Abstract
Schwann cell myelin contains highly compacted layers of membrane as well as noncompacted regions with a visible cytoplasm. One of these cytoplasmic compartments is the Schmidt-Lanterman incisure, which spirals through the compacted layers and is believed to help sustain the growth and function of compact myelin. Incisures contain adherens junctions (AJs), the key components of which are E-cadherin, its cytoplasmic partners called catenins, and F-actin. To explore in vivo the role of cadherin and catenins in incisures, E-cadherin mutant proteins that completely replace endogenous cadherin have been delivered to the cells using adenovirus. When the introduced cadherin lacked its extracellular domain, association of p120 catenin (p120ctn) with the cadherin did not occur, and incisures disappeared. Remarkably, the additional replacement of two phosphorylatable tyrosines by phenylalanine in the cytoplasmic tail of the mutant cadherin restored both p120ctn binding and incisure architecture, indicating that p120ctn recruitment is critical for incisures maintenance and might be regulated by phosphorylations. In addition, the ability of the p120ctn/cadherin complex to support incisures was blocked by mutation of the Rho GTPase regulatory region of the p120ctn, and downregulation of Rac1 activity at the junction reversed this inhibition. Because Rho GTPases regulate the state of the actin filaments, these findings suggest that one role of p120ctn in incisures is to organize the cytoskeleton at the AJ. Finally, developmental studies of Schwann cells demonstrated that p120ctn recruitment from the cytoplasm to the AJ occurs before the appearance of Rac1 GTPase and F-actin at the junction.
Keywords: adherens junction, morphogenesis, Schwann cells, cadherins, p120 catenin, myelin sheath
Introduction
The formation of a myelin sheath around peripheral nerve axons by Schwann cells is essential for the rapid propagation of action potentials. The unique and complex structure of myelin includes not only its characteristic regions of highly compacted membrane but also zones that contain cytoplasm. These noncompacted regions of myelin include the Schmidt-Lanterman incisures, which are conical tube-like cytoplasmic structures that cross the compact myelin and connect the Schwann cell peripheral (abaxonal) cytoplasm to the periaxonal (adaxonal) cytoplasm. It is believed that incisures are important for transport of metabolic substances across the myelin sheath and for the metabolic maintenance and longitudinal growth of the sheath (for review, see Ghabriel and Allt, 1981; Arroyo and Scherer, 2000).
Adherens junctions (AJs) are abundantly distributed in incisures (Ghabriel and Allt, 1981; Fannon et al., 1995), and it has been suggested that they contribute to the architecture of these structures (Fannon et al., 1995). E-cadherin, which is a member of the classical cadherin family of calcium-dependent homophilic adhesion molecules (for review, see Yap et al., 1997), forms the core of the Schwann cell AJs (Fannon et al., 1995). Cadherin function at the AJ requires association with cytoplasmic partners called catenins and the actin cytoskeleton. β-Catenin and γ-catenin bind to the distal domain of the E-cadherin cytoplasmic tail and provide a link to the cytoskeleton via the recruitment of α-catenin, which directly binds to actin (for review, see Yap et al., 1997). p120 catenin (p120ctn), which binds to the E-cadherin juxtamembrane domain (JMD), can regulate cadherin-mediated adhesion (Aono et al., 1999; Thoreson et al., 2000) by a mechanism that appears to involve cadherin transport and stabilization at the membrane (Ireton et al., 2002; Chen et al., 2003; Davis et al., 2003; Xiao et al., 2003). Surprisingly, the p120ctn gene is not required for invertebrate development (Myster et al., 2003; Pettitt et al., 2003), but recent studies using a depletion-rescue strategy in Xenopus indicate an essential role for p120ctn in early development possibly through an interplay with Rho GTPases (Fang et al., 2004).
Although the strategic location of AJ in Schwann cells suggests a key morphogenetic role, little is known about the functional relationship between AJ and myelin architecture. To address this issue, we have developed an experimental system based on the use of dominant-acting mutants of E-cadherin and p120ctn delivered to the Schwann cell in vivo using an adenoviral vector. Our focus has been the Schmidt-Lanterman incisure because of its repeated cellular distribution and relative morphological and molecular simplicity. These attributes are helpful in establishing clear correlations between molecular perturbation of the AJ and cell architecture. The results obtained suggest that AJs are key players in maintenance of incisures and that their function involves association of p120ctn with the cadherin, resulting in regulation of a junctional Rho GTPase that may help to organize the cytoskeleton.
Materials and Methods
Antibodies. Antibodies against the interleukin 2 receptor (IL2R; CD25) extracellular domain were mouse IL2R-1 (NeoMarkers, Fremont, CA) for use in Western blots and rat 33B3 (Immunotech, Marseille, France) and mouse 3G10 (Caltag Laboratories, Burlingame, CA) for use in immunostaining. Antibodies against E-cadherin were the rat monoclonal ECCD-2 (Zymed, San Francisco, CA) against the extracellular domain and the mouse monoclonal 36 (Transduction Laboratories, Lexington, KY) against the intracellular domain. Antibodies against catenins were obtained from Transduction Laboratories. Other antibodies used were against connexin 29 (clone ZMD.81; Zymed), CNPase (clone 11-5B; NeoMarkers), Rac1 (clone 23A8; Upstate Biotechnology, Lake Placid, NY), RhoA (rabbit polyclonal 119; Santa Cruz Biotechnology, Santa Cruz, CA), green fluorescent protein (GFP) (clones 7.1 and 13.1; Roche Molecular Biochemicals, Indianapolis, IN), and phosphotyrosine (clone PT-66; Sigma, St. Louis, MO).
Adenovirus production. Adenoviral preparations were performed and manipulated following National Institutes of Health guidelines. All adenoviral vectors were prepared following published methods (He et al., 1998). Briefly, pAdtrack. CMV vectors containing the constructs were recombined with pAdeasy1 vector in the Adeasy1-BJ5183 strain (Stratagene, La Jolla, CA). The isolated adenoviral DNA was cut with PacI and transfected in 293 cells (Microbix Biosystems, Toronto, Ontario, Canada) using Lipofectamine 2000 (Invitrogen, San Diego, CA). After the first production of adenovirus, three rounds of amplification were performed. In the last step, the viral particles were harvested from the cells by freeze/thaw and ammonium sulfate precipitation (Schagen et al., 2000). Cell and supernatant viral particles were mixed after a first purification on a discontinuous cesium chloride gradient and repurified on another discontinuous gradient. The viral particles were dialyzed against a storage buffer (10 mm Tris, pH 8.0, 2 mm MgCl2, 5% sucrose), concentrated using sterile Microcon minifilters (Millipore, Bedford, MA), aliquoted with 0.01% Fast Green dye (Sigma), and stored at -70°C. The titer of these viral solutions was 109 to 1012 colony-forming units. In parallel, the viral DNA was transfected in 293 cells or Chinese hamster ovary (CHO) cells in 35 mm dishes and the expressed protein analyzed on SDS-PAGE/Western blot to confirm the expression of the correct construct.
Animal surgery and injection. Animals were handled according to protocols approved by the Institutional Animal Care and Use Committee of the Memorial Sloan-Kettering Cancer Center. Seven-week-old Rag2-/- strain mice (Taconic, Germantown, NY) were anesthetized and placed under a Zeiss (Germantown, NY) loop. The gluteus superficialis and biceps femoris muscles were separated to reveal the cavity traversed by the sciatic nerve. A thin glass needle filled with colored viral solution (8 μl) was introduced into the nerve with a micromanipulator. This solution was injected over 10 min with short pressure pulses using a Picospritzer III (Parker Hannifin, Fairfield, NJ), so that regions of the nerve far away from the injection site could be filled. The nerve was replaced in the cavity, the muscles were readjusted, and the wound was closed. Animals were allowed to recover and treated with buprenorphine and antibiotics (Sulfatrim; High-Tech Pharmacal, Amityville, NY) to prevent infection. The clips were removed 1 week after surgery, and the animals were killed by CO2 inhalation 2 weeks later.
Immunohistochemistry. The dissected nerve was washed in L15 medium, fixed in Zamboni's fixative (Stefanini et al., 1967) for 10 min at room temperature, washed in PBS, and incubated in successive glycerol baths (15, 45, 60, 66% in PBS) for 18-24 h each before freezing at -20°C. The nerves were cut in small pieces in 66% glycerol, and the perineurium sheet was removed. Small bundles of fibers were teased in water on polylysine-coated slides, dried overnight at room temperature, and the slides were stored at -20°C. For immunostaining, the slides were incubated in acetone for 10 min at -20°C, washed in PBS, and the samples incubated for 1 h at room temperature in a the blocking solution (10% goat serum, 0.2% Triton X-100, and 0.01% sodium azide in PBS). The samples were then incubated with primary antibodies in blocking solution overnight at 4°C. The next day, the samples were washed in PBS and incubated for 1 h at room temperature with secondary donkey antibodies coupled to cyanine 3 (cy3), cy5 (1:800; Jackson ImmunoResearch, West Grove, PA), or Alexa488 (1:1600; Molecular Probes, Eugene, OR). Myelinated Schwann cells were stained for F-actin by adding tetramethylrhodamine isothiocyanate-phalloidin (5 μg/ml; Sigma) during the first antibody incubation. Finally, the samples were washed in PBS and mounted in Citifluor (Ted Pella, Redding, CA).
For CHO immunostaining, cells were seeded in eight-well permanox slides (Labtek, Campbell, CA) for 1 d, infected with adenoviruses for 2 d, and fixed for 5 min in Zamboni's fixative. After PBS washes, the cells were incubated in blocking buffer for 1 h, then incubated with the first antibody for 1 h at room temperature, washed in PBS, and incubated with the secondary antibody for 45 min at room temperature and with A546-phalloidin (0.33 μm; Molecular Probes) for 20 min. After PBS washes, samples were mounted in Citifluor.
Images were acquired at room temperature using a 63× C-Apochromat objective and a LSM510 Zeiss confocal microscope and its associated software. The pictures were then saved in a tagged image file format and processed with Photoshop (Adobe Systems, San Jose, CA) to build the figures. Entire GFP-labeled cells were reconstructed from five or six images, and the neighboring cells were masked for clarity.
Electron microscopy. Nerves were fixed in situ for 10 min in 2% glutaraldehyde in cacodylate buffer, pH 7.4, dissected, and fixed for an additional 2 h at 4°C in the same solution. The samples were washed in PBS, postfixed in osmium tetroxide 1% for 1 h, dehydrated, and embedded in epoxy resin.
Molecular biology. The IL2R constructs were based on a IL2R-human E-cadherin cytoplasmic domain (CYTO) (without 3xFlag tag), kindly provided by Dr. C. Gottardi (Memorial Sloan-Kettering Cancer Center). In puc19, the intracellular domain of E-cadherin was cut by AflIII to remove the stop codon and to introduce a linker containing the remaining cadherin sequence, additional restriction sites, and a stop codon. A 3xFlag linker was then introduced using the additional restriction sites. To create EcadJMD, the EcadCYTO construct was cut with BsaBI and AflIII, blunted, and religated. To create EcadCYTOΔP755,756, EcadCYTO construct was mutated to replace the tyrosines 755 and 756 of human E-cadherin with phenylalanines using the Transformer site-directed mutagenesis kit (Clontech, Cambridge, UK). All these constructs were transferred from puc19 to pAdtrack-CMV-IL2R in frame.
The human E-cadherin clone mutated on the binding site for p120ctn (glycines 761-763 in alanines) (Thoreson et al., 2000) was tagged by 3xFlag by replacing the nontagged C-terminal (Ct) end (cut with BspHI) by the tagged C-terminal end of EcadCYTO described above.
The mouse p120ctn clone 1 (without splicing A, B, and C) was kindly provided by Dr. A. Reynolds (Vanderbilt University, Nashville, TN). P120ΔN-terminal (Nt) and P120ΔNtΔphosphorylation domain (PD) were generated and Flag-tagged by PCR amplification. Full-length p120ctn with the Flag tag was created by removing the nontagged C-terminal end (XbaI cut) and replacing it by the tagged C-terminal end of P120ΔNt. P120ΔNtΔ PDΔCt was generated by replacing the C-terminal end of P120 (cut with XbaI) by a linker containing a Flag tag and a stop codon. To generate P120Δarmadillo (arm) 3, P120ΔNtΔPDΔarm3, P120Δ622-628, and P120ΔNtΔPDΔ622-628, we first isolated a p120ctn coding sequence (Bsu36I-BamHI) containing part of the armadillo domains in puc 19. From this construct, we deleted the armadillo domain 3 by generating a PCR fragment from each side of the armadillo repeat 3 (amino acids 445 and 487) and religating in frame with newly created XhoI sites. To delete amino acids 622-628, we used the Quickchange mutagenesis kit (Stratagene) according to the manufacturer instructions. The modified fragments of p120ctn were then reintroduced in P120ΔNtΔPD by using the restriction sites BsmI/BamHI. From these constructs, the modified sequences were reintroduced into full-length P120 using the restriction sites BsmI/XbaI.
To generate Rac viruses, Myc-Rac1 wild-type (WT) and mutated clones (Ridley et al., 1992) were cut out of pcDNA3.1 with HindIII/EcoRV and inserted with the same sites in pAdtrack-CMV. To generate P120ΔΔRac constructs, P120Δ622-628 was cut at the C-terminal XbaI site and ligated with Rac1 cut just beyond the myc tag (PstI site) using a linker containing a 3xFlag tag (Sigma).
All mutation and PCR generated sequences were confirmed by sequencing.
Western blot and immunoprecipitation. CHO cells or 293 cells were infected with adenoviruses for 2 d. In some experiments, pCMV5-v-src and mock pCMV5 plasmid were transfected in the cells 6 h previously with Lipofectamine 2000 (Invitrogen). Proteins were extracted in 20 mm Tris, pH 7.5, 0.3% Triton X-100, 150 mm NaCl, 1 mm EDTA, 2.5 mm sodium othovanadate, 250 mm sodium fluoride, and protease inhibitors (Complete; Roche Products) for 20 min at 4°C. Insoluble material was precipitated at 100,000 × g for 20 min. Immunoprecipitation was performed on the supernatant with 1 μg of antibody overnight at 4°C and 20 μl of protein G-agarose for 1 h. The beads were washed four times with extraction buffer, the immunoprecipitated proteins denatured at 98°C, loaded on 7.5% SDS-PAGE, and transferred to polyvinylidene difluoride membranes for immunoblotting.
To pull down Rac1-GTP, the infected cells were solubilized in 25 mm Tris, pH 7.5, 150 mm NaCl, 1% NP-40, 10% glycerol, 10 mm MgCl2, 1 mm EDTA, and protease inhibitors. Insoluble material was pelleted for 20 min at 14,000 × g, and the supernatant was incubated with glutathione S-transferase-p21-activated kinase (PAK)—PAK-binding domain (PBD) agarose beads (40 μl; Chemicon, Temecula, CA) for 45 min at 4°C. The beads were then washed four times with the solubilization buffer, and the proteins were eluted and processed as described above.
Results
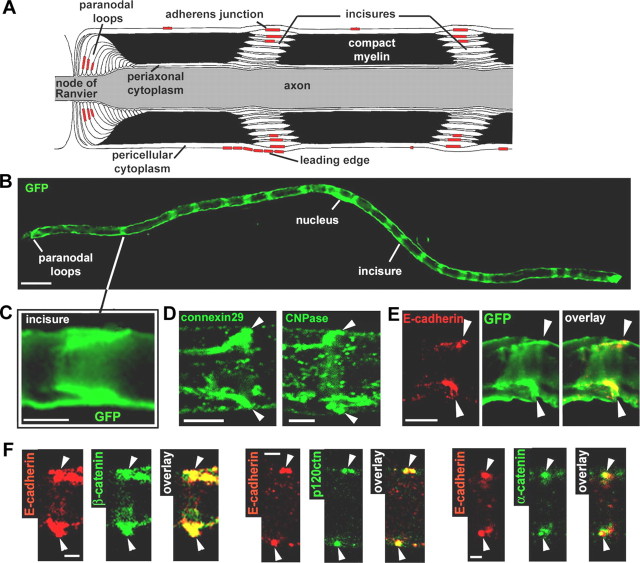
The Schwann cell wraps peripheral axons to produce layers of compact myelin (Fig. 1A). In the mouse, this process begins shortly after birth and continues for ∼6 months. The structure of the compact myelin is interrupted by channels of cytoplasm, the Schmidt-Lanterman incisures (Fig. 1A), which are thought to be required for molecular traffic between the pericellular and the periaxonal cytoplasm. GFP labeling of the cytoplasm in myelinating Schwann cells at 2 months postnatal (mPN) reveals the incisures as repeated bands (Fig. 1B) consisting of two diagonally oriented lines (Fig. 1C) that correspond to the space created by the spiraling tube. These incisures can also be identified by a high level of expression of connexin29 and CNPase (Fig. 1D).
Figure 1.
AJ and architecture of the myelinated Schwann cell. A, Longitudinal view of a myelinated Schwann cell showing the different compartments and the respective location of the AJs. B, Myelinated Schwann cell infected with an adenovirus expressing GFP and showing repeated bands of cytoplasm corresponding to the location of incisures. Scale bar, 50 μm. C, GFP-filled incisure showing the pair of angled cytoplasmic regions representing one incisure as depicted in A. Scale bar, 10 μm. D, Immunostaining of connexin29 (left) and CNPase (right) showing their enrichment in an incisure (arrowheads). Scale bar, 5 μm. E, Pair of E-cadherin clusters (red) that localize at the outer edge of a GFP-filled incisure (arrowheads). Scale bar, 10 μm. F, Coimmunostaining of E-cadherin (red) and β-catenin, p120ctn, and α-catenin (green) on sciatic nerve samples at 2 months postnatal (arrowheads indicate pairs of E-cadherin clusters). Scale bar, 2.5 μm.
E-cadherin-based AJs are formed in the noncompacted regions of the cell (Fig. 1A) and have been detected at incisures in the outer wraps of the myelin sheath by both electron microscopy (Ghabriel and Allt, 1981) and immunostaining (Fig. 1E). These junctions are localized in the apex rather than at the edges of the cytoplasmic bubble and therefore are unlikely to form a physical barrier between the cytoplasm and the compact myelin (Hall and Williams, 1970) (Fig. 1A). They are structurally related to the AJ of epithelial zonula adherens (Landon and Hall, 1976) and contain β-, α-, and p120-catenins (Fig. 1F).
Perturbation of incisures by introduction of E-cadherin lacking its extracellular domain
For experimental manipulations, Schwann cells can be infected in vivo with adenoviral vectors (Guenard et al., 1999; Glatzel et al., 2000). In the present study, the viral vectors were designed to express a dominant-acting protein together with the GFP, used to visualize the cytoplasm, both under the control of two CMV promoters in tandem (He et al., 1998). We chose to introduce dominant-acting proteins into cells of 2mPN mice, because at this age, incisures and AJ are already easily observed, while myelination continues for an additional 4 months.
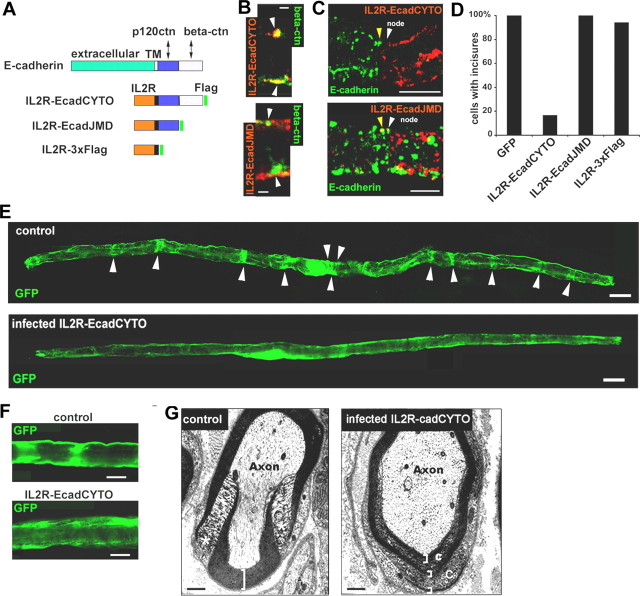
To test whether AJs are essential to incisure architecture, an E-cadherin mutant protein was used in which the extracellular and transmembrane domains had been replaced by the respective domains of the IL2 receptor (Fig. 2A, IL2R-EcadCYTO). Similar constructs are known to act as cadherin dominant negatives by destabilizing the endogenous cadherin through a competition for the binding of β-catenin (Fujimori and Takeichi, 1993; Troxell et al., 1999; Huber et al., 2001). Indeed, we found that IL2R-EcadCYTO colocalizes with β-catenin in Schwann cell (Fig. 2B, top) and that cells expressing this construct no longer immunostain for E-cadherin (Fig. 2C, top).
Figure 2.
Full-length E-cadherin is necessary for maintenance of the cell architecture. A, IL2R/E-cadherin constructs used in this study. The distal domain of E-cadherin cytoplasmic tail (light blue) interacts with β-catenin, whereas the juxtamembrane domain (dark blue) interacts with p120ctn. All constructs are tagged with 3xFlag tag (green). IL2R, Extracellular and transmembrane domains of IL2R. B, Top, IL2R-EcadCYTO construct (red) expressed in a myelinated Schwann cell colocalizes with β-catenin (green) in clusters (arrowheads) at the periphery of the cell. Bottom, IL2R-EcadJMD construct (red) does not colocalize with β-catenin (green) in clusters (arrowheads). Scale bars, 2.5 μm. C, Top, Node of Ranvier having on one side a cell expressing IL2R-EcadCYTO (white arrowhead) but no E-cadherin and on the other side a noninfected cell expressing E-cadherin (yellow arrowhead). Bottom, At a node, a cell (white arrowhead) expressing IL2R-EcadJMD (red) also displays E-cadherin (green) as does a noninfected cell (yellow arrowhead). Scale bars, 10 μm. D, Percentage of cells displaying incisures was determined after infection with viruses expressing different constructs. The presence of incisures was assessed by the presence of transverse bands labeled with GFP or with connexin 29/CNPase immunostaining. Only 16% of the cells infected with IL2R-EcadCYTO maintained one or more incisure. Wounded or dying cells were not counted. Number of cells scored: GFP, 15; IL2R-EcadCYTO, 12; IL2R-EcadJMD, 7; IL2R-3xFlag, 17. E, Myelinated Schwann cells infected with control GFP adenovirus (top) or IL2R-EcadCYTO adenovirus (bottom) as observed by the expression of GFP in the cytoplasm. Scale bars, 20 μm. F, Detail of the GFP distribution in cells infected with GFP virus (top) or IL2R-EcadCYTO virus (bottom). Scale bars, 10 μm. G, Electron micrographs of transversal sections of an IL2R-CcadCYTO-infected nerve (right) and a noninfected nerve (left). Asterisks indicate a normal incisure, brackets indicate the compact myelin, and “C” indicates the cytoplasm inside the compact myelin. Scale bars, 500 nm.
As shown in Figure 2, D and E, replacement of E-cadherin by IL2R-EcadCYTO resulted in a complete loss of incisures in 85% of the cells. Loss of the incisures was also confirmed by the absence of characteristic bands of immunostaining for CNPase and connexin29 (data not shown). The control constructs IL2R-EcadJMD and IL2R-3xflag (Fig. 2A), which do not have the β-catenin-binding domain (Ozawa et al., 1990), did not bind to β-catenin (Fig. 2B, bottom, data shown only for IL2R-EcadJMD). They also did not affect E-cadherin expression (Fig. 2C, bottom) or disrupt incisures (Fig. 2D). Higher-magnification confocal images of the GFP distribution in cells expressing IL2R-EcadCYTO suggested that there was no longer a distinct boundary between the incisure and the compact myelin (Fig. 2F). This finding was supported by electron micrographs showing an abnormal presence of cytoplasm inside the compact myelin of Schwann cells of nerves infected with the IL2R-EcadCYTO virus (Fig. 2G).
Association of p120ctn with E-cadherin is essential for maintenance of incisures
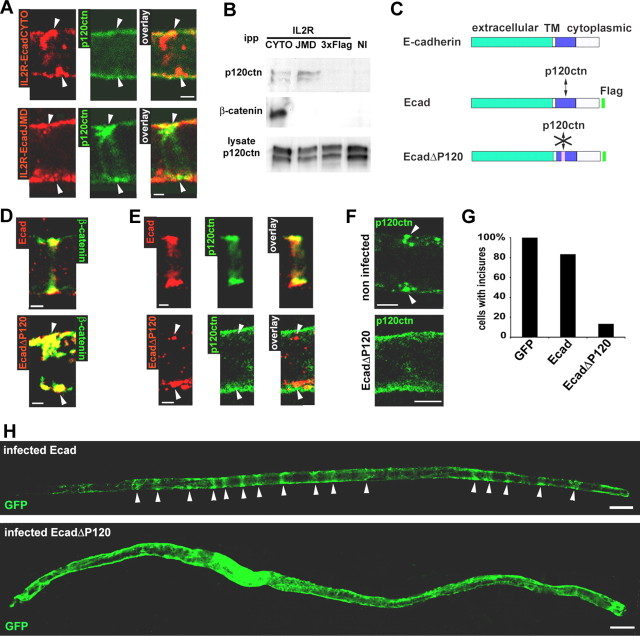
The introduction of IL2R-EcadCYTO in Schwann cells resulted in redistribution of p120ctn from the junction into the cytoplasm. Thus, p120ctn did not colocalize with IL2R-EcadCYTO (Fig. 3A, top) (also see quantitation in supplemental Fig. S1, available at www.jneurosci.org as supplemental material). p120ctn also did not colocalize with IL2R-EcadJMD (Fig. 3A, bottom) (see quantitation in supplemental Fig. S1, available at www.jneurosci.org as supplemental material). However, with IL2R-EcadJMD, p120ctn remained clustered at AJ (Fig. 3A, bottom, arrowheads), because IL2R-EcadJMD does not affect E-cadherin expression (Fig. 2C). This absence of colocalization with p120ctn of either of these cadherin cytoplasmic constructs was surprising because they contain a p120ctn-binding site that is functional in CHO cells (Fig. 3B). This finding suggests that the p120ctn-binding site on IL2R-EcadCYTO is negatively regulated in Schwann cells in vivo.
Figure 3.
Absence of interaction between p120ctn and E-cadherin perturbs the incisures. A, IL2R-EcadCYTO (red; top) and IL2R-EcadJMD (red; bottom) does not colocalize with p120ctn (green). Scale bars, 2.5 μm. B, CHO cells were infected with virus expressing IL2R-EcadCYTO, IL2R-EcadJMD, or IL2R-3xFlag or not infected (NI). The expressed proteins were immunoprecipitated with an antibody against IL2R, and the precipitates were subjected to Western blotting for detection of p120ctn and β-catenin. The same amount of cell lysate was subjected to Western blotting to show that a similar amount of p120ctn was available for coimmunoprecipitation. ipp, Immunoprecipitated proteins. C, Ecad is a full-length E-cadherin construct that is Flag tagged. EcadΔP120 is a full-length human E-cadherin construct Flag tagged and mutated so as to be unable to bind p120ctn. D, Ecad (red; top) and EcadΔP120 (red; bottom) colocalize with β-catenin (green). Scale bars, 2.5 μm. E, Ecad (red; top) but not EcadΔP120 (red; bottom) colocalizes with p120ctn (green). Scale bars, 2.5 μm. F, In a noninfected cell (top), p120ctn (green) forms a pair of clusters (arrowheads) at the periphery of the cell. In an EcadΔP120-infected cell (bottom), p120ctn has a diffuse distribution. Scale bars, 5 μm. G, Percentage of cells displaying incisures determined after infection with viruses expressing GFP alone, Ecad, or EcadΔP120. Thirteen percent of the cells infected with EcadΔP120 and 83% of the cells infected with Ecad virus maintained one or more incisure. Number of cell scored: GFP, 15; Ecad, 22; EcadΔP120, 15. H, GFP distribution in myelinated Schwann cells infected with Ecad (top) or EcadΔP120 (bottom) adenoviruses. Scale bars, 20 μm.
To determine whether the disappearance of incisures produced by IL2R-EcadCYTO was caused by the loss of the extracellular domain of E-cadherin or by the loss of p120ctn binding, a construct encoding a full-length E-cadherin mutated at its p120ctn-binding site (Thoreson et al., 2000) (EcadΔP120) was prepared (Fig. 3C). In CHO cells, EcadΔP120 coimmunoprecipitated with β-catenin but not p120ctn (data not shown). In Schwann cells, both EcadΔP120 and Flag-tagged full-length E-cadherin (Fig. 3C, Ecad) colocalized with β-catenin (Fig. 3D) and probably also replaced endogenous E-cadherin (the small difference between these constructs and E-cadherin did not allow an independent detection of the endogenous protein). However, whereas Ecad colocalized with p120ctn in the junctions (Fig. 3E, top) (see quantitation in supplemental Fig. S1, available at www.jneurosci.org as supplemental material), p120ctn did not colocalize with EcadΔP120 (Fig. 3E, bottom) (quantitation in supplemental Fig. S1, available at www.jneurosci.org as supplemental material). In the latter case, p120ctn appeared to be distributed uniformly in the cytoplasm (Fig. 3F, compare distribution in noninfected cell and EcadΔP120-expressing cells). Remarkably, the cells expressing EcadΔP120, an otherwise intact E-cadherin, did not have any incisure as detected by GFP labeling (Fig. 3H and quantitated in Fig. 3G) or CNPase/connexin29 immunostaining (data not shown).
The perturbation of Schwann cell architecture induced by IL2R-EcadCYTO and EcadΔP120 could have resulted from the absence of p120ctn in cadherin clusters or by a secondary action of the displaced p120ctn in the cytoplasm. Indeed, overexpression of p120ctn in cell lines leads to the presence of the catenin in the cytoplasm, and this has been shown to induce dramatic changes in cell shape (Anastasiadis et al., 2000; Noren et al., 2000; Grosheva et al., 2001). Simple overexpression of p120ctn in Schwann cells in vivo proved to be inadequate as a test for a similar effect in our system, because most of the protein was still sequestered to the cadherin (data not shown). Therefore, a p120ctn mutant was designed that lacks part of its cadherin-binding domain (P120Δarm3) (see supplemental Fig. S3A, available at www.jneurosci.org as supplemental material) (Ireton et al., 2002) but retains its cell shape-associated activity in CHO cells (supplemental Fig. S3B, available at www.jneurosci.org as supplemental material). When expressed in Schwann cells, P120Δarm3 did not colocalize with E-cadherin (supplemental Fig. S3C, available at www.jneurosci.org as supplemental material), was found distributed throughout the cytoplasm (supplemental Fig. S3D, available at www.jneurosci.org as supplemental material), and did not affect endogenous p120ctn expression or distribution (data not shown). Finally, this mutant did not detectably alter incisures (supplemental Fig. S3E and quantitated in Fig. S3F, available at www.jneurosci.org as supplemental material), suggesting that the cytoplasmic distribution of p120ctn by itself is not responsible for the loss of incisures.
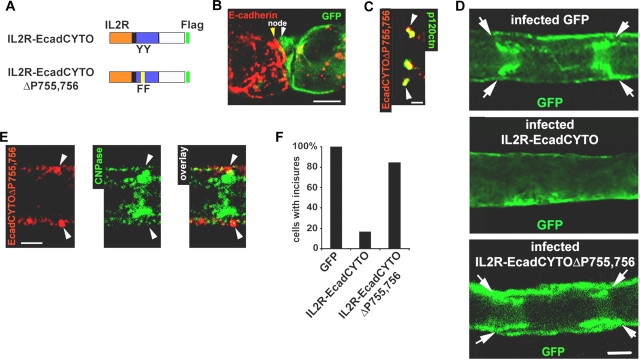
p120ctn interaction with the E-cadherin cytoplasmic domain is regulated by tyrosines 755 and 756 in the cadherin juxtamembrane domain
The above results indicate that regulation of p120ctn binding to E-cadherin is a critical parameter in controlling cellular architecture. Two tyrosine phosphorylations (Y755 and Y756) in the cytoplasmic domain of E-cadherin have been reported to negatively regulate p120ctn binding, possibly through competitive binding of a protein called hakai (Fujita et al., 2002). To investigate a possible role for these phosphorylations in our system, the IL2R-EcadCYTO construct was mutated to phenylalanine at these two tyrosines (Fig. 4A, IL2R-EcadCYTOΔP755,756). When expressed in Schwann cells, IL2R-EcadCYTOΔP755,756 was found to colocalize with β-catenin (data not shown) and to replace endogenous E-cadherin (Fig. 4B) to the same extent as IL2R-EcadCYTO (quantitation in supplemental Fig. S2A,B, available at www.jneurosci.org as supplemental material). As predicted, IL2R-EcadCYTOΔP755,756 regained the ability of the E-cadherin to colocalize with p120ctn (Fig. 4C) (quantitation in supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Most importantly, this construct, which lacks the entire cadherin extracellular domain, was able to restore fully the ability of the cell to maintain incisures as assessed by GFP distribution (Fig. 4D, quantitated in Fig. 4F) as well as CNPase staining (Fig. 4E).
Figure 4.
Removal of the phosphorylation sites in the juxtamembrane region of IL2R-EcadCYTO restores its interaction with p120ctn and the Schwann cell structure. A, IL2R-EcadCYTOΔP755,756 is a IL2R-EcadCYTO construct mutated on the two phosphorylatable tyrosines, 755 and 756 (YY to FF), in the E-cadherin juxtamembrane domain. B, At a node, a cell infected with the IL2R-EcadCYTOΔP755,756 adenovirus (white arrowhead) expresses GFP (green) but very little E-cadherin (red) compared with the noninfected cell (yellow arrowhead). Scale bar, 10 μm. C, IL2R-EcadCYTOΔP755,756 (red) colocalizes with p120ctn (green). Scale bar, 2.5 μm. D, Detail of the GFP distribution in a myelinated Schwann cell expressing GFP alone (top), IL2R-EcadCYTO (middle), and IL2R-EcadCYTOΔP755,756 (bottom). Arrows indicate incisures. Scale bar, 10 μm. E, IL2R-EcadCYTOΔP755,756 (red) forms clusters in the outer region of an incisure (arrowheads) filled with CNPase (green). Scale bar, 5 μm. F, Percentage of cells displaying incisures determined expression of GFP, IL2R-EcadCYTO, or IL2R-EcadCYTOΔP755,756. Incisures are maintained by 84.7% of the cells expressing IL2R-EcadCYTOΔP755,756. Number of cells scored: GFP, 15; IL2R-EcadCYTO, 12; IL2R-EcadCYTOΔP755,756, 13.
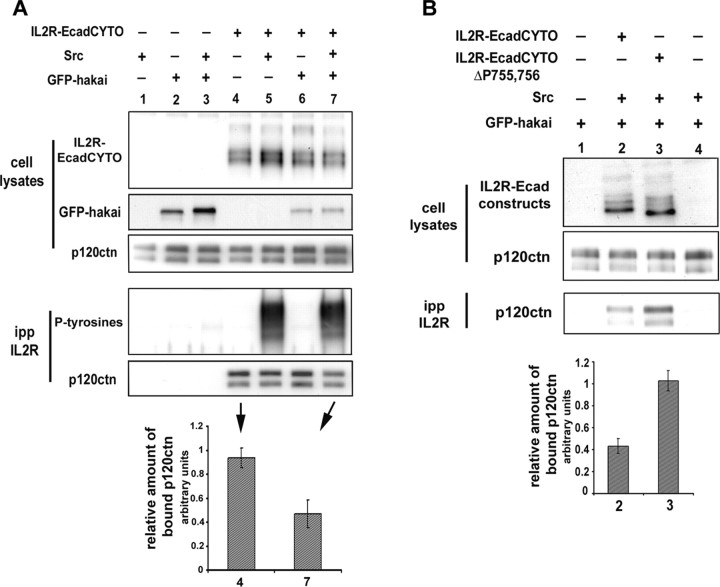
To confirm that p120ctn interaction with IL2R-EcadCYTO can be regulated by tyrosine phosphorylation, IL2R-EcadCYTO and GFP-hakai were coexpressed in 293 cells along with v-src, which phosphorylates the cytoplasmic domain of E-cadherin (Fujita et al., 2002). The IL2R construct was then immunoprecipitated and the coprecipitated p120ctn detected by Western blot. The amount of precipitated p120ctn was found to be significantly reduced when v-src and hakai were coexpressed with the construct (Fig. 5A). The same experiment was then performed using IL2R-EcadCYTOΔP755,756. p120ctn interaction with this mutated construct was not affected by v-src and GFP-hakai coexpression (data not shown), and EcadCYTOΔP755,756 coimmunoprecipitated 2.4 times the amount of p120ctn as did IL2R-EcadCYTO (Fig. 5B), suggesting that the inhibition of p120ctn interaction with IL2R-EcadCYTO can be reversed by the mutation of these two tyrosines.
Figure 5.
p120ctn interaction with IL2R-EcadCYTO is regulated by phosphorylations of the E-cadherin intracellular domain. A, IL2R-EcadCYTO was expressed in 293 cells with or without v-src or GFP-hakai. The cells were solubilized, and equal amounts of solubilized proteins (cell lysates) were immunoprecipitated with an anti-IL2R antibody. The immunoprecitated proteins (ipp) were probed with an anti-phosphotyrosines antibody (P-tyrosines) and an anti-p120ctn antibody. The amount of coimmunoprecipitated p120ctn was quantified by densitometry, normalized to the amount of p120ctn present in the cell lysate (relative amount of bound p120ctn) and the value obtained for lane 4 (IL2R-EcadCYTO alone) and lane 7 (IL2R-EcadCYTO plus v-src and GFP-hakai) plotted in a graph. The amount of bound p120ctn drop of 50% (results from 3 independent experiments). B, IL2R-EcadCYTO (lane2) and IL2R-EcadCYTOΔP755,756 (lane 3) were expressed in 293 cells with v-src and GFP-hakai. The cells were solubilized, and an equal amount of solubilized proteins (cell lysates) was immunoprecipitated with IL2R antibody. The amount of coimmunoprecipitated p120ctn was detected, quantified by densitometry, normalized to the amount of p120ctn present in the cell lysate (relative amount of bound p120ctn), and the value obtained for lanes 2 and 3 plotted in a graph. The amount of bound p120ctn is 2.4 times higher for IL2R-EcadCYTOΔP755,756 than for IL2R-EcadCYTO (results from 3 independent experiments). Error bars represent SD.
p120ctn-mediated effects on cell architecture involve regulation of Rho GTPases activity at the AJ
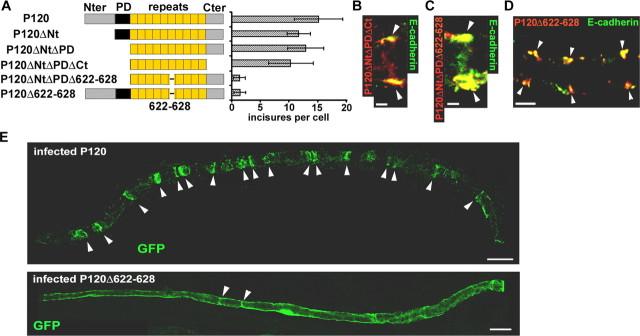
To establish a morphogenetic role for p120ctn when bound to E-cadherin, it was important to identify a domain of the protein responsible for this activity. To this end, a series of constructs were prepared with combinations of deletions of the N-terminal domain, C-terminal domain, phosphorylation domain (Aono et al., 1999), and a basic motif (amino acids 622-628) that has been implicated in p120ctn regulation of RhoA and interaction with the microtubules (Anastasiadis et al., 2000; Franz and Ridley, 2004) (Fig. 6A). Each construct retained the armadillo repeats required for binding to cadherin (Ireton et al., 2002), and all of them colocalized with E-cadherin (for the two most truncated forms, see Fig. 6B, C). Moreover, the normal distribution of AJ in Schwann cells was not perturbed by the expression of any of these constructs (Fig. 6D, P120Δ622-628), suggesting the structural integrity of the junctional structure was not affected. When tested for perturbation of cell architecture, only the two constructs containing the 622-628 deletion produced a clearly reduced number of incisures (data shown for P120 and P120Δ622-628 in Fig. 6E and quantitated in Fig. 6A). This effect was not as complete as obtained with cadherin mutations, with each cell retaining a few incisures (1.5 in average). This difference most likely reflects the fact that despite the high level of expression of the p120ctn mutants, a small amount of endogenous p120ctn remained (data not shown).
Figure 6.
Cadherin-bound p120ctn requires amino acids 622-628 to maintain incisures. A, Left, A series of mutants of p120ctn produced by deleting the N-terminal domain (Nter; P120ΔNt), plus the phosphorylation domain, P120ΔNtΔPD, plus the C-terminal domain (Cter; P120ΔNtΔPDΔCt), and plus amino acids 622-628 in the sixth armadillo repeat (P120ΔNtΔPDΔ622-628). In addition, only the 622-628 region was deleted in full-length p120ctn (P120Δ622-628). All of these constructs were labeled at their C terminus with a Flag tag. Right, Each construct was expressed in myelinated Schwann cells, and the number of incisures displayed by each cell (assessed via GFP labeling or CNPase/connexin29 staining) was determined. Number of cells scored: P120, 27; P120ΔNt, 14; P120ΔNtΔPD, 17; P120ΔNtΔPDΔCt, 13; P120ΔNtΔPDΔ622-628, 5; P120Δ622-628, 22. B, P120ΔNtΔPDΔCt (red) colocalizes with E-cadherin (green). Scale bar, 2.5 μm. C, P120ΔNtΔPDΔ622-628 (red) colocalizes with E-cadherin (green). Scale bar, 2.5 μm. D, Schwann cell expressing P120Δ622-628 (red) displays pairs of E-cadherin clusters (green). Scale bar, 5 μm. E, A Schwann cell expressing P120 (top) displays 17 incisures (arrowheads). A cell expressing P120Δ622-628 (bottom) displays only two GFP-filled incisures (arrowheads). Scale bars, 20 μm.
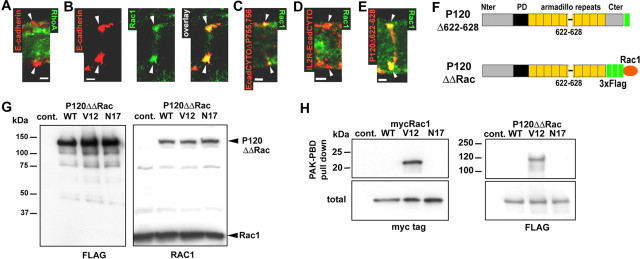
The fact that the 622-628 deletion affects incisures suggests that this effect might involve the regulation of a Rho GTPase at the AJ (Anastasiadis et al., 2000). In Schwann cells, a colocalization of RhoA with E-cadherin clusters could not be detected (Fig. 7A), but Rac1 colocalized with E-cadherin at the AJ (Fig. 7B). Moreover, although Rac1 colocalized with IL2R-EcadCYTOΔP755,756 (Fig. 7C), which has the ability to recruit p120ctn, this colocalization did not occur in the cells expressing E-cadherin mutants that did not recruit p120ctn, namely IL2R-EcadCYTO (Fig. 7D) and EcadΔP120 (data not shown). Together, these results provide consistent but indirect evidence that p120ctn promotes this aspect of morphogenesis by recruiting Rac1 to the AJ. Interestingly, the p120ctn 622-628 deletion, which perturbs incisures (Fig. 6A, E), did not detectably alter the recruitment of Rac1 to the junction (Fig. 7E). This finding suggests that to stabilize cell structure, the cadherin-bound p120ctn must both recruits Rac1 and regulates its activity.
Figure 7.
Rac1 colocalization with AJ correlates with the recruitment of p120ctn to cadherin. A, RhoA (green) does not colocalize with E-cadherin (red) at incisures (arrowheads). Scalebar, 2.5 μm. B, Rac1 (green) partially colocalizes with E-cadherin (red). Scalebar, 2.5 μm. C, IL2R-EcadCYTOΔP755,756 (red) partially colocalizes with Rac1 (green). Scalebar, 2.5 μm. D, IL2R-EcadCYTO (red) does not colocalize with Rac1 (green). Scalebar, 2.5 μm. E, P120Δ622-628 construct (red) colocalizes with Rac1 (green). Scalebar, 2.5 μm. F, The C terminal (Cter) of P120Δ622-628 was fused to a 3xFlag tag and Rac1 wild-type, dominant-active mutant (V12) and dominant-negative mutant (N17) to obtain P120ΔΔRacWT, V12, and N17. G, P120ΔΔRac constructs were expressed in CHO cells and immunoblotted with mouse monoclonal anti-Flag (left) or anti-Rac1 (right) antibodies. Note that anti-Rac1 antibody recognizes both Rac1 and P120ΔΔRac. H, myc-Rac1 (left) and P120ΔΔRac (right) constructs expressed in CHO were subjected to PAK-PBD pull down, and the precipitated proteins were detected by Western blots hybridized with anti-myc and anti-Flag antibodies. A sample of the total amount of protein subjected to precipitation was also processed (total) to show that the same amount of protein was used for each construct. Nter, N terminal; Cont., control.
To investigate the role of Rac1 in incisures maintenance, we first attempted to overexpress Rac1 dominant-active (RacV12) and negative (RacN17) forms in Schwann cells in vivo. RacV12 is stabilized in its active GTP form and permanently stimulates Rac1 effectors, whereas RacN17 preferentially binds GDP and therefore inhibits Rac1 by sequestering Rac1 activators (guanine nucleotide exchange factor) (for review, see Fukata and Kaibuchi, 2001). However, these constructs proved to be highly toxic for Schwann cells (data not shown). To circumvent this problem, we sought to affect Rac1 activity only at the adherens junction by fusing the wild-type Rac1 and the two dominant mutants with the C-terminal domain of a p120ctn construct via a 3xFlag tag. P120Δ622-628 was fused with Rac1, because we anticipated that an associated Rac1 activity would restore the incisure-maintaining activity of p120ctn that is lost in the 622-628 mutant (Fig. 7F, P120ΔΔRac). The expressed fusion proteins were of the expected size (135 kDa) and were recognized with antibodies against Flag (Fig. 7G, left) and against Rac1 (Fig. 7G, right). To test whether the activity of Rac1 was not altered by combination with P120Δ622-628, we expressed the fusion proteins in CHO cells and pulled down active Rac1-GTP with PAK-PBD-coated beads (Benard et al., 1999). P120ΔΔRac fusion proteins behaved in the same way as normal myc-Rac1, in that only the Rac1 dominant-active (V12) form bound to the beads (Fig. 7H).
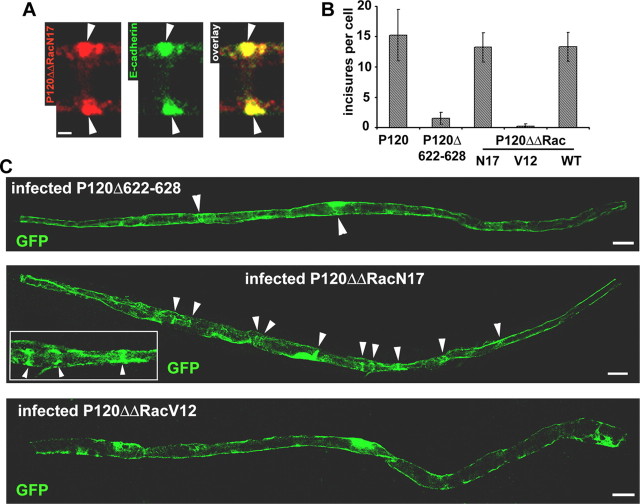
When expressed in Schwann cells, the fusion proteins did not induced toxicity and colocalized exclusively with E-cadherin in the adherens junctions (Fig. 8A, P120ΔΔRacN17). Cells expressing P120ΔΔRacN17 and WT displayed a higher number of GFP-filled incisures than P120Δ622-628-expressing cells (Fig. 8C, quantitated in Fig. 8B). In contrast, P120ΔΔRacV12 cells had fewer incisures (Fig. 8C, quantitated in Fig. 8B). Thus, the local inhibition of Rac1 activity is able to restore the ability of the P120Δ622-628-containing junctions to maintain incisures in Schwann cells.
Figure 8.
Fusion of dominant-negative Rac1 with P120Δ622-628 restores p120ctn ability to maintain the incisures. A, P120ΔΔRacN17 (red) expressed in Schwann cell colocalizes with E-cadherin (green). Scale bar, 2.5 μm. B, Each construct was introduced into myelinated Schwann cells, and the number of incisures displayed by each cell (assessed via GFP labeling or CNPase/connexin29 staining) was determined. Number of cells scored: P120, 27; P120Δ622-628, 22; P120ΔΔRacN17, 17; P120ΔΔRacV12, 24; P120ΔΔRacWT, 11. C, A Schwann cell expressing P120Δ622-628 (top) shows two GFP-filled incisures (arrowheads); a cell expressing P120ΔΔRacN17 (middle) shows 10 incisures (arrowheads), and some of them are shown in detail (inclusion on the right side); a cell expressing P120ΔΔRacV12 (bottom) displays no incisure. Scale bars, 20 μm. Error bars represent SD.
The molecular composition of junctions in developing Schwann cells supports a role for p120ctn-cadherin binding in morphogenesis
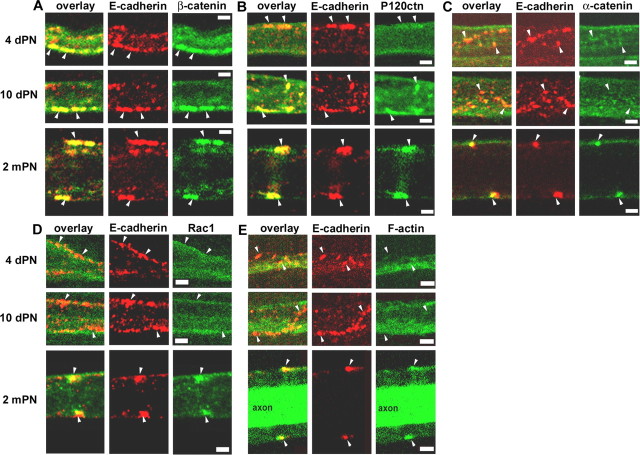
The above results indicate an active role of the AJ in maintaining cell architecture in vivo at 2 mPN and also suggest a hierarchy of functional interactions among components of the AJ. To assess how well these findings reflect molecular events during assembly of the junctions in Schwann cells, it would be useful to monitor the spatial and temporal expression patterns of different junctional components during the early stages of myelination. The developmental stages chosen were 4 d postnatal (dPN), when myelination begins, 10 dPN, when complete incisures start to form (Small et al., 1987), and 2 mPN, when mature structures are present. E-cadherin clusters already were found in Schwann cells at 4 dPN, and there was a clear colocalization of these clusters with β-catenin (Fig. 9A) but not with p120ctn, α-catenin, Rac1, or F-actin, which is the major target of Rac1 (Fig. 9B-E). At 10 dPN, the distributions were similar (Fig. 9A, C-E), except that the larger cadherin clusters began to increase their content of p120ctn (Fig. 9B). At 2 mPN, when incisures had formed, all the catenins plus Rac1 and F-actin were found to be strongly colocalized with E-cadherin clusters (Fig. 9A-E). These findings indicate that the recruitment of the catenins to the E-cadherin-based AJ is precisely regulated in a sequence that is consistent with the functional relationships revealed by the perturbation studies of the cell architecture. That is, β-catenin, which is required to stabilize cadherin in the membrane, appears first; p120ctn, which is required for triggering of local morphogenesis, arrives shortly thereafter; and Rac1, F-actin, and α-catenin, which could ultimately assemble that architecture, arrive last.
Figure 9.
Sequential appearance of AJ components during myelination. A-E, Coimmunostaining of E-cadherin (red) and β-catenin (A), p120ctn (B), α-catenin (C), Rac1 (D), or F-actin (E) on sciatic nerve samples at 4 d postnatal, 10 d postnatal, and 2 months postnatal. Arrowheads indicate cadherin clusters. The staining of F-actin with phalloidin was intense inside the axon at 2 mPN. Scale bars, 2.5 μm.
Discussion
The primary findings of this study are that E-cadherin function is essential for maintenance of Schmidt-Lanterman incisures in myelinating Schwann cells and that this function is mediated by the recruitment of p120ctn to the E-cadherin juxtamembrane domain. Although full-length E-cadherin was able to recruit p120ctn to junctional complexes, the E-cadherin cytoplasmic domain attached to the membrane by IL2R did not colocalize with p120ctn. This result indicates that in normal cells, the cadherin extracellular domain is required for p120ctn recruitment. Remarkably, it is possible to override this requirement by mutations preventing the phosphorylation of the cadherin juxtamembrane domain (tyrosines 755, 756), suggesting that the role of the extracellular domain is manifested through a mechanism leading to dephosphorylation of tyrosines. It will therefore be interesting to investigate a possible role of transmembrane phosphatases such as receptor protein tyrosine phosphatase μ, leukocyte common antigen-related phosphatase, or vascular endothelial-protein tyrosine phosphatase, which are part of the cadherin/catenin complex and can have adhesion-sensitive properties (for review, see Reynolds and Roczniak-Ferguson, 2004).
Interestingly, phosphorylation of tyrosines 755,756 also promotes the binding of a cytosolic protein called hakai that is reported to compete with p120ctn binding (Fujita et al., 2002). Although it can be speculated that such a competition between hakai and p120ctn could underlie the regulation of p120ctn-cadherin binding in Schwann cells in vivo, it should be noted that other proteins have also been reported to bind to this region of cadherin, such as armadillo repeat gene deleted in velo-cardiofacial syndrome, δ-catenin, P0071, and presenilin 1 (for review, see Reynolds and Roczniak-Ferguson, 2004) and could play a similar role.
Recent reports have associated p120ctn with cadherin stability and trafficking (Ireton et al., 2002; Davis et al., 2003; Xiao et al., 2003). These in vitro findings raise the possibility that the effects observed in Schwann cells could also reflect a degradation of endogenous cadherin that in turn destroys incisures, as discussed above for β-catenin. However, the fact that mutation of tyrosines 755,756 in EcadCYTO fully supports incisure maintenance without restoring endogenous cadherin indicates that there is a second regulatory mechanism involved. This second pathway is directly supported by the observation that the Δ622-628 p120ctn mutation, which affects Rho GTPase activity but does not decrease cadherin binding or expression, was not able to maintain incisures.
The events that link E-cadherin, p120ctn, and Rho GTPase with incisure morphology are not as yet clear. Rac1 but not RhoA GTPase was found to be concentrated at Schwann cell adherens junctions, and it was possible to reverse the effects of the P120Δ622-628 mutation by addition of Rac1 to its C-terminal end. Although these finding are consistent with a role for Rac1 in incisure maintenance, other GTPases could be involved as well in that these enzymes can act in cascades, and their regulatory interactions are not completely understood. Nevertheless, it is reasonable to speculate that recruitment and regulation of Rac1 at the junction by p120ctn, perhaps with the help of α-catenin or IQGAP (Noritake et al., 2004), stabilizes the actin cytoskeleton via Arp2/3 complex or formin (Kovacs et al., 2002; Kobielak et al., 2004).
The perturbations of the AJ produced in this study result in the loss of a clear boundary between compact and noncompact myelin. However, ultrastructural studies have shown that AJs are not localized at the border between the two compartments (Hall and Williams, 1970). Therefore it is unlikely that these junctions directly form a “molecular fence” (Pedraza et al., 2001), which would prevent the diffusion of macromolecules between the compartments. However it is possible that AJs control the organization or the stability of such a filter. For example, given that the loss of the boundary after the disruption of the junctions has been related to Rho GTPases and cytoskeleton, it is possible that uncontrolled actin polymerization disrupts the cytoplasmic organization of boundary structures, allowing cytoplasmic components to penetrate into the compact myelin. In this respect, it would be interesting to evaluate cytoskeletal dynamics in incisures and to analyze the effects of AJ perturbation on these dynamics.
It can be noted that the dramatic results obtained in our study by replacement of endogenous with mutated E-cadherin are in contrast to the relatively subtle effects observed in the Schwann cell E-cadherin conditional knock-out mouse (Young et al., 2002). It is possible that an approach that involves sequestration of β-catenin and therefore the simultaneous destabilization of multiple cadherins with potential redundant function at the AJ might be more effective in this context than genetic deletion of a single cadherin.
All of our perturbation studies with cadherin and p120ctn constructs relate to the maintenance of existing incisures. To help relate these findings to the formation of myelin, studies were performed on the timing and distribution of junctional components during development. At early stages of myelination (4 dPN), E-cadherin and β-catenin are present in clusters and possibly already participate in Schwann cell-axonal interactions. At this point, additional effects of the cadherin cytoplasmic domain on junctional maturation and incisure morphogenesis appear to be blocked, possibly by the absence of p120ctn at the complex. The recruitment of p120ctn around 10 dPN, presumably after the dephosphorylation of the cadherin intracellular domain, would trigger events that lead to the formation by 2 mPN of a mature junction containing α-catenin, Rac1, and F-actin. The proposal that recruitment of p120ctn at about 10 dPN is a critical step in junction formation, and thus incisure morphogenesis fits well with electron microscopy studies (Small et al., 1987), which indicate that a substantial change in incisure structure occurs between postnatal day 8 (P8) and P12. Nevertheless, additional experiments are required to demonstrate the role of p120ctn in the formation of the AJ and by extension in the formation of the incisures.
From the perspective of cell biology, perhaps the most surprising implication of this study concerns how one perceives the role of the p120ctn and cadherin in the AJ. Traditionally, adhesion molecules including classical cadherins are defined as homophilic or heterophilic membrane receptors, the function of which is to establish the position, specificity, and stability of cell-cell associations. Instead, the present studies support the idea that a classical cadherin in an AJ is foremost an effector of membrane-cytoskeletal relationships to help structure the cytoplasm, with the extracellular domain serving as a sensor that can trigger the morphogenetic process via recruitment of p120ctn. Of course, as an adhesion molecule, that stimulus is likely to be contact with another cell membrane, which during the process of myelination could reflect the creation of new membrane-membrane appositions during growth of the myelin sheath.
Footnotes
This work was supported in part by National Institutes of Health Grant NS40300. We thank Drs. Y. Fujita and M. Resh for their kind gifts of hakai and v-src cDNAs, C. Gottardi for useful discussions, and the Philippe Foundation and the Fondation Simone and Cino del Duca for their support to N.T.
Correspondence should be addressed to Urs Rutishauser, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: u-rutishauser@mskcc.org.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253259-11$15.00/0
References
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB (2000) Inhibition of RhoA by p120 catenin. Nat Cell Biol 2: 637-644. [DOI] [PubMed] [Google Scholar]
- Aono S, Nakagawa S, Reynolds AB, Takeichi M (1999) p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol 145: 551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo EJ, Scherer SS (2000) On the molecular architecture of myelinated fibers. Histochem Cell Biol 113: 1-18. [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM (1999) Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem 274: 13198-13204. [DOI] [PubMed] [Google Scholar]
- Chen X, Kojima S, Borisy GG, Green KJ (2003) p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol 163: 547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB (2003) A core function for p120-catenin in cadherin turnover. J Cell Biol 163: 525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Ji H, Kim SW, Park JI, Vaught TG, Anastasiadis PZ, Ciesiolka M, McCrea PD (2004) Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J Cell Biol 165: 87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich Jr VL, Colman DR (1995) Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol 129: 189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CM, Ridley AJ (2004) p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem 279: 6588-6594. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Takeichi M (1993) Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol Biol Cell 4: 37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4: 222-231. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kaibuchi K (2001) Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol 2: 887-897. [DOI] [PubMed] [Google Scholar]
- Ghabriel MN, Allt G (1981) Incisures of Schmidt-Lanterman. Prog Neurobiol 17: 25-58. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Bueler H, Aguzzi A (2000) Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci USA 97: 442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosheva I, Shtutman M, Elbaum M, Bershadsky AD, Noren NK, Liu BP, Burridge K, Kreft B, Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB (2001) p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci 114: 695-707. [DOI] [PubMed] [Google Scholar]
- Guenard V, Schweitzer B, Flechsig E, Hemmi S, Martini R, Suter U, Schachner M (1999) Effective gene transfer of lacZ and P0 into Schwann cells of P0-deficient mice. Glia 25: 165-178. [PubMed] [Google Scholar]
- Hall SM, Williams PL (1970) Studies on the “incisures” of Schmidt and Lanterman. J Cell Sci 6: 767-791. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI (2001) The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem 276: 12301-12309. [DOI] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB (2002) A novel role for p120 catenin in E-cadherin function. J Cell Biol 159: 465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E (2004) Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 6: 21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS (2002) Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 12: 379-382. [DOI] [PubMed] [Google Scholar]
- Landon DN, Hall S (1976) The myelinated nerve fibre. In: The peripheral nerve (Landon DN, ed), pp 1-105. London: Chapman and Hall.
- Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M (2003) Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol 160: 433-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B (2000) p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 150: 567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K (2004) Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell-cell contact. Mol Biol Cell 15: 1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Ringwald M, Kemler R (1990) Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA 87: 4246-4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza L, Huang JK, Colman DR (2001) Organizing principles of the axoglial apparatus. Neuron 30: 335-344. [DOI] [PubMed] [Google Scholar]
- Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J (2003) The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol 162: 15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Roczniak-Ferguson A (2004) Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23: 7947-7956. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70: 401-410. [DOI] [PubMed] [Google Scholar]
- Schagen FH, Rademaker HJ, Rabelink MJ, van Ormondt H, Fallaux FJ, van der Eb AJ, Hoeben RC (2000) Ammonium sulphate precipitation of recombinant adenovirus from culture medium: an easy method to increase the total virus yield. Gene Ther 7: 1570-1574. [DOI] [PubMed] [Google Scholar]
- Small JR, Ghabriel MN, Allt G (1987) The development of Schmidt-Lanterman incisures: an electron microscope study. J Anat 150: 277-286. [PMC free article] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L (1967) Fixation of ejaculated spermatozoa for electron microscopy. Nature 216: 173-174. [DOI] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB (2000) Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol 148: 189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell ML, Chen YT, Cobb N, Nelson WJ, Marrs JA (1999) Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am J Physiol 276: C404-C418. [DOI] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP (2003) Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 163: 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM (1997) Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 13: 119-146. [DOI] [PubMed] [Google Scholar]
- Young P, Boussadia O, Berger P, Leone DP, Charnay P, Kemler R, Suter U (2002) E-cadherin is required for the correct formation of autotypic adherens junctions of the outer mesaxon but not for the integrity of myelinated fibers of peripheral nerves. Mol Cell Neurosci 21: 341-351. [DOI] [PubMed] [Google Scholar]