Abstract
Protein kinase Mζ (PKMζ) is a persistently active protein kinase C isoform that is synthesized during long-term potentiation (LTP) and is critical for maintaining LTP. According to “synaptic tagging,” newly synthesized, functionally important plasticity-related proteins (PRPs) may prolong potentiation not only at strongly tetanized pathways, but also at independent, weakly tetanized pathways if synaptic tags are set. We therefore investigated whether PKMζ is involved in tagging and contributes to a sustained potentiation by providing strong and weak tetanization to two independent pathways and then disrupting the function of the kinase by a selective myristoylated ζ-pseudosubstrate inhibitory peptide. We found that persistent PKMζ activity maintains potentiated responses, not only of the strongly tetanized pathway, but also of the weakly tetanized pathway. In contrast, an independent, nontetanized pathway was unaffected by the inhibitor, indicating that the function of PKMζ was specific to the tagged synapses. To further delineate the specificity of the function of PKMζ in synaptic tagging, we examined synaptic “cross-tagging,” in which late LTP in one input can transform early into late long-term depression (LTD) in a separate input or, alternatively, late LTD in one input can transform early into late LTP in a second input, provided that the tags of the weak inputs are set. Although the PKMζ inhibitor reversed late LTP, it did not prevent the persistent depression at the weakly stimulated, cross-tagged LTD input. Conversely, although the agent did not reverse late LTD, it blocked the persistent potentiation of weakly tetanized, cross-tagged synapses. Thus, PKMζ is the first LTP-specific PRP and is critical for the transformation of early into late LTP during both synaptic tagging and cross-tagging.
Keywords: hippocampus, learning, memory formation, synaptic tagging, synaptic cross-tagging, long-term potentiation, long-term depression, late LTP, late LTD, protein kinase Mζ
Introduction
Long-term potentiation (LTP) and long-term depression (LTD) are considered physiological models of learning and memory (Matthies, 1989; Bliss and Collingridge, 1993; Malenka, 1994). Both forms of synaptic plasticity are characterized by early and late phases, the latter dependent on protein translation (Krug et al., 1984; Frey et al., 1988, 1996; Otani et al., 1989; Nguyen and Kandel, 1996; Manahan-Vaughan et al., 2000). How newly synthesized proteins interact with specific activated synapses expressing LTP or LTD, but not with inactive synapses, is fundamental to the synapse specificity thought critical for memory. One hypothesis to explain this specificity is “synaptic tagging,” in which newly synthesized plasticity-related proteins (PRPs) interact with recently potentiated, “tagged” synapses (Frey and Morris, 1997, 1998). This hypothesis explains how a strong tetanus, producing protein synthesis-dependent LTP (late LTP) in one pathway, prolongs the potentiation in an independent, weakly tetanized pathway that would normally have produced only early LTP. Recently, the synaptic tagging model has expanded to include interactions between LTP and LTD, referred to as “cross-tagging” (Sajikumar and Frey, 2004). In cross-tagging, a late LTP/LTD in one synaptic input transforms the opposite protein synthesis-independent early LTD/LTP in an independent input into its long-lasting form (Sajikumar and Frey, 2004). Cross-tagging not only expands the repertoire of interactions between pathways but raises the fundamental question of whether the function of a newly synthesized PRP is to generally prolong the action of a weak stimulus, or whether there are separate PRPs to specifically prolong potentiation or depression.
Elucidating the molecular mechanisms that underlie synaptic tagging has been hampered by our lack of knowledge of specific molecules involved. Many signal transduction molecules have been implicated in the induction of LTP (e.g., protein kinases) or LTD (phosphatases) (Malenka, 1994; Malenka and Bear, 2004). Synaptic tagging, however, is likely to involve the acquisition by a synapse of the molecular mechanisms of maintenance, which are less well characterized than those of induction.
Protein kinase C (PKC) has been implicated in LTP maintenance (Lovinger et al., 1987; Malinow et al., 1988; Reymann et al., 1988). Specifically, an unusual, autonomously active isoform of PKC, called protein kinase Mζ (PKMζ), is both necessary and sufficient for maintaining LTP (Sacktor et al., 1993; Ling et al., 2002). PKC consists of an N-terminal regulatory domain, containing an autoinhibitory pseudosubstrate sequence and second-messenger binding sites, and a C-terminal catalytic domain (Nishizuka, 1995). PKC is normally held in an inactive basal state by interactions between these two domains. Second messengers activate PKC by binding to the regulatory domain and causing a conformational change that temporarily releases the autoinhibition.
PKM, in contrast, consists of an independent PKC catalytic domain, which, lacking the autoinhibitory regulatory domain of PKC, is autonomously active (Schwartz, 1993). In brain, only a single isozyme, the atypical ζ, forms a stable PKM (Sacktor et al., 1993). In LTP, PKMζ increases by new protein synthesis through increased translation from a PKMζ mRNA, producing the independent ζ catalytic domain (Hernandez et al., 2003). The persistent activity of PKMζ sustains the enhancement of AMPA receptor (AMPA-R) responses for hours, thus maintaining LTP (Ling et al., 2002; Serrano et al., 2005).
Several groups have speculated that PKMζ could participate in synaptic tagging (Drier et al., 2002, Martin and Kosik, 2002; Hegde, 2004). We hypothesize that PKMζ is likely to function as a PRP because it demonstrates three critical properties: (1) PKMζ is newly synthesized by strong, but not weak, tetanization (Osten et al., 1996), (2) postsynaptic whole-cell perfusion of the kinase is sufficient to enhance AMPA-R-mediated synaptic transmission (Ling et al., 2002), and (3) inhibition of the activity of the kinase reverses late LTP (Serrano et al., 2005). Here, we studied the function of PKMζ during synaptic tagging. We found that PKMζ plays a critical role in both synaptic tagging and cross-tagging and that the kinase represents an LTP-specific PRP.
Materials and Methods
We used 130 transverse hippocampal slices (400 μm), prepared from 130 male Wistar rats (7 weeks of age), as described previously (Frey and Morris, 1997, 1998; Sajikumar and Frey, 2003, 2004). The animals were killed rapidly by a single blow to the back of the neck using a metallic rod (cervical dislocation) and then decapitated (the entire procedure took ∼3-5 min). Slices were incubated in an interface chamber at 32°C; incubation medium [artificial CSF (ACSF)] contained the following (in mm): 124 NaCl, 4.9 KCl, 1.2 KH2PO4, 2.0 MgSO4, 2.0 CaCl2, 24.6 NaHCO3, 10 d-glucose; carbogen consumption, 18 L/h; flow rate of ACSF, 0.74 ml/min. In all experiments, two monopolar lacquer-coated, stainless-steel electrodes (5 MΩ; A-M Systems, Carlsborg, WA) were positioned within the stratum radiatum of the CA1 region for stimulating two separate independent synaptic inputs, S1 and S2 (see Fig. 1a). For recording the field EPSP (measured as its slope function) and the population spike amplitude (PS), two electrodes (5 MΩ; A-M Systems) were placed in the CA1 dendritic and cell body layer, respectively, of a single neuronal population, and signals were amplified by a custom-made amplifier. The signals were digitized using a Cambridge Electronic Design (Cambridge, UK) 1401 analog-to-digital converter and analyzed with custom-made software (PWIN; Leibniz Institute for Neurobiology). Slices were preincubated for at least 4 h, which is critical for reliable long time recordings of late LTP and late LTD (for more details, see Sajikumar and Frey, 2004).
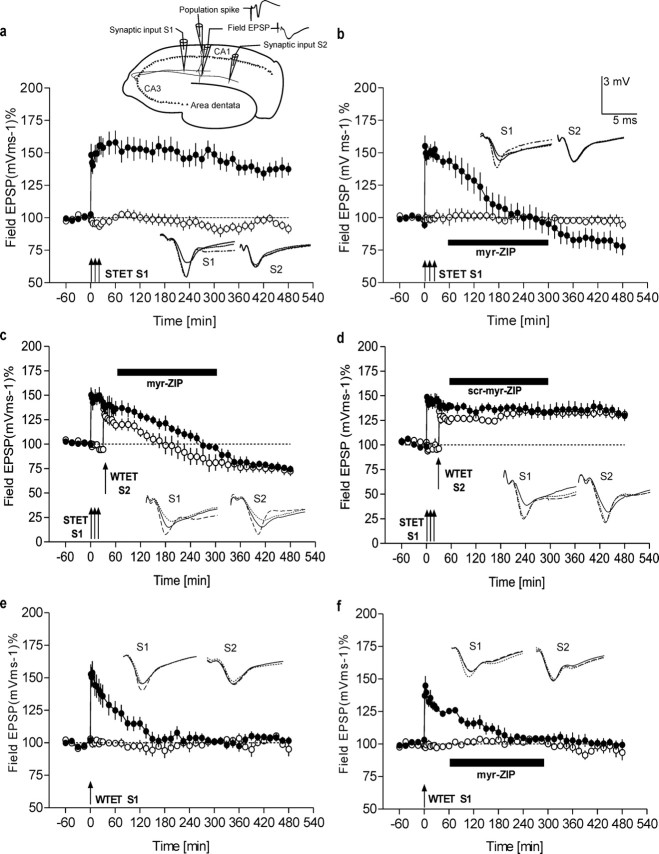
Figure 1.
Effects of PKMζ inhibition on LTP maintenance and synaptic tagging. a, Late LTP. The top scheme represents a transverse hippocampal slice showing the positioning of the electrodes. The two independent synaptic inputs, S1 and S2, to the same neuronal population and the recording sites for the population spike amplitude and the field EPSP, as well as analog recording traces as representative examples, are shown. The graph illustrates that the induction of late LTP in S1 (STET; filled circles) using high-frequency stimulation (HFS) resulted in late LTP that was significantly different for 8 h, compared with the control input S2 (open circles; n = 8; p < 0.05; U test). Control stimulation of S2 showed relatively stable potentials for the time course investigated. b, Inhibition of PKMζ reverses LTP maintenance. Induction of late LTP (n = 7) by applying HFS to S1 (filled circles) and application of the myristoylated ζ-pseudosubstrate peptide inhibitor (1 μm; black bar) 1 h after its induction reversed the late phase of LTP, whereas the potentials of the control path way S2 remained stable throughout the recording period of 8 h (open circles). c, Inhibition of PKMζ reverses late LTP maintenance at tagged synapses. Induction of late LTP (filled circles) in S1, followed by the induction of early LTP in S2 (open circles) by WTET and subsequent application of myr-ZIP 30 min after WTET to S2 (1 μm; black bar; n = 7), is shown. LTP in S1 reversed to baseline 210 min after STET, and the potentiation in S2 reached pretetanization baseline values 120 min after WTET (n = 7; p < 0.05; Wilcoxon test). d, scr-myr-ZIP had no effect on synaptic tagging (1 μm). Late LTP in S1 (filled circles) remained stable over 8.5 h, and synaptic tagging was observed in S2 (open circles). The transformation of early into late LTP in S2 by the previous induction of late LTP in S1 was maintained for 8 h, compared with pretetanization levels (n = 4). e, f, Early LTP. Induction of early LTP by a weak tetanus in S1 (filled circles) revealed a transient potentiation with a duration of 135 min (compared with control input S2; open circles in e; n = 5; p < 0.05; U test). Inhibition of PKMζ after induction of early LTP (f; n = 5) revealed a time course in both inputs similar to that observed in the control series without drug treatment [early LTP was maintained for 135 min in S1 (filled circles) when compared with control input S2 (open circles); p < 0.05; U test]. Analog traces represent typical field EPSPs 30 min before (solid line), 30 min after (hatched line), and 8 h after (dotted line) tetanization of input S1 or, in cases in which S2 was also tetanized, before or after the tetanization of that input. Arrows indicate the time point of tetanization. Bars represent the period of drug application. Error bars represent means ± SEM.
After the preincubation period, the test stimulation strength was determined for each input to elicit a population spike of 40% of its maximal amplitude for all control and LTD-inducing inputs and 25% for LTP-inducing inputs. For stimulation, biphasic constant-current pulses were used. Late LTD was induced using a strong, low-frequency stimulation protocol (SLFS) of 900 bursts [one burst consisted of three stimuli at 20 Hz; interburst interval, 1 s (i.e., f = 1 Hz); stimulus duration, 0.2 ms per half-wave; total number of stimuli, 2700]. This stimulation pattern produced a stable late LTD in vitro for at least 8 h (Sajikumar and Frey, 2003, 2004). In experiments in which a weaker induction of LTD was investigated, a transient early LTD was induced using a weak low-frequency stimulation protocol (WLFS) consisting of 900 pulses at a frequency of 1 Hz, impulse duration of 0.2 ms per half-wave, with a total number of stimuli of 900. Late LTP was induced using three stimulus trains of 100 pulses [strong tetanus (STET), 100 Hz; duration, 0.2 ms per polarity with 10 min intertrain intervals]. Early LTP was induced using a weak tetanization protocol (WTET) consisting of one 100 Hz train (21 biphasic constant-current pulses; 0.2 ms pulse duration per half-wave; stimulus intensity for tetanization, PS threshold). The population spike amplitude and the slope of the field EPSP were monitored on-line. For clarity, only the field EPSP data are shown, because the two recorded parameters showed similar time courses in the experiments, except in cases in which the population spike was abolished during LTD. The baseline was recorded for at least 60 min before LTP/LTD induction; four 0.2 Hz biphasic, constant-current pulses (0.1 ms per polarity) were used for baseline recording and testing 1, 3, 5, 11, 15, 21, 25, and 30 min after tetanus or 21, 25, and 30 min after LFS and thereafter once every 15 min up to 8 h.
The myristoylated ζ-pseudosubstrate peptide (myr-ZIP; myr-SIYRRGARRWRKL-OH; Biosource, Camarillo, CA) and the scrambled control peptide (scr-myr-ZIP; myr-RLYRKRIWRSAGR-OH; Biosource) (Laudanna et al., 1998) were prepared in distilled water as a stock solution (10 mm) and stored at -20°C. The required volume containing the final concentration of 1 μm was dissolved in ACSF immediately before bath application. myr-ZIP is 142-fold more potent on PKMζ than on conventional PKCα and 196-fold more potent on PKMζ than on cAMP-dependent protein kinase [assays described by Ling et al. (2002)].
The average values of the population spike amplitude (measured in millivolts) and the slope function of the field EPSP (in millivolts per millisecond) per time point were subjected to statistical analysis using the Wilcoxon signed rank test when compared within one group or the Mann-Whitney U test when data were compared between groups; p < 0.05 was considered as statistically significant different. Data shown in Figure 2, d and e, and Figure 3, a and e, were partially performed in parallel and completed by additional experiments originally described by Sajikumar and Frey (2004).
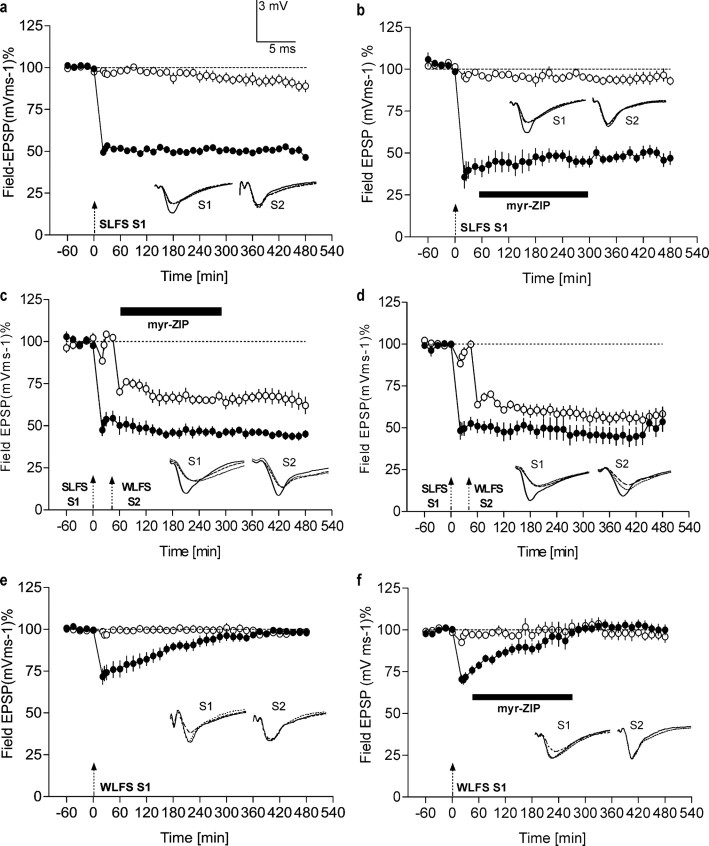
Figure 2.
Effects of PKMζ inhibition on LTD maintenance and synaptic tagging. a, Late LTD. Application of an SLFS to S1 (filled circles) resulted in a significant depression of that input, compared with a control input S2 (open circles; n = 7; p < 0.05; U test). b, Late LTD that was induced using the same protocol as in a was unaffected when the myr-ZIP (1 μm; n = 7) was bath applied 1 h after its induction (filled circles). c, d, Synaptic tagging during LTD under the influence of myr-ZIP. c represents the time course of the field EPSP after SLFS of S1 (filled circles) followed by WLFS in S2 (open circles) and subsequent application of myr-ZIP (n = 5). This protocol resulted in synaptic tagging (i.e., in the transformation of early into late LTD of S2) regardless of the blockade of PKMζ activity by myr-ZIP. d represents a control series of synaptic tagging during LTD without drug treatment (n = 10). e, f, Early LTD. e, Time course of control early LTD in S1 (filled circles) induced by a WLFS (dashed arrow; n = 10) with a duration of 185 min (compared with control input S2; U test; p < 0.05; n = 6). f, This early LTD was unaffected when the myr-ZIP was applied 1 h after its induction. Control stimulation of S2 in e and f showed relatively stable potentials for the time course investigated (open circles). Analog traces represent typical field EPSPs 30 min before (solid line), 30 min after (hatched line), and 8 h after (dotted line) low-frequency stimulation of input S1 or, in cases in which S2 was also treated with LTD-inducing stimulation, before or after the LFS of that input. Dashed arrows represent weak or strong low-frequency stimulation of the corresponding synaptic input. Bars represent the period of drug application. Error bars represent means ± SEM.
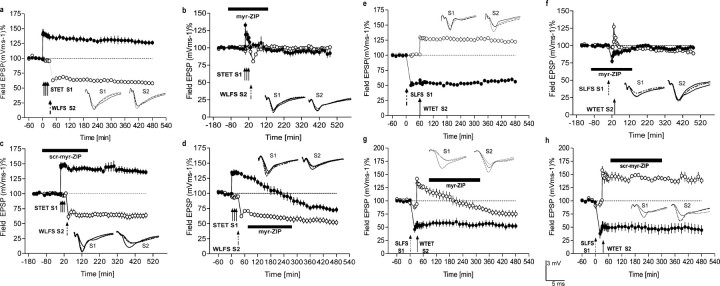
Figure 3.
Effects of PKMζ inhibition on synaptic cross-tagging. a, Late LTP transforms cross-tagged early LTD synapses into persistent LTD. Induction of late LTP in S1 (filled circles) by STET was followed 30 min later by WLFS in S2 (open circles). Here, early LTD in S2 was transformed into late LTD, demonstrating synaptic cross-tagging between LTP and LTD (n = 7). b, Bath application of myr-ZIP 2 h before until 2 h after the tetanization of S1 prevented LTP and LTD induction (n = 7). S1 (filled circles) tetanized with high-frequency stimulation; 30 min later, WLFS was applied to S2 (open circles) to induce early LTD. c, Similar experiment as that in b but with the inactive scrambled peptide (1 μm) instead of myr-ZIP (n = 8). No effect on LTP, LTD induction, or cross-tagging was observed. d, PKMζ inhibition does not affect late LTP to cross-tagged early LTD synapse interactions that establish late LTD. Cross-tagging followed by the application of myr-ZIP 1 h after its induction reversed the maintenance of late LTP in S1 (filled circles); the early LTD in S2 (open circles) was nonetheless transformed into a late LTD regardless of the blockade of late LTP in S1 (n = 7). e, Late-LTD transformation of cross-tagged early LTP synapses. Induction of late LTD in S1 (filled circles) by SLFS was followed 1 h later by WTET in S2 (open circles; n = 7). Here, early LTP in S2 was transformed into late LTP, demonstrating synaptic cross-tagging between LTD and LTP. f, PKMζ inhibition blocks induction of LTD followed by LTP. Similar experiment as that in b but in a reverse manner (n = 7) [i.e., S1 with late-LTD induction (filled circles) and S2 with early LTP (open circles)]. g, PKMζ inhibition prevents LTP maintenance at cross-tagged synapses. The experiment is similar to e but with the subsequent application of myr-ZIP. The ability of late-LTD synapses (filled circles) to interact with and to prolong the maintenance at cross-tagged LTP synapses was prevented by myr-ZIP, revealing early LTP in S2 (open circles) with a subsequent long-lasting depression 5 h after tetanization (compared with baseline values before tetanization; n = 7; p < 0.05; Wilcoxon test). h, scr-myr-ZIP (1 μm) had no effect on cross-tagging (n = 4) [i.e., early LTP in S2 (open circles) was transformed into late LTP by the previous induction of late LTD in S1 (filled circles)]. Analog traces as well as symbols are similar to Figure 1. Error bars represent means ± SEM.
Results
Reversal of late LTP by PKMζ inhibition
Previous work on the role of PKMζ in late LTP has examined a standard, strong, 100 Hz, 1 s, four-train, 5 min-intertrain interval protocol, using hippocampal slices preincubated for 1-2 h (Osten et al., 1996; Ling et al., 2002; Serrano et al., 2005). However, for recording from slices for an extended period of time (∼9 h), we have optimized the hippocampal slice preparation and tetanic stimulation protocols (see Materials and Methods) (Sajikumar and Frey, 2004). To examine the role of persistent PKMζ activity in the maintenance of late LTP under our conditions, we induced late LTP in synaptic input S1 (Fig. 1a, filled circles) and also recorded a control input S2 (Fig. 1a, open circles) and then applied myristoylated ζ-pseudosubstrate peptide inhibitor (Fig. 1b). We used a low concentration of the inhibitor (1 μm), selective for the effects of PKMζ on the consolidation phase of late-LTP maintenance (Serrano et al., 2005), which corresponds to the time of synaptic tagging (Frey and Morris, 1997, 1998). A control nontetanized pathway remained stable at baseline levels for the entire experimental session (Fig. 1b, open circles). In contrast, in the tetanized pathway, the application of myr-ZIP 1 h after the induction of late LTP in S1 (drug application continued up to 5 h) prevented its late maintenance (Fig. 1b, filled circles), returning to baseline responses 135 min after tetanization (p < 0.05; Wilcoxon signed rank test), thus confirming previous studies (Ling et al., 2002; Serrano et al., 2005). Interestingly, under our conditions, after washout of the drug, the potentials further attenuated below baseline levels from 4 h onwards, expressing a lasting depression (p < 0.05; Wilcoxon signed rank test), although no changes were observed during these times with the simultaneously recorded nontetanized control pathway. Although the decrease was specific to the tetanized pathway, we nonetheless excluded changes in general excitability at these late time points by comparing input-output relationships [measured as E/S relationship (i.e., slope function of the EPSP/population spike amplitude); n = 5 for each group] 30 min before, 30 min after, and 8 h after tetanization, in the series with control late LTP (Fig. 1a) and the induction of late LTP and subsequent application by myr-ZIP (Fig. 1b). Input-output characteristics and the evaluation of the E/S coefficient revealed no changes in excitability and normal E/S-shift behavior after LTP induction and its reversal by myr-ZIP [E (in millivolts/millisecond)/S (in millivolts) values at -30 min control, 2.01 ± 0.1/1.65 ± 0.2 vs myr-ZIP, 2.25 ± 0.2/2.06 ± 0.2; +30 min control, 3.54 ± 0.3/4.18 ± 0.3 vs 3.8 ± 0.2/4.31 ± 0.2 in the myr-ZIP group; +8 h control, 3.4 ± 0.3/4.02 ± 0.3 vs myr-ZIP group, 1.65 ± 0.2/1.38 ± 0.2]. In addition, we analyzed tetanus trains (interstimulus interval, 10 ms) with and without drug treatment. No differences in the recorded potentials were detected (data not shown). Thus, the persistent activation of PKMζ not only maintains late LTP, but it appears that strong tetanization alters the baseline properties of AMPA-R-mediated synaptic transmission in those pathways, so that they become partially maintained by PKMζ.
Effects of PKMζ inhibition on synaptic tagging during LTP
We then examined whether the late phase of LTP acquired through synaptic tagging can be influenced by application of myr-ZIP. We induced late LTP in synaptic input S1 and subsequently early LTP in synaptic input S2 and then 30 min later applied myr-ZIP. As seen in Figure 1c, late LTP in S1 was reversed to baseline 210 min after STET, and, in addition, the potentiation in S2 returned to pretetanization baseline values 120 min after WTET (p < 0.05; Wilcoxon test). Thus, the persistence of potentiation after synaptic tagging was prevented by the application of myr-ZIP after tetanization. In contrast, application of an inactive scrambled control peptide (1 μm) did not prevent LTP synaptic tagging between the two separate inputs (Fig. 1d). The transformation of early LTP into late LTP in S2 by the previous induction of late LTP in S1 was maintained for 8 h. The effect of PKMζ was specific to the late phase acquired by the weak tetanization after synaptic tagging, because application of myr-ZIP 60 min after induction of early LTP produced by a weak tetanus alone had no effect on its time course (Fig. 1f), compared with a control series without drug treatment (Fig. 1e). Thus, the persistence of potentiation after synaptic tagging is through acquisition of a PKMζ-dependent late phase.
Effects of PKMζ inhibition on LTD and synaptic tagging during LTD
We next investigated whether myr-ZIP can reverse late LTD or whether its effects are specific to late LTP. Figure 2a represents the time course of late LTD induced by SLFS in S1 (filled circles). The control input S2 remained relatively stable at baseline levels for the entire session (Fig. 2a, open circles). Figure 2b represents the time course of the field EPSP of input S1 when myr-ZIP was applied 1 h after induction of late LTD. Application of the drug up to 5 h after SLFS did not influence either early or late LTD (Fig. 2b, filled circles). The control input S2 (Fig. 2b, open circles) remained stable in the presence of the agent. Thus, in contrast to LTP, the maintenance of late LTD is independent of persistent increased PKMζ activity. Next, we examined the possible effect of myr-ZIP on processes of synaptic tagging during LTD. We studied synaptic tagging between synaptic inputs treated either with SLFS or WLFS and subsequent blockade of PKMζ activity. SLFS of S1 (filled circles) followed by WLFS in S2 (open circles) resulted in synaptic tagging (i.e., in the transformation of early into late LTD of S2) whether in the presence (Fig. 2c) or absence (Fig. 2d) of myr-ZIP. myr-ZIP also had no effect on the maintenance of early LTD induced by WLFS (Fig. 2e,f).
Effects of PKMζ inhibition on cross-tagging
These results show that persistent increased PKMζ activity plays a critical role in the maintenance of LTP and synaptic tagging of potentiated responses but not for the maintenance of LTD. We next asked whether there is a specific role for PKMζ in the processes of synaptic cross-tagging. Is the prolongation of a cross-tagged, weakly depressed synapse by strong LTP, thus transforming it into persistent LTD, caused by the persistent action of PKMζ? Conversely, is the prolongation of a cross-tagged, weakly potentiated synapse by strong LTD caused by acquisition of PKMζ, although the kinase has no apparent role in LTD maintenance?
We first addressed whether cross-tagging from strong LTP to weak LTD requires PKMζ (Fig. 3a). We began by delineating the potential temporal windows within which PKMζ might be involved in this form of cross-tagging. Thus, we first asked whether PKMζ might be important in the induction phase of strong LTP to weak LTD cross-tagging by bath applying myr-ZIP to two independent pathways (Fig. 3b) and then 2 h later delivering STET to S1 (filled circles), followed by WLFS in S2 (open circles). We found that myr-ZIP blocked LTP, supporting the data presented in Figure 1, but, additionally, no cross-tagging was observed (i.e., no transformation of early into late LTD). We confirmed the specificity of this effect by applying the scrambled inactive version of ZIP and observed normal strong LTP to weak LTD cross-tagging responses (Fig. 3c). Interestingly, when PKMζ was inhibited at this early phase of cross-tagging (Fig. 3b), the time course of early LTD appeared modestly shorter than usual (e.g., compared with Fig. 2e,f). This suggested that PKMζ might have an effect on LTD induction, rather than on cross-tagging per se and the acquisition of the maintenance mechanism of LTD.
To address this question, we applied myr-ZIP during the maintenance phase of cross-tagging. We applied STET to S1 (Fig. 3d, filled circles), followed by WLFS in S2 (Fig. 3d, open circles) and then applied myr-ZIP 30 min later (i.e., within the effective time window of 1 h shown in previous studies from our laboratory to be critical for the tagging and cross-tagging processes) (Frey and Morris, 1997, 1998; Sajikumar and Frey, 2004). We found that late LTP in S1 was reversed, similar to that observed in Figure 1, b and c. However, although late LTP was blocked in S1, cross-tagging and persistence of the weak LTD still occurred (i.e., early LTD was transformed into late LTD in S2). This result suggests that a PRP produced by strong tetanization, other than PKMζ, is important for transforming early LTD into late LTD.
The next question was, what would happen if we reverse the order of the induction of plasticity phenomena (i.e., when strong LTD precedes cross-tagged weak LTP) (Fig. 3e)? We first addressed whether reversing the order of the stimuli had any effect on the blockade of induction, which we had observed in Figure 3b. Again, applying myr-ZIP before stimulation blocked the induction processes of both LTP and LTD (Fig. 3f). We then asked whether PKMζ inhibition affected the maintenance processes of strong LTD followed by weak LTP. We first obtained strong LTD in S1 followed by weak LTP in S2 and then 30 min later applied myr-ZIP (Fig. 3g). Late LTD in S1 was not influenced by myr-ZIP. In contrast, PKMζ inhibition completely prevented the ability of late LTD to prolong early LTP (i.e., the WTET of S2 resulted only in early LTP with a late, long-lasting depression below pretetanus baseline levels 5 h after tetanization; see above) (Fig. 1b,c). Application of the inactive scrambled peptide had no effect (Fig. 3h). Together, these results indicate that PKMζ is critical for tagging or cross-tagging interactions with the maintenance mechanism of LTP but not between tagging or cross-tagging and the maintenance mechanism of LTD.
Discussion
Synaptic tagging provides a conceptual basis for characterizing the mechanisms by which newly synthesized proteins that prolong functional changes in synaptic strength may act at specific, recently activated synapses. Although work on synaptic tagging at the descriptive level has advanced since the first report in 1997 by Frey and Morris (Frey and Morris, 1997, 1998; Kauderer and Kandel, 2000; Barco et al., 2002; Dudek and Fields, 2002; Martin, 2002; Fonseca et al., 2004; Kelleher et al., 2004; Navakkode et al., 2004; O'Carroll and Morris, 2004; Sajikumar and Frey, 2004), few core molecules directly involved in the tagging process have been identified. We now find that interaction between synaptic tags and the persistently active isoform of PKC, PKMζ, is critical for the transformation of a weakly potentiated synapse into a persistently potentiated one. Our results extend previous observations on the critical role of PKMζ in the late phase of LTP and, particularly, in the exploration of cross-tagging, demonstrate unexpected specificity for the roles of newly synthesized PRPs.
We first confirmed the work by Ling et al. (2002) and Serrano et al. (2005), who showed a specific role of PKMζ in the maintenance of the late phase of LTP. We applied a low dose of the PKMζ-selective inhibitor myr-ZIP 1 h after tetanization and observed a reversal of potentiated responses, and not of baseline untetanized synaptic transmission, simultaneously recorded within the hippocampal slices. Furthermore, consistent with the role of PKMζ in LTP maintenance, washout of the drug did not result in a return to a potentiated response but in a modest and delayed, but long-lasting, depression of the potentiated input below baseline during very late phases beyond 4 h. We could exclude nonspecific changes in excitability. This suggests that tetanization, in addition to potentiating synapses, may also cause depression in some synapses that is usually masked but now revealed in our experiments when potentiation is specifically reversed by myr-ZIP. Alternatively, tetanization could partially destabilize the mechanisms that maintain baseline synaptic responses. Phosphorylation by PKMζ enhances AMPA-R-mediated synaptic transmission (Ling et al., 2002), and it was shown that AMPA-R function is attenuated during LTD (Carroll et al., 1999; Hirai, 2001; Gutlerner et al., 2002). Therefore, persistently increased PKMζ activity synthesized by the tetanization may thus maintain both enhanced AMPA responses and, in part, the baseline responses specific to the activated pathway. The ability of tetanization to cause modest depression that is masked by potentiation or to destabilize baseline responses has been noted previously (Hrabetova and Sacktor, 2000).
Interestingly, when the PKMζ inhibitor myr-ZIP was applied both during and after the tetanization, the late depression below baseline was not observed (Fig. 3b). This suggests that PKMζ may have dual roles: (1) during induction, it may participate in the destabilization process, which permits plasticity, and (2) during maintenance, it is instructive for potentiating synaptic strength. The block of LTP induction observed here (Fig. 3b) appears stronger than that reported by Serrano et al. (2005). The three-train, 10 min intertrain interval at moderate stimulus intensity used to induce LTP in our study, however, was somewhat weaker than the four-train, 5 min intertrain interval at maximal stimulus intensity used by Serrano et al. (2005). This is supported by the observations that our initial early LTP is ∼150% above the base-line (set at 100%), whereas in the work by Serrano et al. (2005), it is ∼175-200%. The increased early phase in the work by Serrano et al. (2005) may have led to a longer myr-ZIP-independent response. Furthermore, the role of PKMζ in induction may not be specific for the direction of synaptic change, because myr-ZIP also blocked the induction of LTD (Fig. 3f). In contrast, the role of PKMζ in maintenance of potentiation appears remarkably specific (see below).
We next examined the role of PKMζ in synaptic tagging and observed that the persistence of the potentiation of the tagged synapse was dependent on the persistent activity of PKMζ, because the late phase of its potentiation was now reversed by application of myr-ZIP. Because PKMζ directly causes synaptic potentiation in the late phase of LTP (Ling et al., 2002; Serrano et al., 2005), and because PKMζ is synthesized during strong, but not weak, tetanic stimulation (Osten et al., 1996), the simplest interpretation of our results is that newly synthesized PKMζ is a PRP that maintains potentiation at the weakly activated, tagged synapse (Fig. 4, available at www.jneurosci.org as supplemental material). Interestingly, the mRNA that produces PKMζ appears in both the soma and dendrites of hippocampal pyramidal neurons (Muslimov et al., 2004). Future work will be required to determine the source of the newly synthesized PKMζ protein that maintains potentiation at tagged synapses.
The low dose of myr-ZIP used in this study (1 μm) is sufficient to reverse LTP maintenance during the temporal window of synaptic tagging and cross-tagging (Frey and Morris, 1997, 1998; Ling et al., 2002; Sajikumar and Frey, 2004; Serrano et al., 2005). Interestingly, slightly higher doses (2.5-5 μm) are required to reverse established late LTP at later times and to fully block the potentiating effects of whole-cell perfused PKMζ on AMPA-R-mediated synaptic transmission (Serrano et al., 2005). Because myr-ZIP is a competitive substrate, this suggests the possibility that PKMζ may phosphorylate a different, additional substrate during the critical transition period when tagging takes place. We speculate that this substrate could be a component of the potentiation-specific tag complex (see below).
Whole-cell perfusion of PKMζ into CA1 pyramidal cells is also sufficient to cause sustained potentiation at most, if not all, glutamatergic synapses in the cell, suggesting that synaptic tags are not required for potentiation under these conditions. The simplest explanation for this apparent discrepancy is that during LTP the amount of PKMζ synthesized is limiting, and synaptic tags (in their potentiation-specific forms) are high-affinity binding sites required to “capture” the kinase. In contrast, whole-cell perfusion of PKMζ through the recording pipette, even at the low nanomolar concentrations used, might provide an abundant source of the enzyme that obviates the need for a tag.
Inhibition of PKMζ did not affect baseline responses, implying that, before our experimental manipulation, no synapses are persistently potentiated by the kinase. This may be surprising if PKMζ were important for memory storage in vivo. One possibility is that the novel experience of the caged animals used in our experiments is relatively deprived and that the storage of older memories may have declined in the hippocampus, while being maintained elsewhere in the brain. Alternatively, the preparation of hippocampal slices may have eliminated evidence of previous potentiation, perhaps as a result of the glutamate release (Fiala et al., 2003), or subsequent homeostatic mechanisms triggered by the procedure (Kirov et al., 1999), or by a possible shortened half-life of PRPs (such as PKMζ) of ∼2 h (Sajikumar and Frey, 2004).
Additional hypotheses relevant to PKMζ and synaptic tagging are that the preexisting kinase may participate in the tagging process or that the newly synthesized PKMζ may then act as a tag for other downstream proteins or mRNAs important for persistent potentiation. Because recent work has indicated that PKMζ enhances synaptic transmission by increasing the number of postsynaptic AMPA-Rs through regulation of the trafficking of the receptor (Ling et al., 2004), there may be multiple levels of tagging that are essential for the molecular mechanisms of LTP. In addition, other processes relevant to synaptic plasticity, including translation, possibly transcription, and structural changes, may be regulated by persistent PKMζ phosphorylation.
The role of PKMζ was specific to maintaining potentiation. In contrast to its essential role in LTP maintenance, we did not observe any effect of PKMζ on LTD maintenance, because application of myr-ZIP did not reverse early or late LTD. Our results with cross-tagging then provided additional evidence for the functional specificity of PKMζ. We have hypothesized previously (Sajikumar and Frey, 2004) that the induction of either late LTP or late LTD leads to the synthesis of a pool of PRPs. At that time, we proposed that these PRPs could be either one kind of protein or several different kinds of proteins (Kelleher et al., 2004; Sajikumar and Frey, 2004). Our new cross-tagging data with the inhibition of PKMζ after the establishment of LTP and LTD support the hypothesis that late plasticity forms initiate the synthesis of a pool of different and, indeed, process-specific PRPs. We first showed that whereas late LTP was suppressed under the influence of PKMζ inhibition (Fig. 3d), early LTD in the second input was nonetheless transformed into late LTD. Because the induction of early LTD is unable to initiate the synthesis of PRPs but sets its process-specific synaptic tags (Sajikumar and Frey, 2004), the PRPs captured by these tags must be initiated by the strong tetanization in the separate synaptic input, even while late LTP was suppressed by myr-ZIP. This confirms that PRPs other than PKMζ mediate the transformation of early into late LTD.
Furthermore, in the converse experiment (Fig. 3g), applications of myr-ZIP, while not affecting the maintenance of strong LTD, nonetheless prevented the transformation of weakly potentiated synapses into persistently potentiated synapses. This suggests that PKMζ is synthesized during late LTD but functionally interacts, perhaps through phosphorylation, only with the tags of the weakly potentiated synapses (Fig. 4, available at www.jneurosci.org as supplemental material). These results also indicate that PKMζ is fundamental for persistent LTP, even when the sustained enhancement of synaptic efficacy in an input is obtained by dramatically different stimulation paradigms.
Thus, PKMζ is the first LTP-specific PRP. Therefore, we extend our hypothesis of the synthesis of a pool of relatively non-specific PRPs (Sajikumar and Frey, 2004) by the notion that PRPs include process-specific proteins. The pool consists of PRPs specific for either LTP or LTD, as well as process-nonspecific proteins, such as the phosphodiesterase type-4B3, for which we have shown recently a role during both LTP and LTD (Ahmed and Frey, 2003; Ahmed et al., 2004; Navakkode et al., 2004), as determined by the level of the activation of the enzyme. Together, our results suggest that not only does the process-specific tag consist of a complex machinery of molecules (Frey and Morris, 1998; Sajikumar and Frey, 2004), but also PRPs represent a pool of proteins expressing their effector roles by selective interactions with these process-specific tag complexes (Fig. 4, available at www.jneurosci.org as supplemental material). Future work will be required to determine which molecules of the potentiation-specific tag interact with PKMζ.
Footnotes
This work was supported by the German Volkswagenstiftung I/77922 to J.U.F. and National Institutes of Health Grants MH057068 and MH53576 to T.C.S.
Correspondence should be addressed to Prof. Julietta U. Frey, Leibniz Institute for Neurobiology, Department of Neurophysiology, Brenneckestrasse 6, D-39118 Magdeburg, Germany. E-mail: frey@ifn-magdeburg.de.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255750-07$15.00/0
References
- Ahmed T, Frey JU (2003) Expression of the specific type IV phosphodiesterase gene PDE4B3 during different phases of long-term potentiation in single hippocampal slices of rats in vitro. Neuroscience 117: 627-638. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey S, Frey JU (2004) Regulation of the phosphodiesterase PDE4B3-isotype during long-term potentiation in the area dentata in vivo. Neuroscience 124: 857-867. [DOI] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER (2002) Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108: 689-703. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31-39. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC (1999) Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci 2: 454-460. [DOI] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC (2002) Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster Nat Neurosci 5: 316-324. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Fields RD (2002) Somatic action potentials are sufficient for late-phase LTP-related cell signaling. Proc Natl Acad Sci USA 99: 3962-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Kirov SA, Feinberg MD, Petrak LJ, George P, Goddard CA, Harris KM (2003) Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. J Comp Neurol 465: 90-103. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Nagerl UV, Morris RG, Bonhoeffer T (2004) Competing for memory: hippocampal LTP under regimes of reduced protein synthesis. Neuron 44: 1011-1020. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG (1997) Synaptic tagging and long-term potentiation. Nature 385: 533-536. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG (1998) Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology 37: 545-552. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H (1988) Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res 452: 57-65. [DOI] [PubMed] [Google Scholar]
- Frey U, Frey S, Schollmeier F, Krug M (1996) Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol (Lond) 490: 703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutlerner JL, Penick EC, Snyder EM, Kauer JA (2002) Novel protein kinase A-dependent long-term depression of excitatory synapses. Neuron 36: 921-931. [DOI] [PubMed] [Google Scholar]
- Hegde AN (2004) Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Prog Neurobiol 73: 311-357. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC (2003) Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem 278: 40305-40316. [DOI] [PubMed] [Google Scholar]
- Hirai H (2001) Modification of AMPA receptor clustering regulates cerebellar synaptic plasticity. Neurosci Res 39: 261-267. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Sacktor TC (2000) Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci 20: RC81(1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauderer BS, Kandel ER (2000) Capture of a protein synthesis-dependent component of long-term depression. Proc Natl Acad Sci USA 97: 13342-13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher III RJ, Govindarajan A, Tonegawa S (2004) Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 44: 59-73. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM (1999) Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci 19: 2876-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M, Loessner B, Ott T (1984) Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull 13: 39-42. [DOI] [PubMed] [Google Scholar]
- Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC (1998) Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem 273: 30306-30315. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC (2002) Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci 5: 295-296. [DOI] [PubMed] [Google Scholar]
- Ling DS, Sacktor TC, Benardo LS (2004) Enhancement of EPSCs by protein kinase Mζ is due to an increase in postsynaptic AMPA receptor number. Soc Neurosci Abstr 30: 740.1. [Google Scholar]
- Lovinger DM, Wong KL, Murakami K, Routtenberg A (1987) Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res 436: 177-183. [DOI] [PubMed] [Google Scholar]
- Malenka RC (1994) Synaptic plasticity in the hippocampus: LTP and LTD. Cell 78: 535-538. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44: 5-21. [DOI] [PubMed] [Google Scholar]
- Malinow R, Madison DV, Tsien RW (1988) Persistent protein kinase activity underlying long-term potentiation. Nature 335: 820-824. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU (2000) Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci 20: 8572-8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC (2002) Synaptic tagging during synapse-specific long-term facilitation of Aplysia sensory-motor neurons. Neurobiol Learn Mem 78: 489-497. [DOI] [PubMed] [Google Scholar]
- Martin KC, Kosik KS (2002) Synaptic tagging-who's it? Nat Rev Neurosci 3: 813-820. [DOI] [PubMed] [Google Scholar]
- Matthies H (1989) In search of cellular mechanisms of memory. Prog Neurobiol 32: 277-349. [DOI] [PubMed] [Google Scholar]
- Muslimov IA, Nimmrich V, Hernandez AI, Tcherepanov A, Sacktor TC, Tiedge H (2004) Dendritic transport and localization of protein kinase Mzeta mRNA: implications for molecular memory consolidation. J Biol Chem 279: 52613-52622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU (2004) The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. J Neurosci 24: 7740-7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER (1996) A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci 16: 3189-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9: 484-496. [PubMed] [Google Scholar]
- O'Carroll CM, Morris RG (2004) Heterosynaptic co-activation of glutamatergic and dopaminergic afferents is required to induce persistent long-term potentiation. Neuropharmacology 47: 324-332. [DOI] [PubMed] [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC (1996) Protein synthesis-dependent formation of protein kinase Mζ in long-term potentiation. J Neurosci 16: 2444-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Marshall CJ, Tate WP, Goddard GV, Abraham WC (1989) Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience 28: 519-526. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Frey U, Jork R, Matthies H (1988) Polymyxin B, an inhibitor of protein kinase C, prevents the maintenance of synaptic long-term potentiation in hippocampal CA1 neurons. Brain Res 440: 305-314. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E (1993) Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA 90: 8342-8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU (2003) Anisomycin inhibits the late maintenance of long-term depression in rat hippocampal slices in vitro. Neurosci Lett 338: 147-150. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU (2004) Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem 82: 12-25. [DOI] [PubMed] [Google Scholar]
- Schwartz JH (1993) Cognitive kinases. Proc Natl Acad Sci USA 90: 8310-8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC (2005) Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci 25: 1979-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]