Abstract
Abnormal phosphorylation of amyloid-β precursor protein (APP) is a pathologic feature of Alzheimer's disease. To begin to understand the mechanism of APP phosphorylation, we studied this process in differentiating neurons under normal physiological conditions. We found that c-Jun NH2-terminal kinase (JNK), not cyclin-dependent kinase 5, is required for APP phosphorylation, leading to localized accumulation of phosphorylated APP (pAPP) in neurites. We show that JNK-interacting protein-3 (JIP-3), a JNK scaffolding protein that does not bind APP, selectively increases APP phosphorylation, accumulation of pAPP into processes, and stimulates process extension in both neurons and COS-1 cells. Downregulation of JIP-3 by small interfering RNA impairs neurite extension and reduces the amount of localized pAPP. Finally, whereas stress-activated JNK generates pAPP only in the cell body, concomitant expression of JIP-3 restores pAPP accumulation into neurites. Thus, APP phosphorylation, transport of the generated pAPP into neurites, and neurite extension are interdependent processes regulated by JIP-3/JNK, in a pathway distinct from stress-activated JNK signaling.
Keywords: amyloid-β precursor protein, c-Jun NH2-terminal kinase, JNK-interacting protein, Alzheimer's disease, kinesin, axonal transport
Introduction
Alzheimer's disease (AD), the prevalent neurodegenerative disorder in humans later in life (Hedera and Turner, 2002), is a multifactorial syndrome linked to abnormal metabolism of the transmembrane protein, amyloid-β precursor protein (APP) (Selkoe, 2001). The extracellular deposits known as neuritic plaques, a typical neuropathological feature in AD brains, contain large amounts of amyloid-β peptide (Aβ), which is generated from APP by two endoproteolytic cleavages (Sisodia and St George-Hyslop, 2002).
In neurons, newly synthesized APP follows the secretory pathway and is targeted into axons by fast axonal transport (Selkoe, 1998). A fraction of full-length APP reaches the plasma membrane and re-enters the cell via endocytosis. APP is thought to interact via its cytoplasmic domain (ACD) with several proteins that may regulate its metabolism, including Aβ generation, and may participate in APP function (De Strooper and Annaert, 2000; King and Turner, 2004). Many of these proteins (e.g., Mint-1/X11α, mDab1, Fe65, Shc, and Numb) bind to a conserved motif (YENPTY) in ACD via their phosphotyrosine-binding (PTB) domain (De Strooper and Annaert, 2000). The microtubule motor that transports APP into axons, kinesin-1 (Muresan, 2000; Lawrence et al., 2004), may also bind to ACD via its light chain subunit (Kamal et al., 2000).
APP is phosphorylated in its cytoplasmic domain at several residues, including Thr668 (numbering for the major neuronal isoform APP695) (Suzuki et al., 1994). Interestingly, Thr668-phosphorylated APP (pAPP) is preferentially localized to neurite endings (Ando et al., 1999; Iijima et al., 2000), suggesting that phosphorylation might regulate APP transport into neurites. Phosphorylation of APP has also been implicated in neuronal differentiation (Ando et al., 1999), APP processing by secretases (Lee et al., 2003), and localization of an APP fragment to the nucleus (Muresan and Muresan, 2004). Importantly, increased levels of pAPP have been found in AD brains that might be directly linked to the overproduction of Aβ (Lee et al., 2003).
The neuronal kinase that phosphorylates APP in vivo is not known. Potential candidates are cyclin-dependent kinase 5 (Cdk5) (Iijima et al., 2000), c-Jun NH2-terminal kinase (JNK) (Standen et al., 2001; Taru et al., 2002; Scheinfeld et al., 2003), and glycogen synthase kinase 3β (Aplin et al., 1996). These kinases phosphorylate Thr668 in vitro and, under certain experimental conditions, in vivo. Identifying the pathways that lead to APP phosphorylation in vivo, in normal physiological state, could help understand important aspects of APP transport and metabolism.
Using a CNS-derived neuronal cell line, we investigated APP phosphorylation in the absence of external treatments. We found that JNK, not Cdk5, is responsible for APP phosphorylation at Thr668 in differentiated neurons. We also found that JNK-interacting protein-3 (JIP-3), a JNK scaffolding protein that does not directly interact with APP, facilitates this phosphorylation and induces neurite elongation. Finally, we found that, whereas stress activation of JNK can produce pAPP in the cell body, the generation of pAPP in unchallenged cells and the localization of the produced pAPP to process endings require JIP-3. These findings directly implicate JNK and JIP-3 in APP phosphorylation and transport in neurons.
Materials and Methods
Antibodies. Primary antibodies used in this study are as follows: goat anti-JIP-1 (E-19) and anti-JIP-3 (E-18), rabbit anti-Cdk5 (C-8) and p35 (C-19) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit antibodies to phospho-c-Jun (Ser73), stress-activated protein kinase (SAPK)/JNK, phospho-SAPK/JNK (Thr183/Tyr185), and Bcr (Cell Signaling Technology, Beverly, MA); mouse anti-human amyloid-β protein (clone 6E10; Signet, Dedham, MA); rabbit anti-APP (#2452; raised against a polypeptide from the APP cytoplasmic domain; Cell Signaling Technology). The rabbit anti-APP antibody recognizes total APP (phosphorylated and nonphosphorylated) (Muresan and Muresan, 2004) and was used for immunocytochemistry and immunoblotting. The following two antibodies were used to detect pAPP in immunoblots: #2451 (Cell Signaling Technology) and #44-336Z (BioSource International, Camarillo, CA). They recognize specifically the Thr668-phosphorylated, but not nonphosphorylated, forms of APP (Muresan and Muresan, 2004) (data not shown) and were both used in immunoblots. Antibody #44-336Z was also used in immunocytochemistry. As in Western blot analysis, the antibody showed strict specificity for pAPP in immunocytochemistry (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Unlike #2451, the antibody #44-336Z detected strongly cytoplasmic APP but detected poorly the previously described intranuclear pool of phosphorylated APP C-terminal fragment (Muresan and Muresan, 2004). A mouse antibody (MAB1614; clone H2) to kinesin heavy chain was obtained from Chemicon (Temecula, CA), mouse anti-α-tubulin (clone B-5-1-1) was obtained from Sigma (St. Louis, MO), and rabbit anti-actin was obtained from ICN Biomedicals (Aurora, OH). The following mouse antibodies to tags were used: anti-c-myc (OP10; Oncogene Research Products, San Diego, CA) and anti-FLAG epitope (M2; Stratagene, La Jolla, CA).
Cell cultures. The mouse CNS catecholaminergic cell line, CAD (Qi et al., 1997), was grown in 1:1 F-12:DMEM containing 8% FBS and penicillin/streptomycin. Differentiation was induced by culturing cells in serum-free medium. COS-1 cells, originally derived from African green monkey kidney, were grown in high-glucose DMEM containing 10% FBS and penicillin/streptomycin. Human embryonic kidney 293 (HEK293) cells were grown in Roswell Park Memorial Institute-1640 medium containing 8% FBS.
Transfection. CAD, HEK293, and COS-1 cells were transfected either with FuGene 6 (Roche Diagnostics, Indianapolis, IN) or by electroporation using Nucleofector technology (Amaxa, Gaithersburg, MD). Nucleofection gave consistently higher transfection efficiencies. Constructs of human APP695 and green fluorescent protein (GFP)-tagged, dominant-negative Cdk5 (dnk5-GFP) (Niethammer et al., 2000) were obtained from Dr. Li-Huei Tsai (Harvard Medical School, Howard Hughes Medical Institute, Boston, MA). JIP constructs (pcMV-JIP-1-FLAG, pcDNA3-JIP-2-FLAG, and pcDNA3-JIP-3-FLAG) were obtained from Dr. Roger Davis (University of Massachusetts Medical School, Howard Hughes Medical Institute, Worcester, MA) (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). Constructs of FLAG-tagged mouse JIP-1b, mouse JIP-1b lacking the JNK binding domain, and human JIP-1e were obtained from Dr. Luciano D'Adamio (Albert Einstein College of Medicine, Bronx, NY) (Scheinfeld et al., 2002). Myc-tagged Fe65 (cloned into RK5 vector) was obtained from Dr. Ben Margolis (University of Michigan, Howard Hughes Medical Institute, Ann Arbor, MI) (Borg et al., 1996).
After transfections, CAD cells were plated in complete medium (to allow attachment) and then transferred to differentiation medium without serum. At 24-48 h after transfection, cells were fixed for immunocytochemistry or extracted in lysis buffer (10 mm Tris-HCl, pH 7.5, 50 mm NaCl, 4 mm EDTA, 1% NP-40) containing leupeptin, pepstatin, aprotinin (10 μg/ml each), 5 mm benzamidin, 1 mm PMSF, and phosphatase inhibitors (1 mm Na vanadate, 5 mm NaF, 25 mm β-glycerophosphate) and analyzed by immunoblotting (Muresan and Arvan, 1997).
When used, kinase inhibitors were added directly to the culture medium of differentiated CAD cells (transfected or not) as follows: JNK inhibitor SP600125 (Biomol Research Laboratories, Plymouth Meeting, PA), 25 μm, 1-8 h (Bennett et al., 2001; Han et al., 2001); Cdk5 inhibitor roscovitine (Calbiochem, La Jolla, CA), 20 μm, 6 h or overnight (Niethammer et al., 2000). Control cultures received equal amounts of DMSO. For activation of the JNK stress pathway, cells were treated with sorbitol (0.4 m; 30 min at 37°C) (Inomata et al., 2003) or anisomycin (1 μg/ml; 30 min at 37°C) (Kyriakis et al., 1994).
RNA interference experiments. For immunocytochemistry, CAD cells were cotransfected with pmaxGFP (Amaxa) and either JIP-1 or JIP-3-specific small interfering RNA (siRNA) (Santa Cruz Biotechnology). Cells were allowed to attach to the coverslip in the presence of serum and then cultured for 24-48 h in the absence of serum. For biochemistry analysis, cells were cotransfected with FLAG-tagged JIP-1 plus JIP-3 in the presence of either JIP-1 or JIP-3-specific siRNA. After differentiation, cells were extracted in sample buffer, and the expression of JIP-1 or JIP-3 was analyzed by immunoblotting.
Pull-down and coimmunoprecipitation experiments. COS-1 cells, transfected with cDNAs encoding FLAG-tagged JIPs or myc-tagged Fe65, were rinsed with PBS and extracted 30 min at 4°C in lysis buffer. Extracts were centrifuged for 10 min at 16,000 × g and incubated 12-16 h at 4°C with biotinylated polypeptides corresponding to the entire ACD. Protein complexes containing the polypeptides were collected on streptavidin-Sepharose and analyzed by SDS-PAGE and immunoblots with anti-tag antibodies. To test JIP-1 binding to APP in the presence of Fe65, HEK293 cells were cotransfected with JIP-1-FLAG and Fe65-myc. NP-40 cell extracts were subjected to pull-down on biotinylated polypeptides corresponding to ACD, as described above.
Immunocytochemistry. Transfected or nontransfected CAD or COS-1 cells were fixed for 20 min in PBS containing 4% formaldehyde and 4% sucrose, permeabilized with 0.3% Triton X-100 (20 min, 20°C), and processed for antigen labeling as described previously (Muresan et al., 2000). Secondary antibodies coupled to Alexa dyes (488 and 594) were from Molecular Probes (Eugene, OR). The specificity of the anti-pAPP antibodies was tested in the presence of phosphorylated or nonphosphorylated polypeptides (100:1 molar excess over IgG) that encompassed either a 12 amino acid region centered on (p)Thr668 or the entire ACD (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Transfected, nontagged, human APP was selectively detected with the antibody 6E10 that does not detect endogenous mouse APP. Digital images were obtained with a Nikon Optiphot microscope (100× oil, 20×, and 40× objectives) equipped with cooled CCD camera and collected using Optronics Magnafire image analysis software. In some cases, samples were analyzed by confocal microscopy.
Analysis of neurite outgrowth. The ability of exogenous JIPs to promote neurite outgrowth was analyzed in CAD cells that were allowed to attach for 8 h posttransfection in medium containing 8% serum, followed by differentiation for 24 h in medium containing 0.4% serum. Under these conditions, less than one-third of the nontransfected control cells extended processes, which were consistently short. To visualize the processes, cells were stained with an antibody to α-tubulin. Cells were scored as having processes if these were longer than one cell body diameter.
Results
CAD cells as a model system for studying phosphorylation of APP
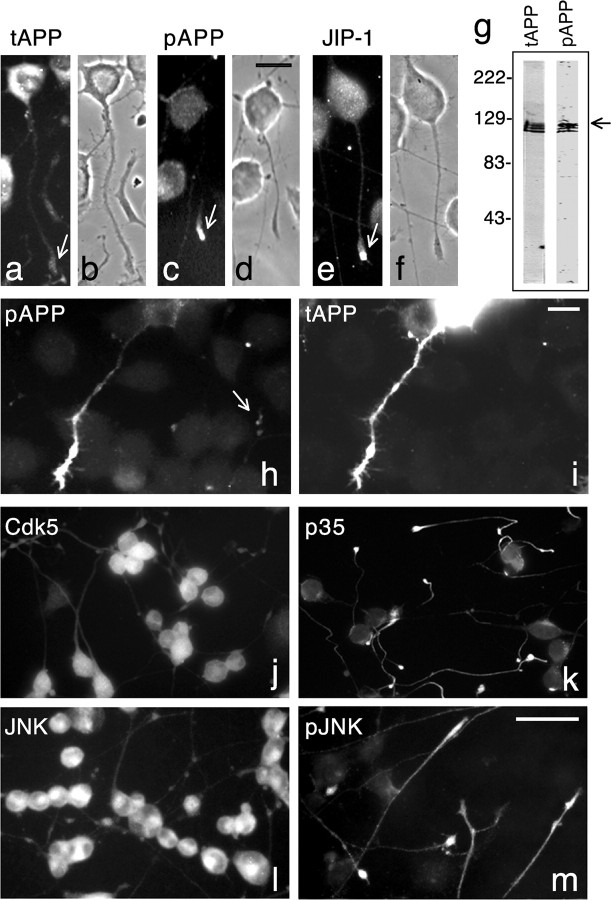
APP is phosphorylated at Thr668 under physiological and pathological conditions, but the mechanism and significance of this phosphorylation are poorly understood. To gain insight into this phenomenon, we used the CNS catecholaminergic cell line CAD. When cultured in serum-free medium, CAD cells stop dividing, grow processes, and acquire a neuronal-like phenotype, a condition that can be maintained for longer than 6 weeks in culture (Qi et al., 1997). Differentiated CAD cells express, transport into neurites, and proteolytically process APP (Muresan and Muresan, 2004). As in primary neurons, the majority of APP is detected in the cell body, whereas a fraction is localized within neurites (Fig. 1a,b). In addition, in CAD cells, APP is phosphorylated at Thr668 (Muresan and Muresan, 2004), and pAPP localizes primarily to neurite endings (Fig. 1c,d), similar to PC12 cells (Ando et al., 1999) and cultured primary neurons (Iijima et al., 2000; Lee et al., 2003; Muresan and Muresan, 2004). Although phosphorylation targets both the mature and immature forms of APP (Liu et al., 2003), the mature form is preferentially phosphorylated (Fig. 1g), as reported previously for PC12 cells and primary neurons (Ando et al., 1999; Iijima et al., 2000). Although only a small fraction of the total APP is phosphorylated (Fig. 1), we found that the machinery responsible for APP phosphorylation is capable to produce a substantial amount of pAPP in cells that overexpress APP695 (Fig. 1h,i). This suggests a tight control of this process. Like endogenous APP, a large amount of the exogenous APP that becomes phosphorylated accumulates in the neurites (Fig. 1h).
Figure 1.
Localization of total APP (tAPP), pAPP, and kinases (Cdk5, JNK) potentially involved in APP phosphorylation in cultured CAD cells. a-f, Immunocytochemical detection of tAPP, pAPP, and JIP-1, a JNK scaffolding protein that interacts with APP. Note that pAPP is preferentially localized to the distal processes (c). Arrows point to the tips of processes. Also note that tAPP is most abundant in the cell body (a), and only a small fraction is detected at the process terminal, where pAPP is concentrated (compare a with c). This implies that only a relatively small fraction of tAPP, which is localized to the neurite tip, might be phosphorylated. g, Immunoblot of extracts of CAD cells transfected with APP695 probed with antibodies to tAPP and pAPP. The distribution pattern of the APP-reactive bands is similar to that reported previously (Buxbaum et al., 1998; Iijima et al., 2000) and consists of immature (bottom band) and mature (partially and fully glycosylated; top two bands) APP forms. Note that the anti-pAPP antibody detects all forms but preferentially the fully matured form of APP (arrow). As reported previously (Ando et al., 1999; Iijima et al., 2000), in nontransfected cells, only the mature endogenous pAPP forms are detected (data not shown). h, i, Increased levels of pAPP are detected throughout the process of a CAD cell transfected with human APP695. Exogenously expressed APP is detected with an antibody to human APP (6E10) that does not cross-react with mouse APP (i). The arrow in h points to terminals containing endogenous pAPP. j-m, Immunocytochemical detection of Cdk5, p35, total JNK, and pJNK. Note that the Cdk5 activator, p35, and active pJNK are preferentially localized within neurites (compare k with j and m with l). b, d, f, Phase-contrast micrographs. Scale bars: (in d) a-f, 20 μm; (in i) h, i, 20 μm; (in m) j-m, 50 μm.
What is the identity of the kinase that phosphorylates APP in CAD cells? Cdk5 and JNK have been proposed to perform this task in cultured neurons (Iijima et al., 2000; Taru et al., 2002). As in primary neurons (Nikolic et al., 1996; Gdalyahu et al., 2004), in CAD cells, these kinases are localized in the cell body and at neurite endings (Fig. 1j,l). Also, the activator of Cdk5, p35, and JIP-1, a scaffolding protein necessary for JNK activity toward some of its substrates (Whitmarsh et al., 1998), localize primarily to the terminals of CAD cell processes (Fig. 1e,f,k). In addition, we found that active, phosphorylated (pThr183/pTyr185) JNK (pJNK) preferentially localizes to distal neurites (Fig. 1m), as does pAPP. Thus, both active Cdk5 and JNK display the spatial distribution that would allow them to phosphorylate APP.
JNK phosphorylates an APP fraction that accumulates in neurites
To determine the implication of Cdk5 and JNK in the phosphorylation of APP in CAD cells, we used drugs and dominant-negative inhibitors of these two kinases (Fig. 2). To block Cdk5 activity, we expressed a well-characterized, dominant-negative construct of Cdk5 (dnk5-GFP) (Niethammer et al., 2000) that has no kinase activity and competes with endogenous Cdk5 for substrates and activators. This construct caused neither significant reduction in pAPP levels nor change in pAPP distribution when expressed in CAD cells (data not shown). Similarly, treatment of cells with the potent Cdk5 inhibitor roscovitine did not block APP phosphorylation and localization to distal neurites, as detected immunocytochemically (Fig. 2a-d,j) or in biochemical experiments (Fig. 2m). These results suggest that Cdk5 plays only a minor role in APP phosphorylation in CAD cells.
Figure 2.
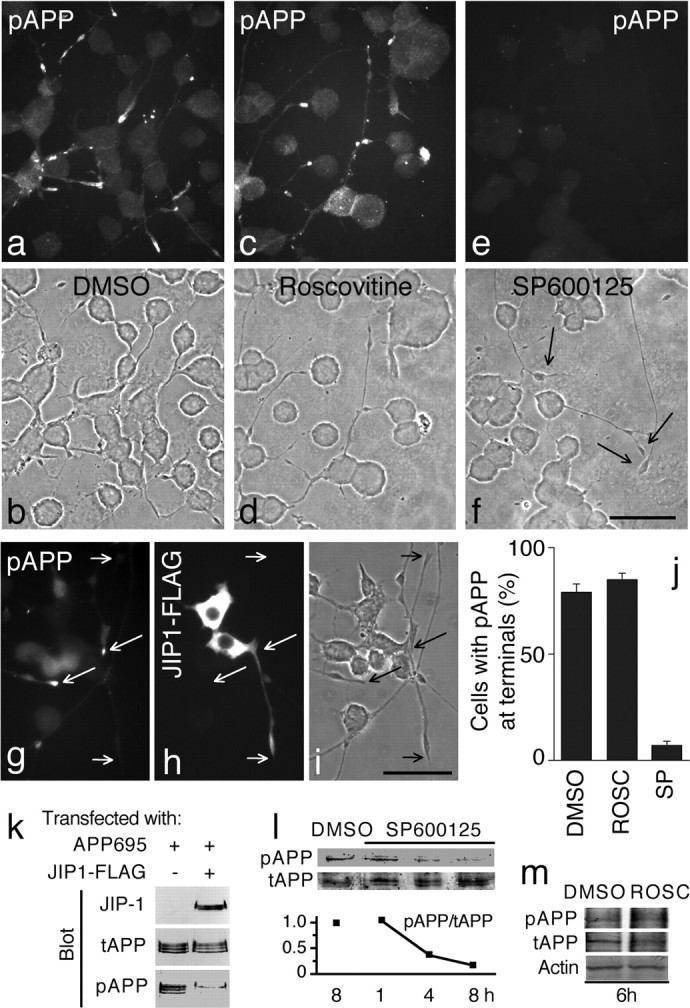
APP is phosphorylated by JNK in CAD cells. a-i, Localization of endogenous pAPP in cells treated with DMSO (control; a, b), the Cdk5 in hibitor roscovitine (c, d), or the JNK inhibitor SP600125 (e, f) and in cells over expressing FLAG-tagged JIP-1 (g-i). Cells were labeled for pAPP (a, c, e) or double labeled for pAPP (g) and the FLAG tag (h). Note that pAPP is not detected in neurites of cells overexpressing JIP-1-FLAG (g). In addition, cells treated with SP600125, but not with roscovitine or DMSO, lack pAPP both in neurites and in the cell body (compare e with a and c). Arrows in f point to neurite terminals. Short and long arrows in g-i point to terminals of processes of transfected and nontransfected cells, respectively. b, d, f, i, Phase-contrast micrographs. Scale bars, 50 μm. j, Quantitative measurement of the effect of roscovitine (ROSC) and SP600125 (SP) on pAPP localization at neurite terminals. CAD cells were treated with the inhibitors or DMSO. Percentages of cells with neurites that showed pAPP at terminals are indicated. Error bars indicate SEM. k, JIP-1 overexpression blocks APP phosphorylation. CAD cells were transfected either with APP695 or with APP695 plus JIP-1-FLAG. Immunoblots of cell lysates were probed with antibodies to total APP (tAPP), pAPP, and FLAG (JIP-1). Note the reduced amount of pAPP in cells transfected with JIP-1-FLAG. Immature (bottom band) and mature (top bands) forms of APP695 are detected. l, m, Phosphorylation of endogenous APP (detected with anti-pAPP antibody) is reduced after treatment of CAD cells with SP600125 but not with roscovitine. Control cells were incubated for 8 h (l) or 6 h (m) in medium containing the vehicle (DMSO). Because the endogenous level of pAPP in CAD cells is low, only the mature phosphorylated form is detected in these experiments. The graph in l shows the pAPP/tAPP ratio measured in relative units, as quantified from densitometric analysis of the immunoblots. The actin blot in m shows equal protein load.
To test whether JNK phosphorylates APP in CAD cells, we used a dominant-negative approach that used overexpression of JIP-1, a scaffold for components of the JNK signaling pathway (Whitmarsh et al., 1998). Overexpressed JIP-1 markedly inhibits JNK by sequestering the kinase away from its substrates (Hall and Davis, 2002). Our results show that overexpression of JIP-1-FLAG did not interfere with cell differentiation, because transfected cells extended normal processes (Fig. 2g-i). However, in sharp contrast with nontransfected cells, where most process terminals contained pAPP, pAPP was undetectable in >85% of the processes of CAD cells expressing JIP-1-FLAG (Fig. 2g-i). This result was confirmed by biochemical analysis of extracts from CAD cells transfected with human APP695 alone or in combination with JIP-1-FLAG. Consistent with a role of JNK in APP phosphorylation, overexpression of JIP-1 caused a dramatic reduction in pAPP, although APP695 was expressed at similar levels (Fig. 2k). This result was further confirmed in experiments in which we used the JNK inhibitor SP600125. Immunocytochemistry showed that treatment with SP600125 significantly reduced the level of pAPP within neurites and in the cell body (Fig. 2e,f,j). Similarly, immunoblots of extracts from treated cells showed a gradual decrease in pAPP, but not total APP, level during incubation with SP600125 (Fig. 2l). Together, these results suggest that generation of most pAPP detected in CAD cells requires the activity of JNK.
JIP-1 and JIP-2, but not JIP-3, bind to APP
To understand the mechanism of APP phosphorylation by JNK, we tested whether proteins that might recruit JNK to APP, such as the JIPs, are involved in this process. Recent reports (Matsuda et al., 2001; Scheinfeld et al., 2002; Taru et al., 2002) have shown that the PTB domain of JIP-1 binds to the YENPTY-containing site in APP. This makes JIP-1 a likely candidate for recruiting JNK in the vicinity of Thr668, thus facilitating its phosphorylation (Inomata et al., 2003). JIP-2, a protein structurally and functionally similar to JIP-1 (Yasuda et al., 1999), also contains a PTB domain and is thus expected to interact with APP. However, the JNK scaffolding protein, JIP-3, is structurally distinct from JIP-1 and JIP-2 and does not contain a PTB domain (Kelkar et al., 2000). First, we tested whether JIPs could be recruited to APP. We expressed FLAG-tagged JIP-1, JIP-2, and JIP-3 in COS-1 cells and tested their interaction with a biotinylated polypeptide representing the entire ACD. Binding of myc-tagged Fe65, a known, high-affinity APP ligand (Borg et al., 1996; Lau et al., 2000), was used as positive control. As shown in supplemental Figure 2, a and b (available at www.jneurosci.org as supplemental material), JIP-2, like JIP-1, interacted with the ACD, as did Fe65 (supplemental Fig. 2d, available at www.jneurosci.org as supplemental material). In contrast, JIP-3 did not bind to ACD (supplemental Fig. 2c, available at www.jneurosci.org as supplemental material). These results suggest that JIP-1 and JIP-2, but not JIP-3, are likely to facilitate phosphorylation of APP. We did not test binding to APP of JIP-4, a ubiquitously expressed protein related to JIP-3 (Lee et al., 2002).
JIP-3, rather than JIP-1, controls phosphorylation of APP and accumulation of pAPP at neurite terminals
As shown in Figure 1, e and f, JIP-1 localizes to the cell body and neurite terminals of CAD cells in a pattern that coincides with that of pAPP (Fig. 1c,d). This localization, considered together with the ability of JIP-1 to bind APP, makes JIP-1 a likely participant in APP phosphorylation by JNK. To test this hypothesis, we transfected CAD cells with a truncated, dominant-negative form of JIP-1 that binds APP but lacks the JNK binding domain (Scheinfeld et al., 2002, 2003) and thus prevents recruitment of JNK to APP. As exemplified in Figure 3, a and c, cells that express dominant-negative JIP-1 show an overall unaltered level of pAPP. This result indicates that, although capable of binding APP, JIP-1 is not the obligatory scaffold used by JNK to phosphorylate APP in neurons.
Figure 3.
JIP-1 does not function in the phosphorylation of endogenous APP. a-h, Overexpression of a dominant-negative JIP-1 lacking the JNK binding domain or of Fe65 does not block APP phosphorylation. a-f, Immunolocalization of pAPP. CAD cells transfected with FLAG-tagged, truncated JIP-1 (a-c) or myc-tagged Fe65 (d-f) were double labeled for endogenous pAPP (a, d) and the tag (b, e). Note that overexpression of either protein does not prevent phosphorylation of APP or localization of pAPP at process endings (long arrows). Also, note that compared with nontransfected cells, the cell body of transfected cells shows a slight increase in anti-pAPP staining. Short arrows in a and d point to the nontransfected cells. g, Immunoblots of CAD cells transfected with APP695 or with APP695 plus Fe65-myc. Cell lysates were probed with antibodies to total APP (tAPP), pAPP, and myc (Fe65). No reduction in the level of pAPP is seen in Fe65-transfected cells. h, JIP-1 does not bind to APP in the presence of Fe65. The lysate of HEK293 cells cotransfected with FLAG-tagged JIP-1 and myc-tagged Fe65 was incubated with biotinylated polypeptide encompassing the APP cytoplasmic domain, which was then collected on streptavidin-Sepharose. Copurified proteins were detected by immunoblotting with antibodies to the FLAG or myc tag. Note that JIP-1 fails to bind to the polypeptide (compare with the experiment described in supplemental Fig. 2, available at www.jneurosci.org as supplemental material). i-n, Downregulation of JIP-1 does not reduce APP phosphorylation. CAD cells were transfected with JIP-1-specific siRNA together with a plasmid encoding GFP to tag transfected cells. Two examples are shown in i-k and l-n. Note that the amount of pAPP detected in the cell bodies of transfected cells appears increased (i, j, l, m, arrows). c, f, k, n, Phase-contrast micrographs. Scale bars, 20 μm. o, Downregulation of JIP-1 by siRNA. CAD cells were transfected with FLAG-tagged JIP-1 cDNA, with or without JIP-1-specific siRNA. Cell lysates were analyzed by immunoblotting with anti-FLAG antibody (JIP-1). The actin blot shows equal protein load.
A similar result was obtained when we overexpressed Fe65, a neuronal protein that competes with JIP-1 for binding APP (Scheinfeld et al., 2002). Fe65 interacts strongly with APP (Lau et al., 2000) (see also supplemental Fig. 2d, available at www.jneurosci.org as supplemental material), with an affinity equal to, if not higher than, that of JIP-1 (Matsuda et al., 2001; Scheinfeld et al., 2002) but does not serve as scaffold for JNK. Confirming displacement of JIP-1 by Fe65, we found that JIP-1 failed to bind to a biotinylated polypeptide corresponding to ACD when coexpressed with Fe65 in HEK293 cells (Fig. 3h). Blocking the binding of JIP-1 to APP by overexpression of Fe65 did not alter the amount (Fig. 3g) and localization pattern of pAPP in CAD cells (Fig. 3d-f). This result supports our conclusion that JIP-1 is not required for APP phosphorylation by JNK.
We further tested the potential role of JIP-1 in APP phosphorylation using RNA interference. To investigate the effect of JIP-1 downregulation on the level of pAPP, we used immunocytochemistry rather than biochemical assays. CAD cells were cotransfected with a mixture of JIP-1-specific siRNAs and GFP as a marker of transfected cells. As shown in Figure 3i-n, pAPP was present in transfected cells at levels that frequently exceeded pAPP levels in nontransfected cells. This was a result of JIP-1 downregulation in transfected cells, because JIP-1 siRNA efficiently reduced expression of JIP-1-FLAG when cotransfected in CAD cells (Fig. 3o). Together, our results indicate that JIP-1 is not involved in APP phosphorylation in neurons.
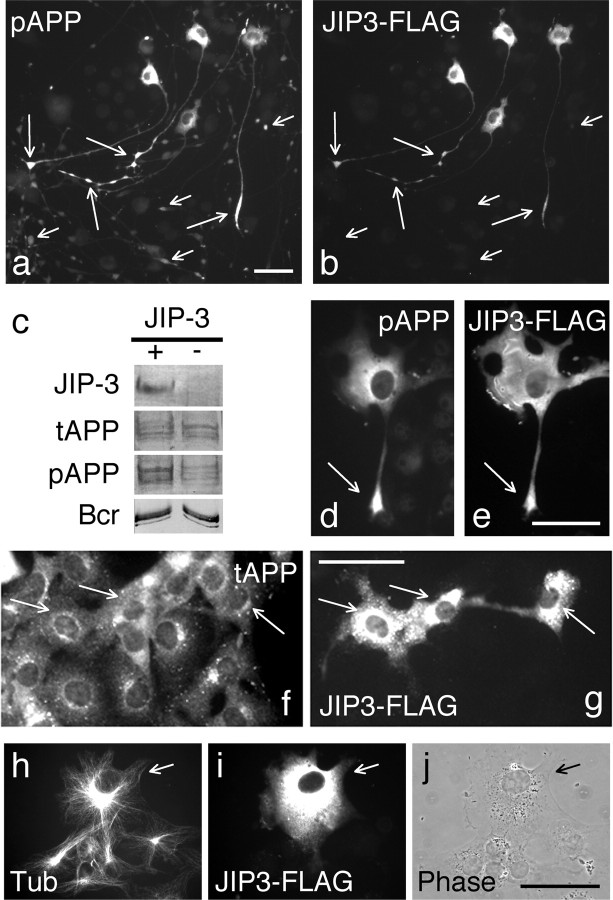
Next, we asked whether JIP-3 might have a role in APP phosphorylation, even though it does not directly bind to ACD (supplemental Fig. 2c, available at www.jneurosci.org as supplemental material). We compared the level of pAPP in extracts of CAD cells expressing APP and JIP-3-FLAG versus APP alone. As shown by immunoblotting (Fig. 4c), compared with controls, JIP-3 overexpression induced extensive phosphorylation of APP. Immunocytochemical analysis confirmed that expression of JIP-3-FLAG increased APP phosphorylation in CAD cells (Fig. 4a,b) without increasing the amount of total APP (Fig. 4f,g). Importantly, a significant fraction of the expressed JIP-3 and the generated pAPP colocalized at the terminals of processes (Fig. 4a,b). The effect of JIP-3 on APP phosphorylation was reproduced in COS-1 cells (Fig. 4d,e), which do not express JIPs (Kelkar et al., 2000). This indicates that JIP-3 activates a pathway that leads to APP phosphorylation, the components of which are generally available, and does not require JIP-1 or JIP-2. Interestingly, similar to its localization in CAD and neuronal cells, the pAPP generated in COS-1 cells by JIP-3 overexpression was often detected at the terminals of the extending protrusions (Fig. 4d). Such protrusions were much less frequent in nontransfected COS-1 cells, which normally phosphorylate APP only during mitosis. This suggests that JIP-3 not only promotes phosphorylation of APP but also seems to facilitate transport of the phosphorylated APP into processes, consistent with its proposed role in vesicular transport (Bowman et al., 2000; Byrd et al., 2001).
Figure 4.
Expression of JIP-3 in CAD and COS-1 cells promotes APP phosphorylation. High levels of pAPP are detected in cell bodies and processes of CAD (a, b) and COS-1 (d, e) cells that express FLAG-tagged JIP-3. Note the increased accumulation of pAPP at the process endings of transfected (a, b, long arrows) compared with nontransfected (a, b, short arrows) cells. c, Immunoblots of CAD cells transfected with APP695 or with APP695 plus JIP-3-FLAG. Cell lysates were probed with antibodies to total APP (tAPP), pAPP, or FLAG (JIP-3). Increased levels of pAPP are detected in JIP-3 transfected cells. An identical blot was probed for the protein Bcr and shows equal protein content of samples. f, g, Immunocytochemical detection of tAPP in COS-1 cells expressing JIP-3-FLAG. Note that transfected cells (arrows) show APP levels similar to nontransfected cells. h-j, A normal microtubule cytoskeleton, emanating from the centrosome, is seen in COS-1 cells overexpressing JIP-3. The arrow points to the transfected cell. Microtubules were labeled with an anti-α-tubulin antibody (Tub). An anti-FLAG antibody was used to detect exogenously expressed JIP-3. j is a phase-contrast micrograph. Scale bars, 50 μm.
Although the effect of JIP-3 on APP phosphorylation was consistently robust, a small fraction of cells that expressed JIP-3 (10-20%; see DMSO column in supplemental Fig. 3g, available at www.jneurosci.org as supplemental material) failed to show increased production of pAPP in both CAD and COS-1 cells. This could be related to the unavailability of the machinery for APP phosphorylation during particular phases of the cell cycle or stages of differentiation.
Because JIP-3 does not directly interact with APP, it is likely that its effect on APP phosphorylation is indirect. For example, JIP-3 might induce cell cycle arrest at the G2/M phase, a condition known to lead to extensive phosphorylation of APP (Suzuki et al., 1994). This was not the case, because JIP-3-transfected COS-1 cells showed intact nuclei and a microtubule network typical for interphase cells (Fig. 4h-j), and CAD cells differentiated into mitotically inactive neurons with long processes (Fig. 4a,b). In addition, the pAPP produced through JIP-3 is localized at neurite terminals, in sharp contrast to the general distribution of pAPP in mitotic cells (Muresan and Muresan, 2004).
Next, because the subcellular distribution of Cdk5/p35 coincides with that of JIP-3-generated pAPP (Fig. 1j,k), we determined whether JIP-3 expression activates Cdk5, rather than JNK, through an unknown pathway. As shown in supplemental Figure 3, a-c and g (available at www.jneurosci.org as supplemental material), the Cdk5 inhibitor roscovitine failed to prevent phosphorylation of APP not only in the nontransfected CAD cells but also in JIP-3 overexpressing cells. This was in sharp contrast to the effect of the JNK inhibitor SP600125, which completely blocked APP phosphorylation in all cells, including those expressing exogenous JIP-3-FLAG (supplemental Fig. 3d-g, available at www.jneurosci.org as supplemental material). It is important to note that the effect of these kinase inhibitors on APP phosphorylation was remarkably similar in nontransfected (Fig. 2) and in JIP-3 overexpressing cells. This supports the notion that JIP-3 overexpression only amplifies a process that occurs normally. Together, these results suggest that JNK phosphorylates APP in CAD cells under normal conditions (i.e., in the absence of stress) and that JIP-3 is implicated in this process.
Exogenous expression of JIP-3 activates JNK but does not lead to global phosphorylation of JNK substrates
To gain insight into the mechanism of JIP-3-facilitated APP phosphorylation, we determined whether exogenous expression of JIP-3 generally activates JNK (e.g., via a stress-induced-like pathway), leading to phosphorylation of known JNK targets such as c-Jun (Kelkar et al., 2000). Immunoblots of lysates from CAD cells transfected with FLAG-tagged JIP-3 showed undetectable levels of phosphorylated c-Jun (pc-Jun), compared with the robust production of pc-Jun caused by treatment with sorbitol (Fig. 5a). This result was confirmed by immunocytochemistry, in which the nuclear labeling for pc-Jun was not increased by expression of JIP-3-FLAG (Fig. 5b-e). In contrast, osmotic stress induced by sorbitol treatment led to massive production of pc-Jun (Fig. 5f,g). This result suggests that, although expressed throughout the cell (Fig. 5b), exogenous JIP-3 appears to activate only a fraction of the total cellular JNK in a spatially restricted manner and toward a specific set of substrates.
Figure 5.
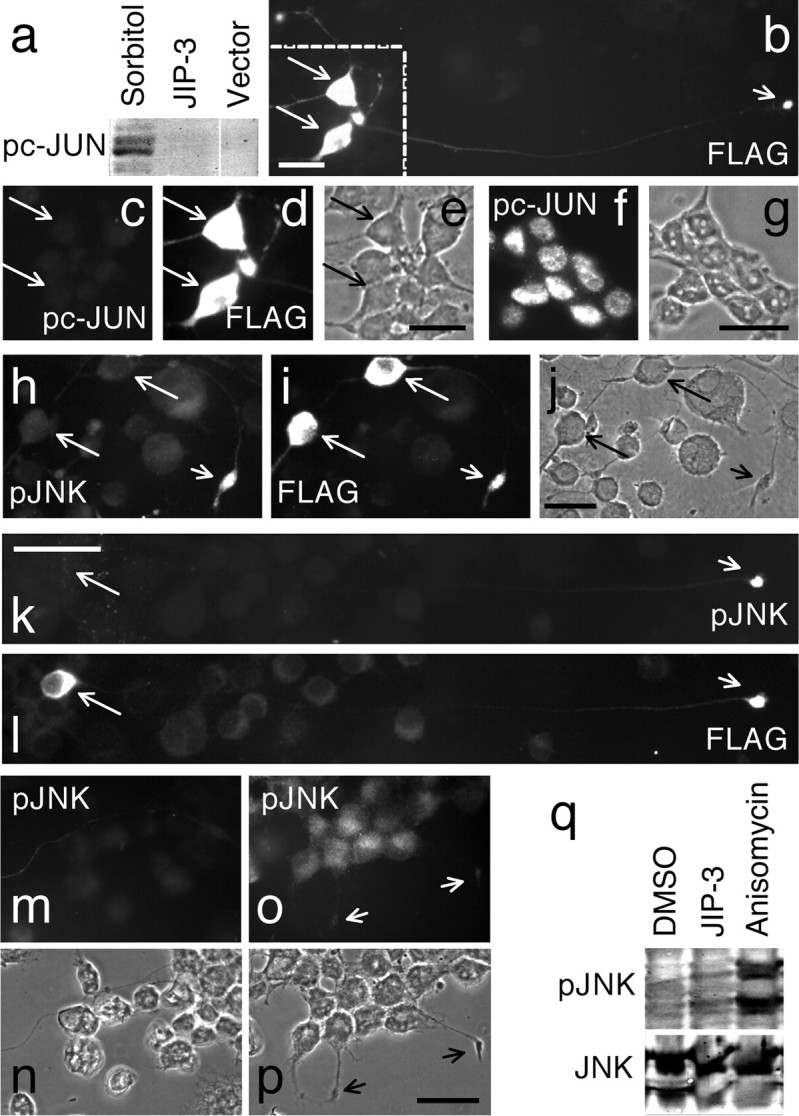
Overexpression of JIP-3 does not lead to phosphorylation of substrates targeted by the stress-activated JNK pathway. a-g, Treatment of CAD cells with sorbitol, but not exogenous expression of JIP-3, causes phosphorylation of c-Jun. a, Immunoblots of cell lysates from cells treated with sorbitol or transfected with JIP-3-FLAG (or vector) were probed with antibodies to pc-Jun. b-g, Immunolocalization of pc-Jun in CAD cells transfected with JIP-3-FLAG (b-e) or treated with sorbitol (f, g). JIP-3-FLAG was detected with an antibody to the tag (b, d). Note that, as in nontransfected cells, pc-Jun is undetectable in JIP-3 overexpressing cells (c, d). In contrast, intranuclear levels of pc-Jun are highly increased in cells treated with sorbitol (f, g). The labeled structures in f are nuclei. The image in b includes the long process of a transfected cell (the short arrow points to its terminal). Images in c-e correspond to the marked area in b and show only the cell bodies of transfected cells. h-p, Immunolocalization of pJNK in CAD cells transfected with JIP-3 (two examples are shown in h-j and k, l) or treated with anisomycin (o, p) or DMSO (control; m, n). Note that in JIP-3-transfected cells, pJNK is found at neurite terminals(h-l, short arrows) but not in the cell body (long arrows). In contrast, pJNK is elevated in cell bodies (o) but decreased at neurite terminals (o, p, short arrows) in anisomycin-treated cells. In all images, long arrows point to the cell bodies of JIP-3-transfected cells. Short arrows indicate neurite terminals. Note that JIP-3-transfected cells consistently extend long processes (b, k, l). e, g, j, n, p, Phase-contrast micrographs. Scale bars: b, (in e) c-e, (in g) f, g, (in j) h-j, (in p) m-p, 30 μm; (in k) k, l, 50 μm. q, Phosphorylation of JNK in JIP-3-transfected and in anisomycin-treated CAD cells. Immunoblots of cell lysates were probed with antibodies to pJNK and total JNK.
To test this notion, we investigated the subcellular localization and the amount of the active JNK, pJNK, in CAD cells transfected with JIP-3-FLAG. As shown in immunoblots (Fig. 5q), in contradistinction to the treatment with anysomycin (an activator of the JNK pathway) (Kyriakis et al., 1994), exogenous expression of JIP-3 only marginally increased the level of pJNK over controls. Although this suggests that JIP-3 expression activates only a small fraction of total JNK, the interpretation of this result is complicated by the low transfection efficiency in these experiments. Nevertheless, immunocytochemistry clearly showed that, whereas anisomycin led to efficient phosphorylation of JNK in the cell body (Fig. 5m-p), JIP-3-FLAG expression increased the level of pJNK specifically at process terminals (Fig. 5h-l). We conclude that JIP-3 does not support the general, stress-induced JNK pathway. Instead, it promotes phosphorylation and, thus, activation of the JNK fraction that is, or becomes, localized to the distal regions of the neurites, similar to pAPP.
Stress-activation of JNK increases APP phosphorylation in the cell body
Because JIP-3 expression and exposure to stress induce activation of JNK in distinct cell compartments (i.e., neurites and cell body, respectively), we determined whether these two different pathways of JNK activation might lead to differential effects on APP phosphorylation. As shown in immunoblots (Fig. 6a), both the expression of JIP-3-FLAG and the treatment with sorbitol increased overall APP phosphorylation. However, in contrast to JIP-3 expression, which promoted accumulation of pAPP at neurite terminals, sorbitol treatment increased the amount of pAPP in the cell body only (Fig. 6d-i). Anisomycin, another activator of the stress pathway (Kyriakis et al., 1994), had an identical effect on APP phosphorylation as the treatment with sorbitol (Fig. 6b,c). The relatively lower amount of pAPP present in the neurites of drug-treated cells was not attributable to an adverse effect of stress on accumulation of pAPP in neurites, because pAPP appeared localized to the distal parts of processes in cells that were treated with anisomycin and also expressed JIP-3-FLAG (Fig. 6j,k). These results suggest that, whereas phosphorylation of APP may be triggered by activation of JNK through different pathways, only the pathway involving JIP-3 leads to localization of the produced pAPP in neurites. Thus, JIP-3 may not only facilitate APP phosphorylation but might also be required for targeting of pAPP into neurites.
Figure 6.
Stress-induced activation of JNK increases APP phosphorylation in the cell body but not in the processes. a, Exogenous expression of JIP-3 or treatment with sorbitol increases APP phosphorylation. Immunoblots of lysates from JIP-3-FLAG-transfected or sorbitol-treated CAD cells were probed with antibodies to pAPP. Control cells were treated with DMSO. The Bcr blot shows equal protein load. b-k, Immunolocalization of pAPP (b, e, h, k), total APP (tAPP; d, g), and FLAG-tagged JIP-3 (j) in CAD cells treated with anisomycin (b, c), DMSO (d-f), or sorbitol (g-i) or transfected with JIP-3-FLAG and then treated with anisomycin (j, k). Note that treatment with either anisomycin or sorbitol causes accumulation of pAPP in the cell body but not at neurite terminals (compare b and h with e), whereas expression of JIP-3 leads to increased accumulation of pAPP both in the cell body and at process terminals (j, k). Also note that drug treatment does not increase the level of total APP in cells (compare g with d). Arrows point to neurite terminals. c, f, i, Phase-contrast micrographs. Scale bars: (in c) b-i, 30 μm; (in k) j, k, 50 μm.
JIP-3 facilitates neurite extension and accumulation of pAPP at process terminals
To directly test the role of JIP-3 in the neuritic localization of pAPP, we investigated the distribution of pAPP in cells that contained reduced amounts of JIP-3. Expression of JIP-3 was downregulated using siRNA. The efficiency and specificity of JIP-3 targeted siRNA in reducing the levels of JIP-3 was tested by immunoblot after cotransfection of CAD cells with FLAG-tagged JIP-3 and JIP-1, with or without JIP-3-specific siRNAs. As shown in Figure 7a, this treatment reduced the level of JIP-3 by ∼90% without affecting the level of JIP-1. This experiment also confirms the high degree of cotransfection of the plasmid-based construct (JIP-3-FLAG) and the oligonucleotide-based siRNA under our transfection conditions.
Figure 7.

JIP-3 is required for accumulation of pAPP in neurites. a, Downregulation of JIP-3, but not JIP-1, by JIP-3-specific siRNA. CAD cells were transfected with a mixture of FLAG-tagged JIP-1 and JIP-3 cDNA with or without JIP-3 siRNA. Cell lysates were analyzed by immunoblotting with anti-FLAG antibody. b-i, Downregulation of JIP-3 leads to diminished accumulation of pAPP in neurites. CAD cells were transfected with JIP-3-specific siRNA together with a plasmid encoding GFP to allow identification of transfected cells (c-f). Control cells were transfected with GFP alone (g-i). Contrast was increased in d to allow clear identification of processes emanating from some transfected cells (c-f, stars). Note that cells transfected with JIP-3 siRNA extend short processes at best (d, f), which do not contain pAPP (c, e). Nontransfected cells show accumulation of pAPP at process terminals, even when the processes are short (arrows; see cells in the left bottom corner in c, e). Also note that in cells transfected with GFP alone, pAPP accumulates at neurite terminals (g, h, arrows). e and g are overlap images of c and d and h and i, respectively. Scale bars, 50 μm. b, Quantitative measurement of the effect of JIP-3 downregulation by siRNA on pAPP localization at neurite terminals. Percentages of cells with neurites that showed pAPP at terminals are indicated. Error bars indicate SEM.
For immunocytochemistry, CAD cells were cotransfected with JIP-3-specific siRNAs and a plasmid encoding GFP (to identify transfected cells). Nontransfected cells (i.e., cells that did not express GFP) (Fig. 7c-f) or cells transfected with GFP alone (Fig. 7g-i) showed normal processes and accumulation of pAPP at process endings. Noticeably, JIP-3 siRNA-transfected cells extended only short processes and contained no pAPP within the neurites (Fig. 7c-f). Statistical analysis of cells that extended neurites revealed that only ∼20% of siRNA-transfected cells contained pAPP in the neurites, a fourfold reduction compared with untreated cells (Fig. 7b). Together, these results indicate that JIP-3 has a major role in the phosphorylation of APP and accumulation of pAPP into neuronal processes under normal physiological conditions.
We noticed that downregulation of JIP-3 in CAD cells was consistently associated with extension of only short processes. This suggested that JIP-3 might be required for normal neurite extension. Consistent with this idea, we found that cells that expressed exogenous JIP-3 constantly extended long processes (Figs. 4a,b,d,e, 5b,k,l). To determine whether JIP-3 facilitates neurite extension, we developed an assay that allows quantification of neurite-extending cells and measurement of neurite length in conditions that only minimally favor process extension. CAD cells were transfected with FLAG-tagged JIP-3 and then cultured for 24 h in the presence of reduced (0.4%) serum. In these culturing conditions, CAD cells still divide, and a fraction extend processes, which are usually short compared with those extended by CAD cells under total serum deprivation. As a control, a second set of cells was subjected to the same treatment after being transfected with JIP-1-FLAG. Both sets were fixed and immunolabeled for the FLAG tag and α-tubulin to allow measurement of process length. Similar to nontransfected cells (29%), ∼21% of JIP-1-expressing cells extended processes. In contrast, >60% of JIP-3-transfected cells showed processes, which were on average at least twofold longer than those from cells expressing JIP-1 (i.e., 96 ± 25 vs 41 ± 16 μm; mean ± SD; p < 0.001). Collectively, these results indicate that, whereas JIP-1 appears to have no effect, JIP-3 positively regulates neurite extension and accumulation of pAPP within neurites under normal physiological conditions.
JIP-3 colocalizes with pAPP, JIP-1, activated JNK, and kinesin-1 within neurites
The finding that JIP-3 participates in the phosphorylation of APP by activating JNK, together with its implication in neurite extension and transport of pAPP into neurites, predicts that JIP-3 is linked to components of the phosphorylation and transport machineries of APP. Indeed, immunolocalization studies showed that in CAD cells, JIP-3-FLAG colocalizes with endogenous pAPP, pJNK, and kinesin-1 (Fig. 8a-h, p-r). JIP-3-FLAG also colocalizes with JIP-1 (Fig. 8k-o), which may be carried along with APP as part of a kinesin-1 cargo, although it does not participate in APP phosphorylation. JIP-3-FLAG also colocalizes with JIP-1 (Fig. 8k-o), which may be carried along with APP as part of a kinesin-1 cargo, although it does not participate in APP phosphorylation. In addition, like overexpressed JIP-3-FLAG, the endogenous JIP-3 colocalizes with pAPP (Fig. 8s-v). Future studies are required to elucidate how these proteins cooperate to phosphorylate and transport APP.
Figure 8.
JIP-3-dependent phosphorylation of APP and transport of pAPP. a-r, Colocalization of overexpressed JIP-3 with endogenous pAPP, pJNK, JIP-1, and kinesin-1, but not kinesin-2, within neurites. CAD cells, transfected with FLAG-tagged JIP-3, were double labeled for either pAPP (a-d), kinesin heavy chain (KHC; e-h), the KIF3A subunit of kinesin-2 (i, j), JIP-1 (k-o), or pJNK (p-r) and the FLAG tag. JIP-3-FLAG also colocalized with the kinesin light chain, but not with total, mostly nonphosphorylated JNK (data not shown).m-o are enlarged images of the neurite terminal shown in k and l. s-v, Colocalization of endogenous JIP-3 with pAPP within neurites. Colocalization is shown in yellow in c, g, o, and u. d, h, r, and v are phase-contrast micrographs. Scale bars: (in d) a-d, (in r, v) p-v, 10 μm; (in h, j, l) e-l, 20 μm; (in o) m-o, 5 μm. The diagrams in w-y show three possible ways of anchoring JIP-3 to APP. In all cases, JNK is recruited and activated via JIP-3, which binds to the light chain (KLC) of kinesin-1 (w, x) or to an unknown receptor (y). The kinesin-1/JIP-3/JNK complex is either anchored to APP directly (w) or via an unknown scaffold (x, ?) or brought in the vicinity of APP by binding to a receptor protein (y). As depicted, the APP phosphorylation and transport machineries are intimately associated.
Discussion
APP is phosphorylated at multiple sites in the 47 amino acid C-terminal cytoplasmic domain (Suzuki et al., 1994). Phosphorylation of Thr668 is particularly important, because it induces conformational changes that affect APP function and metabolism (Ando et al., 2001; Ramelot and Nicholson, 2001). To understand the mechanism of APP phosphorylation at Thr668 in neuronal cells, in this study, we investigated the role of the kinases Cdk5 and JNK and of their scaffolding and activating proteins. Using dominant-negative strategies and small molecule inhibitors, we found that JNK, not Cdk5, phosphorylates APP in differentiating neuronal cells. By preventing the interaction of JIP-1 with JNK or APP, we established that this JNK scaffolding, APP-binding protein does not participate in APP phosphorylation in neurons under normal physiological conditions. Importantly, we found that JIP-3, another JNK adaptor protein, which does not directly interact with APP, participates in the generation of a large fraction of pAPP, which localizes to neurites. Moreover, exogenous expression of JIP-3 leads to a significant increase in APP phosphorylation. Consistent with a function in the phosphorylation of APP by JNK, we further found that JIP-3 colocalizes with activated JNK and pAPP within neuronal processes. Together with the fact that JIP-3 is selectively expressed in neurons (Kelkar et al., 2000), which also produce the highest levels of pAPP, our results identify JIP-3 as the most likely physiological activator/regulator of APP phosphorylation in these cells.
Previous studies investigated the role of JNK in APP phosphorylation in cells lacking essential components of the neuronal JNK signaling pathway, such as the JIPs, or in conditions that forced the process by stress activation or overexpression of the kinase (Taru et al., 2002; Inomata et al., 2003; Scheinfeld et al., 2003). In this study, we used the well-characterized, CNS-derived CAD cells (Qi et al., 1997; Hashemi et al., 2003) that express APP, JNK, and all JIPs and produce detectable levels of endogenous pAPP, in the absence of stress (Muresan and Muresan, 2004). Thus, our system allows for the study of the APP phosphorylation that occurs under normal conditions in neurons.
Yeast two-hybrid screens and in vitro interaction assays found that JIP-1 binds APP directly (Matsuda et al., 2001; Scheinfeld et al., 2002; Taru et al., 2002). Because it also anchors components of the JNK signaling cascade (Whitmarsh et al., 1998), JIP-1 was viewed as the likely JNK scaffold required for APP phosphorylation (Inomata et al., 2003), even though some JIP-1 independent APP phosphorylation may occur in cells that lack JIP-1 (Scheinfeld et al., 2003). Given that JIP-1 is available primarily in neurons, our finding that docking of JNK to APP by JIP-1 is not used for APP phosphorylation in CAD cells was surprising. A likely explanation, supported by our results (Fig. 3), is that JIP-1 binding to APP is competed off by proteins with higher affinity or more abundant than JIP-1 (e.g., Fe65) (Matsuda et al., 2001), or that in neurons, the phosphorylation of APP through the JIP-3-dependent pathway prevails and is distinct from that implicating JIP-1.
Our results unequivocally indicate that the signaling pathway sustained by JIP-3, leading to APP phosphorylation, is qualitatively different from well known stress-activated JNK signaling pathways. Stress stimuli lead to activation of JNK almost exclusively in the cell body and cause phosphorylation of both intranuclear (e.g., c-Jun) and cytoplasmic targets, including APP. However, the stress-generated pAPP remains primarily in the cell body. This is dissimilar to the JIP-3-dependent APP phosphorylation that results in accumulation of a significant fraction of pAPP in neurites. Moreover, in this pathway, JNK seems to be activated locally and does not trigger translocation of pJNK into the nucleus or phosphorylation of nuclear targets such as c-Jun.
Although phosphorylation of APP appears to occur within neurites, where pAPP, pJNK, and a substantial fraction of JIP-3 colocalize, we believe that this distribution is the result of a dynamic process in which pAPP is generated by a JIP-3-dependent mechanism in the cell body and then transported by kinesin-1 into processes, in which it accumulates at the terminals. Indeed, when the interaction of kinesin-1 with its cargos is blocked by dominant-negative strategies, pAPP accumulates in small vesicular structures in cortical areas of the cell body instead of processes (our unpublished results). These results imply that phosphorylation of APP might be connected with the formation of a transport complex that contains pJNK, JIP-3, pAPP, and kinesin-1.
Because JIP-3 does not directly bind APP, how does it facilitate APP phosphorylation and transport into processes? It may do so by interacting with proteins that bind themselves APP (directly or indirectly) or that are in close vicinity to APP on the transport vesicle. In this way, JIP-3 could dock the kinase (JNK) to the substrate (APP) in a supramolecular complex that allows for phosphorylation. Kinesin-1, the microtubule motor that binds and transports APP and JIPs (Bowman et al., 2000; Kamal et al., 2000; Verhey et al., 2001; Whitmarsh et al., 2001), is a likely candidate to provide the link between JIP-3 and APP via its two light chains. The models shown in Figure 8w-y imply that, by facilitating the phosphorylation of its own cargo (i.e., APP), the kinesin-1 machinery that carries pAPP into neurites has a dual role, transport and signaling. Data showing colocalization of pAPP, pJNK, JIP-3, and kinesin-1 within neurites (Fig. 8) support the in vivo existence of such a complex. JIP-1 also colocalizes with these proteins and, although we found that it does not participate in the phosphorylation of APP, it may have other functions that remain to be determined. In the models described above, it is assumed that the increase in the amount of pAPP is attained through increased JIP-3-dependent phosphorylation. However, alternative models in which JIP-3 exercises inhibitory or delocalization effects on a yet unidentified, pAPP-targeted phosphatase cannot be ruled out.
In mammals, JIP-3 is required for normal brain development (Kelkar et al., 2003). Recent reports identified the JIP-3 orthologs Sunday driver protein, in Drosophila melanogaster (Bowman et al., 2000), and uncoordinated locomotion UNC-16, in Caenorhabditis elegans (Byrd et al., 2001) as being essential for axonal transport. Consistent with these studies, we showed that JIP-3 sustains neurite extension, a process that requires active vesicular transport. In addition, we found that JIP-3 is essential for phosphorylation of APP and transport of the generated pAPP into process. Downregulation of JIP-3 in CAD cells concurrently impairs extension of neuronal processes, phosphorylation of APP, and accumulation of phosphorylated APP into neurites. Similarly, extension of protrusions triggered by exogenous JIP-3 in COS-1 cells is accompanied by extensive APP phosphorylation, suggesting that the two events may be part of the same or connected pathways. Thus, JIP-3 recruits JNK into a cascade that phosphorylates APP, and perhaps other not yet identified targets, and promotes vesicular transport resulting in the extension of, and accumulation of pAPP into, cell processes. Such localized activation of signaling cascades that implicate JNK, with end results in rearrangements of the cytoskeletal structures, have been proposed previously in the planar cell polarity pathway that diverges at Dishevelled (Boutros et al., 1998). The partake of JIP-3, pAPP, JIP-1, and kinesin-1 in such a pathway is at present an exciting possibility that needs to be addressed.
The mechanism by which JIP-3 sustains the JNK signaling cascade that leads to APP phosphorylation and transport and to neurite extension remains to be elucidated. Aside from being just an anchor for the JNK signaling module, JIP-3 might play a more active role in coordinating these events (Weston and Davis, 2002). Moreover, JIP-3 might be regulated by phosphorylation (Matsuura et al., 2002), thus leading to the formation of dynamic transport and signaling complexes. Unlike JIP-3, JIP-1 does not appear to be required for the phosphorylation of APP, although we consistently find it colocalized with the transport vesicles enriched in JIP-3 and pAPP. Our unpublished data suggest that JIP-1 may function in the transport of pAPP after its generation by JIP-1-independent mechanisms. Its role in yet unknown signaling pathways, unrelated or downstream from JIP-3 and pAPP, remains to be determined. Thus, JIP-1 and JIP-3 appear to perform distinct but coordinated functions that lead to phosphorylation and transport of APP. Additional studies are required to elucidate the exact mechanisms by which the two proteins integrate JNK signaling with APP phosphorylation, metabolism, and transport by kinesin-1.
Footnotes
This work was supported by National Institutes of Health Grant 1RO1GM068596-01A1, a Mount Sinai Health Care Foundation scholarship, and Pilot Grant AG08012 from the Alzheimer's Disease Research Center at the University Hospitals of Cleveland and Case Western Reserve University (V.M.). We thank Drs. Dona Chikaraishi and James Wang for kindly providing the CAD cell line, Drs. Li-Huei Tsai and Ming-Sum Lee for kindly providing cDNA constructs and an antibody to phosphorylated APP that was used in preliminary experiments, and Drs. Luciano D'Adamio, Roger Davis, and Ben Margolis for kindly providing cDNA constructs.
Correspondence should be addressed to Virgil Muresan, Department of Physiology and Biophysics, Case Western Reserve University School of Medicine, E553, 10900 Euclid Avenue, Cleveland, OH 44106-4970. E-mail: virgil.muresan@case.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253741-11$15.00/0
References
- Ando K, Oishi M, Takeda S, Iijima K, Isohara T, Nairn AC, Kirino Y, Greengard P, Suzuki T (1999) Role of phosphorylation of Alzheimer's amyloid precursor protein during neuronal differentiation. J Neurosci 19: 4421-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T (2001) Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem 276: 40353-40361. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Gibb GM, Jacobsen JS, Gallo JM, Anderton BH (1996) In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3beta. J Neurochem 67: 699-707. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg JP, Ooi J, Levy E, Margolis B (1996) The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol Cell Biol 16: 6229-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109-118. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS (2000) Kinesin-dependent axonal transport is mediated by the Sunday driver (SYD) protein. Cell 103: 583-594. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Thinakaran G, Koliatsos V, O'Callahan J, Slunt HH, Price DL, Sisodia SS (1998) Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J Neurosci 18: 9629-9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y (2001) UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans Neuron 32: 787-800. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 113: 1857-1870. [DOI] [PubMed] [Google Scholar]
- Gdalyahu A, Ghosh I, Levy T, Sapir T, Sapoznik S, Fishler Y, Azoulai D, Reiner O (2004) DCX, a new mediator of the JNK pathway. EMBO J 23: 823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JP, Davis RJ (2002) Analysis of c-Jun N-terminal kinase regulation and function. Methods Enzymol 345: 413-425. [DOI] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108: 73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi SH, Li JY, Faigle R, Dahlstrom A (2003) Adrenergic differentiation and SSR2a receptor expression in CAD-cells cultured in serum-free medium. Neurochem Int 42: 9-17. [DOI] [PubMed] [Google Scholar]
- Hedera P, Turner RS (2002) Inherited dementias. Neurol Clin 20: 779-808. [DOI] [PubMed] [Google Scholar]
- Iijima K, Ando K, Takeda S, Satoh Y, Seki T, Itohara S, Greengard P, Kirino Y, Nairn AC, Suzuki T (2000) Neuron-specific phosphorylation of Alzheimer's beta-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem 75: 1085-1091. [DOI] [PubMed] [Google Scholar]
- Inomata H, Nakamura Y, Hayakawa A, Takata H, Suzuki T, Miyazawa K, Kitamura N (2003) A scaffold protein JIP-1b enhances amyloid precursor protein phosphorylation by JNK and its association with kinesin light chain 1. J Biol Chem 278: 22946-22955. [DOI] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia C, Goldstein LS (2000) Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron 28: 449-459. [DOI] [PubMed] [Google Scholar]
- Kelkar N, Gupta S, Dickens M, Davis RJ (2000) Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol Cell Biol 20: 1030-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar N, Delmotte MH, Weston CR, Barrett T, Sheppard BJ, Flavell RA, Davis RJ (2003) Morphogenesis of the telencephalic commissure requires scaffold protein JNK-interacting protein 3 (JIP3). Proc Natl Acad Sci USA 100: 9843-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Turner RS (2004) Adaptor protein interactions: modulators of amyloid precursor protein metabolism and Alzheimer's disease risk? Exp Neurol 185: 208-219. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369: 156-160. [DOI] [PubMed] [Google Scholar]
- Lau KF, McLoughlin DM, Standen CL, Irving NG, Miller CC (2000) Fe65 and X11beta co-localize with and compete for binding to the amyloid precursor protein. NeuroReport 11: 3607-3610. [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L (2004) A standardized kinesin nomenclature. J Cell Biol 167: 19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Onesime D, Reddy CD, Dhanasekaran N, Reddy EP (2002) JLP: a scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors. Proc Natl Acad Sci USA 99: 14189-14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH (2003) APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol 163: 83-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Su Y, Li B, Zhou Y, Ryder J, Gonzalez-DeWhitt P, May PC, Ni B (2003) Regulation of amyloid precursor protein (APP) phosphorylation and processing by p35/Cdk5 and p25/Cdk5. FEBS Lett 547: 193-196. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Yasukawa T, Homma Y, Ito Y, Niikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, Kouyama K, Yamamoto T, Kyriakis JM, Nishimoto I (2001) c-Jun N-terminal kinase (JNK)-interacting protein-1b/islet-brain-1 scaffolds Alzheimer's amyloid precursor protein with JNK. J Neurosci 21: 6597-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H, Nishitoh H, Takeda K, Matsuzawa A, Amagasa T, Ito M, Yoshioka K, Ichijo H (2002) Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1-JNK signaling pathway. A new mode of regulation of the MAP kinase cascade. J Biol Chem 277: 40703-40709. [DOI] [PubMed] [Google Scholar]
- Muresan V (2000) One axon, many kinesins: what's the logic? J Neurocytol 29: 799-818. [DOI] [PubMed] [Google Scholar]
- Muresan Z, Arvan P (1997) Thyroglobulin transport along the secretory pathway. Investigation of the role of molecular chaperone, GRP94, in protein export from the endoplasmic reticulum. J Biol Chem [Erratum (1997) 272: 30590] 272:26095-26102. [DOI] [PubMed] [Google Scholar]
- Muresan Z, Muresan V (2004) A phosphorylated, carboxy-terminal fragment of (beta)-amyloid precursor protein localizes to the splicing factor compartment. Hum Mol Genet 13: 475-488. [DOI] [PubMed] [Google Scholar]
- Muresan Z, Paul DL, Goodenough DA (2000) Occludin 1B, a variant of the tight junction protein occludin. Mol Biol Cell 11: 627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, Morabito M, Tsai LH (2000) NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron 28: 697-711. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH (1996) The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev 10: 816-825. [DOI] [PubMed] [Google Scholar]
- Qi Y, Wang JK, McMillian M, Chikaraishi DM (1997) Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci 17: 1217-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramelot TA, Nicholson LK (2001) Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J Mol Biol 307: 871-884. [DOI] [PubMed] [Google Scholar]
- Scheinfeld MH, Roncarati R, Vito P, Lopez PA, Abdallah M, D'Adamio L (2002) Jun NH2-terminal kinase (JNK) interacting protein 1 (JIP1) binds the cytoplasmic domain of the Alzheimer's beta-amyloid precursor protein (APP). J Biol Chem 277: 3767-3775. [DOI] [PubMed] [Google Scholar]
- Scheinfeld MH, Ghersi E, Davies P, D'Adamio L (2003) Amyloid beta protein precursor is phosphorylated by JNK-1 independent of, yet facilitated by, JNK-interacting protein (JIP)-1. J Biol Chem 278: 42058-42063. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (1998) The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol 8: 447-453. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741-766. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH (2002) gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci 3: 281-290. [DOI] [PubMed] [Google Scholar]
- Standen CL, Brownlees J, Grierson AJ, Kesavapany S, Lau KF, McLoughlin DM, Miller CC (2001) Phosphorylation of thr(668) in the cytoplasmic domain of the Alzheimer's disease amyloid precursor protein by stress-activated protein kinase 1b (Jun N-terminal kinase-3). J Neurochem 76: 316-320. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oishi M, Marshak DR, Czernik AJ, Nairn AC, Greengard P (1994) Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. EMBO J 13: 1114-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taru H, Iijima K, Hase M, Kirino Y, Yagi Y, Suzuki T (2002) Interaction of Alzheimer's beta-amyloid precursor family proteins with scaffold proteins of the JNK signaling cascade. J Biol Chem 277: 20070-20078. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B (2001) Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol 152: 959-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ (2002) The JNK signal transduction pathway. Curr Opin Genet Dev 12: 14-21. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ (1998) A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281: 1671-1674. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Kuan CY, Kennedy NJ, Kelkar N, Haydar TF, Mordes JP, Appel M, Rossini AA, Jones SN, Flavell RA, Rakic P, Davis RJ (2001) Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev 15: 2421-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ (1999) The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol 19: 7245-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]