Abstract
Respiratory dysfunction after cervical spinal cord injury (SCI) has not been examined experimentally using conscious animals, although clinical SCI most frequently occurs in midcervical segments. Here, we report a C5 hemicontusion SCI model in rats with abnormalities that emulate human post-SCI pathophysiology, including spontaneous recovery processes. Post-C5 SCI rats demonstrated deficits in minute ventilation (Ve) responses to a 7% CO2 challenge that correlated significantly with lesion severities (no injury or 12.5, 25, or 50 mm × 10 g weight drop; New York University impactor; p < 0.001) and ipsilateral motor neuron loss (p = 0.016). Importantly, C5 SCI resulted in at least 4 weeks of respiratory abnormalities that ultimately recovered afterward. Because serotonin is involved in respiration-related neuroplasticity, we investigated the impact of activating 5-HT1A receptors on post-C5 SCI respiratory dysfunction. Treatment with the 5-HT1A agonist 8-hydroxy-2-(di-n-propylmino)tetralin (8-OH DPAT) (250 μg/kg, i.p.) restored hypercapnic Ve at 2 and 4 weeks after injury (i.e., ∼39.2% increase vs post-SCI baseline; p ≤ 0.033). Improvements in hypercapnic Ve response after single administration of 8-OH DPAT were dose dependent and lasted for ∼4 h(p ≤ 0.038 and p ≤ 0.024, respectively). Treatment with another 5-HT1A receptor agonist, buspirone (1.5 mg/kg, i.p.), replicated the results, whereas pretreatment with a 5-HT1A-specific antagonist, 4-iodo-N-[2-[4(methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-benzamide (3 mg/kg, i.p.) given 20 min before 8-OH DPAT negated the effect of 8-OH DPAT. These results imply a potential clinical use of 5-HT1A agonists for post-SCI respiratory disorders.
Keywords: spinal cord injury, cervical, hemicontusion, respiration, serotonin 1A, buspirone
Introduction
Respiratory disorders are the leading cause of morbidity and mortality after spinal cord injury (SCI), affecting approximately half of all patients with a neurological deficit after SCI (Winslow and Rozovsky, 2003). Respiratory impairments resulting from cervical SCI, the most common clinical case, frequently render survivors chronically or permanently ventilator dependent, a sequela that can dramatically compromise quality of life (Winslow and Rozovsky, 2003). However, respiratory abnormalities after cervical injuries have not been studied in conscious animal models, and there are no drug treatments for breathing disorders of SCI.
We reported previously that respiratory dysfunction after SCI is partly attributable to loss of the motoneurons innervating respiratory muscles (Teng et al., 1999). This was demonstrated in a thoracic vertebra 8 (T8) SCI rat model, in which acute treatment with basic fibroblast growth factor (bFGF) mitigated respiratory motoneuron loss and consequently preserved respiratory function. Unfortunately, only a very limited therapeutic window for strategies to reduce post-SCI motoneuron loss exists because of rapid postinjury neuronal degeneration (Teng et al., 1998, 1999). After T8 SCI, death of ventral motoneurons is completed within 4-24 h after SCI, and bFGF has to be given within 5 min after injury (Teng et al., 1998, 1999; Grossman et al., 2001). Considering that 90% of SCIs are initially incomplete, we subsequently designed an alternative therapeutic approach with the aim of enhancing the function of surviving respiratory motoneurons using serotonin 1A (5-HT1A) agonists (Teng et al., 2003). This group of agents was recently identified as respiratory stimulants that can counteract respiratory disturbances produced by hypoxia, morphine overdose, or obstructive sleep apnea (Mendelsohn et al., 1991; Lalley et al., 1994; Sahibzada et al., 2000). Administration of 8-hydroxy-2-(di-n-propylmino)tetralin (8-OH DPAT), the prototypical 5-HT1A-specific agonist, restored respiratory frequency (f), tidal volume (Vt), and minute ventilation (Ve) to pre-SCI levels in T8 SCI rats (Teng et al., 2003). These data suggested that stimulation of surviving respiratory motoneurons via systemic 5-HT1A agonists might become a novel therapy to improve post-SCI respiration.
Agonists of 5-HT1A receptors have yet to be tested in animal models that closely emulate pathophysiology derived from cervical pathology commonly seen in clinical SCI. Of note, respiratory recovery in our T8 SCI model occurred consistently by 7 d after injury (Teng et al., 1999, 2003). Moreover, clinical SCI affects lower thoracic levels far less frequently than midcervical segments. The National Spinal Cord Injury Database (1993-1998) recorded that 47% of SCIs in the cohort were C4-C7 cases, with C5 carrying the greatest frequency (Go et al., 1995). We therefore hypothesized that a C5 hemicontusion model would adequately emulate the pathophysiology of clinical post-SCI respiratory dysfunction while offering practical surgical procedures, postinjury animal care, and survival rates appropriate for laboratory operations. We tested our hypothesis by using a well established whole-body plethysmography (WBP) system (Teng et al., 1999, 2003; Gray et al., 2001) and examined the therapeutic effects of 5-HT1A agonists in conscious post-SCI rats.
Materials and Methods
Spinal cord hemicontusion injury. A 3.0 mm left-sided hemilaminectomy encompassing the caudal end of vertebral level C4 and the rostral end of vertebral level C5 was produced in female Sprague Dawley rats (236-298 g; Charles River Laboratories, Wilmington, MA) after anesthesia with intraperitoneal ketamine (75 mg/kg) and xylazine (10 mg/kg). We used the New York University (NYU) impactor, a standardized device for delivery of contusive SCI in rats (Gruner, 1992), to produce an incomplete hemicontusive injury at the C5 spinal cord level from a height of 12.5 mm (mild SCI; n = 12), 25 mm (moderate SCI; n = 13), or 50 mm (severe SCI; n = 16). Additional animals were given laminectomies only without SCI (n = 10) or no surgery and anesthesia at all (n = 9). We provided postoperative care as described previously (Teng et al., 1999, 2004).
Monitoring of respiratory parameters by plethysmography. Before base-line preoperative respiratory recordings, rats initially completed plethysmograph chamber acclimation training as described previously (Teng et al., 1999, 2003). In addition, a 20 min acclimation phase in the chamber preceded all initial respiratory data acquisition sessions for each rat. After acclimation, rats remained quietly in the chamber, allowing for the acquisition of data without physical signs of stress (e.g., defecation, urination). Respiratory data were measured noninvasively in unanesthetized, conscious, unrestrained, spontaneously breathing rats using a bias flow-ventilated WBP designed for rodents (PLY3213; Buxco Electronics, Wilmington, NC) (Gray et al., 2001). We used the Epstein barometric method to derive lung ventilation parameters from pressure fluctuations within chambers of fixed volume (Epstein et al., 1980). Pressure changes in the chamber were captured by a differential pressure transducer, amplified, and integrated by a software program (Biosystem XA 2.7.9; Buxco Electronics, Wilmington, NC) into volumes. A negative bias flow regulator, set to 1.5 L/min, ensured that fresh room air or alternatively concentrated CO2 gas was steadily supplied into the chambers and that CO2 from breaths expired from the animal did not accumulate. Hypercapnic gas mixtures contained either 7% CO2, 90% O2, and N2 balance or 5% CO2, 90% O2, and N2 balance. Hyperoxic (i.e., 90% O2) gas was used to ensure that the brief respiratory drive surges were initiated by hypercarbia in our long-term studies (i.e., not from hypoxia that also affects the respiratory system) (Feldman et al., 2003). Respiratory parameters from the current study were all within ranges similar to those our laboratory reported previously in which 60% O2 was used (Teng et al., 1999, 2003). Periods when subjects were moving around in the plethysmograph chamber, sniffing, grooming, or otherwise not in a mode of quiet conscious breathing were marked by trained observers during data collection and were excluded from analysis (Teng et al., 1999, 2003; Gray et al., 2001; Stephenson and Gucciardi, 2002).
Drug administration. The 5-HT1A-specific agonists 8-OH DPAT (Tocris Cookson, Ellisville, MO) and buspirone (Sigma, St. Louis, MO) were dissolved in 0.9% saline (adjusted to pH 7.4) and administered intraperitoneally in 0.5 ml of final injection volume per rat at doses of 125 or 250 μg/kg for 8-OH DPAT and 1.5 mg/kg for buspirone (Teng et al., 2003). The 5-HT1A receptor-specific antagonist 4-iodo-N-[2-[4-methoxy-phenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-benzamide (p-MPPI) (Sigma, St. Louis, MO) was dissolved in 0.9% saline and given intraperitoneally at a dose of 3 mg/kg, pH 7.4 (final volume, 0.5 ml), before 8-OH DPAT administration (Teng et al., 2003).
Neurobehavioral studies. The forelimb functional test was done by using a modified Tarlov scale ranging from 0 (no movement) to 5 (normal, weight-bearing forelimb use) as described previously (Gale et al., 1985). To measure hindlimb function, we modified the Basso-Beattie-Bresnahan (BBB) scoring process (the BBB scale ranges from 0 to 21, where 0 indicates total hindlimb paralysis and 21 indicates normal performance) (Basso et al., 1995) by referencing hindlimb movements on the injured side only with those of the forelimb on both the uninjured and injured sides to obtain scores of coordination resulting from dysfunction in the forepaw ipsilateral to the hemicontusion SCI. Because forelimb movement of the injured side alone was not precise enough to evaluate stepping, the intact side forelimb was used as a reference to best judge whether the affected hindlimb moved in combination with the ipsilateral forelimb (i.e., coordination; rats move ipsilateral forelimb and hindlimb simultaneously). Neurobehavioral scores for both the hemicontused and contralateral sides were recorded by two evaluators blinded to the injury doses of the experimental groups.
Histopathology. Rats for histopathological analysis were terminated at 4 weeks after SCI (n = 4 per group; i.e., mild, moderate, severe, or no hemicontusion injury) using intracardiac perfusion with 0.1 m phosphate buffer and 4% paraformaldehyde as described previously (Teng et al., 1999, 2003). Spinal cord histopathology using cresyl violet and solvent blue/hematoxylin and eosin stains were performed as described previously (Teng et al., 2004) to evaluate the dimensions and anatomical profiles of the lesion volume and to evaluate postcontusion motoneuronal loss (Noble and Wrathall, 1989; Teng et al., 1998). All experiments were conducted in strict compliance with the Laboratory Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals after approval by the Animal Care and Use Committee of the Veterans Affairs Boston Healthcare System.
Experimental protocol. A total of 60 animals were used for this study. After 1 week of plethysmograph chamber acclimation training (Teng et al., 1999), preoperative baseline respiratory recording was performed for each rat in room air and 7% CO2. SCI surgery for rats in the surgical groups was performed 24 h after baseline respiratory recording. Postoperative respiratory and neurobehavioral studies were performed at 1 d after SCI and then at 1, 2, 3, 4, and 6 weeks after SCI. At each WBP session, respiratory parameters, including f, Vt, and Ve were collected for 5 min during room air ventilation. Each animal was then exposed to a gas mixture containing 7% CO2 for 7 min, and the same parameters were recorded. At the 2 week post-SCI period, we performed a separate study for all rats using 5% CO2 to confirm a respiratory dose-response to hypercapnia [i.e., to compare respiratory parameters under 0.03% (room air), 5%, and 7% CO2]. As described previously, only the last 2 min of data during either 5 or 7% hypercapnic challenge was used for analysis (Teng et al., 1999).
At 5 d after SCI, four randomly selected rats with severe hemicontusion SCI underwent a washout (i.e., a transformed dose-response) study of the respiratory effect of 8-OH DPAT (250 μg/kg, i.p.). Respiratory parameters in room air (for 5 min) and under 7% CO2 (7 min, with the last 2 min used for data analysis) were recorded 15 min after an intraperitoneal 8-OH DPAT injection, with the 12 min (5 plus 7 min) recording period repeated at the end of every consecutive hour for up to 5 h as described previously (Teng et al., 2003). Studies to evaluate the effects of 8-OH DPAT (250 μg/kg) across injury dose groups were performed at 2 and 4 weeks after SCI in subgroups of rats randomly sampled from each uninjured and hemicontused group (mild, n = 9; moderate, n = 9; severe, n = 12; uninjured controls, n = 13). Rats were allowed to rest at least 60 min in room air before being injected intraperitoneally with 8-OH DPAT (250 μg/kg). After a 15 min postinjection period, room air respiratory data were recorded for 5 min. Immediately after room air recording, each rat was then exposed to 7% CO2 for 7 min, and the last 2 min were used for data analysis.
Twelve rats in the severely injured group underwent additional testing with buspirone (1.5 mg/kg), p-MPPI (3 mg/kg), and a lower dose of 8-OH DPAT (125 μg/kg) separately. Because of the large number of tests performed at the 2 week post-SCI period and the lengthy waiting periods necessary to washout 8-OH DPAT, these tests were randomly divided over a 3 d period that corresponded to days 13-15 after SCI. We delivered p-MPPI 20 min before 8-OH DPAT injection and recorded 5 min of room air respiratory data 5 min after the p-MPPI injection. Fifteen minutes after the 8-OH DPAT injection, respiratory parameters were recorded in room air for 5 min, followed by 7% CO2 for 7 min. Respiratory parameters were recorded 15 min after buspirone (1.5 mg/kg, i.p.) injection.
Statistics. Respiratory study data for the overall C5 hemicontusion model were analyzed by a generalized linear model (GLM) resulting from dissimilarities in the number of observations per subject (i.e., some rats were terminated at 4 weeks for histopathological study), constraining the use of standard ANOVA techniques (Kleinbaum et al., 1998). Post hoc linear regression was performed when general analysis showed a significant difference. The dependent variables were f, Vt, and Ve, whereas injury dose, body weight, and CO2 level were tested as predictors. Nonlongitudinal multiple linear regression was used to examine short-term changes in f during the acute post-SCI period. To examine the effects of the pharmacological agents, respiratory parameters were compared using repeated-measures ANOVA, with post hoc Tukey's test (Teng et al., 2003). Correlations between Ve at the time the animals were killed and lesion size or motoneuronal counts were examined using Pearson correlation. Correlations between injury dose and lesion size or motoneuronal counts were examined using Spearman correlation. Statistics were performed using the STATA 8.2 statistical package (StataCorp, College Station, TX). We considered differences in data to be significant when p < 0.05. All interventions and primary data collection were performed in a blinded manner.
Results
C5 hemicontusion SCI produces lesion dose-dependent respiratory deficits
Clinical studies have revealed that deficits in post-SCI respiratory parameters correlate with injury severity (Linn et al., 2001). We therefore subjected rats to varying grades of contusive SCI at the C5 level, delegated as mild (10 g × 12.5 mm; n = 12), moderate (10 g × 25 mm; n = 13), or severe (10 g × 50 mm, n = 16) injury using an NYU impactor (Gruner, 1992). Because midline cervical SCI in rats dramatically impairs respiration and food procurement to cause very poor postoperative survival, we administered a left-sided hemicontusion injury. In anticipation of potentially greater morbidity and mortality in groups receiving more severe injury doses, we assigned higher numbers of subjects to these groups. Only one rat each in the moderate and severely injured groups died postoperatively, however, with subsequent removal of their data from the study. Control rats received C5 left hemilaminectomy only without SCI (n = 10) or no surgery (n = 9).
To avoid the potential artifacts stemming from physical restraints (Teng et al., 1999, 2003), anesthesia (Teng and Wrathall, 1996), or instrumentation (Teng and Wrathall, 1996), we measured respiratory parameters noninvasively in conscious, spontaneously breathing rats using WBP after sufficient acclimation (Fig. 1) (Teng et al., 1999, 2003). Respiratory parameters (i.e., f, Vt, and Ve) for controls and for rats before surgery in room air and under hypercapnic conditions (data not shown) were consistent with the literature (Lai et al., 1978; Teng et al., 1999, 2003). No significant differences in body weight or respiratory function recordings were detected between the group that received C5 hemilaminectomy only and the nonoperative group at any time point (data not shown), and their data were therefore combined to form a single control group (n = 19; Teng et al., 1998). Body weights in rats with hemicontusion injury decreased during the initial post-SCI period in correlation with injury dose (e.g., |r| = 0.616; p < 0.001 at 7 d; Spearman correlation) (Fig. 2A). By week 6 after SCI, there was only a weak correlation between body weight and injury dose (|r| = 0.255; p = 0.024; Spearman correlation); rats given mild or moderate injury recovered their body weights to levels comparable with controls.
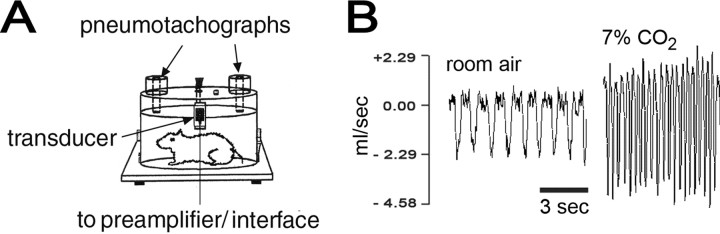
Figure 1.
Noninvasive measurement of respiratory function in conscious rats using WBP. A, Diagram of a fixed-volume (3.9 L) plethysmograph chamber for WBP testing of unrestrained conscious rats. After acclimation, rats remained calm within the chambers for prolonged periods (up to 5 h). B, WBP flow tracings (ml/s) of an uninjured rat exposed to room air (left) and 7% CO2 (right). The tracings were converted to respiratory parameters such as f, Vt, and Ve by software.
Figure 2.
More severe grades of C5 hemicontusion injury resulted in greater loss of postinjury body weight and higher degrees of respiratory dysfunction. A, The effect of lesion dose (uninjured control vs 12.5, 25, or 50 mm C5 hemicontusion injury) is shown to correlate inversely with body weight during the 6 week post-SCI course. Note that rats with 50 mm injuries did not recover to control levels, even after 6 weeks (*p < 0.05; Spearman correlation). ctrl, Control; p.i., postinjury. B, Representative ventilatory parameters at 2 weeks after SCI versus uninjured controls. Error bars represent SEM. *p < 0.05; GLM, post hoc multiple linear regression. mod, Moderate injury. Left, Respiratory frequency as a function of lesion dose (uninjured control vs 12.5, 25, or 50 mm C5 hemicontusion injury) and CO2 exposure (room air, 5% CO2, or 7% CO2). The CO2 level was a significant covariate across all lesion dose groups (p < 0.001). Lesion dose was a significant covariate during room air ventilation (p = 0.037). Rats with more severe lesions had a tendency for greater resting respiratory frequency while breathing room air but had a diminished augmentation of response to hypercapnic stimulus. Middle, Tidal volume as a function of lesion dose and CO2 exposure. The CO2 level was a significant covariate across all lesion dose groups (p < 0.001). Lesion dose was a significant covariate during room air ventilation and 5% and 7% CO2 ventilation (p ≤ 0.017). Right, Minute ventilation as a function of lesion dose and CO2 exposure. The CO2 level was a significant covariate across all lesion dose groups (p < 0.001). Rats with all grades of C5 hemicontusion injury, resulting from a combination of higher f and diminished Vt, had resting Ve similar to those of controls while breathing room air. When challenged with hypercapnic stimulus, rats with C5 hemicontusion injury displayed a lesion dose-dependent diminished augmentation of minute ventilation response compared with uninjured controls (p = 0.035 at 5% CO2; p < 0.001 at 7% CO2).
Primary data analysis using a GLM revealed that lesion dose (i.e., uninjured controls or mild, moderate, or severe hemicontusion injury) had a significant effect on Vt and Ve (p < 0.001), but not f (p = 0.116), over the 6 week post-SCI course (Table 1). Importantly, post hoc multiple linear regression analyses showed that groups receiving hemicontusion injury had a statistically significant injury dose-dependent reduction of Vt during room air exposure during the first 2 weeks after injury (p ≤ 0.037) (Fig. 2B, Table 1). Additionally, the data revealed a lesion dose-dependent posthemicontusion increase in room air f, resulting in a shallow, rapid breathing pattern during the first 2 weeks after injury (p ≤ 0.044; GLM with post hoc multiple linear regression) (Table 1), which was consistent with our previous reports (Teng et al., 1999, 2003). Room air ventilatory parameters (f, Vt, and Ve) in hemicontused rats recovered to levels not statistically different from controls 2 weeks after injury.
Table 1.
Lesion dose-dependent respiratory parameters after C5 hemicontusion SCI
|
|
Day 1 |
Week 1 |
Week 2 |
Week 4 |
Week 6 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injury dose |
0.03% CO2
|
7% CO2
|
0.03% CO2
|
7% CO2
|
0.03% CO2
|
5% CO2
|
7% CO2
|
0.03% CO2
|
7% CO2
|
0.03% CO2
|
7% CO2
|
||||||
| f (bpm) | |||||||||||||||||
| Control | 103.0 ± 2.2 | 172.5 ± 4.9 | 108.0 ± 3.3 | 171.6 ± 3.5 | 105.2 ± 2.8 | 145.3 ± 4.2 | 174.8 ± 3.8 | 100.6 ± 2.4 | 178.6 ± 4.4 | 99.2 ± 2.2 | 178.8 ± 4.5 | ||||||
| 12.5 mm | 112.8 ± 1.5 | 160.6 ± 2.0 | 112.0 ± 2.0 | 163.2 ± 2.2 | 108.7 ± 1.8 | 144.2 ± 2.6 | 170.4 ± 2.4 | 106.1 ± 1.7 | 173.6 ± 2.9 | 101.6 ± 1.5 | 180.7 ± 3.0 | ||||||
| 25 mm | 122.5 ± 1.6 | 148.7 ± 2.9 | 116.0 ± 2.2 | 154.9 ± 2.4 | 112.2 ± 1.9 | 143.2 ± 2.6 | 166.1 ± 2.6 | 108.1 ± 3.3 | 168.6 ± 3.2 | 104.0 ± 1.7 | 182.6 ± 3.4 | ||||||
| 50 mm | 132.2 ± 2.4 | 136.9 ± 4.4 | 120.0 ± 3.6 | 146.6 ± 3.9 | 115.7 ± 3.1 | 142.1 ± 4.2 | 161.7 ± 4.3 | 103.0 ± 3.1 | 163.6 ± 5.2 | 106.3 ± 2.7 | 184.5 ± 5.4 | ||||||
| p < 0.001† | p < 0.001† | p = 0.044† | p < 0.001† | p = 0.037† | p = 0.651 | p = 0.057 | p = 0.771 | p = 0.063 | p = 0.084 | p = 0.476 | |||||||
| Vt (ml) | |||||||||||||||||
| Control | 1.35 ± 0.03 | 2.74 ± 0.05 | 1.32 ± 0.03 | 2.75 ± 0.08 | 1.33 ± 0.03 | 2.37 ± 0.06 | 2.97 ± 0.06 | 1.47 ± 0.03 | 3.08 ± 0.06 | 1.52 ± 0.03 | 3.20 ± 0.06 | ||||||
| 12.5 mm | 1.24 ± 0.02 | 2.36 ± 0.04 | 1.28 ± 0.02 | 2.58 ± 0.05 | 1.30 ± 0.02 | 2.29 ± 0.04 | 2.78 ± 0.04 | 1.46 ± 0.02 | 2.98 ± 0.04 | 1.49 ± 0.02 | 3.15 ± 0.04 | ||||||
| 25 mm | 1.14 ± 0.02 | 1.98 ± 0.04 | 1.25 ± 0.02 | 2.41 ± 0.05 | 1.25 ± 0.02 | 2.20 ± 0.04 | 2.59 ± 0.04 | 1.44 ± 0.02 | 2.89 ± 0.04 | 1.47 ± 0.02 | 3.10 ± 0.05 | ||||||
| 50 mm | 1.02 ± 0.03 | 1.60 ± 0.06 | 1.18 ± 0.04 | 2.24 ± 0.09 | 1.22 ± 0.03 | 2.12 ± 0.06 | 2.40 ± 0.06 | 1.43 ± 0.04 | 2.79 ± 0.07 | 1.44 ± 0.04 | 3.04 ± 0.07 | ||||||
| Adjusted r2 | 0.432 | 0.784 | 0.196 | 0.375 | 0.150 | 0.249 | 0.424 | 0.092 | 0.332 | 0.124 | 0.092 | ||||||
| p < 0.001* | p < 0.001* | p = 0.028* | p = 0.001* | p = 0.017* | p = 0.017* | p < 0.001* | p = 0.477 | p = 0.007* | p = 0.194 | p = 0.165 | |||||||
| Ve (ml/min) | |||||||||||||||||
| Control | 139.6 ± 3.2 | 475.1 ± 14.3 | 145.1 ± 3.7 | 476.8 ± 18.7 | 138.9 ± 3.7 | 345.2 ± 12.4 | 519.4 ± 15.9 | 152.3 ± 4.4 | 540.5 ± 18.4 | 149.5 ± 4.7 | 578.6 ± 20.2 | ||||||
| 12.5 mm | 138.4 ± 2.1 | 387.7 ± 9.5 | 143.3 ± 2.5 | 426.4 ± 11.3 | 140.0 ± 2.3 | 330.2 ± 7.7 | 476.4 ± 10.0 | 151.1 ± 2.9 | 516.1 ± 12.0 | 151.7 ± 3.1 | 571.7 ± 13.3 | ||||||
| 25 mm | 137.1 ± 2.3 | 300.3 ± 10.2 | 141.6 ± 2.3 | 376.0 ± 12.4 | 140.2 ± 2.5 | 315.2 ± 7.7 | 433.5 ± 10.9 | 150.0 ± 3.3 | 491.8 ± 13.8 | 153.9 ± 3.5 | 564.8 ± 15.2 | ||||||
| 50 mm | 135.8 ± 3.5 | 213.0 ± 15.6 | 138.1 ± 4.7 | 325.6 ± 20.6 | 140.3 ± 4.1 | 300.1 ± 12.5 | 390.5 ± 17.7 | 148.8 ± 5.3 | 467.4 ± 21.8 | 156.1 ± 5.5 | 557.9 ± 23.9 | ||||||
| Adjusted r2 | 0.025 | 0.737 | 0.029 | 0.434 | 0.026 | 0.216 | 0.374 | 0.029 | 0.146 | 0.002 | 0.038 | ||||||
|
|
p = 0.475 |
p < 0.001*
|
p = 0.335 |
p < 0.001*
|
p = 0.952 |
p = 0.035*
|
p < 0.001*
|
p = 0.669 |
p = 0.031*
|
p = 0.429 |
p = 0.568 |
||||||
Respiratory frequency (bpm, breaths per minute), tidal volume, and minute ventilation measured by WBP in conscious, unrestrained rats with no SCI (n = 19) or after 12.5 (n = 12), 25 (n = 12), or 50 (n = 15) mm C5 hemicontusion injury (× 10 g; NYU impactor) at 1 d and 1, 2, 4, and 6 weeks after SCI in room air (0.03% CO2) and under hypercapnic conditions (5 or 7% CO2 plus 90% O2 and N2 balance). Week 3 data did not differ significantly from week 4 data and are not presented. Four rats from each group were killed after collection of respiratory data at week 4 for histological studies; thus, week 6 data represent 15,8,8, and 11 animals for the control, 12.5, 25, and 50 mm groups, respectively. All parameters represent means (corrected by linear regression for group differences in body weight) ± SE of the linear prediction of the mean. The asterisk indicates primary GLM positive (p< 0.05; post hoc linear regression). The dagger symbol indicates nonlongitudinal multiple linear regression (p < 0.05).
To document respiratory dysfunction of a more chronic nature, rats were also challenged with 7% CO2. Hypercapnic challenge has been demonstrated to elicit respiratory deficits with greater sensitivity than room air experimentally (Teng et al., 1999, 2003; Golder et al., 2001). Moreover, profoundly reduced Vt, f, and Ve responses to hypercapnia have been well documented in chronic tetraplegics in the clinical literature (Manning et al., 1992). We found a significantly diminished ability in hemicontused rats to augment Vt when challenged by 7% CO2 that was injury dose dependent (p ≤ 0.007; GLM with post hoc multiple linear regression), resulting in a severely mitigated Ve response during the first 4 weeks after SCI (p ≤ 0.031; GLM with post hoc multiple linear regression) (Table 1). For instance, there was a 24.8% decrease in hypercapnic Ve in severely injured rats at 2 weeks after SCI and a 13.5% decrease at 4 weeks after SCI compared with uninjured controls. Moreover, there was a clear injury dose-related trend in the loss of the ability to augment f when breathing 7% CO2 (p ≤ 0.063 GLM with post hoc multiple linear regression). Overall, these data indicated that there were injury dose-dependent chronic abnormalities in the post-SCI respiratory parameters in our C5 hemicontusion model.
Respiratory Deficits After C5 Hemicontusion Sci Correlate With Lesion Pathology
At 4 weeks after SCI, rats with behavioral scores representative of the corresponding group average were selected from each study group (i.e., mild, moderate, severe, or no hemicontusion; n = 4 per group) for histopathological analysis of the spinal cords (Teng et al., 1999, 2004). This end number had been determined adequate for functional data analysis resulting from the high consistency in our data collection (Teng et al., 1999, 2003). All rats undergoing histopathological analysis received 8-OH DPAT (250 μg/kg, i.p.) at 2 and 4 weeks after injury and buspirone (1.5 mg/kg, i.p.) and p-MPPI (3 mg/kg, i.p.) at 2 weeks after injury while undergoing respiratory studies. In addition, the severely injured rats received 8-OH DPAT (250 μg/kg) at 5 d after SCI for the dose washout study and 8-OH DPAT (125 μg/kg) at 2 weeks after SCI during the dose-response study. For general lesion morphology, we examined transverse sections of spinal cord at the C5 epicenter of hemicontusion injury and in 1 mm increments rostral and caudal to the epicenter that were stained with solvent blue/hematoxylin and eosin (Teng et al., 2004) (Fig. 3A). The cross-sectional profiles of injury epicenters of rats with severe hemicontusion injuries were characterized by the occasional presence of the most peripheral elements of gray matter, as well as a peripheral, often incomplete, rim of residual hypomyelinated white matter in the affected half spinal cord (Fig. 3A5). White matter degeneration at the epicenter included the ventrolateral, ventral, and medial funiculi, which are important descending respiratory pathways in the rat (Newsom Davis and Plum, 1972), the ventromedial white matter, which is the most critical region for hindlimb locomotion in rats (Blight, 1983), and the basal part of the dorsal column, a sector that houses the rat corticospinal tract, which is crucial in controlling precise forelimb movement (Raineteau et al., 2001). Injury dose-dependent areas of white and gray matter pathology extended up to 3 mm rostral and caudal to the epicenter (r ≥ 0.537; p ≤ 0.028; Spearman correlation), with partial involvement of the C4 and C6 levels in the most severe cases.
Figure 3.
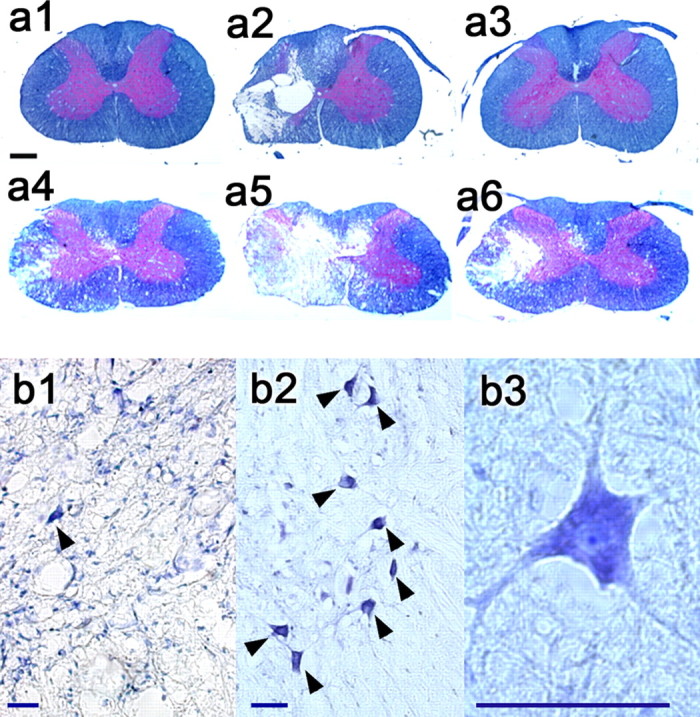
Histopathological changes after C5 hemicontusion injury. a, Solvent blue plus hematoxylin and eosin transverse sections 2 mm rostral from the epicenter (a1, a4), at the epicenter (a2, a5), and 2 mm caudal from the epicenter (a3, a6) in rats representative of mild injury (top; a1-a3) and severe injury (bottom; a4-a6) at 4 weeks after SCI. Scale bar, (in a1) 500 μm. b, Cresyl violet transverse sections 2 mm rostral to the epicenter in a representative severe hemicontusion injury (b1) at 4 weeks after SCI versus an uninjured control (b2) rat. Arrowheads indicate ventral motoneurons. b3, A representative ventral motoneuron (oil immersion) displaying Nissl substance, a euchromatic nucleus, and a distinct nucleolus. Scale bars: b1-b3, 20 μm.
To explore whether neuronal loss is responsible for postinjury respiratory abnormalities (Teng et al., 1999), we stained the immediately adjacent transverse sections with cresyl violet (n = 13; four uninjured controls and three of each injury severity). We observed an injury dose-dependent loss of ventral motoneurons (r ≥ 0.768; p ≤ 0.006; Spearman correlation) (Fig. 3B) on the hemicontused side in each section up to 2 mm rostral and 1 mm caudal to the epicenter relative to controls. Note that the numbers of ventral motoneurons at 2 mm rostral to the epicenter on the hemicontused side or its corresponding counts in controls significantly correlated with Ve under 7% CO2 at week 4 after injury (r = 0.701; p = 0.016; Pearson correlation). Ventral motoneurons ipsilateral to the injury at this segment numbered from 8.8 ± 1.9 (mean ± SEM) in uninjured controls, 4.0 ± 0.6 in mildly injured animals, 3.3 ± 1.5 in moderately injured animals, to 1.3 ± 0.7 in the severely injured group. Ipsilateral motoneuronal counts in other transverse sections did not correlate with hypercapnic Ve before the animal was killed (p ≥ 0.127; Pearson correlation). Contralateral ventral motoneuronal counts were not significantly different in rats with hemicontusion injury compared with controls (on average, 8.4 ± 0.4 vs 9.4 ± 0.7; p > 0.05; Student's t test).
Effect of C5 hemicontusion SCI on general somatic neurobehavioral function
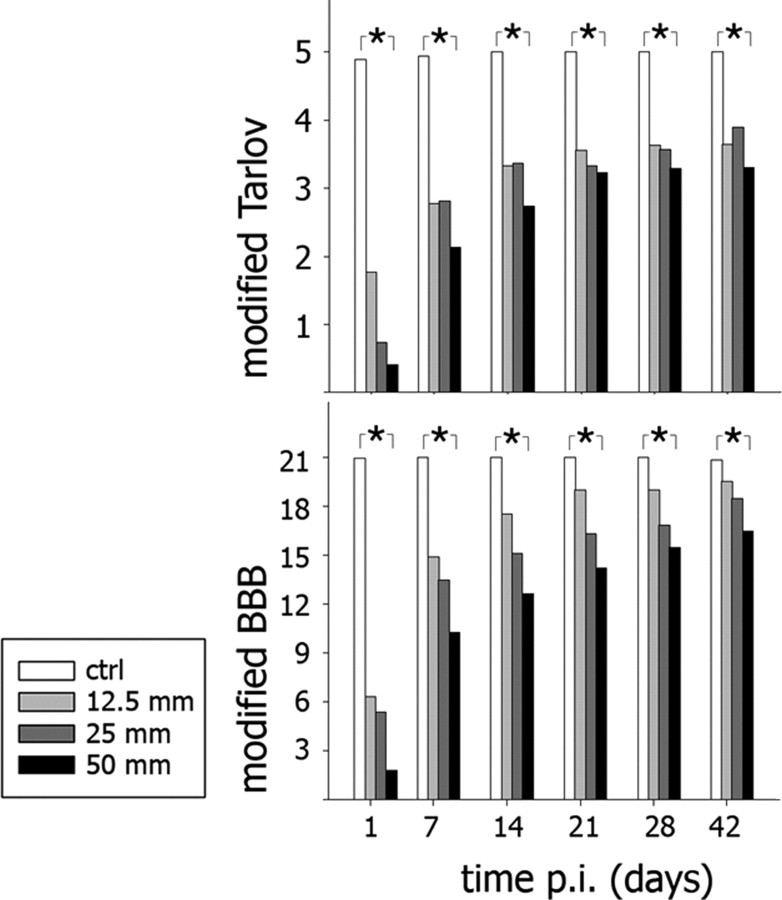
We recorded the neurobehavioral effects of our C5 hemicontusion model using modified Tarlov (Gale et al., 1985) and modified BBB scales (Basso et al., 1995) for the forelimbs and hind-limbs, respectively (Fig. 4). Forelimb and hindlimb neurobehavioral scores contralateral to the hemicontusion injury recovered to preoperative baselines during the first week after SCI. In contrast, ipsilateral locomotor function remained abnormal for all 6 weeks after injury. For the forelimb, there was a significant inverse correlation between injury dose and ipsilateral modified Tarlov scores at all time points from 1 d to 6 weeks after SCI (r ≤ -0.695; p < 0.001; Spearman correlation). Injury dose and ipsilateral hindlimb modified BBB scores were also inversely correlated at all time points from 1 d to 6 weeks after injury (r ≤ -0.735; p < 0.001; Spearman correlation). Overall, these data demonstrated chronic somatomotor deficits in the ipsilateral forelimbs and hindlimbs, which did not recover to baseline preoperative levels even after 6 weeks after injury. Respiratory function, in contrast, improved to levels comparable with uninjured controls at 2 and 4 weeks after injury, in room air or hypercapnic ventilation, respectively.
Figure 4.
Forelimb and hindlimb neurobehavioral function declined after C5 hemicontusion injury in lesion dose-dependent manners. Top, Neurobehavioral function of the forelimb (measured by a modified Tarlov scale) on the side ipsilateral to the hemicontused spinal cord correlated inversely with lesion dose (uninjured control vs 12.5, 25, or 50 mm C5 hemicontusion injury) from 1 d to 6 weeks after SCI (*p < 0.05; Spearman correlation). Forelimb neurobehavioral function on the side opposite of the spinal cord hemicontusion recovered to preoperative levels during the first week after SCI. Bottom, Neurobehavioral function of the hindlimb (measured by a modified BBB scale) on the side ipsilateral to the hemicontused spinal cord correlated inversely with lesion dose (uninjured control vs 12.5, 25, or 50 mm C5 hemicontusion injury) from 1 d to 6 weeks after SCI (*p < 0.05; Spearman correlation). ctrl, Control; p.i., postinjury.
5-HT1A agonists ameliorate respiratory deficits resulting from C5 hemicontusion SCI
Serotonin is well known to be a key modulator of respiration in the CNS (Zhou and Goshgarian, 2000; Fuller et al., 2005) (for review, see Mitchell and Johnson, 2003). In particular, agonists of the 5-HT1A subreceptor have been shown to improve respiratory function experimentally (Lalley et al., 1994; Sahibzada et al., 2000; Teng et al., 2003) and clinically (Mendelsohn et al., 1991). Although the precise mechanisms are not clear, antibody studies have demonstrated the presence of 5-HT1A on the axon hillocks of ventral horn motoneurons in the cervical spinal cord of primates (Kheck et al., 1995), possibly indicating a direct stimulatory pathway (Takahashi and Berger, 1990; Richmonds and Hudgel, 1996). Because the greatest density of 5-HT1A receptors is found in the superficial layers of the dorsal horns of the spinal cord (Thor et al., 1993; Kheck et al., 1995), we also reasoned that 5-HT1A agonists may, alternatively, act on a pathway involving the afferents from respiratory muscles to supraspinal respiratory centers (Teng et al., 1999).
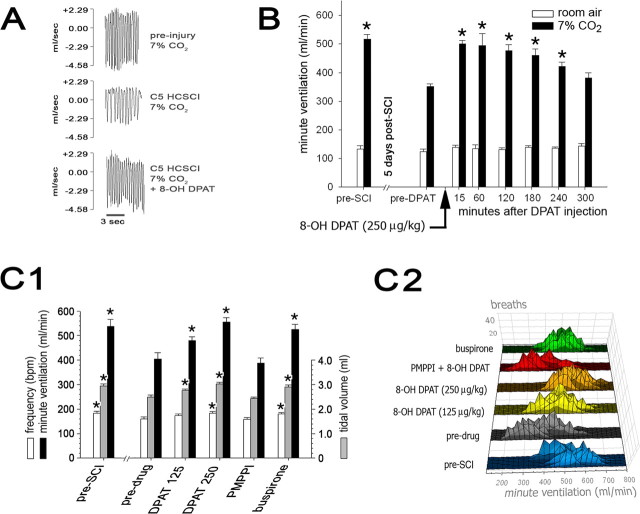
To further investigate the putative role of 5-HT1A receptor agonists in post-SCI respiration in C5 hemicontusion SCI, a subset of rats with mild (n = 9), moderate (n = 9), or severe (n = 12) injuries and 13 controls underwent testing with 8-OH DPAT at 2 and 4 weeks after SCI. Although long-term effects of the 8-OH DPAT treatments in SCI rats were not systematically examined, no significant differences were noted between the pretreatment respiratory parameters of rats that received occasional 5-HT1A treatments and those that did not receive treatment during the 2 or 4 week postinjury course (data not shown). This indicated that 5-HT1A agonists did not produce early tolerance. The present data revealed that 8-OH DPAT (250 μg/kg, i.p.) reversed respiratory abnormalities for all rats with hemicontusion injury at both 2 and 4 weeks after injury (Fig. 5A, Table 2). During room air ventilation at 2 weeks after SCI, we observed that 8-OH DPAT corrected Vt deficits for all groups with hemicontusion SCI (p ≤ 0.038; repeated-measures ANOVA with post hoc Tukey's tests) and significantly reduced f in rats in the severely injured group (p = 0.047; repeated measures ANOVA with post hoc Tukey's tests), eliminating the shallow, rapid breathing pattern noted before treatment. During 7% CO2 challenge, 8-OH DPAT treatment significantly improved Ve for all hemicontused groups at 2 weeks (p ≤ 0.001) and 4 weeks (p ≤ 0.033; repeated-measures ANOVA with post hoc Tukey's tests) after injury relative to pretreatment Ve to levels comparable with unlesioned control rats. For instance, severely injured rats at 2 weeks after injury mounted a Ve response to hypercapnic conditions that was ∼77% of uninjured controls, whereas after the severely injured rats received 8-OH DPAT, their response improved by 37.6% (p < 0.001; repeated-measures ANOVA with post hoc Tukey's tests) compared with before treatment or ∼105% compared with uninjured controls. At 2 weeks after SCI, moderately injured rats improved their hypercapnic Ve by 39.2% (p < 0.001) and mildly injured rats by 27.1% (p < 0.001; repeated-measures ANOVA with post hoc Tukey's tests) compared with predrug baselines to levels comparable with uninjured controls. At 4 weeks after SCI, when predrug baseline hypercapnic Ve for hemicontused animals were improved but still not normal, 8-OH DPAT improved Ve by 8.4% in mildly injured rats (p = 0.033), 9.2% in moderately injured rats (p = 0.025), and 28.5% in severely injured rats (p = 0.001; repeated-measures ANOVA with post hoc Tukey's tests). Rats without hemicontusion SCI did not display any statistically significant change in ventilatory parameters after 8-OH DPAT treatment at the equivalent time points. As documented previously (Teng et al., 2003), no significant neurobehavioral changes in any group were noted after 8-OH DPAT administration. No discernible adverse effects to any of the drugs under the current dose regimens were noted (Bjorvatn et al., 1997).
Figure 5.
Effects of 5-HT1A agents on minute ventilation in hypercapnic conditions after C5 hemicontusion injury. A, WBP respiratory flow tracings of a representative uninjured rat breathing 7% CO2 (top) and then breathing 7% CO2 2 weeks after severe C5 hemicontusion injury (HCSCI) before (middle) and after administration of 8-OH DPAT (250 μg/kg, i.p.; bottom). B, The effects of 8-OH DPAT on Ve in rats (n = 4) with severe (50 mm) hemicontusion injury at 5 d after SCI. Systemic 8-OH DPAT (250 μg/kg) restored Ve responses to 7% CO2 stimulus in severely injured rats to a level comparable with preinjury baseline, an effect that lasted up to 4 h after a single intraperitoneal dose (*p < 0.05, significantly different from predrug levels; repeated-measures ANOVA with post hoc Tukey's tests). C1, Effects of 5-HT1A agonists and antagonists on respiratory parameters at 2 weeks after SCI in severely injured rats (*p < 0.05; repeated-measures ANOVA with post hoc Tukey's tests). Note that Ve responses to a hypercapnic stimulus were markedly diminished at 2 weeks after SCI before drug treatment compared with preinjury baselines. 8-OH DPAT treatment resulted in a dose-dependent increase in Ve response; treatment with buspirone also improved Ve response compared with predrug levels. The augmentation of Ve after 8-OH DPAT was blocked by preadministration of the 5-HT1A antagonist p-MPPI (PMPPI). C2, Histogram of Ve responses to 7% CO2 stimulus in rats with severe hemicontusion injury (50 mm; n = 12) after 8-OH DPAT (125 μg/kg), 8-OH DPAT (250 μg/kg), p-MPPI (3 mg/kg), p-MPPI plus 8-OH DPAT (250 μg/kg), or buspirone (1.5 mg/kg) treatment at 2 weeks after SCI compared with predrug Ve at 2 weeks after SCI and preoperative baselines. Each black horizontal line within each larger colored line represents the distribution of per-breath Ve responses for each rat during hypercapnic challenge.
Table 2.
Effects of 8-OH DPAT on respiration after SCI
|
Time after SCI |
DPAT |
CO2 |
Control |
12.5 mm |
25 mm |
50 mm |
|---|---|---|---|---|---|---|
| 2 weeks | ||||||
| f(bpm) | − | 0.03% | 100.4 ± 3.2 | 111.0 ± 4.2 | 116.4 ± 3.4 | 110.8 ± 4.4 |
| + | 0.03% | 101.1 ± 1.9 | 108.3 ± 2.1 | 113.4 ± 5.7 | 101.6 ± 2.6 | |
| p = 0.702 | p = 0.422 | p = 0.638 | p = 0.027* | |||
| Vt (ml) | − | 0.03% | 1.34 ± 0.04 | 1.31 ± 0.03 | 1.26 ± 0.03 | 1.22 ± 0.04 |
| + | 0.03% | 1.31 ± 0.03 | 1.47 ± 0.04 | 1.46 ± 0.07 | 1.48 ± 0.06 | |
| p = 0.203 | p = 0.002* | p = 0.016* | p < 0.001* | |||
| Ve (ml/min) | − | 0.03% | 133.7 ± 4.3 | 144.8 ± 6.2 | 146.2 ± 3.3 | 134.8 ± 5.5 |
| + | 0.03% | 131.7 ± 2.8 | 159.6 ± 5.1 | 164.9 ± 9.5 | 149.6 ± 5.8 | |
| p = 0.468 | p = 0.069 | p = 0.043* | p = 0.099 | |||
| f(bpm) | − | 7% | 181.1 ± 4.2 | 163.7 ± 5.5 | 169.7 ± 3.8 | 160.7 ± 7.3 |
| + | 7% | 182.0 ± 2.5 | 188.4 ± 3.8 | 194.5 ± 6.2 | 183.7 ± 6.7 | |
| p = 0.155* | p = 0.023* | p < 0.001* | p < 0.001 | |||
| Vt (ml) | − | 7% | 2.91 ± 0.06 | 2.73 ± 0.08 | 2.42 ± 0.08 | 2.49 ± 0.07 |
| + | 7% | 2.86 ± 0.05 | 3.04 ± 0.10 | 2.94 ± 0.16 | 3.03 ± 0.06 | |
| p = 0.494* | p < 0.001* | p = 0.023* | p = 0.001 | |||
| Ve (ml/min) | − | 7% | 527.1 ± 18.3 | 449.4 ± 27.3 | 412.0 ± 20.6 | 403.3 ± 26.4 |
| + | 7% | 535.8 ± 10.4 | 571.5 ± 13.8 | 573.6 ± 38.9 | 555.1 ± 17.9 | |
| p = 0.494* | p < 0.001* | p = 0.005* | p < 0.001 | |||
| 4 weeks | ||||||
| f(bpm) | − | 0.03% | 99.1 ± 3.0 | 105.1 ± 2.1 | 106.8 ± 3.8 | 101.2 ± 4.0 |
| + | 0.03% | 107.1 ± 3.9 | 104.3 ± 2.6 | 101.7 ± 3.0 | 99.1 ± 3.3 | |
| p = 0.063 | p = 0.833 | p = 0.350 | p = 0.504 | |||
| Vt (ml) | − | 0.03% | 1.52 ± 0.05 | 1.50 ± 0.05 | 1.40 ± 0.06 | 1.45 ± 0.05 |
| + | 0.03% | 1.45 ± 0.06 | 1.55 ± 0.05 | 1.53 ± 0.04 | 1.48 ± 0.06 | |
| p = 0.306 | p = 0.217 | p = 0.085 | p = 0.783 | |||
| Ve (ml/min) | − | 0.03% | 151.3 ± 7.9 | 158.4 ± 6.7 | 148.2 ± 4.9 | 147.3 ± 7.9 |
| + | 0.03% | 154.7 ± 7.4 | 160.6 ± 4.7 | 155.8 ± 6.0 | 146.4 ± 7.0 | |
| p = 0.689 | p = 0.713 | p = 0.171 | p = 0.928 | |||
| f(bpm) | − | 7% | 176.8 ± 7.1 | 174.9 ± 6.7 | 171.8 ± 7.3 | 165.0 ± 5.8 |
| + | 7% | 182.3 ± 4.7 | 177.6 ± 3.5 | 176.6 ± 5.9 | 187.2 ± 4.6 | |
| p = 0.255 | p = 0.639 | p = 0.387* | p < 0.001 | |||
| Vt (ml) | − | 7% | 3.09 ± 0.08 | 3.01 ± 0.09 | 3.12 ± 0.07 | 2.64 ± 0.09 |
| + | 7% | 3.05 ± 0.07 | 3.23 ± 0.09 | 3.32 ± 0.06 | 2.99 ± 0.15 | |
| p = 0.533 | p = 0.015* | p < 0.001* | p = 0.023* | |||
| Ve (ml/min) | − | 7% | 544.6 ± 23.6 | 530.6 ± 32.7 | 536.2 ± 25.2 | 437.9 ± 26.5 |
| + | 7% | 556.6 ± 21.4 | 575.2 ± 24.0 | 585.6 ± 21.2 | 562.7 ± 36.4 | |
|
|
|
|
p = 0.449 |
p = 0.048*
|
p = 0.038*
|
p < 0.001*
|
Effects of systemic 8-OH DPAT (250 μg/kg) administration on respiratory parameters (f, Vt, and Ve) for rats without SCI (n = 13) or mild (n = 9), moderate (n = 9), or severe (n = 12) hemicontusion injury at 2 or 4 weeks after SCI. Data revealed that 8-OH DPAT restored minute ventilation during hypercapnic challenge (7% CO2) in rats with all grades of hemicontusion injury to levels similar to uninjured controls at both 2 and 4 weeks after SCI (p ≤ 0.033; repeated-measures ANOVA). Respiratory parameters for uninjured rats were not significantly affected by 8-OH DPAT administration at either 2 or 4 weeks after the baseline period (*p < 0.05; repeated-measures ANOVA with post hoc Tukey's tests).
To confirm that 5-HT1A receptor agonism plays a central role in respiratory function recovery after 8-OH DPAT administration, a dose washout study, a dose-response study, and additional tests using another 5-HT1A agonist buspirone and 5-HT1A-specific antagonist p-MPPI were performed with randomly selected subsets of rats receiving severe hemicontusion injury (Table 3) (Teng et al., 2003). At 5 d after SCI, an 8-OH DPAT dose washout study (250 μg/kg; n = 4) demonstrated a restoration of Ve response to hypercapnia, which peaked during the first hour after injection, lasted up to 4 h, and then subsequently returned to pretreatment levels (p ≤ 0.024; repeated-measures ANOVA with post hoc Tukey's test) (Fig. 5B). These results were consistent with our previous data that showed a prolonged (i.e., 4 h), but not permanent, amelioration of post-SCI respiratory dysfunction after a single intraperitoneal administration of 8-OH DPAT (Teng et al., 2003). The four rats that received 8-OH DPAT at 5 d after SCI did not display differences in respiratory parameters on subsequent WBP testing for the remainder of their studies compared with the other severely injured rats, suggesting no long-term effect on respiratory function after the single 8-OH DPAT injection at 5 d after SCI (data not shown). Finally, 8-OH DPAT administration did not enhance respiratory response to 7% CO2 in control rats.
Table 3.
Effects of various agents with 5-HT1A receptor activity on respiration after SCI
|
|
f(bpm) |
Vt (ml) |
Ve (ml/min) |
|---|---|---|---|
| Pre-SCI | 182.9 ± 8.5 | 2.94 ± 0.09 | 537.7 ± 29.6 |
| Predrug (2 weeks after SCI) | 160.7 ± 7.3† | 2.50 ± 0.08† | 404.3 ± 26.8† |
| 8-OH DPAT (125 μg/kg) | 173.9 ± 6.3 | 2.77 ± 0.06*† | 479.7 ± 15.6*† |
| 8-OH DPAT (250 μg/kg) | 183.7 ± 5.0* | 3.03 ± 0.06* | 555.1 ± 17.9* |
| p-MPPI (3 mg/kg) plus 8-OH DPAT (250 μg/kg) | 158.7 ± 7.3† | 2.43 ± 0.06† | 380.7 ± 24.9† |
| Buspirone (1.5 mg/kg) | 180.7 ± 5.6* | 2.91 ± 0.08* | 524.8 ± 21.7* |
| Repeated-measures ANOVA |
p < 0.001 |
p < 0.001 |
p < 0.001 |
Respiratory responses to hypercapnic stimulus (7% CO2) for rats with severe hemicontusion injury (50 mm; n = 12) at preinjury baseline or 2 weeks after SCI under predrug, 8-OH DPAT (125 μg/kg), 8-OH DPAT (250 μg/kg), p-MPPI (3 mg/kg), p-MPPI (3 mg/kg) plus 8-OH DPAT (250 μg/kg), or buspirone (1.5 mg/kg). All numbers are presented as mean ± SEM. The asterisk indicates a significant difference from predrug on post hoc Tukey's test (p < 0.05), and the dagger symbol indicates a significant difference from 8-OH DPAT (250 μg/kg) on post hoc Tukey's test (p < 0.05) after significant differences were found on repeated-measures ANOVA.
In the dose-response study performed at 2 weeks after SCI (n = 12), a dose-dependent restoration of hypercapnic Ve response was observed. The 250 μg/kg dose of 8-OH DPAT produced the greatest improvement in Ve versus predrug baseline (37.2% increase; p < 0.001), followed by the 125 μg/kg dose (18.6% increase; p < 0.038; repeated-measures ANOVA with post hoc Tukey's tests) (Fig. 5C, Table 3). In other separate studies during the second week after injury, preadministration of p-MPPI (3 mg/kg, i.p.), given 20 min before 8-OH DPAT, blocked the beneficial effects of 8-OH DPAT (250 μg/kg). Whereas 8-OH DPAT (250 μg/kg) significantly improved hypercapnic Ve compared with the predrug baseline (p < 0.001; see above) when p-MPPI was given before 8-OH DPAT, hypercapnic Ve did not significantly differ from predrug baseline (p > 0.05; repeated-measures ANOVA with post hoc Tukey's test). Buspirone (1.5 mg/kg, i.p.; n = 12), like 8-OH DPAT, also improved Ve under hypercapnic conditions significantly greater than the predrug baseline (29.8% increase; p = 0.003) to levels indiscernible from the pre-SCI response (p > 0.05; repeated-measures ANOVA with post hoc Tukey's tests). Together, the results strongly supported the proposition that the respiratory benefits seen in our model were mediated through 5-HT1A receptors.
Discussion
Previous reports examining respiratory dysfunction after SCI examined pathophysiology in either thoracic models or anesthetized rats (El-Bohy et al., 1998; Teng et al., 1999, 2003; Golder et al., 2001). These studies were pivotal but showed only transient dysfunction or were necessarily terminal. This is the first report of the impact of a contusive cervical SCI on respiratory function in conscious rats with chronic clinical manifestations. Our model offers feasible laboratory procedures while emulating the epidemiology of disease and the clinical features of post-SCI respiratory dysfunction (Gruner, 1992; Manning et al., 1992). Using this model, we demonstrated that systemic 5-HT1A agonists counteracted post-SCI respiratory abnormalities without discernable adverse effects.
Greater chronicity of respiratory dysfunction after C5 hemicontusion than thoracic SCI likely to be associated with phrenic nucleus involvement in the C5 model
We speculate that involvement of the phrenic nucleus ipsilateral to the hemicontusion was accountable for the greater chronicity of respiratory deficits in our cervical model than after T8 SCI. The phrenic nucleus of the rat is a longitudinal column of cells located between the ventromedial and ventrolateral cell groups of the ventral horn, extending from C3 to C5 or sometimes C6 (Kuzuhara and Chou, 1980; DeVries and Goshgarian, 1989). Here, we demonstrated an obliteration of this midventral region at the C5 epicenters of all hemicontused groups, with the loss extending partially through the C4 and C6 levels. Thus, the hemidiaphragm ipsilateral to the hemicontusion was, at least in part, denervated.
Although the precise contribution of the rat diaphragm to various respiratory parameters has yet to be described, indubitably, the diaphragm plays a more significant role than the lower thoracic intercostals in rodent respiration (e.g., the human diaphragm normally contributes ∼65% of vital capacity) (Ledsom and Sharp, 1981). Thus, after midcervical injury, potential natural compensatory mechanisms involving the intercostals are not likely to fully compensate for diminished diaphragmatic function, whereas in the thoracic injury model, increased diaphragmatic function might overcome respiratory deficits attributable to intercostal muscle dysfunction. A more fruitful compensatory measure after C5 hemicontusion is likely to be increased diaphragmatic drive via supraspinal pathways. Our observation of an increased f during the early postinjury period was compatible with this postulation. However, this rapid breathing pattern can be detrimental to gas exchange and culminate in hypoxemia or frank respiratory failure (Ledsom and Sharp, 1981; Rochester, 1993).
Eventual recovery of respiratory function
The eventual recovery of respiratory dysfunction observed in this study is consistent with clinical observations (Bluechardt et al., 1992). Although mechanisms underlying this recovery are essentially unknown, it is well documented that a hemiparetic diaphragm ipsilateral to a C2 hemisected cord can regain function shortly after the contralateral phrenic nerve is transected, or spontaneously ∼4 weeks after injury (O'Hara and Goshgarian, 1991; Nantwi et al., 1999; Golder et al., 2003). The so-called “crossed phrenic phenomenon” works by reactivating silent synapses of the bulbospinal pathways that cross the spinal midline caudal to the C2 lesion (O'Hara and Goshgarian, 1991). Because our hemicontusion SCI obliterated the majority of the ipsilateral phrenic area, the recovery was unlikely to be mediated by crossed phrenic pathways.
Alternatively, respiratory recovery may have been partially caused by reinnervation of the hemicontusion SCI-denervated hemidiaphragm through axonal sprouting from spared ipsilateral phrenic neurons, contralateral phrenic neurons, and/or phrenic terminals. This reinnervation may potentially have been enhanced by our 5-HT1A agonist administrations, because 5-HT1A activation triggers functional respiratory neural plasticity (Giroux et al., 1999; Mantilla and Sieck, 2003). However, the delay in our initial administration of 5-HT1A agonists fell far beyond the therapeutic windows for tissue preservation after SCI (Teng et al., 1997, 1998, 2004), precluding any hope for 5-HT1A agonist-triggered neuroprotection in our study. Nevertheless, it remains to be examined whether earlier and/or more frequent administration of serotoninergic agonists can further improve or elicit a more prompt neuroplasticity for respiratory recovery.
Amelioration of respiratory dysfunction by 5-HT1A agonists
In our study, we observed a temporary restoration (lasting up to 4 h) of respiratory function in C5-injured rats after systemic 5-HT1A agonists at 2 and 4 weeks after injury. The mechanism by which 5-HT1A agonists improve respiration after SCI is not clear (Teng et al., 2003). One possibility is through a dorsal horn afferent-supraspinal respiratory center pathway (Thor et al., 1993; Jankowska et al., 1997; Teng et al., 2003). Briefly, respiratory muscle afferents can influence brainstem respiratory centers and ultimately affect motor output to the skeletal muscles responsible for breathing. It has been suggested that the transfer of information from these respiratory muscle afferents to supraspinal centers may be gated by descending serotonergic, presumably 5-HT1A, pathways (Jankowska et al., 1997; Teng et al., 2003). Buspirone and 8-OH DPAT may act on the dorsal horns of rats with SCI to modulate these pathways and restore normal respiratory function (Teng et al., 2003). We observed a correlation between hypercapnic Ve responses and motoneuronal count in the rostral, but not caudal, margins of the lesion epicenter. It is our speculation that any positive modulation of the dorsal spinal-supraspinal pathway should trigger a corresponding increase in Ve response to hypercapnia, because the rostral marginal motoneurons would primarily have synaptic continuity with supraspinal centers.
Another possible mechanism explaining the effect of 5-HT1A agonists is via diaphragmatic-thoracic reflex loops. Such reflexes increase accessory respiratory neuronal output (i.e., thoracic motor neurons that control intercostal and abdominal muscles) after their activation by phrenic sensory input from diaphragmatic proprioceptors and mechanoreceptors (Goshgarian and Roubal, 1986). After hemicontusion SCI, certain ipsilateral proprioceptive fibers could still be stimulated by contralateral hemidiaphragmatic contraction (Monteau and Hilaire, 1991). Serotonin 1A agonists may have stimulated this reflex loop, a testable hypothesis for future experiments.
It might be postulated that our observed respiratory recovery could have been augmented through 5-HT2 receptor activation and expression, as reported after C2 hemisection (Zhou et al., 2001; Fuller et al., 2005) and cervical dorsal rhizotomy (CDR) (Kinkead et al., 1998). Post-CDR rats displayed increased serotonin-immunoreactive terminals on phrenic motor neurons, and the consequent long-lasting enhancement of phrenic motor output triggered by hypoxia was blocked by pretreatment with a 5-HT2-specific antagonist. However, 8-OH DPAT and buspirone used in our work are 5-HT1A specific. 8-OH DPAT, for instance, has an ∼1000-fold selectivity for the 5-HT1A over the other 5-HT receptors (Middlemiss and Fozard, 1983), and its respiratory effect observed in this study was completely blocked by p-MPPI, a highly specific 5-HT1A antagonist (Ki5-HT1A = 1.0 vs Ki5-HT2 = 270 nm) (Kung et al., 1994). Nevertheless, we are currently testing whether coadministration of 5HT1A and 5HT2A agonists would synergistically or additively improve post-SCI respiratory recovery.
The evidence supporting a direct supraspinal action for 5-HT1A agonists in post-SCI respiratory dysfunction is less clear. Although 5-HT1A presynaptic and postsynaptic receptors are well known to be distributed in supraspinal areas such as the limbic regions, dorsal raphe nuclei, and various cortical regions and have complex interactions (Blier and Ward, 2003), the respiratory effects after 5-HT1A agonist exposure are unclear. Respiratory frequencies, for instance, have increased in some reports (Mandal et al., 1990; Rose et al., 1995) while remaining level in others (King and Holtman, 1990). Moreover, complexities in the study designs, including dose and route of drug administration (e.g., intravenous, intraventricular, or direct parenchymal injection), anesthesia use, or use of decerebrate models make interpretations of these studies difficult. Anesthesia is a particularly troublesome factor in the study of serotonin and respiration, because anesthetics may easily reduce the excitatory serotonergic input to motoneurons (Rose et al., 1995). In vitro brainstem-spinal cord preparations of neonatal rats have shown that addition of 8-OH DPAT to the superfusate increased respiratory frequency to 28 ± 7% greater than controls (Monteau et al., 1994). Other studies have shown that 8-OH DPAT has little effect on output from key brainstem respiratory centers (Johnson et al., 1996, 2001). However, emphasis must be made that none of these models incorporated a clinically relevant SCI in their designs.
Additional studies will be needed to precisely define the mechanisms through which 5-HT1A agonists ameliorate respiratory dysfunction in this experiment and others. In light of the pressing need for clinical therapeutics applicable to post-SCI respiratory dysfunction and the availability of buspirone, a well tolerated clinical 5-HT1A agonist used as an anxiolytic, we believe that 5-HT1A agonists may offer a novel strategy for treating post-SCI respiratory dysfunction. These data, together with the fact that 5-HT1A agonists corrected respiratory deficits in rats with a significant loss of one of their primary phrenic nuclear regions, suggest that strategies aimed at augmenting function and/or expression levels of 5-HT receptors should be further explored to develop treatments for SCI via functional repair of the gray matter (Teng et al., 1999, 2003; Reier et al., 2002) in addition to the well studied tactics for white matter protection (Teng and Wrathall, 1997; Stys, 1998; Waxman, 2002). Importantly, our model will allow additional investigations of other promising pharmaceutical avenues for this highly morbid and occasionally fatal complication of SCI (Nantwi et al., 2003).
Footnotes
This work was supported by the United Spinal Association, Veterans Affairs (VA) Biomedical Research Grant VA B2958O, National Institutes of Health Grant 1-R01 NS40822, the Foundation for Physical Medicine and Rehabilitation (FPM&R), and Project Amyotrophic Lateral Sclerosis. H.C. is a graduate of the VA Advanced Spinal Cord Injury Medicine Fellowship and a recipient of the FPM&R New Investigator Award. We declare that we have no competing financial interests.
Correspondence should be addressed to Dr. Yang D. Teng, 300 Longwood Avenue, Bader 3, Boston, MA 02115. E-mail: yang_teng@hms.harvard.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/254550-10$15.00/0
References
- Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1-21. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Fagerland S, Eid T, Ursin R (1997) Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res 770: 81-88. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM (2003) Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry 53: 193-203. [DOI] [PubMed] [Google Scholar]
- Blight AR (1983) Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience 10: 521-543. [DOI] [PubMed] [Google Scholar]
- Bluechardt MH, Wiens M, Thomas SG, Plyley MJ (1992) Repeated measurements of pulmonary function following spinal cord injury. Paraplegia 30: 768-774. [DOI] [PubMed] [Google Scholar]
- DeVries KL, Goshgarian H (1989) Spinal cord localization and characterization of the neurons which give rise to the accessory phrenic nerve in the adult rat. Exp Neurol 104: 88-90. [DOI] [PubMed] [Google Scholar]
- El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG (1998) Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol 150: 143-152. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Epstein MAF, Haddad GG, Mellins RB (1980) Practical implementation of the barometric method for measurement of tidal volume. J Appl Physiol 49: 1107-1115. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE (2003) Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS (2005) Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma 22: 203-213. [DOI] [PubMed] [Google Scholar]
- Gale K, Kerasidis H, Wrathall JR (1985) Spinal cord contusion in the rat: behavioral analysis of functional neurological impairment. Exp Neurol 88: 123-134. [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA (1999) Autoradiographic study of alpha 1 and alpha 2 noradrenergic and serotonin 1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol 406: 402-414. [PubMed] [Google Scholar]
- Go BK, DeVivo MJ, Ricards JS (1995) The epidemiology of spinal cord injury. In: Spinal cord injury: clinical outcomes of the model systems (Stover SL, DeLisa JA, Whiteneck GG, eds), pp 21-55. Gaithersburg, MD: Aspen.
- Golder FJ, Reier PJ, Bolser DC (2001) Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci 21: 8680-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC (2003) Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Roubal PJ (1986) Origin and distribution of phrenic primary afferent nerve fibers in the spinal cord of the adult rat. Exp Neurol 92: 624-638. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL (2001) Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR (2001) Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 168: 273-282. [DOI] [PubMed] [Google Scholar]
- Gruner JA (1992) A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 9: 123-128. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo-Lackberg Z, Yin XK (1997) Modulation of responses to four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci 9: 1375-1387. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL (1996) Modulation of respiratory rhythm in vitro: role of Gi/O protein-mediated mechanisms. J Appl Physiol 80: 2120-2133. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JER, Henderson DR, Wenninger MR, Mitchell GS (2001) Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro J Appl Physiol 91: 2703-2712. [DOI] [PubMed] [Google Scholar]
- Kheck NM, Gannon PJ, Azmitia EC (1995) 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J Comp Neurol 355: 211-220. [DOI] [PubMed] [Google Scholar]
- King KA, Holtman Jr JR (1990) Characterization of the effects of activation of ventral medullary serotonin receptor subtypes on cardiovascular activity and respiratory motor outflow to the diaphragm and larynx. J Pharmacol Exp Ther 252: 665-674. [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS (1998) Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, Nizam A (1998) Analysis of repeated measures data. In: Applied regression analysis and other multivariable methods, Chap 21, Ed 3, pp 589-638. Pacific Grove, CA: Duxbury.
- Kung MP, Zhang ZP, Frederick D, Kung HF (1994) In vivo binding of [123I] 4-(2′-methoxyphenyl)-1-[2′-(N-2′-pyridinyl)-p-iodobenzamido-] ethyl-piperazine, p-MPPI, to 5-HT1A receptors in rat brain. Synapse 18: 359-366. [DOI] [PubMed] [Google Scholar]
- Kuzuhara S, Chou SM (1980) Localization of the phrenic nucleus in the rat: a HRP study. Neurosci Lett 16: 119-124. [DOI] [PubMed] [Google Scholar]
- Lai YL, Tsuya Y, Hildebrandt J (1978) Ventilatory responses to acute CO2 exposure in the rat. J Appl Physiol 45: 611-618. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW (1994) Serotonin 1A receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett 172: 59-62. [DOI] [PubMed] [Google Scholar]
- Ledsom JR, Sharp JM (1981) Pulmonary function in acute cervical cord injury. Am Rev Respir Dis 124: 41-44. [DOI] [PubMed] [Google Scholar]
- Linn WS, Spungen AM, Gong Jr H, Adkins RH, Bauman WA, Waters RL (2001) Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord 39: 263-268. [DOI] [PubMed] [Google Scholar]
- Mandal AK, Zhong PY, Kellar KJ, Gillis RA (1990) Ventrolateral medulla: an important site of action for the hypotensive effect of drugs that activate the serotonin-1A receptors. J Cardiovasc Pharmacol 15 [Suppl 7]: S49-S60. [PubMed] [Google Scholar]
- Manning HL, Brown R, Scharf SM, Leith DE, Weiss JW, Weinberger SE, Schwartzstein RM (1992) Ventilatory and P0.1 response to hypercapnia in quadriplegia. Respir Physiol 89: 97-112. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC (2003) Plasticity in respiratory motor control: invited review: mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol 94: 1230-1241. [DOI] [PubMed] [Google Scholar]
- Mendelsohn WB, Maczaj M, Holt J (1991) Buspirone administration to sleep apnea patients. J Clin Psychopharmacol 11: 71-72. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR (1983) 8-Hydroxy-2-(di-N-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol 90: 151-153. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM (2003) Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358-374. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hilaire G (1991) Spinal respiratory motoneurons. Prog Neurobiol 37: 83-144. [DOI] [PubMed] [Google Scholar]
- Monteau R, Pasquale ED, Hilaire G (1994) Further evidence that various 5-HT receptor subtypes modulate central respiratory activity: in vitro studies with SR 46349B. Eur J Pharmacol 259: 71-74. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG (1999) Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair 13: 225-234. [Google Scholar]
- Nantwi KD, Basura GJ, Goshgarian HG (2003) Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected adult rats. Exp Neurol 182: 232-239. [DOI] [PubMed] [Google Scholar]
- Newsom Davis J, Plum F (1972) Separation of descending spinal pathways to respiratory motoneurons. Exp Neurol 34: 78-94. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR (1989) Correlative analysis of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol 103: 34-40. [DOI] [PubMed] [Google Scholar]
- O'Hara TE, Goshgarian GH (1991) Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol 111: 244-250. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Noth P, Thallmair M, Schwab ME (2001) Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat. Proc Natl Acad Sci USA 98: 6929-6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ, Golder FJ, Bolser DC, Hubscher C, Johnson R, Schrimsher GW, Velardo MJ (2002) Gray matter repair in the cervical spinal cord. Prog Brain Res 37: 49-70. [DOI] [PubMed] [Google Scholar]
- Richmonds CR, Hudgel DW (1996) Hypoglossal and phrenic motoneuron responses to serotonergic active agents in rats. Respir Physiol 106: 153-160. [DOI] [PubMed] [Google Scholar]
- Rochester DF (1993) Respiratory muscles and ventilatory failure: 1993 perspective. Am J Med Sci 305: 394-402. [DOI] [PubMed] [Google Scholar]
- Rose D, Khater-Boidin J, Toussaint P, Duron B (1995) Central effects of 5-HT on respiratory and hypoglossal activities in the adult cat. Respir Physiol 101: 59-69. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA (2000) Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine (1A) receptors. J Pharmacol Exp Ther 292: 704-713. [PubMed] [Google Scholar]
- Stephenson R, Gucciardi EJ (2002) Theoretical and practical considerations in the application of whole body plethysmography to sleep research. Eur J Appl Physiol 87: 207-219. [DOI] [PubMed] [Google Scholar]
- Stys PK (1998) Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab 18: 2-25. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ (1990) Direct excitation of rat spinal motoneurons by serotonin. J Physiol (Lond) 423: 63-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Wrathall JR (1996) Evaluation of cardiorespiratory parameters in rats after spinal cord trauma and treatment with NBQX, an antagonist of excitatory amino acid receptors. Neurosci Lett 209: 5-8. [DOI] [PubMed] [Google Scholar]
- Teng YD, Wrathall JR (1997) Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J Neurosci 17: 4359-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Wrathall JR (1998) Basic and acidic fibroblast growth factors protect spinal motor neurons in vivo after experimental spinal cord injury. Eur J Neurosci 10: 798-802. [DOI] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR (1999) Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci 19: 7037-7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR (2003) Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci 23: 4182-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM (2004) Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA 101: 3071-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Nickolaus S, Helke CJ (1993) Autoradiographic localization of 5-hydroxytryptamine 1A, 5-hydroxytryptamine 1B and 5-hydroxytryptamine 1C binding sites in the rat spinal cord. Neuroscience 55: 235-252. [DOI] [PubMed] [Google Scholar]
- Waxman SG (2002) Ion channels and neuronal dysfunction in multiple sclerosis. Arch Neurol 59: 1377-1380. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J (2003) Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehab 82: 803-814. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian H (2000) 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol 89: 1528-1536. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG (2001) Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol 91: 2665-2673. [DOI] [PubMed] [Google Scholar]