Abstract
Downregulation of oligodendrocyte-related genes, referred to as oligodendrocyte dysfunction, in schizophrenia has been revealed by DNA microarray studies. Because oligodendrocyte-specific transcription factors regulate the differentiation of oligodendrocytes, genes encoding them are prime candidates for oligodendrocyte dysfunction in schizophrenia. We found that the cytosine-guanine dinucleotide (CpG) island of sex-determining region Y-box containing gene 10 (SOX10), an oligodendrocyte-specific transcription factor, tended to be highly methylated in brains of patients with schizophrenia, correlated with reduced expression of SOX10. We also found that DNA methylation status of SOX10 also was associated with other oligodendrocyte gene expressions in schizophrenia. This may be specific to SOX10, because the CpG island of OLIG2, which encodes another oligodendrocyte-specific transcription factor, was rarely methylated in brains, and the methylation status of myelin-associated oligodendrocytic basic protein, which encodes structural protein in oligodendrocytes, did not account for their expressions or other oligodendrocyte gene expressions. Therefore, DNA methylation status of the SOX10 CpG island could be an epigenetic sign of oligodendrocyte dysfunction in schizophrenia.
Keywords: oligodendrocyte, postmortem, schizophrenia, DNA methylation, epigenetics, SOX10
Introduction
Schizophrenia is a severe mental disorder with symptoms such as hallucination, delusion, disorganized thought, and impairment of cognitive functions. Twin, family, and adoption studies have revealed that complex interactions between hereditary and environmental factors are involved in the etiology of schizophrenia (Gottesman, 1991).
Several lines of evidence suggest the alteration of oligodendrocytes in schizophrenia. Electron microscopic studies revealed the ultrastructural alteration of oligodendroglias (Uranova et al., 2004). Immunohistochemical analysis revealed the downregulation of oligodendrocyte proteins in gray matter (Honer et al., 1999; Flynn et al., 2003) and alteration in number and density of oligodendrocytes in layer III of the cortex in schizophrenia (Hof et al., 2003). Although the results remain inconclusive, magnetic resonance imaging studies suggested some changes in white matter in patients with schizophrenia, and these changes are considered related to the above findings (Davis et al., 2003; Stewart and Davis, 2004).
Gene expression analyses using DNA microarray also support the alteration of oligodendrocytes in schizophrenia (Bunney et al., 2003; Mirnics et al., 2004). Coordinated downregulation of a subset of oligodendrocyte-related genes (referred to as oligodendrocyte dysfunction) in prefrontal [Brodmann area (BA) 46 (Hakak et al., 2001; Tkachev et al., 2003) or BA47 (Sugai et al., 2004)] and temporal [BA21 (Aston et al., 2004)] cortices of patients with schizophrenia have been revealed.
Among the downregulated oligodendrocyte genes, we focused on sex-determining region Y-box containing gene 10 (SOX10), because a combination of transcription factors such as SOX10 and oligodendrocyte lineage transcription factor 1 (OLIG1) and OLIG2 regulates the differentiation of oligodendrocytes (Kessaris et al., 2001), and that SOX10 is responsible for terminal differentiation of oligodendrocytes (Stolt et al., 2002). Here, we examined the DNA methylation status of the SOX10 cytosine-guanine dinucleotide island in brains of patients with schizophrenia. We found that the DNA methylation status of SOX10 can, but that of OLIG2 or myelin-associated oligodendrocytic basic protein (MOBP) cannot, account for its downregulation and oligodendrocyte dysfunction in schizophrenia.
Materials and Methods
Postmortem brains. Postmortem prefrontal cortices (BA10) were provided by the Stanley Foundation Brain Collection (The Stanley Medical Research Institute, Bethesda, MD). The materials originally were provided as frozen tissues, consisted of gray matter and a small portion of white matter, and were coded blindly to diagnostic and demographic variables. Homogenate of the frozen tissue was used for extraction of total RNA and genomic DNA. Information about postmortem brains has been described previously (Torrey et al., 2000). Briefly, controls and patients were matched for age, gender, postmortem interval, and sample pH. We previously obtained gene expression profiles using an Affymetrix U95Av2 chip (Affymetrix, Santa Clara, CA) from 50 subjects, including controls (n = 15) and patients with schizophrenia (n = 13) (Iwamoto et al., 2004). Genetic and epigenetic analyses were performed after the blinded codes were opened.
Three additional brain samples (BA10), including two controls and one with psychotic disorder, were also provided by the Stanley Foundation Brain Collection. These three specimens were divided into gray and white matter portions. Genomic DNA and total RNA were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA).
Cell culture. A lymphoblastoid cell line, TC42, was established from the lymphocytes of a 28-year-old Japanese male with no history of neuropsychiatric diseases using standard techniques (Kato et al., 2002). SHSY5Y (a human neuroblastoma), 1321N1 (a human astrocytoma; a gift from Dr. Norimichi Nakahata, Tohoku University, Miyagi, Japan), and A2058 (a human melanoma) cell lines were maintained in DMEM containing 10% fetal bovine serum. A melanoma cell line, A2058, was selected as a SOX10-expressing cell line (Su et al., 2002) using the SOURCE website, (http://source.stanford.edu) (Diehn et al., 2003). Total RNA and genomic DNA were extracted from the harvested cells using TRIzol reagent.
Expression studies. After the DNase I treatment, 5 μg of total RNA was used for cDNA synthesis by oligo(dT) primer and SuperScript II reverse transcriptase (RT) (Invitrogen). Quantitative RT-PCR (qRT-PCR) using SYBER/GREEN I dye (Applied Biosystems, Foster city, CA) was performed with ABI7900 (Applied Biosystems). The comparative threshold cycle (Ct) method was used for quantification according to the protocol of the manufacture (Applied Biosystems). Measurement of δ Ct was performed at least three times per sample. Amplification of the single product was confirmed by monitoring the dissociation curve and by electrophoresis. In addition to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), we used two other genes [β actin (ACTB) and cofilin 1 (CFL1)] for normalization to control for possible fluctuations in quantitative values of the target transcripts. Primer pairs for GAPDH, ACTB, and CFL1 have been shown previously (Iwamoto et al., 2004). Primer pairs used for qRT-PCR are as follows: SOX10, 5′-CCAGTA CCCGCACCTGCAC-3′ and 5′-CTTTCGTTCAGCAGCCTCCAG-3′; OLIG2, 5′-AAGCTTTCCAAGATCGCCACG-3′ and 5′-TAGATCTCGCTCACCAGTCGCTTC-3′; MOBP, 5′-ACTCCGAACACTTCAGCATACACT-3′ and 5′-GATCCAGTCCTCCTCTTTCTTCTG-3′.
Genetic studies. For mutation screening of SOX10, we used genomic DNA extracted from postmortem liver samples of the 13 patients with schizophrenia whose brain RNA was used for the DNA microarray. All exons, exon-intron boundaries, and 1 kb upstream regions from the first exons in the splice variants were sequenced. Primer pairs and PCR conditions are available on request.
Epigenetic studies. To confirm biallelic expression in SOX10, we amplified a part of SOX10 containing a single-nucleotide polymorphism (SNP) (rs139883) with the primers 5′-ACCACTCCTATGACTCCTGTTTTCTC-3′ and 5′-ATAGAGCCTAGTAAGGGAAGAGGGA-3′ using brain-derived cDNA as a template. PCR products were directly sequenced or cloned using a TOPO TA cloning kit (Invitrogen). Single bacterial colonies were subjected to sequencing analysis.
Bisulfite modification of genomic DNA derived from postmortem brains (BA10) or cell lines was done as follows. After denaturation, 1-2 μg of genomic DNA was treated with 3.6 m sodium bisulfite. The reaction was performed at 55°C for 16 h. Genomic DNA was then purified with a Wizard DNA Clean-up kit (Promega, Madison, MI) and eluted with 50 μl of water. We typically used 2-5 μl of bisulfite-modified DNA for PCR. The CpG islands (Gardiner-Garden and Frommer, 1987) of oligodendrocyte genes were obtained through the University of California, Santa Cruz Genome Browser (http://www.genome.ucsc.edu/index). Primer pairs determined using MethPrimer software (Li and Dahiya, 2002) were as follows: SOX10, 5′-TGGGTAAGGTTAAGAAGGAGTAGTAG-3′ and 5′-CTACCTAAACCCACACCATAAAAAC-3′; OLIG2-1, 5′-TTTTAAGTTTTTGTTTTTAGTTGGG-3′ and 5′-AATCTCCTCCCTAACTCTTCCTCTAT-3′; OLIG2-2, 5′-TAGAGGAAGAGTTAG-GGAGGAGATT-3′ and 5′-ACCACCACAAAATCAAATTAAAAAA-3′; OLIG2-3, 5′TTAAAGAAAGGTTTTTATTTTTTATT-3′ and 5′-TCTCTAACCCTCCTTTTAACTACAC-3′; MOBP, 5′-TTAGAAGAAAGAGGAGGATTG-GATT-3′ and 5′-CTTCCAATCTCCCTAAAATACCTTC-3′. After the separation by agarose gel electrophoresis, PCR products were excised, purified, and TA cloned. Single-bacterial colonies were subject to sequencing analysis.
Results
Downregulation of SOX10 in schizophrenia
Using our previous DNA microarray data (Iwamoto et al., 2004), we confirmed the downregulation of oligodendrocyte-related genes, including SOX10 in schizophrenia (supplemental Table 1, available at www.neurosci.org as supplemental material). Downregulation of SOX10 was confirmed by qRT-PCR (Fig. 1A). Expression of SOX10 was not significantly correlated with age (r = 0.037; p = 0.851; n = 28), postmortem interval (r = -0.168; p = 0.392; n = 28), or pH (r = 0.034; p = 0.863; n = 28). Furthermore, expression of SOX10 did not significantly differ according to gender (male, n = 17; female, n = 11; p = 0.327) or the side of the brain (right, n = 11; left, n = 17; p = 0.423). These results were confirmed when we used qRT-PCR data or the remaining DNA microarray data of patients with other mental disorders for calculation (data not shown).
Figure 1.

Expression and epigenetic analyses of SOX10. A, qRT-PCR of SOX10. p values were calculated by the Mann-Whitney U test. C, Control subjects (n = 14); SZ, schizophrenic patients (n = 13). The original data set (Torrey et al., 2000) was composed of 15 control subjects and 15 schizophrenic patients. One control subject was omitted from qRT-PCR data analysis, because we could not obtain appropriate amplification curves. Samples of two schizophrenic patients were omitted from DNA microarray analysis and qRT-PCR, because they showed poor quality, as revealed by denatured gel electrophoresis and the results of Test2chip (Affymetrix). B, Examples of DNA methylation status in a control subject (left) and a schizophrenic patient (right). Results of all subjects can be found in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). Open circle, Nonmethylated CpG; closed circle, methylated CpG. C, Biallelic expression of SOX10. The arrow indicates an SNP at the 3′-untranslated region (reverse orientation of rs139883) in the postmortem brain cDNA sequence. Biallelic expression was also confirmed by cloning and sequence analysis of three independent subjects who had heterozygous alleles with regard to rs139883. D, Inverse correlation between methylation status and expression level of SOX10.
Mutation screening of SOX10
Genetic mutations in SOX10 cause neurological diseases such as peripheral demyelinating neuropathy, central demyelinating leukodystrophy, Waardenburg syndrome, and Hirschprung disease (known collectively as PCWH) (Inoue et al., 2004). Because some mutations result in the generation of unstable mRNAs that are rapidly degraded by the nonsense-mediated decay (NMD) pathway, downregulation of SOX10 in schizophrenia may be accounted for by such mutations. To explore this possibility, we performed mutation screening by sequencing all exons, exon-intron boundaries, and 1 kb upstream regions from the first exons of the splice variants in 13 patients with schizophrenia. This analysis was performed using liver-derived genomic DNA, the brain RNA of which was used for the DNA microarray study. We did not find the putative functional SNPs that change amino acid sequences or cause degradation by the NMD pathway (Inoue et al., 2004). There were also no significant differences in the expression levels of SOX10 among genotypes or haplotypes of polymorphisms detected in these patients (data not shown).
Epigenetic analysis of SOX10
To further examine the cause of SOX10 downregulation, we investigated its DNA methylation status in schizophrenic brains, because hypermethylation is known to correlate with silencing of gene expression in normal and pathological conditions. The brain-derived genomic DNA (BA10) was bisulfite modified and then a part of the CpG island of SOX10 was amplified (Fig. 2A). The amplicon was TA cloned and subjected to sequencing analysis. We performed this analysis in each of 23 subjects (12 controls, 11 schizophrenics). Genomic DNA from the rest of the subjects (three controls, two schizophrenics) did not show sufficiently good quality for methylation analysis. We found that clones contained either densely or nonmethylated alleles (Fig. 1B) (supplemental Fig. 1, available at www.neurosci.org as supplemental material). This methylation pattern cannot be explained by monoallelic expression, because expression from both alleles was confirmed (Fig. 1C). We arbitrarily defined a methylated allele as one in which 30% or more of CpG dinucleotides are methylated in the examined region (Fig. 1B). We then determined for each subject the percentage of the total methylated alleles. Patients with schizophrenia showed a tendency of higher percentage of methylated alleles and lower expression levels of SOX10 compared with control subjects. There was an inverse correlation between the percentage of methylated alleles and expression level of SOX10 (r = -0.586; p = 0.003; n = 23), suggesting that downregulation of SOX10 was associated with an increased percentage of the methylated allele in brains (Fig. 1D). The inverse correlation between percentage of methylated alleles and expression level was not dependent on the definition of methylated allele (Table 1). There were no significant differences of DNA methylation status among genotypes or haplotypes of polymorphisms detected in these patients (data not shown). Additionally, we found that the methylation status of SOX10 was also related to downregulations of other oligodendrocyte genes that were differentially expressed in schizophrenia (Table 2).
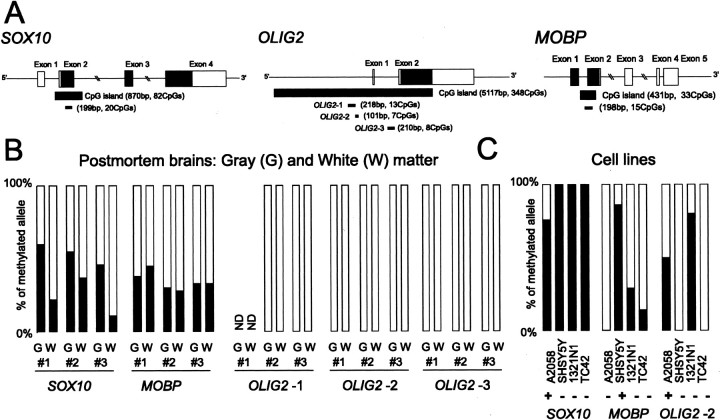
Figure 2.
DNA methylation status of oligodendrocyte genes. A, Genomic structure and CpG island of SOX10, OLIG2, and MOBP. Exons are denoted by boxes, with untranslated regions in white and translated regions in black. PCR-amplified regions for methylation analysis are underlined. B, DNA methylation status in postmortem brains. The percentage of methylated alleles is indicated in black. ND, Not determined. C, DNA methylation status in human cell lines. A2058, SHSY5Y, 1321N1, and TC42 are melanoma, neuroblastoma, astrocytoma, and lymphoblastoid cell lines, respectively. Expression levels of each oligodendrocyte gene were measured (+, detected; -, not detected). The relative expression levels in relation to GAPDH are as follows: 0.03363 (SOX10 in A2058), 0.00025 (OLIG2 in A2058), and 0.00005 (MOBP in SHSY5Y).
Table 1.
Definition of methylated allele and correlation with expression levels
|
|
Definition of a methylated allele |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
20% |
30% |
40% |
50% |
60% |
70% |
80% |
||||||
| SOX10 | |||||||||||||
| r | −0.534 | −0.586 | −0.579 | −0.574 | −0.485 | −0.419 | −0.366 | ||||||
| p | 0.009 | 0.003 | 0.004 | 0.004 | 0.019 | 0.047 | 0.086 | ||||||
| MOBP | |||||||||||||
| r | 0.139 | 0.005 | 0.005 | 0.019 | 0.007 | 0.104 | −0.021 | ||||||
|
p
|
0.528 |
0.981 |
0.981 |
0.931 |
0.974 |
0.636 |
0.924 |
||||||
A methylated allele was defined as an allele that gave a percentage or more of methylated CpGs of the total number of CpGs in the examined region. There were 20 and 15 CpGs in SOX10 and MOBP, respectively, in the examined region. Pearson's r and significance (p) values are given.
Table 2.
Correlation between methylation status and expression levels of oligodendrocyte genes
|
|
Methylation status |
||||||
|---|---|---|---|---|---|---|---|
|
SOX10 (n = 23) |
MOBP (n = 23) |
||||||
|
|
r
|
p
|
r
|
p
|
|||
| SOX10 | −0.586 | 0.003 | −0.139 | 0.527 | |||
| OLIG2 | −0.533 | 0.009 | −0.130 | 0.554 | |||
| MAG | −0.592 | 0.003 | −0.109 | 0.620 | |||
| PLP1 | −0.640 | 0.001 | −0.141 | 0.520 | |||
| MOBP | −0.527 | 0.010 | 0.005 | 0.981 | |||
| OMG | −0.074 | 0.738 | −0.073 | 0.740 | |||
| PMP22 | −0.605 | 0.002 | −0.146 | 0.507 | |||
| MAL | −0.618 | 0.002 | −0.138 | 0.529 | |||
| CNP | −0.485 | 0.019 | −0.147 | 0.505 | |||
| TF | −0.531 | 0.009 | 0.044 | 0.842 | |||
|
GSN
|
−0.513 |
0.012 |
−0.061 |
0.783 |
|||
Pearson's correlation coefficient (r) and p values are given. CNP, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; GSN, gelsolin; MAL, myelin and lymphocyte protein; OMG, oligodendrocyte-myelin glycoprotein; PLP1, myelin proteolipid protein; PMP22, peripheral myelin protein 22; TF, transferrin.
To test whether the DNA methylation status of SOX10 is different between gray and white matters, we divided another set of postmortem samples into gray and white matter portions and then examined their methylation status. The percentage of methylated alleles was significantly greater in gray matter than in white matter (p = 0.02; t test) (Fig. 2B) (supplemental Fig. 2, available at www.neurosci.org as supplemental material), consistent with the SOX10 expression in oligodendrocytes. We next examined the SOX10 methylation status in human cell lines. The SOX10-expressing cell line had nonmethylated alleles, whereas the SOX10-nonexpressing cell lines had only methylated alleles (Fig. 2C) (supplemental Fig. 3, available at www.neurosci.org as supplemental material). Together, these results suggest that expression of SOX10 is associated with the presence of nonmethylated CpG island alleles.
Epigenetic analysis of OLIG2 and MOBP
To test whether correlation between methylation and gene expression was also observed in other genes, we examined the methylation status of OLIG2 and MOBP. OLIG2 plays a role in the specification of oligodendrocytes and motor neurons (Lu et al., 2002), and MOBP is one of the major protein components of myelin. Both genes were consistently downregulated in schizophrenia, as revealed by a previous DNA microarray study (Tkachev et al., 2003) and by our results. Because the CpG island of OLIG2 spans >5 kb, we examined its methylation status in three regions (OLIG2-1 to OLIG2-3) (Fig. 2A). We found that the CpG island of OLIG2 was rarely methylated in gray or white matter in brains (Fig. 2B) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). We further examined the methylation status in several control and schizophrenia samples and also found that none of the subjects had a densely methylated CpG allele (supplemental Fig. 4, available at www.neurosci.org as supplemental material). In the case of MOBP, both gray and white matter portions contained the methylated alleles (Fig. 2B) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). However, there are no apparent differences in the percentage of methylated alleles between gray and white matter. In addition, the methylation status of MOBP did not correlate with its expression level (Table 1) or the expression levels of other oligodendrocyte genes (Table 2) (supplemental Fig. 5, available at www.neurosci.org as supplemental material). In cell lines, the DNA methylation status of the CpG islands of OLIG2 and MOBP were not associated with their expression patterns, suggesting that their expressions were not regulated through DNA methylation in these cell lines (Fig. 2C) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
Discussion
Accumulating evidence suggests that complex mental disorders may be mediated through aberrant epigenetic processes (Costa et al., 2003; Abdolmaleky et al., 2004; Petronis, 2004). However, there are few studies that document the epigenetic status directly in the brains of patients. To date, DNA methylation of the reelin promoter was suggested to be involved in its downregulation in schizophrenia (Veldic et al., 2004; Abdolmaleky et al., 2005), and site-specific DNA methylation of catechol-O-methyltransferase promoter was proposed to be altered in schizophrenia (Murphy et al., 2005). Our present findings provide the first evidence concerning the epigenetic aspects of oligodendrocyte dysfunction in schizophrenia. We found that the DNA methylation status of SOX10 inversely correlates with expression levels of SOX10 and other oligodendrocyte genes. Considering that expression of oligodendrocyte genes are regulated by a combination of oligodendrocyte-specific transcription factors (Kessaris et al., 2001) and the role of SOX10 in the oligodendrocyte differentiation (Stolt et al., 2002), it would be reasonable to assume that the majority of the oligodendrocyte-expressed genes, including those listed in Table 2, are under the direct or indirect control of SOX10. It is not known whether observed oligodendrocyte dysfunction was accompanied by substantial loss or increase of specific cell populations. However, variations in the sampling step of postmortem brains were not likely to be the cause for this dysfunction, because not all of oligodendrocyte-specific genes or glial genes were downregulated in schizophrenia by independent DNA microarray studies, including ours, and some reports revealed the oligodendrocyte dysfunction in gray matter of patients with schizophrenia (Honer et al., 1999; Hakak et al., 2001; Flynn et al., 2003).
Our findings at the mRNA levels were in good accordance with previous reports at the protein levels such as downregulation of 2′, 3′-cyclic nucleotide 3′-phosphodiesterase in schizophrenia (Flynn et al., 2003). However, the results of some genes such as myelin basic protein (Honer et al., 1999) or myelin-associated glycoprotein (MAG) (Flynn et al., 2003) were not compatible with mRNA findings. This was partly caused by the presence of complex splicing isoforms (Tkachev et al., 2003).
We cannot completely rule out the effect of medication on oligodendrocyte gene expressions in this study. Our sample set included three medication-free patients with schizophrenia (including a patient who was treated with electroconvulsive treatment). Consistent with a previous report (Tkachev et al., 2003), these medication-free patients also showed the typical downregulations of oligodendrocyte genes (Fig. 1D), suggesting that these expression changes were not caused by the medication. In addition, expression and DNA methylation levels of SOX10 were not significantly correlated with a lifetime antipsychotic dose (fluphenazine-equivalent dose) in the schizophrenia group (r = -0.078 and r = 0.066, respectively).
Oligodendrocyte dysfunction has also been reported in bipolar disorder (Tkachev et al., 2003) and major depression (Aston et al., 2005). Our analysis of BA10 (Iwamoto et al., 2004) failed to detect oligodendrocyte dysfunction in bipolar disorder and major depression, whereas that of BA46 (Iwamoto et al., 2005) revealed the downregulation of oligodendrocyte genes in both diseases (data not shown). Therefore, contrary to the case of schizophrenia, there might be regional differences in oligodendrocyte dysfunction in other mental disorders. Alternatively, the discrepancy between our data sets with respect to bipolar disorder may be caused by the difference in patient population.
We do not know which factors affect the percentage of methylated SOX10 alleles in schizophrenic brains at this stage. Mutation screening suggests that genetic variations in SOX10 were not involved in its expression level or DNA methylation status. The increased DNA methylation of SOX10 in gray matter, compared with that in white matter, and results from cell line experiments suggest the dysfunction and/or decreased number of SOX10-expressing cells in brains of patients with schizophrenia. Considering previous DNA microarray results (Hakak et al., 2001; Tkachev et al., 2003; Aston et al., 2004) along with our results, which show the downregulation of a subset of oligodendrocyte genes, specific gene expression deficits within oligodendrocytes are probable. Because postmortem samples included both gray and a small portion of white matter, it is not clear whether possible defects were restricted in gray matter.
Importantly, the DNA methylation status of MOBP and OLIG2 cannot explain their expression levels or expressions of other oligodendrocyte genes. Although the expression of SOX10, a gene that is important for oligodendrocyte differentiation, is regulated through global DNA methylation of the CpG island, other oligodendrocyte genes may not be tightly regulated at that level. Therefore, the DNA methylation status of the SOX10 CpG island provides a clue in elucidating the regulation of oligodendrocyte gene expressions, and it could be an epigenetic sign of oligodendrocyte dysfunction in schizophrenia.
Footnotes
Postmortem brains and livers were donated by the Stanley Foundation Brain Collection, courtesy of Drs. Michael B. Knable, E. Fuller Torrey, Maree J. Webster, and Robert H. Yolken. We thank Dr. Kazuhiko Ikeda for helpful discussions. We are indebted to the Research Resource Center at our institute for DNA microarray and sequencing analyses.
Correspondence should be addressed to Dr. Kazuya Iwamoto, Laboratory for Molecular Dynamics of Mental Disorders, RIKEN Brain Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: kaziwamoto@brain.riken.jp.
Copyright © 2005 Society for Neuroscience 0270-6474/05/255376-06$15.00/0
References
- Abdolmaleky HM, Smith CL, Faraone SV, Shafa R, Stone W, Glatt SJ, Tsuang MT (2004) Methylomics in psychiatry: modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet 127: 51-59. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT (2005) Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet 134: 60-66. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP (2004) Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res 77: 858-866. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP (2005) Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 10: 309-322. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG, Vawter MP, Tomita H, Li J, Evans SJ, Choudary PV, Myers RM, Jones EG, Watson SJ, Akil H (2003) Microarray technology: a review of new strategies to discover candidate vulnerability genes in psychiatric disorders. Am J Psychiatry 160: 657-666. [DOI] [PubMed] [Google Scholar]
- Costa E, Grayson DR, Guidotti A (2003) Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention? Mol Interv 3: 220-229. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V (2003) White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 60: 443-456. [DOI] [PubMed] [Google Scholar]
- Diehn M, Sherlock G, Binkley G, Jin H, Matese JC, Hernandez-Boussard T, Rees CA, Cherry JM, Botstein D, Brown PO, Alizadeh AA (2003) SOURCE: a unified genomic resource of functional annotations, ontologies, and gene expression data. Nucleic Acids Res 31: 219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG (2003) Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry 8: 811-820. [DOI] [PubMed] [Google Scholar]
- Gardiner-Garden M, Frommer M (1987) CpG islands in vertebrate genomes. J Mol Biol 196: 261-282. [DOI] [PubMed] [Google Scholar]
- Gottesman I (1991) Schizophrenia genesis: the origins of madness. New York: Freeman.
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA (2001) Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 98: 4746-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich Jr VL, Byne W, Buitron C, Perl DP, Davis KL (2003) Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 53: 1075-1085. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Chen C, Arango V, Mann JJ, Dwork AJ (1999) Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience 91: 1247-1255. [DOI] [PubMed] [Google Scholar]
- Inoue K, Khajavi M, Ohyama T, Hirabayashi S, Wilson J, Reggin JD, Mancias P, Butler IJ, Wilkinson MF, Wegner M, Lupski JR (2004) Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet 36: 361-369. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Kakiuchi C, Bundo M, Ikeda K, Kato T (2004) Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol Psychiatry 9: 406-416. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T (2005) Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet 14: 241-253. [DOI] [PubMed] [Google Scholar]
- Kato T, Ishiwata M, Nagai T (2002) Mitochondrial calcium response in human transformed lymphoblastoid cells. Life Sci 71: 581-590. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Pringle N, Richardson WD (2001) Ventral neurogenesis and the neuron-glial switch. Neuron 31: 677-680. [DOI] [PubMed] [Google Scholar]
- Li LC, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18: 1427-1431. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109: 75-86. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Levitt P, Lewis DA (2004) DNA microarray analysis of postmortem brain tissue. Int Rev Neurobiol 60: 153-181. [DOI] [PubMed] [Google Scholar]
- Murphy BC, O'Reilly RL, Singh SM (2005) Site-specific cytosine methylation in S-COMT promoter in 31 brain regions with implications for studies involving schizophrenia. Am J Med Genet B Neuropsychiatr Genet 133: 37-42. [DOI] [PubMed] [Google Scholar]
- Petronis A (2004) The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry 55: 965-970. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Davis KL (2004) Possible contributions of myelin and oligodendrocyte dysfunction to schizophrenia. Int Rev Neurobiol 59: 381-424. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M (2002) Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 16: 165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB (2002) Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99: 4465-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C, Nakamura R, Kakita A, Takahashi H, Nawa H (2004) Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann NY Acad Sci 1025: 84-91. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S (2003) Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362: 798-805. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH (2000) The stanley foundation brain collection and neuropathology consortium. Schizophr Res 44: 151-155. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI (2004) Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 67: 269-275. [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E (2004) DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA 101: 348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]