pretreatment with naloxone on plasma ACTH and parvocellular PVN CRH mRNA expression after intravenous IL-1β in virgin and pregnant (day 21) rats. a, One basal blood sample (B) was collected before administration of either naloxone (5 mg/kg, i.v.; 10 mg/ml) or vehicle (0.5 ml/kg, i.v.; 0.9% saline). A blood sample was withdrawn 15 min after naloxone/vehicle injection and then rhIL-1β (500 ng/kg, i.v.; 1 μg/ml) was given to all rats. Sequential blood samples were taken after IL-1β as shown. Values are group means ± SEM. Group numbers are as follows: virgin/vehicle, n = 7; pregnant/vehicle, n = 6; virgin/naloxone, n = 8; pregnant/naloxone, n = 6. Two-way ANOVA for repeated measures was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus basal values in the same group; #p < 0.02 versus all other groups at the same time point; +p < 0.004 versus virgin groups at the same time point. b, c, Four hours after the IL-1β injection, the rats were killed. Cryostat 15 μm coronal brain sections were hybridized with an 35S-labeled oligo-probe complementary to CRH mRNA. b, Representative film autoradiographs, bright-field, exposed for 21 d from group: A, virgin/vehicle; B, virgin/naloxone; C, pregnant/vehicle; D, pregnant/naloxone. 3V, Third ventricle; pPVN, dorsomedial parvocellular subdivision of PVN. Scale bar, 100 μm. c, Measurements of grain area per unit area of pPVN profile (grain density) in film autoradiographs. Values are group means ± SEM. Group numbers are as follows: virgin/vehicle, n = 6; pregnant/vehicle, n = 6; virgin/naloxone, n = 6; pregnant/naloxone, n = 7. Two-way ANOVA was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus all other groups.
Experiment 4: effects of naloxone on PVN CRH mRNA responses to IL-1β
CRH mRNA expression in the pPVN 4 h after IL-1β treatment was 7.4-fold greater in the virgin vehicle-pretreated rats than in the pregnant vehicle-pretreated rats (Fig. 5b,c) (p < 0.001; two-way ANOVA). Naloxone pretreatment significantly increased CRH mRNA expression in response to IL-1β in the pregnant group (p < 0.001 vs pregnant/vehicle group; two-way ANOVA) but significantly reduced CRH mRNA expression in response to IL-1β in the virgin group (p < 0.001 vs virgin/vehicle group; two-way ANOVA). There were no differences in CRH mRNA expression between the virgin and pregnant IL-1β-treated groups pretreated with naloxone (p = 0.457; two-way ANOVA).
Experiment 5: pENK-A mRNA expression in the pPVN, perifornical region and NTS, and μ-opioid receptor mRNA expression in the NTS in pregnancy
pENK-A mRNA expression in the NTS was significantly greater on day 21 of pregnancy compared with virgin and lactating rats (Fig. 6a,b) (p < 0.01; one-way ANOVA). There was no significant difference in the level of pENK-A mRNA expression in either the pPVN or the perifornical region between virgin and late-pregnant rats (Table 1) (p = 0.50 and p = 0.58, respectively; Student's t test). μ-Opioid receptor mRNA expression in the NTS was significantly increased in pregnant, parturient, and lactating rats versus virgin controls; peak expression was at parturition (Fig. 6c,d) (p < 0.001; one-way ANOVA).
Figure 6.
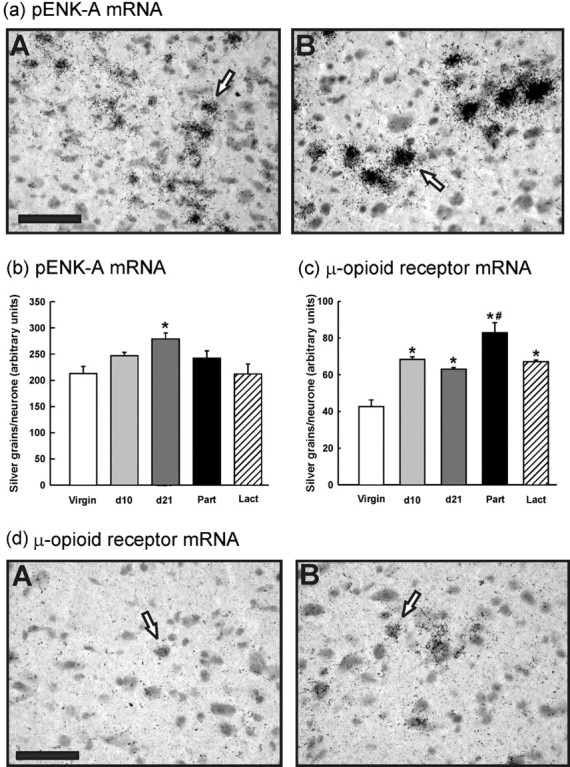
pENK-A and μ-opioid receptor mRNA expression in the NTS. Cryostat 15 μm coronal brainstem sections were hybridized with 35S-labeled oligonucleotides probes complementary to pENK-A or μ-opioid receptor mRNA. a, Representative autoradiographs in bright-field showing cells in the NTS-expressing pENK-A mRNA from: A, virgin; B, day 21 pregnant groups. Open arrows indicate examples of a pENK-A mRNA-expressing cell. Midline is to the right of the field. Scale bar, 50 μm. b, Measurements of silver grains per neuron. Values are group means ± SEM. Group numbers are as follows: virgin, n = 6; day 10 pregnant, n = 6; day 21 pregnant, n = 6; parturient, n = 6; lactating, n = 5. One-way ANOVA was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.01 versus virgin and lactating group. c, μ-Opioid receptor mRNA expression in the NTS: measurements of silver grains per neuron. Values are group means ± SEM. Group numbers are as follows: virgin, n = 6; day 10 pregnant, n = 6; day 21 pregnant, n = 6; parturient, n = 6; lactating, n = 6. One-way ANOVA was used followed by Student-Newman-Keuls multiple-comparison test: *p < 0.001 versus virgin group; #p < 0.01 versus all other groups. d, Representative autoradiographs showing cells in the NTS-expressing μ-opioid receptor mRNA from the following: A, virgin; B, day 21 pregnant groups. Open arrows indicate an example of a μ-opioid receptor mRNA-expressing cell. Scale bar, 50 μm.
Table 1.
Proenkephalin-A mRNA expression in the pPVN and perifornical region in virgin and pregnant (day 21) rats
|
|
pPVN |
Perifornical region |
||||
|---|---|---|---|---|---|---|
| Group |
Number of positive cells |
Grain density (arbitrary units) |
Number of positive cells |
Grain density (arbitrary units) |
||
| Virgin | 36.1 ± 6.5 (n=10) | 0.249 ± 0.02 (n=8) | 29.1 ± 3.2 (n= 9) | 0.317 ± 0.03 (n=8) | ||
| Pregnant (day 21) |
40.9 ± 3.2 (n=11) |
0.234 ± 0.01 (n=8) |
31.9 ± 3.6 (n=11) |
0.310 ± 0.03 (n=8) |
||
Coronal brain sections (15 μm) were hybridized with an 35S-labeled oligonucleotide probe complementary to pENK-A mRNA. Values are the mean (±SEM) number of cells in the pPVN and perifornical region expressing pENK-A mRNA and mean (±SEM) grain density over these regions.
Experiment 6: effects of naloxone on IL-1β-evoked noradrenaline release in the PVN in virgin and pregnant rats
Effect of IL-1β on noradrenaline release in the PVN of virgin and pregnant rats
Basal noradrenaline levels were not significantly different between the virgin group (513 ± 149.8 pg/ml) and the pregnant group (618.5 ± 206.3 pg/ml; Student's t test; p = 0.466). Statistical analysis of log transformed basal noradrenaline data (to reduce variance) also showed no significant difference between virgin and pregnant rats (Student's t test; p = 0.20). Systemic IL-1β administration evoked a significant increase in noradrenaline release in the PVN of virgin rats but not pregnant rats (Fig. 7a) (two-way RM ANOVA; p < 0.001); to control for interanimal variability, data were normalized as a percentage of within-rat mean basal values calculated from the six samples before IL-1β.
Effect of naloxone treatment on IL-1β-evoked noradrenaline release in the PVN of virgin and pregnant rats
In the pregnant rats, naloxone treatment alone had no significant effect on noradrenaline release in the PVN (Fig. 7b) (for analysis of effects of naloxone, data before naloxone and after IL-1β were normalized to the mean of the three post-naloxone microdialysate noradrenaline contents). After stimulation with IL-1β, noradrenaline release in the PVN was significantly increased in the pregnant rats pretreated with naloxone (two-way RM ANOVA; p < 0.02 at peak), contrasting with the lack of a significant effect of IL-1β on noradrenaline release in the PVN of the pregnant rats pretreated with vehicle (Fig. 7b).
Naloxone treatment alone had no significant effect on noradrenaline release in the virgin rats. In virgin rats, naloxone had no significant effect on noradrenaline release in the PVN after IL-1β administration, which was indistinguishable from that observed in the virgin rats treated with IL-1β only (data not shown).
Discussion
Our results demonstrate that in late pregnancy, the HPA axis is almost nonresponsive to immune challenge with systemic endotoxin or IL-1β as a result of endogenous opioid inhibition of pPVN CRH neurons. This inhibition may be exerted by enkephalins produced by NTS neurons and acting via μ-opioid receptors on noradrenergic terminals in the PVN. The results complement findings of reduced HPA responses in pregnancy to emotional stressors (Douglas et al., 1998; Neumann et al., 1998; Johnstone et al., 2000) and to orexin (Brunton and Russell, 2003), indicating global suppression of CRH neuron responses to stressors in late pregnancy.
We showed HPA axis activation by intravenous LPS in virgin females as in male rats (Turnbull et al., 1998). In late pregnancy, the ACTH secretory response to LPS was strongly attenuated. It is not known whether direct LPS actions on the brain (Chakravarty and Herkenham, 2005) are involved in HPA axis responses or altered in pregnancy. The reduced ACTH response to LPS in late pregnancy was not a result of reduced cytokine generation by immune cells, because the ACTH and corticosterone responses to IL-1β were also suppressed. The attenuated ACTH response to LPS or IL-1β is not attributable to failure of corticotrophs to respond to secretagogues in pregnancy (Ma et al., 2005), nor is metabolic clearance of corticosterone enhanced (Waddell and Atkinson, 1994). The attenuated ACTH and corticosterone responses to immune challenge result from reduced CRH drive (and/or vasopressin, although CRH predominates) (Turnbull et al., 1998) by the pPVN, because CRH mRNA expression in the pPVN was not stimulated by intravenous IL-1β in late pregnancy in contrast with virgin rats. Increased pPVN CRH mRNA expression after IL-1β indicates stimulation of the CRH neurons, which releases CRH from the axon terminals in the median eminence (Herman and Morrison, 1996). As CRH increases anterior pituitary POMC mRNA expression (Bruhn et al., 1984), the increase after IL-1β only in virgin rats indicates greater corticotroph exposure to CRH in virgin rats than in late-pregnant rats. Moreover, intracerebroventricular IL-1β depletes median eminence CRH in virgin rats but not in pregnant rats (Nakamura et al., 1998).
Reduced activation of CRH mRNA expression in late pregnancy after IL-1β is probably a consequence of altered input from brainstem noradrenergic neurons. Noradrenaline excites CRH neurons (Cole and Sawchenko, 2002), and CRH mRNA expression is tonically stimulated by noradrenergic and adrenergic pathways (Kiss et al., 1996). Here, increased noradrenaline release in the PVN of virgin rats, but not late-pregnant rats, after IL-1β may explain the differences in CRH neuron activation. A putative source of this noradrenaline is the brainstem NTS neurons, which project directly to the PVN, relaying peripheral information to CRH neurons after immune activation (Ericsson et al., 1994; Buller et al., 2001b). Lesioning this noradrenergic projection (with 6-hydroxydopamine) abolishes increased PVN CRH mRNA expression and reduces ACTH secretion after intravenous IL-1β (Melik Parsadaniantz et al., 1995). Our findings of suppressed noradrenaline release in the PVN after immune challenge in late pregnancy are consistent with studies in lactation, when the HPA axis is also hyporesponsive (Toufexis et al., 1998). We found similar numbers of Fos-expressing neurons in the NTS A2 region (the location of noradrenaline cells projecting to the dorsomedial PVN) after intravenous IL-1β in virgin and late-pregnant rats, indicating intact IL-1β signaling to the NTS in late pregnancy. Thus, signaling from brainstem blood vessels after systemic IL-1β [involving prostaglandin generation via cyclooxygenase (COX)] (Rivest et al., 2000) is unlikely to be suppressed in late pregnancy. We propose that reduced signaling by systemic IL-1β is at the level of the pPVN CRH neurons, including the terminals of the NTS projection. Altered intrahypothalamic COX signaling in pregnancy, underlying reduced LPS-induced fever responses (Mouihate et al., 2002), might contribute to reduced pPVN CRH neuron activation after systemic IL-1β. NTS and VLM catecholamine cells may also mediate HPA responses to systemic IL-1β indirectly via the central amygdala and bed nucleus of stria terminalis (Buller et al., 2001a), and signaling through this pathway might be suppressed in pregnancy.
Naloxone reinstated an ACTH response to IL-1β in late-pregnant rats, which strongly indicates a role for endogenous opioids in the attenuated response to systemic IL-1β. Naloxone also increases ACTH secretion after forced swimming in late pregnancy (Douglas et al., 1998) and during parturition (Wigger et al., 1999). Reduced corticotroph responses to CRH may explain the still-reduced ACTH secretory response to IL-1β in late-pregnant rats after naloxone (Neumann et al., 1998; Ma et al., 2005). However, a central inhibitory action of opioids in late pregnancy was clearly indicated, because naloxone triggered a pPVN CRH mRNA response to IL-1β and, infused directly into the PVN, naloxone restored IL-1β-evoked noradrenaline release in late-pregnant rats. In contrast, naloxone reduced the pPVN CRH mRNA response to IL-1β in virgin rats but not the ACTH response; naloxone reduces the ACTH response to swim stress in virgin rats (Douglas et al., 1998). The inferred excitation by endogenous opioids of HPA axis responses in virgins is consistent with potentiation of HPA stress responses by morphine (Buckingham, 1982) and stimulation of CRH release by enkephalins (Buckingham, 1982). Hence, we deduce that endogenous opioids promote pPVN CRH neuron stress responses in virgin rats; however, in late pregnancy, a dominant inhibitory opioid mechanism is activated, attenuating pPVN CRH neuron and, hence, ACTH secretory responses.
Naloxone does not distinguish the opioid receptor type(s) mediating the opposing effects on HPA activity in virgin and pregnant rats. However, the hypothalamus is almost devoid of δ-receptors (Mansour et al., 1987), and selective μ-receptor antagonists abolish ACTH stress responses in male rats (Cover and Buckingham, 1989), whereas blockade of κ-receptors exaggerates stress responses (Cover and Buckingham, 1989). Thus, in the present study, naloxone may have attenuated pPVN CRH mRNA responses to IL-1β in virgins via μ-receptors. However, the central endogenous opioid inhibition of oxytocin secretion that emerges in pregnancy is exerted via μ- not κ-receptors (Douglas et al., 1995). Furthermore, μ-receptors mediate attenuated HPA responses to IL-1β in male rats treated chronically with morphine (Chang et al., 1996), indicating the potential for inhibitory actions via μ-receptors.
The present finding of increased μ-opioid receptor mRNA expression in the NTS of pregnant rats is consistent with μ-receptor mediation of endogenous opioid inhibition of pPVN CRH neuron activation by IL-1β. Upregulated μ-opioid receptor production in NTS neurons could lead to increased transport to their terminals in the hypothalamus, where μ-opioid inhibits noradrenaline release in the supraoptic nucleus (Onaka et al., 1995). HPA axis hyporesponsiveness to immunological stressors in lactation (Shanks et al., 1999) and late pregnancy is likely a result of different mechanisms, because in the present study, proenkephalin-A mRNA expression in the brainstem was similar in virgin and lactating rats.
There are several possible sources of central endogenous opioid restrain that CRH neuron responses in pregnancy, including the arcuate nucleus, pPVN CRH neurons, perifornical region, and the NTS neurons themselves. Arcuate nucleus β-endorphin cells project directly to the PVN (Sawchenko et al., 1982), and in late pregnancy, POMC mRNA and β-endorphin expression in the arcuate nucleus are increased (Douglas et al., 2002). However, arcuate neurons are unlikely to mediate the immediate opioid actions on responses to systemic IL-1β, because they respond later than NTS and pPVN neurons (Brady et al., 1994). Enkephalin is expressed in the perifornical region and is coexpressed with CRH in pPVN cells, but here we showed that pENK-A mRNA expression is not increased in either the pPVN or perifornical region in late pregnancy. Furthermore, because these pPVN cells are not activated in pregnant rats after IL-1β, autoinhibition of pPVN neurons by enkephalin seems implausible.
The NTS is a more likely source of opioids that inhibit pPVN CRH neuron responses to IL-1β. Enkephalins and dynorphins are synthesized by NTS neurons (Ceccatelli et al., 1989, 1992). Thus, if the NTS neurons showing upregulated pENK-A mRNA expression in pregnancy include noradrenergic neurons projecting to the PVN (Ericsson et al., 1994), their activation by IL-1β is expected to release more enkephalin from their terminals to act on the upregulated μ-opioid receptors. Hence, the proposed site of naloxone action in pregnancy is the brainstem noradrenergic nerve terminals in the PVN, as supported by increased noradrenaline release after systemic IL-1β during local naloxone application in pregnant rats.
In conclusion, in late pregnancy, HPA axis responsiveness to LPS and IL-1β is markedly attenuated, resulting from reduced drive by pPVN CRH neurons. This seems not to be a consequence of interrupted signaling to NTS neurons in pregnancy but is parsimoniously proposed to be through upregulated presynaptic autoinhibitory endogenous opioid action on the brainstem noradrenergic projection to the CRH neurons, suppressing noradrenaline release. Reduced HPA responses to immune challenge in pregnancy will protect the fetuses from excessive glucocorticoid exposure, which can cause adverse programming and may also underlie the altered incidence of autoimmune disorders in pregnancy (Dunna and Finlay, 1989; Runmarker and Andersen, 1995; Ostensen and Villiger, 2002).
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council, the British Council, and Komitet Badaṅ Naukowych (State Committee for Scientific Research, Poland). We thank Prof. Keith Kendrick and colleagues at The Babraham Institute (Cambridge, UK) for performing the HPLC analysis and Dr. Karen Francis, Valerie Bishop, Claire Wilkinson, and Helen Cameron for excellent technical advice and assistance.
Correspondence should be addressed to Dr. P. J. Brunton, School of Biomedical and Clinical Laboratory Sciences, University of Edinburgh, Hugh Robson Building, George Square, Edinburgh EH8 9XD, UK. E-mail: P.J.Brunton@ed.ac.uk.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255117-10$15.00/0
References
- Andersson J, Nagy S, Bjork L, Abrams J, Holm S, Andersson U (1992) Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev 127: 69-96. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H (1987) Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 238: 524-526. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, Del Rey A (1992) Immune-neuroendocrine circuits: integrative role of cytokines. Front Neuroendocrinol 13: 61-94. [PubMed] [Google Scholar]
- Brady LS, Lynn AB, Herkenham M, Gottesfeld Z (1994) Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci 14: 4951-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn TO, Sutton RE, Rivier CL, Vale WW (1984) Corticotropin-releasing factor regulates proopiomelanocortin messenger ribonucleic acid levels in vivo. Neuroendocrinology 39: 170-175. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA (2003) Hypothalamic-pituitary-adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J Neuroendocrinol 15: 633-637. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Ma S, Shipston MJ, Wigger A, Neumann ID, Douglas AJ, Russell JA (2000) Central mechanisms underlying reduced ACTH stress responses in pregnant rats: attenuated acute gene activation in the parvocellular paraventricular nucleus (pPVN). Eur J Neurosci 12: 184.17. [Google Scholar]
- Buckingham JC (1982) Secretion of corticotrophin and its hypothalamic releasing factor in response to morphine and opioid peptides. Neuroendocrinology 35: 111-116. [DOI] [PubMed] [Google Scholar]
- Buller KM, Crane JW, Day TA (2001a) The central nucleus of the amygdala: a conduit for modulation of HPA axis responses to an immune challenge? Stress 4: 277-287. [DOI] [PubMed] [Google Scholar]
- Buller KM, Xu Y, Dayas CV, Day TA (2001b) Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1beta-induced HPA axis responses. Neuroendocrinology 73: 129-138. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Millhorn DE, Hokfelt T, Goldstein M (1989) Evidence for the occurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Exp Brain Res 74: 631-640. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Seroogy KB, Millhorn DE, Terenius L (1992) Presence of a dynorphin-like peptide in a restricted subpopulation of catecholaminergic neurons in rat nucleus tractus solitarii. Brain Res 589: 225-230. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M (2005) Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 25: 1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Moldow RL, House SD, Zadina JE (1996) Morphine affects the brain-immune axis by modulating an interleukin-1 beta dependent pathway. Adv Exp Med Biol 402: 35-42. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol 44: 8-12. [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE (2002) Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci 22: 959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover PO, Buckingham JC (1989) Effects of selective opioid-receptor blockade on the hypothalamo-pituitary-adrenocortical responses to surgical trauma in the rat. J Endocrinol 121: 213-220. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Neumann I, Meeren HKM, Leng G, Johnstone LE, Munro G, Russell JA (1995) Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci 15: 5049-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone HA, Wigger A, Landgraf R, Neumann ID (1998) The role of endogenous opioids in neurohypophysial and hypothalamo-pituitary-adrenal axis hormone secretory responses to stress in pregnant rats. J Endocrinol 158: 285-293. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Bicknell RJ, Leng G, Russell JA, Meddle SL (2002) Beta-endorphin cells in the arcuate nucleus: projections to the supraoptic nucleus and changes in expression during pregnancy and parturition. J Neuroendocrinol 14: 768-777. [DOI] [PubMed] [Google Scholar]
- Dunna SF, Finlay AY (1989) Psoriasis: improvement during and worsening after pregnancy. Br J Dermatol 120: 584. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE (1994) A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 14: 897-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Liu C, Hart RP, Sawchenko PE (1995) Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol 361: 681-698. [DOI] [PubMed] [Google Scholar]
- Givalois L, Siaud P, Mekaouche M, Ixart G, Malaval F, Assenmacher I, Barbanel G (1995) Early hypothalamic activation of combined Fos and CRH41 immunoreactivity and of CRH41 release in push-pull cannulated rats after systemic endotoxin challenge. Mol Chem Neuropathol 26: 171-186. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL (1989) Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol 122: 705-711. [DOI] [PubMed] [Google Scholar]
- Herman JP, Morrison DG (1996) Immunoautoradiographic and in situ hybridization analysis of corticotropin-releasing hormone biosynthesis in the hypothalamic paraventricular nucleus. J Chem Neuroanat 11: 49-56. [DOI] [PubMed] [Google Scholar]
- Hodgkinson SC, Allolio B, Landon J, Lowry PJ (1984) Development of a non-extracted two-site immunoradiometric assay for corticotropin utilizing extreme amino- and carboxy terminally directed antibodies. Biochem J 218: 703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Fahrenkrug J, Tatemoto K, Mutt V, Werner S, Hulting AL, Terenius L, Chang KJ (1983) The PHI (PHI-27)/corticotropin-releasing factor/enkephalin immunoreactive hypothalamic neuron: possible morphological basis for integrated control of prolactin, corticotropin, and growth hormone secretion. Proc Natl Acad Sci USA 80: 895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingami H, Mizuno N, Takahashi H, Shibahara S, Furutani Y, Imura H, Numa S (1985) Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett 191: 63-66. [DOI] [PubMed] [Google Scholar]
- Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, Russell JA (2000) Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol 12: 811-822. [DOI] [PubMed] [Google Scholar]
- Kakucska I, Qi Y, Clark BD, Lechan RM (1993) Endotoxin-induced corticotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is mediated centrally by interleukin-1. Endocrinology 133: 815-821. [DOI] [PubMed] [Google Scholar]
- Keith LD, Winslow JR, Reynolds RW (1978) A general procedure for estimation of corticosteroid response in individual rats. Steroids 31: 523-531. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guevara-Guzman R, de la Riva C, Christensen J, Ostergaard K, Emson PB (1996) NMDA and kainate-evoked release of nitric oxide and classical transmitters in the rat striatum: in vivo evidence that nitric oxide may play a neuroprotective role. Eur J Neurosci 8: 2619-2634. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Watson SJ (1983) Enkephalin systems in diencephalon and brainstem of the rat. J Comp Neurol 220: 310-320. [DOI] [PubMed] [Google Scholar]
- Kiss A, Palkovits M, Aguilera G (1996) Neural regulation of corticotropin releasing hormone (CRH) and CRH receptor mRNA in the hypothalamic paraventricular nucleus in the rat. J Neuroendocrinol 8: 103-112. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Ludwig M (1991) Vasopressin release within the supraoptic and paraventricular nuclei of the rat brain: osmotic stimulation via microdialysis. Brain Res 558: 191-196. [DOI] [PubMed] [Google Scholar]
- Ma S, Shipston MJ, Morilak D, Russell JA (2005) Reduced hypothalamic vasopressin secretion underlies attenuated adrenocorticotropin stress responses in pregnant rats. Endocrinology 146: 1626-1637. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1987) Autoradiographic differentiation of μ, δ, and κ opioid receptors in the rat forebrain and midbrain. J Neurosci 7: 2445-2464. [PMC free article] [PubMed] [Google Scholar]
- Melik Parsadaniantz S, Gaillet S, Malaval F, Lenoir V, Batsche E, Barbanel G, Gardier A, Terlain B, Jacquot C, Szafarczyk A, Assenmacher I, Kerdelhue B (1995) Lesions of the afferent catecholaminergic pathways inhibit the temporal activation of the CRH and POMC gene expression and ACTH release induced by human interleukin-1beta in the male rat. Neuroendocrinology 62: 586-595. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Clerget-Froidevaux MS, Nakamura K, Negishi M, Wallace JL, Pittman QJ (2002) Suppression of fever at near term is associated with reduced COX-2 protein expression in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol 283: R800-R805. [DOI] [PubMed] [Google Scholar]
- Mulla A, Buckingham JC (1999) Regulation of the hypothalamo-pituitary-adrenal axis by cytokines. Best Pract Res Clin Endocrinol Metab 13: 503-521. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Seto T, Hatta K, Matsuzaki I, Nagase H, Yoshida M, Ogino K (1998) Central administration of interleukin-1beta reduces natural killer cell activity in non-pregnant rats, but not in pregnant rats. Psychoneuroendocrinology 23: 651-659. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S (1979) Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature 278: 423-427. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ (1998) Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol (Lond) 508: 289-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka T, Luckman SM, Guevara-Guzman R, Ueta Y, Kendrick K, Leng G (1995) Presynaptic actions of morphine: blockade of cholecystokinin-induced noradrenaline release in the rat supraoptic nucleus. J Physiol (Lond) 482: 69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostensen M, Villiger PM (2002) Immunology of pregnancy-pregnancy as a remission inducing agent in rheumatoid arthritis. Transpl Immunol 9: 155-160. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1996) The rat brain in stereotaxic coordinates, Ed 3. New York: Academic. [DOI] [PubMed]
- Rivest S, Rivier C (1991) Influence of the paraventricular nucleus of the hypothalamus in the alteration of neuroendocrine functions induced by intermittent footshock or interleukin. Endocrinology 129: 2049-2057. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N (2000) How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Exp Biol Med 223: 22-38. [DOI] [PubMed] [Google Scholar]
- Rosen H, Douglass J, Herbert E (1984) Isolation and characterization of the rat proenkephalin gene. J Biol Chem 259: 14309-14313. [PubMed] [Google Scholar]
- Runmarker B, Andersen O (1995) Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain 118: 253-261. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W (1987) Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238: 522-524. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Joseph SA (1982) The distribution and cells of origin of ACTH (1-39)-stained varicosities in the paraventricular and supraoptic nuclei. Brain Res 232: 365-374. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL (1999) The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J Neuroendocrinol 11: 857-865. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K (1991) c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci 11: 2321-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N, Walker CD (1998) Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. J Neuroendocrinol 10: 417-427. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C (1995) Regulation of the HPA axis by cytokines. Brain Behav Immun 9: 253-275. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Lee S, Rivier C (1998) Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann NY Acad Sci 840: 434-443. [DOI] [PubMed] [Google Scholar]
- Waddell BJ, Atkinson HC (1994) Production rate, metabolic clearance rate and uterine extraction of corticosterone during rat pregnancy. J Endocrinol 143: 183-190. [DOI] [PubMed] [Google Scholar]
- Wigger A, Lorscher P, Oehler I, Keck ME, Naruo T, Neumann ID (1999) Nonresponsiveness of the rat hypothalamo-pituitary-adrenocortical axis to parturition-related events: inhibitory action of endogenous opioids. Endocrinology 140: 2843-2849. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rivest S (1999) Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. Eur J Neurosci 11: 2651-2668. [DOI] [PubMed] [Google Scholar]