Abstract
Brain-derived neurotrophic factor (BDNF) is best characterized for critical roles in neuronal survival, differentiation, and synaptic modulation mediated by the TrkB receptor tyrosine kinase. Developmentally regulated death signaling by BDNF has also been demonstrated via activation of p75NTR. Because recent studies suggest that proNGF, the precursor form of NGF, is more active than mature NGF in inducing apoptosis after binding to p75NTR and a coreceptor, sortilin, we asked whether the precursor of BDNF (proBDNF) is also a proapoptotic ligand in the nervous system. proBDNF is secreted by cultured neurons, and recombinant proBDNF binds to sortilin. In sympathetic neurons coexpressing sortilin and p75NTR, we found that proBDNF is an apoptotic ligand that induces death at subnanomolar concentrations. In contrast, mature BDNF, but not proBDNF, is effective in inducing TrkB phosphorylation. proBDNF effects are dependent on cellular coexpression of both p75NTR and sortilin, because neurons deficient in p75NTR are resistant to proBDNF-induced apoptosis, and competitive antagonists of sortilin block sympathetic neuron death. Moreover, addition of preformed complexes of soluble sortilin and proBDNF failed to induce apoptosis of cells coexpressing both sortilin and p75NTR, suggesting that interaction of proBDNF with both receptors on the cell surface is required to initiate cell death. Together with our past findings, these data suggest that the neurotrophin family is capable of modulating diverse biological processes via differential processing of the proneurotrophins.
Keywords: proBDNF, apoptosis, p75 receptor, sortilin, neuron, neurotrophin
Introduction
Brain-derived neurotrophic factor (BDNF) is the most functionally diverse member of the neurotrophin family. In addition to critical developmental actions that promote the differentiation and migration of peripheral and central neurons and glia (McAllister et al., 1997; Cosgaya et al., 2002; Carter et al., 2003), BDNF exhibits survival and synaptic actions in adulthood with distinct behavioral effects on memory, food intake and energy balance, and mood (Lyons et al., 1999; Kernie et al., 2000; Hariri et al., 2003; Xu et al., 2003; Pang et al., 2004). Indeed, even modest impairment in BDNF secretion, which occurs secondary to a fairly prevalent polymorphism in humans, has been correlated with neuropsychiatric conditions and abnormalities in episodic memory (Egan et al., 2003; Jiang et al., 2005). Although BDNF binds to both the TrkB receptor tyrosine kinase and to p75NTR, a member of the tumor necrosis factor receptor family, many of the effects of BDNF have been attributed to TrkB activation (for review, see Huang and Reichardt, 2001). However, distinct actions of BDNF mediated by p75NTR activation include promotion of myelination (Cosgaya et al., 2002), neuronal migration (Carter et al., 2003), neuronal process retraction (Cahoon-Metzger et al., 2001; Gehler et al., 2004), and neuronal apoptosis (Bamji et al., 1998; Boyd and Gordon, 2002; Troy et al., 2002).
All neurotrophins are synthesized as precursor forms (proneurotrophins), which dimerize after translation (Kolbeck et al., 1994). Proneurotrophins, including the precursor of BDNF (proBDNF), may be secreted from cells (Heymach and Shooter, 1995; Lee et al., 2001; Mowla et al., 2001; Chen et al., 2004) or cleaved intracellularly by furin or proconvertases to yield C-terminal mature neurotrophin dimers. To date, only mature BDNF is considered as the biologically active form that elicits TrkB-dependent signaling (Huang and Reichardt, 2001). Our recent studies indicate that the precursor form of a neurotrophin family member, proNGF, can be secreted by cells and act via a dual receptor system of p75NTR and the type I transmembrane protein sortilin to mediate cell apoptosis (Lee et al., 2001; Nykjaer et al., 2004). proNGF interacts via its pro-domain with sortilin, whereas interactions with p75NTR are most likely mediated by the mature domain. However, binding of proNGF to both receptors on the cell surface is necessary to generate high-affinity sites and mediate apoptosis (Nykjaer et al., 2004).
Although the pro-domains of BDNF and NGF exhibit only regions of similarity (Suter et al., 1991), previous studies support the hypothesis that local BDNF can promote neuronal apoptosis in vivo (Bamji et al., 1998; Kohn et al., 1999; Boyd and Gordon, 2002). When overexpressed, proBDNF is released from neuroendocrine cells (Chen et al., 2004). However, the significance of proBDNF secretion has not been directly examined. In the present study, we thus characterize the properties of proBDNF in terms of proteinase sensitivity, receptor requirements, as well as biological effects on neuronal and glial populations. Our findings reveal a role for proBDNF as a proapoptotic ligand for sympathetic neurons and support the hypothesis that the diversity of neurotrophin functions might in part be modulated by regulated release of the mature versus pro-isoforms in the nervous system.
Materials and Methods
Cell cultures. Human embryonic kidney (HEK) 293 cells, 293T cells, and 293 cells stably expressing human sortilin (Nykjaer et al., 2004) were maintained in DMEM supplemented with 10% fetal bovine serum, 1% glutamine, 1% penicillin/streptomycin, and 1% pyruvate. TrkB-expressing PC12 cells, a generous gift from P. Tsoulfas (University of Miami, Miami, FL) were maintained in DMEM, 10% calf serum, 5% horse serum, 1% glutamine, 1% penicillin/streptomycin, 1% pyruvate, and 0.5 μg/ml G418.
Generation of viral vectors and recombinant proteins. Murine proBDNF was amplified by reverse transcription-PCR from murine BDNF exon V (kindly provided by L. Tessarollo, National Cancer Institute, Frederick, MD) using primers to introduce a hexahistidine tag, stop codon, and XhoI site at the 3′terminus and a 5′ BglII site. In parallel, constructs were generated lacking the hexahistidine tag. Point mutation of RR (amino acids 129 and 130) to AA was performed using PCR-based mutagenesis to generate furin-resistant proBDNF. Constructs encoding native or furin-resistant His-tagged BDNF cDNAs, subcloned in pcDNA3.1 (pcDNA BDNF-His or pcDNA proBDNF-His, respectively), were bidirectionally sequenced. Recombinant adenovirus (serotype 5) encoding native or furin-resistant proBDNF with or without a C-terminal His tag, was propagated, purified by ultracentrifugation, and documented to be replication incompetent in the Weill-Cornell Gene Therapy Core Facility. Additionally, recombinant baculoviral expression vectors were generated encoding native or furin-resistant His-tagged BDNF using the Bac-to-Bac baculovirus expression system. Baculoviral stocks were amplified and propagated using Spodoptera frugiperda (Sf9) cells cultured in Sf-900 II SFM for 72 h, whereas High Five cells cultured in Express Five SFM were used for protein purification. All baculovirus expression system-related reagents and cells were from Invitrogen (San Diego, CA).
An adenovirus expressing the soluble ectodomain of human sortilin was generated (amino acids 33-725) in PAC-CMVpLqA. The titer of the purified recombinant adenovirus was ∼5 × 108 pfu/ml and was used to infect 293 cells at a multiplicity of infection (MOI) of 5 for 2 h. Cells were then washed and incubated in serum-free DMEM containing insulin, transferrin, and selenium, before harvest of media at 48 h after infection.
Purification of recombinant BDNF. Three approaches were used to generate furin-resistant His-tagged proBDNF or mature BDNF. In some experiments, media from adenovirus-infected 293 cells or baculovirus-infected High Five cells were used as sources (see above, Generation of viral vectors and recombinant proteins). Alternatively, HEK 293 cells were transfected with pcDNA BDNF-His or proBDNF-His, and stable clones were generated after selection in media containing 0.5 μg/ml G418. Media from cells cultured in DMEM, 0.1% FBS, 1% glutamine, 1% penicillin/streptomycin, and 1% pyruvate for 2-3 d were harvested for purification.
To purify proBDNF or mature BDNF lacking a His-tag, conditioned media from adenoviral-infected cells were purified using two successive cation exchange columns, following a procedure modified from Petrides and Shooter (1986). Briefly, conditioned media were acidified to pH 4, and proteinase inhibitors were added (1 mm PMSF, 1 mm 1, 10-phenanthroline, 10 μg/ml leupeptin, and 1 μg/ml aprotonin). Media were then loaded onto a CM-52 column (Whatman, Maidstone, UK) and washed with 50 mm Na-acetate, pH 4.0, and 150 mm NaCl. proBDNF was eluted with 50 mm Tris-Cl, pH 8.4, and 400 mm NaCl, and mature BDNF was eluted with 50 mm Tris-Cl, pH 9.0, and 400 mm NaCl. Fractions containing proBDNF or BDNF were pooled and diluted 1:1 with 50 mm Na-acetate, pH 4.0, and then rechromatographed on a Resource S column (Amersham Biosciences, Arlington Heights, IL) and eluted with 50 mm Tris-Cl, pH 8.4, 400 mm NaCl (for proBDNF) or 50 mm Tris-Cl, pH 9.0, and 400 mm NaCl (for mature BDNF). Chromatography was then performed using a Superdex 200 gel filtration column (Amersham Biosciences) pre-equilibrated in 25 mm Tris-Cl, pH 7.0, and 200 mm NaCl. Protein peaks were collected, resolved by SDS-PAGE, and analyzed by Western blotting using anti-mature BDNF antibody (SC-546; Santa Cruz Biotechnology, Santa Cruz, CA).
His-tagged proBDNF or His-tagged native BDNF was purified from conditioned media of 293 cells stably expressing these cDNAs or from 293 cells infected with adenovirus encoding these cDNAs. Proteinase inhibitors and NaCl were added (final concentration, 300 mm NaCl), and media were incubated with SwellGel Nickel Chelated Discs (Pierce, Rockford, IL) at 4°C for 18 h. After washing in 50 mm Na-phosphate, pH 7.8, 300 mm NaCl, and 25 mm imidazole, proBDNF-His was eluted with 50 mm Na-phosphate, pH 7.8, 300 mm NaCl, and 350 mm imidazole, and mature BDNF-His was eluted with 50 mm Na-phosphate, pH 8.4, and 500 mm imidazole. His-tagged proBDNF was also purified from conditioned media of High Five cells infected with recombinant baculovirus at an MOI of 5, at 66 h after infection. Proteinase inhibitors were added, and media were subjected to tangential flow dialysis (Minimate TFF Capsule; Pall Corporation, East Hills, NY) against PBS, followed by purification using HisTrap HP column chromatography (Amersham Biosciences), with 20 mm Na-phosphate, pH 7.0, 500 mm NaCl, and 1 m imidazole to elute proBDNF-His.
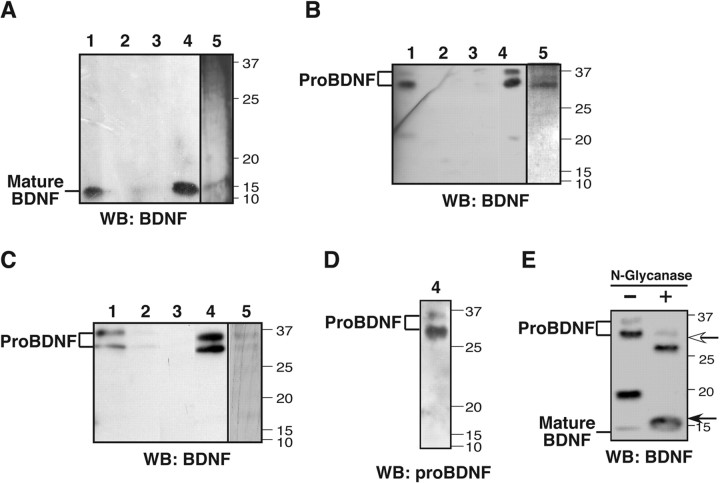
Recombinant proteins were dialyzed against HBSS (Invitrogen) and stored at -80°C until use. Unless otherwise stated, imidazole-eluted mature BDNF or proBDNF that corresponded to fraction 4 of Figure 2 A-C were used for additional experiments. Purification was monitored by Western blot analysis using anti-mature BDNF and by silver staining.
Figure 2.
Purification of proBDNF and mature BDNF. A, B, Recombinant adenovirus encoding BDNF-His (A) or proBDNF-His (B) was used to infect HEK 293 cells, and conditioned media were purified using Ni-chromatography. Lane 1, Conditioned media before chromatography. Lane 2, Column flow-through. Lane 3, Column wash. Lane 4, Column eluate. Lane 5, Column eluate subjected to silver stain analysis. C, Purification of proBDNF-His from media of baculovirus-infected insect cells. Lane 1, Conditioned media before chromatography. Lane 2, Column flow-through. Lane 3, Column wash. Lane 4, Column eluate. Lane 5, Column eluate subjected to silver stain analysis. D, ProBDNF-His column eluates (from lane 4, B) were reprobed with antibody specific for the pro-domain of BDNF. E, Medium from 293 cells expressing proBDNF-His was harvested and purified using Ni-chromatography. Purified proteins were incubated without or with N-glycanase (25 mU/ml) at 20°C for 12 h. Reactions were terminated by addition of SDS sample buffer and boiling. Proteins were separated by SDS-PAGE and Western blotted with anti-mature BDNF. When applicable, positions of proBDNF, mature BDNF, and their deglycosylated forms are indicated by arrows. WB, Western blot.
Stability and proteinase sensitivity assay of BDNF. To examine the effects of secreted sortilin on proteinase sensitivity of proBDNF, conditioned media from cells infected with adeno-soluble sortilin or adeno-green fluorescent protein (GFP) were mixed with media from stable 293 clones secreting proBDNF and then incubated with plasmin (0-4 μg/ml; American Diagnostica, Greenwich, CT) for 1 h at 37°C. Reactions were terminated by addition of SDS sample buffer, followed by SDS-PAGE and anti-BDNF Western blotting.
Deglycosylation of proBDNF. Media containing secreted proBDNF-His were incubated with N-glycanase (25 mU/ml; ProZyme, San Leandro, CA) for 18 h at 20°C. Reactions were terminated by addition of SDS sample buffer, followed by anti-BDNF immunoblotting analysis.
TrkB activation and neuritogenesis assay. TrkB-expressing PC12 cells were treated with 1 nm proBDNF, 1 nm mature BDNF, or diluent control. Ten minutes after buffer treatment, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer. Trk was immunoprecipitated with a polyclonal anti-Trk antibody (SC-139; Santa Cruz Biotechnology), followed by Western blotting with a monoclonal anti-Trk antibody (SC-7268; Santa Cruz Biotechnology) or polyclonal anti-phospho-Trk (pTyr490) antiserum (Cell Signaling Technology, Beverly, MA).
ProBDNF-His and BDNF-His were added to replicate cultures of TrkB-expressing PC12 cells at the indicated concentrations in DMEM containing 0.1% FBS. Neurites more than two cell body diameters in length were scored as positive 48 h later. At least 100 cells were counted from randomly chosen fields per culture conditions, by an observer blinded to the treatments.
Surface plasmon resonance analysis. The luminal domain of human sortilin was expressed in Chinese hamster ovary cells and purified as described previously (Munck Petersen et al., 1999). The surface plasmon resonance (SPR) analysis was performed essentially as described previously (Nykjaer et al., 2004). Briefly, the receptor was immobilized on a CM5 chip (BIAcore, Neuchātel, Switzerland) at a concentration of 10 μg/ml, and remaining binding sites were blocked with 1 m ethanolamine, pH 8.5. Sample and running buffer was 10 mm HEPES, pH 7.4, 150 mm (NH4)2SO4, 1.5 mm CaCl2, 1 mm EGTA, and 0.005% Tween 20. Regeneration of the sensor chip after each analysis cycle was performed with 10 mm glycine-HCl, pH 4.0, 500 mm NaCl, and 20 mm EDTA. The BIAcore signal is expressed in relative response units (i.e., the difference in response between protein and a control flow channel). Kinetic parameters were determined using BIAevaluation 3.1 software.
Primary cultures and assessment of apoptosis. Dissociated superior cervical ganglion (SCG) neurons were prepared from either postnatal day 0 (P0) or P1 rats or from p75NTR-null mice and their wild-type littermates (Lee et al., 1992). Neurons were plated on laminin-coated slides and maintained for 5 d in NGF as described previously (Bamji et al., 1998; Nykjaer et al., 2004). On the day of the experiment, replicate cultures were rinsed five times with NGF-free medium and treated with mature BDNF, proBDNF, or equivalent quantities of diluent. Where appropriate, parallel cultures were concomitantly treated with 20 μm neurotensin. After 36 h, SCG cultures were processed for terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) analysis (Roche Molecular Biochemicals, Basel, Switzerland) and counterstained with anti-neuronal-specific β-tubulin (Tuj1; Covance, Berkeley, CA) and 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei. TUNEL-positive neurons were scored blinded as to condition by the observer, and at least 100 cells were counted for each culture condition.
The ability of proBDNF to induce apoptosis in p75NTR-expressing vascular smooth muscle cells was assessed by TUNEL as described previously (Lee et al., 2001). When appropriate, statistical significance was determined by Student's t test.
Rat primary Schwann cells were plated on poly-l-lysine-coated plates in DMEM supplemented with 10% FCS, glutamine, penicillin and streptomycin, and forskolin and bovine pituitary extract as described previously (Nykjaer et al., 2004). Cells were transfected with pcDNA encoding human sortilin or pcDNA alone using calcium phosphate. Forty-eight hours after transfection, cells were treated with mature BDNF, proBDNF, proBDNF complexed to soluble sortilin, or diluent alone. After 18 h, cells were fixed, and immunofluorescence detection was performed with a mouse anti-sortilin antibody (catalog #612101; BD Biosciences Transduction Laboratories, Lexington, KY) and rhodamine-conjugated goat anti-mouse IgG, followed by DAPI staining and TUNEL analysis. At least 1000 cells per condition were counted in a blinded manner, and results are representative of two independent experiments.
Cortical neurons cultures and endogenous proBDNF immunoprecipitation. Timed-pregnant Sprague Dawley rats were purchased from Taconic Farms (Germantown, NY). Dissociated embryonic day 18 (E18) cortical neurons were plated on poly-l-lysine-coated 150 mm tissue culture plates and maintained in Neurobasal medium (containing 1× B27 supplement and 0.5 μm glutamine). On 3 d in vitro (DIV), cultures were treated with 5-fluorodeoxy-uridine for 2 d to eliminate contaminating non-neuronal cells. Anti-proBDNF immunoprecipitation experiments were performed on DIV 7 from neuronal detergent lysates or from conditioned media as indicated. To prevent proteolytic degradation of secreted proBDNF, a cell-impermeant α2 anti-plasmin inhibitor (Calbiochem, La Jolla, CA) was added to the cultures for the last 48 h. Harvested media were supplemented with proteinase inhibitors and immunoprecipitated with agarose-conjugated anti-proBDNF antiserum (Pang et al., 2004) at 4°C overnight. Immunoprecipitates were washed five times with RIPA buffer before Western blotting with an anti-BDNF antibody (Santa Cruz Biotechnology).
Results
Several recent studies demonstrate that proNGF effectively activates p75NTR-dependent cellular events, including apoptosis and chemotaxis, and that it may play a causal role in neuronal or oligodendroglial death in the injured brain and spinal cord (Beattie et al., 2002; Shonukan et al., 2003; Harrington et al., 2004; Nykjaer et al., 2004). In contrast, although a role for the prodomain of proBDNF has been characterized in terms of intracellular trafficking and mature BDNF secretion (Egan et al., 2003; Chen et al., 2004), proBDNF itself has not been considered an active ligand. We therefore sought to determine whether endogenous proBDNF is released from neurons and, if so, its physiological effects in the nervous system.
Endogenous ProBDNF secretion from cortical neurons
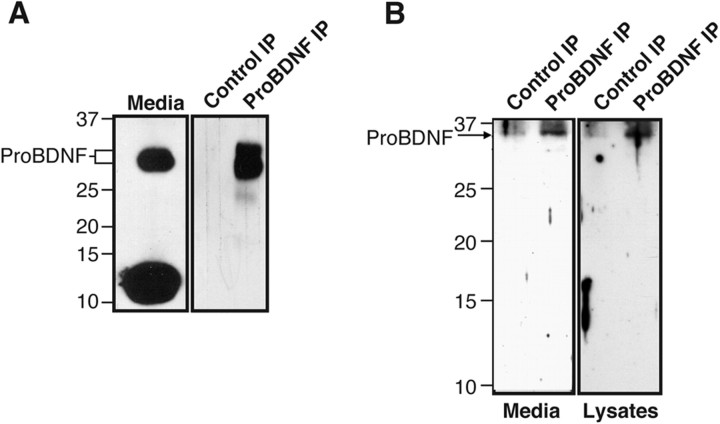
A recent work (Pang et al., 2004) reported that proBDNF is cleaved by the extracellular proteinase plasmin, resulting in its conversion to mature BDNF, which is well know to modulate synaptic plasticity (Patterson et al., 2001). This study suggests that proBDNF might be released at the hippocampal synapse. To directly examine whether endogenous proBDNF is released from CNS neurons, conditioned media from cultured cortical neurons were immunoprecipitated with an antiserum that was generated against the pro-domain of proBDNF [referred to as anti-proBDNF (Beattie et al., 2002)]. HEK 293 cells infected with adenoviral vector harboring full-length wild-type BDNF cDNA secreted both mature BDNF and proBDNF at an ∼4:1 ratio. To document antibody specificity, immunoprecipitation of conditioned media from these cells using the anti-proBDNF antibody (Beattie et al., 2002) followed by anti-BDNF Western blotting revealed that only the higher molecular weight species is recognized by the proBDNF antibody (Fig. 1A).
Figure 1.
proBDNF is released from cultured cortical neurons. A, Specificity of proBDNF antisera. Media from 293 cells infected with adeno-BDNF were incubated with proBDNF-specific IgY coupled to Sepharose (proBDNF IP) or comparably treated Sepharose in which IgY was not included in the coupling reaction (Control IP). Immunoprecipitated proteins were separated by SDS-PAGE and Western blotted with anti-mature BDNF. B, Conditioned media (40 ml from 1 × 108 cells) and detergent lysates (2 mg; prepared in RIPA buffer) from cultured cortical neurons were immunoprecipitated with anti-proBDNF-coupled Sepharose beads. Immunoprecipitated proteins were separated by SDS-PAGE and Western blotted with anti-mature BDNF antiserum. The positions of proBDNF along with molecular weight markers (numbers in kilodaltons) are indicated. IP, Immunoprecipitation.
Next, cellular lysates or conditioned media from replicate cultures of E18 cortical neurons were immunoprecipitated with the anti-proBDNF antiserum. Western blotting analysis using an anti-BDNF antibody (which recognizes both proBDNF and mature BDNF) indicated that proBDNF is released from cultured cortical neurons (Fig. 1B).
Purification of recombinant proBDNF
To evaluate the biochemical properties of secreted proBDNF, point mutation of the dibasic site used by furin or proconvertases (Mowla et al., 2001) was generated to impair intracellular proteolysis, and an in-frame C-terminal His-tag was added to facilitate purification using Ni-bead chromatography. Using recombinant adenovirus encoding this construct, furin-resistant, His-tagged proBDNF was produced and secreted by 293 cells. In parallel cultures, media were collected from 293 cells infected with recombinant adenovirus encoding native BDNF with a C-terminal His-tag. ProBDNF-His and native BDNF-His were purified using Ni-bead chromatography. By Western blot analysis using antibodies against the mature domain, mature BDNF was detectable with the predicted molecular mass of 13 kDa (Fig. 2A), whereas purified proBDNF exhibited immunoreactivity of predominantly 34 and 32 kDa, as well as a less prominent 19 kDa product (Fig. 2B). ProBDNF purified from the media of baculovirus-infected insect cells yielded similar 34 and 32 kDa species (Fig. 2C). Furthermore, the 34 and 32 kDa products were recognized by an antibody specific for the prodomain of BDNF (Fig. 2D), indicating that these two higher molecular species were indeed proBDNF. N-glycanase treatment of purified proBDNF resulted in all three species shifting to lower molecular mass entities (to 32, 30, and 16 kDa), suggesting that these secreted products had been N-glycosylated during synthesis (Fig. 2E). These results suggest that the 19 kDa species retains a used glycosylation site. This observation, and the identification of an abundant proteolytic product of BDNF encoding the mature domain with a 19 amino acid N-terminal extension (Kolbeck et al., 1994), suggest that the observed 19 kDa form may correspond to this proteolytic product.
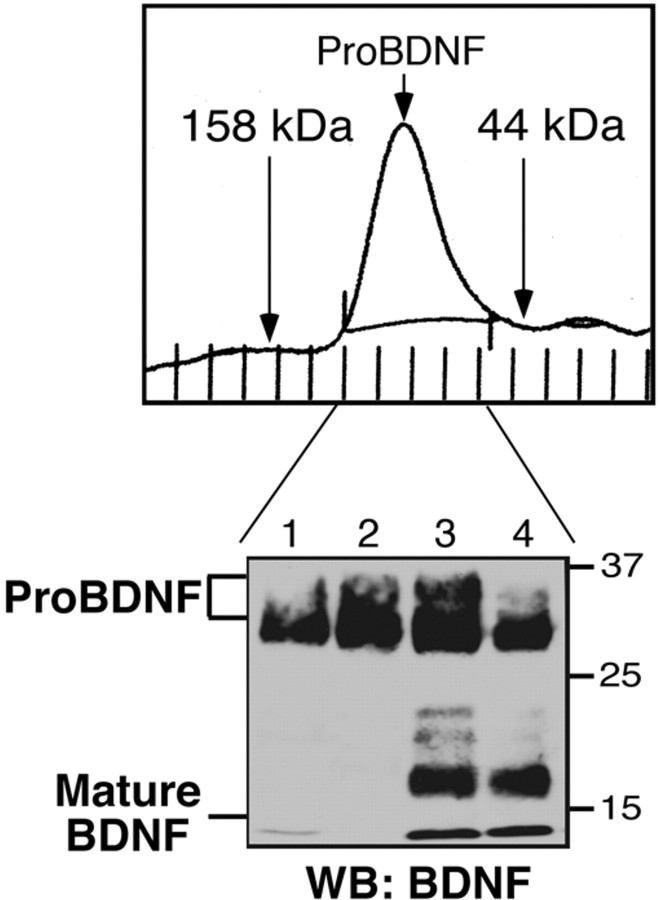
To assess the monomeric or dimeric nature of secreted proBDNF, gel filtration analysis was performed. As shown in Figure 3, proBDNF elutes as a peak of ∼60-70 kDa. Western blot analysis of column fractions demonstrated that this peak consists of 32 or 34 kDa dimers (lanes 1, 2), as well as lower molecular mass species consisting of dimers of intact and proteolyzed proBDNF (dimers of 32 and 19 kDa; lanes 3, 4). Notably, no significant peaks with molecular masses of <40 kDa (indicative of proBDNF monomers) were observed by chromatographic separation, consistent with previous analyses of proneurotrophin dimerization (Kolbeck et al., 1994; Heymach and Shooter, 1995; Mowla et al., 2001; Rattenholl et al., 2001).
Figure 3.
Secreted proBDNF is dimeric. ProBDNF (non-His-tag) from recombinant adenovirus-infected cell media was purified using cation-exchange chromatography as described. Purified proBDNF was fractionated by Superdex 200 gel filtration in 25 mm Tris-Cl, pH 7.0, and 200 mm NaCl. The indicated fractions were analyzed by Western blotting with anti-BDNF antiserum. Top, Chromatogram of proBDNF elution profile. Native molecular weight standards are indicated by arrows. The peak of eluted proBDNF (arrow) has a calculated molecular weight of 68 kDa. Bottom, Western blot (WB) of eluates containing proBDNF.
Characterization of sortilin-proBDNF interactions
Our recent studies have identified the sortilin Vps10p receptor as a coreceptor with p75NTR that is required to mediate proNGF binding (Nykjaer et al., 2004) via a direct interaction of the prodomain of NGF with sortilin. Although the pro-domains of proNGF and proBDNF have limited sequence identity, areas of similarity have been proposed (Suter et al., 1991). Therefore, using SPR analysis, we examined binding of proBDNF to the luminal/extracellular domain of sortilin. ProBDNF binds to soluble sortilin with a predicted affinity of ∼0.4 nm (Fig. 4A). The binding of proBDNF to sortilin was completely inhibited in the presence of sortilin antagonist neurotensin. In parallel conditions, proNGF binds to soluble sortilin with a predicted affinity of ∼5 nm, comparable with previous results (Nykjaer et al., 2004) (data not shown). However, the binding of proBDNF and proNGF to p75NTR-Fc fusion protein were more similar, with predicted affinities of 20 or 15 nm, respectively. The results obtained with proNGF are comparable with those published previously (Nykjaer et al., 2004).
Figure 4.
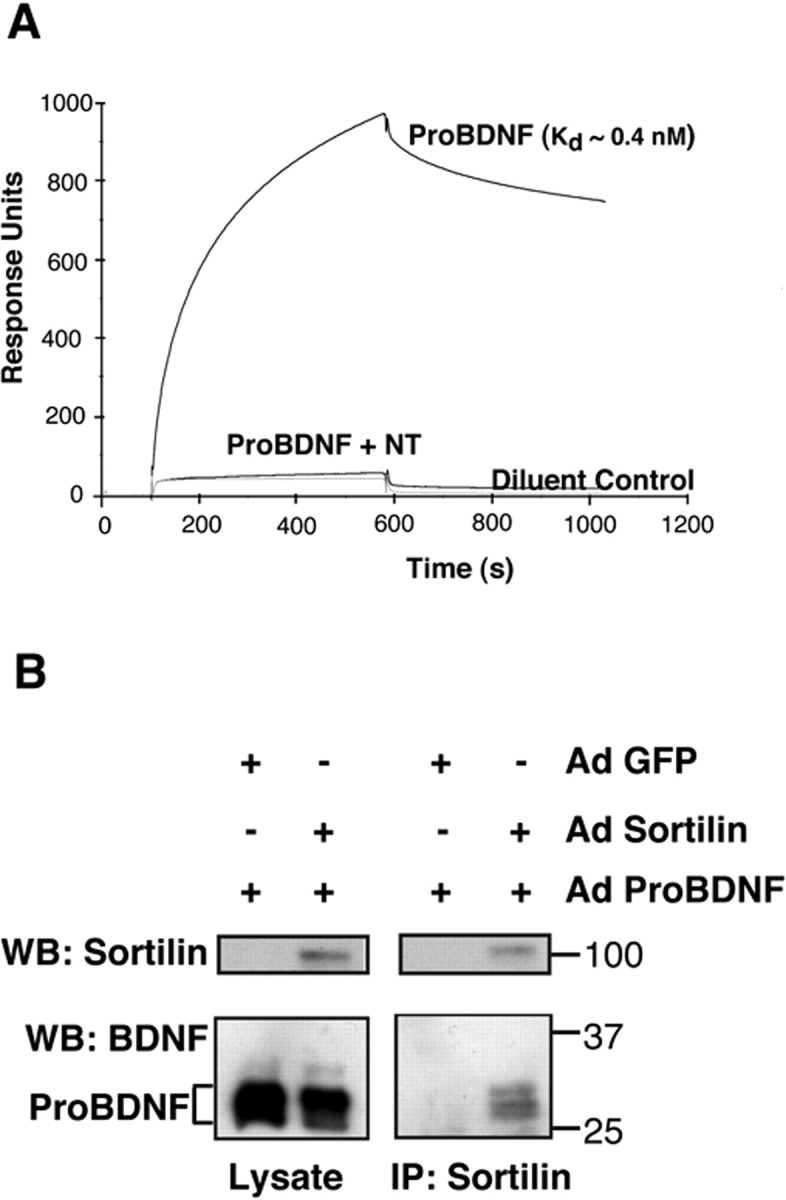
ProBDNF binds to sortilin. A, Surface plasmon resonance analysis of proBDNF binding to sortilin. Purified extracellular domain of sortilin was immobilized on a biosensor chip (120 fmol/mm2), followed by incubation with proBDNF purified from baculovirus-infected insect cell media at the indicated concentrations. The on and off rates were recorded, and the calculated Kd value is indicated. Inhibition of proBDNF (10 nm) binding to sortilin by 10 μm neurotensin (NT) is indicated, as are the response units obtained with diluent alone. B, 293 cells stably expressing proBDNF-His were infected with adenovirus (Ad) expressing GFP or soluble sortilin. At 2 d after infection, cell lysates were harvested and subjected to immunoprecipitation (IP) with sortilin antisera. Immunoprecipitated proteins were resolved by SDS-PAGE and Western blotted with anti-sortilin (top panels) or anti-mature BDNF (bottom panels) antisera. Cell lysates were resolved and Western blotted in parallel. WB, Western blot.
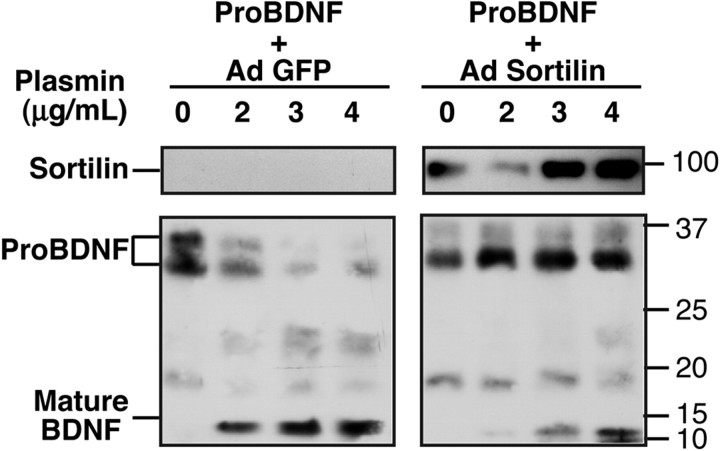
To further probe the interaction between proBDNF and sortilin, secreted proBDNF was incubated with soluble secreted luminal/extracellular domain of sortilin to assess the stability of this interaction. Complexes composed of proBDNF-His and soluble sortilin were stable to exposure to high ionic strength (0.5 m NaCl) and can be coeluted after Nichromatography (see Fig. 8A below). Furthermore, complexes of soluble sortilin and proBDNF can be immunoprecipitated using antisera to the extracellular domain of sortilin (Fig. 4B). Last, interactions of proBDNF with soluble sortilin appear to protect proBDNF from proteolysis or degradation. During incubation with plasmin, proBDNF is cleaved to 24 and 13 kDa products over 4 h, whereas proBDNF-sortilin complexes exhibited reduced degradation of proBDNF under comparable conditions (Fig. 5). These studies suggest that the interaction between the proBDNF and sortilin receptor is stable and may protect the more readily proteolyzed pro-domain of proBDNF from cleavage.
Figure 8.
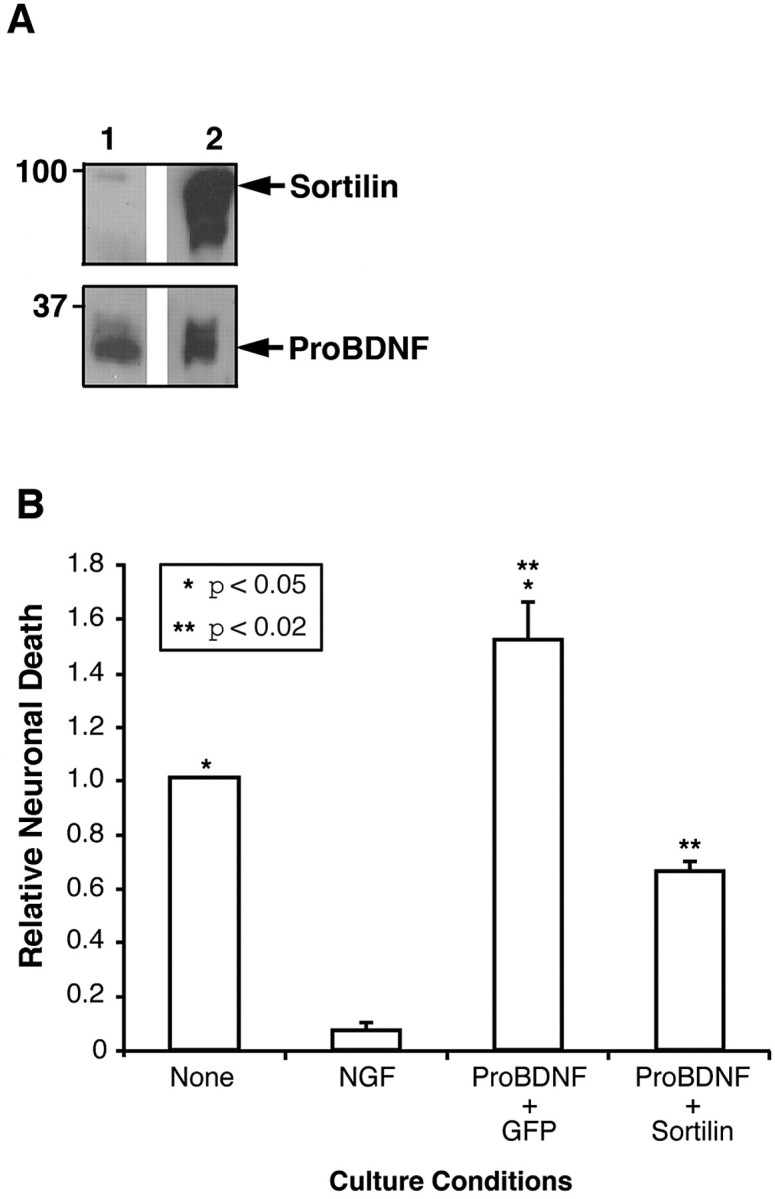
Effect of proBDNF and the proBDNF-soluble sortilin complex on neuronal apoptosis. Conditioned media from 293 cells stably expressing proBDNF-His were incubated with conditioned media from cells infected with adenovirus encoding GFP (lane 1) or soluble sortilin (lane 2) for 4 h at 4°C. ProBDNF and proBDNF-sortilin complexes were purified by Ni-chromatography. A, Eluates were resolved by SDS-PAGE and Western blotted with anti-sortilin (top) or anti-BDNF (bottom) antisera. The positions of soluble sortilin and proBDNF are indicated on the right (arrows). B, Rat SCG neurons were treated with the indicated ligands and processed for TUNEL analysis as described in Figure 7B. Error bars indicate SEM from three independently conducted experiments. When applicable, statistical significance between the indicated pairs of samples was presented.
Figure 5.
Secreted sortilin reduces degradation of proBDNF. Conditioned media from 293 cells infected with adenovirus (Ad) expressing GFP or soluble sortilin were mixed (4 h at 4°C) with conditioned media from cells expressing proBDNF-His. These mixtures were then incubated with plasmin at the indicated concentration for 1 h, followed by SDS-PAGE and Western blot analysis using anti-sortilin (top panels) or anti-BDNF (bottom panels) antisera.
In vivo receptor requirement for proBDNF actions
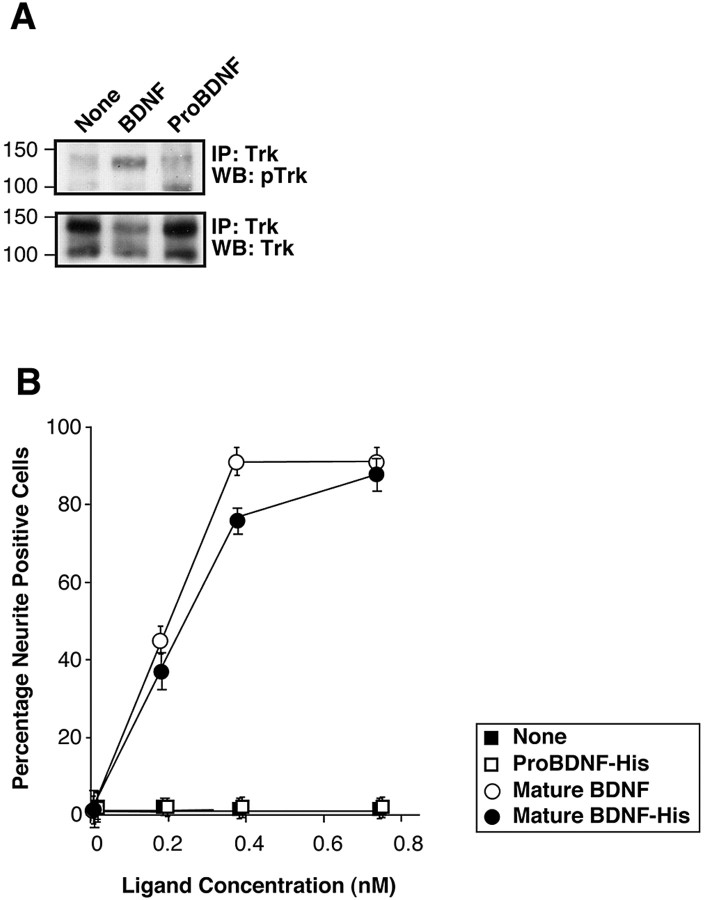
To assess the biological actions of proBDNF, we first examined whether recombinant proBDNF retains the ability to activate the TrkB receptor tyrosine kinase. Using TrkB-expressing PC12 cells as a model for TrkB-specific differentiative signaling, we found that, unlike mature BDNF, proBDNF does not effectively activate TrkB, as assessed by tyrosine phosphorylation of the receptor (Fig. 6A). This inability of proBDNF to induce the phosphorylation of TrkB also correlates with a lack of neuritogenic effect on TrkB-PC12 cells, whereas mature BDNF is effective at subnanomolar concentrations (Fig. 6B).
Figure 6.
Mature BDNF is more effective than proBDNF in eliciting TrkB activation. Secreted proBDNF-His or mature BDNF-His was purified by Ni-chromatography. A, TrkB-expressing PC12 cells were cultured in serum-free media for 6 h, followed by treatment with 1 nm mature BDNF, 1 nm proBDNF, or diluent control (None) as indicated for 10 min. Cell lysates were prepared and immunoprecipitated (IP) with anti-Trk antisera, followed by SDS-PAGE, and Western blotted (WB) with the indicated antisera. B, TrkB-expressing PC12 cells were treated, or not, with proBDNF-His, mature BDNF-His, or commercial mature BDNF in media containing 1% serum. Forty-eight hours later, the percentages of neurite-bearing cells were scored by an observer blinded to the treatment conditions. Error bars indicate the values obtained from two independent experiments.
Next, we sought to evaluate whether proBDNF might elicit p75NTR-dependent cellular events. To evaluate the relative activity of mature BDNF and proBDNF in inducing p75NTR-mediated apoptosis, vascular smooth muscle cells expressing both sortilin and p75NTR were used. These cells have been characterized previously as susceptible to apoptosis to mature BDNF in a dose-dependent manner (Wang et al., 2000). Cells were treated with varying concentrations of recombinant, purified mature BDNF or recombinant purified proBDNF and subjected to TUNEL analysis (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Whereas concentrations of 2 nm mature BDNF were required to induce a fourfold increase in TUNEL detection, parallel cultures treated with 0.1-0.2 nm proBDNF exhibited similar increases in TUNEL positivity.
ProBDNF-induced neuronal apoptosis requires p75NTR and sortilin
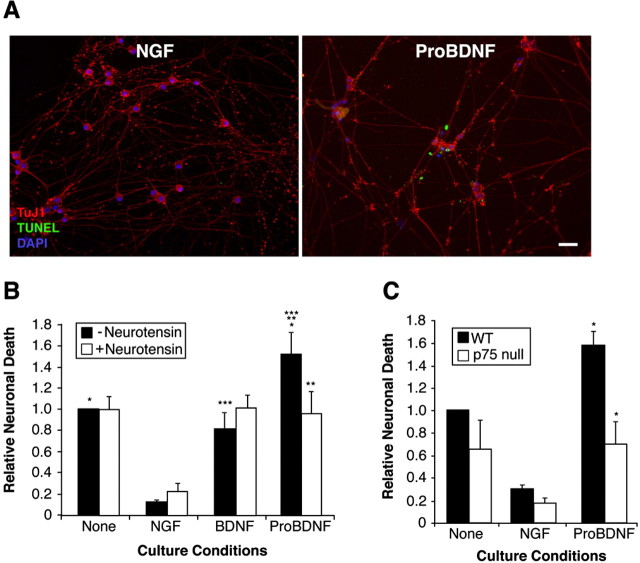
Past studies have demonstrated that BDNF induces neuronal apoptosis when p75NTR is activated in the absence of TrkB signaling (Bamji et al., 1998; Palmada et al., 2002). Because proBDNF interacts with p75NTR and sortilin but does not activate TrkB, we posited that proBDNF treatment of SCG neurons would lead to cell death in a p75NTR- and sortilin-dependent manner. To test this hypothesis, we first examined whether proBDNF induces SCG apoptosis. As shown in Figure 7, replicate cultures of P0-P1 SCG neurons were deprived of NGF in the presence or absence of recombinant cleavage-resistant proBDNF. Assessment of TUNEL positivity and nuclear morphology revealed that proBDNF, at subnanomolar concentrations, significantly increases the proportion of apoptotic SCG when compared with non-proBDNF-treated (but NGF-deprived) controls (Fig. 7A, B). Interestingly, mature BDNF, when applied at an identical concentration to that of proBDNF (4 ng/ml), did not enhance apoptosis of the NGF-deprived cultures (Fig. 7B), suggesting that proBDNF is at least 10-20 times more effective than mature BDNF in inducing SCG death where concentrations of 50-100 ng/ml are required (Bamji et al., 1998; Palmada et al., 2002).
Figure 7.
Sortilin and p75NTR are required for the proapoptotic action of proBDNF. A, Cultured rat SCG neurons were incubated with either mature BDNF or proBDNF as indicated for 48 h. The cultures were then fixed and processed for immunohistochemistry with Tuj1 (red), followed by TUNEL analysis (green). Nuclei were counterstained with DAPI (blue). Scale bar, 100 μm. B, Replicate cultures of NGF-deprived rat SCG neurons were treated, or not, with NGF (10 ng/ml), mature BDNF (4 ng/ml), or proBDNF (4 ng/ml) in the presence or absence of 20 μm neurotensin as indicated. Forty-eight hours later, cells were processed for TUNEL analysis as in A. The percentage of TUNEL-positive neurons was scored under each culture condition. The numbers were then normalized to those neurons that were NGF deprived (varied between 10 and 20% in different experiments). Error bars indicate SEM from three independently conducted experiments. Statistical significance (p < 0.05) between each paired sample is indicated by asterisks. C, Sympathetic neurons from p75NTR-null or wild-type (WT) mice were established as described previously (Bamji et al., 1998). Replicate cultures were treated as in B and processed for TUNEL analysis. Error bars indicate SEM from three independently conducted experiments. Statistical significance (p < 0.05) between the paired sample is indicated by asterisks.
Because neurotensin blocks the interaction of proBDNF to sortilin in vitro (Fig. 4A), neurotensin might compete with proBDNF for binding to a p75NTR-sortilin coreceptor complex in vivo. Indeed, pretreatment of SCG cultures with neurotensin completely prevents the apoptotic actions of proBDNF on cultured SCG neurons (Fig. 7B). Importantly, neurotensin alone has no survival-promoting effects on NGF-deprived SCG, consistent with past findings (Unsicker and Stogbauer, 1992). These data implicate sortilin as a critical coreceptor for the apoptotic effects of proBDNF.
To evaluate the role of p75NTR in proBDNF-elicited neuronal apoptosis, we compared the effects of proBDNF on SCG cultures derived from p75NTR-null mice versus wild-type littermates (Fig. 7C). Our previous work has shown that both wild-type and p75NTR-/- SCG neurons retain sortilin expression (Nykjaer et al., 2004). Consistent with the data obtained with rat SCG, proBDNF induces an ∼50% increase in neuronal apoptosis of wild-type mouse SCG cultures. In contrast, treatment of p75NTR-null SCG neurons with proBDNF did not enhance cell death, indicating that proBDNF-induced apoptosis occurs in an p75NTR-dependent manner. The reduced apoptotic response of growth factor-deprived p75NTR-null neurons is consistent with past findings (Bamji et al., 1998). Together, these in vivo data suggest that the biological actions of proBDNF require coexpression of both sortilin and p75NTR receptors.
Because previous studies have demonstrated that TrkA activation can antagonize p75NTR-dependent apoptotic signaling (Yoon et al., 1998), we evaluated whether proBDNF-induced neuronal apoptosis can be similarly modulated by NGF (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Indeed, NGF treatment was found to significantly reduce proBDNF-elicited SCG neuron death and can do at low concentrations of NGF (0.1 ng/ml), consistent with activation of high-affinity NGF binding sites on SCG (Lee et al., 1994). These results suggest that, in cells that express a full complement of Trk, p75NTR, and sortilin receptors, competitive signaling can occur during exposure to pro-survival and apoptotic ligands.
Neuronal protective effect of soluble sortilin
Because a previous report (Navarro et al., 2002) indicates that the extracellular domain of sortilin can be cleaved by metalloproteinases and thus may be shed from cells, we sought to determine whether preformed complexes of proBDNF and soluble extracellular domain of sortilin was capable of initiating apoptosis of sympathetic neurons that express endogenous p75NTR and sortilin. To this end, secreted proBDNF-His was incubated with media from cells infected with adenovirus encoding soluble sortilin, or as a control, adenovirus encoding GFP, and proBDNF was purified by metal ion chromatography. As demonstrated by Western blot analysis, purified proBDNF deficient in soluble sortilin or complexes of proBDNF-sortilin were purified (Fig. 8A) and used in apoptosis assays. Whereas proBDNF was capable of initiating apoptosis at subnanomolar concentrations, complexes of proBDNF with soluble sortilin were not (Fig. 8B). Comparable results were obtained using rat Schwann cells coexpressing sortilin and p75NTR; treatment with sortilin-deficient proBDNF (0.1 nm) induced a twofold increase in apoptosis, whereas proBDNF complexed to soluble sortilin was significantly less effective (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These results suggest that proBDNF-specific biological actions are preferentially activated when both receptors are coexpressed on the cell surface, and that a preformed complex of proBDNF and soluble sortilin might be a means to restrict proBDNF signaling (supplemental Fig. 4, available at www.jneurosci.org as supplemental material).
Discussion
Like many other growth factor families, neurotrophins are synthesized as larger precursor forms that undergo intracellular enzymatic cleavage to yield mature, biologically active ligands. In the case of NGF and BDNF, previous studies have identified that the pro-domains of these proteins serve important roles in the proper folding, dimerization, and targeting of the mature neurotrophins (Kolbeck et al., 1994; Rattenholl et al., 2001; Egan et al., 2003; Chen et al., 2004). However, more recent studies have suggested that the precursor of NGF can be released under pathological conditions (Harrington et al., 2004) and detected in the brains from Alzheimer's disease patients (Peng et al., 2004; Pedraza et al., 2005) and that it is the preferred ligand for p75NTR-dependent but not TrkA-dependent cellular events (Lee et al., 2001). An obvious issue raised by these findings is whether they reflect a unique property of proNGF or whether the proforms of other neurotrophin family members are similarly active in modulating p75NTR signaling. To address this question, we generated a cleavage-resistant mutant of BDNF and show that it is an effective proapoptotic ligand for cultured sympathetic neurons. Evidence is also presented here that proBDNF requires both p75NTR and sortilin, a recently identified coreceptor for proNGF (Nykjaer et al., 2004), to initiate cell death. These results together raise the possibility that proneurotrophins are not merely intracellular precursors for the mature forms but that they are bona fide ligands with distinct physiological functions.
Properties of recombinant proBDNF
Consistent with previous reports (Kolbeck et al., 1994; Heymach and Shooter, 1995), secreted proBDNF is a dimer of ∼60 kDa and is glycosylated in mammalian cells. The presence of two immunoreactive proBDNF species may reflect differences in the degree or composition of N-linked glycosylation, or N-terminal cleavage (Kolbeck et al., 1994; Mowla et al., 2001) that occurs within the cell. Studies with proNGF suggest that interactions of mature domains strongly promote the dimerization of proNGF (Rattenholl et al., 2001). Preformed dimers of either proNGF or proBDNF are tightly associated and are not in rapid equilibrium with the monomeric forms (Kolbeck et al., 1994; Rattenholl et al., 2001). Our results with secreted mammalian proBDNF are consistent with these previous findings, and, indeed, we find that proBDNF dimers are stable to high ionic strength and exposure to a wide pH range.
ProBDNF exhibits high-affinity binding to sortilin, which is impaired by the sortilin antagonist neurotensin. Based on structure-function analysis of proNGF and neurotensin binding to the cysteine-rich region of the sortilin ectodomain (Westergaard et al., 2004), it is likely that proBDNF interacts with a similar region of sortilin. Indeed, the affinities of proNGF and proBDNF with sortilin suggest that proBDNF is the preferred ligand (Nykjaer et al., 2004 and this study). The association of proBDNF with the soluble ectodomain of sortilin is also remarkably stable: it is maintained after exposure to high ionic strength, physiologic, and high pH, with preformed complexes stable for up to 1 week in solution (data not shown). Despite an observed preference of proBDNF for sortilin binding, it is presently unclear whether proBDNF is a higher-affinity ligand than proNGF for inducing cellular apoptosis: comparison of these two proneurotrophins using cultured SCG neurons did not yield a significant difference (Nykjaer et al., 2004 and this study). However, these in vitro experiments cannot rule out the possibility that constitutive shedding of the ectodomain of sortilin occurs (Navarro et al., 2002), and that this soluble form of sortilin binds to proBDNF to impair proapoptotic activity. Indeed, preformed complexes of proBDNF and sortilin are far less active than proBDNF in initiating cell death (Fig. 8) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These observations suggest that the bioactivity of proBDNF may be determined by the local concentration of soluble sortilin. Finally, our finding that mature NGF can counter the apoptotic activity of proBDNF further supports the notion that neuronal death/survival decision in vivo is modulated by multiple extrinsic signals.
Neuronal release of endogenous proBDNF
Mature BDNF is remarkably conserved throughout evolution, with human and chicken BDNF sharing over 90% identity in amino acid sequence. Surprisingly, this high degree of conservation also extends to the pro-domain of BDNF across species. In contrast, human and chicken epidermal growth factor precursors are only 50% identical. One interpretation of these observations is that the pro-domain of BDNF is required not only for a structural role in protein folding (Kolbeck et al., 1994; Heymach and Shooter, 1995) but perhaps serves additional functions that are critical for the organism.
Consistent with the above possibility, we now find that proBDNF is constitutively released from cultured cortical neurons. Although proBDNF immunoreactivity has been detected in neurons as well as postmortem human brain (Michalski and Fahnestock, 2003), secretion of endogenous proBDNF from neuronal populations has not been reported previously. Our study thus indicates that proBDNF might be an alternative ligand to mature BDNF in modulating CNS function. Indeed, Pang et al. (2004) recently demonstrated that hippocampal long-term potentiation (LTP) requires the conversion of secreted proBDNF to mature BDNF by the extracellular tPA/plasmin proteases and that proBDNF protein is accumulated in the hippocampi of tPA- or plasminogen-null mice. The inability of the proBDNF to mediate LTP in a TrkB-dependent manner (Pang et al., 2004) is consistent with the observed reduced ability of proBDNF to induce TrkB phosphorylation or promote neurite outgrowth compared with mature BDNF (Fig. 6).
Coreceptor interaction for proBDNF
The relative ineffectiveness of proBDNF in activating TrkB (Fig. 6), the cognate receptor for mature BDNF, is consistent with the structural constraint predicted by x-ray crystallographic studies of neurotrophin/Trk pairing (Wiesmann et al., 1999; Banfield et al., 2001). Instead, proBDNF induces sympathetic neuron and glia apoptosis that is dependent on the p75NTR and sortilin coreceptors (Fig. 7) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Although mature BDNF can also elicit SCG death in the mismatched neurotrophin-receptor paradigm (Bamji et al., 1998; Palmada et al., 2002), proBDNF is at least 10 times more effective than mature BDNF in inducing comparable apoptosis (cf. Bamji et al., 1998 and this study). Although the full spectrum of proBDNF biological activities has not yet been established, the enhanced sensitivity of p75NTR and sortilin coexpressing cells to proBDNF-induced apoptosis suggests that the access of proBDNF to its physiological targets must been strictly regulated. Accordingly, we found that soluble sortilin forms a high-affinity complex with proBDNF and that sortilin stabilizes/protects proBDNF from proteolytic degradation. It is also interesting to note that proBDNF, when in a preformed complex with soluble sortilin, is ineffective in eliciting cell death of cells coexpressing p75NTR and sortilin (Fig. 8) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Because the ectodomain of sortilin can be cleaved from cells by metalloproteinases (Navarro et al., 2002), it is possible that a proportion of secreted proBDNF may be bound to sortilin in vivo, and this interaction may regulate the biological activity of proBDNF. The significance of these considerations is not without precedence: the TNF receptor family, of which p75NTR is a founding member, is well known to consist of decoy receptors that modulate the biological activity of the transmembrane counterparts (for review, see Ashkenazi, 2002). Whether the soluble ectodomain of sortilin serves to stabilize proBDNF or to restrict the biological activity of proBDNF (or both) in vivo awaits additional investigation.
In summary, we have characterized a recombinant cleavage-resistant form of proBDNF in terms of proapoptotic activity in peripheral neurons and glia as well as coreceptor requirements. While these studies were in progress, proBDNF was shown to be an ineffective ligand in initiating TrkB-dependent LTP in CNS neurons, whereas the processive conversion of plasminogen to plasmin, which in turn promotes the conversion of proBDNF to mature BDNF, was required for LTP (Pang et al., 2004), suggesting distinct functions of proBDNF in specific neuronal and non-neuronal cell types. Thus, previously characterized roles of mature BDNF notwithstanding, a comprehensive analysis of neurotrophin functions now merits additional consideration with respect to the mechanisms that regulate pro-domain cleavage.
Footnotes
This work was supported by National Institutes of Health Grants NS30687 and HL46403 (B.L.H.) and MH68850 (F.S.L.), the Burroughs Wellcome Fund (B.L.H.), the Danish Medical Research Council, the Lundbeck Foundation and Novo Nordisk Foundation (A.N.), the James Birrell Neuroblastoma Research Fund (R.T.), and Fundação para a Ciência e a Tecnologia (R.D.A.). We thank Peter Fischer for technical assistance and Patrice Maurel and Juan-Carlos Arevelo for valuable reagents.
Correspondence should be addressed to B. L. Hempstead, Weill Medical College of Cornell University, 1300 York Avenue, Room C-606, New York, NY 10021. E-mail: blhempst@med.cornell.edu.
R. Lee's present address: Center for Neurobiology and Behavior, Columbia University, New York, NY 10032.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255455-09$15.00/0
References
- Ashkenazi A (2002)) Targeting death and decoy receptors of the tumournecrosis factor superfamily. Nat Rev Cancer 2: 420-430. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD (1998) The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol 140: 911-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield MJ, Naylor RL, Robertson AG, Allen SJ, Dawbarn D, Brady RL (2001) Specificity in Trk receptor:neurotrophin interactions: the crystal structure of TrkB-d5 in complex with neurotrophin-4/5. Structure 9: 1191-1199. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO (2002) ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 36: 375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JG, Gordon T (2002) A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci 15: 613-626. [DOI] [PubMed] [Google Scholar]
- Cahoon-Metzger SM, Wang G, Scott SA (2001) Contribution of BDNF-mediated inhibition of patterning avian skin innervation. Dev Biol 232: 246-254. [DOI] [PubMed] [Google Scholar]
- Carter AR, Berry EM, Segal RA (2003) Regional expression of p75NTR contributes to neurotrophin regulation of cerebellar patterning. Mol Cell Neurosci 22: 1-13. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS (2004) Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24: 4401-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM (2002) The neurotrophin receptor p75NTR as a positive modulator of myelination. Science 298: 1184-1186. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257-269. [DOI] [PubMed] [Google Scholar]
- Gehler S, Gallo G, Veien E, Letourneau PC (2004) p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J Neurosci 24: 4363-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23: 6690-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM (2004) Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA 101: 6226-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymach Jr JV, Shooter EM (1995) The biosynthesis of neurotrophin heterodimers by transfected mammalian cells. J Biol Chem 270: 12297-12304. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF (2000) BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19: 1290-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD (1999) Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci 13: 5393-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck R, Jungbluth S, Barde YA (1994) Characterisation of neurotrophin dimers and monomers. Eur J Biochem 225: 995-1003. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R (1992) Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69: 737-749. [DOI] [PubMed] [Google Scholar]
- Lee KF, Davies AM, Jaenisch R (1994) p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development 120: 1027-1033. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945-1948. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L (1999) Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 96: 15239-15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC (1997) Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18: 767-778. [DOI] [PubMed] [Google Scholar]
- Michalski B, Fahnestock M (2003) Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer's disease. Brain Res Mol Brain Res 111: 148-154. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276: 12660-12666. [DOI] [PubMed] [Google Scholar]
- Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P (1999) Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 18: 595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro V, Vincent JP, Mazella J (2002) Shedding of the luminal domain of the neurotensin receptor-3/sortilin in the HT29 cell line. Biochem Biophys Res Commun 298: 760-764. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM (2004) Sortilin is essential for proNGF-induced neuronal cell death. Nature 427: 843-848. [DOI] [PubMed] [Google Scholar]
- Palmada M, Kanwal S, Rutkoski NJ, Gustafson-Brown C, Johnson RS, Wisdom R, Carter BD, Gufstafson-Brown C (2002) c-jun is essential for sympathetic neuronal death induced by NGF withdrawal but not by p75 activation. J Cell Biol 158: 453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306: 487-491. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER (2001) Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 32: 123-140. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arevalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C (2005) Pro-NGF isolated from the human brain affected by Alzheimer's disease induces neuronal apoptosis mediated by p75NTR Am J Pathol 166: 533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M (2004) Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol 63: 641-649. [DOI] [PubMed] [Google Scholar]
- Petrides PE, Shooter EM (1986) Rapid isolation of the 7S-nerve growth factor complex and its subunits from murine submaxillary glands and saliva. J Neurochem 46: 721-725. [DOI] [PubMed] [Google Scholar]
- Rattenholl, A. Lilie H, Grossmann A, Stern A, Schwarz E, Rudolph R (2001) The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem 268: 3296-3303. [DOI] [PubMed] [Google Scholar]
- Shonukan O, Bagayogo I, McCrea P, Chao M, Hempstead B (2003) Neurotrophin-induced melanoma cell migration is mediated through the actin-bundling protein fascin. Oncogene 22: 3616-3623. [DOI] [PubMed] [Google Scholar]
- Suter U, Heymach Jr JV, Shooter EM (1991) Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J 10: 2395-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ (2002) Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem 277: 34295-34302. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Stogbauer F (1992) Screening of adrenal medullary neuropeptides for putative neurotrophic effects. Int J Dev Neurosci 10: 171-179. [DOI] [PubMed] [Google Scholar]
- Wang S, Bray P, McCaffrey T, March K, Hempstead BL, Kraemer R (2000) p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am J Pathol 157: 1247-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard UB, Sorensen ES, Hermey G, Nielsen MS, Nykjaer A, Kirkegaard K, Jacobsen C, Gliemann J, Madsen P, Petersen CM (2004) Functional organization of the sortilin Vps10p domain. J Biol Chem 279: 50221-50229. [DOI] [PubMed] [Google Scholar]
- Wiesmann C, Ultsch MH, Bass SH, de Vos AM (1999) Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature 401: 184-188. [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF (2003) Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6: 736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV (1998) Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci 18: 3273-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]