Abstract
CaV2.1 channels conduct P/Q-type Ca2+ currents that are modulated by calmodulin (CaM) and the structurally related Ca2+-binding protein 1 (CaBP1). Visinin-like protein-2 (VILIP-2) is a CaM-related Ca2+-binding protein expressed in the neocortex and hippocampus. Coexpression of CaV2.1 and VILIP-2 in tsA-201 cells resulted in Ca2+ channel modulation distinct from CaM and CaBP1. CaV2.1 channels with β2a subunits undergo Ca2+-dependent facilitation and inactivation attributable to association of endogenous Ca2+/CaM. VILIP-2 coexpression does not alter facilitation measured in paired-pulse experiments but slows the rate of inactivation to that seen without Ca2+/CaM binding and reduces inactivation of Ca2+ currents during trains of repetitive depolarizations. CaV2.1 channels with β1b subunits have rapid voltage-dependent inactivation, and VILIP-2 has no effect on the rate of inactivation or facilitation of the Ca2+ current. In contrast, when Ba2+ replaces Ca2+ as the charge carrier, VILIP-2 slows inactivation. The effects of VILIP-2 are prevented by deletion of the CaM-binding domain (CBD) in the C terminus of CaV2.1 channels. However, both the CBD and an upstream IQ-like domain must be deleted to prevent VILIP-2 binding. Our results indicate that VILIP-2 binds to the CBD and IQ-like domains of CaV2.1 channels like CaM but slows inactivation, which enhances facilitation of CaV2.1 channels during extended trains of stimuli. Comparison of VILIP-2 effects with those of CaBP1 indicates striking differences in modulation of both facilitation and inactivation. Differential regulation of CaV2.1 channels by CaM, VILIP-2, CaBP1, and other neurospecific Ca2+-binding proteins is a potentially important determinant of Ca2+ entry in neurotransmission.
Keywords: facilitation, inactivation, neuromodulation, voltage clamp, synaptic plasticity, calcium current
Introduction
P/Q-type Ca2+ currents initiate exocytosis of neurotransmitters (Takahashi and Momiyama, 1993; Regehr and Mintz, 1994). Because the efficiency of synaptic transmission is proportional to the third power of the local Ca2+ concentration (Dodge and Rahamimoff, 1967; Mintz et al., 1995), small changes in Ca2+ influx and residual free Ca2+ cause multiple forms of short-term synaptic plasticity that have an important influence on synaptic function (Zucker and Regehr, 2002). CaV2.1 channels mediate P/Q-type currents (Llinás et al., 1989; Starr et al., 1991; Wheeler et al., 1994), and these channels are localized in high density in presynaptic active zones of central neurons (Westenbroek et al., 1995; Sakurai et al., 1996; Wu et al., 1999).
Ca2+ binding to calmodulin (CaM) causes facilitation and enhances inactivation of CaV2.1 channels through binding to a site in the C-terminal domain (Lee et al., 1999, 2000; Pate et al., 2000; DeMaria et al., 2001). Ca2+-binding protein 1 (CaBP1), a neurospecific CaM-like Ca2+ binding protein, accelerates inactivation, prevents facilitation, and binds at the same site as CaM in aCa2+-independent manner (Lee et al., 2002). These results raise the possibility that multiple CaM-related neuronal CaBPs (nCaBPs) may interact with the C-terminal regulatory site and differentially regulate CaV2.1 channels.
Visinin-like proteins (VILIPs) belong to the neuronal Ca2+ sensor superfamily (Haeseleer et al., 2000; Burgoyne and Weiss, 2001) and are expressed in retinal (Lenz et al., 1992) and brain neurons (Saitoh et al., 1995; Bernstein et al., 1999, 2003; Hamashima et al., 2001; Spilker et al., 2002). They sensitize G-protein signaling cascades to Ca2+ and enhance desensitization of G-protein-coupled receptors (De Castro et al., 1995; Ames et al., 1997; Sallese et al., 2000), which can regulate CaV2.1 channels through Gβγ subunits (Herlitze et al., 1996; Ikeda, 1996). VILIPs reversibly translocate to membranes in response to Ca2+ fluctuations in neurons (Spilker et al., 2002), suggesting that they may shuttle between the plasma membrane and intracellular compartments in response to Ca2+ channel activity.
The kinetics and voltage dependence of activation and inactivation of Ca2+ channels are strongly influenced by their CaVβ subunits (Hofmann et al., 1999; Lee et al., 2000; Arikkath and Campbell, 2003). The β1b subunit causes rapid voltage-dependent inactivation, similar to β3 and β4 (De Waard and Campbell, 1995), whereas the β2a subunit slows channel inactivation (Olcese et al., 1994; Chien et al., 1996). The rapid inactivation of CaV2.1 channels with β1b subunits substantially reduces Ca2+/CaM-dependent facilitation compared with β2a subunits (Lee et al., 1999). Both β1b and β2a are coexpressed with CaV2.1 (Stea et al., 1994; Tanaka et al., 1995; Ludwig et al., 1997; Burgess et al., 1999) and are likely to form functional channels in vivo. In this report, we describe modulation of CaV2.1 channels having β1b or β2a subunits by VILIP-2 and compare these results with modulation by CaBP1. Our results show that VILIP-2 modulates CaV2.1 channels in a manner distinct from CaM and CaBP1, supporting the conclusion that CaV2.1 channel properties are fine-tuned by interaction of multiple neuronal Ca2+-binding proteins at a common C-terminal regulatory site.
Materials and Methods
Cloning of VILIP-2. A full-length cDNA clone of VILIP-2 was isolated from total rat brain mRNA. VILIP-2-specific cDNA was generated with reverse primer TCTAGACTACTTCTGCATG using Superscript First-Strand Synthesis system for reverse transcription-PCR (Invitrogen, San Diego, CA). Using the same reverse primer and the forward primer CTCGAGATGGGGAAGAACAATAGC, we amplified a single band that was cloned via the TOPO TA cloning kit (Invitrogen). This was sequence verified and subcloned into pcDNA 3.1+. Construction of a carboxyl myc-tagged form of VILIP-2 (VILIP-2 myc) was accomplished by PCR using the same forward primer and the reverse primer TCTAGACTACAAGTCCTCTTCAGAAATGAGCTTTTGCTCCTTCTGCATG, which was amplified and cloned via TOPO TA cloning kit and subcloned into pcDNA 3.1+. The myc tag was detected with an anti-myc antibody from Invitrogen.
cDNA expression constructs. Expression vectors for rat CaV2.1 channels were prepared by subcloning wild-type and mutant subunit cDNAs into the following plasmids: α12.1(rbA-II) in pcDNA 3.1+, α12.1ΔCBD (CaM-binding domain), α12.1 1965 stop, α12.1 IQ-AA, α12.1 IQ-AA/ΔCBD in pMT2, β1b and β2a in pMT2XS, and α2δ in pZEM228 (Ellis et al., 1988; Starr et al., 1991; Perez-Reyes et al., 1992; Stea et al., 1994), as described previously (Lee et al., 1999, 2000, 2003). CaBP1 constructs were prepared as described previously (Lee et al., 2002).
Cell culture and transfection. tsA-201 cells were maintained in DMEM/F-12 supplemented with 10% fetal bovine serum (Invitrogen) at 37°C under 10% CO2. Cells plated in 35 mm culture dishes for electrophysiological recording were grown to 70% confluence and transfected by the CaPO4 method with a total of 5 μg of DNA, including a 1:1 molar ratio of Ca2+ channel subunits (α12.1, β1b or β2a, and α2δ), 0.3 μg of CD8 expression plasmid for identification of transfected cells, and 1 μg of VILIP-2 cDNA. Biochemical studies used 150 mm dishes with a maximum of 70 μg of DNA using the same ratios as above, but without CD8.
Electrophysiology. tsA-201 cells were incubated with CD8 antibody-coated microspheres (Dynal Biotech, Oslo, Norway) to allow visual identification of transfected cells 48-72 h after transfection. Ca2+ currents were recorded in the whole-cell configuration of the patch-clamp technique using an Axopatch 200A patch-clamp amplifier (Molecular Devices, Union City, CA) and were filtered at 5 kHz. Voltage protocols were applied with Pulse software (HEKA Elektronik, Lambrecht/Pfalz, Germany), and data were analyzed using IGOR Pro 4.0 software (Wave-Metrics, Lake Oswego, OR). Leak and capacitive transients were subtracted using P/-4 protocol. The extracellular recording solution contained the following (in mm): 150 Tris, 1 MgCl2, and 10 CaCl2 or BaCl2, adjusted to pH 7.3 with methanesulfonic acid, except where differences are noted in Figures 1, 2, 3, 4, 5, 6, 7, 8. Intracellular solutions contained the following (in mm): 120 N-methyl-d-glucamine, 60 HEPES, 1 MgCl2, 2 Mg-ATP, and either 0.5 or 10 EGTA, adjusted to pH 7.3 with methanesulfonic acid. Because extracellular Ba2+ caused positive shifts in the voltage dependence of activation of 10 mV, voltage protocols were adjusted to compensate for this difference, as noted in Figures 1, 2, 3, 4, 5, 6, 7, 8. All averaged data represent the mean ± SEM. The current traces presented in Figures 1, 2, 3, 4, 5, 6, 7, 8 represent the means of normalized current traces from the indicated number of samples for each experimental condition.
Figure 1.

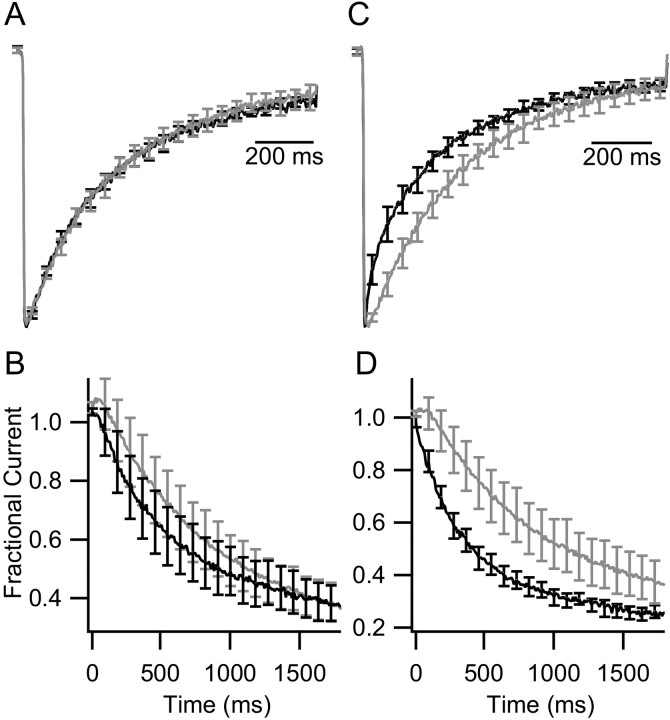
Effect of coexpression of VILIP-2 on CaV2.1 channels. A, Voltage dependence of activation. CaV2.1/β2a channels was activated by 5 ms pulses to the indicated potentials from a holding potential of -80 mV, and Ca2+ tail currents were recorded after repolarization to -40 mV without (gray) or with (black) coexpression of VILIP-2 (±SEM; n ≥ 10). B, ICa was evoked by 1 s depolarizing test pulses to +10 mV from a holding potential of -80 mV in tsA-201 cells expressing CaV2.1/β2a channels without (gray) or with (black) VILIP-2. Current records were normalized to the peak inward current and averaged (±SEM; n ≥ 10). C, IBa was evoked and analyzed as in B. D, ICa was evoked and analyzed as in B for CaV2.1ΔCBD/β2a. E, ICa conducted by CaV2.1/β1b channels was evoked and analyzed as in B. F, IBa conducted by CaV2.1/β1b channels was evoked and analyzed as in B. G, IBa conducted by CaV2.1ΔCBD/β1b channels was evoked and analyzed as in B. H, I, Residual current amplitude at the end of the 1 s pulse (IRes) was divided by peak current from cells transfected with CaV2.1 alone (gray) or with VILIP-2 (black) for the indicated experimental conditions. WT, Wild type. Error bars represent SEM.
Figure 2.

Effect of coexpression of VILIP-2 on paired-pulse facilitation of CaV2.1/β2a channels. Test pulses to potentials ranging from -40 to +80 mV were applied without a prepulse (open symbols) or with a 50 ms prepulse to +10 mV and an 8 ms period at -80 mV before the test pulse (filled symbols). A, Peak inward calcium currents for cells expressing CaV2.1/β2a channels without (gray circles) or with (black squares) VILIP-2. B, Same protocol as that in A, with 10 mm barium as the charge carrier. Insets, Representative calcium or barium currents at 70 mV. Calibration: horizontal, 2 ms; vertical, A, B, left insets, 1 nA; A, B, right insets, 500 pA. Error bars represent SEM.
Figure 3.

Effect of VILIP-2 on facilitation and inactivation of CaV2.1 channels during trains of repetitive depolarizations. Test pulses to +10 mV (0 mV for IBa) for 5 ms at a frequency of 100 Hz were applied to transfected tsA-201 cells expressing CaV2.1 channels only (gray) or CaV2.1 channels plus VILIP-2 (black). Peak current amplitudes were normalized to the first pulse in the series and plotted against time (±SEM; n ≥ 10; every 10th SEM is plotted). A, Representative ICa measured for the first pulse, the pulse at 355 ms, and the last pulse in a train of stimuli for CaV2.1/β2a alone. B, Representative ICa measured for the first pulse, the pulse at 355 ms, and the last pulse in a train of stimuli for CaV2.1/β2a plus VILIP-2. C, ICa, CaV2.1/β2a. D, ICa, CaV2.1/β1b. E, IBa,CaV2.1/β2a. F, IBa, CaV2.1/β1b. Error bars represent SEM.
Figure 4.

Effect of VILIP-2 on CaV2.1/β2a channels after trains of repetitive depolarizations. CaV2.1/β2a channel currents were evoked by 1 s test pulses to +10 mV before (gray) or after (black) a 600 ms train of 5 ms depolarizations to +20 mV (+10 mV for IBa) at 100 Hz. A 2 min interval was maintained between applications of this protocol to allow the effects of the previous stimuli to return to baseline. Current amplitudes were normalized to the peak current of the first test pulse. Mean normalized currents during test pulse 1 (gray) and test pulse 2 (black) are overlaid. A, ICa, CaV2.1/β2a alone. B, IBa, CaV2.1/β2a alone. C, ICa, CaV2.1/β2a plus VILIP-2. D, IBa, CaV2.1/β2a plus VILIP-2. E, Bar graph presenting facilitation as the pulse ratio (P2/P1) at the time of the peak current in each panel (mean ± SEM). Error bars represent SEM.
Figure 5.

Effect of VILIP-2 on CaV2.1/β1b channels after trains of repetitive depolarizations. CaV2.1/β1b channel currents were evoked by 1 s test pulses to +10 mV before (gray) or after (black) a 600 ms train of repetitive depolarization to +20 mV (+10 mV for IBa) for 5 ms at 100 Hz. A 2 min interval was maintained between applications of this protocol to allow the effects of the previous stimuli to return to baseline. Current amplitudes were normalized to the peak current of the first test pulse. Mean normalized currents during test pulse 1 (gray) and test pulse 2 (black) are overlaid. A, ICa, CaV2.1/β1b alone. B, IBa, CaV2.1/β1b alone. C, ICa, CaV2.1/β1b plus VILIP-2. D, IBa, CaV2.1/β1b plus VILIP-2. E, Bar graph presenting facilitation as the pulse ratio (P2/P1) at the time of the peak current in each panel (mean ± SEM). Error bars represent SEM.
Figure 6.
Association of VILIP-2 with CaV2.1 channels via the IQ-like domain and CBD. Transfected cells were cross-linked in situ with DSP, as described in Materials and Methods. A, Lysates from cells transfected with CaV2.1 plus VILIP-2 myc or CaV2.1ΔCBD plus VILIP-2 myc were subjected to immunoprecipitation by anti-CaV2.1 antibodies or control IgG as indicated in the presence of 10 mm EGTA. B, Lysates from cells transfected with CaV2.1, CaV2.1/1965ST, CaV2.1/IM-AA, and CaV2.1ΔCBD/IM-AA plus VILIP-2 myc were subjected to immunoprecipitation by anti-CaV2.1 antibodies or control IgG in the presence of 10 mm EGTA. Blots were probed with either anti-CaV2.1 (top) or anti-myc (bottom). WT, Wild type.
Figure 7.
Effect of CaBP1 on CaV2.1/β1b channels. A, Average, smoothed ICa conducted by CaV2.1 channels evoked by 1 s depolarizing pulses from a holding potential of -80 mV to +30 mV without (gray) or with (black) coexpression of CaBP1 (±SEM; n = 5-7). B, From a holding potential of -80 mV, test pulses to +10 mV for 5 ms at a frequency of 100 Hz were applied to transfected tsA-201 cells expressing CaV2.1 channels alone (gray) or CaV2.1 channels plus CaBP1 (black). Peak current amplitudes were normalized to the first pulse in the series and plotted against time of stimulation (±SEM; n = 8-10; every 10th SEM is plotted). C, Averaged, smoothed IBa was measured as in A with a test pulse to +20 mV for CaV2.1/β1b channels without (gray) or with (black) coexpression of CaBP1 (±SEM; n = 5-8). D, From a holding potential of -80 mV, test pulses to 0 mV for 5 ms at a frequency of 100 Hz were applied to transfected tsA-201 cells expressing CaV2.1/β1b channels without (gray) or with (black) coexpression of CaBP1. Peak current amplitudes were normalized to the first pulse in the series and plotted against time (±SEM; n = 6-8; every 10th SEM is plotted). Error bars represent SEM.
Figure 8.

Comparison of the structures and modulatory effects of CaM, VILIP-2, and CaBP1. A, Schematic illustration of the structures of CaM, CaBP1, and VILIP-2. EF-hands that are active in binding Ca2+ are indicated in gray, and inactive EF-hands are indicated in white. Circles denote the central α helix connecting EF-hands 2 and 3. Bent lines indicate N-terminal myristoylation. The lengths of the line segments approximately correspond to the length of the amino acid sequences. B, Ca2+-dependent inactivation of CaV2.1/β2a channels with CaM, CaBP1, or VILIP-2. Overlapped Ca2+ and Ba2+ currents are plotted. The shaded area indicates the Ca2+-dependent increase in inactivation caused by CaM. C, Ca2+-dependent facilitation and inactivation of CaV2.1/β2a channels with CaM, CaBP1, or VILIP-2. The CaM and CaBP1 data in B and C were modified from Lee et al. (2000) and (2002), respectively. Error bars represent SEM.
Biochemical studies. tsA-201 cells were cultured and transfected as described above and harvested 48 h after transfection. Dithiobis (succinimidyl propionate) (DSP; 2 mm; Pierce, Rockford, IL) was dissolved in DMSO and applied for 30 min in PBS, followed by quenching in Tris. Cells were harvested in lysis buffer containing 150 mm NaCl, 10 mm EGTA, 20 mm TrisHCl, and 1% NP-40. Lysates were passed through a syringe needle (3 ml of 25G 5/8) four times, followed by centrifugation at 14,000 rpm for 10 min to remove the nuclear fraction. Anti-CNA4 (10 μg) directed against α12.1 (Sakurai et al., 1996) or IgG control antibody was incubated for 1 h, followed by 15 min protein A-Sepharose bead precipitation. Beads were washed three times in lysis buffer with 0.1% NP-40, heated to 95°C for 10 min in loading buffer containing 0.1 m DTT, and separated by SDS-PAGE in a 4-20% acrylamide gel. Proteins were transferred onto nitrocellulose membranes, blocked in 3% nonfat milk and 0.5% SDS for 2 h, and probed with either anti-CNA4 (1:100) or anti-myc (1:5000), followed by secondary labeling with horseradish peroxidase-linked protein A (Amersham Biosciences, Piscatway, NJ) or horseradish peroxidase-linked sheep anti-mouse (Amersham Biosciences). Bound horseradish peroxidase was detected by ECL reaction (Amersham Biosciences) followed by exposure to film.
Results
Coexpression of VILIP-2 with CaV2.1/β2achannels
We first examined the effect of coexpression of VILIP-2 on Ca2+ currents (ICa) in whole-cell voltage-clamp recordings of transfected tsA-201 cells expressing CaV2.1 channels with β2a subunits. Ca2+ currents were observed in test pulses to potentials more positive than -10 mV, approached maximum at +40 mV, and inactivated slowly during long pulses (Fig. 1A,B, gray). Coexpression of VILIP-2 did not affect the voltage dependence of activation of CaV2.1 channels in response to depolarizing test pulses to different membrane potentials (Fig. 1A, black). ICa inactivates ∼53% during a 1 s test pulse (Fig. 1B, gray) compared with 31% for Ba2+ current (IBa) (Fig. 1C, gray). This Ca2+-dependent component of inactivation is caused by endogenous CaM, which binds entering Ca2+ and interacts with the CBD in the C terminus to inactivate CaV2.1 channels (Lee et al., 1999, 2003). When VILIP-2 is coexpressed with CaV2.1/β2a channels, the rate of inactivation of ICa is slowed considerably (Fig. 1B, black). In contrast to ICa, VILIP-2 has no effect on the rate of inactivation of IBa conducted by CaV2.1/β2a channels (Fig. 1C). The block of Ca2+-dependent inactivation by VILIP-2 suggests that it may prevent binding of endogenous Ca2+/CaM to the CBD of CaV2.1 channels.
A deletion mutant of CaV2.1 lacking the CBD (CaV2.1ΔCBD) conducts Ca2+ currents that do not undergo rapid CaM-dependent inactivation (Fig. 1D, gray), confirming that CaM association with the CBD is required for Ca2+-dependent inactivation. Expression of VILIP-2 with CaV2.1ΔCBD has no effect on its inactivation (Fig. 1D, black), supporting the conclusion that VILIP-2 acts primarily to negate the effect of endogenous Ca2+/CaM on CaV2.1/β2a channels. Because VILIP-2 has no effect on inactivation of the CaV2.1ΔCBD channel, either VILIP-2 does not bind to CaV2.1ΔCBD or it binds but has no effect on inactivation.
Coexpression of VILIP-2 with CaV2.1/β1b channels
When CaV2.1 is coexpressed with the β1b subunit, voltage-dependent inactivation of ICa is faster than for CaV2.1/β2a (Fig. 1, compare E, gray with B, gray), and it is unaffected by coexpression of VILIP-2 (Fig. 1E, black). Inactivation of IBa conducted by CaV2.1/β1b is also more rapid than inactivation of IBa of CaV2.1/β2a channels (Fig. 1, compare F, gray with C, gray), and it is substantially slowed by coexpression of VILIP-2 (Fig. 1F, black), without effect on the voltage dependence of activation (data not shown). In contrast, the CaV2.1ΔCBD/β1b mutant channel is unaffected by VILIP-2 (Fig. 1G), indicating that VILIP-2 acts at the same site as CaM to control the rate of inactivation. Because VILIP-2 alters the rate of inactivation of IBa, it must associate with CaV2.1 channels at the resting level of intracellular Ca2+ and slow voltage-dependent inactivation. However, although VILIP-2 is bound to the CaV2.1/β1b channel, it is unable to slow the inactivation of ICa appreciably, presumably because of the strong driving force for inactivation with both β1b and Ca2+ bound to the channel complex. Overall, VILIP-2 appears to act by negating rapid Ca2+/CaM-dependent inactivation and slowing voltage-dependent inactivation in a β subunit-dependent manner.
Paired-pulse facilitation of CaV2.1/β2a channels coexpressed with VILIP-2
ICa conducted by CaV2.1/β2a channels is significantly facilitated after a previous depolarizing pulse, resulting from Ca2+/CaM interaction with the IQ-like domain and CBD (Lee et al., 2000, 2003; DeMaria et al., 2001). With 10 mm EGTA in the recording pipette, Ca2+ facilitation can be recorded in isolation without interference from Ca2+-dependent inactivation (Lee et al., 2000). Applying a similar protocol, we found that paired-pulse facilitation of ICa was unchanged by coexpression of VILIP-2 (Fig. 2A). As expected, no facilitation of IBa was observed without or with VILIP-2 (Fig. 2B). Evidently, although VILIP-2 modulates inactivation differently from CaM, it can mediate Ca2+-dependent paired-pulse facilitation similarly.
Ca2+-dependent facilitation and inactivation during trains of depolarizations
Previous work demonstrated that activity-dependent increases in Ca2+ entry cause facilitation attributable to a local increase in Ca2+, followed by inactivation attributable to a more global increase in Ca2+ during extended trains of depolarizations (Lee et al., 2000). These trains of brief depolarizations more closely resemble electrical activity in nerve terminals than single long depolarizing pulses. This dual regulation is caused by stepwise Ca2+ binding to C- and N-terminal lobes of CaM and sequential binding of Ca2+/CaM to the IQ-like domain and CBD of CaV2.1 channels (Lee et al., 1999, 2000, 2003; DeMaria et al., 2001). To investigate the effects of VILIP-2 coexpression on CaV2.1 channels during trains of brief stimuli, we depolarized tsA-201 cells expressing CaV2.1/β2a channels repetitively to +10 mV for 5 ms at a frequency of 100 Hz and recorded ICa during each stimulation. Initially, we observed that VILIP-2 prevented both facilitation and subsequent inactivation (data not shown). However, on more careful analysis, we realized that the residual increase in Ca2+ concentration from previous depolarization protocols was occluding facilitation. By recording from cells that either had not experienced stimulated Ca2+ influx or were allowed to recover from previous depolarizations for at least 2 min, we were able to accurately record both facilitation and inactivation by CaM (Fig. 3A,C, gray) and facilitation by VILIP-2 (Fig. 3B,C, black). VILIP-2 coexpression increased the amount of facilitation and decreased the rate of pulse-wise inactivation of ICa compared with control cells not expressing VILIP-2 (Fig. 3C). As expected from the lack of effect of VILIP-2 on paired-pulse facilitation, it had insignificant effects during the first few pulses but reduced the pulse-wise inactivation of ICa thereafter. In contrast, when Ba2+ replaced Ca2+ as the charge carrier, insignificant facilitation was observed with endogenous CaM in control cells or after coexpression of VILIP-2 (Fig. 3E). These results are in agreement with our observation (Fig. 1) that VILIP-2 slows the rate of Ca2+-dependent inactivation of CaV2.1/β2a channels during single long depolarizations and show further that VILIP-2 enhances facilitation during extended trains of stimuli in a Ca2+-dependent manner. Because VILIP-2 has no effect on facilitation by a single pulse, it is likely that the increase in facilitation during trains of stimuli reflects slowing of cumulative Ca2+-dependent inactivation.
When the β1b subunit was coexpressed with CaV2.1, and Ca2+ was the charge carrier, trains of stimuli elicited brief facilitation followed by rapid, pulse-wise inactivation (Fig. 3D, gray). VILIP-2 had no effect on facilitation or inactivation of ICa during repetitive depolarizations (Fig. 3D, black). However, when Ba2+ replaced Ca2+ as the charge carrier, minimal facilitation of the current was observed, and inactivation was reduced. Coexpression of VILIP-2 reduced the rate of the remaining pulse-wise inactivation (Fig. 3F, black) compared with control cells (Fig. 3F, gray), similar to the reduced inactivation of IBa we observed during single long depolarizations (Fig. 1F). The effects of VILIP-2 on facilitation and inactivation of both CaV2.1/β2a channels and CaV2.1/β1b channels are consistent with a model in which VILIP-2 associates with the CBD at resting levels of Ca2+, blocks the effects of CaM, slows inactivation, and thereby enhances facilitation in a β subunit-dependent manner.
Modulation of CaV2.1 channels by VILIP-2 after trains of depolarizations
To further characterize the effects of trains of depolarizations on the modulation of CaV2.1 channels by VILIP-2, we used a double-pulse protocol in which a 1 s depolarizing test pulse was followed by a 600 ms train of depolarizations to +20 mV for 5 ms at 100 Hz and finally by a second identical test pulse. This protocol is expected to cause accumulation of Ca2+ in the vicinity of Ca2+ channels during the train of depolarizations, which will modulate the activation and inactivation of the CaV2.1 channels in the second test pulse compared with the first. For CaV2.1 channels containing the β2a subunit, the rate of activation and the peak amplitude of ICa during 1 s test pulses to +10 mV are significantly increased after the pulse train (Fig. 4A, black, E) compared with before the train of stimuli (Fig. 4A, gray, E). These effects are not observed for IBa, indicating that they are caused by Ca2+ entering the cells during the train of stimuli (Fig. 4B,E). For cells coexpressing VILIP-2, the peak amplitude of ICa is further enhanced after the train of depolarizing stimuli (Fig. 4C), and this effect is also lost when Ba2+ is the current carrier (Fig. 4D,E). Thus, VILIP-2 increases the Ca2+-dependent facilitation of ICa conducted by CaV2.1/β2a channels after a train of depolarizations, as observed in the experiments shown in Figure 2 for ICa recorded during the brief depolarizations within the train. As for depolarizations within the train, it is likely that the enhanced facilitation caused by VILIP-2 reflects slowing of cumulative inactivation of ICa during the pulse train.
We next examined the effect of a train of repetitive depolarizing stimuli on CaV2.1 channels containing the β1b subunit (Fig. 5). In contrast to CaV2.1/β2a channels, previous stimulation of CaV2.1/β1b channels with a train of depolarizing pulses reduces peak ICa (Fig. 5A, black) compared with currents recorded during an identical test pulse immediately before the train (Fig. 5A, gray), yielding a pulse ratio [pulse 2 (P2)/P1] significantly <1.0 (Fig. 5E). Similar results are obtained for IBa (Fig. 5B), indicating that these effects of the train of prepulses are independent of Ca2+ and therefore reflect cumulative voltage-dependent inactivation. Coexpression of VILIP-2 has little effect on the response of IBa conducted by CaV2.1/β1b channels to a train of depolarizations (Fig. 5C,D). Evidently, the effects of VILIP-2 on facilitation and inactivation after trains of prepulses are specific for CaV2.1 channels containing β2a subunits, which have substantial Ca2+-dependent facilitation and inactivation.
Association of VILIP-2 with CaV2.1 channels
We measured the association of VILIP-2 and CaV2.1 channels in coimmunoprecipitation studies by coexpressing a myc-tagged form of VILIP-2 with CaV2.1 and the β1b subunit. Our initial experiments failed to demonstrate interaction of VILIP-2 with CaV2.1 channels after detergent solubilization and immunoprecipitation, indicating that the complex formed is reversible and dissociates during isolation. In contrast to these results, in situ protein cross-linking with DSP applied to intact cells (see Materials and Methods), followed by detergent extraction and immunoprecipitation, resulted in detection of VILIP-2 binding to CaV2.1 channels at resting levels of Ca2+ (Fig. 6A, WT). Coimmunoprecipitation of VILIP-2 was specific, because VILIP-2 was not coimmunoprecipitated with control IgG in cells transfected with CaV2.1 and VILIP-2.
CaM binds to two closely spaced motifs in the C terminus of CaV2.1 channels, the CBD and the IQ-like domain (Lee et al., 1999, 2000, 2003; DeMaria et al., 2001; Erickson et al., 2003). Deletion of the CBD or deletion of the carboxyl-half of the C terminus containing the CBD at amino acid 1965 caused a variable decrease in VILIP-2 binding (Fig. 6A, ΔCBD, B, 1965ST), suggesting that VILIP-2 bound to a second site or subsite in addition to the CBD. Mutation of the first two amino acid residues of the IQ-like domain had little effect on binding of VILIP-2 (Fig. 6B, IMAA). In contrast, combined mutation of the CBD and the IQ-like domain consistently prevented VILIP-2 binding (Fig. 6B, ΔCBD-IMAA). These results indicate that VILIP-2, like CaM (Lee et al., 1999, 2000, 2003; DeMaria et al., 2001; Erickson et al., 2003), binds to both the IQ-like domain and the CBD of CaV2.1 channels.
Modulation of CaV2.1/β1b channels by CaBP1
In previous studies, we examined the effects of CaBP1 on CaV2.1/β2a channels and found that it enhanced voltage-dependent inactivation and prevented facilitation (Lee et al., 2002), in marked contrast to the effects of VILIP-2 reported here. To complete the comparison of modulation of CaV2.1 channels by CaBP1 and VILIP-2, we also studied the effects of CaBP1 on CaV2.1/β1b channels. Under these experimental conditions, coexpression of CaBP1 had no effect on the rate of activation or inactivation of ICa conducted by CaV2.1/β1b channels during 1 s depolarizations (Fig. 7A) and also had no effect on their voltage dependence of activation (data not shown). Although there was a trend toward increased decay of ICa during trains of repetitive pulses (Fig. 7B), this difference did not reach statistical significance. In contrast to these results for ICa, replacement of Ca2+ with Ba2+ eliminates the small effects of Ca2+/CaM-dependent inactivation of CaV2.1/β1b channels and reveals Ca2+-independent effects of CaBP1. Coexpression of CaBP1 enhanced inactivation of IBa during single 1 s test pulses (Fig. 7C) and during extended trains of depolarizations (Fig. 7D). Thus, coexpression of CaBP1 has little effect on ICa conducted by CaV2.1/β1b channels but accelerates inactivation of IBa, opposite to the effect of VILIP-2.
Discussion
Modulation of CaV2.1 channels by VILIP-2
Our results show that VILIP-2 modulates Cav2.1 channels in a biphasic manner that depends on the associated β subunit and on Ca2+ entry. CaV2.1 channels with associated β2a subunits have slow voltage-dependent inactivation (Olcese et al., 1994; Chien et al., 1996). VILIP-2 decreases Ca2+/CaM-dependent inactivation of the Ca2+ current through CaV2.1/β2a channels during 1 s depolarizations. Replacing Ca2+ with Ba2+ as the charge carrier eliminates Ca2+/CaM-dependent inactivation and eliminates the effect of VILIP-2. Similarly, CaV2.1ΔCBD/β2a channels, which have impaired CaM binding, do not undergo rapid Ca2+/CaM-dependent inactivation, and inactivation of ICa is not further slowed by VILIP-2 coexpression. These results are consistent with the conclusion that VILIP-2 occupies the same binding site as endogenous CaM and thereby prevents rapid Ca2+/CaM-dependent inactivation. When bound in place of CaM, VILIP-2 reduces inactivation of CaV2.1/β2A channels.
Coexpression of VILIP-2 does not alter Ca2+-dependent facilitation of CaV2.1/β2a channels after a single pulse in paired-pulse facilitation experiments. Evidently, VILIP-2 can bind Ca2+ and induce facilitation like CaM. However, the effect of VILIP-2 to slow inactivation allows enhanced facilitation during and after extended trains of depolarizations by reducing cumulative inactivation during the train.
CaV2.1/β1b channels exhibit rapid voltage-dependent inactivation (De Waard and Campbell, 1995; Hofmann et al., 1999; Lee et al., 2000; Arikkath and Campbell, 2003) and comparatively little acceleration of inactivation by Ca2+/CaM (Lee et al., 1999, 2000). In contrast to CaV2.1/β2a channels, coexpression of VILIP-2 has no effect on the rapid inactivation of ICa but slows voltage-dependent inactivation of IBa to give a rate comparable with inactivation of IBa through CaV2.1/β2a channels. Deletion of the CBD blocks the effect of VILIP-2 to slow the rate of inactivation of IBa, identifying this site as being necessary for VILIP-2 modulation of CaV2.1/β1b. VILIP-2 coexpression also reduces inactivation of IBa during trains of depolarizations. These results are consistent with the conclusion that VILIP-2 occupies the same binding site as CaM. When bound in place of CaM, VILIP-2 slows inactivation of IBa but does not enhance facilitation of CaV2.1/β1b channels during extended trains of depolarizations.
Interaction of VILIP-2 with CaV2.1 channels
CaM binds to at least two interacting subsites on the carboxyl tail of CaV2.1, the IQ-like domain and the CBD (Lee et al., 1999, 2003; DeMaria et al., 2001). Sequential Ca2+-dependent interactions with these subsites are required for Ca2+/CaM-dependent facilitation and inactivation (Lee et al., 2003). In contrast, we found that VILIP-2 binds to CaV2.1 channels at the resting level of Ca2+ by interaction with the IQ-like domain and the CBD. VILIP-2 binding was weaker than CaM and was not stable through solubilization and immunoprecipitation; however, covalent cross-linking in situ with DSP stabilized this in vivo interaction so that it could be measured by solubilization and coimmunoprecipitation. Because mutation of both the IQ-like motif and the CBD were required to prevent binding of VILIP-2, it likely displaces CaM interactions from both of those subsites on CaV2.1 channels. Evidently, binding of VILIP-2 at resting Ca2+ levels and displacement of CaM from the IQ-like domain and the CBD combine to produce the effects that we have recorded in our electrophysiological studies.
Colocalization of VILIP-2 and CaV2.1 channels in the brain
VILIP-2 is expressed in the neocortex, caudate-putamen, and hippocampus, including both pyramidal neurons and dentate granule cells, and in the Purkinje neurons of the cerebellum (Paterlini et al., 2000). CaV2.1, β2a, and β1b are also expressed in these regions (Stea et al., 1994; Tanaka et al., 1995; Westenbroek et al., 1995; Sakurai et al., 1996). The CaV2.1/β2a currents reported here are similar to slowly inactivating P/Q-type Ca2+ currents recorded in hippocampal CA1 neurons (Hillyard et al., 1992; Mintz, 1994), cerebellar granule neurons (Randall and Tsien, 1995), and Purkinje neurons (Mintz et al., 1992a,b). Thus, VILIP-2 is present in neurons in which CaV2.1/β2a is expressed and is therefore able to modulate these channels in vivo. Immunocytochemical studies of VILIP-2 localization show broad distribution in the cell bodies and dendrites of hippocampal pyramidal neurons, dentate granule neurons, cortical neurons, and cerebellar Purkinje neurons (Saitoh et al., 1994, 1995). In addition, punctate labeling in the neuropil of the hippocampus and cerebral cortex suggests localization of VILIP-2 in nerve terminals as well (Saitoh et al., 1994, 1995), together with CaV2.1 channels (Westenbroek et al., 1995). These results indicate that VILIP-2 is colocalized with CaV2.1 channels in neuronal cell bodies and dendrites and probably in nerve terminals. Our previous results show that CaBP1 and CaV2.1 channels are colocalized in cell bodies, dendrites, and nerve terminals in the hippocampus and cerebellum (Lee et al., 2002), thereby placing both nCaBPs and CaV2.1 channels in the same subcellular locations. CaV2.1 channels in these neuronal subcellular compartments would be modulated in a reciprocal calcium-dependent manner by CaM, CaBP1, and VILIP-2 acting at the same site.
Differential modulation of CaV2.1 channels by neuronal Ca2+-binding proteins
This study provides new evidence that different Ca2+-binding proteins fine-tune the functional properties of CaV2.1 channels. Figure 8 illustrates the structural features of CaM, CaBP1, and VILIP-2 and shows their effects on CaV2.1 channels during single depolarizations and repetitive stimulation as an example of their differential actions. CaM has four EF-hands, which are all functional in binding Ca2+.An α helix connects EF-hands 2 and 3 and divides the molecule into two halves, which function differentially in facilitation and inactivation of CaV2.1 channels (DeMaria et al., 2001; Lee et al., 2003). High-affinity binding of local Ca2+ to EF-hands 3 and 4 initiates facilitation by enhancing interaction with the IQ-like domain, whereas lower-affinity binding of globally increased Ca2+ to EF-hands 1 and 2 initiates Ca2+-dependent inactivation by interaction with the CBD (Lee et al., 2000, 2003; DeMaria et al., 2001).
CaBP1 and VILIP-2 have distinct molecular features and functional effects (Fig. 8). EF-hand 2 of CaBP1 has amino acid sequence changes that prevent high-affinity binding of Ca2+, the long N-terminal has a myristoyl lipid anchor, and the central α helix is lengthened by an additional turn compared with CaM (Fig. 8A). In contrast, EF-hand 1 of VILIP-2 does not bind Ca2+, the N-terminal is shorter but also has a myristoyl lipid anchor, and the central α helix is similar in length to CaM (Fig. 8A). These molecular differences cause different functional effects on CaV2.1 channels (Fig. 8B,C). CaM mediates Ca2+-dependent inactivation, CaBP1 causes rapid inactivation regardless of whether Ca2+ is the permeant ion, and VILIP-2 causes slow inactivation regardless of whether Ca2+ is the permeant ion (Fig. 8B). The differences in the effects of these neuronal Ca2+-binding proteins on inactivation during single long depolarizations (Fig. 8B) is mirrored in their effects on facilitation and inactivation during trains of stimuli (Fig. 8C). CaM causes facilitation followed by inactivation, VILIP-2 causes only enhanced facilitation, and CaBP1 causes only enhanced inactivation. These differential effects on presynaptic Ca2+ channels would alter the encoding properties of the presynaptic terminal by changing its response to repetitive stimuli from facilitation followed by inactivation to either facilitation only or inactivation only (Fig. 8). Thus, in nerve terminals where these Ca2+-binding proteins are colocalized with CaV2.1 channels, the input-output relationships of the corresponding synapses would be influenced in an important way by regulation of the presynaptic Ca2+ channels by differentially expressed neurospecific Ca2+-binding proteins.
Footnotes
This work was supported by National Institutes of Health Grants R01 NS 22625 (W.A.C.), F32 NS 11099 (N.J.L.), and T32 GM07270 (A.P.F.). We thank Dr. Amy Lee (Emory University, Atlanta, GA) for cDNA constructs and for valuable discussions.
Correspondence should be addressed to William Catterall, Department of Pharmacology, University of Washington School of Medicine, 1959 Northeast Pacific Street, Room F427, Seattle, WA 98195. E-mail: wcatt@u.washington.edu.
N. J. Lautermilch's present address: MDS Pharma Services, 22011 30th Drive Southeast, Bothell, WA 98021-4444.
Copyright © 2005 Society for Neuroscience 0270-6474/05/257062-•$15.00/0
References
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M (1997) Molecular mechanics of calcium-myristoyl switches. Nature 389: 198-202. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Campbell KP (2003) Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298-307. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Baumann B, Danos P, Diekmann S, Bogerts B, Gundelfinger ED, Braunewell KH (1999) Regional and cellular distribution of neural visinin-like protein immunoreactivities (VILIP-1 and VILIP-3) in human brain. J Neurocytol 28: 655-662. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Becker A, Keilhoff G, Spilker C, Gorczyca WA, Braunewell KH, Grecksch G (2003) Brain region-specific changes in the expression of calcium sensor proteins after repeated applications of ketamine to rats. Neurosci Lett 339: 95-98. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Biddlecome GH, McDonough SI, Diaz ME, Zilinski CA, Bean BP, Campbell KP, Noebels JL (1999) Beta subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain. Mol Cell Neurosci 13: 293-311. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL (2001) The neuronal calcium sensor family of calcium-binding proteins. Biochem J 353: 1-12. [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM (1996) Identification of palmitoylation sites within the L-type calcium channel β2a subunit and effects on channel function. J Biol Chem 271: 26465-26468. [DOI] [PubMed] [Google Scholar]
- De Castro E, Nef S, Fiumelli H, Lenz SE, Kawamura S, Nef P (1995) Regulation of rhodopsin phosphorylation by a family of neuronal calcium sensors. Biochem Biophys Res Commun 216: 133-140. [DOI] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT (2001) Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411: 484-489. [DOI] [PubMed] [Google Scholar]
- De Waard M, Campbell KP (1995) Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. J Physiol (Lond) 485: 619-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge Jr FA, Rahamimoff R (1967) Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol (Lond) 193: 419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM (1988) Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science 241: 1661-1664. [DOI] [PubMed] [Google Scholar]
- Erickson MG, Liang H, Mori MX, Yue DT (2003) FRET two-hybrid mapping reveals function and location of L-type calcium channel CaM preassociation. Neuron 39: 97-107. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CL, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K (2000) Five members of a novel calcium-binding protein (CaBP) subfamily with similarity to calmodulin. J Biol Chem 275: 1247-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamashima H, Tamaru T, Noguchi H, Kobayashi M, Takamatsu K (2001) Immunochemical assessment of neural visinin-like calcium-binding protein 3 expression in rat brain. Neurosci Res 39: 133-143. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA (1996) Modulation of Ca2+ channels by G protein βγ subunits. Nature 380: 258-262. [DOI] [PubMed] [Google Scholar]
- Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, McIntosh JM, Cruz LJ, et al. (1992) A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron 9: 69-77. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Lacinová L, Klugbauer N (1999) Voltage-dependent calcium channels: from structure to function. Rev Physiol Biochem Pharmacol 139: 33-87. [DOI] [PubMed] [Google Scholar]
- Ikeda SR (1996) Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature 380: 255-258. [DOI] [PubMed] [Google Scholar]
- Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA (1999) Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 399: 155-159. [DOI] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA (2000) Ca2+-calmodulin dependent inactivation and facilitation of P/Q-type Ca2+ channels. J Neurosci 20: 6830-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA (2002) Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci 5: 210-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Zhou H, Scheuer T, Catterall WA (2003) Molecular determinants of calcium/calmodulin-dependent regulation of CaV2.1 channels. Proc Natl Acad Sci USA 100: 16059-16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz SE, Henschel Y, Zopf D, Voss B, Gundelfinger ED (1992) VILIP, a cognate protein of the retinal calcium binding proteins visinin and recoverin, is expressed in the developing chicken brain. Brain Res Mol Brain Res 15: 133-140. [DOI] [PubMed] [Google Scholar]
- Llinás R, Sugimori M, Lin JW, Cherksey B (1989) Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci USA 86: 1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F (1997) Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci 17: 1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM (1994) Block of calcium channels in rat central neurons by the spider toxin ω-Aga-IIIA. J Neurosci 14: 2844-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME (1992a) P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature 355: 827-829. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP (1992b) P-type calcium channels in rat central and peripheral neurons. Neuron 9: 85-95. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG (1995) Calcium control of transmitter release at a cerebellar synapse. Neuron 15: 675-688. [DOI] [PubMed] [Google Scholar]
- Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L (1994) The amino terminus of a calcium channel beta subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron 13: 1433-1438. [DOI] [PubMed] [Google Scholar]
- Pate P, Mochca-Morales J, Wu Y, Zhang JZ, Rodney GG, Serysheva II, Williams BY, Anderson ME, Hamilton SL (2000) Determinants for calmodulin binding on voltage-dependent Ca2+ channels. J Biol Chem 275: 39786-39792. [DOI] [PubMed] [Google Scholar]
- Paterlini M, Revilla V, Grant AL, Wisden W (2000) Expression of the neuronal calcium sensor protein family in the rat brain. Neuroscience 99: 205-216. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei X, Birnbaumer L (1992) Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem 267: 1792-1797. [PubMed] [Google Scholar]
- Randall A, Tsien RW (1995) Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci 15: 2995-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Mintz IM (1994) Participation of multiple calcium channel types in transmission at single climbing fiber to Purkinje cell synapses. Neuron 12: 605-613. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takamatsu K, Kobayashi M, Noguchi T (1994) Immunohistochemical localization of neural visin-like calcium binding protein-2. Neurosci Lett 171: 155-158. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Kobayashi M, Kuroki T, Noguchi T, Takamatsu K (1995) The development of neural visinin-like calcium-binding protein-2 immunoreactivity in the rat neocortex and hippocampus. Neurosci Res 23: 383-388. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Westenbroek RE, Rettig J, Hell J, Catterall WA (1996) Biochemical properties and subcellular distribution of the BI and rbA isoforms of α1A subunits of brain calcium channels. J Cell Biol 134: 511-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, De Blasi A (2000) Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta 1498: 112-121. [DOI] [PubMed] [Google Scholar]
- Spilker C, Dresbach T, Braunewell KH (2002) Reversible translocation and activity-dependent localization of the calcium-myristoyl switch protein VILIP-1 to different membrane compartments in living hippocampal neurons. J Neurosci 22: 7331-7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TVB, Prystay W, Snutch TP (1991) Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci USA 88: 5621-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP (1994) The localization and functional properties of a rat brain α1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci USA 91: 10576-10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A (1993) Different types of calcium channels mediate central synaptic transmission. Nature 366: 156-158. [DOI] [PubMed] [Google Scholar]
- Tanaka O, Sakagami H, Kondo H (1995) Localization of mRNAs of voltage-dependent Ca2+-channels: four subtypes of α1- and β-subunits in developing and mature rat brain. Mol Brain Res 30: 1-16. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TVB, Snutch TP, Catterall WA (1995) Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. J Neurosci 15: 6403-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW (1994) Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science 264: 107-111. [DOI] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B (1999) Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci 19: 726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64: 355-405. [DOI] [PubMed] [Google Scholar]