Abstract
Sensitization and dishabituation of the defensive withdrawal reflex in Aplysia have been ascribed to presynaptic mechanisms, particularly presynaptic facilitation of transmission at sensorimotor synapses in the CNS of Aplysia. Here, we show that facilitation of sensorimotor synapses in cell culture during and after serotonin (5-HT) exposure depends on a rise in postsynaptic intracellular Ca2+ and release of Ca2+ from postsynaptic stores. We also provide support for the idea that postsynaptic AMPA receptor insertion mediates a component of synaptic facilitation by showing that facilitation after 5-HT offset is blocked by injecting botulinum toxin, an exocytotic inhibitor, into motor neurons before application of 5-HT. Using a reduced preparation, we extend our results to synaptic facilitation in the abdominal ganglion. We show that tail nerve shock-induced facilitation of siphon sensorimotor synapses also depends on elevated postsynaptic Ca2+ and release of Ca2+ from postsynaptic stores and recruits a late phase of facilitation that involves selective enhancement of the AMPA receptor-mediated synaptic response. To examine the potential role of postsynaptic exocytosis of AMPA receptors in learning in Aplysia, we test the effect of injecting botulinum toxin into siphon motor neurons on dishabituation of the siphon-withdrawal reflex. We find that postsynaptic injections of the toxin block dishabituation resulting from tail shock. Our results indicate that postsynaptic mechanisms, particularly Ca2+-dependent modulation of AMPA receptor trafficking, play a critical role in synaptic facilitation as well as in dishabituation and sensitization in Aplysia.
Keywords: Aplysia, sensitization, dishabituation, serotonin, intracellular calcium stores, AMPA receptor trafficking
Introduction
The gill- and siphon-withdrawal reflex (SWR) in the marine snail Aplysia, together with its underlying neuronal circuitry, has been a valuable model system for the cellular analysis of learning and memory (Kandel, 2001). Neurobiological investigations of two related forms of nonassociative learning in Aplysia, sensitization and dishabituation, have focused primarily on plasticity at the monosynaptic connection between the central sensory and motor neurons that mediate the withdrawal reflex (Walters et al., 1983b; Cohen et al., 1997; Antonov et al., 1999). Noxious stimulation of the tail of Aplysia, which can induce either sensitization or dishabituation of the withdrawal reflex depending on factors such as the age of the animal and the amount of opposing inhibition recruited by the tail stimulation (Mackey et al., 1987; Marcus et al., 1988; Rankin and Carew, 1988; Antonov et al., 1999), activates serotonergic interneurons within the snail's CNS (Glanzman et al., 1989; Mackey et al., 1989; Marinesco and Carew, 2002). Serotonin (5-HT), released from these monoaminergic interneurons, facilitates transmitter release from the sensory neurons by processes that depend on the second messengers cAMP and protein kinase C (PKC) (for review, see Byrne and Kandel, 1996). As well as facilitating presynaptic release of transmitter, 5-HT may have postsynaptic facilitatory actions. Previously, we (Chitwood et al., 2001) found that a single, 10 min application of 5-HT to isolated siphon motor neurons in dissociated cell culture caused prolonged (≥40 min) enhancement of the response of motor neurons to glutamate, the sensory neuron transmitter (Dale and Kandel, 1993; Zhu et al., 1997) (but see Trudeau and Castellucci, 1995). The facilitation of the glutamate response in isolated motor neurons is mediated by functional upregulation of AMPA-type receptors. This modulatory action depends on a rise in intracellular Ca2+ and exocytosis, because it is blocked by the rapid Ca2+ chelator 1,2-bis(O-aminophenoxy)ethane-N, N,N′,N′ acid (BAPTA), as well as by botulinum toxin, which inhibits exocytosis (Südhof, 1995). Based on these results, we hypothesized that persistent 5-HT-dependent facilitation of sensorimotor synapses in Aplysia depends on a postsynaptic rise in intracellular Ca2+, postsynaptic exocytosis, and modulation of AMPA receptor trafficking. Here, we provide additional support for this hypothesis using sensorimotor synapses in dissociated cell culture (Rayport and Schacher, 1986; Lin and Glanzman, 1994a). We also show, using a reduced preparation, that sensitization/dishabituation-related facilitation of sensorimotor synapses in the abdominal ganglion of Aplysia depends on postsynaptic modifications, including selective enhancement of the AMPA receptor-mediated synaptic response. Finally, we extend our cellular results to the behavioral level by showing that inhibition of exocytosis in identified siphon motor neurons disrupts that dishabituation of the SWR. The results from the present study support a novel view of the cellular mechanisms that underlie behavioral enhancement in Aplysia. Some of our results have been reported previously in abstract form (Li et al., 2001; Roberts and Glanzman, 2001, 2002, 2003a; Li and Glanzman, 2002).
Materials and Methods
Animals. Adult Aplysia californica were obtained from local suppliers (Alacrity Marine Biological, Redondo Beach, CA and M-REP, Escon-dido, CA.) Animals were housed in a 50 gallon aquarium filled with cooled (14°C), aerated artificial seawater (ASW; Instant Ocean; Aquarium Systems, Mentor, OH). All animals were housed for at least 24 h before the start of the experiment. Note that for all of the comparisons described here, the controls for a given set of experiments that tested the effects of a specific pharmacological inhibitor on synaptic facilitation/dishabituation were always performed at the same time as the experiments involving that inhibitor. Moreover, all of the experimental tests involved in a comparison of the effects of a drug were performed with synapses/preparations made from the same shipments of animals. This was done to avoid the confounding effects of physiological variability attributable to season or to the variable health of animals from different shipments, which can be significant.
Electrophysiological experiments on synapses in cell culture. Sensorimotor cocultures, each comprising a single presynaptic sensory neuron and a single postsynaptic motor neuron, were fabricated using small siphon (LFS) motor neurons (Frost et al., 1988; Hickie and Walters, 1995) and pleural sensory neurons (Walters et al., 1983a). The neurons were dissociated from Aplysia (60-100 g; Alacrity Marine Biological) and cultured as described previously (Lin and Glanzman, 1994a). Cultures were maintained at 18°C for 3-5 d before the experiments. During experiments, the cultures were perfused with 50% sterile ASW and 50% Leibowitz-15 (1.5-2.0 cc per min). All experiments were performed at room temperature (18-22°C), except those involving botulinum toxin. Here, the temperature was maintained 28-30°C to maximize efficacy of the toxin. (Control experiments for the botulinum toxin experiments were also performed at 28-30°C.) A single presynaptic sensory neuron and a single postsynaptic motor neuron were impaled with sharp microelectrodes [20-30 MΩ in the experiments using BAPTA and 2-aminoethoxy-diphenyl borate (2-APB), 12-15 MΩ in the experiments using heparin, dantrolene, and botulinum toxin]. The basic microelectrode solution included 2.0 m potassium acetate, 0.5 m potassium chloride, and 0.01 m HEPES, pH 7.2. In the experiments involving type B botulinum toxin (Botox B), the microelectrode solution consisted of 1.5 m potassium acetate, 0.01 m HEPES, pH 7.2, 2.5 mm ditheothreitol, 5 mm sodium chloride, 10 mm sodium phosphate, and 0.5 μm Botox B. (In the control experiments, motor neurons received injections of the same solution minus the toxin.) The electrophysiological recording methods were similar to those described previously (Chitwood et al., 2001). Motor neurons were held at -80 to -85 mV throughout the experiment by passing negative current (0.3-0.8 nA) into the cell via the bridge circuit of the microelectrode amplifier; this was done to prevent spontaneous firing of the motor neurons during testing and to minimize stimulus-evoked firing. Sensory neurons were activated with brief depolarizing current pulses (40 ms, 0.2-0.8 nA), and the resulting monosynaptic EPSPs were recorded. Immediately after impalement of the sensory neuron and motor neuron, the sensory neuron was fired once and the size of the EPSP was determined. After this initial test, there was a period of 30-45 min before the start of the experiment. If the size of the EPSP declined by ≥50% during this period, the experiment was discontinued.
Note that, in some cases, stimulation of a presynaptic sensory neuron during testing resulted in the elicitation of an action potential in the motor neuron, despite the injection of constant hyperpolarizing current into the postsynaptic cell. In those cases, a value of 75-80 mV was assigned to the “EPSP,” depending on exactly how much below its resting potential the motor neuron was hyperpolarized [see Bailey et al. (2000) for use of a similar method]. It should be emphasized, however, that the triggering of an action potential during a trial was a relatively rare event in the present experiments. For example, in the experiments involving BAPTA (see Fig. 1), a presynaptic action potential elicited a postsynaptic spike in just two trials in only a single experiment (out of eight) in the 5-HT control group and in only two trials in two experiments (of eight) in the 5-HT/BAPTA group. We have recalculated the statistics for all of our experiments after using a lower value (60 mV) for the EPSP in those trials in which postsynaptic action potentials were elicited. This made no significant difference in our results.
Figure 1.

Postsynaptic injection of BAPTA, a rapid chelator of intracellular Ca2+, disrupts 5-HT-dependent facilitation of the sensorimotor synapse in culture. A, Representative EPSPs for each of the experimental groups. (The eliciting presynaptic action potentials are not shown in this or subsequent figures.) Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of EPSPs in the three experimental groups: synapses treated with 5-HT without postsynaptic BAPTA (5-HT control; n = 8); synapses treated with 5-HT after BAPTA had been injected into the motor neuron (5-HT/BAPTA; n = 8); synapses that received the postsynaptic BAPTA injection but were not treated with 5-HT (test-alone/BAPTA; n = 8). A repeated-measures ANOVA for the trials during which 5-HT was present indicated that there was a significant main effect of experimental treatment (F(2,21) = 19.09; p < 0.0001) as well as a significant interaction (F(2,21) = 4.81; p < 0.02). Accordingly, one-way ANOVAs were performed on the 10 and 15 min trials, followed by Bonferroni's post hoc tests. The differences among the three experimental groups (5-HT control, 5-HT/BAPTA, and test-alone/BAPTA) were significant on the 10 min trial (F(2,21) = 11.36; p < 0.001). The mean normalized EPSP for the 10 min trial was 200 ± 25% in the 5-HT control group and 185 ± 24% in the 5-HT/BAPTA group; both values were significantly greater than that for the test-alone/BAPTA group (77 ± 5%; p < 0.003 for each comparison). The difference between the mean EPSP values in the 5-HT control and 5-HT/BAPTA groups was not significant for the 10 min trial. Analysis of the 15 min trial also revealed significant differences among the three experimental groups (F(2,21) = 17.23; p < 0.001). The mean normalized EPSP for the 5-HT control group for this trial (280 ± 42%) was significantly greater than the mean normalized EPSPs in both the 5-HT/BAPTA group (177 ± 20%) and the test-alone/BAPTA group (58 ± 6%; p < 0.05 for each comparison). The 5-HT/BAPTA EPSP was significantly greater than the test-alone/BAPTA EPSP (p < 0.02). A repeated-measures ANOVA performed on the group means for the 20-55 min trials indicated a significant main effect of experimental treatment (F(2,21) = 8.98; p < 0.002). SNK post hoc tests showed that the post-5-HT EPSPs in the 5-HT control group were significantly greater than those for both the 5-HT/BAPTA and test-alone groups (p < 0.05 for each comparison). The difference between the EPSPs in the 5-HT/BAPTA and test-alone groups, however, was not significant during the post-5-HT period. The asterisks indicate significant differences between the 5-HT control and 5-HT/BAPTA data, the # symbol indicates the significant difference between the 5-HT/BAPTA and test-alone data, and the crosses indicate significant differences between the 5-HT control and the test-alone data. Error bars represent SEM. Interstimulus interval, 5 min.
EPSP amplitude is typically used in measurements of synaptic strength in experiments on Aplysia sensorimotor synapses in culture. However, given the occasional elicitation of a postsynaptic action potential to a test stimulus, as sometimes happened in the experiments, particularly during 5-HT treatment, one might question whether EPSP amplitude was an appropriate measure. An alternative measure of synaptic strength is EPSP slope. To test whether these two measures yielded different results, we compared the values for EPSP amplitude and EPSP slope for all of the EPSPs from six of our cell culture experiments. In four of these experiments, the synapse was treated with 5-HT; in the other two, the synapse received only the test stimuli. We calculated both the peak amplitude (in millivolts) and the maximum slope (in millivolts per second) for each EPSP and statistically compared the two sets of data using the coefficient of determination (R2) (StatView; SAS Institute, Cary, NC). The R2 for the six experiments ranged from 0.858 to 0.988, and the correlation between amplitude and maximum slope was highly significant (p < 0.0001 for all six experiments, as determined by t tests). We therefore conclude that EPSP amplitude was an appropriate measure of synaptic strength in the present experiments.
The input resistances of the sensory and motor neurons were monitored throughout the experiment by injecting 300 ms pulses of negative current (0.1 nA) into the neurons once every 5 min. The data from the experiments were discarded if the input resistance of either cell dropped by ≥50% during the experiment. 5-HT was prepared fresh daily as a 2 mm stock solution dissolved in ASW. The 5-HT was diluted to a final concentration of 10-20 μm just before an experiment in the perfusion medium and applied to the cultured synapses for 10 min, after which it was rapidly washed out with normal perfusion medium. In experiments in which drugs were applied to the motor neuron intracellularly, the drugs were added to the microelectrode solution and allowed to diffuse into the neuron for 30-45 min before the start of the experiment. Diffusion was assisted by passing negative current (-0.3 to -0.8 nA) through the microelectrode. (In control experiments, the same hyperpolarizing current was applied to the standard microelectrode solution for 30-45 min.) All drugs were from Sigma (St. Louis, MO), except 2-APB (Calbiochem, San Diego, CA) and Botox B (List Biological, Campbell, CA).
Electrophysiological experiments on synapses the abdominal ganglion. Reduced preparations were made from Aplysia (100-200 g; Alacrity Marine Biological or M-REP). The experimental preparation, which comprised the CNS of Aplysia, together with two peripheral pedal nerves (P9) that innervate the animal's tail, has been described previously (Murphy and Glanzman, 1999). Before the start of the experiment, one siphon (LE) sensory neuron and one siphon (LFS) motor neuron in the abdominal ganglion were impaled with sharp microelectrodes (14-22 MΩ, basic microelectrode solution as above). The membrane potential of the motor neurons was maintained at -90 mV throughout the experiment by passing negative current through the bridge circuit of the amplifier. During testing, action potentials were elicited from the sensory neurons by injecting a brief (20 ms) pulse of positive current. Two types of perfusion media were used: (1) normal ASW (in mm: 460 NaCl, 11 CaCl2,10 KCl, 55 MgCl2, 10 HEPES buffer, pH 7.6) and (2) 2:1 ASW (in mm: 368 NaCl, 13.8 CaCl2, 8 KCl, 101 MgCl2, 20 MgSO4, 10 HEPES buffer, pH 7.6) (Trudeau and Castellucci, 1992; Murphy and Glanzman, 1999). The purpose of the 2:1 ASW was to eliminate the polysynaptic component from the sensorimotor EPSP (Liao and Walters, 2002). Before experimentation, the dissecting medium was replaced with 2:1 perfusion medium. All groups then received five tests at one per minute in the 2:1 solution. Afterward, the preparation was perfused with normal ASW for 2.5-3 min, and some preparations received extracellular stimulation of the P9 nerves (tail nerve shock) via suction electrodes. The extracellular nerve stimulation comprised either one or three shocks (1 s, 25 Hz; 30 s intershock interval when multiple shocks were used). The intensity of the shocks was three to six times the threshold for eliciting an ESPS in the motor neuron. (Note that the controls and experimental preparations for each experiment received the identical regimen of nerve shocks.) After delivery of the tail nerve shocks, the preparation was perfused for 2.5-3.0 min with the 2:1 solution and testing continued. In those experiments in which the preparation received only the test stimuli (“test-alone” experiments), the identical regimen of alternating perfusion of 2:1 and normal ASW was followed.
In the experiments in which glutamate receptor antagonists were used to isolate the effects of nerve shock on the AMPA and NMDA receptor-mediated components of the sensorimotor EPSP (see Fig. 8), solutions with normal divalent cations were used throughout. We did not use synapses in which the initial EPSP in normal ASW was <2or >10 mV in these experiments; the latter condition was imposed to reduce the possibility of a significant contribution to the EPSPs from interneurons. The first pretest was in normal ASW; the second pretest, which was performed with dl-2-amino-5-phosphonovalerate (APV), 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX), or 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulfonamide (NBQX) added to the ASW, occurred 15 min later. Then the drug was washed out of the experimental chamber for 15 min with normal ASW, after which three shocks (1 s, 25 Hz; 30 s intershock interval) were applied to the tail nerves. Immediately after the last shock, ASW containing the same glutamate receptor antagonist that had been used in the second pretest was perfused into the experimental chamber for 4 min, and 10 postshock tests were performed at one per minute.
Figure 8.

Differential facilitation of the AMPA receptor-mediated and NMDA receptor-mediated components of the sensorimotor EPSP after tail nerve shock. A, Representative EPSPs for the different experimental groups. Trial times correspond to those indicated on the abscissa in B and C. B, Mean normalized amplitude of the EPSPs in the three groups that received only the test stimuli (test-alone groups). The mean initial (0 min) non-normalized EPSP value, determined in perfusion with normal ASW, was 4.5 ± 1.2 mV for the test-alone/APV group (n = 5), 4.9 ± 1.4 mV for the test-alone/CNQX group (n = 6), and 5.0 ± 1.3 mV for the test-alone/NBQX group (n = 4). The differences among the initial EPSPs in the three test-alone groups were not significant, as indicated by a one-way ANOVA (p > 0.9). The EPSPs were normalized to the second (15 min) trial, which took place during perfusion with one of the three glutamate receptor antagonists (APV, CNQX, or NBQX). After the 15 min trial, normal ASW was washed back into the experimental chamber for 16 min, followed by perfusion with the same glutamate receptor antagonist that had been used for the 15 min trial; the remainder of the tests were performed in this antagonist. (See C for an explanation of the experimental protocol.) The differences among the test-alone groups with respect to their normalized EPSP values for the 35-44 min trials were not significant (repeated-measures ANOVA; p > 0.3). The data from the three groups were combined for the test-alone comparison curve in C. C, Mean normalized amplitude of the EPSPs in the three groups that received nerve shock. The mean non-normalized EPSP on the 0 min trial in normal ASW was 4.7 ± 0.7 mV for the APV-treated group (TNS/APV; n = 11), 5.3 ± 0.7 mV for the CNQX-treated group (TNS/CNQX; n = 11), and 5.3 ± 1.3 mV for the NBQX group (TNS/NBQX; n = 7). The initial EPSPs were not significantly different among the groups (p > 0.8; one-way ANOVA). The second (15 min) trial took place during perfusion with one of the three glutamate receptor antagonists; the EPSPs were normalized to this trial. The glutamate receptor antagonist was then washed from the experimental chamber for 15 min with normal ASW, after which nerve shocks (arrow) were initiated. There were three shocks (1 s in duration, 25 Hz; 30 s intershock interval; see Materials and Methods). Immediately after the third shock, ASW containing the same glutamate receptor antagonist (APV, CNQX, or NBQX) that had been used in the second trial was perfused into the experimental chamber for 4 min, and then there were 10 postshock tests at one per minute. There were no differences in the overall data from the TNS/CNQX and TNS/NBQX groups (repeated-measures ANOVA; p > 0.4), so the data from these groups were combined for statistical comparisons with the TNS/APV group. A repeated-measures ANOVA indicated that there was a significant main group effect (F(2,41) = 12.33; p < 0.0001) as well as a significant interaction (F(2,18) = 7.85; p < 0.0001). Post hoc assessments of the group differences with SNK tests revealed that the EPSPs in the TNS/APV group were significantly greater than the combined TNS/CNQX/NBQX group, as well as the combined test-alone group (p < 0.05 for each comparison). The difference between the postshock EPSPs in the combined TNS/CNQX/NBQX group and the combined test-alone group, however, was not statistically significant. To look for possible differences between these two groups during the postshock trials, we performed one-way ANOVAs on each of the 35-44 min trials. There were no significant differences between the combined TNS/CNQX/NBQX and the test-alone groups on any individual trial. We attribute this lack of statistical significance to the severe correction for multiple comparisons used in the Bonferroni's tests (see also Fig. 7). The asterisk indicates the statistically significant difference between the postshock data for the TNS/APV and TNS/CNQX/NBQX groups, and the cross indicates the significant difference between the TNS/APV and the test-alone postshock data. Error bars represent SEM.
Behavioral experiments. Reduced preparations of Aplysia (100-200 g; Alacrity Marine Biological and M-REP) were used in the experiments. Animals were anesthetized with intrahemocoel injections of isotonic MgCl2 (equal to approximately one-half their body weight), and the dissection was performed in 50% MgCl2 and 50% ASW. The dissection was similar to that used for the electrophysiological experiments, except that the tail and siphon were preserved and left attached to the CNS via the P9 nerves and the siphon nerve. The siphon was partially cut in half approximately one-third of the way along its longitudinal axis. One-half of the siphon was pinned to the bottom of a Sylgard-lined experimental chamber. The tail was left unpinned. The unpinned part of the siphon was attached to a force transducer (MLT0201; AD Instruments, Colorado Springs, CO) via a silk suture. The output of the force transducer was amplified (Bridge Amp ML110; AD Instruments) and recorded digitally (MacLab 4; AD Instruments). The force transducer was calibrated with known weights. Pairs of Teflon-coated platinum wires (0.005 in diameter; no. 73000; A-M Systems, Carlsborg, WA) were implanted into the tail and siphon for electrical stimulation, and the abdominal ganglion was desheathed. Afterward, the 50% MgCl2/50% ASW in the experimental chamber was replaced with normal ASW; normal ASW was also injected into the siphon and tail to flush out the MgCl2. The preparation was then allowed to rest for at least 1 h. After this rest period, two LFSB siphon motor neurons (Frost et al., 1988; Hickie and Walters, 1995) were impaled with sharp microelectrodes (9-14 MΩ). The microelectrodes contained either the control electrode solution (above) or Botox B (1 μm in control solution). The motor neurons were depolarized and made to fire a train of action potentials, thereby enabling us to determine what portion of the siphon the neurons innervated. If a motor neuron was found to strongly innervate the unpinned portion of the siphon, the motor neuron was selected for injection of either toxin-containing or control solution. The solution was injected into the motor neuron using 0.7 nA of negative current for 2 h. After the motor neuron injections were completed, the microelectrodes were withdrawn. The threshold level for producing movement of the unpinned portion of the siphon was determined through shocks (25 Hz, 500 ms) of gradually increasing intensity delivered to the implanted electrodes via a Master-8 electronic stimulator (A.M.P.I., Jerusalem, Israel). At least 15 min after the threshold level of stimulus intensity was determined, six tests (trials) were performed (5 min intertrial interval), during which the siphon was stimulated at threshold intensity. Two minutes after the sixth trial, three shocks were delivered to the tail (40 Hz, 1 s, 75 V; 30 s intershock interval) via the implanted electrodes with an electronic stimulator (S88; Grass-Telefactor, West Warwick, RI). Behavioral testing resumed at 7 min after the last tail shock; there were four postshock trials in which the siphon was again stimulated at the threshold intensity level at a rate of once per 5 min.
Statistical analyses. The peak amplitudes of the evoked monosynaptic EPSPs, or of the SWRs, were measured and normalized as indicated in the figure legends. The normalized data were expressed as means ± SEM. Statistical comparisons were performed using StatView and SPSS (SPSS, Chicago IL). For multiple group comparisons, repeated-measures ANOVAs were first performed, followed by between-group comparisons with Student-Newman-Keuls (SNK) tests. If the repeated-measures ANOVA indicated a significant interaction, the interaction was investigated with the Bonferroni's correction, which adjusts for multiple comparisons. All reported levels of statistical significance represent two-tailed values.
In the statistical analyses for the experiments involving 5-HT, we treated the two experimental trials during which 5-HT was present in the bath (the 10 and 15 min trials) separately from the trials that occurred after washout of the drug. For each of these experiments, two repeated-measures ANOVAs were performed: one for the two trials performed in 5-HT and the other for the trials (20-55 min trials) after 5-HT washout. In the experiments involving nerve/tail shock, we performed a single repeated-measures ANOVA for the data from the postshock tests. For the test-alone experiments, in which the synapses/preparations received only the test stimuli, a single repeated-measures ANOVA was performed on all of the trials.
Results
The EPSPs recorded from sensorimotor synapses characteristically exhibit significant homosynaptic depression at the rates of test stimulation used in the present experiments (Castellucci and Kandel, 1974; Rayport and Schacher, 1986). These were once per 5 min for the experiments using sensorimotor synapses in dissociated cell culture (Figs. 1, 2, 3, 4, 5) and once per minute for the experiments using synapses in the abdominal ganglion (Figs. 6, 7, 8). The homosynaptic depression produced by our stimulation protocols means that the effect of a pharmacological inhibitor on plasticity-related changes in synaptic transmission was superimposed on a decrementing baseline response. The accepted method used to control for the decrement in the sensorimotor synaptic response attributable to the homosynaptic depression is to include separate sets of experiments in which the synapses received only the test stimuli (test-alone experiments) to control for the effect of short-term homosynaptic depression (Trudeau and Castellucci, 1993; Bao et al., 1997, 1998; Jin and Hawkins, 2003). We have followed this practice in the present study.
Figure 2.
Postsynaptic injection of heparin, an inhibitor of IP3 receptors, disrupts 5-HT-dependent facilitation of the sensorimotor synapse in culture. A, Representative EPSPs for three of the experimental groups: 5-HT control, 5-HT/heparin, and test-alone/heparin. Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of the EPSPs in the four experimental groups: synapses in the 5-HT control group (n = 7); synapses treated with 5-HT after heparin had been injected into the motor neuron (5-HT/heparin; n = 5); synapses that received the test stimuli but were not treated with 5-HT (test-alone; n = 6); synapses that received the test stimuli together with postsynaptic heparin (test-alone/heparin; n = 5). A repeated-measures ANOVA on the data from the 5-HT treatment period (10-15 min trials) indicated that there was a significant main effect for experimental treatment (F(2,20) = 34.76; p < 0.0001). The mean normalized EPSPs for the 10 min trial were 344 ± 44% in the 5-HT control group, 225 ± 23% in the 5-HT/heparin group, and 80 ± 6% in the combined test-alone group (n = 11). The mean normalized EPSPs for the 15 min trial were 331 ± 43% in the 5-HT control group, 186 ± 19% in the 5-HT/heparin group, and 68 ± 4% in the combined test-alone group. SNK post hoc tests indicated that the EPSPs in the 5-HT control group were significantly greater than those in the other two experimental groups during the 5-HT treatment period (p < 0.05 for each test) and that the EPSPs in the 5-HT/heparin group were significantly greater than those in the combined test-alone group during 5-HT treatment (p < 0.05). A repeated-measures ANOVA on the data from the period after washout of 5-HT (20-55 min trials) revealed that there was a highly significant main effect for experimental treatment during this period (F(2,20) = 21.78; p < 0.0001). SNK post hoc tests showed that the mean value for the EPSPs after drug washout was significantly greater for the 5-HT control group than those for the other two experimental groups (p < 0.05 for each test). The difference between the EPSP data for the 5-HT/heparin and combined test-alone groups during this period, however, was not significant. The asterisks indicate significant differences between the 5-HT control and 5-HT/heparin data, the # symbol indicates the significant difference between the 5-HT/heparin and test-alone data, and the crosses indicate significant differences between the 5-HT control and the test-alone data. Error bars represent SEM. Interstimulus interval, 5 min.
Figure 3.

Bath application of 2-APB, a cell membrane-permeant inhibitor of IP3 receptors, disrupts 5-HT-dependent facilitation of the sensorimotor synapse in culture. A, Representative EPSPs for three of the experimental groups: 5-HT control, 5-HT/2-APB, and test-alone/2-APB. Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of the EPSPs in the four experimental groups: 5-HT control synapses (n = 6); synapses treated with 5-HT in the presence of 2-APB (5-HT/2-APB; n = 7); synapses that received the test stimuli but not 5-HT (test-alone; n = 6); synapses that received the test stimuli in the presence of 2-APB (test-alone/2-APB; n = 5). A repeated-measures ANOVA performed on the data from the 5-HT treatment period (10-15 min trials) indicated that there was a significant main effect for experimental treatment (F(2,21) = 44.78; p < 0.0001). For the 10 min trial, the mean normalized EPSPs were 270 ± 35% in the 5-HT control group, 223 ± 21% in the 5-HT/2-APB group, and 68 ± 5% in the combined test-alone group (n = 11). For the 15 min trial, the EPSP values in the three groups were 284 ± 30, 229 ± 20, and 63 ± 6%, respectively. SNK post hoc tests indicated that during the 5-HT treatment period, the EPSPs in the 5-HT control group were significantly greater than those in the combined test-alone group, as were the EPSPs in the 5-HT/2-APB group (p < 0.05 for each test). However, there was no significant difference between the EPSPs in the 5-HT control and 5-HT/2-APB groups. Regarding the data after the washout of 5-HT, a repeated-measures ANOVA on the data from the 20-55 min trials indicated a highly significant main effect for experimental treatment (F(2,21) = 20.45; p < 0.0001). As indicated by SNK post hoc tests, the EPSPs in the 5-HT control group were significantly greater than those in both the 5-HT/2-APB groups and the combined test-alone group. The difference between the EPSP values for the 5-HT/2-APB and those for the combined test-alone group were also significant during the 20-55 min trials (p < 0.05). The asterisk indicates the significant difference between the 5-HT control and 5-HT/2-APB data, the # symbols indicate significant differences between the 5-HT/2-APB and test-alone data, and the crosses indicate significant differences between the 5-HT control and the test-alone data. Error bars represent SEM. Interstimulus interval, 5 min.
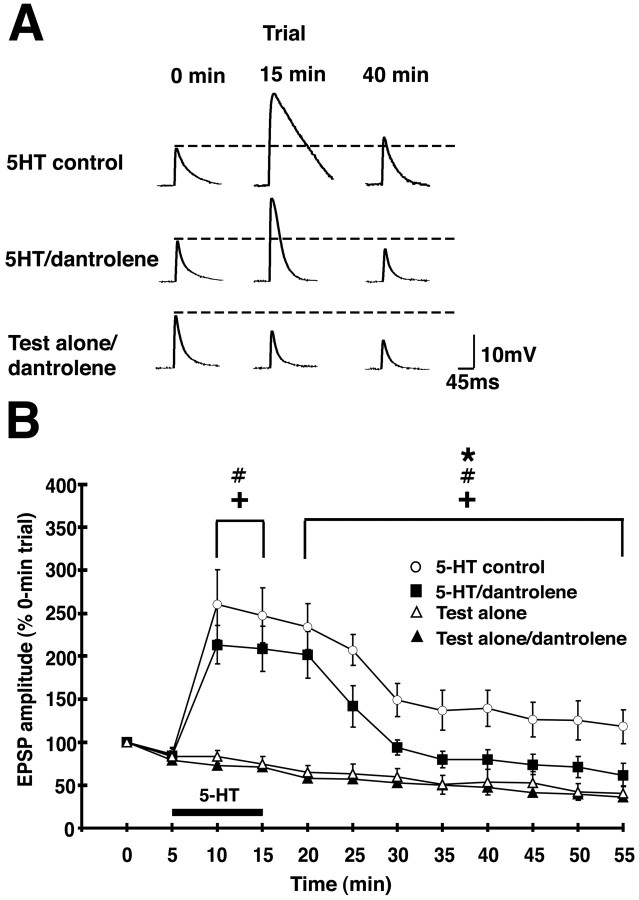
Figure 4.
Postsynaptic injection of dantrolene, an inhibitor of RyRs, disrupts 5-HT-dependent facilitation of the sensorimotor synapse in culture. A, Representative EPSPs for three of the experimental groups: 5-HT control, 5-HT/dantrolene, and test-alone/dantrolene. Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of the EPSPs in the four experimental groups: 5-HT control synapses (n = 7); synapses treated with 5-HT after dantrolene had been injected into the motor neuron (5-HT/dantrolene; n = 7); synapses that received the test stimuli but not postsynaptic dantrolene (test-alone; n = 7); synapses that received the test stimuli with postsynaptic dantrolene (test-alone/dantrolene; n = 7). There was a significant main effect for experimental treatment during the 5-HT treatment, as revealed by a repeated-measures ANOVA (F(2,25) = 27.30; p < 0.0001). The mean normalized EPSPs for the 10 min trial were 260 ± 41% in the 5-HT control group, 213 ± 22% in the 5-HT/dantrolene group, and 78 ± 4% in the combined test-alone group (n = 14). For the 15 min, trial the mean normalized EPSPs were 248 ± 32% in the 5-HT control group, 209 ± 26% in the 5-HT/dantrolene group, and 73 ± 5% in the combined test-alone group. SNK post hoc tests for the 5-HT application period indicated that the amplitudes of the EPSPs in the 5-HT control group and in the 5-HT/dantrolene groups were significantly facilitated compared with those in the combined test-alone group (p < 0.05 for each comparison). There was no significant difference between the 5-HT control and 5-HT/dantrolene EPSP values when 5-HT was present in the bath. A repeated-measures ANOVA on the data from the period after washout of 5-HT (20-55 min trials) also showed a significant main effect for experimental treatment (F(2,25) = 31.67; p < 0.0001). The EPSPs in the 5-HT control group were significantly greater than those in 5-HT/dantrolene and combined test-alone groups (p < 0.05 for each comparison), as revealed by SNK post hoc tests. Also, the EPSPs in the 5-HT/dantrolene group were significantly greater than those in the combined test-alone group (p < 0.05), indicating that facilitation persisted, to some extent, in the former group. The asterisk indicates the significant difference between the 5-HT control and 5-HT/dantrolene data, the # symbols indicate significant differences between the 5-HT/dantrolene and test-alone data, and the crosses indicate significant differences between the 5-HT control and the test-alone data. Error bars represent SEM. Interstimulus interval, 5 min.
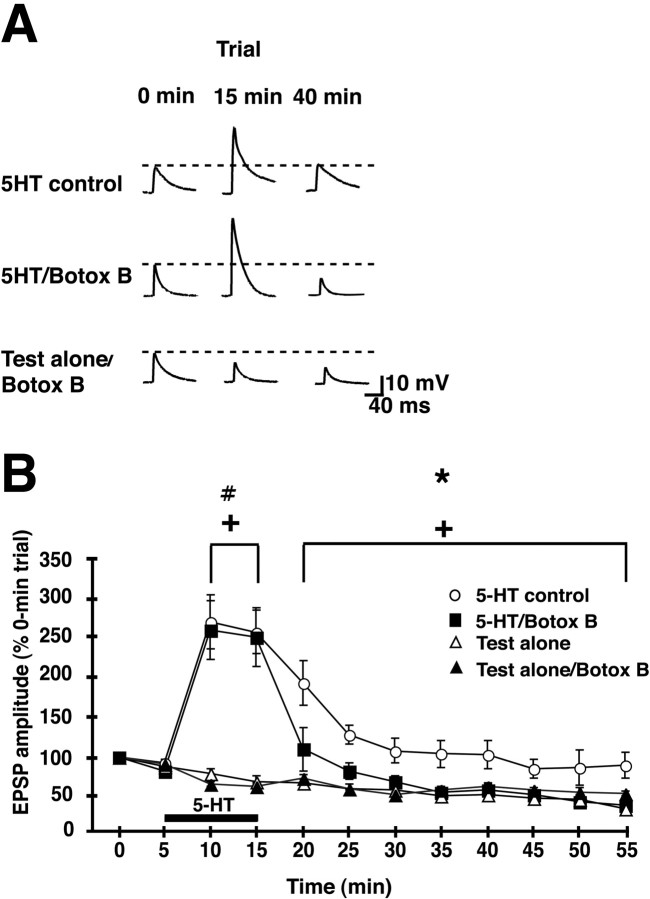
Figure 5.
Postsynaptic injection of Botox B, an inhibitor of exocytosis, disrupts 5-HT-dependent facilitation of the sensorimotor synapse in culture. A, Representative EPSPs for three of the experimental groups: 5-HT control, 5-HT/Botox B, and test-alone/Botox B. Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of the EPSPs in the four experimental groups: synapses in the 5-HT control group (n = 6); synapses were treated with 5-HT after Botox B had been injected into the motor neuron (5-HT/Botox B; n = 5); synapses that received the test stimuli, but not postsynaptic Botox B (test-alone; n = 6); synapses that received the test stimuli together with postsynaptic Botox B (test-alone/Botox B; n = 3). There was a significant main effect for experimental treatment during the period of 5-HT application (10-15 min trials), as revealed by a repeated-measures ANOVA (F(2,17) = 18.29; p < 0.0001). The mean normalized EPSPs for the 10 min trial were 267 ± 39% in the 5-HT control group, 257 ± 46% in the 5-HT/Botox B group, and 75 ± 5% in the combined test-alone group (n = 9). For the 15 min trial, the mean normalized EPSPs were 255 ± 31% in the 5-HT control group, 248 ± 47% in the 5-HT/Botox B group, and 68 ± 6% in the combined test-alone group. SNK post hoc tests for the 5-HT treatment period showed that the amplitudes of the EPSPs in the 5-HT control group and in the 5-HT-Botox B groups were significantly facilitated compared with those in the combined test-alone group (p < 0.05 for each comparison). The difference between the 5-HT control and 5-HT/Botox B EPSP values was not significant during 5-HT application. A repeated-measures ANOVA on the data from the post-5-HT period (20-55 min trials) showed a significant main effect for experimental treatment (F(2,17) = 9.11; p < 0.003). As revealed by SNK post hoc tests for the post-5-HT trials, the mean EPSPs in the 5-HT control group were significantly greater than those in the 5-HT/Botox B and combined test-alone groups (p < 0.05 for each comparison). There was no significant difference between the EPSP data for the 5-HT/Botox B and combined test-alone groups during the post-5-HT period. The asterisk indicates the significant differences between the 5-HT control and 5-HT/Botox B data, the # symbol indicates the significant differences between the 5-HT/Botox B and test-alone data, and the crosses indicate significant differences between the 5-HT control and the test-alone data. Error bars represent SEM. Interstimulus interval, 5 min.
Figure 6.

Postsynaptic injection of BAPTA disrupts tail nerve shock-induced facilitation of siphon sensorimotor synapses in the abdominal ganglion. A, Representative EPSPs for each of the experimental groups. Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of the EPSPs in the experimental groups. The EPSPs were normalized to the 4 min trial results. All of the tests, which occurred at one per minute, except during the period of nerve shock, were performed while the preparation was perfused in ASW with twofold normal Mg2+ and slightly elevated Ca2+ (2:1 ASW; see Materials and Methods). The tail nerve shock (arrow) was delivered in normal ASW. In the experiments involving nerve shock, a single shock (1 s, 25 Hz) was delivered to the P9 nerves (see Materials and Methods) 3 min after the 4 min trial. Immediately after the occurrence of the shock, the bathing medium was returned to the 2:1 ASW, and testing resumed 3 min later. A repeated-measures ANOVA performed on the post shock trials (10-19 min trials) indicated that there was a significant main group effect (F(2,18) = 13.38; p < 0.0004) as well as a significant interaction (F(2,18) = 3.37; p < 0.0001). SNK post hoc tests on the group differences for the main treatment effect revealed that EPSPs elicited in the TNS group (n = 7) were significantly greater than those in TNS/BAPTA group (n = 7) as well as in the test-alone group (n = 7; p < 0.05 for each comparison). The overall difference between the EPSPs in the TNS/BAPTA and test-alone groups during the postshock period was not significant. To further probe the interaction obtained from the repeated-measures ANOVA, we performed one-way ANOVAs with corrected Bonferroni post hoc tests on each of the postshock trials. Although several of the one-way ANOVAs indicated that there was a significant difference between the TNS and test-alone groups, only the ANOVA for the 10 min trial showed a significant difference between the TNS/BAPTA and test-alone groups (p < 0.05). The asterisk indicates the significant difference between the postshock data for the TNS and TNS/BAPTA groups, the cross indicates the significant difference between the TNS and the test-alone postshock data. Error bars represent SEM.
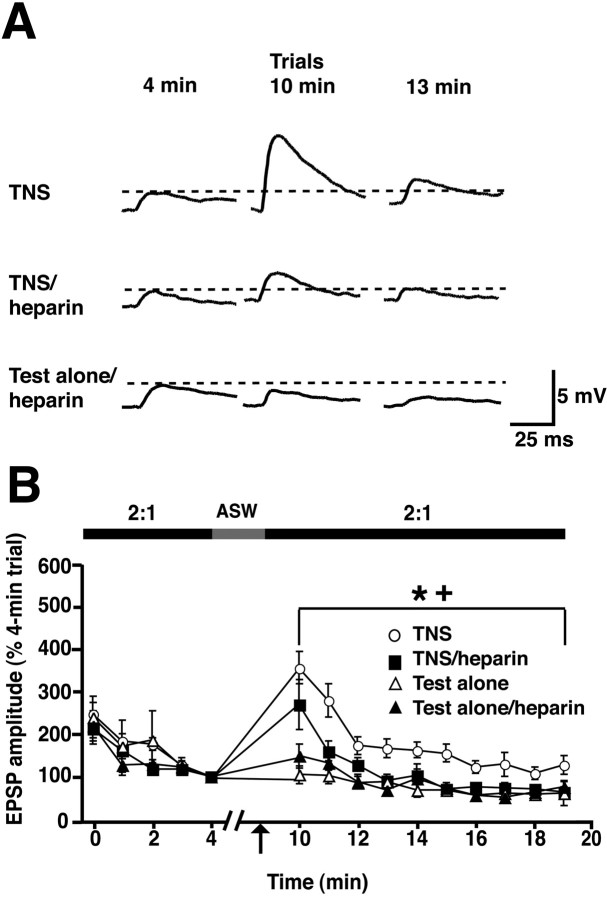
Figure 7.
Postsynaptic injection of heparin disrupts tail nerve shock-induced facilitation of siphon sensorimotor synapses in the abdominal ganglion. A, Representative EPSPs for three of the experimental groups: TNS, TNS/heparin, and test-alone/heparin. Trial times correspond to those indicated on the abscissa of the graph in B. B, Mean normalized amplitude of the EPSPs in the experimental groups. Test stimuli were delivered at one per minute in 2:1 ASW, except during the period of nerve shock. The EPSPs were normalized to the 4 min trial results. See Figure 6 for the testing protocol. The nerve shock protocol differed slightly from that used in the BAPTA experiments (Fig. 6). Three shocks (1 s each, 25 Hz; see Materials and Methods) were delivered at 30 s intervals in normal ASW, beginning at 2.5 min after the 4 min trial in normal ASW. The perfusion medium was switched back to 2:1 ASW immediately after the third shock (which occurred at 3.5 min after the 4 min trial), and testing resumed 2.5 min later. There was a highly significant main group effect, as indicated by the repeated-measures ANOVA on the postshock data (F(2,18) = 2.74; p < 0.0003). Group differences were assessed with SNK post hoc tests. These indicated that the postshock EPSPs for the TNS group (n = 8) were significantly greater than those for both the TNS/heparin group (n = 8) and the combined test-alone group (n = 14; p < 0.05 for each comparison). [The combined test-alone group comprised synapses that received the test stimuli without postsynaptic heparin (test-alone; n = 7) and synapses that received the test stimuli with postsynaptic heparin (test-alone/heparin group, n = 7).] The EPSPs in the TNS/heparin and the combined test-alone groups did not differ for the postshock period. To investigate whether these two groups differed significantly on any individual postshock trial, we performed one-way ANOVAs with corrected Bonferroni's post hoc tests on each postshock trial. No significant differences were observed between the TNS/heparin and test-alone groups for any trial. The absence of significance between the TNS/heparin and test-alone EPSPs on this trial was probably attributable to the severe correction for multiple comparisons involved in the Bonferroni's tests. The asterisk indicates the statistically significant difference between the postshock data for the TNS and TNS/heparin groups, and the cross indicates the significant difference between the TNS and the test-alone postshock data. Error bars represent SEM.
Prolonged 5-HT-dependent facilitation of the sensorimotor synapse requires elevated postsynaptic Ca2+
In our previous experiments performed on isolated siphon motor neurons in vitro, we observed that 5-HT-induced enhancement of the evoked glutamate response was triggered by elevated postsynaptic Ca2+ (Chitwood et al., 2001). To test whether 5-HT-dependent synaptic facilitation involves a rise in postsynaptic intracellular Ca2, we injected the rapid Ca2+ chelator BAPTA (200 mm in the recording micropipette) into the postsynaptic motor neuron before the start of an experiment. Application of 5-HT (10 μm, 10 min) produced significant facilitation of the sensorimotor EPSP in synapses in which BAPTA was not present in the motor neuron (5-HT control data) (Fig. 1), both during the period in which 5-HT was present in the bath (the 10-15 min trials) (Figs. 1, 2, 3, 4, 5, black bar) and during the period after washout of the drug (20-55 min trials), as indicated by planned comparisons between the 5-HT control and test-alone/BAPTA data. Chelating Ca2+ in the motor neuron significantly reduced facilitation, both while 5-HT was present and after 5-HT washout, as indicated by comparisons between the 5-HT control and 5-HT/BAPTA EPSPs. Furthermore, the overall difference between the 5-HT/BAPTA and the test-alone/BAPTA groups was not significant for the post-5-HT period. Analyses of individual tests during 5-HT exposure indicated that the amplitudes of the EPSPs in the 5-HT control and 5-HT/BAPTA groups did not differ significantly on the 10 min trial but did differ significantly on the 15 min trial. Thus, the presence of BAPTA in the motor neuron did not impair facilitation ≤5 min after the onset of 5-HT but did thereafter. These data indicate that persistent facilitation requires elevated postsynaptic Ca2+.
Note that we did not include a separate control group in which synapses received just the test stimuli without the postsynaptic BAPTA injection. We and others have previously found that postsynaptic BAPTA does not affect homosynaptic depression of sensorimotor synapses caused by test stimuli delivered at low frequencies (Murphy and Glanzman, 1996; Bao et al., 1997) (see below).
Evidence that prolonged 5-HT-dependent facilitation of the sensorimotor synapse requires release of Ca2+ from postsynaptic intracellular stores
A possible source of the rise in postsynaptic Ca2+ required for persistent facilitation of the sensorimotor synapse is release from intracellular stores (Berridge, 1993). As an initial test of this possibility, we injected heparin (5 mm in the recording micropipette), a competitive antagonist of inositol-1,4,5-trisphosphate (IP3) receptors (Ghosh et al., 1988), into the motor neuron. Postsynaptic heparin had no effect on either basal synaptic transmission or homosynaptic depression (repeated-measures ANOVA on the overall data for the two test-alone groups; p > 0.6) (Fig. 2). Accordingly, the data from the test-alone and test-alone/heparin experiments were combined for the statistical comparisons with the two groups that received 5-HT treatment. Application of 5-HT (10-20 μm, 10 min) produced significant facilitation of the sensorimotor EPSP in the 5-HT control group during both the 5-HT treatment period and the post-5-HT period, as indicated by statistical comparisons between the 5-HT control and combined test-alone groups. Postsynaptic heparin significantly reduced the facilitation while 5-HT was present in the bath, as well as after 5-HT washout, as shown by comparisons between the 5-HT control and 5-HT/heparin groups. The presence of heparin in the motor neuron did not eliminate the facilitation elicited in the presence of 5-HT, because there was a significant difference between the 5-HT/heparin and test-alone groups during the 10-15 min trials. However, postsynaptic heparin blocked the facilitation that persisted after washout of 5-HT.
IP3 receptors play a significant role in 5-HT-dependent facilitation of the sensorimotor synapse
Heparin can have nonspecific effects (Taylor and Broad, 1998). We therefore tested the effect of another, more specific, inhibitor of IP3 receptor-mediated release of intracellular Ca2+, 2-APB (Maruyama et al., 1997; Ma et al., 2000), on 5-HT-dependent facilitation. This cell membrane-permeant inhibitor was bath applied (20-80 μm in perfusion medium with 0.1% DMSO, beginning 5 min before the first trial) and was present throughout the experiment. [Note that 0.1% DMSO was also present in the perfusion medium during the control experiments in which 2-APB was not used. This concentration of DMSO has no effect on basic synaptic transmission, the input resistance of the neurons or on synaptic facilitation induced by 5-HT (Q. Li and D. L. Glanzman, unpublished observations)]. The overall data for the test-alone and test-alone/2-APB groups did not differ (repeated-measures ANOVA; p > 0.1) (Fig. 3), and the data from these two groups were combined for statistical comparisons with the other experimental groups. 5-HT (10 μm, 10 min) produced significant facilitation in both the 5-HT control and 5-HT/2-APB groups, compared with the combined test-alone group, during 5-HT exposure. After washout of 5-HT, significant facilitation persisted in the 5-HT control group, as indicated by the comparison between the 5-HT control and combined test-alone groups. Inhibition of IP3 receptors did not significantly disrupt facilitation while 5-HT was present. However, the 2-APB impaired post-5-HT facilitation: the EPSPs in the 5-HT/2-APB group were significantly reduced during this period compared with those in the 5-HT control group. Although disruptive of facilitation, inhibition of IP3 receptors did not eliminate facilitation after washout of 5-HT, as indicated by the significant difference between the 5-HT/2-APB data and the test-alone data during the 20-55 min trials.
The two inhibitors of IP3 receptors that we used, heparin and 2-APB, are known to have nonspecific effects. For example, heparin can inhibit both the formation of inositol 1,3,4,5-tetrakiphosphate (IP4) and the binding of IP4 to its receptor; in addition, heparin can activate ryanodine receptors (RyRs) and uncouple receptors from their G-proteins (Taylor and Broad, 1998). 2-APB has been reported to block Ca2+ entry through store-operated Ca2+ channels (Ma et al., 2001; Peppiatt et al., 2003). 2-APB can also enhance Ca2+ release and Ca2+ entry. Prakriya and Lewis (2001) reported that, at low concentrations (1-5 μm), 2-APB enhanced Ca2+ entry into cells via the Ca2+ release-activated Ca2+ current (ICRAC), whereas at higher concentrations (≥10 μm, similar to those used in the present experiments), 2-APB caused brief enhancement of ICRAC, followed by inhibition. We found that low concentrations of 2-APB (1-10 μm) significantly potentiated the 5-HT-induced facilitation that persists after 5-HT washout (data not shown). This effect may be attributable to a facilitatory effect of 2-APB on either presynaptic or postsynaptic ICRAC. Despite the potential nonspecific effects of heparin and 2-APB, however, the inhibitors had similar, albeit not identical, disruptive effects on facilitation of the sensorimotor synapse. Because the nonspecific effects of these two inhibitors are attributable to effects on different cellular targets, the overall similarity of their inhibitory actions here supports the idea that their impairment of synaptic facilitation was caused by a common action, namely inhibition of IP3 receptor-mediated Ca2+ release in the motor neuron. We do not have an explanation for why the effect of heparin on facilitation was quantitatively greater than that of 2-APB. One possibility is that 2-APB had a stimulatory effect on Ca2+ influx (Prakriya and Lewis, 2001), perhaps in the sensory neuron, in addition to its antagonism of postsynaptic IP3 receptors. Another possibility is that the more drastic effect of heparin on facilitation may have partly been attributable to uncoupling of 5-HT receptors with their G-proteins (Taylor and Broad, 1998). These possibilities are, of course, not mutually exclusive.
Postsynaptic RyRs also mediate 5-HT-dependent facilitation of the sensorimotor synapse
Elevated intracellular Ca2+ within neurons can also cause release of Ca2+ from intracellular stores mediated by RyRs (Berridge et al., 2000). To investigate whether Ca2+-induced Ca2+ release from RyR-mediated internal stores within motor neurons contributes to 5-HT-dependent facilitation, we injected dantrolene (500 μm in the recording micropipette), an inhibitor of RyRs (Xu et al., 1998), into the motor neuron. A repeated-measures ANOVA indicated that postsynaptic injection of dantrolene had no effect on either basal synaptic transmission or on homosynaptic depression (p > 0.5) (Fig. 4). The data for the two test-alone groups were therefore combined for subsequent tests. As in the previous experiments, application of 5-HT (20 μm, 10 min) facilitated transmission of the in vitro sensorimotor synapse. The EPSPs in the 5-HT control group were significantly greater than those in the combined test-alone group, both during the presence of the drug and after its washout. Postsynaptic dantrolene did not significantly alter the 5-HT-induced facilitation while the drug was present in the bath, but thereafter inhibition of RyRs in the motor neuron significantly degraded facilitation. Some facilitation persisted after washout of 5-HT, however, despite postsynaptic dantrolene, as indicated by the comparison between the 5-HT/dantrolene and test-alone data.
5-HT-induced facilitation depends on postsynaptic exocytosis
We previously observed that the light chain of Botox B blocks facilitation of the glutamate response in isolated motor neurons of Aplysia (Chitwood et al., 2001). Botox B blocks exocytosis by selectively cleaving vesicle-associated membrane protein/synaptobrevin (Südhof, 1995). We therefore hypothesized that facilitation of the glutamate response in isolated motor neurons might be attributable to exocytotic insertion of additional AMPA receptors into the cell membrane of the neurons. To determine whether 5-HT-induced facilitation of the sensorimotor synapse depends on postsynaptic exocytosis, we injected Botox B (0.5 μm in the recording micropipette) into the motor neuron (Poulain et al., 1996). Neither synaptic transmission nor homosynaptic depression was altered by postsynaptic Botox B (repeated-measures ANOVA on the overall EPSP data for the test-alone and test-alone/Botox B groups; p > 0.5) (Fig. 5). Subsequent statistical comparisons therefore used the combined data from the two test-alone groups. The EPSPs in the 5-HT control group were significantly facilitated by 5-HT (10 μm, 10 min), compared with the EPSPs in synapses that only received the test stimuli, both while the 5-HT was present in the bath and after washout of the drug. Blocking postsynaptic exocytosis did not affect facilitation during the 5-HT application period but blocked facilitation thereafter. There was no significant difference between the 5-HT/Botox B EPSPs and the test-alone EPSPs during the 20-55 min trials.
The lack of any effect on facilitation of postsynaptic Botox B while 5-HT was present in the bath contrasts with the effects of chelating postsynaptic intracellular Ca2+ (Fig. 1) and of antagonizing postsynaptic IP3 receptors (Figs. 2, 3). This difference suggests that 5-HT stimulates early processes within the motor neuron that depend on elevated intracellular Ca2+ and that can support synaptic facilitation independent of postsynaptic exocytosis.
Facilitation of siphon sensorimotor synapses in the abdominal ganglion caused by tail nerve shock depends on elevated postsynaptic Ca2+
Noxious stimulation, such as tail or tail nerve shock, causes activation of identified serotonergic neurons in Aplysia (Mackey et al., 1989) and the release of 5-HT within the CNS of Aplysia (Marinesco and Carew, 2002). Furthermore, serotonergic processes have been shown to contact both sensory and motor neurons within the central ganglia of Aplysia (Kistler et al., 1985; Marinesco and Carew, 2002; Zhang et al., 2003). Finally, release of 5-HT is required for dishabituation and sensitization of the defensive withdrawal reflex in Aplysia (Glanzman et al., 1989). These facts, together with the data from our experiments on sensorimotor synapses (above), suggest that 5-HT-dependent postsynaptic processes might be critical for behavioral dishabituation and/or sensitization. To test this idea, we used a so-called “cellular analog” of dishabituation/sensitization of the SWR. Electrical shocks delivered to two tail (P9) nerves of a reduced preparation served as dishabituating/sensitizing stimuli (see Materials and Methods). The electrophysiological readout of dishabituation/sensitization was facilitation of siphon sensorimotor synapses within the abdominal ganglion. Test stimuli were delivered to presynaptic siphon sensory neurons at a rate of one per minute, except during the period in which the nerves were shocked. To suppress the activity of interneurons, and thereby ensure that the EPSPs evoked in response to our test stimuli were monosynaptic, ASW containing approximately twofold extracellular Mg2+ and slightly elevated extracellular Ca2+ (2:1 solution; see Materials and Methods) was perfused over the preparation during testing (Trudeau and Castellucci, 1992; Murphy and Glanzman, 1996; Liao and Walters, 2002). The perfusion medium was changed to normal ASW just before the delivery of nerve shock; afterward, the perfusion medium was switched back to the 2:1 solution, and testing continued (Fig. 6). During these exchanges of solution, the amount of normal ASW/2:1 solution washed through the experimental chamber was three to four times the volume of the chamber.
We initially tested whether elevation of postsynaptic intracellular Ca2+ is required for facilitation of sensorimotor synapses caused by tail nerve shock. BAPTA (200 mm in the recording micropipette) was injected into the LFS motor neuron of a siphon sensorimotor synapse ≥30 min before the start of an experiment. There were three experimental groups: a test-alone group that received only the test stimuli; a tail nerve shock (TNS) group that received tail nerve shock in addition to the test stimuli; and a group that received the same stimuli as the TNS group, but with BAPTA present in the motor neuron (TNS/BAPTA group). All three groups exhibited homosynaptic depression (Castellucci and Kandel, 1974) during the preshock trials (Fig. 6), and there were no significant differences among the EPSPs for the groups during the preshock trials (repeated-measures ANOVA; p > 0.5). Tail nerve shock produced significant facilitation of the monosynaptic EPSP, as indicated by the comparison between the TNS and test-alone data. Chelating Ca2+ in the motor neuron blocked the shock-induced facilitation, as indicated by the comparison between TNS and TNS/BAPTA data. Although the overall postshock data for the TNS/BAPTA and test-alone groups did not differ, one-way ANOVAs with Bonferroni's corrections on the individual postshock trials revealed that the 10 min EPSP for the TNS/BAPTA group was significantly greater than that of the test-alone group. This result indicates that, after tail shock, there is a brief contribution to facilitation from processes, presumably presynaptic (Byrne and Kandel, 1996), that are independent of postsynaptic Ca2+.
In a separate experiment, we tested whether postsynaptic BAPTA affected the response of sensorimotor synapses to the test stimuli. There were two groups: a test-alone group (n = 4) and a test-alone/BAPTA group (n = 4). The experimental protocol was identical to that of the experiments that involved nerve shock, including the exchanges of perfusion medium, except that no nerve shock was used. A repeated-measures ANOVA indicated that there was no difference between the EPSPs for the test-alone and test-alone/BAPTA groups (p > 0.5; data not shown). Postsynaptic BAPTA treatment therefore did not alter either basal synaptic transmission or short-term homosynaptic depression, at least at the rates of test stimulation used here (Murphy and Glanzman, 1996; Bao et al., 1997).
Tail nerve shock-induced facilitation of siphon sensorimotor synapses requires release from postsynaptic intracellular stores of Ca2+
To investigate whether nerve shock-induced facilitation depends on release of Ca2+ from postsynaptic intracellular stores, we injected heparin (5-7.5 mm in the recording micropipette) into motor neurons before the start of the experiments. The experimental protocol was similar to that in the BAPTA experiments, except that three nerve shocks were delivered in the TNS groups, rather than a single shock (Fig. 7). There were four experimental groups: test-alone, test-alone/heparin, TNS, and TNS/heparin. The synapses in the test-alone and test-alone/heparin groups (n = 7 in each) did not differ with respect to their responses to the test stimuli (repeated-measures ANOVA; p > 0.2). Subsequent statistical comparisons used the combined results from the two test-alone groups. Postsynaptic heparin had no significant effect on the response of the synapse to the test stimuli, as indicated by a repeated-measures ANOVA on the preshock trials for the three groups (p > 0.6) (Fig. 7). Nerve shock produced significant facilitation of the sensorimotor synapse, and this nerve shock-induced facilitation was disrupted by the presence of heparin in the motor neuron. There was no significant difference between the postshock data for the TNS/heparin and the combined test-alone groups. These data indicate that, like 5-HT-dependent facilitation, dishabituation/sensitization-related facilitation in Aplysia depends on release of Ca2+ from postsynaptic internal stores for its full expression. Our data are consistent with a role for postsynaptic IP3 receptors in dishabituation/sensitization, although they do not rule out an additional role for RyRs.
Tail nerve shock produces greater facilitation of the AMPA component of the sensorimotor EPSP than of the NMDA component
NMDA-type receptors have previously been implicated in plasticity of Aplysia sensorimotor synapses (Lin and Glanzman, 1994a,b; Murphy and Glanzman, 1997, 1999; Antonov et al., 2003). Moreover, experiments on sensorimotor synapses in culture (Glanzman, 1994; Conrad et al., 1999) and in the abdominal ganglion (Antonov et al., 2003) have shown that, under some circumstances, sensorimotor EPSPs contain an APV-sensitive component. Antonov et al. (2003) reported that the AMPA receptor antagonist CNQX selectively antagonizes the peak, but not the late phase, of the EPSP for siphon sensorimotor synapses in the abdominal ganglion, whereas APV selectively antagonizes the late phase, but not the peak, of the EPSP. [Note that the experiments by Antonov et al. (2003) were performed in ASW having normal extracellular Mg2+, with the postsynaptic motor neuron hyperpolarized by ∼30 mV.] The results of Antonov et al. indicate that the AMPA receptor-mediated and NMDA receptor-mediated components of the sensorimotor EPSP can be pharmacologically separated for synapses in the CNS of Aplysia. Accordingly, we used APV, CNQX, and another, more selective AMPA receptor antagonist, NBQX (Sheardown et al., 1990), to test whether the AMPA receptor-mediated and NMDA receptor-mediated components of the sensorimotor EPSP are differentially facilitated by nerve shock. Because of the homosynaptic depression exhibited by the sensorimotor synapse to low-frequency stimulation, we had to modify our experimental protocol to obtain measurable EPSPs in CNQX/NBQX. Therefore, there were only two tests before the nerve shock, the first in ASW alone and the second in ASW containing one of the three glutamate receptor antagonists: APV (200 μm), CNQX (50 μm), or NBQX (200 μm). The drugs were washed out of the experimental chamber immediately after the second test and washed back in after the nerve shock (Fig. 8A,C). The EPSPs elicited on the first trial in normal ASW ranged from 2 to 10 mV. There were no significant differences among the three experimental groups with respect to the starting amplitudes of the EPSPs. To determine whether the nerve shocks produced significant synaptic facilitation, we also performed parallel experiments in each of the glutamate receptor antagonists using the identical protocol as shown in Figure 8C, except that no nerve shocks were delivered. There were no significant differences among the three sets of normalized test-alone data (Fig. 8B), and therefore the data from the test-alone experiments were combined for comparison with the data from the nerve shock experiments. Tail nerve shock produced significant facilitation of the EPSP in the TNS/APV group, as shown by the comparison with the combined test-alone group (Fig. 8C, open diamonds). The postshock EPSPs in the TNS/APV group were also significantly greater than those in the combined TNS/CNQX/NBQX group. There was no significant difference, however, between the postshock EPSPs in the CNQX/NBQX and the test-alone EPSPs for the equivalent trials. Thus, tail nerve shock produced significant enhancement of the AMPA receptor-mediated component of the EPSP, but not of the NMDA receptor-mediated component. These results appear inconsistent with the idea that dishabituation/sensitization in Aplysia is mediated either exclusively or predominately by presynaptic facilitation (see Discussion).
It might be argued, however, that our data permit an alternative interpretation, one compatible with an exclusively presynaptic model of facilitation. The alternative interpretation depends on the notion that AMPA and non-AMPA receptors might be localized opposite different presynaptic terminals. If postsynaptic AMPA receptors were present opposite release sites that were somehow more sensitive to 5-HT than the release sites opposite non-AMPA receptors, then nerve shock-released 5-HT (or other facilitatory transmitter) would be expected to selectively enhance the AMPA receptor-mediated component of the EPSP, even if 5-HT simply increased presynaptic release. However, this alternative interpretation seems unlikely given the substantial similarity between our present results and those from our previous experiments on isolated motor neurons in cell culture (Chitwood et al., 2001). Any potential contribution from presynaptic processes to facilitation was excluded in those experiments. Moreover, as we show below (see Discussion) the deduced time course of the postsynaptic process that underlies facilitation of the sensorimotor EPSP closely parallels the time course of the facilitation of the glutamate response in isolated motor neurons. Given these facts, the most reasonable interpretation of the data in Figure 8 is that nerve shock results in a postsynaptic facilitatory action, specifically selective enhancement of the AMPA receptor-mediated synaptic response, in addition to its presynaptic effects.
Although our results represent convincing evidence that dishabituation-related facilitation of the sensorimotor synapse involves postsynaptic changes, they may underestimate the contribution of presynaptic changes to this form of learning. This is because the severe corrections for multiple comparisons involved in our post hoc statistical tests may have caused a failure to detect a real difference between the postshock EPSPs in the AMPA receptor antagonists and the test-alone EPSPs (type II error).
Analysis of the non-normalized EPSPs in APV indicated that the NMDA receptor antagonist had little effect on the peak of the EPSP in 9 of the 11 synapses in the TNS/APV group, consistent with the results of Antonov et al. (2003). But in two of the synapses, APV significantly antagonized the peak of the EPSP. These results may indicate that for a minority of sensorimotor synapses in the abdominal ganglion, NMDA receptor-mediated currents contribute to the peak, as well as the late phase, of the EPSP. Note, however, that because we did not include an ASW control group in this set of experiments, we are unable to statistically disentangle the effects of homosynaptic depression from the effects of the glutamate receptor antagonists on the EPSPs. We also observed that CNQX appeared to produce greater reduction of the EPSP peak than did NBQX, a result that we have confirmed in experiments on sensorimotor synapses in cell culture (Li and Glanzman, unpublished observations). This observation is consistent with findings from the mammalian CNS that indicate that NBQX is a more selective antagonist of AMPA receptors than CNQX (Sheardown et al., 1990; Randle et al., 1992).
Behavioral dishabituation depends on postsynaptic exocytosis
Previous evidence from our laboratory (Chitwood et al., 2001) and results from the present study (Fig. 5) implicate an exocytotic process in 5-HT-induced enhancement of AMPA receptor function. To test for a role for postsynaptic exocytosis in actual learning in Aplysia, we examined the effect of injection of Botox B into siphon motor neurons on dishabituation of the SWR (Hawkins et al., 1998; Antonov et al., 1999). For these behavioral experiments, we used a reduced preparation similar to that developed by Antonov et al. (1999, 2001, 2003) (Fig. 9A). This preparation permitted us to monitor the SWR while recording electrophysiologically from siphon motor neurons in the abdominal ganglion. At the beginning of each experiment, two siphon motor neurons (LFSB type) in the abdominal ganglion were unambiguously identified by their electrophysiological properties and their contribution to movement of the siphon (Frost et al., 1988; Hickie and Walters, 1995) (Fig. 9B). Botox B (1 μm) or the control electrode solution (see Materials and Methods) was iontophoresed into the two motor neurons by applying constant negative current for 2 h (Hunt et al., 1994). Behavioral experiments were then performed on the preparation. Six weak test shocks were delivered to the siphon at one per 5 min, and the resulting SWR was measured. As expected, the preparations exhibited habituation of the SWR in response to the test stimuli (Antonov et al., 1999). After the sixth test stimulus, strong electrical shocks were delivered to the tail preparation, and testing of the siphon response resumed 7 min afterward. In control preparations without Botox B injections, the tail shocks produced significant dishabituation of the withdrawal reflex (Fig. 9C,D). Preparations in the Botox B group did not exhibit dishabituation. Furthermore, the averaged postshock SWR was significantly greater in the control group than in the Botox B group.
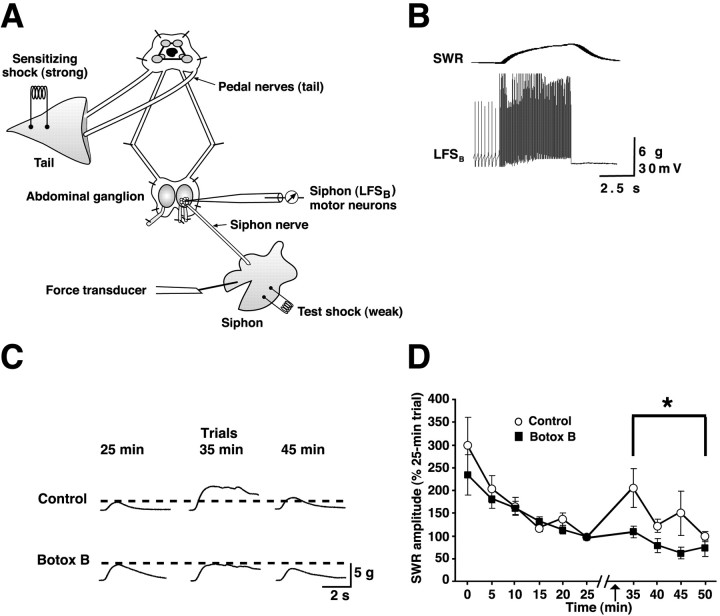
Figure 9.
Effect of loading siphon motor neurons with Botox B on dish abituation of the SWR. A, Experimental preparation. Note that the abdominal ganglion is shown artificially enlarged relative to the other ganglia. B, Intracellular activation of an LFSB-type motor neuron (bottom) produced contraction of the siphon, as indicated by the force transducer record (top). This motor neuron was subsequently loaded with Botox B (light chain of type B; see Materials and Methods for details). C, Sample records of SWRs from the experiments. Trial times correspond to those indicated on the abscissa in D. D, Mean amplitude of the SWR in the control and Botox B groups, normalized to the 25 min trial (the trial that immediately preceded the tail shocks). Tests were delivered at one per 5 min. A repeated-measures ANOVA on the first five trials showed that there was no difference in the normalized SWR for the control (n = 7) and Botox B (n = 7) groups (p > 0.5). Two minutes after the sixth trial, electrical shocks (arrow) were delivered to the tail via implanted electrodes. There were three shocks; each shock (40 Hz, 75 V) was 1 s in duration, and there was a 30 s intershock interval. Testing of the SWR resumed 7 min after the third tail shock. There were four postshock trials (intertrial interval, 5 min). The mean normalized SWR for the first postshock (35 min) trial in control preparations (207 ± 42%) was significantly greater than for the trial immediately preceding the tail shocks (25 min trial; SWR, 100%; p < 0.05). The preparations with the Botox B-injected LSFB neurons did not exhibit significant dishabituation on the 35 min trial (mean normalized SWR, 110 ± 13%; p > 0.4), and the mean SWR in this group was <100% for each of the other three postshock trials. A repeated-measures ANOVA on the data from the postshock trials indicated that the SWR was significantly stronger in the control group than in the Botox B group (F(1,12) = 6.20; p < 0.03). The asterisk indicates the significant difference between the postshock data for the control and Botox B groups. The non-normalized magnitude of the initial SWR to the first test stimulus was 6.14 ± 0.76 g in control preparations and 5.50 ± 0.99 g in the Botox B preparations (p > 0.6). The intensity of the electrical shock required to elicit a threshold SWR was 10.1 ± 0.3 V in the control group and 9.3 ± 0.4 V in the Botox B group (p > 0.1). Error bars represent SEM.
It might be expected that Botox B would interfere with the SWR itself by blocking presynaptic release from the terminals of the LFSB neurons treated with the toxin (Hunt et al., 1994). However, the normalized SWR averaged across the first five trials (0-20 min trials) was not significantly different in the control and Botox B groups (Fig. 9D). Also, there was no difference in the non-normalized magnitude of the initial SWR to the first test stimulus. Finally, the intensity of the electrical shock required to elicit a threshold SWR in the two groups did not differ significantly. These results indicate that the Botox B injections did not disrupt transmitter release from LFSB neurons, probably because to reach the terminals of these neurons, the toxin would have had to diffuse a distance of over 20 mm, the approximate distance from the abdominal ganglion to the siphon via the siphon nerve. Nonetheless, it might be argued that the reason the preshock data did not differ for the control and Botox B groups, whereas the postshock data did, was that the toxin finally arrived at the terminals of the injected motor neurons at the time of the 35-50 min trials. We therefore performed an additional set of experiments using a protocol identical to that of the dishabituation experiments presented in Figure 9, with the difference that no tail shocks were given to the preparations. In these test-alone experiments, there was no significant difference between the control group (n = 5) and Botox B group (n = 5), either for the averaged group data for all of the trials (p > 0.5) or for the four trials corresponding to the postshock trials in the dishabituation experiments (35-50 min trials; p > 0.5; data not shown). Together, these results provide strong support for the idea that a postsynaptic exocytotic process is required for normal behavioral dishabituation of the SWR.
Discussion
Our data indicate that postsynaptic facilitatory mechanisms, particularly upregulation of the function of AMPA-type receptors, play critical roles in synaptic facilitation and behavioral dishabituation in Aplysia. The present results are reminiscent of those reported for long-term potentiation (LTP) of synapses in the CA1 region of the hippocampus (Malinow and Malenka, 2002). During LTP, the trafficking of AMPA receptors in CA1 neurons is modulated; specifically, LTP induction causes additional AMPA receptors to be delivered to the postsynaptic plasma membrane of CA1 neurons, possibly by exocytosis (Lledo et al., 1998; Lüscher et al., 1999; Shi et al., 1999; Hayashi et al., 2000). However, work on AMPA receptor trafficking in the mammalian hippocampus has been concerned primarily with synaptic plasticity. Our results, in contrast, link modulation of AMPA receptor trafficking with actual learning in a simple model system (Takahashi et al., 2003; Bocchiaro and Feldman, 2004; Rumpel et al., 2005).
Our in vitro experiments indicate that buffering of postsynaptic intracellular Ca2+ and antagonism of IP3 receptors produce earlier disruption of facilitation than does antagonism of postsynaptic RyRs and blockade of postsynaptic exocytosis (Figs. 1, 2, 3, 4, 5). This suggests that release of Ca2+ from ryanodine-sensitive stores may be the cellular event most closely related to insertion of AMPA receptors into the postsynaptic membrane and, furthermore, that there are Ca2+-dependent postsynaptic facilitatory mechanisms that do not involve exocytosis.
Do the postsynaptic facilitatory processes described here participate in sensitization, the enhancement of a nonhabituated response, as well as in dishabituation? Previous data indicate a possible mechanistic distinction between dishabituation and sensitization of the SWR in Aplysia (Hochner et al., 1986; Marcus et al., 1988; Rankin and Carew, 1988). Nevertheless, 5-HT appears to play a critical role in both of these forms of nonassociative learning (Glanzman et al., 1989; Marinesco and Carew, 2002). We therefore presume that sensitization, like dishabituation, depends on 5-HT-induced postsynaptic processes.
A related question is whether postsynaptic processes play critical roles in facilitation of nondepressed synapses. 5-HT recruits an additional presynaptic facilitatory component in synapses that have undergone severe short-term homosynaptic depression due to rates of stimulation (e.g., 0.1 Hz) significantly higher than those used here (Hochner et al., 1986). Furthermore, 5-HT-dependent facilitation of sensorimotor synapses appears to involve somewhat different processes depending on whether the synapses are nondepressed or moderately depressed (Ghirardi et al., 1992). The synapses in our electrophysiological experiments were, at most, moderately depressed by the stimulation frequencies that we used (once per 1 or 5 min). For example, in the experiments on cultured synapses, 5-HT was applied to synapses that were depressed by only ∼25% (Figs. 1, 2, 3, 4, 5). Moreover, both of the facilitatory treatments, application of 5-HT and tail nerve shock (Figs. 6, 7), increased the amplitude of the sensorimotor EPSP beyond its initial level in the control experiments. Thus, the postsynaptic processes described here appear to be important for facilitation of nondepressed as well as of moderately depressed synapses, a conclusion supported by the overall similarity between the present results and those from our previous experiments involving facilitation of the glutamate response in isolated motor neurons (Chitwood et al., 2001).
Previous studies (for review, see Byrne and Kandel, 1996) concluded that sensitization/dishabituation-related facilitation in Aplysia depends on exclusively presynaptic mechanisms. Castellucci and Kandel (1976) performed a quantal analysis on transmission at sensorimotor synapses before and after nerve shock. They reported that nerve shock produced an increase in m (quantal content), a parameter classically associated with presynaptic transmitter release, without altering q (quantal size), a parameter associated with postsynaptic sensitivity. Nevertheless, the interpretation of results from studies of LTP expression in the hippocampus using similar statistical analyses has been problematic (Nicoll, 2003). One might suppose that our results could be reconciled with the previous results (Castellucci and Kandel, 1976) if the facilitation were induced, at least in part, postsynaptically (i.e., by release of Ca2+ from intracellular stores) but expressed entirely presynaptically, as enhanced presynaptic release. But the results from the present experiments using AMPA receptor antagonists (Fig. 8) show that nerve shock-induced facilitation of the sensorimotor synapse is expressed postsynaptically as enhanced AMPA receptor function.
What is the reason for the contrast between our conclusions and those of previous studies of synaptic facilitation in Aplysia? One potential source of the discrepancy may be methodological: we applied 5-HT for 10 min, which is significantly longer than the briefer 5-HT treatment periods generally used to investigate short-term facilitation of sensorimotor synapses (Byrne and Kandel, 1996). The longer 5-HT treatment is likely to have recruited processes involved in intermediate-term facilitation (Sutton et al., 2001), and postsynaptic processes may play a more significant role in intermediate-term than in short-term facilitation. The indication from our results that exclusively presynaptic facilitation is relatively brief (below) supports this idea. But this explanation does not account for the results from our experiments involving nerve shock, which used a standard training protocol. Moreover, postsynaptic mechanisms have not been implicated previously in cellular studies of (activity-independent) intermediate-term facilitation, although there is evidence for a role for postsynaptic mechanisms in long-term facilitation (Glanzman et al., 1990; Sherff and Carew, 2004). An ambiguity inherent in many previous studies of synaptic facilitation in Aplysia arises from the use of cell membrane-permeant inhibitors, of protein kinase A (PKA) or PKC, for example, to disrupt intracellular signaling pathways. These inhibitors have frequently been applied to sensorimotor cocultures or to central ganglia (Ghirardi et al., 1992; Nakanishi et al., 1997; Sutton et al., 2004). Blockade of synaptic facilitation resulting from application of the inhibitors has been previously interpreted as evidence for the involvement of specific presynaptic signaling pathways in facilitation; however, the results might actually reflect, at least in part, disruption of postsynaptic facilitatory pathways. Even in those instances in which disruption of facilitation has been produced by presynaptic injection of cell membrane-impermeant inhibitors, the available results do not permit one to distinguish between a direct modulatory effect of 5-HT (or a sensitizing stimulus) on a presynaptic cellular pathway and an indirect effect attributable to stimulation of a retrograde signal that actually originates in the motor neuron (Antonov et al., 2003; Roberts and Glanzman, 2003b).
Using data from the present experiments, we have estimated the relative contributions of presynaptic and postsynaptic processes to 5-HT-dependent facilitation of the sensorimotor synapse (Fig. 10A). Our estimate indicates that exclusively presynaptic facilitation has a rapid onset, peaking ≤5 min after the introduction of 5-HT. The putative presynaptic component of facilitation, although robust while 5-HT is present in the bath, declines rapidly after the drug is washed out. By 15 min after 5-HT washout, exclusively presynaptic processes no longer make a major contribution to facilitation. In contrast, the postsynaptic component has a slow onset but persists after washout of 5-HT, exhibiting little decrement for the next 40 min. Beginning at ∼5 min after washout of 5-HT, the postsynaptic component makes the dominant contribution to facilitation. For comparison, we also present data from the experiments of Chitwood et al. (2001); these data depict facilitation of the glutamate-evoked response in isolated motor neurons in cell culture. Both the time course and magnitude of this unambiguously “postsynaptic” form of facilitation parallel our estimate of the postsynaptic component of facilitation from the present experiments. This parallel reinforces our conclusion that persistent facilitation of the sensorimotor synapse, at least for the first hour after 5-HT application, is mediated primarily by postsynaptic processes. Our data suggest a model (Fig. 10B) in which purely presynaptic facilitatory mechanisms predominate during a very early stage of facilitation (≤5-10 min after the onset of 5-HT). Thereafter, postsynaptic facilitatory processes predominate.
Figure 10.

Cellular model for facilitation of the sensorimotor synapse. A, Time course of the presynaptic and postsynaptic processes that mediate 5-HT-induced facilitation of the sensorimotor synapse. The estimates of the duration of the presynaptic and postsynaptic processes in the graph are based on data from the present experiments. We derived a mathematical curve that represents the putatively presynaptic component of facilitation by averaging the mean normalized data for the experiments on cultured sensorimotor synapses that used BAPTA, heparin, 2-APB, dantrolene, and Botox B (data from Figs. 1 B, 2 B, 3B, 4 B, 5B). The data were then corrected for homosynaptic depression by subtracting the mean of the test-alone data for each trial from the corresponding experiment. Therefore, a value of 0% equals no facilitation. We then subtracted the data represented by the presynaptic curve (blue) from the average of the 5-HT control data in Figures 1 B, 2 B, 3B, 4 B, and 5B. The resulting curve (red) depicts our estimate of the postsynaptic contribution to facilitation. Data from the experiments of Chitwood et al. (2001) is also presented for comparison; these data depict facilitation of the glutamate-evoked response in isolated motor neurons in cell culture (green curve) [Chitwood et al., (2001), their Fig. 1 D]. The glutamate PSP data from Chitwood et al. (2001) were adjusted for comparison with the present data by subtracting 1.0 from each of the values. The resulting values were then multiplied by 100%. B, Model of synaptic facilitation during dishabituation in Aplysia. Stimuli that produce dishabituation of the withdrawal reflex, such as tail shock, activate facilitatory interneurons, some of which are serotonergic (Glanzman et al., 1989; Mackey et al., 1989; Marinesco and Carew, 2002). Release of 5-HT onto sensory and motor neurons within the CNS of Aplysia (Kistler et al., 1985; Marinesco and Carew, 2002; Zhang et al., 2003) causes rapid-onset presynaptic facilitation via processes that involve PKA and PKC [see Byrne and Kandel (1996) for details]. The strength of the presynaptic facilitatory processes wanes rapidly after washout of 5-HT. 5-HT also stimulates a slower, more prolonged, postsynaptic facilitatory process that involves release of Ca2+ from intracellular stores within the motor neuron via activation of IP3 receptors and RyRs. The rise in intracellular Ca2+ results in enhanced postsynaptic AMPA receptor function, possibly via exocytotic delivery of additional receptors to the postsynaptic membrane. Postsynaptic PKC activity may play a critical role in this modulation of AMPA receptor trafficking (see Discussion).
Byrne and Kandel (1996) have proposed that persistent activation of presynaptic PKC might account for persistent facilitation produced by prolonged applications (> 5 min) of 5-HT. Our data indicate that PKC activation attributable exclusively to presynaptic signaling pathways makes, at most, a minor contribution to prolonged facilitation of sensorimotor synapses. The data are consistent, however, with a role for postsynaptic PKC (Villareal et al., 2003) and for presynaptic PKC activated by a retrograde signal. Much of the previous data taken as evidence that activation of presynaptic PKC mediates persistent facilitation in Aplysia are actually ambiguous with respect to whether the critical site of PKC activity is presynaptic or postsynaptic. In particular, biochemical assays that demonstrate prolonged activation of PKC by 5-HT have used central ganglia that contain motor neurons and interneurons as well as sensory neurons (Sossin and Schwartz, 1992; Sossin et al., 1994), or the entire CNS (Sutton et al., 2004). Manseau et al. (2001) have reported that injection of mutant “dominant-negative” PKC constructs into sensory neurons blocks short-term facilitation (lasting <5 min) of sensorimotor synapses. This result does not contradict the present findings, which do not exclude a significant role for presynaptic mechanisms in very early facilitation (Fig. 10).
Tail shock is used as the unconditioned stimulus (US) in classical conditioning in Aplysia (Carew et al., 1981, 1983). Therefore, Ca2+-dependent enhancement of postsynaptic AMPA receptor function may partly mediate classical conditioning. Consistent with this idea, injection of BAPTA into the postsynaptic motor neuron has been shown to block conditioning-related enhancement of sensorimotor synapses (Murphy and Glanzman, 1996; Bao et al., 1998). Furthermore, injection of BAPTA into siphon motor neurons reduces conditioned enhancement of the SWR itself (Antonov et al., 2003). Previously, the site of convergence for the cellular processes triggered by US-induced release of 5-HT and those triggered by NMDA receptor activation during classical conditioning in Aplysia (Lin and Glanzman, 1994b; Murphy and Glanzman, 1997, 1999; Antonov et al., 2003) has been presumed to be the sensory neuron (Lechner and Byrne, 1998; Antonov et al., 2003). But the present data suggest a potential postsynaptic site of convergence for the effects of the conditioned stimulus-induced release of glutamate and the US-induced cellular processes. The postsynaptic interaction of 5-HT-dependent and NMDA receptor-dependent mechanisms may therefore make a crucial contribution to classical conditioning in Aplysia (Roberts and Glanzman, 2003b).
Footnotes
This work was supported by National Institutes of Health Grants NS29563 (D.L.G.) and MH19384 (A.C.R.). We thank Drs. Daniel Fulton, Marc Klein, Kelsey Martin, Felix Schweitzer, and William Wright for critical reading of previous versions of this manuscript and for helpful discussions. We also thank Dr. Barbara Ehrlich for insights into intracellular Ca2+ release.
Correspondence should be addressed to Dr. David L. Glanzman, Gonda (Goldschmied) Neuroscience and Genetics Research Center, 695 Young Drive South, Box 951761, University of California Los Angeles, Los Angeles, CA 90095-1761. E-mail: dglanzman@physci.ucla.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255623-15$15.00/0
Q.L. and A.C.R. contributed equally to this work
References
- Antonov I, Kandel ER, Hawkins RD (1999) The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex. J Neurosci 19: 10438-10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Antonova I, Kandel ER, Hawkins RD (2001) The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia J Neurosci 21: 6413-6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Antonova I, Kandel ER, Hawkins RD (2003) Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia Neuron 37: 135-147. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER (2000) A novel function for serotonin-mediated short-term facilitation in Aplysia: conversion of a transient, cell-wide homosynaptic Hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc Natl Acad Sci USA 97: 11581-11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J-X, Kandel ER, Hawkins RD (1997) Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science 275: 969-973. [DOI] [PubMed] [Google Scholar]
- Bao JX, Kandel ER, Hawkins RD (1998) Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci 18: 458-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ (1993) Inositol trisphosphate and calcium signalling. Nature 361: 315-325. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11-21. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL (2004) Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA 101: 4292-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER (1996) Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Walters ET, Kandel ER (1981) Classical conditioning in a simple withdrawal reflex in Aplysia californica J Neurosci 1: 1426-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, Kandel ER (1983) Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica Science 219: 397-400. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER (1974) A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia Proc Natl Acad Sci USA 71: 5004-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER (1976) Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia Science 194: 1176-1178. [DOI] [PubMed] [Google Scholar]
- Chitwood RA, Li Q, Glanzman DL (2001) Serotonin facilitates AMPA-type responses in isolated siphon motor neurons of Aplysia in culture. J Physiol (Lond) 534: 501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TE, Kaplan SW, Kandel ER, Hawkins RD (1997) A simplified preparation for relating cellular events to behavior: mechanisms contributing to habituation, dishabituation, and sensitization of the Aplysia gill-withdrawal reflex. J Neurosci 17: 2886-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P, Wu F, Schacher S (1999) Changes in functional glutamate receptors on a postsynaptic neuron accompany formation and maturation of an identified synapse. J Neurobiol 39: 237-248. [PubMed] [Google Scholar]
- Dale N, Kandel ER (1993) l-Glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci USA 90: 7163-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Clark GA, Kandel ER (1988) Parallel processing of short-term memory for sensitization in Aplysia J Neurobiol 19: 297-334. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N (1992) Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron 9: 479-489. [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Eis PS, Mullaney JM, Ebert CL, Gill DL (1988) Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem 263: 11075-11079. [PubMed] [Google Scholar]
- Glanzman DL (1994) Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture. J Neurobiol 25: 666-693. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER (1989) Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci 9: 4200-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S (1990) Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science 249: 799-802. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Cohen TE, Greene W, Kandel ER (1998) Relationships between dishabituation, sensitization, and inhibition of the gill- and siphon-withdrawal reflex in Aplysia californica: effects of response measure, test time, and training stimulus. Behav Neurosci 112: 24-38. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262-2267. [DOI] [PubMed] [Google Scholar]
- Hickie C, Walters ET (1995) Motor neuronal control of tail-directed and head-directed siphon responses in Aplysia californica J Neurophysiol 74: 307-321. [DOI] [PubMed] [Google Scholar]
- Hochner B, Klein M, Schacher S, Kandel ER (1986) Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia Proc Natl Acad Sci USA 83: 8794-8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JM, Bommert K, Charlton MP, Kistner A, Habermann E, Augustine GJ, Betz H (1994) A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron 12: 1269-1279. [DOI] [PubMed] [Google Scholar]
- Jin I, Hawkins RD (2003) Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at Aplysia sensory-motor neuron synapses. J Neurosci 23: 7288-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030-1038. [DOI] [PubMed] [Google Scholar]
- Kistler Jr HB, Hawkins RD, Koester J, Steinbusch HWM, Kandel ER, Schwartz JH (1985) Distribution of serotonin-immunoreactive cell bodies and processes in the abdominal ganglion of mature Aplysia J Neurosci 5: 72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Byrne JH (1998) New perspectives on classical conditioning: a synthesis of Hebbian and non-Hebbian mechanisms. Neuron 20: 355-358. [DOI] [PubMed] [Google Scholar]
- Li Q, Glanzman DL (2002) The role of IP3 receptor-mediated calcium release from postsynaptic intracellular stores in serotonin-induced facilitation of Aplysia sensorimotor synapses. Soc Neurosci Abstr 28: 376.8. [Google Scholar]
- Li Q, Villareal G, Glanzman DL (2001) The role of postsynaptic calcium and postsynaptic excocytosis in serotonin-induced facilitation of Aplysia sensorimotor synapses. Soc Neurosci Abstr 27: 2533. [Google Scholar]
- Liao X, Walters ET (2002) The use of elevated divalent cation solutions to isolate monosynaptic components of sensorimotor connections in Aplysia J Neurosci Methods 120: 45-54. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL (1994a) Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc R Soc Lond B Biol Sci 255: 113-118. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL (1994b) Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proc R Soc Lond B Biol Sci 255: 215-221. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Seudhof TC, Malenka RC, Nicoll RA (1998) Postsynaptic membrane fusion and long-term potentiation. Science 279: 399-403. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA (1999) Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24: 649-658. [DOI] [PubMed] [Google Scholar]
- Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL (2000) Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 287: 1647-1651. [DOI] [PubMed] [Google Scholar]
- Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, Gill DL (2001) Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem 276: 18888-18896. [DOI] [PubMed] [Google Scholar]
- Mackey SL, Glanzman DL, Small SA, Dyke AM, Kandel ER, Hawkins RD (1987) Tail shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc Natl Acad Sci USA 84: 8730-8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey SL, Kandel ER, Hawkins RD (1989) Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci 9: 4227-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. [DOI] [PubMed] [Google Scholar]
- Manseau F, Fan X, Hueftlein T, Sossin W, Castellucci VF (2001) Ca2+-independent protein kinase C Apl II mediates the serotonin-induced facilitation at depressed Aplysia sensorimotor synapses. J Neurosci 21: 1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus EA, Nolen TG, Rankin CH, Carew TJ (1988) Behavioral dissociation of dishabituation, sensitization, and inhibition in Aplysia Science 241: 210-213. [DOI] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ (2002) Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci 22: 2299-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122: 498-505. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL (1996) Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+ Proc Natl Acad Sci USA 93: 9931-9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL (1997) Mediation of classical conditioning in Aplysia californica by LTP of sensorimotor synapses. Science 278: 467-471. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL (1999) Cellular analog of differential classical conditioning in Aplysia: disruption by the NMDA receptor-antagonist dl-2-amino-5-phosphonovalerate. J Neurosci 19: 10595-10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Zhang F, Baxter DA, Eskin A, Byrne JH (1997) Role of calcium-calmodulin-dependent protein kinase II in modulation of sensorimotor synapses in Aplysia J Neurophysiol 78: 409-416. [DOI] [PubMed] [Google Scholar]
- Nicoll RA (2003) Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B Biol Sci 358: 721-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL (2003) 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 34: 97-108. [DOI] [PubMed] [Google Scholar]
- Poulain B, De Paiva A, Deloye F, Doussau F, Tauc L, Weller U, Dolly JO (1996) Differences in the multiple step process of inhibition of neurotransmitter release induced by tetanus toxin and botulinum neurotoxins type A and B at Aplysia synapses. Neuroscience 70: 567-576. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS (2001) Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol (Lond) 536: 3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle JC, Guet T, Cordi A, Lepagnol JM (1992) Competitive inhibition by NBQX of kainate/AMPA receptor currents and excitatory synaptic potentials: importance of 6-nitro substitution. Eur J Pharmacol 215: 237-244. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Carew TJ (1988) Dishabituation and sensitization emerge as separate processes during development in Aplysia J Neurosci 8: 197-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayport SG, Schacher S (1986) Synaptic plasticity in vitro: cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J Neurosci 6: 759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Glanzman DL (2001) Role of postsynaptic calcium and postsynaptic exocytosis in sensitization of the siphon-withdrawal reflex in Aplysia Soc Neurosci Abstr 27: 2533. [Google Scholar]
- Roberts AC, Glanzman DL (2002) The role of calcium release from postsynaptic intracellular calcium stores in behavioral sensitization of the siphon-withdrawal reflex in Aplysia Soc Neurosci Abstr 28: 376.5. [Google Scholar]
- Roberts AC, Glanzman DL (2003a) Behavioral sensitization of the siphon withdrawal reflex in Aplysia depends upon postsynaptic exocytosis. Soc Neurosci Abstr 29: 291.12. [Google Scholar]
- Roberts AC, Glanzman DL (2003b) Learning in Aplysia: looking at synaptic plasticity from both sides. Trends Neurosci 26: 662-670. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R (2005) Postsynaptic receptor trafficking underlying a form of associative learning. Science 308: 83-88. [DOI] [PubMed] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T (1990) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science 247: 571-574. [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ (2004) Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia Proc Natl Acad Sci USA 101: 7463-7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811-1816. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Schwartz JH (1992) Selective activation of Ca2+-activated PKCs in Aplysia neurons by 5-HT. J Neurosci 12: 1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS, Sacktor TC, Schwartz JH (1994) Persistent activation of protein kinase C during the development of long-term facilitation in Aplysia Learn Mem 1: 189-202. [PubMed] [Google Scholar]
- Sutton MA, Masters SE, Bagnall MW, Carew TJ (2001) Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia Neuron 31: 143-154. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ (2004) Intermediate-term memory for site-specific sensitization in Aplysia is maintained by persistent activation of protein kinase C. J Neurosci 24: 3600-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (1995) The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 375: 645-653. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R (2003) Experience strengthening transmission by driving AMPA receptors into synapses. Science 299: 1585-1588. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Broad LM (1998) Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci 19: 370-375. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF (1992) Contribution of polysynaptic pathways in the mediation and plasticity of Aplysia gill and siphon withdrawal reflex: evidence for differential modulation. J Neurosci 12: 3838-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF (1993) Excitatory amino acid neurotransmission at sensory-motor and interneuronal synapses of Aplysia californica J Neurophysiol 70: 1221-1230. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF (1995) Postsynaptic modifications in long-term facilitation in Aplysia: upregulation of excitatory amino acid receptors. J Neurosci 15: 1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal GJ, Fink A, Cai D, Glanzman DL (2003) Serotonin-dependent enhancement of the glutamate response in isolated siphon motor neurons: the potential role of postsynaptic protein kinase C in synaptic facilitation in Aplysia Soc Neurosci Abstr 29: 291.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER (1983a) Mechanoafferent neurons innervating tail of Aplysia I. Response properties and synaptic connections. J Neurophysiol 50: 1522-1542. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER (1983b) Mechanoafferent neurons innervating tail of Aplysia II. Modulation by sensitizing stimuli. J Neurophysiol 50: 1543-1559. [DOI] [PubMed] [Google Scholar]
- Xu L, Tripathy A, Pasek DA, Meissner G (1998) Potential for pharmacology of ryanodine receptor/calcium release channels. Ann NY Acad Sci 853: 130-148. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wainwright M, Byrne JH, Cleary LJ (2003) Quantitation of contacts among sensory, motor, and serotonergic neurons in the pedal ganglion of Aplysia Learn Mem 10: 387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wu F, Schacher S (1997) Site-specific and sensory neuron-dependent increases in postsynaptic glutamate sensitivity accompany serotonin-induced long-term facilitation at Aplysia sensorimotor synapses. J Neurosci 17: 4976-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]