Abstract
Voltage-gated sodium channels (VGSCs) ensure the saltatory propagation of action potentials along axons by acting as signal amplifiers at the nodes of Ranvier. In the retina, activity mediated by VGSCs is important for the refinement of the retinotectal map. Here, we conducted a full-field electroretinogram (ERG) study on mice null for the sodium channel NaV1.6. Interestingly, the light-activated hyperpolarization of photoreceptor cells (the a-wave) and the major “downstream” components of the ERG, the b-wave and the oscillatory potentials, are markedly reduced and delayed in these mice. The functional deficit was not associated with any morphological abnormality. We demonstrate that Scn8a is expressed in the ganglion and inner nuclear layers and at low levels in the outer nuclear layer beginning shortly before the observed ERG deficit. Together, our data reveal a previously unappreciated role for VGSCs in the physiological maturation of photoreceptors.
Keywords: voltage-gated sodium channel, NaV1.6, Scn8a, retina, photoreceptors, electroretinogram, development
Introduction
By allowing sodium to enter excitable cells such as neurons and muscle in response to increases in membrane potential, voltage-gated sodium channels (VGSCs) are essential to the generation and propagation of action potentials. The NaV1.6 channel, encoded by the Scn8a gene (Catterall, 2000; Goldin et al., 2000), is one of the most abundant VGSCs in the brain. It is the primary sodium channel expressed at the nodes of Ranvier in the CNS and peripheral nervous system (Caldwell et al., 2000), thus highlighting the importance of this particular VGSC isoform in saltatory conduction. NaV1.6 is also present in presynaptic and postsynaptic membranes of the neocortex and cerebellum (Caldwell et al., 2000), suggesting that it may also play an important role in modulating synaptic plasticity. In the adult retina, major sites of NaV1.6 expression include the ganglion cell and inner nuclear layers (Fjell et al., 1997; Krzemien et al., 2000). In the optic nerve, NaV1.6 is concentrated specifically at the nodes of Ranvier of myelinated axons and is absent from the unmyelinated axons (Boiko et al., 2001).
Several strains of mice harbor mutations in Scn8a (Meisler et al., 2001). In the original motor endplate disease (med) mouse (Scn8amed) described previously by Duchen (1970), a null mutation in Scn8a results in reduced neuromuscular transmission leading to paralysis and lethality approximately at the time of weaning (Burgess et al., 1995). Nerve conduction velocity is reduced and the refractory period is prolonged in these animals (Angaut-Petit et al., 1982). Additionally, degenerating muscle (dmu)(Scn8admu), a recently identified sporadic mutant allelic to Scn8amed, displays cardiac and skeletal muscle degeneration (De Repentigny et al., 2001).
Here, we demonstrate that mice with null mutations in Scn8a display profound anomalies in a Ganzfeld flash electroretinogram (ERG), in both the a- and b-waves. We did not, however, detect abnormalities in retinal histology, ultrastructure, cellular contribution, or vasculature. Our demonstration that Scn8a transcript is expressed in the nuclear layers shortly before the elongation of the photoreceptor outer segments suggests that this channel directly influences the physiological maturation of photoreceptors.
Materials and Methods
Animals. The degenerating muscle allele arose spontaneously in our colony of (C57BL/6 × C3H) F1 hybrid mice and was mapped to the motor endplate disease locus (De Repentigny et al., 2001). To eliminate the possibility of interference from the rd1 (retinal degeneration 1) locus on the C3H genetic background, the mice were bred for three to four generations onto the DBA/2J or C57BL/6J backgrounds. We also obtained med mice from The Jackson Laboratory (C3HeB/FeJ-Scn8amed; stock number 3798; Bar Harbor, ME) and bred them onto the DBA/2J background for three generations.
The mice were housed under a 12 h light/dark cycle with ad libitum access to food and water. Unless indicated otherwise, the animals were killed by cervical dislocation at postnatal day 16 (P16). The mice were maintained according to the guidelines of the Canadian Council on Animal Care, and all procedures were approved by the University of Ottawa Animal Care Committee.
Genotyping assay for Scn8admu. The dmu mutation consists of a single nucleotide deletion in exon 10A of the Scn8a coding sequence (position 1538 of GenBank accession number NM_011323). This mutation results in the destruction of a BslI restriction site. A 215 bp fragment of Scn8a containing the mutation is PCR amplified from genomic DNA isolated from tail biopsies using the primers cgggcaccgtctcagaagat (forward) and tccttctcatgccatcttcc (reverse). The PCR product is purified using the MinElute kit (Qiagen, Valencia, CA) and incubated with BslI. Only the wild-type sequences will be digested with BslI.
In situ hybridization. Murine Scn8a digoxigenin (DIG)-labeled “sense” and “antisense” RNA probes were produced. Two different templates were used, one using an NcoI fragment corresponding to position 1233-2045 of the Scn8a cDNA sequence according to GenBank accession number NM_323011, and another using a PstI-BamHI fragment corresponding to position 2988-3459 of the same sequence. The antisense probe to murine patched was described previously (Jensen and Wallace, 1997). In situ hybridization was performed as described previously by Jensen and Wallace (1997). All probes were used at a 1:1000 dilution in hybridization buffer. After in situ hybridization and incubation with anti-DIG fab fragments (Roche Diagnostics, Indianapolis, IN), specimens were incubated overnight in staining buffer containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Roche Diagnostics).
Histology and morphometry. Retinas were dissected and placed in fixative (4% paraformaldehyde in PBS) overnight, incubated in cryoprotectant (30% sucrose in PBS), frozen in liquid nitrogen-cooled isopentane in a mixture of OCT compound (Sakura, Torrance, CA) and sucrose (15%), and sectioned in a cryostat in the plane of the optic nerve. Sections were stained with hematoxylin and eosin, and only sections in which the optic nerve was visible were used for quantification. Using NIH Image software, measurements were taken in wild-type (n = 3) and dmu (n = 3) retinas at random locations within a distance of >50 μm but <200 μm from the optic nerve stalk by a person unaware of the nature of the samples.
Immunohistochemistry. Eyes were dissected in PBS and processed as follows: after 30 min of fixation (4% paraformaldehyde and 0.1 m phosphate buffer, pH 7.4), the lens was removed through an opening in the anterior chamber, and the eye was fixed for an additional 30 min and then incubated for 8 h in a cryoprotective compound (30% sucrose in 0.1 m phosphate buffer, pH 7.4). After a 30 min incubation at 4°C in a matrix (50% OCT, 15% sucrose, and 50 mm phosphate buffer, pH 7.4), the eyes were frozen in the matrix with liquid nitrogen-cooled isopentane, sectioned at a thickness of 10 μm using a cryostat, dried at room temperature, and stored at -80°C. Sciatic nerves isolated from P15 dmu homozygotes and wild-type littermates were processed as described previously (Caldwell et al., 2000). The following primary antibodies were used in this study: mouse monoclonal anti-caspr (contactin-associated protein) (clone 275) (Poliak et al., 1999), “pan-specific” antiserum directed against an epitope present in all vertebrate NaV1 isoforms (Dugandzija-Novakovic et al., 1995), mouse monoclonal anti-rhodopsin (clone B630) (Rohlich et al., 1989), Chx10 antiserum (gift from Dr. R. Bremner, University of Toronto, Toronto, Ontario, Canada) (Burmeister et al., 1996), CRALBP (cellular retinaldehyde-binding protein) antiserum (gift from J. Saari, University of Washington, Seattle, WA) (De Leeuw et al., 1990), mouse monoclonal HPC-1 against syntaxin 1 (Sigma, St. Louis, MO) (Barnstable et al., 1985), and Brn3b antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) (Xiang et al., 1993); the FITC-labeled lectin peanut agglutinin (Sigma) was used for the detection of cone photoreceptors. For the detection of NaV1.6, antibody 848 was used in sciatic nerves (Caldwell et al., 2000). 4′,6-Diamidino-2-phenylindole dihydrochloride (1 μg/ml; Molecular Probes, Eugene, OR) was used on occasion as a chromatin stain. The primary antibodies were detected using cyanine 3, Alexa 488, Alexa 546, or FITC-conjugated secondary antibodies (Molecular Probes) or a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) in conjunction with the ABC Vectastain Elite peroxidase system (Vector Laboratories) in accordance with the instructions of the manufacturer. For imaging of the vasculature, retinas were dissected as whole mounts, flattened, and stained with FITC-labeled Griffonia simplicifolia lectin (Sigma), as described previously (Zhang and Stone, 1997). Photomicrographs were captured using an Axioplan2 microscope (Zeiss, Thornwood, NY) equipped with an Axiocam digital camera (Zeiss).
Electroretinography. Mice were dark adapted for a minimum of 1 h before ERG recordings. The animals were anesthetized using avertin (2,2,2-tribromoethanol; 275 mg/kg, i.p.), and a topical analgesic (0.5% proparacaine hydrochloride) was applied to the eye before the positioning of the electrode. Body temperature was maintained at 37°C using a heated pad, and pupils were dilated using a 1% tropicamide solution. Both eyes were positioned within the lambertian sphere range of a ColorBurst handheld Ganzfeld stimulator (Diagnosys, Littleton, MA). The reference electrode consisted of a gold-plated connector placed in the mouth. A silver needle was placed subcutaneously in the tail to ground the animal, and the active electrodes placed on the corneas were constructed from Dawson-Trick-Litzkow-plus microconductive fiber (Diagnosys). A 2.5% hydroxypropyl methylcellulose solution was applied to maintain hydration and to ensure proper conductance. Stimulation and recording were automated using the Espion software suite version 2.37.37 (Diagnosys). Flashes of white light were presented for 4 ms over a black background at increasing intensities of -3.0, -2.6, -2.2, -1.8, -1.4, -1.0, -0.6, -0.2, 0.6, 1.0, and 1.4 log cd·s/m2. Between four and six responses were averaged with an interflash interval of 5 s for stimulus intensities below -1.0 log cd·s/m2 and increased to 10 s for intensities of -1.0 log cd·s/m2 and above. The marker for the a-wave was placed on the second peak of the trace when more than one peak was apparent, and the marker for the b-wave was placed on the third oscillatory potential. In each animal, the eye that produced the strongest a-wave response at 1.4 log cd·s/m2 was chosen for subsequent analysis. Data were processed using Microsoft (Redmond, WA) Excel.
Electron microscopy. P16 wild-type and homozygous dmu mice were anesthetized by intraperitoneal injection of avertin. The mice were transcardially perfused with 10 ml of PBS followed by 20 ml of Karnovsky's fixative (4% paraformaldehyde, 2% glutaraldehyde, and 0.1 m cacodylate in PBS, pH 7.4). The eyes were dissected in PBS, the lens was removed, and the eyes were incubated in Karnovsky's fixative for 4 h. The eyes were hemisected, and “pie slices” were cut around the optic nerve. The samples were washed in cacodylate buffer, treated with 2% osmium tetroxide, dehydrated, and embedded in Spurr resin (Electron Microscopy Sciences, Hatfield, PA). Sections (60 nm) were collected onto grids, pretreated with 2% aqueous uranyl acetate, stained with lead citrate, and observed using a model 1010 transmission electron microscope (Jeol, Peabody, MA).
Results
NaV1.6-null mice have a strongly reduced sensitivity to light
We conducted a full-field flash ERG study on two spontaneous mutant alleles of Scn8a. Neither the Scn8admu (De Repentigny et al., 2001) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) nor the Scn8amed (Burgess et al., 1995) alleles express functional NaV1.6. Various components of the ERG, the retinal field potential elicited by light stimulation, provide information on the activity of distinct cell populations within the retina. In dark-adapted animals, the a-wave, the early negative potential, reflects rod photoreceptor activity (Penn and Hagins, 1969), whereas the later positive b-wave originates from the associated activity of depolarizing (ON) bipolar cells (Stockton and Slaughter, 1989; Green and Kapousta-Bruneau, 1999) and Müller cells (Miller and Dowling, 1970). At P16, the amplitude of the a-wave in Scn8admu mice is reduced at flash intensities ranging from 0.6 to 1.4 log cd·s/m2, and the b-wave is reduced throughout compared with littermate controls (Fig. 1a). Furthermore, the latency times (from flash onset to peak) for both the a- and b-waves are significantly increased at intensities above -1.0 log cd·s/m2 (Fig. 1b). Synaptic transmission occurs in the retina of Scn8admu mice, because oscillatory potentials can occasionally be observed (Fig. 1a and supplemental Fig. 2a, available at www.jneurosci.org as supplemental material), and, after stimulation with bright flashes, Scn8admu retinas express the intermediate early gene c-fos in bipolar cells and in ganglion cells (data not shown). Similar ERG results were obtained in Scn8admu mice bred onto the C57BL/6 genetic background and in the Scn8amed mice (supplemental Fig. 2a,b, available at www.jneurosci.org as supplemental material). Interestingly, however, of 15 Scn8a-null mice tested by ERG, we observed in one instance (an Scn8amed mouse) the presence of an a-wave and a b-wave of intermediate amplitude and latency time relative to controls and affected individuals (supplemental Fig. 2c, available at www.jneurosci.org as supplemental material), raising the possibility that compensation against the effect of NaV1.6 depletion can occur. The ERG response from P16 Scn8admu mice is very similar to that of P12 wild-type animals (Fig. 1) and suggests that NaV1.6 is required during a critical period of retinal development.
Figure 1.
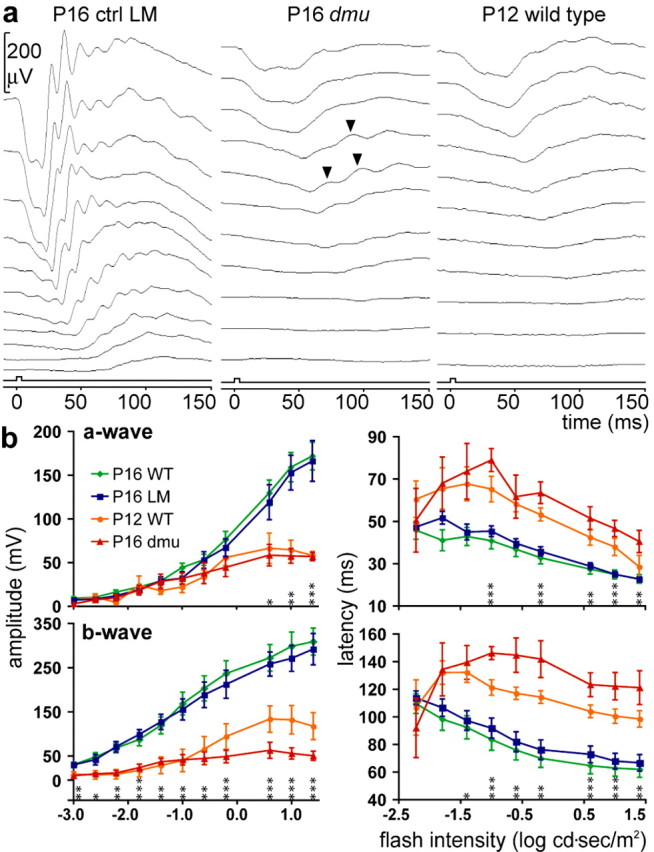
Scn8a-null mice have a strongly reduced sensitivity to light. a, Representative ERG response in a P16 control littermate (ctrl LM) of Scn8admu mice bred onto the DBA/2J background (left), a P16 Scn8admu homozygous mouse (dmu) bred onto the DBA/2J background (middle), and a P12 wild-type DBA/2J mouse (right). The ERGs were elicited by flashes of white light of increasing intensities ranging from -3.0 (bottom) to 1.4 (top) log cd·s/m2 (square box indicates flash onset and duration). Although ERG components, such as, from left to right, a pronounced a-wave, oscillatory potentials, and a b-wave, can be clearly distinguished in the P16 wild-type mouse, these components are strongly reduced and delayed in Scn8admu mice. Oscillatory potentials can occasionally be seen, indicating that synaptic transmission can occur (arrowheads). The amplitude and general appearance of the Scn8admu ERG is similar to that of the immature P12 wild-type ERG. b, Quantitative analysis of the amplitudes and latencies of the a-wave (absolute values) and b-wave relative to the flash onset from P16 wild-type DBA/2J mice (P16 WT; n = 18), normal littermates of Scn8admu mice (P16 LM; n = 6), P12 wild-type DBA/2Jmice (P12WT; n=3), and P16 Scn8admu mice (P16dmu; n = 6). Error bars represent ± SEM. P16 Scn8admu mice are compared with their littermates using an unpaired Student's t test, and significance is assigned as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
Scn8a expression in the retina begins shortly before the time of the observed ERG deficit
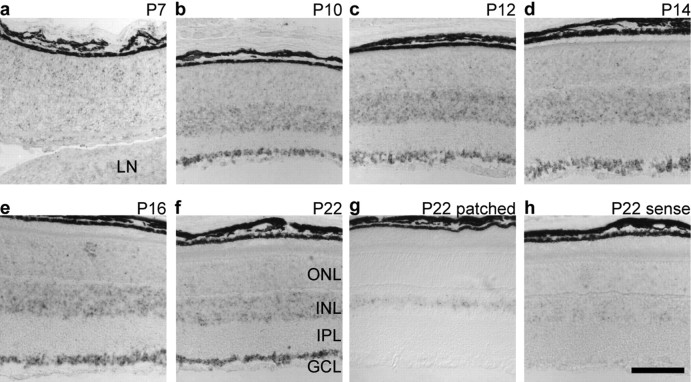
Although no difference was detected between antisense and sense Scn8a probes at stages ranging from P0 (data not shown) to P7 (Fig. 2a), in situ hybridization of retinas isolated at various postnatal time points thereafter produced a signal above background, with the earliest being at P10 (Fig. 2b). From P10 to P22, staining for Scn8a transcripts was particularly strong in the ganglion cell and inner nuclear layers (Fig. 2b-f). Comparison of the Scn8a staining with the pattern of patched expression (Fig. 2g), which is restricted to the narrow band of Müller glia nuclei, indicates that all cell types residing in the inner nuclear layer, including amacrine, bipolar, horizontal, and Müller cells, are candidates for Scn8a expression. Furthermore, it is noteworthy that, in the outer nuclear layer, the staining for Scn8a was slightly but consistently above background levels. These results were confirmed using a different probe to Scn8a (data not shown) and are consistent with those of Fjell et al. (1997).
Figure 2.
Developmental time course of Scn8a expression in the retina. a-f, In situ hybridization of wild-type retinas during postnatal development with a digoxigenin-labeled Scn8a antisense probe reveals Scn8a expression in the ganglion cell and inner nuclear layers that begins between P7 and P10. The broad expression of Scn8a in the inner nuclear layer is corroborated by comparing with patched (g), a marker for Müller cell nuclei, which displays a more restricted expression pattern. h, Scn8a sense control. LN, Lens; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 100 μm.
The functional deficit does not correlate with morphological abnormalities
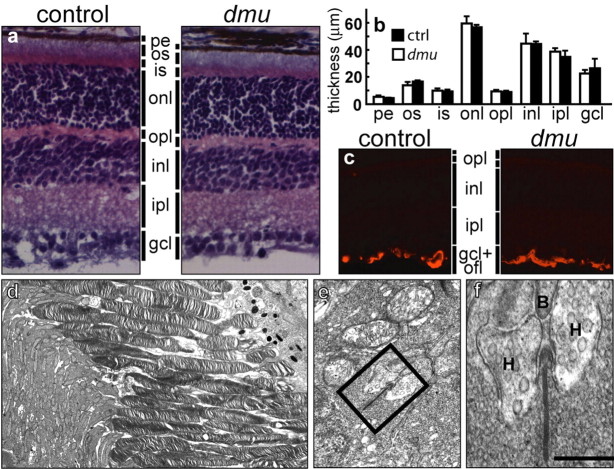
To determine whether the observed electrophysiological deficits were attributable to a developmental defect or to retinal degeneration, we initially compared the retinal morphology of P16 Scn8admu mice with littermate controls. No differences were detected using a number of criteria (Fig. 3a,b). In addition, ultrastructural analysis indicated that the outer segments of Scn8admu photoreceptors have a normal appearance with the presence of ordered stacks of disk membranes (Fig. 3d). In addition, synaptic ribbons are present at the junction of bipolar and horizontal cell processes within rod spherules, indicating that Scn8admu photoreceptors form structurally normal synapses with second-order neurons (Fig. 3e,f). Markers for retinal cell types indicated the normal presence of rods, cones, bipolar cells, horizontal cells, amacrine cells, and Müller glia (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
Figure 3.
Absence of NaV1.6 does not affect retinal morphology. a, Hematoxylin and eosin staining of P16 control and P16 Scn8admu (dmu) retinas does not reveal overt abnormalities. b, Quantification of the layer thickness. Error bars represent SEM. c, In Scn8admu mice, GFAP is restricted to the end feet of Müller cells, indicating the absence of reactive gliosis. d-f, A survey of the Scn8admu photoreceptor ultrastructure at P16 shows normal outer and inner segments with no signs of degeneration (d). e, f, In addition, in the outer plexiform layer, bipolar (B) and horizontal (H) dendritic processes invaginate rod spherules to form synaptic clefts that have the normal triad appearance with the electron-dense ribbon located between the horizontal cells. Scale bar: (in f) a, c, 60 μm; d, 3 μm; e, 0.5 μm; f, 0.2 μm. pe, Pigment epithelium; os, outer segment; is, inner segment; onl, outer nuclear layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer; ofl, optic fiber layer.
Null mutants for Scn8a have a reduced lifespan (∼20 d) and at P16 have reduced motility and generalized muscle atrophy (De Repentigny et al., 2001). This raises the possibility that the observed loss of function is caused by a systemic factor (such as hypoxia attributable to labored breathing for example). The retinal vasculature and Müller cells are exquisitely sensitive to oxygen and nutrient deprivation (Stone et al., 1999). Therefore, to address the possibility that the reduced ERG is attributable to an abnormal oxygen or nutrient supply, we stained Müller cells for glial fibrillary acidic protein (GFAP) and the vasculature with a chromophore-conjugated lectin. GFAP staining remained restricted to the end feet of the Müller cells (Fig. 3c), indicating the absence of reactive gliosis. Furthermore, the density and caliber of the capillaries in Scn8admu retinas were equivalent to those of control animals (supplemental Fig. 4, available at www.jneurosci.org as supplemental material), excluding hypoxia as a causative agent for the retinal defect.
Discussion
Acute blockade of VGSCs using pharmacological agents has revealed that they are dispensable in the mature retina for the generation of the a-wave (Dong and Hare, 2000; Bui and Fortune, 2003). Furthermore, although action potentials have been reported in mature photoreceptors from human retinal explants (Kawai et al., 2001), the blockade of VGSCs does not affect the photocurrent, and the function of the detected action potentials is unclear. This, in conjunction with the similarity of the P16 Scn8a-null ERG to the P12 wild-type ERG, favors a developmental function for NaV1.6.
Mode of action of NaV1.6 influence on photoreceptor cell maturation
The function that the NaV1.6 sodium channel may have in retinal development is unknown, but its expression in the outer and inner nuclear layers and in ganglion cells in combination with our ERG assessment of Scn8a-null mice suggests the existence of an activity-mediated signaling mechanism necessary for normal photoreceptor maturation.
The observations we made could result from a paracrine effect mediated by neuromodulators or neurotrophins. For example, the absence of NaV1.6 may reduce evoked and/or spontaneous activity (Raman et al., 1997; Chen et al., 1999) in retinal ganglion cells, in which this channel is strongly expressed (Fig. 2) (Fjell et al., 1997; Krzemien et al., 2000). In turn, stimulus-induced and spontaneous activity in retinal ganglion cells has been shown to be necessary for the normal expression (Karlsson and Hallbrook, 1998) and trafficking (Chytrova and Johnson, 2004) of brain-derived neurotrophic factor (BDNF), respectively.
A recent study on utricular hair cells, another type of sensory receptor thought to be devoid of VGSCs, has shown that VGSC-mediated activity is responsible for BDNF release from the hair cells but only during the early postnatal period (Chabbert et al., 2003). In turn, BDNF and its specific receptor TrkB (tyrosine receptor kinase B) (Rohrer et al., 1999) are required for the normal maturation of photoreceptors and of the rod pathway. Therefore, the detection of low levels of Scn8a expression in the nuclei of photoreceptors in the outer nuclear layer (Fig. 2b-f) raises another possibility. Activity mediated by NaV1.6 may be required in photoreceptors during a restricted period of development for its normal maturation via the action of a neurotrophin, possibly BDNF.
In summary, mice with null mutations in Scn8a produce an abnormal ERG with reduced and delayed components (a-wave, b-wave, and oscillatory potentials). The absence of obvious morphological abnormalities in the retina leads us to conclude that the physiological defect does not result from degeneration, abnormal cell migration, or oxygen or nutrient deprivation. Because previous studies have shown that TTX-sensitive VGSCs, such as NaV1.6, are not required for mature photoreceptors to function properly, this work is, to our knowledge, the first demonstration that a VGSC is required in the retina for normal photoreceptor maturation.
Footnotes
This work was supported by the Muscular Dystrophy Association and the Human Frontier Science Program. P.D.C. is a fellow of the Canadian Institutes of Health Research. We are grateful to D. Petrin for technical assistance. We also thank V. Wallace for use of equipment and reagents and for helpful advice during the course of this work (others who have contributed reagents are acknowledged in Materials and Methods).
Correspondence should be addressed to Rashmi Kothary, Ottawa Health Research Institute, 501 Smyth Road, Ottawa, Ontario, Canada K1H 8L6. E-mail: rkothary@ohri.ca.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255046-05$15.00/0
References
- Angaut-Petit D, McArdle JJ, Mallart A, Bournaud R, Pinçon-Raymond M, Rieger F (1982) Electrophysiological and morphological studies of a motor nerve in `motor endplate disease' of the mouse. Proc R Soc Lond B Biol Sci 215: 117-125. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K (1985) A marker of early amacrine cell development in rat retina. Brain Res 352: 286-290. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G (2001) Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30: 91-104. [DOI] [PubMed] [Google Scholar]
- Bui B, Fortune B (2003) Ganglion cell contributions to the rat full-field electroretinogram. J Physiol (Lond) 555: 153-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH (1995) Mutation of a new sodium channel gene, Scn8a, in the mouse mutant “motor endplate disease.” Nat Genet 10: 461-465. [DOI] [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR (1996) Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet 12: 376-384. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR (2000) Sodium channel Na(v)1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci USA 97: 5616-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13-25. [DOI] [PubMed] [Google Scholar]
- Chabbert C, Mechaly I, Sieso V, Giraud P, Brugeaud A, Lehouelleur J, Couraud F, Valmier J, Sans A (2003) Voltage-gated Na+ channel activation induces both action potentials in utricular hair cells and brain-derived neurotrophic factor release in the rat utricle during a restricted period of development. J Physiol (Lond) 553: 113-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Sprunger LK, Meisler MH, Waller HJ, Godfrey DA (1999) Reduced spontaneous activity in the dorsal cochlear nucleus of Scn8a mutant mice. Brain Res 847: 85-89. [DOI] [PubMed] [Google Scholar]
- Chytrova G, Johnson JE (2004) Spontaneous retinal activity modulates BDNF trafficking in the developing chick visual system. Mol Cell Neurosci 25: 549-557. [DOI] [PubMed] [Google Scholar]
- De Leeuw AM, Gaur VP, Saari JC, Milam AH (1990) Immunolocalization of cellular retinol-, retinaldehyde- and retinoic acid-binding proteins in rat retina during pre- and postnatal development. J Neurocytol 19: 253-264. [DOI] [PubMed] [Google Scholar]
- De Repentigny Y, Côté PD, Pool M, Bernier G, Girard S, Vidal SM, Kothary R (2001) Pathological and genetic analysis of the degenerating muscle (dmu) mouse: a new allele of Scn8a. Hum Mol Genet 10: 1819-1827. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Hare WA (2000) Contribution to the kinetics and amplitude of the electroretinogram b-wave by third-order retinal neurons in the rabbit retina. Vision Res 40: 579-589. [DOI] [PubMed] [Google Scholar]
- Duchen LW (1970) Hereditary motor end-plate disease in the mouse: light and electron microscopic studies. J Neurol Neurosurg Psychiatry 33: 238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P (1995) Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J Neurosci 15: 492-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell J, Dib-Hajj S, Fried K, Black JA, Waxman SG (1997) Differential expression of sodium channel genes in retinal ganglion cells. Brain Res Mol Brain Res 50: 197-204. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA (2000) Nomenclature of voltage-gated sodium channels. Neuron 28: 365-368. [DOI] [PubMed] [Google Scholar]
- Green DG, Kapousta-Bruneau NV (1999) A dissection of the electroretinogram from the isolated rat retina with microelectrodes and drugs. Vis Neurosci 16: 727-741. [DOI] [PubMed] [Google Scholar]
- Jensen AM, Wallace VA (1997) Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development 124: 363-371. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Hallbrook F (1998) Kainic acid, tetrodotoxin and light modulate expression of brain-derived neurotrophic factor in developing avian retinal ganglion cells and their tectal target. Neuroscience 83: 137-150. [DOI] [PubMed] [Google Scholar]
- Kawai F, Horiguchi M, Suzuki H, Miyachi E (2001) Na+ action potentials in human photoreceptors. Neuron 30: 451-458. [DOI] [PubMed] [Google Scholar]
- Krzemien DM, Schaller KL, Levinson SR, Caldwell JH (2000) Immunolocalization of sodium channel isoform NaCh6 in the nervous system. J Comp Neurol 420: 70-83. [PubMed] [Google Scholar]
- Meisler MH, Kearney J, Escayg A, MacDonald BT, Sprunger LK (2001) Sodium channels and neurological disease: insights from Scn8a mutations in the mouse. The Neuroscientist 7: 136-145. [DOI] [PubMed] [Google Scholar]
- Miller RF, Dowling JE (1970) Intracellular responses of the Muller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol 33: 323-341. [DOI] [PubMed] [Google Scholar]
- Penn RD, Hagins WA (1969) Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 223: 201-204. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E (1999) Caspr2, a new member of the neurexin super-family, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 24: 1037-1047. [DOI] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP (1997) Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19: 881-891. [DOI] [PubMed] [Google Scholar]
- Rohlich P, Adamus G, McDowell JH, Hargrave PA (1989) Binding pattern of anti-rhodopsin monoclonal antibodies to photoreceptor cells: an immunocytochemical study. Exp Eye Res 49: 999-1013. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B (1999) Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci 19: 8919-8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM (1989) B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol 93: 101-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Peer J (1999) Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res 18: 689-735. [DOI] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J (1993) Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron 11: 689-701. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stone J (1997) Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci 38: 1653-1666. [PubMed] [Google Scholar]