Abstract
Staphylococcal enterotoxin A (SEA) is a microbial superantigen that activates T-lymphocytes and induces production of various cytokines, including tumor necrosis factor-α (TNFα). Previously, it was shown that SEA activates the hypothalamic-pituitary-adrenal axis and augments gustatory neophobic behaviors. In the present study, it was hypothesized that these effects involve neuronal activation in forebrain regions mediating fear and/or anxiety and are dependent on the production of TNFα. Male C57BL/6J mice were given intraperitoneal injections of 10 μg of SEA and 5 μg of lipopolysaccharide (LPS) or saline and perfused 2 h later for histochemical determination of brain c-Fos immunoreactivity (IR). The results showed increased c-Fos IR in the paraventricular nucleus, arcuate nucleus, central nucleus of the amygdala, bed nucleus of the stria terminalis, and lateral septum. Challenge of TNF-/- mice with SEA did not produce a significant increase in brain c-Fos IR, although c-Fos was increased after exposure to a psychogenic stressor (i.e., open field). In additional experiments, the elevated corticosterone response to SEA was abrogated in TNF-/- mice and was shown to be corticotropin-releasing hormone dependent. Finally, the augmented reduction in novel food intake after SEA challenge was attenuated in TNF-/- mice as well as in wild-type mice administered antibody to TNFα. In conclusion, challenge with SEA recruits brain regions mediating stress and anxiety responses, an effect that requires endogenous TNFα. Whether this is indicative of all T-cell superantigens remains to be determined, although it stands in contrast to other models of neuroimmunomodulation (e.g., LPS) that involve multiple cytokine influences.
Keywords: anorexia, corticosterone, immunity, limbic, neuromodulation, tumor necrosis factor, staphylococcal enterotoxin A
Introduction
Superantigens (SAgs) are microbial products implicated in toxic-shock syndrome and autoimmune disease (Torres et al., 2001). The best characterized SAgs are the staphylococcal enterotoxins (SEs) (Proft and Fraser, 2003) and, in particular, staphylococcal enterotoxin A (SEA) and B (SEB). These SEs display affinity for distinct Vβ regions on human and murine T-lymphocyte receptors (Marrack et al., 1993; Torres et al., 2001; Petersson et al., 2004) and in the mouse, stimulate Vβ3+ and Vβ8+ T-cells, respectively, in a major histocompatiblity complex class II restricted manner (Gonzalo et al., 1993). Moreover, challenge with SEA and SEB induces high plasma levels of cytokines, such as tumor necrosis factor-α (TNFα), interleukin-2 (IL-2), and interferon-γ (IFNγ) (Bette et al., 1993; Rosendahl et al., 1997).
Considerable evidence shows that administration of SEA or SEB exerts neurobiological effects. This includes activation of the hypothalamic-pituitary-adrenal (HPA) axis, sympathetic nervous system, anorexic behavior, altered nociception, and pyrogenesis (Gonzalo et al., 1993; Shurin et al., 1997; Kusnecov et al., 1999; Goehler et al., 2001; Del Rey et al., 2002; Kawashima and Kusnecov, 2002; Kawashima et al., 2002; Pacheco-Lopez et al., 2004). Studies in rats also showed that SEB activates a number of limbic brain regions, including the lateral septum (LS), central amygdala (ceA), and paraventricular nucleus (PVN) (Goehler et al., 2001; Wang et al., 2004), which is consistent with murine studies in which SEB increased hypothalamic and amygdaloid corticotropin-releasing hormone (CRH) mRNA (Kusnecov et al., 1999). These neuroanatomical data imply the induction of anxiety/fear-like responses and anorexia (Davis, 1998; Sheehan et al., 2004) after superantigenic challenge. Indeed, although SEA and SEB induce anorexia, this appears to be independent of malaise and more likely is a result of augmented novelty-induced arousal (Kusnecov et al., 1999; Kawashima and Kusnecov, 2002; Rossi-George et al., 2004).
The mechanism by which SAgs impact the CNS likely involves endogenous cytokine production. In mice, IL-2 does not activate the HPA axis (Zalcman et al., 1994; Kusnecov et al., 1999), whereas the more likely candidate, IL-1β, is not detected in plasma after SAg challenge (Kawashima and Kusnecov, 2002). Alternatively, SEA elevates circulating TNFα (Kawashima and Kusnecov, 2002), a cytokine capable of increasing plasma corticosterone, hypophagia, and brain c-fos (Bluthe et al., 1994; Ando and Dunn, 1999; Hayley et al., 2001, 2002). Therefore, it was hypothesized that the primary influence for the CNS effects of SAg challenge may reside with TNFα.
To test this hypothesis, the present study used TNFα knock-out animals generated on a C57BL/6J background. Consequently, SEA was used because it reliably activates T-cells and TNFα production in C57BL/6 mice (Rosendahl et al., 1997; Kawashima and Kusnecov, 2002). Assessment of neuronal activation in limbic forebrain regions involved immunohistochemical detection of the immediate-early gene c-fos. We report for the first time that SEA challenge increased c-Fos immunoreactivity (IR) in key brain regions implicated in stress and regulation of ingestive behavior. Moreover, the findings revealed that in contrast to lipopolysaccharide (LPS) (Perlstein et al., 1993; Ebisui et al., 1994; Turnbull and Rivier, 1998), neurobehavioral and endocrine changes pursuant to SEA challenge were fully dependent on the production of TNFα.
Materials and Methods
Animals
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 5-6 weeks of age and housed four per cage. TNFα-deficient and IL-1 receptor I (IL-1RI)-deficient mice bearing a C57BL/6 background were also obtained from The Jackson Laboratory. The TNF-/- population was expanded by backcrossing with C57BL/6J mice, followed by maintenance of breeding between F1 heterozygous (TNF-/+) males and females, with the knock-out and wild-type (TNF+/+) members of the F2 generation selected for experimentation. Animals were weaned at postnatal day 21 and housed in same-sex groups until genotyping (within 1-2 weeks). Subsequent to genotyping, animals were further subdivided into groups matched for age and genotype. For all experiments involving C57BL/6J mice ordered from the vendor (i.e., not bred in our animal facility), ∼2 weeks of acclimation was provided before testing. In some experiments, animals were subjected to a daily food deprivation schedule (see below). Otherwise, animals were housed four per cage under 12 h light/dark illumination (lights on at 5:00 A.M.) and ad libitum access to food and water. All experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Rutgers University.
Genotyping of TNFα-deficient mice
Shortly after weaning, 2-3 mm tail sections were excised and subjected to DNA extraction by overnight digestion at 54°C in 200 μl of lysis buffer containing 100 mm Tris-HCl, pH 8.5, 5 mm EDTA, 200 mm NaCl, 0.2% SDS, and 1 mg/ml proteinase K. The samples were then centrifuged at 1200 rpm for 5 min, and the DNA was precipitated from the supernatant with isopropanol, rinsed with 70% ethanol, and resuspended in 100 μl of Tris-EDTA buffer, pH 8. Five microliters of DNA were devoted to a 50 μl PCR using the TaqPCR Master Mix kit (Qiagen, Valencia, CA). The primer sequences and protocol for the TNF tm1Gk1 were obtained from The Jackson Laboratory, and the oligonucleotides were synthesized by the DNA synthesis facility at the University of Medicine and Dentistry of New Jersey. PCR-amplified DNA was separated by gel electrophoresis on a 1.5% agarose gel containing ethidium bromide and read using the EDAS120 Kodak Gel Imaging System (Eastman Kodak, Rochester, NY). The genotype of the subjects was determined as follows: knock-out, single product of 146 bp; heterozygous, two products of 146 and 280 bp; wild-type, single product of 280 bp. Once the animals were genotyped, they were housed four per cage corresponding to their genotype and allowed to acclimate for at least 2 weeks.
Reagents
SEA, LPS (055:B5), and astressin were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant murine IL-1β was purchased from R&D Systems (Minneapolis, MN). Hamster anti-murine TNFα IgG antibody was kindly provided by the Biological Resources Branch, National Cancer Institute (Bethesda, MD). Hamster IgG isotype control was purchased from Fisher Scientific (Pittsburgh, PA). Antibodies were administered intraperitoneally.
Immunological and psychogenic stressor exposure
Immunological stressors. In experiments examining the effects of immunological challenge on CNS activation, animals were challenged with 10 μg of SEA, 5 μg of LPS, or 100 ng of IL-1β. After injection, wild-type and transgenic mice were (1) perfused for c-Fos immunohistochemistry (only for experiments involving SEA and LPS treatment), (2) killed by decapitation for measurement of corticosterone, or (3) tested for food intake. In most experiments, killing the mice or testing took place 2 h after injection, because this was previously shown to be the optimal time for increased pituitary-adrenal activation (Kawashima and Kusnecov, 2002). For experiments involving IL-1β, ingestive behavior was assessed 1 h after injection, whereas in other experiments, corticosterone measures were taken 2 h after injection. In all experiments, vehicle controls consisted of animals that were given injections of pyrogen-free saline.
Exposure to an open field. To confirm whether TNF-/- mice were capable of responding to a psychogenic stressor with increased recruitment of neuronal activity in limbic brain regions, both wild-type and knock-out mice were exposed for 15 min to an open field as described previously (Kawashima and Kusnecov, 2002) and returned to their home cage for 45 min. Subsequently, mice were perfused, and the brains were assayed for c-Fos IR as described below.
c-Fos immunocytochemistry
The procedure for immunohistochemical detection of c-Fos-immunoreactive cells conformed to previously published methods (Pezzone et al., 1992). Animals were anesthetized with 50 mg/kg sodium pentobarbitol (Nembutal) and perfused transcardially for 3-5 min with isotonic saline containing 2% sodium nitrite. This was followed by a 5-10 min perfusion with 4% paraformaldehyde containing 2.5% Acrolein (Polysciences, Warrington, PA). After an additional 5 min rinse with sodium nitrite, the brains were removed and stored at 4°C in 5 ml of 25% sucrose solution until ready to be sectioned for immunocytochemistry. Anterior-to-posterior coronal brain sections (25 μm thickness) were collected using a freezing microtome. Sectioning commenced from the prefrontal cortical region anterior to the anterior commissure and terminated at the posterior end of the diencephalon. The sections were cryopreserved as free-floating sections in a -20°C freezer until ready for assay.
For the assay, tissue sections were incubated initially for 72 h at 4°C in 0.4% Triton X-100 in PBS, pH 7.2, containing a 1:15,000 final dilution of rabbit anti-human Fos antibody (Oncogene Science, Cambridge, MA). This antibody is directed at the N-terminal region of Fos. After rinsing in PBS, the tissues were incubated for 1 h at room temperature in PBS/0.4% Triton X-100 containing a 1:500 final dilution of biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA). After an additional rinse, tissues were treated for 1 h at room temperature with a avidin-biotin-peroxidase complex solution from the Vector Elite ABC kit (Vector Laboratories), followed by sequential washes in PBS and 0.175 m sodium acetate (NaOAc). The enzyme-substrate reaction was subsequently generated by the addition of a 3,3-diaminobenzedine (DAB) substrate solution consisting of 0.175 m NaOAc containing 25 mg/ml NiSO4, 0.2 mg/ml DAB, and 0.28% H2O2. The addition of NiSO4 allowed for dark blue to black stains to become visible during the reaction process, which were allowed to proceed for 10-20 min. Termination of the reaction was achieved by rinsing the tissues in 0.175 m NaOAc and then in PBS, after which the tissues were mounted on Superfrost slides (Fisher Scientific), dehydrated in a graded series of alcohols, and clarified and coverslipped using Histoclear and Histomount (VWR Scientific, Westchester, PA).
For quantitation of c-Fos-immunoreactive cells, stained slides were examined under a Nikon Eclipse E400 microscope equipped with a high-resolution CCD camera. Neuroanatomically distinct regions as defined by the mouse atlas of Franklin and Paxinos (1997) were digitally captured, and immunopositive cells were enumerated using the NIH Image software program. Up to four to six sections per animal for each region of interest were quantified bilaterally and then averaged. Demarcation of nuclei was conducted by two observers who were blind to group treatments. Confirmation of software accuracy was conducted by two observers hand-counting randomly selected regions of interest. Variation in number between human and software counts was not >1-2%.
Behavioral testing for food consumption
Consumption of a liquid diet. Animals were tested for consumption of Prosobee, a commercially available baby liquid formula (Mead Johnson, Evansville, IN). This solution is highly palatable and readily consumed by mice without the need for food and water deprivation (Kusnecov et al., 1999; Kawashima and Kusnecov, 2002; Kaneta and Kusnecov, 2005). This approach is consistent with other laboratories that have used short-term exposure to chocolate milk or sweetened condensed milk to examine ingestive responses to immunological stimuli (Dunn and Swiergel, 1998; Hayley et al., 2002). Preparation of full-strength Prosobee was according to instructions provided on the product label. Before giving animals Prosobee, the solution was diluted 1:2 using distilled water. For measurement of consumption, each animal was placed for 1 h in an opaque box with a preweighed bottle of Prosobee, after which the animals were returned to their home cages. The bottles were then weighed to determine the amount consumed. Exposure to the bottle of Prosobee occurred 1-2 h after injection. That is, testing for the effects of SEA commenced 2 h after injection, whereas 1 h was allowed to elapse before animals were tested after IL-1β injection (see Fig. 6 B).
Figure 6.
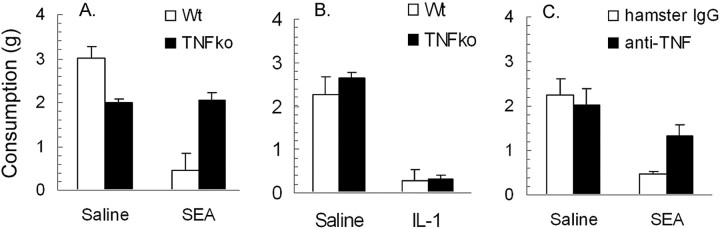
Consumption of a novel liquid diet (Prosobee) in wild-type (TNF+/+) and TNFα knock-out (TNF-/-) mice. A, Mean in take (±SE) of Prosobee in wild-type and knock-out mice after injection of 10 μg of SEA (n = 6 per strain) or saline (n = 5-7 per strain). B, Mean intake (±SE) of Prosobee in wild-type and knock-out mice after injection of 100 ng of murine recombinant IL-1β (n = 6 per strain) or saline (n = 5 per strain). C, Effect of pretreatment with hamster IgG (SEA, n = 5; saline, n = 4) or hamster anti-mouse TNFα antibody (SEA, n = 6; saline, n = 4) on mean intake (±SE) of Prosobee in wild-type C57BL/6J mice that were given injections of 10 μg of SEA or saline. *p < 0.05 (unpaired Student's t test) relative to corresponding saline control. Wt, Wild-type; ko, knock-out.
Consumption of food pellets. This experiment tested the effect of SEA challenge on consumption of small 20 mg food pellets (Noyes pellets; Research Diets, New Brunswick, NJ) to which animals were previously oriented (familiar group, n = 20) or remained naive (novel group, n = 20). Wild-type C57BL/6 mice were housed four per cage after arriving in the laboratory and during acclimation received ad libitum access to food and water. After commencement of the experiment, cages were allocated randomly to the different food preexposure conditions, and food was restricted for 3 d to a daily 4 h period that occurred before the onset of darkness. There was no restriction of availability to water. Subjects in the familiar group received in their home cage exposure to a glass Petri dish containing 10 g of the Noyes 20 mg food pellets. No other food was provided. Animals that were not oriented (i.e., the novel group) to the Noyes pellets ate regular laboratory chow (a pile of 3.5-4.5 g LabDiet pellets) accessible through the metal cage top. On the test day, one-half of the animals in the familiar and novel groups was given injections of 10 μg of SEA, and the other half was given injections of saline, after which they were returned to the home cage without any exposure to food (i.e., the deprivation period was still in effect). Two hours after injection, the animals were removed from their home cage and placed individually in a test apparatus containing at one end 10 g of Noyes food pellets in the glass Petri dish. The apparatus was a metal chamber fitted with a metal grid floor and measuring 38 × 25 × 18 cm (length by width by height). Multiple test chambers were available to allow for cohorts of SEA- and saline-injected animals to be tested at the same time. Each animal was placed in the apparatus initially at the end opposite the location of the Petri dish. Animals were allowed to investigate and consume the food pellets for 30 min, after which they were returned to the home cage. After removal of the animal, the contents of the Petri dish were weighed, and adjustments were made after weighing spilled food captured by a sheet of white paper (∼19 × 25 cm) placed immediately below the area where the Petri dish was located. Because of the large number of animals, the experiment involved testing two cohorts containing equal numbers of animals (i.e., n = 20). Each cohort was tested on succeeding days, although equal representation of all injection and novelty/familiarity conditions was present in each cohort: SEA/novel pellets (n = 5); SEA/familiar pellets (n = 5); saline/novel pellets (n = 5); saline/familiar pellets (n = 5). There were no differences between the cohorts, and therefore the results were pooled (see Results and Fig. 7).
Figure 7.
A, Consumption of novel and familiar food pellets after challenge of wild-type mice (TNF+/+) with 10 μg of SEA or saline (n = 10/group). B, Effect of injection with SEA (n = 8) or saline (n = 7) on intake of novel food pellets by TNF knock-out mice (TNF-/-). The knock-out mice were not tested for consumption of familiar food pellets, because SEA did not reduce consumption of familiar pellets in wild-type mice. In both experiments, testing commenced 2 h after injection. Data represents mean intake ± SE.
Testing of TNF-/- animals. The preceding experiment was repeated using TNF-deficient animals. However, no groups were included that received home-cage preexposure to the pellets. Therefore, animals were subjected to the same regimen of daily restricted food intake of regular laboratory chow. On the test day, they were given injections of SEA (n = 8) or saline (n = 7) as described above and tested 2 h later for consumption of novel pellets presented in the test apparatus.
Measurement of plasma corticosterone, TNFα, and IFNγ
Trunk blood was collected by rapid decapitation into heparin-treated vacutainer tubes (Becton Dickinson, Rutherford, NJ). The blood was centrifuged immediately at 2000 rpm for 15 min, and the plasma was collected and stored at -70°C. Corticosterone was measured by radioimmunoassay (ICN Biomedicals, Costa Mesa, CA), and TNFα and IFNγ were measured using a Quantikine ELISA kit from R&D Systems.
Reverse transcription and real-time PCR for splenic IFN-γ and IL-2 mRNA
Confirmation of T-cell activation in TNF-deficient mice challenged with SEA was conducted by real-time PCR. Relative quantitation of spleen mRNA for the T-cell cytokines IFNγ and IL-2 used a validated quantitative reverse transcription (RT)-PCR method as described previously by others at the Keck Center for Collaborative Neuroscience, Rutgers University (Pan et al., 2004). Wild-type (n = 8) and mutant (n = 8) mice were given injections of saline (n = 4/strain) or 10 μg of SEA (n = 4/strain), and the spleens removed 2 h later. Total RNA was extracted from all 16 spleens in a single run using a Totally RNA extraction kit (Ambion, Austin, TX) followed by quantification using Ribogreen (Molecular Probes, Eugene, OR). Generation of cDNA from 1 μg of RNA was accomplished with the Advantage RT-for-PCR kit (BD Biosciences Clontech, Palo Alto, CA), followed by real-time PCR using mRNA primers for IFNγ, IL-2, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR reactions were performed in a 10 μl volume using 5 ng of cDNA, 25 nm of reverse and forward primers (see Table 1 for PCR primer sequences), and PCR master mix containing the fluorescence agent SYBR Green (Applied Biosystems, Warrington, UK). Real-time PCR was conducted using an Applied Biosystems 7900HT system, with threshold cycles for each sample being compared with a standard curve. The standard curve was generated using a fourfold dilution series of cDNA from spleen RNA obtained from an animal that was given an injection of SEA (undiluted control cDNA, 10 arbitrary units). This allowed for comparative or relative quantification of starting gene-of-interest cDNA in each of the samples. The same control cDNA was used to generate separate standard curves for each primer set. Analysis of the melting point for each sample revealed the presence of only a single amplified product. For each gene of interest, the sample data were expressed in arbitrary units based on the standard curve.
Table 1.
PCR primers (all sequences are 5′ to 3′)
|
Gene amplified |
Forward primer |
Reverse primer |
|---|---|---|
| IFNγ | agctcatccgagtggtccac | gcttcctgaggctggattcc |
| IL-2 | gtcaacagcgcacccactt | tgcttccgctgtagagcttg |
| GAPDH |
aactccctcaagattgtcagcaa |
ggctaagcagttggtggtgc |
Data analysis
Most experiments conformed to a factorial design and were therefore analyzed by ANOVA using Statview, a statistical software package. The c-Fos data were expressed as the number of c-Fos-immunoreactive cells per brain region, whereas food intake was measured in grams. Finally, corticosterone was expressed as nanograms of plasma per milliliter. Post hoc comparisons were conducted using the Fisher's least significant difference test when the omnibus F achieved a 0.05 level of significance. In some cases, in which a priori predictions based on previous data were warranted, an unpaired t test was conducted.
Results
Brain c-Fos IR
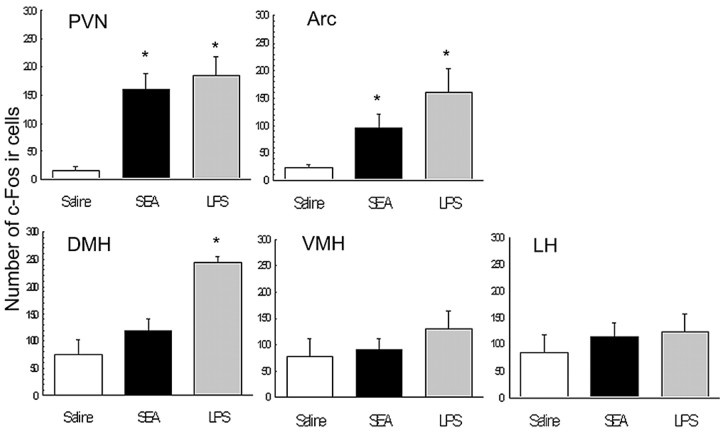
Mice were challenged with either saline, 10 μg of SEA, or 5 μg of LPS and killed 2 h later for assessment of brain c-Fos IR. A group challenged with LPS was used as a positive comparison, because LPS has been shown to increase brain c-Fos expression in C57BL/6 mice (Laflamme et al., 1997; Matsuoka et al., 2003; Reyes et al., 2003). Hypothalamic nuclei were examined that are associated with ingestive behavior and stress responses. Of the five hypothalamic nuclei that were analyzed [PVN, arcuate nucleus, dorsomedial hypothalamic nucleus (DMH), ventromedial hypothalamic nucleus (VMH), lateral hypothalamus (LH)], the PVN (F(2,12) = 11.9; p < 0.001) and arcuate nucleus (F(2,12) = 6.15; p < 0.05) showed significantly increased numbers of c-Fos-immunoreactive cells after SEA and LPS challenge (Fig. 1). However, in neither SEA- nor LPS-treated animals did the VMH and LH neurons show increased c-Fos IR relative to saline controls (Fig. 1). Interestingly, although a one-way ANOVA revealed a main effect of toxin treatment on c-Fos IR in the DMH (F(2,12) = 13.7; p < 0.001), post hoc analyses revealed this to be attributable to LPS treatment (Fig. 1).
Figure 1.
Mean number (±SE) of c-Fos-immunoreactive cells in hypothalamic nuclei 2 h after intraperitoneal injection with saline (n = 5), 10 μg of SEA (n = 6), or 5 μg of LPS (n = 4). Each bar represents the mean ± SE. Arc, Arcuate nucleus. *p < 0.05 relative to saline.
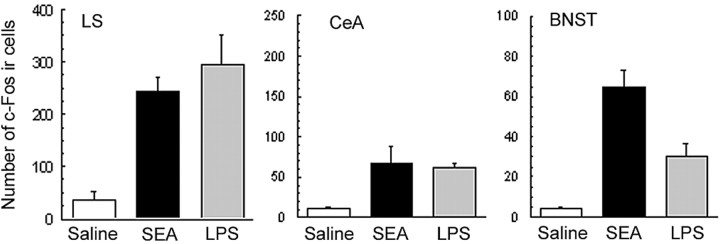
Extrahypothalamic forebrain regions that mediate emotional responses such as fear and anxiety were also examined, with the focus directed on the lateral septum (LS), bed nucleus of the stria terminalis (BNST), and ceA. As shown in Figure 2, the results revealed that both SEA and LPS induced significant increases in the number of c-Fos-immunoreactive cells in all three regions (BNST: F(2,12) = 11.15, p < 0.005; LS: F(2,12) = 16.1, p < 0.001; ceA: F(2,12) = 4.73, p < 0.05).
Figure 2.
Mean number (±SE) of c-Fos-immunoreactive cells in the LS, ceA, and BNST 2 h after intraperitoneal injection with saline (n = 5), 10 μg of SEA (n = 6), or 5 μg of LPS (n = 4). Each bar represents the mean ± SE. *p < 0.05 relative to saline.
Administration of 1-10 μg of SEA to C57BL/6J mice produces by 1 h after injection a significant elevation in plasma TNFα that persists for several hours (Table 2). This is consistent with our previous observations and occurs in the absence of detectable IL-1β in plasma (Kawashima and Kusnecov, 2002), which typically rises coincidentally with TNFα in response to LPS challenge (Netea et al., 1996). Therefore, a second experiment was conducted in which TNF-/- mice were challenged with either 10 μg of SEA or saline. The results showed that in TNF-/- mice, exposure to SEA did not increase the number of c-Fos-immunoreactive cells in brain regions that were shown to be activated in wild-type TNF+/+. This is shown in Figures 3 and 4, with the latter showing the percentage of change from saline controls in the number of c-Fos-immunoreactive cells from SEA-challenged TNF+/+ and TNF-/- mice. Corresponding photomicrographs of c-Fos expression in representative wild-type and knock-out mice challenged with SEA or saline are presented in Figure 3 (see Fig. 5 for the PVN).
Table 2.
Time course of plasma TNF α elevations after SEA challenge
|
Dosea |
Time after challenge (h) |
Plasma TNF α (pg/ml ± SE) |
|---|---|---|
| 1 μg (n = 3/time point) | 1 | 44.2 ± 7.2 |
| 2 | 66.8 ± 7.5 | |
| 4 | 17.3 ± 3.7 | |
| 5 μg (n = 3/time point) | 1 | 79.5 ± 19.7 |
| 2 | 87.7 ± 12.6 | |
| 4 | 26.8 ± 8.8 | |
| 10 μg (n = 3/time point) | 1 | 58.6 ± 7.6 |
| 2 | 80.8 ± 8.5 | |
|
|
4 |
20.9 ± 1.5 |
Control animals (n = 3/time point) were given injections of saline. ELISA results revealed no detectable TNF.
Figure 3.

Brain c-Fos IR in SEA-challenged (10 μg/mouse) wild-type (TNF+/+) and TNFα knock-out (TNF-/-) mice. Representative images of the LS (A, D), ceA (B, E), and BNST (C, F) are shown. The images display the greatest numbers of c-Fos-immunoreactive cells in wild-type mice (A-C) compared withknock-out mice (D-F). Magnification, 10×. LV, Lateral ventricle; ac, anterior commissure.
Figure 4.
Percentage increase in the number of c-Fos-immunoreactive cells in the brains of SEA-challenged wild-type (TNF+/+; n = 6) and TNFα knock-out (TNF-/-; n = 5) mice. For each individual animal that was given an injection of SEA, the quantitation for each brain region was expressed as a percentage above the group mean of the corresponding saline-injected control of the same strain. The mean number of c-Fos-positive cells in wild-type saline-injected (n = 5) (see Fig. 1) and saline-injected TNFα knock-out mice (n = 5) did not differ (mean ± SE for TNF knock-out saline: PVN, 60 ± 34; arcuate nucleus, 55 ± 12; LS, 58 ± 36; BNST, 10.7 ± 2.2; ceA, 8.9 ± 1.9). *p < 0.05 (unpaired Student's t test) relative to the mean percentage of change of the TNFα knock-out group. Arc, Arcuate nucleus.
Figure 5.
Increased c-Fos IR in the PVN of wild-type and TNFα knock-out mice exposed to a psychogenic stressor (open field). Relative to wild-type mice challenged with SEA (A), similarly challenged TNFα knock-out mice failed to increase c-Fos IR in the PVN (B) but displayed a robust increase after exposure to the open field (OF; C). D, Quantitation of the number of c-Fos-immunoreactive cells in wild-type and TNFα knock-out mice revealed no differences in magnitude of responsiveness to open-field exposure (each bar represents mean ± SE for n = 5 animals). Magnification, A-C, 10×.
To confirm that TNF-/- mice were not deficient in c-Fos induction, an experiment was conducted in which knock-out and wild-type mice were exposed to a psychogenic stressor (i.e., an open field) and killed 1 h later. Increased c-Fos IR was widespread throughout the brain. Quantitative data for the PVN is presented in Figure 5, where it can be seen that both TNF+/+ and TNF-/- mice displayed significantly increased numbers of c-Fos-immunoreactive cells after exposure to the open field (F(1,14) = 12.89; p < 0.05).
Food intake
Previously, it was demonstrated that 5 μg of SEA administered to male C57BL/6J mice reduced consumption of a novel liquid diet (Kawashima and Kusnecov, 2002). The foregoing c-Fos data suggest that TNFα production in response to SEA may be important to the effects of SEA on food intake.
Liquid food experiments
The anorexic (or hypophagic) effect of SEA was assessed by challenging wild-type TNF+/+ and mutant TNF-/- mice with SEA or saline and testing 2 h later for food intake. The results revealed a main effect of SEA treatment (F(1,20) = 20.84; p < 0.001) that interacted with mouse genotype (F(1,20) = 10.29; p < 0.001). Figure 6A presents these results, where it can be seen that wild-type mice challenged with SEA showed a reduction in food intake, whereas SEA-challenged TNF-/- knock-out mice did not differ from corresponding saline-injected controls.
This lack of SEA effect in TNF-/- mice may have been a result of an inherent failure to respond to anorexigenic signals. However, as shown in Figure 6B, both knock-out and wild-type mice that were given injections of 100 ng of recombinant murine IL-1β displayed a similar reduction in food intake (F(1,18) = 100.1; p < 0.0001). There was no interaction between IL-1β treatment and genotype (F(1,18) = 0.706; p = 0.4). Hence, these data ruled out the possibility that TNF-/- mice do not respond to anorexigenic signals.
In an additional assessment of the role of TNFα in SEA-induced hypophagic behavior, wild-type C57BL/6J mice were intraperitoneally administered hamster anti-murine TNFα antibody before challenge with SEA. The results are presented in Figure 6C. Antibody treatment significantly attenuated the reduction in Prosobee consumption after SEA, as revealed by significant main effects (F(1,15) = 27.3; p < 0.001) and interaction effects (F(1,15) = 9.0; p < 0.01). These results complemented the consumption data obtained with TNF-/- mice and strongly suggest that the hypophagic influence of SEA on food intake is dependent on TNFα.
Food pellet experiments
An additional experiment was conducted to determine whether SEA challenge would alter ingestion of solid food pellets, as opposed to a liquid diet. To this end, wild-type TNF+/+ mice were familiarized to small (20 mg) food pellets in their home cages or encountered the food pellets for the first time during testing. Animals were tested for food pellet intake 120 min after injection with 10 μg of SEA. The results are presented in Figure 7A. Statistical analysis revealed a main effect of pellet novelty/familiarity (F(1,36) = 180.0; p < 0.0001), in that greater consumption was observed in animals that had been familiarized with the food pellets. In addition, there was a significant interaction between SEA treatment and pellet novelty/familiarity (F(1,36) = 13.3; p < 0.001). Because we had observed previously that Prosobee consumption was reduced in response to SEA, a planned unpaired t test was conducted comparing SEA- and saline-treated animals in the novel pellet condition. As shown in Figure 7A, the difference between SEA- and saline-treated animals was found to be significant (t(18) = -2.71; p = 0.015). These data support previous experiments using Prosobee consumption, in that exposure to unfamiliar food substances elicits a greater neophobic response in SEA-treated animals (Kawashima and Kusnecov, 2002).
An additional experiment was conducted using TNF-/- mice that had not been preexposed in their home cage to the small food pellets. As shown in Figure 7B, SEA- and saline-injected animals did not differ significantly in the amount of novel pellet ingestion. This supported the results of the Prosobee ingestion experiments involving TNF-/- mice (Fig. 6A).
Role of endogenous IL-1
Previously, we had reported an inability to detect (by ELISA) plasma IL-1β in SEA-challenged mice (Kawashima and Kusnecov, 2002). However, mRNA for IL-1β was increased in the spleen after 1-2 h (data not shown). Consequently, we explored the possibility that low concentrations of IL-1β may still be neuromodulatory in SEA-induced hypophagia. The effects of SEA on food intake were tested in mice lacking IL-1RI (IL-1R-/-), which mediates the behavioral effects of IL-1β (Bluthe et al., 2000). The results showed that IL-1R-/- mice responded to 10 μg of SEA with a marked reduction in consumption of Prosobee solution [mean consumption: saline (n = 4), 2.18 ± 0.29 g; SEA (n = 5), 0.43 ± 0.07 g; t(7) = -1.75; p < 0.001]. Therefore, it is unlikely that reduced food intake after SEA involves engagement of IL-1RI.
Corticosterone
Challenge with SEA activates the pituitary-adrenal axis, producing increased plasma levels of ACTH and corticosterone (Kawashima and Kusnecov, 2002; Kaneta and Kusnecov, 2005). To determine whether the lack of PVN c-Fos expression in TNF-/- mice (Figs. 4, 5) also resulted in corresponding endocrine changes, plasma corticosterone was measured 2 h after injection of 10 μg of SEA into wild-type and knock-out mice. The results showed a significant increase in plasma corticosterone in wild-type mice challenged with SEA (F(1,20) = 20.68; p < 0.001). This was attenuated in SEA-challenged TNF-/- mice, as supported by a genotype and toxin interaction (F(1,20) = 13.18; p < 0.01) (Fig. 8A). Furthermore, it was shown that TNF-/- mice are not deficient in adrenocorticoid reactivity, as tested by injection of 100 ng of recombinant murine IL-1β, which significantly elevated plasma corticosterone in both mouse strains (Fig. 8B).
Figure 8.
Plasma corticosterone levels (nanograms per milliliter) in SEA- and saline-treated wild-type (TNF+/+) and TNFα knock-out (TNF-/-) mice. A, Mean plasma corticosterone (±SE) in wild-type and knock-out mice after injection of 10 μg of SEA (n = 5-6 per strain) or saline (n = 6 per strain). B, Mean plasma corticosterone (±SE) in wild-type and knock-out mice after injection of 100 ng of murine recombinant IL-1β (n = 5-6 per strain) or saline (n = 6 per strain). C, Effect of pretreatment with astressin (500 μg/kg) or vehicle (0.2 ml of saline) on mean plasma corticosterone (±SE) in wild-type C57BL/6J mice that were given injections of 10 μg of SEA (n = 5 astressin; n = 6 vehicle) or saline (n = 6 astressin; n = 6 vehicle). Wt, Wild-type; ko, knock-out.
Finally, the likelihood that SEA challenge increased plasma corticosterone via hypothalamic release of CRH into the hypophyseal portal system was tested by pretreating wild-type mice with 500 μg/kg astressin, a nonselective CRH receptor antagonist. Figure 8C shows that astressin significantly attenuated the increase in plasma corticosterone levels after SEA injection (toxin treatment: F(1,20) = 26.96, p < 0.0001; astressin-by-toxin interaction: F(1,20) = 8.83; p < 0.01). Therefore, it is likely that SEA activation of the PVN (Figs. 1, 4) results in an increased release of neurosecretory CRH.
T-cell activation by SEA in TNF-/- mice
It was possible that the failure of SEA challenge to increase neuronal c-Fos IR in TNF-/- mice was attributable to deficient activation of T-cells. Therefore, mice were challenged with 10 μg of SEA and killed 2 h later, and the spleens were removed for assessment of mRNA changes for IL-2 and IFNγ, cytokines exclusively produced by T-cells (Rosendahl et al., 1997). The results are presented in Table 3. Quantitation of mRNA revealed no difference in the amount of IL-2 nor IFNγ mRNA in the spleens of wild-type and mutant mice challenged with SEA. Interestingly, there was a difference in the GAPDH values between TNF-/- and TNF+/+ animals, in that mutant mice (whether SEA or saline treated) had higher values than wild-type animals. However, within each strain, GAPDH did not change as a function of SEA challenge. This allowed amplified cytokine product to be compared accurately with GAPDH (Table 3, Normalization) without the possibility of distortion by SEA treatment. As seen in Table 3, the relationship of the normalizing gene (GAPDH) to the cytokine genes did not change the fact that spleens from SEA-treated animals had dramatically higher cytokine responses relative to saline-treated animals. Moreover, this was equivalent in mutant and wild-type mice.
Table 3.
SEA treatment induces T-cell cytokine mRNA and plasma IFNγ in TNF+/+ and TNF−/− mice
|
|
TNF+/+ (n = 4/treatment) |
TNF−/− (n = 4/treatment) |
||||
|---|---|---|---|---|---|---|
|
|
SEA |
Saline |
SEA |
Saline |
||
| Real-time RT-PCR | ||||||
| Target cDNAa | ||||||
| IFNγ | 54.93 ± 13.5 | 0.438 ± 0.11 | 115.0 ± 22.13 | 1.32 ± 0.52 | ||
| IL-2 | 41.52 ± 11.8 | 0.11 ± 0.16 | 106 ± 40.4 | 0.11 ± 0.02 | ||
| GAPDH | 8.1 ± 0.98 | 6.76 ± 1.6 | 21.24 ± 6.4 | 18.3 ± 4.7 | ||
| Normalizationb | ||||||
| IFNγ/GAPDH | 651.9 ± 79.6 | 6.4 ± 0.4 | 624.5 ± 94.0 | 6.8 ± 1.0 | ||
| IL-2/GAPDH | 483.5 ± 81.7 | 1.8 ± 0.2 | 559.9 ± 164.4 | 2.7 ± 1.4 | ||
| ELISA | ||||||
| IFNγ (ng/ml)c
|
136.74 ± 18.5 |
27.0 ± 4.6 |
177.3 ± 28.0 |
16.8 ± 2.4 |
||
The threshold cycle for each sample was converted to arbitrary units calculated using a standard curve as described in Materials and Methods.
For each individual animal, the calculated arbitrary units for each gene of interest were expressed as a ratio of the corresponding GAPDH units for the same animal and multiplied by 100.
Plasma was assayed for IFNγ by commercial ELISA as described in Materials and Methods.
The latter observation was reinforced by measurement of plasma for IFNγ (insufficient plasma was available for IL-2 determinations). This revealed a similar lack of difference between the two strains (Table 3). Therefore, the immune compartment of TNF-/- mice was competent in the ability to present SEA and activate T-lymphocytes, ruling out the possibility that these mice were immunologically unresponsive.
Discussion
Previous studies had confirmed that SEA, a T-cell SAg, exerts significant effects on pituitary-adrenal activation and neophobic reactivity to gustatory and contextual stimuli (Kawashima and Kusnecov, 2002; Kawashima et al., 2002). The present results suggest that these behavioral and endocrine effects involve underlying recruitment of brain nuclei that coordinate the CNS response to psychogenic and systemic stressors. Moreover, they suggest that production of TNFα is a necessary condition for these and other behavioral and endocrine effects to be observed after T-cell activation with SEA.
In so far as immune reactivity to SEA recruited key forebrain regions involved in stress, the hypothalamus was especially reactive in the PVN and arcuate subdivisions. Interestingly, c-Fos IR remained unchanged in the VMH and LH, areas traditionally implicated in appetite regulation (Elmquist et al., 1999; Schwartz et al., 2000). Therefore, SEA-induced hypophagia may use alternative neural substrates regulating food intake, such as the arcuate, which is pivotal to controlling food intake (Schwartz et al., 2000). Because the arcuate provides afferent connections to the PVN, it is plausible that populations of cells expressing anorexigenic peptides (e.g., CRH) were activated by SEA challenge. However, despite activation by nonimmunological (e.g., immobilization or restraint) and immunological (e.g., IL-1) stressors, the relevance of the arcuate to immune-derived anorexia has been questioned (Reyes and Sawchenko, 2002).
The activation of PVN neurons by SEA suggests possible involvement of CRH-positive neurons. Because the nonselective CRH receptor antagonist astressin (Gulyas et al., 1995) blocked the corticosterone response to SEA, it is likely that CRH-secreting neurons in the PVN were activated. Consideration should also be given to the BNST, another region activated by SEA that influences hypophagia via local CRH receptor engagement (Ciccocioppo et al., 2003). Indeed, central CRH receptor blockade abrogates anorexia induced by SEA (Kaneta and Kusnecov, 2005), although the relevant sites of CRH receptor engagement are not known.
The c-Fos data replicate the results of another study involving rats administered SEB (Wang et al., 2004), which stimulates a different subpopulation of T-cells than SEA (Gonzalo et al., 1993). Administration of SEB stimulated brain regions that we showed to be activated in mice given SEA (Wang et al., 2004). This is also in keeping with our previous data that SEB showed increased c-Fos and CRH mRNA expression in the PVN and ceA (Shurin et al., 1997; Kusnecov et al., 1999). Interestingly, the impact of SEB on central c-Fos expression in rats was attenuated by subdiaphragmatic vagotomy (Wang et al., 2004). This suggests that superantigenic effects on CNS function involve communication by vagal afferents, although it remains to be determined whether the effects of SEA in mice are similarly dependent on this mechanism. Indeed, given that the present results reveal a dependence on TNFα production, it is noteworthy that the neuroendocrine effects of exogenous TNFα rely on an intact vagus (Fleshner et al., 1998).
The hypophagia induced by SEA (as well as SEB) appears to be dependent on the novelty of palatable liquid diet (Kusnecov et al., 1999; Kawashima and Kusnecov, 2002). We extended this observation, demonstrating that SEA reduced voluntary ingestion of novel, but not familiar, food pellets, and it should also be noted that SEA does not affect operant responding for familiar food pellets (our unpublished observations). Therefore, illness-induced anorexia is unlikely to be an explanation for the reduction in food intake. More likely, the data suggest that anxiety/fear-like processes underlying neophobic reactivity may be augmented by SEA treatment, thereby promoting anorexia and/or greater avoidance of a novel diet. This may involve central CRH receptors (Kaneta and Kusnecov, 2005), because CRH also attenuates ingestion of novel foods (Heinrichs and Koob, 1992). Moreover, the SEA-induced activation of neural substrates for anxiety and/or fear, in particular the ceA, BNST, and lateral septum (Davis, 1998; Sheehan et al., 2004), also substantiates this hypothesis. Therefore, activation of these regions provides neuroanatomical support for the contention that the behavioral changes observed in response to SEA involve a neophobic and/or anxiogenic component.
The systemic impetus for SEA-induced CNS activation involves TNFα production, because SEA did not increase c-Fos expression in TNFα-deficient mice. This was not an intrinsic failure to activate the c-fos gene, because exposure to a psychogenic stressor (i.e., an open field) increased brain c-Fos in TNFα-deficient mice. Complementary experiments also showed that TNFα-deficient mice responded to IL-1β with corticosterone and hypophagic responses. Therefore, endogenous TNFα is required to observe increased brain c-Fos IR after exposure to SEA, which is consistent with previous data showing that exogenous TNFα increases c-Fos IR in the PVN and ceA (Hayley et al., 2001). In additional experiments, TNFα-deficient mice failed to show corticosterone and hypophagic responses to SEA, and antibody to TNFα blocked SEA-induced reduction in food intake in wild-type mice. Collectively, these data strongly implicate TNFα as a critical mediator of SEA-induced neuromodulation.
How TNFα contributes to the neural effects of SEA is unknown but could involve transport of TNFα into the brain via TNFα receptor-mediated processes (Pan and Kastin, 2002). Indeed, the type I (p55) TNFα receptor is expressed in various circumventricular and parenchymal regions of the brain, including the PVN (Nadeau and Rivest, 1999). Moreover, in p55 TNFα receptor-deficient mice, there is a significant attenuation of the corticosterone elevation induced by centrally administered TNFα (Benigni et al., 1996), whereas in rats undergoing turpentine-induced inflammation, the ACTH and corticosterone response is attenuated by central antagonism of TNFα (Turnbull et al., 1997). Consequently, circulating levels of endogenous TNFα may pass into the brain and stimulate p55 type I TNFα receptors on parenchymal cells, including those in the PVN. Whether this occurs in the hypothalamus remains to be determined. However, future studies can use double-label immunohistochemistry to determine whether c-Fos-positive cells also express TNFα receptors, as well as CRH.
The induction of secondary cytokines dependent on TNFα production cannot be ruled out, although in the present study, it does not appear that IL-1 is critical. For example, previous studies had suggested that TNFα exerts behavioral effects in part through IL-1 induction (Bluthe et al., 1991, 1994), although SEA injection does not result in measurable IL-1β in plasma (Kawashima and Kusnecov, 2002). Although trace amounts of IL-1 could still exert biological effects, this was contraindicated by IL-1RI-deficient mice still showing hypophagia after SEA challenge. Because the anorectic effects of IL-1 rely on the presence of IL-1RI (Bluthe et al., 2000), these data question the involvement of IL-1 in the anorexic effects of SEA.
The absence of a corticosterone response to SEA in TNFα-deficient mice was consistent with the lack of PVN activation. Astressin treatment blocked corticosterone elevation in response to SEA, suggesting that CRH neurosecretory cells in the PVN are responsive to endogenous production of TNFα. This is consistent with reports of increased CRH IR in the PVN and median eminence after administration of TNFα to CD-1 mice (Hayley et al., 2001). Interestingly, use of the strong TNFα inducer LPS has implicated TNFα in HPA axis activation. However, in contrast to the present findings, the CNS effects of LPS do not rely entirely on TNFα. For example, blockade of TNFα only partially attenuated the ACTH and corticosterone increase induced by LPS (Perlstein et al., 1993; Ebisui et al., 1994; Turnbull and Rivier, 1998), and in TNF receptor I knock-out mice, attenuation of the corticosterone response to LPS occurred only at a lower dose (Hayley et al., 2004).
These observations raise questions concerning the relative neuromodulatory contributions of different cytokines elaborated by various bacterial toxins. In the case of SEA, and possibly other SAgs, TNFα is produced together with a range of other T-cell-derived cytokines, although potent neuromodulatory cytokines like IL-1β are not elevated (Shurin et al., 1997; Kawashima and Kusnecov, 2002). In contrast, LPS induces not only TNFα but also IL-1 and IL-6, which all have considerable effects on the CNS. Hence, it can be hypothesized that the primacy of TNFα as a neuromodulator depends on the presence and/or absence of other coreleased neuromodulatory cytokines. Whereas any further discussion is speculative, the contrasting reactivity of the CNS to TNFα induced by LPS and SEA suggests that distinct patterns of cytokine production may influence how the brain responds to each individual cytokine. It is notable that circulating levels of TNFα achieved in response to SEA were in the subnanogram range, whereas that to LPS is tenfold higher (our unpublished observations). Furthermore, preliminary results have suggested that TNFα knock-out animals retain anorexia and central c-Fos induction in response to 1-5 μg of LPS (our unpublished observations). Hence, the potency of endogenous TNFα may vary depending on concomitant production of other cytokines that may serve either restrictive and/or permissive roles in CNS activation. This concept was already advanced through demonstrations of IL-1, TNFα, and IL-6 synergism (Brebner et al., 2000). In the case of LPS, the presence of other neuromodulatory cytokines may mitigate TNFα exerting an excessive influence. With SAgs, such as SEA, the induction of T-cell-derived cytokines and the relative lack of IL-1 may increase the likelihood that TNFα ensures optimal stimulation of the CNS. Indeed, it should be noted that increases in the number of brain c-Fos-immunoreactive cells were comparable between SEA- and LPS-challenged mice (Fig. 1), despite the fact that LPS induces much higher concentrations of TNFα.
In conclusion, the current results demonstrate that activation of T-lymphocytes by a SE elicits functional recruitment of brain areas devoted to the processing of stress-related information. This is dependent on the elaboration of TNFα, the presence of which is also necessary for the endocrine and anorexic responses to SEA. The precise pathway of influence exerted by TNFα remains to be determined. Multiple extant mechanisms are possible, such as the vagus, additional TNFα induction in the brain, and TNFα extravasation into brain parenchyma (Pan and Kastin, 2002). Whatever the relevant downstream processes, the present results strongly identify TNFα production as a necessary step in the alteration of brain and behavioral function after challenge with a T-cell SAg.
Footnotes
This work was supported by United States Public Health Service Grants MH60706 and DA141186 and National Institute of Environmental Health Sciences Center Grant P30 ES05022.
Correspondence should be addressed to Dr. Alexander W. Kusnecov, Department of Psychology, Rutgers, The State University of New Jersey, Piscataway, NJ 08854. E-mail: kusnecov@rci.rutgers.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255314-09$15.00/0
References
- Ando T, Dunn AJ (1999) Mouse tumor necrosis factor-alpha increases brain tryptophan concentrations and norepinephrine metabolism while activating the HPA axis in mice. Neuroimmunomodulation 6: 319-329. [DOI] [PubMed] [Google Scholar]
- Benigni F, Faggioni R, Sironi M, Fantuzzi G, Vandenabeele P, Takahashi N, Sacco S, Fiers W, Buurman WA, Ghezzi P (1996) TNF receptor p55 plays a major role in centrally mediated increases of serum IL-6 and corticosterone after intracerebroventricular injection of TNF. J Immunol 157: 5563-5568. [PubMed] [Google Scholar]
- Bette M, Schafer MK, Van Rooijen N, Weihe E, Fleischer B (1993) Distribution and kinetics of superantigen-induced cytokine gene expression in mouse spleen. J Exp Med 178: 1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW (1991) Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J Pharmacol 209: 281-283. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R (1994) Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 19: 197-207. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P (2000) Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci 12: 4447-4456. [PubMed] [Google Scholar]
- Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H (2000) Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 22: 566-580. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M (2003) The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci 23: 9445-9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M (1998) Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry 44: 1239-1247. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Kabiersch A, Petzoldt S, Besedovsky HO (2002) Involvement of noradrenergic nerves in the activation and clonal deletion of T cells stimulated by superantigen in vivo. J Neuroimmunol 127: 44-53. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergel AH (1998) The role of cytokines in infection-related behavior. Ann NY Acad Sci 840: 577-585. [DOI] [PubMed] [Google Scholar]
- Ebisui O, Fukata J, Murakami N, Kobayashi H, Segawa H, Muro S, Hanaoka I, Naito Y, Masui Y, Ohmoto Y, (1994) Effect of IL-1 receptor antagonist and antiserum to TNF-alpha on LPS-induced plasma ACTH and corticosterone rise in rats. Am J Physiol 266: E986-E992. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB (1999) From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221-232. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Goehler LE, Schwartz BA, McGorry M, Martin D, Maier SF, Watkins LR (1998) Thermogenic and corticosterone responses to intravenous cytokines (IL-1beta and TNF-alpha) are attenuated by subdiaphragmatic vagotomy. J Neuroimmunol 86: 134-141. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. San Diego: Academic.
- Goehler LE, Gaykema RP, Hansen MK, Kleiner JL, Maier SF, Watkins LR (2001) Staphylococcal enterotoxin B induces fever, brain c-Fos expression, and serum corticosterone in rats. Am J Physiol Regul Integr Comp Physiol 280: R1434-R1439. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Gonzalez-Garcia A, Martinez C, Kroemer G (1993) Glucocorticoid-mediated control of the activation and clonal deletion of peripheral T cells in vivo. J Exp Med 177: 1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas J, Rivier C, Perrin M, Koerber SC, Sutton S, Corrigan A, Lahrichi SL, Craig AG, Vale W, Rivier J (1995) Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci USA 92: 10575-10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Staines W, Merali Z, Anisman H (2001) Time-dependent sensitization of corticotropin-releasing hormone, arginine vasopressin and c-fos immunoreactivity within the mouse brain in response to tumor necrosis factor-alpha. Neuroscience 106: 137-148. [DOI] [PubMed] [Google Scholar]
- Hayley S, Wall P, Anisman H (2002) Sensitization to the neuroendocrine, central monoamine and behavioural effects of murine tumor necrosis factor-alpha: peripheral and central mechanisms. Eur J Neurosci 15: 1061-1076. [DOI] [PubMed] [Google Scholar]
- Hayley S, Kelly O, Anisman H (2004) Corticosterone changes in response to stressors, acute and protracted actions of tumor necrosis factor-alpha, and lipopolysaccharide treatments in mice lacking the tumor necrosis factor-alpha p55 receptor gene. Neuroimmunomodulation 11: 241-246. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF (1992) Corticotropin-releasing factor modulates dietary preference in nutritionally and physically stressed rats. Psychopharmacology (Berl) 109: 177-184. [DOI] [PubMed] [Google Scholar]
- Kaneta T, Kusnecov AW (2005) The role of central corticotropin-releasing hormone in the anorexic and endocrine effects of the bacterial T cell superantigen, staphylococcal enterotoxin A. Brain Behav Immun 19: 138-146. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Kusnecov AW (2002) Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunol 123: 41-49. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Fugate J, Kusnecov AW (2002) Immunological challenge modulates brain orphanin FQ/nociceptin and nociceptive behavior. Brain Res 949: 71-78. [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, Liang R, Shurin G (1999) T-lymphocyte activation increases hypothalamic and amygdaloid expression of CRH mRNA and emotional reactivity to novelty. J Neurosci 19: 4533-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Barden N, Rivest S (1997) Corticotropin-releasing factor and glucocorticoid receptor (GR) gene expression in the paraventricular nucleus of immune-challenged transgenic mice expressing type II GR anti-sense ribonucleic acid. J Mol Neurosci 8: 165-179. [DOI] [PubMed] [Google Scholar]
- Marrack P, Winslow GM, Choi Y, Scherer M, Pullen A, White J, Kappler JW (1993) The bacterial and mouse mammary tumor virus superantigens; two different families of proteins with the same functions. Immunol Rev 131: 79-92. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, Mizoguchi A, Shichi H, Fukuda Y, Nakao K, Narumiya S (2003) Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc Natl Acad Sci USA 100: 4132-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Rivest S (1999) Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience 93: 1449-1464. [DOI] [PubMed] [Google Scholar]
- Netea MG, Demacker PN, Kullberg BJ, Boerman OC, Verschueren I, Stalenhoef AF, van der Meer JW (1996) Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest 97: 1366-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Niemi MB, Kou W, Harting M, Del Rey A, Besedovsky HO, Schedlowski M (2004) Behavioural endocrine immune-conditioned response is induced by taste and superantigen pairing. Neuroscience 129: 555-562. [DOI] [PubMed] [Google Scholar]
- Pan JZ, Jornsten R, Hart RP (2004) Screening anti-inflammatory compounds in injured spinal cord with microarrays: a comparison of bioinformatics analysis approaches. Physiol Genomics 17: 201-214. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ (2002) TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol 174: 193-200. [DOI] [PubMed] [Google Scholar]
- Perlstein RS, Whitnall MH, Abrams JS, Mougey EH, Neta R (1993) Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology 132: 946-952. [DOI] [PubMed] [Google Scholar]
- Petersson K, Forsberg G, Walse B (2004) Interplay between superantigens and immunoreceptors. Scand J Immunol 59: 345-355. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Rabin BS (1992) Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res 597: 41-50. [DOI] [PubMed] [Google Scholar]
- Proft T, Fraser JD (2003) Bacterial superantigens. Clin Exp Immunol 133: 299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Sawchenko PE (2002) Involvement of the arcuate nucleus in interleukin-1 induced anorexia. J Neurosci 22: 5091-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE (2003) Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23: 5607-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl A, Hansson J, Antonsson P, Sekaly RP, Kalland T, Dohlsten M (1997) A mutation of F47 to A in staphylococcus enterotoxin A activates the T-cell receptor Vbeta repertoire in vivo. Infect Immun 65: 5118-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, LeBlanc F, Kaneta T, Urbach D, Kusnecov AW (2004) Effects of bacterial superantigens on behavior of mice in the elevated plus maze and light-dark box. Brain Behav Immun 18: 46-54. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404: 661-671. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS (2004) Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev 46: 71-117. [DOI] [PubMed] [Google Scholar]
- Shurin G, Shanks N, Nelson L, Hoffman G, Huang L, Kusnecov AW (1997) Hypothalamic-pituitary-adrenal activation by the bacterial superantigen staphylococcal enterotoxin B: role of macrophages and T cells. Neuroendocrinology 65: 18-28. [DOI] [PubMed] [Google Scholar]
- Torres BA, Kominsky S, Perrin GQ, Hobeika AC, Johnson HM (2001) Superantigens: the good, the bad, and the ugly. Exp Biol Med 226: 164-176. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL (1998) Intracerebroventricular passive immunization. I. The effect of intracerebroventricular administration of an antiserum to tumor necrosis factor-alpha on the plasma adrenocorticotropin response to lipopolysaccharide in rats. Endocrinology 139: 119-127. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Pitossi FJ, Lebrun JJ, Lee S, Meltzer JC, Nance DM, del Rey A, Besedovsky HO, Rivier C (1997) Inhibition of tumor necrosis factor-alpha action within the CNS markedly reduces the plasma adrenocorticotropin response to peripheral local inflammation in rats. J Neurosci 17: 3262-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Duan XL, Guo X, Ju G (2004) Fos expression in the rat brain after intraperitoneal injection of staphylococcus enterotoxin B and the effect of vagotomy. Neurochem Res 29: 1667-1674. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, Greenberg AH (1994) Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res 643: 40-49. [DOI] [PubMed] [Google Scholar]