Abstract
Glutamate neurotransmission was recently implicated in the action of stress and in antidepressant mechanisms. We report that chronic (not acute) treatment with three antidepressants with different primary mechanisms (fluoxetine, reboxetine, and desipramine) markedly reduced depolarization-evoked release of glutamate, stimulated by 15 or 25 mm KCl, but not release of GABA. Endogenous glutamate and GABA release was measured in superfused synaptosomes, freshly prepared from hippocampus of drug-treated rats. Interestingly, treatment with the three drugs only barely changed the release of glutamate (and of GABA) induced by ionomycin. In synaptic membranes of chronically treated rats we found a marked reduction in the protein-protein interaction between syntaxin 1 and Thr286-phosphorylated αCaM kinase II (α-calcium/calmodulin-dependent protein kinase II) (an interaction previously proposed to promote neurotransmitter release) and a marked increase in the interaction between syntaxin 1 and Munc-18 (an interaction proposed to reduce neurotransmitter release). Furthermore, we found a selective reduction in the expression level of the three proteins forming the core SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex. These findings suggest that antidepressants work by stabilizing glutamate neurotransmission in the hippocampus and that they may represent a useful tool for the study of relationship between functional and molecular processes in nerve terminals.
Keywords: release, glutamate, GABA, antidepressant, CaMKII, SNARE protein, neuroplasticity
Introduction
Recent neuroimaging and histopathological studies in brains of patients with major depression and bipolar disorder revealed the presence of morphometric/volumetric modifications, including ventricular enlargement and hippocampal and cortical volumetric reduction, and of reduced neurons and glia density (Drevets et al., 1997; Rajkowska et al., 1999; Sheline et al., 1999). In many regions of interest, glutamatergic neurons and synapses predominate, suggesting an involvement of glutamate neurotransmission in the pathophysiology of mood disorders. Indeed, in the last few years, numerous lines of evidence accumulated in favor of a role for glutamate in psychiatric pathophysiology, including the following: (1) higher glutamate plasma levels in patients with mood disorders (Altamura et al., 1993); (2) abnormal elevation of glutamate neurotransmission and glutamate levels in cortical/limbic brain areas of depressed patients (Drevets, 2000, 2004; Sanacora et al., 2004); (3) atrophy of apical dendrites in CA3 hippocampal neurons induced by chronic stress, a major factor in pathogenesis of mood disorders (Watanabe et al., 1992); (4) increased amplitude and reduced decay kinetics of NMDA current induced by chronic stress (Kole et al., 2002); (5) impaired long-term potentiation (LTP) and facilitated long-term depression induced by stress (Kim et al., 1996). Conversely, antidepressant treatments were also shown to affect glutamate neurotransmission: (1) antidepressants downregulate NMDA receptor subunits and dampen NMDA function (Skolnick, 1999); (2) antidepressants overcome the effects of stress on LTP (Shakesby et al., 2002); (3) fluoxetine, an antidepressant that selectively inhibits serotonin reuptake, reduces 4-aminopyridine (4-AP)-evoked glutamate release from cerebrocortical synaptosomes on acute exposure in vitro (Wang et al., 2003). As a consequence, now several compounds are under development for the treatment of mood disorders (depression, bipolar disorder, anxiety) that modulate glutamate receptors or neurotransmission at various levels (Holden, 2003). Some of these putative drugs may work by stabilizing glutamate release when its synaptic level becomes too high, a feature that is now considered to be part of the pathophysiology of mood disorders (Sapolsky, 2000; Zarate et al., 2002). However, very little is known about the effect of chronic treatments with typical antidepressants on glutamate release, although such information may be particularly valuable in the design of new drugs and therapies.
Here, we monitored the release of endogenous glutamate and GABA from synaptosomes under continuous superfusion, by using an original technique that has become the method of choice for the analysis of neurotransmitter release from a given family of nerve terminals without the interfering action of other neurotransmitters (M. Raiteri et al., 1974; Raiteri and Raiteri, 2000; L. Raiteri et al., 2002). We found that three different antidepressant drugs markedly reduce depolarization-evoked release of glutamate, but not of GABA, after chronic but not acute treatment. This functional change was accompanied by dramatic modifications of protein-protein interactions in the presynaptic machinery. These findings suggest that antidepressants work by stabilizing glutamate neurotransmission in the hippocampus, and that they may represent a useful tool for the study of relationship between functional and molecular processes in nerve terminals.
Materials and Methods
Animals and drug treatments. Experiments complied with guidelines for use of experimental animals of European Community Council Directive 86/609/EEC, and with the policy of the Society for Neuroscience on use of animals in research. Groups of 12 male Sprague Dawley rats (170-200 g) (Charles River, Calco, Italy) were anesthetized and subcutaneously implanted with osmotic minipumps (Alzet 2ML2; Charles River, Palo Alto, CA) (release, 5 μl/h; capacity, 2 ml), containing either vehicle (5% ethanol) or reboxetine, a selective norepinephrine reuptake inhibitor (NRI), fluoxetine, a selective serotonin reuptake inhibitor (SSRI), or desipramine, a tricyclic antidepressant (TCA) primarily inhibiting norepinephrine release. Drug dosage was 10 mg/kg daily for each drug. Acute treatment was performed injecting rats (250-270 g) intraperitoneally. Animals were killed after 3 h for acute and after 14 d for chronic treatment.
Preparation of synaptosomes. Animals were killed, and the hippocampus was quickly removed. Purified synaptosomes were prepared essentially according to Dunkley et al. (1986), with minor modifications. The tissue was homogenized in 10 vol of 0.32 m sucrose, buffered at pH 7.4 with Tris, using a glass Teflon tissue grinder (clearance, 0.25 mm). The homogenate was centrifuged (5 min; 1000 × g at 4°C) to remove nuclei and debris, and the supernatant was gently stratified on a discontinuous Percoll gradient (2, 6, 10, and 20% v/v in Tris-buffered sucrose) and centrifuged at 33,500 × g for 5 min. The layer between 10 and 20% Percoll (synaptosomal fraction) was collected and washed by centrifugation. When used for neurotransmitter release experiments, synaptosomes were resuspended in physiological medium with the following composition (in mm): 125 NaCl, 3 KCl, 1.2 MgSO4, 1.2 CaCl2, 1 NaH2PO4, 22 NaHCO3, 10 glucose (aeration with 95% O2 and 5% CO2), pH 7.2-7.4. For all of the other experiments, synaptosomes were resuspended in lysis buffer: 120 mm NaCl, 20 mm HEPES, pH 7.4, 0.1 mm EGTA, 0.1 mm DTT, containing 20 mm NaF, 5 mm Na4P2O7, 1 mm Na2VO4, and 2 μl/ml protease inhibitor mixture (Sigma, St. Louis, MO). Additional fractionation of purified synaptosomes into synaptic membrane fraction (LP1) and synaptic vesicle fraction (LP2) was performed by differential centrifugation and ultracentrifugation as reported previously (Celano et al., 2003).
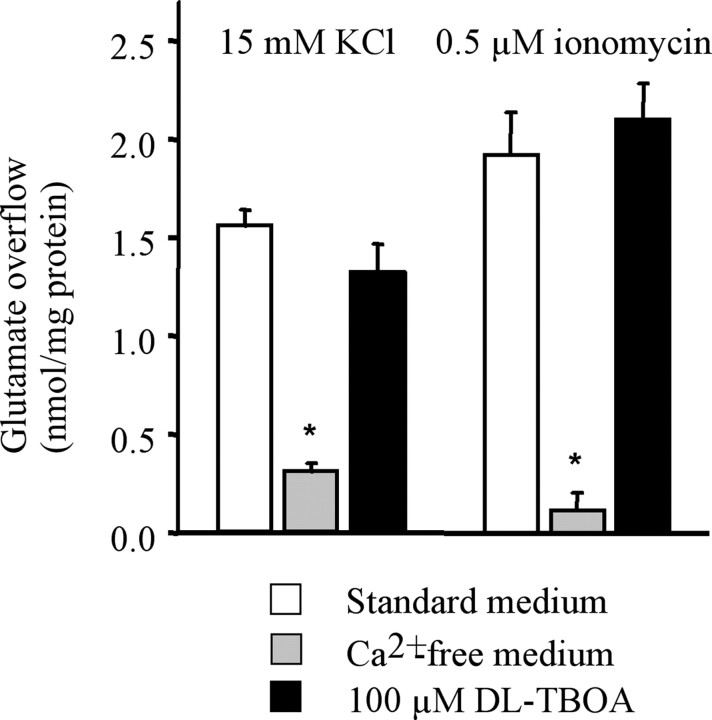
Release experiments. Synaptosomes were incubated at 37°C for 15 min with gentle shaking in a 95% O2 and 5% CO2 atmosphere. At the end of the incubation period, aliquots of the synaptosomal suspensions (∼100 μg of protein) were layered on microporous filters at the bottom of a set of parallel superfusion chambers maintained at 37°C (Raiteri et al., 1974; Raiteri and Raiteri, 2000). Superfusion was started at a rate of 0.5 ml/min with standard medium aerated with 95% O2 and 5% CO2. After 36 min of superfusion, to equilibrate the system, samples were collected according to the following scheme: two 3 min samples (t = 36-39 min and t = 45-48 min; basal outflow) before and after one 6 min sample (t = 39-45 min; stimulus-evoked release). A 90 s period of stimulation was applied at t = 39 min, after the first sample had been collected. Stimulation of synaptosomes was performed with 0.1 or 0.5 μm ionomycin and 15 or 35 mm KCl, the latter substituting for an equimolar concentration of NaCl. dl-Threo-β-benzyloxyaspartic acid (dl-TBOA; 100 μm) was added at t = 30 min; when used, the Ca2+-free medium was introduced at t = 20 min. The collected fractions as well as the superfused synaptosomes were analyzed for their endogenous glutamate and GABA content. The endogenous amino acid release was expressed as picomoles per milligram of protein. The stimulus-evoked overflow was estimated by subtracting the transmitter content of the two 3 min samples (basal outflow) from the release evoked in the 6 min sample collected during and after the depolarization pulse (stimulus-evoked release). Drug treatment effects were evaluated by comparing the stimulus-evoked overflow in drug-treated animals versus that calculated in vehicle-treated rats. Data in Figure 5 were evaluated by comparing the depolarization-evoked overflow in the presence of the drug versus that calculated under control conditions. Appropriate controls were always run in parallel.
Figure 5.
Effect of Ca2+ omission or glutamate transporter block on the release of glutamate from rat hippocampal synaptosomes. Synaptosomes were exposed in superfusion to a 90s pulse of 25 mm KCl or 1 μm ionomycin, and the stimulus-evoked overflow of endogenous glutamate was measured. The stimulus-evoked overflow was calculated by subtracting the neurotransmitter content in the basal outflow from the release evoked by stimulation (see the legend to Fig. 1). dl-TBOA (100 μm) was introduced 9 min before KCl. Ca2+ was omitted 19 min before KCl. Data represent the means ± SEM of three to four separate experiments run in triplicate. *p < 0.001 versus the overflow induced by KCl or ionomycin in the absence of drugs (two-tailed Student's t test). Open bars, Controls; gray bars, Ca2+-free; filled bars, 100 μm dl-TBOA.
Endogenous glutamate and GABA determination. Endogenous glutamate and GABA were measured by HPLC analysis after precolumn derivatization with o-phthalaldehyde and separation on a C18 reverse-phase chromatographic column (10 × 4.6 mm, 3 μm; at 30°C; Chrompack, Middleburg, The Netherlands) coupled with fluorometric detection (excitation wavelength, 350 nm; emission wavelength, 450 nm) (Raiteri and Raiteri, 2000). Buffers and the gradient program were as follows: solvent A, 0.1 m sodium acetate (pH 5.8)/methanol, 80:20; solvent B, 0.1 m sodium acetate (pH 5.8)/methanol, 20:80; solvent C, sodium acetate (pH 6.0)/methanol, 80:20; gradient program, 100% C for 4 min from the initiation of the program; 90% A and 10% B in 1 min; isocratic flow, 2 min; 78% A and 22% B in 2 min; isocratic flow, 6 min; 66% A and 34% B in 3 min; 42% A and 58% B in 1 min; 100% B in 1 min; isocratic flow, 2 min; 100% C in 3 min; flow rate, 0.9 ml/min. Homoserine was used as internal standard.
Western blot. Western blot analysis was performed as described previously (Verona et al., 2000; Tiraboschi et al., 2004a) by incubating polyvinylidene difluoride membranes containing electrophoresed proteins with monoclonal or polyclonal antibodies in appropriate dilutions. Monoclonal antibodies used were as follows: antibody for α-calcium/calmodulin-dependent protein kinase II (αCaM kinase II), 1:1000 (Chemicon, Temecula, CA); synaptophysin (syph), 1:1000; soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP)-25, 1:2000; and synaptobrevin (syb), 1:4000 (Synaptic Systems, Goettingen, Germany); synaptotagmin (syt), 1:500 (Popoli, 1993); syntaxin (stx), 1:5000; and β-actin, 1:5000 (Sigma). Polyclonal antibody was as follows: antibody for phospho-Thr286 αCaM kinase II, 1:500 (Promega, Milan, Italy). The membranes were blocked with 5% milk and incubated with primary antibody. After incubation with peroxidase-coupled secondary antibodies, protein bands were detected by using ECL (Amersham Biosciences, Piscataway, NJ) or Super Signal Dura West (Pierce, Rockford, IL). Standard curves were obtained by loading increasing amounts of samples on gels as described previously (Verona et al., 2000). All of the protein bands used were within linear range of standard curves, and normalized for actin level in the same membrane. Quantity One software (Bio-Rad, Hercules, CA) was used for standardization and quantitation, as reported previously (Tiraboschi et al., 2004a).
Coimmunoprecipitations of αCaM kinase II/syntaxin 1 and Munc-18/syntaxin 1. Aliquots of 100 μg of synaptic membrane fraction (LP1) were incubated in immunoprecipitation (IP) buffer containing (final concentration): 200 mm NaCl, 10 mm EDTA, 10 mm Na2HPO4, 0.5% SDS, pH 7.4, in a final volume of 200 μl with protease inhibitor mixture (2 μl/ml) (Sigma) and antibody against either αCaM kinase II or Munc-18 (Becton Dickinson, Rutherford, NJ) overnight at 4° with slow end-over-end rotation. Protein A-Sepharose (Sigma; 10 mg/tube) washed in the same buffer was added, and incubation was continued for 2 h at 4°C. The beads were collected by centrifugation and washed four times with 500 μl of the same buffer. Sample buffer (2×) for SDS-PAGE was added, and the mixture was boiled for 3 min. The beads were pelleted by centrifugation, and 25 μl of supernatant was applied to 10% polyacrylamide gels for SDS-PAGE and electroblotted on Hibond-P membrane (Amersham Biosciences) for 2 h at 250 mA. Coimmunoprecipitated proteins were detected with primary antibodies for Munc-18 (Becton Dickinson; 1:3000), αCaM kinase II (Chemicon; 1:3000), and syntaxin 1 (Sigma; 1:1000). The membranes were then incubated with secondary antibodies (1:5000) and revealed with ECL (Amersham Biosciences).
In vitro activation of αCaM kinase II. A total of 100 μg of lysed synaptosomes from hippocampus was incubated with phosphorylation buffer (25 mm HEPES, 10 mm Mg(C2H3O2)2, 0.1 mm DTT, pH 7.4) and phosphatase inhibitors (5 mm Na4P2O7, 20 mm NaF, and 1 mm NaVO3), final volume of 100 μl, in the presence of the following: (1) 20 mm EGTA; (2) 1 mm CaCl2, 20 μg/ml calmodulin (CaM); (3) 1 mm CaCl2, 20 μg/ml CaM, 500 μm ATP. The reaction was performed at 37°C for 5 min and stopped by addition of 100 μl of IP buffer (see above); αCaM kinase II was immunoprecipitated as described above. Immunoprecipitates were separated by SDS-PAGE on 10% acrylamide gels and electroblotted on Hibond-P membrane (Amersham Biosciences) for 2 h at 250 mA. The membranes were processed as above, and proteins were recognized with primary antibodies for phospho-αCaM kinase II (Promega, Milan, Italy; 1:500), αCaM kinase II (Chemicon; 1:3000), and syntaxin 1 (Sigma; 1:1000). The membranes were then incubated with secondary antibodies (1:3000) and revealed with ECL (Amersham Biosciences).
Materials. dl-TBOA was obtained from Sigma. All of the other reagents were of laboratory or HPLC grade, as appropriate.
Results
Reduction of depolarization-evoked release of glutamate but not GABA by chronic antidepressants
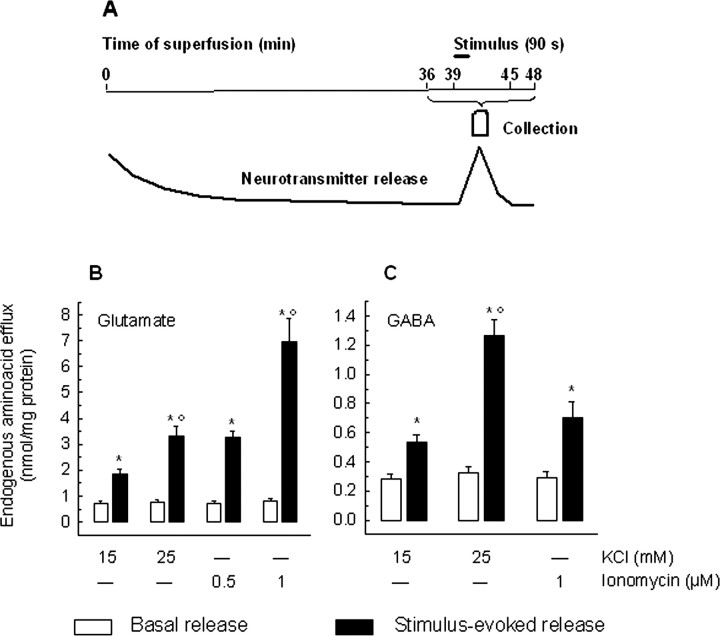
Synaptosomes were purified from the hippocampi of chronically drug- or vehicle-treated rats, as well as of acutely treated rats, and exposed in superfusion to KCl or ionomycin; then the release of endogenous glutamate and GABA was monitored. The time course of release experiments and a schematic pattern of transmitter release are depicted in Figure 1A. During the first 36 min of superfusion with standard medium at 37°C, to equilibrate the system, the spontaneous outflow asymptotically diminishes to a constant level; a 90 s exposure to the stimulus at t = 39 min produces a transient increase of neurotransmitter release that soon returns to basal levels.
Figure 1.
A, Schematic time course of the release of a putative neurotransmitter from synaptosomes stimulated in superfusion by a depolarizing pulse. Synaptosomes were stratified on microporous filter, and neurotransmitter release was monitored during superfusion. After 36 min were allowed to equilibrate the system, two 3 min fractions (t = 36-39 min and t = 45-48 min) were collected before and after one 6 min fraction (t = 39-45 min). Synaptosomes were exposed to the stimulus (90 s) at the end of the first fraction collected (t = 39 min; see line). B, C, Characteristics of the release of glutamate and GABA from hippocampal synaptosomes exposed in superfusion to KCl or ionomycin. Synaptosomes were stratified on microporous filters, superfused, and stimulated by a 90 s pulse of KCl (15 or 25 mm) or ionomycin (0.5 or 1 μm) at t = 39 min of superfusion: the spontaneous or the stimulus-evoked release of endogenous glutamate (B) and GABA (C) was monitored. Basal outflow represents the neurotransmitter content in the two 3 min fractions collected before and after the stimulation fraction; the stimulus-evoked release represents the neurotransmitter content in the 6 min stimulation fraction, collected during and after application of the releasing pulse. *p < 0.005, when compared with respective basal outflow value; °p < 0.005, when compared with the release evoked by 15 mm KCl or 0.1 μm ionomycin, as appropriate (two-tailed Student's t test). Open bars, Basal release; filled bars, stimulus-evoked release. Error bars indicate SEM.
Figure 1 also shows the absolute values of the basal outflow and of total release (induced by the KCl or ionomycin stimulus) of glutamate (B) and GABA (C). The amount of glutamate released appeared to be approximately three times higher than that of GABA; nevertheless, both glutamate and GABA release were directly related to the strength of the stimulus applied.
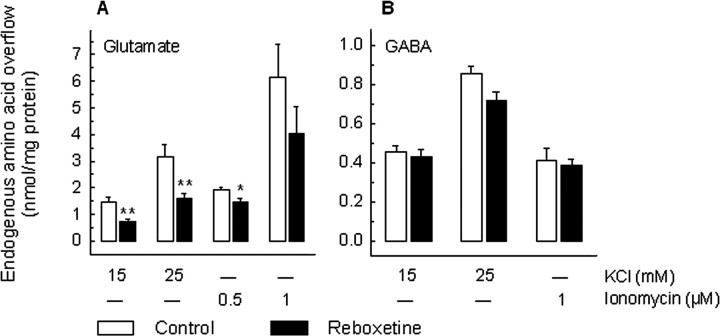
The stimulus-evoked overflow (total release minus basal outflow) of glutamate and that of GABA were differently affected by chronic treatment with the selective NE reuptake inhibitor reboxetine. As reported in Figure 2A, both the 15 and 25 mm KCl-induced glutamate overflow were reduced after chronic treatment with reboxetine by ∼50%. Interestingly, the release of glutamate induced by ionomycin (0.5 and 1 μm) was much less affected by reboxetine treatment; indeed, only the overflow evoked by 0.5 μm ionomycin was significantly (∼20%) diminished (Fig. 2A). On the contrary, the stimulus-induced overflow of GABA was not modified by reboxetine, both when using KCl (15 and 25 mm) and ionomycin (1 μm) as releasing stimuli (Fig. 2B).
Figure 2.
Effect of chronic treatment with reboxetine on the release of glutamate and GABA from rat hippocampal synaptosomes. Reboxetine was administered by means of osmotic minipumps (10 mg/kg daily). After 14 d of treatment, animals were killed for release experiments. Synaptosomes were exposed in superfusion to a 90 s pulse of KCl (15 or 25 mm) or ionomycin (0.5 or 1 μm) at t = 39 min, and the stimulus-evoked overflow of endogenous glutamate (A) and GABA (B) was monitored. The stimulus-evoked overflow was calculated by subtracting the neurotransmitter content in the basal outflow from the release evoked by stimulation (see the legend to Fig. 1). Data represent the means ± SEM of four to six separate experiments run in triplicate. *p < 0.05, **p < 0.01 versus the respective control value (two-tailed Student's t test). Open bars, Vehicle-treated animals; filled bars, reboxetine-treated animals.
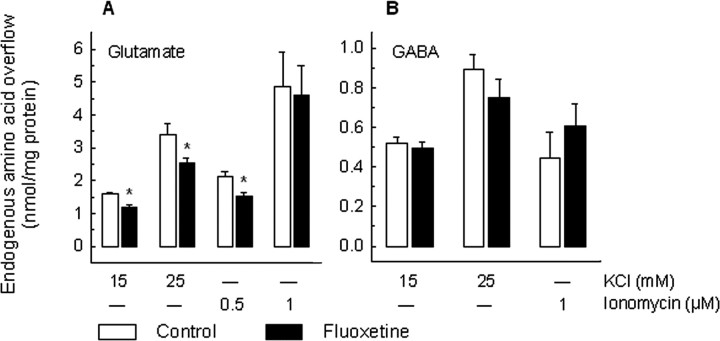
Experiments with the selective serotonin reuptake inhibitor fluoxetine gave similar results (Fig. 3): the 15 and 25 mm KCl-induced overflow of glutamate was significantly diminished by the chronic treatment, although the modulation was less pronounced than in the case of reboxetine (∼25% reduction vs 50%). Again, only the 0.5 μm, but not 1 μm, ionomycin-induced glutamate overflow was modified by the drug treatment. As for reboxetine, the KCl- or ionomycin-induced overflow of GABA was not modified by fluoxetine (Fig. 2B). No significant difference in the basal outflow of the two amino acids was observed after chronic treatment with reboxetine or fluoxetine (data not shown).
Figure 3.
Effect of chronic treatment with fluoxetine on the release of glutamate and GABA from rat hippocampal synaptosomes. Fluoxetine was administered by means of osmotic minipumps (10 mg/kg daily). After 14 d of treatment, animals were killed for release experiments. Synaptosomes were exposed in superfusion to KCl (15 or 25 mm) or ionomycin (0.5 or 1 μm), and the stimulus-evoked overflow of endogenous glutamate (A) and GABA (B) was monitored. The stimulus-evoked overflow was calculated by subtracting the neurotransmitter content in the basal outflow from the release evoked by stimulation (see the legend to Fig. 1). Data represent the means ± SEM of four to five separate experiments run in triplicate. *p < 0.05 versus the respective control value (two-tailed Student's t test). Open bars, Vehicle-treated animals; filled bars, fluoxetine-treated animals.
We also tested the effect of chronic treatment with the tricyclic drug desipramine on the release of glutamate and GABA evoked by KCl or ionomycin. As reported in Table 1 and similar to what was shown above, the release of glutamate evoked by 25 mm KCl, but not that induced by 1 μm ionomycin, was decreased by the drug treatment. Desipramine did not modify either the KCl- or ionomycin-evoked overflow of GABA (Table 1) or the spontaneous outflow of the two neurotransmitters (data not shown).
Table 1.
Effect of chronic treatment with desmethylimipramine (DMI) on the release of glutamate and GABA from rat hippocampal synaptosomes
|
|
25 mm KCl |
1 μm lonomycin |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Control |
|
DMI |
Control |
|
DMI |
||||
| Glutamate | 4.03 ± 0.270 (4) | 2.66 ± 0.309 (4) | 5.62 ± 0.803 (6) | 4.31 ± 0.721 (6) | ||||||
| p <0.05 | NS | |||||||||
| GABA | 1.15 ± 0.083 (6) | 1.21 ± 0.089 (6) | 0.677 ± 0.128 (5) | 0.675 ± 0.120 (5) | ||||||
|
|
|
NS |
|
|
NS |
|
||||
DMI was administered by means of osmotic minipumps (10 mg/kg daily). After 14 d of treatment, animals were killed for release experiments. Synaptosomes were exposed in superfusion to a 90 s pulse of 25 mm KCl or 1 μm ionomycin at t = 39 min, and the stimulus-evoked overflow of endogenous glutamate and GABA was monitored. The stimulus-evoked overflow was calculated by subtracting the neurotransmitter content in the basal release from the release evoked by stimulation (see the legend to Fig. 1). Data represent the means ± SEM of the number of experiments indicated in parentheses. Values are expressed in nanomoles per milligram of protein. NS, Nonsignificant.
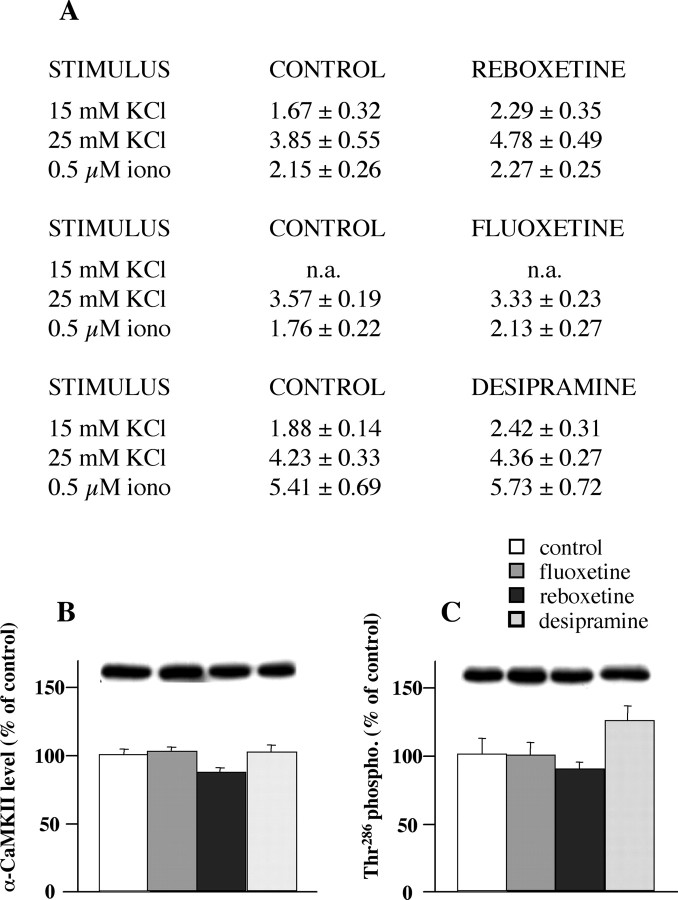
As a control, we performed release experiments after acute treatment with reboxetine, fluoxetine, or desipramine. Animals were treated once with 10 mg/kg, i.p., of each drug and killed after 3 h, and the KCl- or ionomycin-evoked release of glutamate was measured. None of the drugs modified significantly the stimulus-evoked overflow of glutamate (Fig. 4A).
Figure 4.
The effect of acute treatment (A) with fluoxetine, reboxetine, and desipramine on the release of glutamate from rat hippocampal synaptosomes. The three drugs were administered intraperitoneally (10 mg/kg), and the rats were killed after 3 h. Synaptosomes were superfused and stimulated as in previous figures; the stimulus-evoked overflow is reported. Data represent the means ± SEM of three to four separate experiments run in triplicate. No significant differences were found (two-tailed Student's t test). Expression levels of total αCaM kinase II (B) and αCaM kinase II phosphorylated on Thr286 (C) in synaptosomes from rats acutely treated with antidepressants are shown. Expression levels were normalized for level of β-actin in the same blotted membrane. Representative immunoreactive bands are shown. Data represent the means ± SEM (percentage treated vs control) of three separate experiments run in triplicate; n.a., not assessed; iono, ionomycin. No significant differences were found (two-tailed Student's t test).
Depolarization-evoked release of glutamate and GABA can be sustained by different mechanisms, including exocytosis, supported by both external Ca2+ entry and mobilization of Ca2+ from internal stores, and reversal of the transporter (Raiteri et al., 2002). To test what mechanism(s) is involved in the reduction of glutamate release after chronic treatment, we analyzed the Ca2+ dependence and the transporter dependence of the release induced by KCl or ionomycin. As shown in Figure 5, both KCl- and ionomycin-induced overflow were strictly dependent on the presence of Ca2+ in the extracellular milieu; accordingly, the glutamate transport blocker dl-TBOA did not modify the overflow of the neurotransmitter. Therefore, we concluded that the reduction of glutamate release attributable to the drug treatments above is accounted for only by an impairment of glutamate exocytosis.
Reduced expression level of soluble N-ethylmaleimide-sensitive factor attachment protein receptors in synaptic membranes after chronic antidepressants
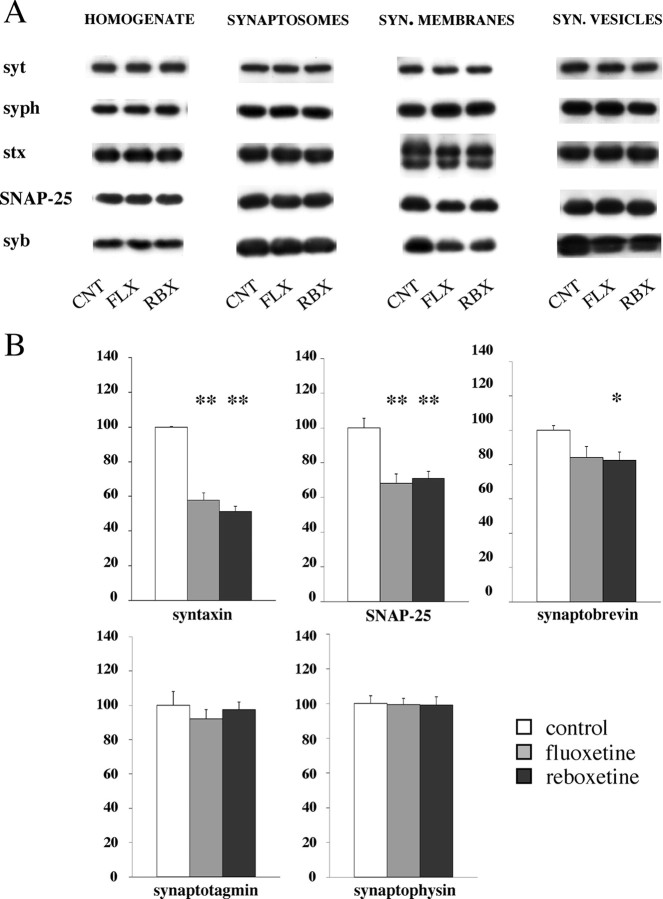
To investigate the molecular changes that could account for the large reduction in depolarization-dependent glutamate release induced by chronic antidepressants, we measured expression level of presynaptic proteins. These and the following experiments were performed with samples from rats treated with either fluoxetine or reboxetine. We assumed that a correlation between functional changes in glutamate release and molecular changes in presynaptic machinery could be established because of the large number of glutamatergic terminals in hippocampus, estimated to represent 40-50% of the total number. Furthermore, because we found that antidepressant treatments selectively reduced glutamate release without altering the release of GABA (second most abundant neurotransmitter in hippocampus), we envisaged that major changes observed in the presynaptic release machinery of drug-treated animals would reflect changes in glutamatergic terminals. We looked at the expression of the three presynaptic proteins (syb, stx, and SNAP-25) forming the SNARE core complex that mediates synaptic exocytosis, and of two proteins that regulate the function of the SNAP receptor (SNARE) complex (syt and syph) (Chen and Scheller, 2001). Previous work suggested that expression of some of them is affected by chronic antidepressants, although with somewhat conflicting results (Lesch and Schmitt, 2002; Yamada et al., 2002; Rapp et al., 2004). In contrast with previous findings, we found no significant changes in the level of the five proteins examined in total hippocampal homogenate from both fluoxetine- and reboxetine-treated rats (Fig. 6A). To assess whether expression levels were changed at specific synaptic location, we fractionated Percoll gradient-purified synaptosomes, by means of differential centrifugation and ultracentrifugation, into fractions enriched in synaptic membranes and synaptic vesicles (Celano et al., 2003). Again, we found no changes in synaptosomes and synaptic vesicles (Fig. 6A, B), but in synaptic membranes, the level of the three SNAREs was significantly decreased, whereas the two regulatory proteins (syt and syph) were unchanged. According to previous studies, the synaptic membrane fraction contains the so-called readily releasable pool (RRP) of vesicles that is exocytosed during electrical stimulation or application of hypertonic sucrose, and at the same time the trans-SNARE core complex formed by syb from vesicle membrane and stx/SNAP-25 from synaptic membrane (Rosenmund and Stevens, 1996; Chen and Scheller, 2001). It has been suggested that the amount of preformed SNARE core complex in presynaptic membranes may regulate the size of the RRP (Lonart and Sudhof, 2000). To assess whether the number of SNARE core complexes was decreased in synaptic membranes of drug-treated rats, we measured the amount of complexes, which were detected by loading unboiled synaptic membrane proteins on SDS gels and developing Western blots with stx or syb antibody (Sollner et al., 1993). We found a similar amount of SNARE complexes in vehicle- as well as in fluoxetine- or reboxetine-treated rats (data not shown), apparently ruling out a decrease in SNARE complex as a causative factor in drug-induced reduction of glutamate release.
Figure 6.
Expression levels of presynaptic proteins, measured by Western analysis, in total homogenate, whole synaptosomes, synaptic membranes, synaptic vesicles from hippocampus of vehicle- and drug-treated rats (chronic treatment). CNT, Control; FLX, fluoxetine; RBX, reboxetine. Expression levels were normalized for level of β-actin in the same blotted membrane. A, Representative immunoreactive bands. SYN., Synaptic; syt, synaptotagmin I; syph, synaptophysin; stx, syntaxin 1; syb, VAMP (vesicle-associated membrane protein)-synaptobrevin 2. B, Quantitation of protein expression level for each single protein in synaptic membrane fraction. Data represent the means ± SEM (percentage treated vs control) of six separate experiments run in triplicate. *p < 0.05, **p < 0.01 versus the respective control value (two-tailed Student's t test).
Reduced Thr286 phosphorylation of αCaM kinase II in synaptic membranes after chronic antidepressants
We showed in previous work that chronic antidepressants markedly alter the function of CaM kinase II (Popoli et al., 2000; Celano et al., 2003), a serine/threonine kinase highly expressed in hippocampus and cerebral cortex and crucial for plasticity of glutamate neurotransmission (Giese et al., 1998; Lisman et al., 2002). We found that enzymatic activity and Thr286 phosphorylation of αCaM kinase II (the major isoform in forebrain) are upregulated by antidepressants in neuronal cell bodies and in synaptic vesicles (Celano et al., 2003; Tiraboschi et al., 2004b). Autophosphorylation of Thr286 is a pivotal regulatory event for this kinase, because it generates calcium-independent kinase activity necessary for synaptic plasticity (Giese et al., 1998; Lisman et al., 2002). However, the role of CaM kinase II in presynaptic terminals, with special regard to the mechanisms of neurotransmitter release, is still unclear (Hinds et al., 2003).
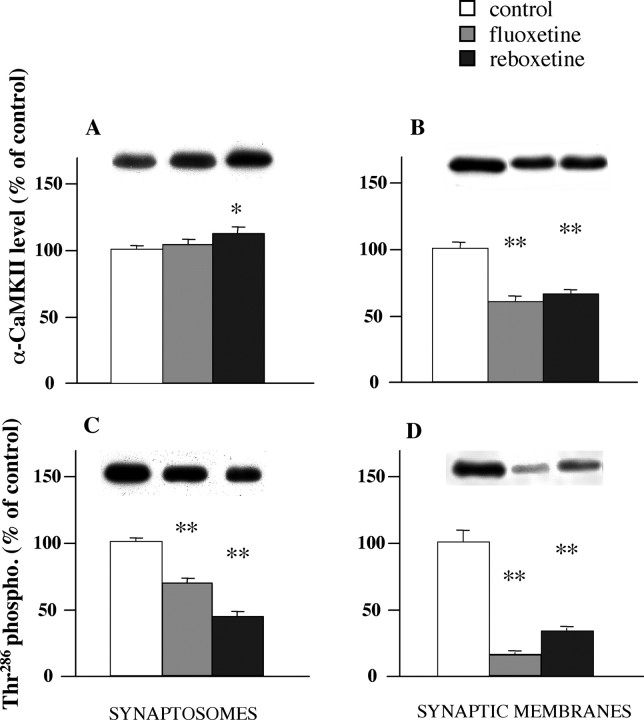
Here, we measured protein expression level and Thr286 phosphorylation of αCaM kinase II in synaptosomes and found a significant reduction of phosphorylation in chronically treated (Fig. 7B), but not acutely treated rats (Fig. 4B). The phosphorylation reduction was even more marked in synaptic membranes, although this was partly attributable to a reduction in the protein level of αCaM kinase II (Fig. 7A). It is likely that the synaptic membrane fraction accounts for this change in synaptosomes, because Thr286 phosphorylation in synaptic vesicles was increased after antidepressants, as we showed previously (Popoli et al., 2000; Celano et al., 2003). We found that the change in phosphorylation of αCaM kinase II in synaptic membranes has a deep impact on certain protein-protein interactions in presynaptic machinery regulating neurotransmitter release.
Figure 7.
Expression levels of total αCaM kinase II (A, B) and αCaM kinase II phosphorylated on Thr286 (C, D) in synaptosomes and synaptic membranes (chronic treatment). Expression levels were normalized for level of β-actin in the same blotted membrane. Representative immunoreactive bands are shown. Data represent the means ± SEM (percentage treated vs control) of six separate experiments run in triplicate. *p < 0.05, **p < 0.01 versus the respective control value (two-tailed Student's t test).
Reduced syntaxin 1-αCaM kinase II and increased syntaxin 1-Munc-18 interaction in synaptic membranes after chronic antidepressants
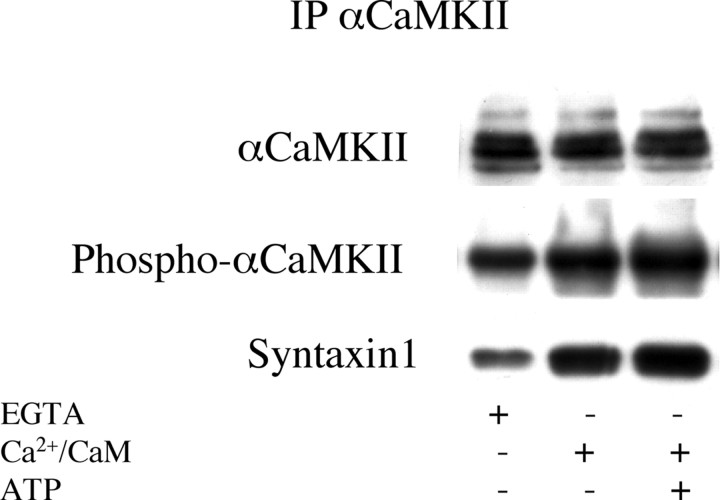
It was recently shown that syntaxin 1 reversibly binds αCaM kinase II and that the affinity of this interaction is greatly increased when the kinase is phosphorylated at Thr286 (Ohyama et al., 2002; Nomura et al., 2003). It was found that the αCaM kinase II-binding domain of syntaxin 1, injected into presynaptic neurons, inhibits synaptic transmission, and that syntaxin 1 binds alternatively either αCaM kinase II or Munc-18, a presynaptic protein that was shown to block syntaxin from interacting with other SNAREs and forming the SNARE complex (Rizo and Sudhof, 2002). Therefore, it was proposed that the complex αCaM kinase II-syntaxin is an intermediate toward, and favors the formation of the SNARE complex (Ohyama et al., 2002; Colbran, 2004). We investigated whether activation of CaM kinase II in synaptosomes effectively increases the binding of syntaxin 1, and found, by coimmunoprecipitation, that indeed activating the kinase by adding calcium/CaM/ATP markedly increased the amount of syntaxin 1 bound to the kinase (Fig. 8). Therefore, we speculated that the large reduction of αCaM kinase II phospho-Thr286 in synaptic membranes of drug-treated rats could lead to a reduced interaction between αCaM kinase II and syntaxin 1, and in turn to an increased interaction of syntaxin 1 with Munc-18, a modification that would seemingly reduce the efficiency of exocytosis.
Figure 8.
Activation of αCaM kinase II in vitro increases phosphorylation of Thr286 and binding of syntaxin 1 to αCaM kinase II. αCaM kinase II was activated by adding to lysed synaptosomes 1 mm CaCl2, 20 μg/ml CaM, or 1 mm CaCl2, 20 μg/ml CaM, and 500 μm ATP. Control samples contained 20 mm EGTA. The reaction was performed at 37°C for 5 min and stopped by adding immunoprecipitation buffer. After immunoprecipitation with antibody for αCaM kinase II, immunoprecipitated protein was separated by SDS-electrophoresis and electroblotted on membrane. The membrane was probed with antibodies for αCaM kinase II, phospho-αCaM kinase II, and syntaxin 1. Representative of four separate experiments run in duplicate.
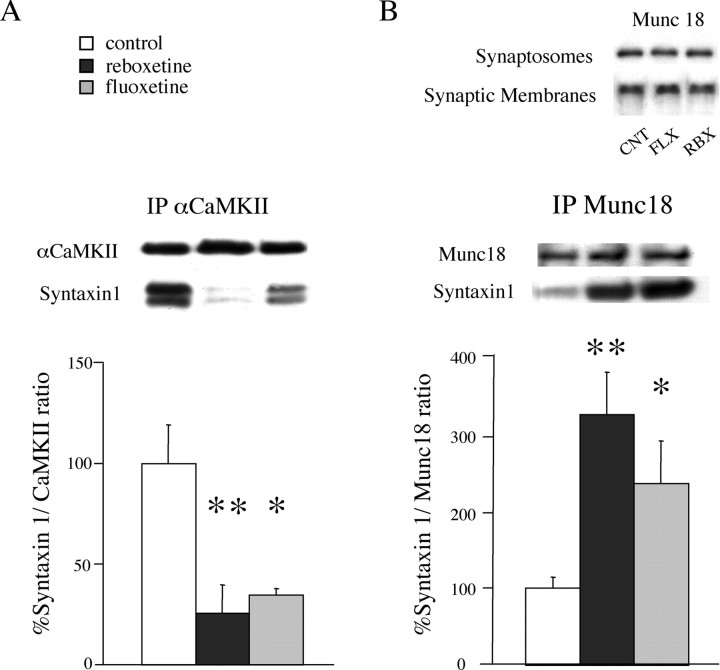
To verify this hypothesis, we immunoprecipitated αCaM kinase II from synaptic membranes of control, reboxetinetreated, and fluoxetine-treated rats, and measured by Western blot the amount of syntaxin 1 coimmunoprecipitated with the kinase (Fig. 9A). Although, as shown above (Fig. 7), the expression level of αCaM kinase II in synaptic membranes was reduced after chronic drug treatments, the amount of αCaM kinase II immunoprecipitated from control and drug-treated animals was consistent and reproducible (Fig. 9A). For each immunoprecipitated sample, the quantitated signal of syntaxin 1 band was normalized to the corresponding αCaM kinase II band in the same lane. The kinase-bound syntaxin 1 was reduced 73 ± 14% by reboxetine and 64 ± 3% by fluoxetine. Then, we immunoprecipitated Munc-18 and measured the amount of syntaxin 1 bound to Munc-18 by the same procedure as above (Fig. 9B). We verified previously the expression level of Munc-18 in synaptosomes and synaptic membranes of drug-treated rats, and found it was unchanged (Fig. 9B, inset). On coimmunoprecipitation, we found a dramatic increase in the amount of Munc-18-bound syntaxin 1 (229 ± 56% by reboxetine and 138 ± 57% by fluoxetine). These results showed that, in synaptic membranes of drug-treated rats, syntaxin 1 undergoes a major redistribution between αCaM kinase II and Munc-18, suggesting that antidepressants induce major changes in presynaptic machinery and in the efficiency of glutamate release.
Figure 9.
Chronic treatment with fluoxetine or reboxetine increases the binding of syntaxin 1 to αCaM kinase II and decreases the binding of syntaxin 1 to Munc-18 in synaptic membranes. A, αCaM kinase II was immunoprecipitated; αCaM kinase II and syntaxin 1 in the immunoprecipitate were analyzed by Western analysis. Representative immunoreactive bands are shown. Data represent the means ± SEM (percentage ratio syntaxin 1/αCaM kinase II) of four separate experiments in duplicate. *p < 0.05, **p < 0.01 versus the respective control value (two-tailed Student's t test). B, Munc-18 was immunoprecipitated; Munc-18 and syntaxin 1 in the immunoprecipitate were analyzed by Western analysis. CNT, Control; FLX, fluoxetine; RBX, reboxetine. Data represent the means ± SEM (percentage ratio syntaxin 1/Munc-18) of four separate experiments in duplicate. *p < 0.05, **p < 0.01 versus the respective control value (two-tailed Student's t test). Inset, Protein expression level of Munc-18 in synaptosomes and synaptic membranes.
Discussion
Chronic antidepressant treatments reduce depolarization-evoked release of glutamate: functional and pharmacological consequences
The hippocampal formation, a brain area involved in declarative, spatial, and contextual memory, particularly sensitive to stressful insults, undergoes atrophy in prolonged depression along with impairment of cognitive functions (Burt et al., 1995; Sheline et al., 1999). Glutamate excitotoxicity in hippocampus was implicated in pathophysiology of mood disorders (Sapolsky, 2000; Zarate et al., 2002). Indeed, both stress and glucocorticoid hormones (often elevated in depressed patients) increase extracellular glutamate concentration (Moghaddam et al., 1994; Lowy et al., 1995). Furthermore, augmentation of NMDA receptor expression and activation seems to mediate some cellular effects of stress, whereas antidepressants reverse these effects to some extent (Bartanusz et al., 1995; Skolnick, 1999; Kole et al., 2002). In this work, for the first time, we obtained clear evidence that chronic but not acute antidepressants of three different classes (SSRI, TCA, NRI) selectively reduce depolarization-evoked release of glutamate in hippocampus. It is interesting that basal release of glutamate and basal/evoked release of GABA from nerve terminals were not changed by the drug treatments. First, this suggests that release of glutamate evoked by neuronal activation but not basal (unstimulated) release is affected. Although there is no direct evidence that stress increases glutamate release, both microdialysis measurements and electrophysiological recordings strongly suggest this possibility (Moghaddam et al., 1994; Kole et al., 2002). If, as proposed, consequences of stress at molecular and cellular levels have a primary role in the induction of morphological/functional modifications in mood disorders (McEwen, 1999; Sapolsky, 2000), then antidepressants might work by limiting excessive release of glutamate when this is induced by stressful neuronal activation. Our observation that this effect is measurable only after repeated drug administration is also in line with the well known property of these drugs to be therapeutically efficient only after prolonged treatment (Stahl, 1998). Second, our results show that glutamatergic neurotransmission is selectively inhibited (GABA was not affected); therefore, release of glutamate evoked by neuronal activation will be decreased in the face of unchanged constraint exerted by GABA. This would induce a marked alteration in the balance between excitatory and inhibitory neurotransmission, contributing to dampening of excessive neuronal activation after stressful stimuli (Liu, 2004).
An intriguing result of this study is that drug treatments selectively inhibited the release of glutamate evoked by K+ depolarization, while having little or no effect on release induced by the calcium ionophore ionomycin; in our hands, only the release induced by the lowest concentration of ionomycin was significantly decreased by drug treatments (Figs. 2, 3; Table 1). The most likely explanation for this difference is that the two kinds of stimuli affect pools of synaptic vesicles of different origin. It is generally agreed that depolarization, electrical stimulation, and hypertonic sucrose exocytose the RRP of vesicles, morphologically corresponding to the vesicles docked to the presynaptic membrane and primed for release (Schikorski and Stevens, 2001). We and others recently showed that, although exocytosis of RRP induced by either K+ depolarization or hypertonic sucrose is sensitive to clostridial neurotoxins, a late phase of neurotransmitter release induced by calcium ionophores is not blocked by clostridial neurotoxins and is probably attributable to the involvement of vesicles set into the reserve pool (Ashton and Dolly, 2000; Stigliani et al., 2003). Therefore, our present results would suggest that antidepressant treatments particularly affect the release of glutamate from the RRP, thereby altering a physiologically relevant pool of neurotransmitter. In this view, the reduction of 0.5 μm ionomycin-evoked glutamate release by antidepressants can be easily interpreted to be attributable to a significant contribution of the RRP to the total release induced by this low concentration of the calcium ionophore. Furthermore, the changes in protein-protein interactions observed here in drug-treated rats displaying reduced glutamate release were found in the synaptic membrane fraction, shown previously to contain docked, release-competent vesicles bound to the membrane (Mehta et al., 1996).
Two previous works investigated the action of antidepressants on glutamate release. In the first (Wang et al., 2003), the effect of in vitro application of fluoxetine was studied (see below). In the second, two different drugs (imipramine and phenelzine) were found to reduce depolarization-evoked glutamate overflow from slices of prefrontal cortex after both acute and chronic treatment (Michael-Titus et al., 2000). In our hands, only chronic drug treatments reduced glutamate release from synaptic terminals, whereas acute treatments were devoid of any effect. Several differences in the methodology may account for this, the primary one being that we used purified synaptosomes from hippocampus rather than cortical slices, in which indirect effects are likely to occur. Our release procedure, using synaptosomes stratified as a monolayer on a microporous filter and stimulated in superfusion, allows measuring the release of a given neurotransmitter from a single population of synaptic boutons without interference by the myriad of other transmitters secreted from neighboring structures (Raiteri et al., 1974; Raiteri and Raiteri, 2000).
A mechanism for modulation of glutamate release: interaction of syntaxin 1 with phospho-αCaM kinase II and Munc-18
Different mechanisms can lie behind the decrease of glutamate exocytosis observed in chronically treated animals. As reported above, in vitro application of fluoxetine reduces 4-AP-evoked release of glutamate from cerebrocortical synaptosomes, reportedly by inhibiting P/Q-type calcium channels (Wang et al., 2003). The fact that none of the drugs used here, including fluoxetine, exerted any acute effect on glutamate release suggests that this mechanism is not involved. To understand whether changes in the presynaptic machinery regulating release are involved, we first looked at the expression level of SNAREs, synaptobrevin, syntaxin 1, SNAP-25, and associated proteins. We found a selective decrease of expression for the three SNAREs in synaptic membranes, reportedly involved in the assembly of trans-SNARE core complex. However, this did not lead to a decrease in the net amount of SNARE complex measured in the membranes of drug-treated rats, suggesting that more subtle changes, such as in the kinetics of SNARE complex assembly, may be involved.
Indeed, we found that the Thr286 autophosphorylation of αCaM kinase II associated with synaptic membranes was reduced by 70-80% in drug-treated rats (membrane expression of kinase was also reduced, to a lower extent). In addition to its regulation of presynaptic proteins by phosphorylation (Popoli, 1993; Hilfiker et al., 1999), αCaM kinase II was also implicated in a more direct regulation of the exocytotic machinery, because the autophosphorylated kinase interacts with syntaxin 1 and promotes exocytosis, probably by favoring the formation of the SNARE complex (Colbran, 2004). In fact, it was shown previously that only the open conformation of syntaxin 1 (that is able to assemble into the SNARE complex) binds autophosphorylated αCaM kinase II, whereas the closed conformation (unable to assemble into the SNARE complex) binds Munc-18, with an interaction competing with SNARE complex formation (Yang et al., 2000; Ohyama et al., 2002). We found that the interaction syntaxin 1/αCaM kinase II was reduced by 60-70% and the interaction syntaxin 1/Munc-18 was augmented up to 200% in drug-treated rats, strongly suggesting that, in hippocampal glutamatergic nerve terminals, a reduced autophosphorylation of the kinase downregulates the availability of syntaxin 1 for the SNARE complex, thereby reducing depolarization-evoked release of neurotransmitter.
If changes in presynaptic protein-protein interactions are likely to be a mechanistic reason for reduction of glutamate exocytosis, complex receptor-mediated events, induced by chronic transporter blockade, could induce the presynaptic protein changes (for review, see Artigas et al., 1996; Millan et al., 2000; Popoli et al., 2000). In particular, whereas noradrenergic and serotonergic neurons possess autoreceptors mediating functional inhibition (Starke et al., 1989), glutamatergic terminals are endowed with release-inhibiting α2-adrenergic (Kamisaki et al., 1992) and 5-HT1B/D serotonergic (Maura et al., 1998) heteroreceptors. When chronically activated, release-regulating autoreceptors undergo downregulation (Maura and Raiteri, 1984; Raiteri et al., 1986), whereas heteroreceptors in general seem not to downregulate (Raiteri et al., 1983), possibly because they are involved in distant nonsynaptic or volume transmission (for review, see Vizi, 2000). Therefore, one can speculate that, if autoreceptors are downregulated after chronic antidepressants, NE and 5-HT exocytosis is disinhibited and the consequent heteroreceptor activation is transduced into presynaptic protein changes and inhibition of glutamate release. A central role in this mechanism seems to be played by αCaM kinase II: chronic blockade of 5-HT or NE transporter induces complex adaptive changes in autophosphorylation and function of the kinase in synaptosomes, which in turn modify relevant interactions of αCaM kinase II with presynaptic machinery [for a complete discussion of relationship between receptor changes and changes in αCaM kinase II, see Popoli et al. (2000)]. A relevant question is whether the effect we observed is unique to antidepressants. In a previous study, we addressed this question by measuring enzymatic activity, expression, and Thr286 phosphorylation of αCaM kinase II in synaptic vesicles from rats chronically treated with antidepressants, haloperidol, or lithium (Celano et al., 2003). We found that, whereas antidepressants upregulated the kinase, haloperidol did not change any of the kinase parameters, and lithium down-regulated the kinase. These results suggest that the effect of antidepressants on αCaM kinase II is not shared by other major classes of psychotropic drugs.
Significance of present results for pathophysiology and treatment of mood disorders
The present and previous results clearly indicate that dampening glutamate neurotransmission is a common effect of antidepressants, likely representing a component in the therapeutic action of these compounds (Skolnick, 1999; Popoli et al., 2002; Zarate et al., 2002; Holden, 2003). In a companion study (investigating expression of genes related to the glutamate system), we found that chronic antidepressants markedly reduced the expression of NR1 subunit of NMDA receptor in synaptic membranes of hippocampus, with no changes in NR1 mRNA/protein in total extract (A. Barbon, S. Barlati, M. Gennarelli, E. Tiraboschi, and M. Popoli, unpublished observations). Overall, it appears that these drugs may stabilize glutamate neurotransmission at various levels. The intriguing finding of a redistribution of syntaxin 1 between αCaM kinase II and Munc-18 defines selected mechanisms in the presynaptic machinery, as well as αCaM kinase II and glutamate receptors, as possible targets for new pharmacological strategies.
Footnotes
This work was supported by grants from the National Alliance for Research on Schizophrenia and Depression and the Ministry of University (Italy) (Progetti di Ricerca di Interesse Nazionale 2001054224 and 2003053993). We are indebted to Valentina Barbiero for help with immunoprecipitation experiments. This study was presented in preliminary form at the 34th Annual Meeting of the Society for Neuroscience (2004).
Correspondence should be addressed to Dr. Maurizio Popoli, Center of Neuropharmacology, Department of Pharmacological Sciences, University of Milan, Via Balzaretti 9, 20133 Milan, Italy. E-mail: maurizio.popoli@unimi.it.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253270-10$15.00/0
R.G. and L.R. contributed equally to this work.
References
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F (1993) Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry 150: 1731-1733. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P (1996) Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 19: 378-383. [DOI] [PubMed] [Google Scholar]
- Ashton AC, Dolly JO (2000) A late phase of exocytosis from synaptosomes induced by elevated [Ca2+]i is not blocked by Clostridial neurotoxins. J Neurochem 74: 1979-1988. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Aubry JM, Pagliusi S, Jezova D, Baffi J, Kiss JZ (1995) Stress-induced changes in messenger RNA levels of N-methyl-d-aspartate and AMPA receptor subunits in selected regions of the rat hippocampus and hypothalamus. Neuroscience 66: 247-252. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G (1995) Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 117: 285-305. [DOI] [PubMed] [Google Scholar]
- Celano E, Tiraboschi E, Consogno E, D'Urso G, Mbakop MP, Gennarelli M, de Bartolomeis A, Racagni G, Popoli M (2003) Selective regulation of presynaptic calcium/calmodulin-dependent protein kinase II by psychotropic drugs. Biol Psychiatry 53: 442-449. [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH (2001) SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98-106. [DOI] [PubMed] [Google Scholar]
- Colbran RJ (2004) Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J 378: 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC (2000) Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 126: 413-431. [DOI] [PubMed] [Google Scholar]
- Drevets WC (2004) Neuroplasticity in mood disorders. Dialogues Clin Neurosci 6: 199-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson Jr JR, Todd RD, Reich T, Vannier M, Raichle ME (1997) Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386: 824-827. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA (1986) A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res 372: 115-129. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the α-calcium-calmodulin kinase II in LTP and learning. Science 279: 870-873. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P (1999) Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354: 269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, Goussakov I, Nakazawa K, Tonegawa S, Bolshakov VY (2003) Essential function of α-calcium/calmodulin-dependent protein kinase II in neurotransmitter release at a glutamatergic central synapse. Proc Natl Acad Sci USA 100: 4275-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden C (2003) Psychiatric drugs. Excited by glutamate. Science 300: 1866-1868. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Hamahashi T, Hamada T, Maeda K, Itoh T (1992) Presynaptic inhibition by clonidine of neurotransmitter amino acid release in various brain regions. Eur J Pharmacol 217: 57-63. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF (1996) Behavioral stress modifies hippocampal plasticity through N-methyl-d-aspartate receptor activation. Proc Natl Acad Sci USA 93: 4750-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Swan L, Fuchs E (2002) The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci 16: 807-816. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Schmitt A (2002) Antidepressants and gene expression profiling: how to SNARE novel drug targets. Pharmacogenomics J 2: 346-348. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175-190. [DOI] [PubMed] [Google Scholar]
- Liu G (2004) Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7: 373-379. [DOI] [PubMed] [Google Scholar]
- Lonart G, Sudhof TC (2000) Assembly of SNARE core complexes prior to neurotransmitter release sets the readily releasable pool of synaptic vesicles. J Biol Chem 275: 27703-27707. [PubMed] [Google Scholar]
- Lowy MT, Wittenberg L, Yamamoto BK (1995) Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem 65: 268-274. [DOI] [PubMed] [Google Scholar]
- Maura G, Raiteri M (1984) Functional evidence that chronic drugs induce adaptive changes of central autoreceptors regulating serotonin release. Eur J Pharmacol 97: 309-313. [DOI] [PubMed] [Google Scholar]
- Maura G, Marcoli M, Tortarolo M, Andrioli GC, Raiteri M (1998) Glutamate release in human cerebral cortex and its modulation by 5-hydroxytryptamine acting at h5-HT1D receptors. Br J Pharmacol 123: 45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1999) Stress and hippocampal plasticity. Annu Rev Neurosci 22: 105-122. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Battenberg E, Wilson MC (1996) SNAP-25 and synaptotagmin involvement in the final Ca2+-dependent triggering of neurotransmitter exocytosis. Proc Natl Acad Sci USA 93: 10471-10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Titus AT, Bains S, Jeetle J, Whelpton R (2000) Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex: a possible mechanism of neuroprotection in major depression? Neuroscience 100: 681-684. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A (2000) Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex: relevance to the actions of antidepressant agents. J Psychopharmacol 14: 114-138. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R (1994) Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res 655: 251-254. [DOI] [PubMed] [Google Scholar]
- Nomura K, Ohyama A, Komiya Y, Igarashi M (2003) Minimal residues in linker domain of syntaxin 1A required for binding affinity to Ca2+/calmodulin-dependent protein kinase II. J Neurosci Res 72: 198-202. [DOI] [PubMed] [Google Scholar]
- Ohyama A, Hosaka K, Komiya Y, Akagawa K, Yamauchi E, Taniguchi H, Sasagawa N, Kumakura K, Mochida S, Yamauchi T, Igarashi M (2002) Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J Neurosci 22: 3342-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M (1993) Synaptotagmin is endogenously phosphorylated by Ca2+/calmodulin protein kinase II in synaptic vesicles. FEBS Lett 317: 85-88. [DOI] [PubMed] [Google Scholar]
- Popoli M, Brunello N, Perez J, Racagni G (2000) Second messenger-regulated protein kinases in the brain: their functional role and the action of antidepressant drugs. J Neurochem 74: 21-33. [DOI] [PubMed] [Google Scholar]
- Popoli M, Gennarelli M, Racagni G (2002) Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord 4: 166-182. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M (2000) Synaptosomes still viable after 25 years of superfusion. Neurochem Res 25: 1265-1274. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M, Bonanno G (2002) Coexistence and function of different neurotransmitter transporters in the plasma membrane of CNS neurons. Prog Neurobiol 68: 287-309. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Angelini F, Levi G (1974) A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur J Pharmacol 25: 411-414. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Marchi M, Maura G (1983) Chronic drug treatments induce changes in the sensitivity of presynaptic autoreceptors but not of presynaptic heteroreceptors. Eur J Pharmacol 91: 141-143. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Bonanno G, Maura G (1986) Changes of sensitivity of presynaptic α2-adrenoceptors induced by chronic clonidine in rat brain. J Hypertension 4: S122-S124. [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA (1999) Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45: 1085-1098. [DOI] [PubMed] [Google Scholar]
- Rapp S, Baader M, Hu M, Jennen-Steinmetz C, Henn FA, Thome J (2004) Differential regulation of synaptic vesicle proteins by antidepressant drugs. Pharmacogenomics J 4: 110-113. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC (2002) SNAREs and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci 3: 641-653. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF (1996) Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16: 1197-1207. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF (2004) Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61: 705-713. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2000) The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry 48: 755-765. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF (2001) Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci 4: 391-395. [DOI] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ (2002) Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci 22: 3638-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999) Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19: 5034-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P (1999) Antidepressants for the new millennium. Eur J Pharmacol 375: 31-40. [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409-418. [DOI] [PubMed] [Google Scholar]
- Stahl SM (1998) Basic psychopharmacology of antidepressants, part 1: antidepressants have seven distinct mechanisms of action. J Clin Psychiatry 59 [Suppl 4]: 5-14. [PubMed] [Google Scholar]
- Starke K, Göthert M, Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69: 864-989. [DOI] [PubMed] [Google Scholar]
- Stigliani S, Raiteri L, Fassio A, Bonanno G (2003) The sensitivity of catecholamine release to botulinum toxin C1 and E suggests selective targeting of vesicles set into the readily releasable pool. J Neurochem 85: 409-421. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M (2004a) Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology 29: 1831-1840. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Giambelli R, D'Urso G, Galietta A, Barbon A, de Bartolomeis A, Gennarelli M, Barlati S, Racagni G, Popoli M (2004b) Antidepressants activate CaMKII in neuron cell body by Thr286 phosphorylation. Neuro-Report 15: 2393-2396. [DOI] [PubMed] [Google Scholar]
- Verona M, Zanotti S, Schafer T, Racagni G, Popoli M (2000) Changes of synaptotagmin interaction with t-SNARE proteins in vitro after calcium/calmodulin-dependent phosphorylation. J Neurochem 74: 209-221. [DOI] [PubMed] [Google Scholar]
- Vizi ES (2000) Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev 52: 63-89. [PubMed] [Google Scholar]
- Wang SJ, Su CF, Kuo YH (2003) Fluoxetine depresses glutamate exocytosis in the rat cerebrocortical nerve terminals (synaptosomes) via inhibition of P/Q-type Ca2+ channels. Synapse 48: 170-177. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS (1992) Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 588: 341-345. [DOI] [PubMed] [Google Scholar]
- Yamada M, Takahashi K, Tsunoda M, Nishioka G, Kudo K, Ohata H, Kamijima K, Higuchi T, Momose K (2002) Differential expression of VAMP2/synaptobrevin-2 after antidepressant and electroconvulsive treatment in rat frontal cortex. Pharmacogenomics J 2: 377-382. [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez Jr LC, Scheller RH (2000) nSec1 binds a closed conformation of syntaxin1A. J Cell Biol 148: 247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Quiroz J, Payne J, Manji HK (2002) Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull 36: 35-83. [PubMed] [Google Scholar]