Abstract
Cholinergic neurons innervating the cortex have been conceptualized as a major component of the attention system of the brain. Because of recent evidence indicating plastic mechanisms regulating choline transporter (CHT)-mediated high-affinity choline uptake, which is the rate-limiting step of acetylcholine synthesis, the present experiment determined the capacity of cholinergic terminals to transport choline, and the proportion of choline transporters localized in the membrane of synaptic terminals, in several brain regions of rats performing a cognitive vigilance task (CVT) and a simple reaction time task (SRTT) and nonperforming (NP) rats. Compared with evidence from NP rats, increased choline transporter capacity [as indicated by maximum transporter velocity (Vmax)] and an increased density of CHTs situated in synaptic plasma membrane, relative to intracellular locations, were observed in the medial prefrontal cortex of the right but not left hemisphere of CVT-performing animals. Furthermore, right medial prefrontal Vmax values of CVT-performing rats correlated positively and left medial Vmax values correlated negatively with the animals' performance in signal trials. Measures of CHT function in the brains of SRTT-performing animals did not differ significantly from those in NP rats. The present data support the hypothesis that an increased capacity of choline transporters in the right medial prefrontal cortex, primarily attributable to increased trafficking of transporters from intracellular compartments to the terminal membrane, represents a cellular mechanism contributing to the mediation of attentional performance.
Keywords: choline transporter, attention, prefrontal cortex, acetylcholine, synaptosome, trafficking
Introduction
Attentional capacities are mediated via distributed prefrontal and parietal circuits (Posner and Dehaene, 1994; Coull, 1998; Corbetta and Shulman, 2002). Cholinergic neurons originating in the basal forebrain and projecting to these cortical regions represent a crucial component of the attention system of the brain (Everitt and Robbins, 1997; Sarter et al., 2005). Past experiments demonstrated that the integrity of cortical cholinergic inputs is necessary for attentional performance (McGaughy et al., 1996). Several studies documented increases in cortical acetylcholine (ACh) release in animals performing tasks involving explicit demands on attentional processing; such increases were not observed in rats performing behavioral control procedures not involving such demands (Himmelheber et al., 1997; Passetti et al., 2000; Himmelheber et al., 2001; Arnold et al., 2002).
The transport of choline into presynaptic terminals by the high-affinity choline transporter (CHT) influences the rate of ACh synthesis. Recent evidence has indicated that changes in CHT function may be regulated in part by altering the rate of trafficking of CHTs between intracellular domains and synaptic membranes (Apparsundaram et al., 2000; Ferguson et al., 2003; Ferguson and Blakely, 2004). However, relatively little is known concerning the CHT in behavioral or cognitive contexts (Sarter and Parikh, 2005). The present experiment was designed to test the hypotheses that, in animals performing an operant cognitive vigilance task (CVT) (McGaughy and Sarter, 1995), measures of choline uptake and the proportion of CHTs present in synaptic plasma membrane reveal enhanced CHT capacity in cortical regions but not the striatum. Evidence from CVT-performing animals was compared with data from nonperforming (NP) rats and from animals trained to perform a simple reaction time task (SRTT) (Moore et al., 1992). The SRTT was identical to the CVT except that only the correct response lever was made available; thus, this task lacked the requirement to process propositional response rules (hence the term CVT to depict the former) (Parasuraman et al., 1987) (see Fig. 1). Measures of CHT function were determined in the medial prefrontal cortex (mPFC) because of evidence indicating the crucial role of cholinergic inputs to this region in attentional performance (Sarter et al., 2001; Dalley et al., 2004). Furthermore, the posterior parietal cortex (PPC) was selected for analysis in view of evidence from human imaging studies that consistently indicated activation of this area in target detection tasks (Corbetta et al., 2000). Moreover, cholinergic depletion of the PPC of rats was demonstrated to impair attentional processes (Bucci et al., 1998). Because performance was not expected to alter striatal CHT function, striatal tissues from both hemispheres were pooled. The main results indicate that CVT performance increased the capacity of CHTs to transport choline in the right but not left mPFC. Furthermore, increased CHT capacity in the right mPFC was associated with an increased proportion of CHTs located in plasma membranes obtained from this region.
Figure 1.
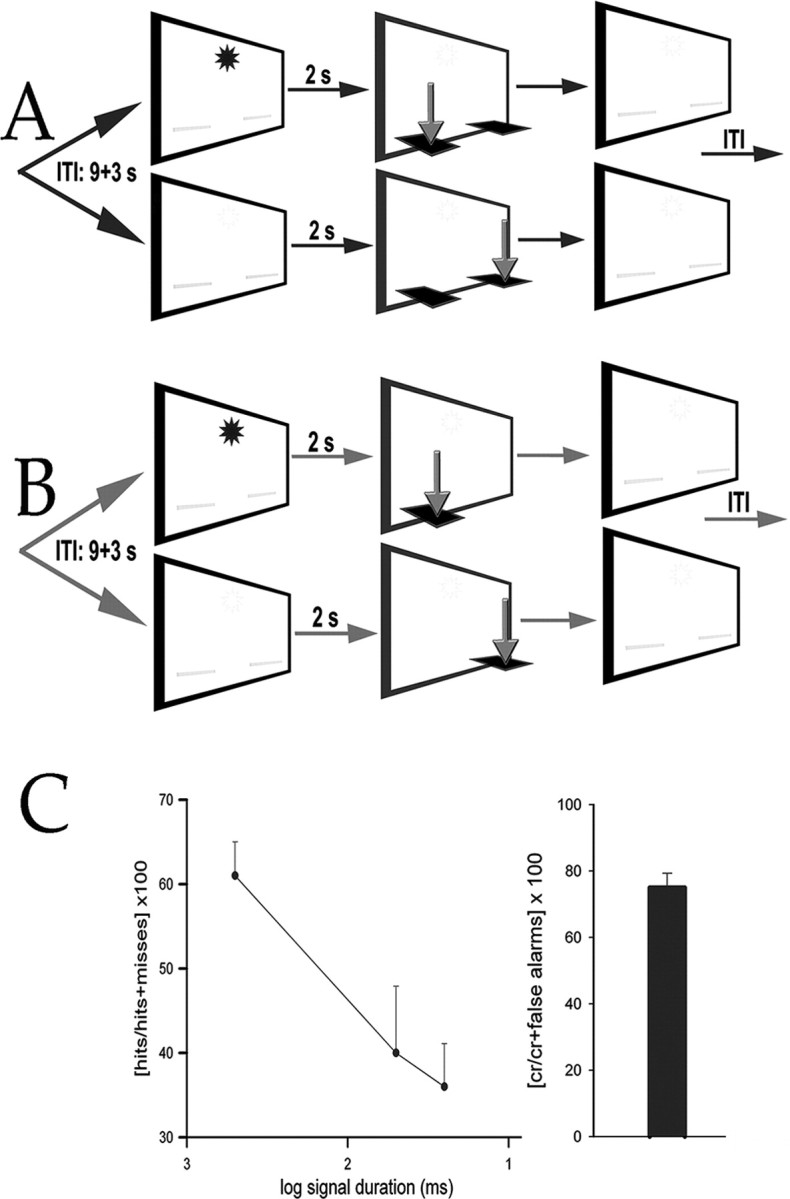
Main events of the CVT (A) and SRTT (B) and illustration of performance (C). A, After a variable ITI, a signal (illumination of a panel light for 500, 50, or 25 ms; see asterisk) was presented or not (nonsignal). In the CVT, both levers were extended 2 s later, and animals were required to press one lever to report a hit (after signals) and the other one to report a correct rejection (after nonsignals) to receive a water reward (see arrows exemplifying 1 set of rules). Incorrect responses (misses and false alarms) and omissions were not rewarded. Levers were withdrawn after a lever press or after 4 s. B, The SRTT differed from the CVT by making only the correct lever available. C, The ability of CVT-performing animals to detect signals was signal duration dependent (line graph), and they responded correctly in >75% of all nonsignal trials (bar).
Materials and Methods
Subjects. Subjects were adult male Fisher/Brown Norway hybrid rats (Harlan, Indianapolis, IN; n = 40) weighing 200-250 g at the beginning of the experiment. Animals were individually housed in a temperature-controlled (23°C) and humidity-controlled (45%) environment and on a 12 h light/dark cycle (lights on at 6:30 A.M.). Animals were extensively handled before the beginning of training and were water-deprived by restricting access to water to an 8 min period after each daily behavioral session. Food was available ad libitum (Rodent Chow; Harlan Teklad, Madison, WI). Animals were randomly assigned to CVT (n = 16), NP (n = 16), and SRTT (n = 8) groups with half the number of animals from each group subjected to either CHT function or CHT expression studies. All procedures were conducted in adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols approved by institutional committees.
Behavioral apparatus. Behavioral training and testing were conducted using a set of 12 operant chambers (Med-Associates, St. Albans, VT) located inside larger sound-attenuating chambers. Each operant chamber was equipped with an intelligence panel consisting of three panel lights (2.8 W), two retractable levers, and a water dispenser (0.05 ml of water per delivery).
CVT training, testing, and measures of performance. The task and evidence in support of the validity of performance measures in terms of reflecting sustained attention were described previously (McGaughy and Sarter, 1995). Animals were initially shaped to lever press in accordance with a modified fixed ratio-1 schedule for water reinforcement. After at least three consecutive sessions per day of >100 reinforced lever presses, animals were trained to discriminate between signal (1 s illumination of the central panel light) and nonsignal (no illumination) events. Each response period was cued by extension of the two levers into the chamber 2 s after a signal or nonsignal event (see Fig. 1). On signal trials, a response on the left lever was reinforced (“hit”), whereas a response on the right lever was not reinforced (“miss”). On nonsignal trials, a response on the right lever was reinforced (“correct rejection”), whereas a response on the left lever was termed a “false alarm” and not reinforced. If no response occurred within 4 s, levers were retracted, and an omission was recorded. Signal and nonsignal events were presented in pseudorandom order for a total of 81 trials per trial type and session (162 trials total per session). The intertrial interval (ITI) was 12 ± 3 s. House lights were not illuminated during this training step. After at least 5 consecutive days of stable performance, defined as >70% hits to longest signals and >70% correct rejections, multiple signal durations (500, 50, and 25 ms) were introduced. Trial type and signal duration continued to be pseudorandomly determined for each trial. The event rate was increased by reducing the ITI to 9 ± 3 s. After at least 7 d of stable performance (at least 70% hits to 500 ms signals and at least 70% correct rejections), house lights were illuminated throughout the session. This important final modification requires the animals to constrain their behavior and presumably to maintain persistent attention to the intelligence panel for signal detection. Final criterion performance was defined as >65% hits to 500 ms signals, >65% correct rejections, and <20% omissions for three consecutive sessions.
Measures of performance included hits, misses, correct rejections, and false alarms. The relative number of hits (hits/hits + misses) was calculated for each signal length, and the relative number of correct rejections (correct rejections/correct rejections + false alarms) was also computed. Finally, errors of omission and response latencies (time from the extension of the levers to a lever press) were recorded.
SRTT and NP rats. The SRTT was identical to the CVT task except that only the correct lever was extended after an event (see Fig. 1). Thus, the SRTT controlled for the effects of the cognitive processing of the propositional task rules in the CVT. The number of lever presses or its inverse, the number of omissions, and response latencies were obtained from these animals. NP rats were water-deprived and transferred daily to the operant chambers, where they spent equivalent amounts of time before returning back to the home cages, where they were able to consume water for a 10 min period ad libitum.
Administration of saline or urethane and tissue dissection. To minimize the potentially confounding effects of stress and novelty associated with the decapitation procedure on ex vivo neurochemical measures, animals were anesthetized with urethane immediately after completion of the final behavioral test and left in the chambers until sedated. To habituate animals to this event, they received daily intraperitoneal injections of saline immediately after the test session and remained in the chambers for an additional 15 min. After the final test session, urethane (Sigma, St. Louis, MO; 1.5 g · kg-1 · ml-1, i.p.) was administered. Brains were quickly removed and dissected on an ice-cold surface. The left and right mPFCs (prelimbic, infralimbic, and anterior cingulate cortices), left and right PPCs (posterior parietal and visual cortices), and striatum were prepared for choline uptake assays and immunoblotting procedures.
Choline uptake assay. Briefly, for choline transport assays (Ferguson et al., 2003), aliquots containing 25-50 μg of crude synaptosomes (P2 fraction) were incubated with [3H]choline (0.03-9 μm) in Krebs' bicarbonate buffer (200 μl) in the presence and absence of 10 μm hemicholinium-3 for 5 min. Transport assays were terminated by washing the crude synaptosomes with ice-cold wash buffer. Accumulated radioactivity was determined using a liquid scintillation counter. CHT-mediated choline uptake was determined as total choline uptake minus the uptake in the presence of hemicholinium-3. Furthermore, maximum transporter velocity (Vmax) and the affinity for choline (Km) were determined.
Subcellular fractionation and immunoblotting assays. Assays were conducted as described previously (Ferguson et al., 2003). Brain tissue was homogenized and centrifuged at 1000 × g for 10 min. The supernatant was centrifuged at 13,000 × g to yield a crude synaptosomal pellet (P2). Synaptosomes in this P2 fraction were lysed by homogenization in 5 mm HEPES-NaOH plus protease inhibitors (1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin, and 250 μm phenylmethylsufonyl fluoride). Synaptic plasma membranes (LP1) and other large membranes were collected at 15,000 × g for 20 min. The LP2 pellet was obtained after centrifugation of the resulting supernatant (LS1 fraction) for 30 min at 200,000 × g and thus was enriched with vesicular membranes. Proteins were extracted from each fraction. Protein concentrations were determined, and equal quantities of protein, 50 μg for P2 fractions and 25 μg for LP1 and LP2 fractions, were subjected to immunoblot analysis using a rabbit polyclonal antibody recognizing CHT (Gates et al., 2004). CHT-immunoreactive bands were quantified using densitometry and Scion Image software. The relative proportion of CHTs in plasma membrane was expressed by calculating the ratio LP1/LP1 + LP2. To validate the subfractionation method used in these experiments, the blots were also examined for the distribution of vesicular acetylcholine transporter (VAChT), synaptophysin, and NMDA-receptor subunit 2A/B (NR2A/B) using goat antisera against VAChT, mouse antisera against synptophysin, and rabbit antisera against NR2A/B, respectively (Chemicon, Temecula, CA).
Statistical analyses. Data were analyzed using ANOVAs or, when appropriate, repeated measures ANOVAs, followed by post hoc multiple comparisons. Correlations between behavioral and neurochemical measures were analyzed by calculating Spearman's r and testing correlation coefficients for statistical significance (α = 0.05 for all tests). Statistical analyses were performed using SPSS/PC (version +12.01; SPSS, Chicago, IL).
Results
CVT and SRTT performance
The hit rate of CVT-performing animals was signal duration-dependent (F(2,30) = 36.53; p < 0.001) (Fig. 1), and >75% of their responses in nonsignal trials were correct rejections. CVT-performing animals completed 141.69 ± 5.74 (mean ± SEM) trials per session, and SRTT-performing animals pressed the extended lever in 115.06 ± 4.88 trials per session. The response latencies (time between lever extension and lever press) did not differ between CVT- and SRTT-performing animals (F(1,22) = 1.65; p > 0.05) or lever (F(1,22) = 1.19; p > 0.05), and the two factors did not interact significantly (F(1,22) = 1.05; p > 0.05). After the extension of levers, animals required 1.29 ± 0.57 s to press a lever. CVT or SRTT performance did not differ between animals assigned to choline uptake or immunoblotting experiments, respectively (all p > 0.05).
Choline uptake and correlation with performance
Compared with NP controls, the capacity of CHTs to transport choline in CVT-performing rats (as indicated by Vmax) was increased in the right mPFC (F(2,19) = 8.60; p < 0.005) but not in the left mPFC, left or right PPC, or striatum (all p > 0.3) (Fig. 2, Table 1).
Figure 2.
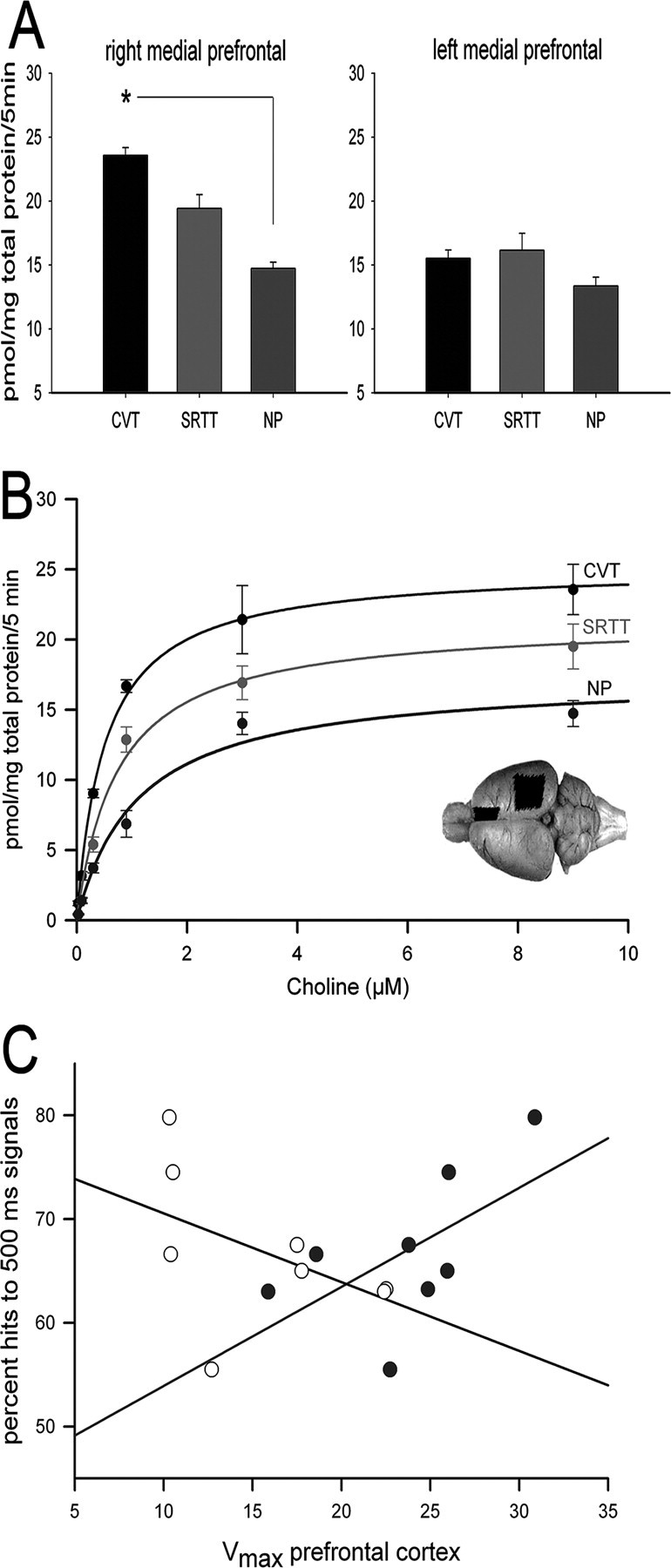
Results from choline uptake assays and correlations with performance. A, Compared with NP controls, the capacity of the CHT to transport choline (as indicated by Vmax) was increased in the right but not left mPFC (the asterisk depicts the significant difference indicated by post hoc multiple comparisons using Tukey's honestly significant difference) of CVT-performing rats. B, Results from saturation analyses depicting hemicholinium-sensitive choline uptake by synaptosomes obtained from right mPFC from animals of the three groups. The inset shows the approximate location and extent of the prefrontal and parietal cortical tissue extracted for these experiments (the more ventral portions of the mPFC are not visible). C, Right mPFC Vmax values correlated significantly with the animal's ability to detect 500 ms signals (filled circles and positive slope regression line), whereas left PFC Vmax scores correlated negatively with this measure (open circles and negative slope regression line; see Results for statistical results).
Table 1.
Results from choline uptake assays and CHT immunoblotting analyses (mean ± SEM)
|
|
Right mPFC |
Left mPFC |
Right posterior cortex |
Left posterior cortex |
Striatum |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Task |
Vmax (pmol·mg−1·5 min−1) |
Km2 (μm) |
LP1/LP1 + LP2 (AU) |
Vmax (pmol·mg−1·5 min−1) |
Km2 (μm) |
LP1/LP1 + LP2 (AU) |
Vmax (pmol·mg−1·5 min−1) |
Km2 (μm) |
LP1/LP1 + LP2 (AU) |
Vmax (pmol·mg−1·5 min−1) |
Km2 (μm) |
LP1/LP1 + LP2 (AU) |
Vmax (pmol·mg−1·5 min−1) |
Km2 (μm) |
LP1/LP1 + LP2 (AU) |
||||||||||
| NP | 14.72 ± 0.48 | 0.91 ± 0.02 | 0.31 ± 0.03 | 13.36 ± 0.68 | 1.05 ± 0.02 | 0.40 ± 0.02 | 15.25 ± 0.25 | 1.16 ± 0.02 | 0.40 ± 0.02 | 13.64 ± 0.45 | 0.88 ± 0.05 | 0.43 ± 0.01 | 37.13 ± 2.53 | 0.78 ± 0.10 | 0.42 ± 0.03 | ||||||||||
| SRTT | 19.43 ± 1.07 | 1.03 ± 0.04 | 0.43 ± 0.01 | 16.15 ± 1.32 | 1.16 ± 0.06 | 0.33 ± 0.01 | 16.04 ± 1.00 | 1.18 ± 0.05 | 0.36 ± 0.02 | 14.25 ± 0.54 | 1.12 ± 0.06 | 0.42 ± 0.02 | 28.11 ± 1.74 | 1.19 ± 0.03 | 0.37 ± 0.03 | ||||||||||
| CVT |
23.58 ± 0.58*
|
1.12 ± 0.03 |
0.50 ± 0.03*
|
15.52 ± 0.65 |
1.05 ± 0.03 |
0.34 ± 0.02 |
17.07 ± 0.92 |
0.83 ± 0.04 |
0.40 ± 0.02 |
13.73 ± 0.57 |
1.11 ± 0.03 |
0.46 ± 0.02 |
38.75 ± 1.91 |
0.96 ± 0.04 |
0.46 ± 0.03 |
||||||||||
AU, Arbitrary units.
Significantly different from NP data, based on Tukey's honestly significant difference (p < 0.05).
Vmax data from animals performing the SRTT did not differ from those of NP controls. Furthermore, Km values did not differ between groups (Table 1) (all p > 0.05).
Right mPFC Vmax values correlated significantly with the animal's ability to detect 500 ms signals (Spearman's r = 0.69; p < 0.03). In contrast, left PFC Vmax scores correlated negatively with this measure (r = -0.64; p < 0.05) (Fig. 2). Vmax or Km values obtained from the PFC did not correlate with other measures of performance (all p > 0.05).
Density of CHTs located in plasma membrane versus intracellular domains
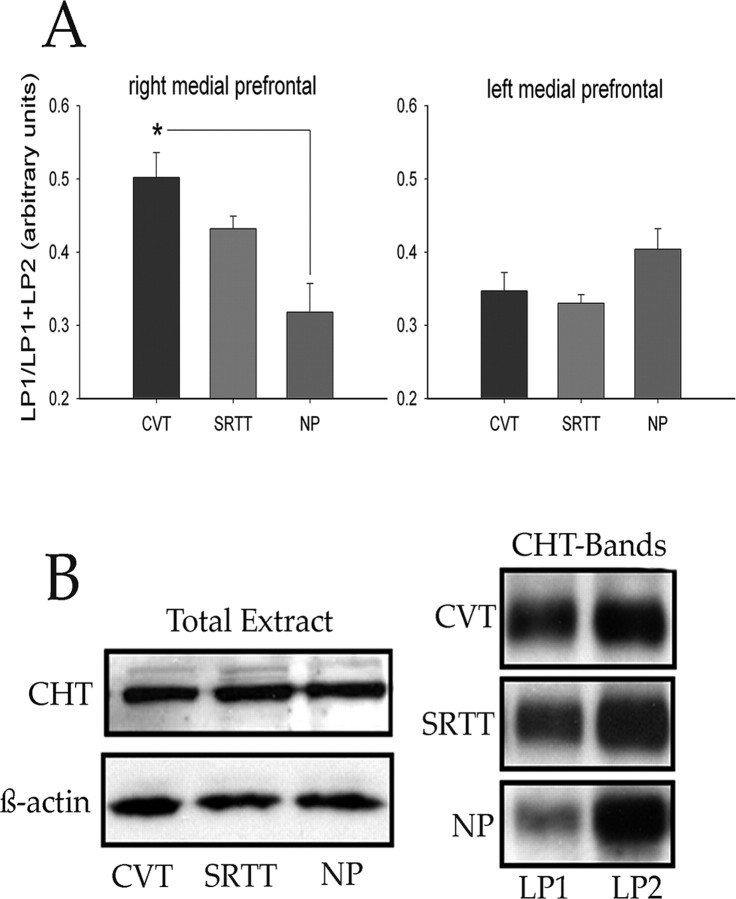
An increased proportion of CHTs in the plasma membrane-enriched fraction, relative to the vesicular membrane-enriched fraction, was found in the right mPFC of CVT-performing rats when compared with NP rats (F(2,19) = 7.52; p < 0.01) (Fig. 3, Table 1). Left mPFC CHT immunoreactivity values did not differ between groups; likewise, CHT density scores did not differ with respect to any other brain region assessed (all p > 0.05). Total protein levels did not differ between the groups (Fig. 3B), and CHT immunoreactivity values did not correlate with behavioral performance (all p > 0.05).
Figure 3.
A, An increased proportion of CHTs was found in the plasma membrane-enriched fraction obtained from the right mPFC of CVT-performing rats when compared with NP rats (the asterisk depicts the significant difference indicated by post hoc multiple comparisons using Tukey's honestly significant difference). Data from the left mPFC, left or right posterior cortex, or striatum did not differ between groups. B, Left bands, Total CHT protein levels were not affected by performance in either task. β-Actin levels were monitored to verify the amount of samples used for immunoblot analysis. Right bands, CHT activity in LP1 and LP2 fractions, indicating the greater proportion of CHTs in the plasma membranes in the right mPFC of CVT-performing rats when compared with NP rats.
Consistent with the previous findings (Ferguson et al., 2003), the synaptic vesicle markers VAChT and synaptophysin were predominantly present in the (vesicular membrane-enriched) LP2 fraction, whereas NR2A/B was predominantly present in the (plasma membrane-enriched) LP1 fraction. However, the distribution of these markers in the total, LP1, and LP2 fractions of assayed brain regions did not differ between the three groups of animals. Likewise, there was no significant difference in the distribution of these proteins when expressed as LP1/LP1 + LP2 ratios (all p > 0.05).
Discussion
CVT performance resulted in significant increases in the capacity of the CHT to transport choline into the presynaptic terminal in the right mPFC. Right mPFC Vmax values correlated positively and left mPFC Vmax values correlated negatively with the animals' hit rate to longest signals. Second, immunoblot analyses indicated an increased proportion of CHTs in presynaptic plasma membrane-enriched fractions from the right but not left mPFC of CVT-performing rats.
By necessity, the two neurochemical assays were conducted using tissues from separate groups of rats. Although this procedure prohibited the calculation of correlations between measures of choline uptake and CHT immunoreactivity, it is noteworthy that both analyses indicated that CVT performance-associated upregulation of CHT function was restricted to the right mPFC.
The present data support the hypothesis that the increased capacity of right mPFC CHTs to transport choline was based primarily on the availability of an increased number of CHTs located in plasma membranes. Km values did not differ between the groups, and, generally, there is little evidence in the literature in support of the possibility that changes in the affinity of CHTs for choline underlie major changes in choline uptake (Gates et al., 2004; Sarter and Parikh, 2005). Presumably, the increased density of CHTs in plasma membranes was a result of increased translocation of CHTs from intracellular locations to plasma membranes (Ferguson et al., 2003; Ferguson and Blakely, 2004). However, a comparison of the absolute CHT immunoreactivities observed in right mPFC LP2 fractions generated for the three groups failed to support this assumption directly, although there was a clear trend toward lower CHT densities in the LP2 fractions of task-performing rats (Fig. 3B, CHT LP2 bands) (mean ± SEM arbitrary units: NP, 24,769 ± 2878; CVT, 17,371 ± 1698; SRTT, 18,404 ± 1398; F(2,19) = 3.17; p = 0.068).
The exact source of CHTs translocated to plasma membranes of the right mPFC of CVT-performing animals remains unsettled. Ferguson et al. (2003) observed that a proportion of intracellular CHTs are located on VAChT-positive vesicles. Because the present data indicated a performance-induced increase in the relative proportion of CHTs in plasma membranes but not of VAChTs, vesicular membrane-situated proteins may not generally be delivered to plasma membranes. Alternatively, either other intracellular CHT pools were redistributed into the plasma membrane of the right mPFC of CVT-performing animals, or translocated proteins indeed originated from vesicular pools, but only CHTs were retained in the plasma membrane.
The intermediate Vmax and CHT expression data observed in the right mPFC of SRTT-performing animals deserve comment. SRTT performance entails rudimentary demands on sustained attention because animals are required to monitor lever movements. Furthermore, the observation that response latencies did not differ between CVT- and SRTT-performing rats suggests the possibility that SRTT-performing rats responded to signals. CVT-performing rats typically position themselves in front of the misscorrect rejection lever (while withdrawn) and move to the opposite lever on detection of a signal, thereby minimizing or abolishing lever-based differences in response latencies (see Results). Although SRTT performance did not require signal detection, the absence of differences between the response latencies of CVT and SRTT animals suggests that SRTT-performing animals likewise monitored signal events and responded to a signal by switching over to the hit lever. Thus, SRTT performance may have involved a greater degree of attentional processing than originally assumed. However, only CVT animals were able to commit misses, and, indeed, their detection rate depended on signal duration (Fig. 1C). [The term “detection” describes a cognitive process that consists of “... the entry of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal by an arbitrary response indicated by the experimenter...” (Posner et al., 1980).] Although SRTT animals may have used signals to switch positions, the decisional components of the detection processes required for CVT performance were not taxed by the SRTT. Thus, significantly enhanced CHT trafficking and, as a result, enhanced CHT capacity in the right mPFC are hypothesized to mediate the performance of attention-taxing tasks, which also involve demands on the cognitive processing of response rules.
The finding that CVT performance was exclusively associated with enhancement of CHT capacity and density in the right mPFC corresponds to evidence from several lines of research. Right but not left hemispheric loss of cortical cholinergic inputs, which was not restricted to the PFC, impaired rats' ability to detect signals in the CVT (Martinez and Sarter, 2004). Human imaging studies have consistently indicated that sustained attention performance activates primarily right hemispheric cortical regions (Coull, 1998; Corbetta and Shulman, 2002). The present data support the general concept that cholinergic inputs to the right mPFC represent a crucial component of this circuitry (Sarter et al., 2001, 2005). Moreover, the present results suggest that increases in CHT capacity and the underlying trafficking of CHTs represent molecular mechanisms involved in the attention-associated activation of right prefrontal regions.
The interpretation of the negative correlation between choline uptake and left medial prefrontal Vmax values remains speculative. Left prefrontal deactivation has been occasionally observed in human imaging studies and in association with highly demanding cognitive performance (Iidaka et al., 2000). Because the detection process in attention-demanding situations appears to be mediated primarily by the right hemispheric, particularly prefrontal, systems, it may be speculated that suppression of left cholinergic inputs to the prefrontal cortex limits potentially interfering cognitive activity, thereby promoting sustained attentional performance.
The finding that CVT performance enhanced CHT function in the prefrontal but not posterior parietal cortex corresponds with the hypothesis that (cholinergic) activation of the prefrontal cortex is required to sustain attentional performance in the presence of additional cognitive demands such as competing response alternatives (Gill et al., 2000; Bunge et al., 2002; Sarter et al. 2005). In contrast, cholinergic inputs to other cortical regions may primarily be activated to optimize signal detection and input processing under challenging conditions. For example, to attenuate declining attentional performance, the prefrontal cortex may activate cholinergic inputs to other cortical, including parietal (Nelson et al., 2005), regions, thereby improving the detection and processing of signals (Sarter et al., 2001, 2005). Ongoing experiments are designed to test the role of prefrontal cholinergic inputs in the regulation of parietal cholinergic inputs in situations that impede the detection process.
Footnotes
This work was supported by United States Public Health Service Grants RO1NS37026, KO2MH01072 (M.S.), 5P20RR155592, and K12DA14040 and by the National Alliance for Research on Schizophrenia and Depression (S.A.). We thank Dr. Randy Blakely for kindly providing the polyclonal CHT antibody and Arpana Sali and Kasturi Sirisha Yalamanchili for technical assistance.
Correspondence should be addressed to Martin Sarter, Department of Psychology, University of Michigan, 525 East University Avenue, 4032 East Hall, Ann Arbor, MI 48109-1109. E-mail: msarter@umich.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253851-06$15.00/0
S.A. and V.M. contributed equally to this work.
References
- Apparsundaram S, Ferguson SM, George Jr AL, Blakely RD (2000) Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun 276: 862-867. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP (2002) Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 114: 451-460. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M (1998) Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci 18: 8030-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE (2002) Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage 17: 1562-1571. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201-215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292-297. [DOI] [PubMed] [Google Scholar]
- Coull JT (1998) Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55: 343-361. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW (2004) Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex 14: 922-932. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (1997) Central cholinergic systems and cognition. Annu Rev Psychol 48: 649-684. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD (2004) The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv 4: 22-37. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD (2003) Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci 23: 9697-9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates Jr J, Ferguson SM, Blakely RD, Apparsundaram S (2004) Regulation of choline transporter surface expression and phosphorylation by protein kinase C and protein phosphatase 1/2A. J Pharmacol Exp Ther 310: 536-545. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B (2000) Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci 20: 4745-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP (1997) Operant performance and cortical acetylcholine release: role of response rate, reward density, and non-contingent stimuli. Brain Res Cogn Brain Res 6: 23-36. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP (2001) The effects of manipulations of attentional demand on cortical acetylcholine release. Brain Res Cogn Brain Res 12: 353-370. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FIM (2000) The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography. J Cognit Neurosci 12: 267-280. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M (2004) Lateralized attentional functions of cortical cholinergic inputs. Behav Neurosci 118: 984-991. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M (1995) Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 117: 340-357. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M (1996) Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci 110: 247-265. [DOI] [PubMed] [Google Scholar]
- Moore H, Dudchenko P, Bruno JP, Sarter M (1992) Toward modeling age-related changes of attentional abilities in rats: simple and choice reaction time tasks and vigilance. Neurobiol Aging 13: 759-772. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP (2005) Prefrontal cortical modulation of acetylcholine release in the posterior parietal cortex. Neuroscience, in press. [DOI] [PubMed]
- Parasuraman R, Warm JS, Dember WN (1987) Vigilance: taxonomy and utility. In: Ergonomics and human factors. (Mark LS, Warm JS, Huston RL, eds), pp 11-32. New York: Springer.
- Passetti F, Dalley JW, O'Connell MT, Everitt BJ, Robbins TW (2000) Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci 12: 3051-3058. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S (1994) Attentional networks. Trends Neurosci 17: 75-79. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ (1980) Attention and the detection of signals. J Exp Psychol Gen 109: 160-174. [PubMed] [Google Scholar]
- Sarter M, Parikh V (2005) Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci 6: 48-56. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP (2001) The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev 35: 146-160. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B (2005) Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and top-down cholinergic modulation of signal detection. Brain Res Rev 48: 98-111. [DOI] [PubMed] [Google Scholar]