Abstract
A key aspect of postsynaptic function, also important for plasticity, is the segregation within dendritic spines of Ca2+ rises attributable to release from intracellular stores. Previous studies have shown that overexpression in hippocampal neurons of two postsynaptic density (PSD) scaffold proteins, Shank1B and Homer1b, induces spine maturation, including translocation of the intracellular Ca2+ channel inositol trisphosphate receptor (IP3R). The structural and functional significance of these processes remained undefined. Here, we show that in its relocation, IP3R is accompanied by other endoplasmic reticulum (ER) proteins: the Ca2+ pump sarcoendoplasmic reticulum calcium ATPase, the lumenal Ca2+-binding protein calreticulin, the ER lumen-addressed green fluorescent protein, and, to a lesser extent, the membrane chaperone calbindin. The specificity of these translocations was demonstrated by their inhibition by both a Shank1 fragment and the dominant-negative Homer1a. Activation in Shank1B-transfected neurons of the metabotropic glutamatergic receptors 1/5 (mGluRs1/5), which induce IP3 generation with ensuing Ca2+ release from the stores, triggered considerable increases in Ca2+-dependent responses: activation of the big K+ channel, which was revealed by patch clamping, and extracellular signal-regulated protein kinase (ERK) phosphorylation. The interaction of Shank1B and Homer1b appears as the molecular mechanism linking mGluRs1/5, strategically located in the spines, to IP3R with the integration of entire ER cisternas in the PSD and with consequences on both local Ca2+ homeostasis and overall neuronal signaling.
Keywords: postsynaptic density, ERK1/2, mGluR, ER calcium stores, big K+channel, Shank, Homer
Introduction
The properties of synapses depend mainly on their molecular and structural architecture. In particular, at most CNS excitatory synapses, a key role is attributed to postsynaptic densities (PSDs) localized within dendritic spines (Kennedy, 2000; Hering and Sheng, 2001). Each spine corresponds to a compartment mostly distinct from the dendritic shaft, and different types of spines are characterized by peculiar homeostatic properties (Nimchinsky et al., 2002).
PSDs are tridimensional complexes composed of numerous proteins, most of which act as scaffolds anchoring both membrane and soluble proteins to the cytoskeleton and intracellular signaling pathways (Garner et al., 2000; Kennedy, 2000; Kim and Sheng, 2004). Among PSD proteins, those of the Shank1-3 family [also called ProSAP (proline-rich synapse-associated protein), SSTRIP (somatostatin receptor-interacting protein), cortBP (cortactin-binding protein), Synamon, and Spank] are characterized by their dual interactions: with the plasma membrane NMDA receptor/PSD-95 complex via SAPAP/GKAP (synapse-associated protein/PSD-associated protein/guanylate kinase associated protein) and with the group I metabotropic glutamate receptors (mGluRs1/5) via the intracellular Homer1b protein, which in turn interacts with the intracellular Ca2+ [Ca2+]i channel, the inositol 1,4,5-trisphosphate receptor (IP3R) (Boeckers et al., 1999; Naisbitt et al., 1999; Tu et al., 1999; Zitzer et al., 1999). Shank proteins also interact with several actin regulatory proteins such as cortactin, IRSp53 (insulin receptor substrate p53), and β-PIX (β-p21-associated kinase-interacting exchange factor) (Kim and Sheng, 2004).
In previous studies, the spines of cultured hippocampal neurons were found to lack IP3Rs. However, overexpression of Shank1B together with Homer1b induced synapse maturation, with spine enlargement and an increase in IP3R localization (Sala et al., 2001). Here, the structural and functional significance of these changes was investigated. In particular, we studied whether overexpression of Shank, alone or together with Homer, and the appearance of IP3Rs correspond to the accumulation of complete endoplasmic reticulum (ER) cisternas in the spines and whether these events have any functional consequence in cultured neurons.
Materials and Methods
Hippocampal neuron cultures and recombinant DNA. Hippocampal cultures were prepared from embryonic day 18 (E18) to E19 rats (Charles River, Calco, Italy), as described previously (Sala et al., 2003), and transfected by using calcium phosphate precipitation. Full-length N-terminal hemagglutinin (HA)-tagged Shank1B (HA-Shank1B) and N-terminal Myc-tagged Homer1b and Homer1a cDNAs were prepared as described previously (Sala et al., 2001). ER-green fluorescent protein (ER-GFP) (Truong et al., 2001) was subcloned in a GW1 vector (British Biotechnology, Oxford, UK).
Immunostaining and antibodies. Neurons were fixed and stained (Sala et al., 2003). The following antibodies were used: mouse monoclonal anti-HA (Hoffmann-La Roche, Basel, Switzerland), guinea pig anti-Shank 1123 and rabbit anti-Homer 1133 (gifts from E. Kim, Institute of Science and Technology, Taejon, Korea), rabbit calreticulin (gift from H. D. Söling, Max Planck Institute of Biophysical Chemistry, Gottingen, Germany), mouse monoclonal anti-calbindin (Swant, Bellinzona, Switzerland), rabbit anti-sarcoendoplasmic reticulum calcium ATPase 2b (anti-SERCA2b; J. Meldolesi), rabbit phospho-extracellular signal-regulated protein kinase (pERK1/2; New England Biolabs, Beverly, MA), rabbit anti-mGluR5 (Chemicon, Temecula, CA), and FITC- and cyanine 3-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA).
Image acquisition and quantification. Neurons were selected randomly, and confocal images were acquired with a Bio-Rad (Hercules, CA) MRC1024 confocal microscope using a Nikon (Tokyo, Japan) 60× objective with sequential acquisition settings that were maintained as constants during the acquisition from different coverslips. Each image was a z-series projection taken at 0.75-μm-deep intervals. Images also were acquired with a Zeiss (Oberkochen, Germany) Axicam high-resolution mode CCD camera, using a Zeiss Axioplan microscope and a 63× objective. Morphometric analyses were made by using MetaMorph image analysis software (Universal Imaging Corporation, West Chester, PA). The staining intensity of endogenous proteins on dendritic spines was measured in transfected and nontransfected neurons as the mean intensity of synaptic areas, defined by Shank staining, and expressed as the means ± SD relative to controls.
Electrophysiology. Neurons were perfused continuously with external medium containing the following (in mm): 140 NaCl, 2 CaCl2, 3 KCl, 10 HEPES, 10 d-glucose, and 0.3 × 10-3 tetrodotoxin, pH 7.4, at 330 mOsm. Neurons transfected with GFP, and Shank1 expression plasmids (1:2 ratio) were selected on the basis of their fluorescence and recorded at room temperature under the cell-attached patch-clamp configuration. With the external medium, the recording pipette resistance was 2-3 MΩ. The Ca2+-dependent big K+ (BK) single-channel currents were recorded through an Axopatch 200B amplifier (Molecular Devices, Union City, CA), filtered at 1 kHz, stored on a tape recorder, and then digitized at 3 kHz with the use of the Axotape and analyzed with Axograph software from Molecular Devices. The open probability (Po) of BK channels was measured at +20 mV membrane potential, calculated with respect to the reversal potential of the recorded K+ channel unitary current taken as the resting potential of the neuron. The mGluR1/5 agonist dihydrophenylglycine (DHPG) was applied by a fast gravity perfusion system, with complete exchange of the cell environment in <50 ms.
Results
Shank1B overexpression induces spine recruitment of ER cisternas
In cultured hippocampal neurons, the overexpression of Shank1B and Homer1b induces IP3R accumulation in dendritic spines (Sala et al., 2001). These results could be attributable to increased density of the receptor in preexisting ER membranes, to its transfer to a heterologous type of membrane, or to recruitment of entire ER cisternas from the dendritic shaft to the spine. To distinguish among these alternatives, we transfected hippocampal neurons with HA-tagged Shank1B, with or without Homer1b, and stained them for three endogenous ER proteins: the membrane-bound and lumenal chaperone Ca2+-binding proteins, calbindin and calreticulin, and the Ca2+ pump SERCA2b. Previous studies have shown these proteins in the dendritic ER of hippocampal neurons (Villa et al., 1994; Baba-Aissa et al., 1998).
In nontransfected or vector-transfected neurons, the staining of the endogenous ER proteins was distributed in small patches along the dendritic shaft and was inappreciable in the Shank1B-containing spines (Fig. 1D1-D3,G1-G3,J1-J3), whereas in neurons overexpressing Shank1B, the colocalizations appeared in the spines. This effect was reinforced clearly when Shank1B was transfected with Homer1b. The results, however, were not identical for the three ER proteins. Calreticulin (Fig. 1A1-B3) and SERCA2b (Fig. 1H1-I3) appeared to be highly concentrated in the dendritic spines, together with Shank1B, whereas calbindin immunolabeling, although clearly appreciable, was of variable intensity (Fig. 1E1-F3): moderate in many and high in only some spines (Fig. 1F1-F3, arrows and arrowheads, respectively). Although mGluRs1/5 also bind to Homer1b, we did not observe any increased staining of the endogenous protein in the spines of neurons overexpressing both Shank1B and Homer1b (Fig. 1K1-L3).
Figure 1.
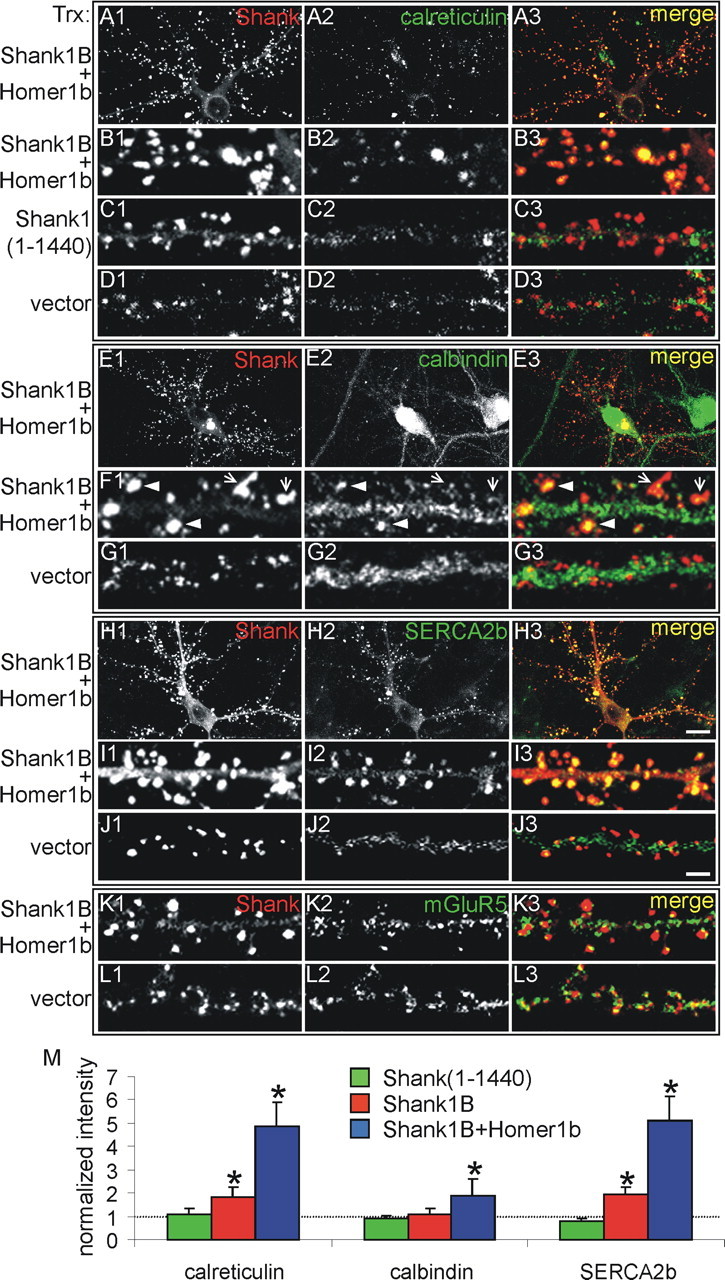
Shank1B and Homer1b recruit calreticulin, calbindin, and SERCA2b to dendritic spines but not mGluRs1/5. Neurons were transfected (Trx) at 11 d in vitro (DIV) with HA-Shank1B plus Homer1b, with the HA-Shank1 (1-1440) fragment, or with an empty vector, as indicated on the left side of the panels, and the neurons were stained at 18 DIV for Shank (on the red channel; left column) together with calreticulin (on the green channel) (A2, B2, C2, D2; middle column), calbindin (E2, F2, G2; middle column), SERCA2b (H2, I2, J2; middle column), or mGluR5 (K2, L2; middle column). Merged images are shown in the right column. In Shank1B plus Homer1b-cotransfected neurons, the calreticulin and SERCA2b colocalize with Shank. Only a few spines of transfected neurons show intense staining for calbindin (F1-F3, arrow heads), although most show a moderate staining (F1-F3, arrows). Endogenous mGluR5 staining is colocalized with Shank partially (K1-K3) but is not recruited further to synapses by Shank1B and Homer1b overexpression (L1-L3). B1-B3, F1-F3, and I1-I3 are higher magnifications of A1-A3, E1-E3, and H1-H3, respectively. Scale bars: (in H3) A1-A3, E1-E3, H1-H3, 10 μm; (in J3) B1-D3, F1-G3, I1-L3, 2.8 μm. M, Quantification of the changes in synaptic staining of the indicated proteins induced by the overexpression of Shank1B plus Homer1b. At least six neurons were analyzed for each endogenous protein (30-50 synapses scored per neuron). Histograms and error bars show the means ± SD, normalized to the staining intensity in nontransfected and vector-transfected neurons; *p < 0.05.
The immunofluorescence results were confirmed by quantitative analyses. As can be seen in Figure 1M, increases in both calreticulin and SERCA2b immunofluorescence were already significant in neurons transfected with Shank1B alone (1.8 ± 0.4 and 2.0 ± 0.3, respectively), whereas those of calbindin were appreciable but remained below significance (1.1 ± 0.3). When Shank1B was transfected with Homer1b, the spine increase in calreticulin and SERCA2b was fourfold (4.9 ± 1.0 and 5.1 ± 1.1, respectively). Also, in these neurons, the increase in calbindin (1.9 ± 0.7) was significantly larger than in neurons transfected with Shank1B only.
We then investigated the effects of Shank1B overexpression on the distribution of a GFP-tagged ER retention sequence (ER-GFP). When transfected alone, ER-GFP localized mostly in the cell body and proximal dendrites and not in the spines (Fig. 2A1-B3,Ea1-Ea3). In contrast, when coexpressed with Shank1B plus Homer1b, ER-GFP colocalized with Shank1B in the spines, up to the distal dendrites (Fig. 2C1-D3,Eb1-Eb3), to an extent (3.9 ± 0.9) similar to that observed with calreticulin and SERCA2b. Interestingly, the distribution of ER-GFP within dendritic spines was often not homogeneous but, rather, was concentrated in a few internal structures visible at high magnification (Fig. 2Eb1-Eb3). Together with the results obtained with the endogenous ER proteins, these observations strongly suggest that overexpression of Shank1B and Homer1b induces recruitment to the spines of entire ER cisternas. However, because of the lower accumulation of calbindin, these cisternas might not be identical to those predominant in the cell bodies and dendritic shafts.
Figure 2.

A-D, The ER compartment is recruited to dendritic spines by Shank1B plus Homer1b transfection. Neurons were transfected (Trx) at 11 d in vitro (DIV) with ER-GFP alone or in combination with HA-Shank1B plus Homer1b, as indicated on the left side of the panels. Then they were stained at 18 DIV for Shank (A1, B1, C1, D1 on the red channel) and GFP (A2, B2, C2, D2 on the green channel). Merged images are shown in the right panels (A3, B3, C3, D3). When transfected alone, ER-GFP is localized mostly on the cell body and proximal dendrites. When transfected with Shank1B plus Homer1b, it colocalizes with Shank in large dendritic spines. Ea1-Ea3 and Eb1-Eb3 are higher magnifications of A1-A3 and C1-C3, respectively. Ec1-Ec3 show high magnification of a dendrite from aneuron triple transfected with ER-GFP plus HA-Shank1B and Homer1a. F, Quantification of changes in synaptic staining intensity of ER-GFP induced by the overexpression of Shank1B (Sh1B) plus Homer1b (Ho1b) or Shank1B plus Homer1a (Ho1a). At least six neurons were analyzed for each endogenous protein (30-50 synapses scored per neuron). Histograms and error bars show the means ± SD normalized to the staining intensity in neurons transfected with ER-GFP alone (*p < 0.05). Scale bars: (in C3) A1-A3, C1-C3, 10 μm; (in D3) B1-B3, D1-D3, 5 μm; (in Ec3) Ea1-Ec3, 1.5 μm.
The role of direct interactions between Shank and Homer in the spine accumulation of ER was tested by using Shank1 (1-1440), a mutant that does not interact with Homer, and Homer1a, a dominant-negative form of Homer that disrupts the Shank1B/Homer1b interaction as well as that between Homer1b and IP3R. In previous studies, we have shown that Homer1a inhibits the Shank1B-induced enlargement of dendritic spines and causes redistribution of endogenous Shank1B to dendritic shafts (Sala et al., 2003). The defective Shank construct did not induce spine accumulation in either of the endogenous ER proteins (Fig. 1C1-C3,M) (data not shown) or in the transfected ER-GFP (Fig. 2, compare Ec1-Ec3,D1-D3, Eb1-Eb3) (for quantitation, see Figs. 1M, 2F). We conclude therefore that direct interaction of Shank1B and Homer1b is needed for the recruitment of ER cisternas to dendritic spines, presumably via the binding of Homer1b to the IP3R.
Functional effects of Shank1B expression
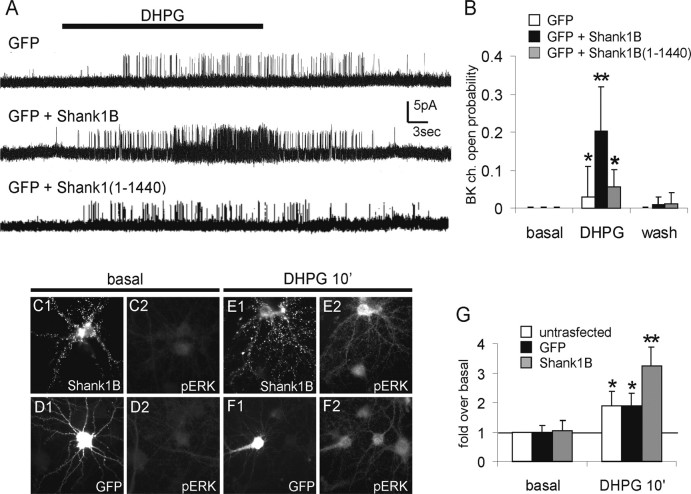
The functional implications of the structural changes induced by Shank1B also were investigated. ER is the major source of intracellular Ca2+ release in response to activation of receptors such as the mGluRs1/5 and stimulation of IP3Rs. Thus, it is possible that the recruitment of ER by Shank1B facilitates the mGluR1/5-mediated Ca2+ release response. The ensuing rise of [Ca2+]i then can activate the Ca2+-sensitive BK. The activity of this channel, measured in the cell-attached configuration of the patch-clamp technique, has been used previously to detect mGluR1/5 activity in neurons (Ango et al., 2001).
Because performing cell-attached patch clamp in spines poses unresolved problems, we made recordings from the cell soma. Neurons were transfected with Shank1B together with GFP. We did not transfect Homer1b, because this protein has been reported to induce intracellular retention of mGluR5 (Roche et al., 1999; Ango et al., 2002). Figure 3A (top trace) shows that in controls, the selective mGluR1/5 agonist DHPG reversibly increased BK Po [from 0.001 ± 0.001 (n = 10) to 0.029 ± 0.080 (n = 10)]. In neurons transfected with Shank1B (Fig. 3A, middle trace, B), this effect of DHPG was 10-fold more pronounced [Po increased from 0.001 ± 0.001 (n = 10) to 0.203 ± 0.115 (n = 10)]. Interestingly, in neurons transfected with Shank1 (1-1440), a mutant that does not interact with Homer, the BK Po values (Po increase from 0.001 ± 0.001 to 0.056 ± 0.045; n = 10) were similar to those measured in neurons transfected with GFP only (Fig. 3A, bottom trace, B).
Figure 3.
Shank1B overexpression increases BK currents, an mGluR1/5-induced Ca2+-dependent response, and ERK1/2 phosphorylation in neurons. A, BK unitary currents were recorded in cell-attached patches from GFP-transfected (top), GFP plus Shank1B-transfected (middle), and GFP plus Shank1 (1-1440)-transfected (bottom) neurons. The membrane patch was held at +20 mV. The horizontal bar represents the DHPG application (400 μm). B, Open probability of cell-attached recorded BK current obtained from GFP, GFP plus Shank1B, and GFP plus Shank1 (1-1440) cotransfected neurons before, during, and after DHPG application. Error bars and the histograms represent the means ± SEM of n = 10 neurons. C-F, Neurons were transfected at 11 d in vitro (DIV) with HA-Shank1B (C1, C2, E1, E2) or GFP (D1, D2, F1, F2) and stained at 18 DIV for HA (C1, E1) and pERK1/2 (C2, D2, E2, F2) before or after incubation for 10 min with 100 μm DHPG, as indicated at the top of each panel. G, Fluorescent intensity of pERK1/2 staining was quantified and compared in transfected versus nontransfected neurons. Histograms and error bars are the means ± SD of at least 20 neurons (*p < 0.05; **p < 0.01).
The effect of Shank1B overexpression also was tested on the phosphorylation of ERK1/2, taking place after mGluR1/5 stimulation (Roberson et al., 1999). Neurons were fixed and stained for pERK before or 10 min after treatment with the specific agonist DHPG (100 μm). With respect to basal, DHPG induced almost twofold increases in pERK immunostaining in both nontransfected and GFP-transfected neurons (1.9 ± 0.4 and 1.8 ± 0.5, respectively) (Fig. 3D1,D2,F1,F2,G). In Shank1B-transfected neurons, the increases were significantly higher (3.2 ± 0.7) (Fig. 3C1,C2,E1,E2,G). Interestingly, the neuronal levels of ERK phosphorylation induced by depolarization with 50 mm KCl or activation of the NMDA receptor (with 100 μm NMDA) were not significantly higher in Shank1B-transfected neurons with respect to nontransfected neurons or neurons transfected with GFP alone (data not shown). Thus, the increase in ERK phosphorylation in Shank1B-transfected neurons is specific to mGluRs1/5.
Discussion
Appropriate functioning of most excitatory synapses depends on the presence within dendritic spines of an elaborate tridimensional scaffold structure, the PSD. Numerous protein-protein interactions, identified and characterized at the molecular level within the PSD, are required for the integration of postsynaptic signals also at the level of organelles. Detailed ultrastructural studies of the hippocampal tissue have demonstrated that at least the large spines of pyramidal neurons contain ER cisternas (Spacek and Harris, 1997), whereas studies in cultured brain slices have revealed the generation of signals such as IP3 and Ca2+ release in the spines in response to receptor activation (Emptage et al., 1999, 2001). In cerebellar Purkinje neurons, the spines contain even more elaborate ER systems highly enriched in IP3 receptors (Satoh et al., 1990), which are essential for controlling Ca2+ homeostasis and synaptic signaling (Miyata et al., 2000; Wang et al., 2000).
Compared with those of the brain tissue, most spines of cultured neurons appear to be smaller, less elaborate in structure, and lacking in IP3R. These marked differences suggested the existence of specific maturation mechanisms that are necessary to convert the immature in vitro spines to a mature state. Previous data showing enlargement of spines and recruitment of IP3Rs after neuron transfection with Shank1B, together with Homer1b (Sala et al., 2001), suggested an important role for the latter two proteins in spine maturation. However, these previous data did not exclude the possibility that the spine accumulation of IP3Rs was attributable to redistribution of IP3Rto non-ER-type membranes, to increased density of IP3Rs in predistributed ER cisternas, or to both. Moreover, the functional importance of the Shank/Homer-induced IP3R accumulation in the spines remained undefined.
Our present results provide answers to these questions. The accumulation of ER proteins, both lumenal and transmembrane, in parallel with the IP3Rs is a strong indication of the recruitment of ER cisternas to the spines. This effect, observed previously after transfection of Shank1B alone most likely interacting with the endogenous Homer1b, was enhanced further when the two proteins were cotransfected. The ER lumen is known to be continuous all along the cell. Therefore, the concentration of lumenal proteins is expected to be homogenous unless local aggregation into specific subcellular compartments occurs. This does not appear to be the case for calreticulin (Pezzati et al., 1997) and ER-GFP (Rizzuto et al., 1998; Truong et al., 2001). As far as the membrane-bound proteins were concerned, a lesser degree of accumulation was observed only with calbindin, an ER chaperone active in the folding of newly synthesized proteins exposed to the ER lumen. This could be attributable to ER heterogeneity (Sitia and Meldolesi, 1992). In the spines, the ER is smooth surfaced; thus, a high density of a chaperone might be unnecessary.
In contrast, the experiments with the truncated Shank1 (1-1440) and Homer1a proteins provided indications about the mechanisms of ER recruitment to the spines. The lack of this process, which was observed any time the binding of Shank1 to Homer1b was prevented, strongly suggests the need of the two proteins to form a complex, with Homer1b binding to IP3R and Shank1 interacting with PSD proteins and with the cytoskeleton via actin-associated proteins. The tendency of Shank1 to dimerize could enlarge and reinforce this complex further (Naisbitt et al., 1999; Romorini et al., 2004). The establishment of the complex also might have a role in the Shank1/Homer1b-induced maturation of the spines. Alternatively, an early enlargement of the spines induced by Shank1 could be instrumental to a subsequent ER recruitment. Whatever the sequence, it suggests that the mature spine organization depends on the Shank1/Homer1b interaction. Interestingly, although mGluRs1/5 bind Homer1b, their overexpression in neurons appears to be insufficient to recruit the ER markers, including IP3R, to the dendritic spines (C. Sala, unpublished observations).
In view of their equipment with the SERCA2b Ca2+ pump, the Ca2+-binding protein calreticulin, and the Ca2+ channel IP3R, the ER cisternas recruited to the spines by Shank1B/Homer1b were expected to be functional. Consistent with this prediction, the activation of the Ca2+-dependent BK in response to mGluR1/5 activation was much larger in neurons transfected with Shank1B than in control neurons. Although this Ca2+-dependent response was studied in the cell body of transfected neurons, one can assume that it also occurred in dendritic spines, because these are the structures in which the transfected Shank1B accumulates and in which mGluRs1/5 and IP3-sensitive Ca2+ stores are expressed (Fagni et al., 2000).
Signal transduction from mGluRs1/5 to ERK1/2 phosphorylation has been investigated previously, and both Ca2+-dependent and Ca2+-independent mechanisms have been proposed (Ferraguti et al., 1999; Roberson et al., 1999; Choe and Wang, 2001; Wang et al., 2004). In our hippocampal neurons transfected with Shank1B, the density of mGluRs1/5 in the postsynaptic membrane was unchanged, yet the ERK phosphorylation in response to the activation of this receptor was increased considerably. In contrast, ERK phosphorylations occurring after other types of stimulation, involving not the Ca2+ stores but rather plasma membrane channels and receptors (depolarization, activation of NMDA receptors), were unchanged. We conclude that the expression of Shank1B induces a signaling facilitation specifically concerning the mGluR1/5 stimulation of Ca2+ stores and probably not other signaling pathways.
In conclusion, our data demonstrate that the PSD scaffold proteins Shank1B and Homer1b play major roles in the molecular composition, structural organization, and functional properties of dendritic spines in hippocampal neurons. The specific protein-protein interactions of the Shank1/Homer1b complex with various spine components, in particular the mGluRs1/5 and IP3R, appear to be necessary not only to recruit ER cisternas to the spines but also to establish optimal signaling conditions and thus to generate larger functional responses. The physiological relevance of these processes is still mostly unknown. However, in cerebellar Purkinje cells and hippocampal pyramidal neurons, the ER accumulation in the spines (Miyata et al., 2000) and phosphorylation of ERK1/2 after mGluR1/5 activation (Gallagher et al., 2004), respectively, have been reported previously to play a role in the development of long-term depression. Therefore, the Shank1B/Homer1b complex with PSD and other proteins may be important not only for the functional organization of synapses but also for synaptic plasticity.
Footnotes
This work was supported by the European Community (LSHM-CT-2004-511995, SYNSCAFF). C.S. is supported by the Giovanni Armenise-Harvard Foundation Career Development Program. G.R. holds a grant from the Ministere de l'Education Nationale de la Recherche et de la Technologie. J.M. is supported by the European Union program APOPIS, Telethon (GGP030234), and the Consiglio Nazionale delle Ricerca program “Physiopathology of the Nervous System.” L.F. is supported by the Centre National de la Recherche Scientifique and the Institut National de la Santé et de la Recherche Médicale. We thank Stefano Romorini and Maria Noemi Tonna for technical help.
Correspondence should be addressed to Carlo Sala, Cellular and Molecular Pharmacology Section, Institute of Neuroscience, Consiglio Nazionale delle Ricerche, and Department of Pharmacology, University of Milan, Via Vanvitelli 32, 20129 Milan, Italy. E-mail: c.sala@in.cnr.it.
Copyright © 2005 Society for Neuroscience 0270-6474/05/254587-06$15.00/0
References
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962-965. [DOI] [PubMed] [Google Scholar]
- Ango F, Robe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2002) Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci 20: 323-329. [DOI] [PubMed] [Google Scholar]
- Baba-Aissa F, Raeymaekers L, Wuytack F, Dode L, Casteels R (1998) Distribution and isoform diversity of the organellar Ca2+ pumps in the brain. Mol Chem Neuropathol 33: 199-208. [DOI] [PubMed] [Google Scholar]
- Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, Wex H, Langnaese K, Bockmann J, Garner CC, Gundelfinger ED (1999) Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ domain protein highly enriched in the postsynaptic density. J Neurosci 19: 6506-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Wang JQ (2001) Group I metabotropic glutamate receptors control phosphorylation of CREB, Elk-1, and ERK via a CaMKII-dependent pathway in rat striatum. Neurosci Lett 313: 129-132. [DOI] [PubMed] [Google Scholar]
- Emptage N, Bliss TV, Fine A (1999) Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22: 115-124. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A (2001) Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron 29: 197-208. [DOI] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J (2000) Complex interactions between mGluRs, intracellular Ca2+ stores, and ion channels in neurons. Trends Neurosci 23: 80-88. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C (1999) Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci 11: 2073-2082. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM (2004) Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci 24: 4859-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL (2000) PDZ domains in synapse assembly and signaling. Trends Cell Biol 10: 274-280. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M (2001) Dendritic spines: structure, dynamics, and regulation. Nat Rev Neurosci 2: 880-888. [DOI] [PubMed] [Google Scholar]
- Kennedy MB (2000) Signal-processing machines at the postsynaptic density. Science 290: 750-754. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771-781. [DOI] [PubMed] [Google Scholar]
- Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M (2000) Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28: 233-244. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M (1999) Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23: 569-582. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K (2002) Structure and function of dendritic spines. Annu Rev Physiol 64: 313-353. [DOI] [PubMed] [Google Scholar]
- Pezzati R, Bossi M, Podini P, Meldolesi J, Grohovaz F (1997) High-resolution calcium mapping of the endoplasmic reticulum-Golgi-exocytic membrane system. Electron energy loss imaging analysis of quick-frozen freeze-dried PC12 cells. Mol Biol Cell 8: 1501-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763-1766. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD (1999) The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element-binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 19: 4337-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF (1999) Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem 274: 25953-25957. [DOI] [PubMed] [Google Scholar]
- Romorini S, Piccoli G, Jiang M, Grossano P, Tonna N, Passafaro M, Zhang M, Sala C (2004) A functional role of postsynaptic density-95-guanylate kinase-associated protein complex in regulating Shank assembly and stability to synapses. J Neurosci 24: 9391-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M (2001) Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31: 115-130. [DOI] [PubMed] [Google Scholar]
- Sala C, Futai K, Yamamoto K, Worley PF, Hayashi Y, Sheng M (2003) Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. J Neurosci 23: 6327-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J (1990) The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol 111: 615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Meldolesi J (1992) Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell 3: 1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Harris K (1997) Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci 17: 190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong K, Sawano A, Mizuno H, Hama H, Tong KI, Mal TK, Miyawaki A, Ikura M (2001) FRET-based in vivo Ca2+ imaging by a new calmodulin-GFP fusion molecule. Nat Struct Biol 8: 1069-1073. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF (1999) Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23: 583-592. [DOI] [PubMed] [Google Scholar]
- Villa A, Podini P, Panzeri MC, Racchetti G, Meldolesi J (1994) Cytosolic Ca2+ binding proteins during rat brain ageing: loss of calbindin and calretinin in the hippocampus, with no change in the cerebellum. Eur J Neurosci 6: 1491-1499. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L (2004) Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol Neurobiol 29: 1-14. [DOI] [PubMed] [Google Scholar]
- Wang SS, Denk W, Hausser M (2000) Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci 3: 1266-1273. [DOI] [PubMed] [Google Scholar]
- Zitzer H, Honck HH, Bachner D, Richter D, Kreienkamp HJ (1999) Somatostatin receptor interacting protein defines a novel family of multidomain proteins present in human and rodent brain. J Biol Chem 274: 32997-33001. [DOI] [PubMed] [Google Scholar]