Abstract
The primate anterior hippocampus (which corresponds to the rodent ventral hippocampus) receives inputs from brain regions involved in reward processing, such as the amygdala and orbitofrontal cortex. To investigate how this affective input may be incorporated into primate hippocampal function, we recorded neuronal activity while rhesus macaques performed a reward-place association task in which each spatial scene shown on a video monitor had one location that, if touched, yielded a preferred fruit juice reward and a second location that yielded a less-preferred juice reward. Each scene had different locations for the different rewards. Of 312 neurons analyzed in the hippocampus, 18% responded more to the location of the preferred reward in different scenes, and 5% responded to the location of the less-preferred reward. When the locations of the preferred rewards in the scenes were reversed, 60% of 44 hippocampal neurons tested reversed the location to which they responded, showing that the reward-place associations could be altered by new learning in a few trials. The majority (82%) of these 44 hippocampal neurons tested did not respond to reward associations in a visual discrimination, object-reward association task. Thus, the primate hippocampus contains a representation of the reward associations of places “out there” being viewed. By associating places with the rewards available, the concept that the primate hippocampus is involved in object-place event memory is now extended to remembering goals available at different spatial locations. This is an important type of association memory.
Keywords: hippocampus, episodic memory, macaque, spatial view cells, place cells, reward, associative learning
Introduction
The primate anterior hippocampus corresponds to the rat ventral hippocampus and has inputs from regions such as the amygdala, orbitofrontal cortex, and olfactory areas, which reach the hippocampus via the entorhinal cortex (Amaral et al., 1992; Carmichael et al., 1994; Stefanacci et al., 1996; Petrovich et al., 2001). For example, the lateral nucleus of the amygdala projects to the rostral part of the primate entorhinal cortex (Pitkanen et al., 2002) and also to the perirhinal cortex (Stefanacci et al., 1996), which projects on to the entorhinal cortex. There are also direct projections from the basal accessory nucleus of the amygdala to the CA1-CA3 regions of the hippocampus (Amaral et al., 1992). In addition, the orbitofrontal cortex projects to the entorhinal cortex and perirhinal cortex (Van Hoesen et al., 1975; Suzuki and Amaral, 1994). Given the functions of the primate amygdala and orbitofrontal cortex in representing different rewards and punishers (Rolls, 1992, 1999a, 2000, 2004), including the taste, smell, texture, and sight of food (Rolls, 2004, 2005; Kadohisa et al., 2005), it is possible that reward-related information may reach the primate hippocampus. It is known that the primate hippocampus contains spatial view cells, which provide a representation of space “out there” (Rolls et al., 1997a, 1998; Robertson et al., 1998; Rolls, 1999b), and it is a very interesting hypothesis that the spatial and reward information might be combined associatively in the hippocampus (Marr, 1971; Rolls, 1989, 1996; Treves and Rolls, 1994; Rolls and Treves, 1998; Rolls et al., 2002) to provide a representation of the rewards available at different spatial locations. The hypothesis that reward-related information may reach the primate hippocampus and be used in its memory functions has not been investigated neurophysiologically in previous research. Consistent with this hypothesis, neurotoxic hippocampal lesions impair spatial scene memory, which is tested by requiring a monkey to remember which location in each of a number of scenes to touch to obtain reward (Murray et al., 1998). In rodents, there is some evidence that reward can influence ventral hippocampal neurons (Hölscher et al., 2003; Tabuchi et al., 2003). However, the rat hippocampus contains place cells that respond to the place where the rat is located (McNaughton et al., 1983; O' Keefe and Speakman, 1987), and place cells are not useful for the primate (including human) memory task of remembering the rewards available at different locations in a scene that is being viewed, because they respond to where the animal is located. In contrast, primate hippocampal spatial view cells represent allocentric information about the spatial location being viewed and respond to the spatial view provided that the monkey looks at the location in space (Georges-François et al., 1999). Association between spatial view representations and rewards might provide a basis for remembering where different rewards are located in space, and it is the encoding of this type of information that we describe in this study in a reward-place association learning task and its reversal.
Materials and Methods
The reward-place memory task. We designed a memory task in which monkeys must associate different locations in an entire scene with different rewards, in what is formally a reward-place association memory task. The monkey is shown an entire scene on a video monitor and can touch one of two locations in the scene to obtain one of two rewards. One location, when touched, leads to a more-preferred reward (e.g., black-currant juice), and the other location leads to a less-preferred reward (orange juice). The rewards are delivered to the mouth through a lick tube so that the monkey cannot see the reward. This is thus a place out there-to-taste reward association task.
The task is illustrated in Figure 1c. Spatial scenes, of which examples are shown in Figure 1a, were shown on a touch-screen video monitor at a distance of 24 cm from the monkey subtending 64 × 50° at the retina. The images of spatial scenes had a resolution of 256 × 256 and 256 grayscale levels. In the different scenes, the preferred reward [reward 1 (R1)] was available at one of four possible locations, P1-P4, and the less-preferred reward [reward 2 (R2)] was available at a second location, as shown in Figure 1. (The firing rate graphs superimposed on the scene in Fig. 1 were not part of what was seen by the monkey.) Two faint circles in each scene indicated where a touch would lead to either R1 or R2. On any one trial, only one location had a faint circle, and R1 or R2 was obtainable on that trial depending on which location was circled and touched. The area within which a touch led to reward was ∼13° at the retina. The monkey could touch a location in a scene up to three times on any trial to obtain three rewards. Three scenes were typically used and presented in permuted random sequence. Scenes 1 and 2 shown in Figure 1 had R1 and R2 in opposite egocentric locations, showing that it was the location in each scene of each reward that had to be learned, and not the egocentric locations of the rewards. Scene three used two locations that were not used in scenes 1 and 2. The presentation of a scene was preceded by a 0.5 s tone cue, the scene was presented for 4 s, and the intertrial interval was 3 s. At the time of the tone cue, the monkey typically saccaded to look at that monitor if he was not already doing so, as shown by the scleral search coil eye position recordings (Judge et al., 1980). When the scene was shown, the monkey typically made saccades until he chose a location to touch and then typically kept fixating that location while he guided his hand to touch the correct location. Because multiple licks could be made for fruit juice during the period in which the visual scene was on, the monkey typically worked quickly for the three touches, so that he could obtain three licks of fruit juice. Although both of the fruit juices were palatable to the monkey, the blackcurrant juice (R1) was preferred, as shown by the fact that the location at which it was available was touched on ∼82% of the trials, independently of which scene was being shown. When the location of R2 was indicated with a faint circle, the monkey touched this on ∼50% of trials, and this was part of the evidence that R2 was less preferred. The monkey learned the locations of the rewards in each scene well, because the same scenes were used daily.
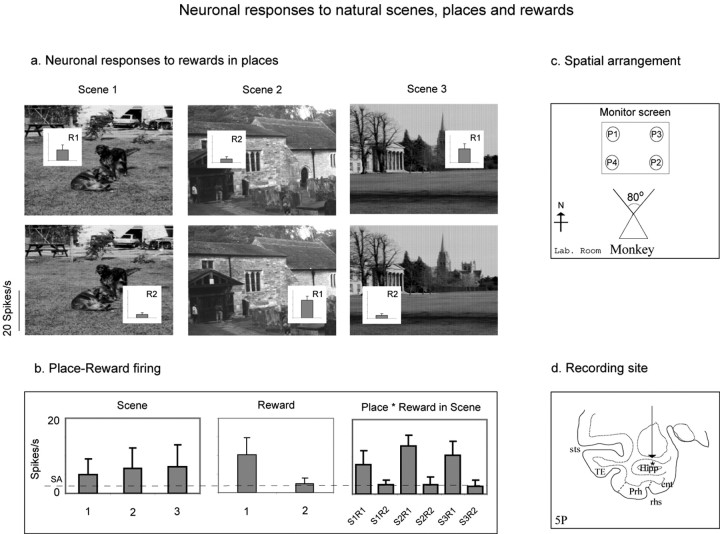
Figure 1.
A neuron with place-reward-related firing. a, Firing rate insets to show the firing in three different scenes (S1-S3) of the locations associated with R1 (preferred) and R2. The mean responses ± SEM are shown in all figures. b, The firing rates, sorted by scene, by reward (1 vs 2), and by scene-reward combinations (e.g., scene 1, reward 1 = S1R1). SA, Spontaneous firing rate. c, The spatial arrangement on the screen of the four spatial locations (P1-P4). d, The recording site of the neuron. ent, Entorhinal cortex; Hipp, hippocampal pyramidal cell field CA3/CA1 and dentate gyrus; Prh, perirhinal cortex; rhs, rhinal sulcus; sts, superior temporal sulcus; TE, inferior temporal visual cortex.
Reversal of the reward-place memory task. To test whether the hippocampal neurons could rapidly learn new reward-place locations, we set up a reversal of the reward-place associations from the standard associations. The type of reward available at the locations in each scene was reversed. The monkey was able to learn to do this reversal with practice in ∼20 trials. (The criterion was 7 of the last 10 responses were to the new location in each scene of the preferred reward.) For some neurons, after the main recording investigation described above, it was possible to test the monkey with the reversed contingencies, to determine whether the neurons could relearn the reward-place associations.
Object-reward task. For comparison with place-reward learning, we also tested whether object-reward association learning was encoded by the same neurons. It is known that visual object-taste reward associations are learned, and reversed rapidly, in the primate orbitofrontal cortex (Thorpe et al., 1983; Deco and Rolls, 2005), which may be seen as an output of “what” systems for vision, touch, taste, smell, and probably audition (Rolls, 2004, 2005). It is likely that the hippocampus is involved particularly in associations of space with other inputs, and it was therefore of interest to investigate whether place-reward hippocampal neurons are not involved in object-reward encoding. This was also a useful control to check that the place in a scene-reward association described here was not as a result of associations between objects in the scenes and reward. The object-reward task was also useful in providing evidence on whether the hippocampal neurons described here with reward-place encoding could be activated just by reward, which was being provided in the object-reward association task.
In the object-reward task, a single object subtending 28° at the retina was shown in the middle of the screen in a Go/NoGo visual discrimination task. To one object (typically a triangle), a lick would lead to the preferred reward being delivered from the lick tube. To the other object (typically a square), a lick would lead to a drop of saline being delivered from the lick tube. This task was chosen to be identical to that used in investigations of neuronal activity in the orbitofrontal cortex in which neurons do respond to objects based on their taste reward or saline association (Rolls et al., 1996), to enable a direct comparison with activity in the hippocampus. The aim was to garner evidence on whether the hippocampus is involved in reward associations in general or more with spatial-reward than object-reward associations.
Recordings. Single neurons were recorded with epoxylite-insulated tungsten microelectrodes (FHC, Bowdinham, MA) with impedances of 1-5 MΩ, and giving a high signal/noise ratio of at least 3:1 with well isolated neurons from one rhesus macaque (5 kg), with methods that have been described previously (Rolls et al., 1989, 1996). [All procedures, including preparative and subsequent ones, were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were licensed under the United Kingdom Animals (Scientific Procedures) Act, 1986.] For every time that the cell fired, the time was recorded with an accuracy of 0.1 ms. Datawave (Boulder, CO) Discovery spike acquisition software was used during the recordings, and its spike cluster cutting software was used off-line to ensure that the spikes of well isolated neurons were analyzed.
Procedure. The single neuron microelectrode was lowered until just above the hippocampus (as determined by the recording depth and the background noise that is low in the ventricle), and each neuron encountered was monitored to test whether it had the large-amplitude, well isolated action potential with a low spontaneous firing rate (Rolls et al., 1998) and peak firing rate of typically 10 but up to 20 spikes/s, which is typical of hippocampal spatial view cells and is thought to be produced by hippocampal pyramidal cells (Rolls et al., 1997b, 1998; Robertson et al., 1998; Georges-François et al., 1999). Then the reward-place task was run for at least 60 trials, and typically 100 trials. If the neuron was still being recorded, then the reward-reversal paradigm, the object-reward paradigm, or both were run.
After recording in the hippocampus, recording typically continued until the microelectrode reached the base of the brain, so that neuronal activity in the perirhinal cortex and related areas such as TF could be sampled and analyzed during the same task.
Statistics. The neuronal activity for each cell was analyzed for a 1 s period starting 100 ms after the visual stimulus was turned on, as the monkey was processing the visual stimulus and making the decision about whether to respond in this period. The firing rate for every trial was included in the analyses. There were the same number of R1 and R2 trials, and for each neuron, there were typically 50 trials of each type (and a minimum of 30 trials), with approximately one-third of these trials in each of the three different scenes. A two-way ANOVA was then performed with one factor the reward type and the second factor the scene. A significant effect on only the first factor indicated that a neuron had significantly different firing to R1 versus R2 independently of the scene, and wherever R1 and R2 were in each scene. A significant effect on only the second factor indicated that the neuron had significantly different firing for the different scenes. This was the main statistical test considered to avoid the need for statistics corrected for multiple comparisons. Fisher's exact probability tests, as described in Results, were performed to check that the number of significant neuronal responses found could not have arisen by chance. [The Fisher (1932) probability combination (or generalized significance or exact probability) test is well established and asymptotically Bahadur optimal (Littell and Folks, 1971; Zaykin et al., 2002)]. Two-tailed least significant difference post hoc tests as implemented in SPSS (Chicago, IL) software and corrected for multiple comparisons were performed to check whether, for example, the differential firing of neurons to R1 versus R2 was consistent across every scene. The level of significance taken was p < 0.05, and tests not significant at this level are termed NS.
Recording sites. X-radiography was used to determine the position of the microelectrode after each recording track relative to permanent reference electrodes and to the anterior sphenoidal process, a bony landmark with a position that is relatively invariant with respect to deep brain structures. Microlesions (60-100 μA, 100 s) made through the tip of the recording electrode during the final tracks were used to mark the location of typical neurons. These microlesions, together with the associated X-radiographs, allowed the position of all cells to be reconstructed in the 50 μm brain sections with the methods described by Feigenbaum and Rolls (1991). As described previously, the spatial view cells typically had low spontaneous firing rates, low peak firing rates, and large-amplitude broad spikes (Rolls et al., 1997b). All of the hippocampal neurons described here had the large spike amplitude and relatively low firing rate type of activity and were recorded in regions where there are pyramidal cells. They are sometimes referred to, for brevity, as pyramidal cells here.
Results
It was possible to complete the data collection required for the detailed analyses described below for 312 neurons in the hippocampus and 93 neurons in the perirhinal cortex. First, we describe the types of neuronal response found in the task and then summarize the findings for the population of neurons analyzed.
Neurons with differential firing to places depending on the reward available at that place
An example of a neuron with differential reward-place firing is shown in Figure 1. The neuron had a higher firing rate to the location in each of the three scenes where R1 could be obtained than to the location where R2 could be obtained, as shown in Figure 1a (t = 2.02; df = 43; p < 10-9) and in the middle and right panels of Figure 1b. The mean firing rate to the locations of R1 across the three scenes was 18.1 ± 1.2 spikes/s, and the mean firing rate to the locations of R2 was 4.6 ± 1.3 spikes/s.
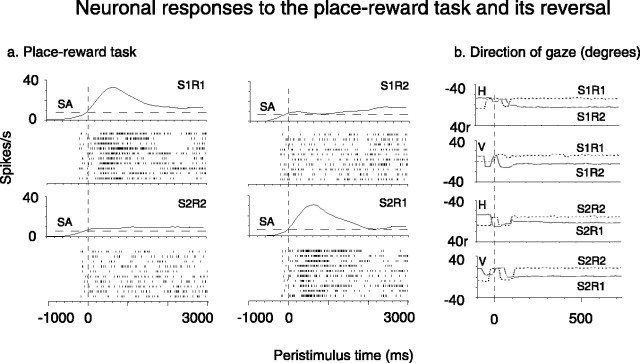
An example of another neuron with responses that depended on the reward available at a location is shown at the top of Figure 2. The neuron had a higher firing rate to the location in each of the two scenes where R1 could be obtained than to the location where R2 could be obtained, as shown in Figure 2a (t = 2.01; df = 54; p < 10-6). The mean firing rate to the locations of R1 across the two scenes was 20.8 ± 1.2 spikes/s, and the mean firing rate to the locations of R2 was 11.7 ± 1.2 spikes/s. Peristimulus rastergrams and histograms and eye position recordings of this same data (Fig. 2, top) are shown in Figure 3. The latency of the neuronal responses is ∼200 ms, and typically one or two saccades are made to acquire the correct location, as shown in Figure 3.
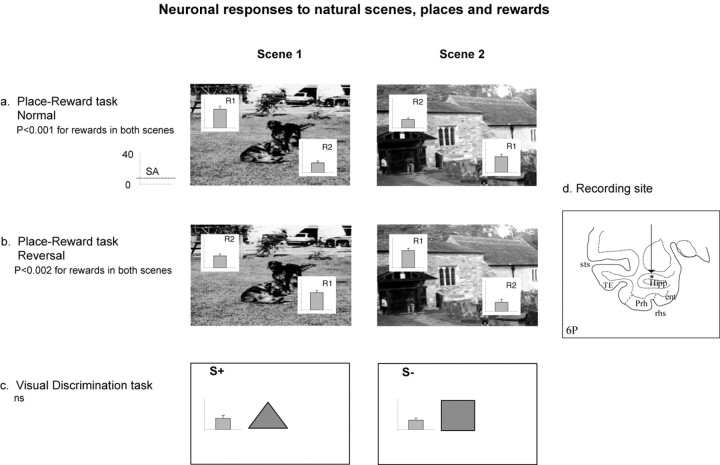
Figure 2.
a, A neuron (BP063) with reward-place-related firing. b, Data for the same neuron after reversing the places at which R1 and R2 were available. c, The lack of response of the neuron in the visual discrimination (or object-reward association) task, in which S+ is the rewarded stimulus and S- is the punished stimulus. d, The recording site of the neuron in the CA3 region. SA, Spontaneous firing rate; ent, entorhinal cortex; Hipp, hippocampal pyramidal cell field CA3/CA1 and dentate gyrus; Prh, perirhinal cortex; rhs, rhinal sulcus; sts, superior temporal sulcus; TE, inferior temporal visual cortex.
Figure 3.
a, Peristimulus time histograms and rastergrams showing the response of neuron 63a04 in the reward-place memory task. Each rastergram shows the firing on a single trial. The rastergrams start 500 ms before the onset of the visual stimuli at time = 0, and the peristimulus time histogram was smoothed with a Gaussian filter with an SD of 150 ms. SA, Spontaneous activity of the neuron. b, Eye-position recordings made during the reward-place task. The axis for the horizontal eye position (H) is from left -40° to right (r) +40°. The axis for the vertical eye position (V) is from down -40° to up +40°. S1R1, Scene1, reward 1; S1R2, scene 1, reward 2.
Hippocampus
Of the 312 neurons analyzed in the hippocampus, 70 had responses that were significantly different from R1 versus R2 locations (Table 1). Of these 70 neurons, 56 had larger responses to the locations where R1 (mean across neurons, 18.6 ± 1.6 SEM spikes per second) was presented than where R2 was presented (11.0 ± 1.2 spikes/s; paired t test; p < 10-12). Fourteen neurons had larger responses to the locations where R2 was presented than where R1 was presented (10.3 ± 3.5 vs 7.7 ± 3.0 spikes/s; p < 0.0006). Thus, although the majority of these reward-place neurons fired more for the place of the preferred reward in all scenes, a proportion responded to the locations of the less-preferred reward. Thus, the locations of both types of reward are encoded by primate hippocampal neurons. This is also a useful control finding, because it shows that nonspecific motivational effects cannot account for the types of neuronal response found, in that some neurons code for the locations of the less-preferred reward.
Table 1.
Numbers of neurons with significantly different responses to locations in different scenes that depend on the reward available at the location and with different responses to different scenes
|
|
|
Reward |
Scene |
||||
|---|---|---|---|---|---|---|---|
| Region |
Total |
n
|
% |
n
|
% |
||
| Hippocampus | 312 | 70 | 22.4 | 24 | 7.7 | ||
| Perirhinal cortex |
93 |
10 |
10.8 |
3 |
3.2 |
||
We checked that the significant results for R1 versus R2 locations obtained for these neurons were not attributable to chance by calculating a Fisher's exact probability of obtaining the number of significant results across the R1 versus R2 ANOVAs performed on the entire population of 312 neurons. This check showed that across the 312 neurons, the Z value was 13.53, associated with the hypothesis that this number of results with the significance level of each test that was obtained in the ANOVA would have been obtained by chance, and thus the hypothesis that the results were as a result of chance statistical fluctuations can be rejected with p ≪ 10-15. (For the 70 neurons, the response of 19 neurons to the location of R1 was significantly different from the response at the location of R2 at p < 0.001, with 26 neurons significant at p < 0.01 and 25 neurons significant at p < 0.05.)
The mean spontaneous firing rate of these neurons was 5.9 ± 0.3 spikes/s
A different population of hippocampal neurons, 24 in the sample of 312 neurons, did have significantly different firing rates to the different scenes (Table 1). A Fisher's exact probability test showed that this did not occur by chance with p = 0.01. The mean firing rate to the most effective scene was 13.2 ± 2.4 spikes/s and to the least effective scene was 9.6 ± 2.0 spikes/s (paired t = 2.07; df = 23; p < 10-7). In addition, 5 neurons in the sample of 312 neurons responded significantly differently to the same position on the screen independently of which reward was available at each position or of which scene was being shown. These five neurons thus appeared to code for position on the screen. The significance levels of the differences in firing rates for different positions of most of these five neurons were <0.007. However, a Fisher's exact probability test showed that this set of results (for the five neurons responding to position on the screen independently of scene or reward) could have occurred across the entire population of 312 neurons by chance (Z = 0.9).
Perirhinal cortex
Of the 93 neurons analyzed in the perirhinal cortex, 10 had responses that were significantly different from R1 versus R2 locations (Table 1). Of these 10 neurons, 9 had larger responses to the locations where R1 (mean across neurons, 20.0 ± 3.9 SEM spikes per second) was presented than where R2 was presented (15.0 ± 3.3 spikes/s; paired t test; p = 0.004). One neuron had larger responses to the location where R2 was presented than where R1 was presented.
We checked that the significant results for R1 versus R2 locations obtained for these neurons were not attributable to chance by calculating a Fisher's exact probability of obtaining the number of significant results across the R1 versus R2 ANOVAs performed on the entire population of 93 neurons. This check showed that across the 93 neurons, the Z value was 2.88, associated with the hypothesis that this number of results with the significance level of each test that was obtained in the ANOVA would have been obtained by chance, and thus the hypothesis that the results were as a result of chance statistical fluctuations can be rejected with p < 0.004.
The mean spontaneous firing rate of these perirhinal cortex neurons was 12.2 ± 0.5 spikes/s.
Reversal of the reward-place memory task
An example of a reversal experiment is shown in Figure 2. In Figure 2a, the results before reversal (called “direct”), already described, are shown. The neuron responded more to the location of R1 than to the location of R2 in both scenes with p < 0.001 in each case. After reversal, the firing changed as shown in Figure 2b, with more firing now to different locations in the scenes. In particular, there was more firing to the new locations in both scenes that now are associated with R1 (t = 2.02; df = 54; p < 0.001), with p < 0.002 in each case. Before reversal, the mean firing rate to the locations of R1 across the two scenes was 20.8 ± 1.2 spikes/s, and the mean firing rate to the locations of R2 was 11.7 ± 1.2 spikes/s. After reversal, the mean firing rate to the locations of R1 across the two scenes was 23.0 ± 3.1 spikes/s, and the mean firing rate to the locations of R2 was 12.0 ± 3.9 spikes/s.
The majority of the 44 hippocampal neurons tested in reversal did reverse the locations to which they responded after reversal. For example, of the 34 neurons with larger responses to R1 than R2 before reversal, 24 reversed the location to which they responded after reversal, such that their response continued to be larger to the new location of R1 (Table 2). [For these neurons, the mean response before reversal to R1 was 16.4 ± 2.2 spikes/s and to R2 was 11.0 ± 21.7 spikes/s (p < 10-6), and after reversal, the mean rate of the 24 reversing neurons to R1 was 16.2 ± 2.3 spikes/s and to R2 was 10.7 ± 1.8 spikes/s (p < 10-6).] Of the 34 neurons, 6 stopped responding significantly differently to the two locations after reversal (Table 2). Of the 10 neurons with the response to R1 less than that to R2 (R1 < R2), two reversed and three stopped discriminating after reversal. Thus, overall, for hippocampal neurons, of 44 tested in reversal, 26 showed significant reversal and 9 stopped responding differently to the different locations after reversal (Table 2). As shown in Table 2, 9 of the 44 hippocampal neurons tested in reversal did not reverse the locations to which they responded when the reward associations of the locations were reversed, and these neurons thus represented spatial view independently of reward association.
Table 2.
Numbers of neurons with more activity to R1 or R2 before reversal (direct) and after reversal a
|
|
|
Hippocampus |
Perirhinal cortex |
||||
|---|---|---|---|---|---|---|---|
| Experiments | R1 > R2 | R1 < R2 | R1 > R2 | R1 < R2 | |||
| Direct | 34 | 10 | 5 | 1 | |||
| Reversal | R1 > R2 | 24 | 5 | 3 | 1 | ||
| R1 < R2 | 4 | 2 | 1 | 0 | |||
| R1 = R2 | 6 | 3 | 1 | 0 | |||
| Visual discrimination | S+ > S− | 6 | 2 | 1 | 0 | ||
| S+ < S− | 2 | 0 | 0 | 1 | |||
|
|
S+ = S− |
26 |
8 |
4 |
0 |
||
The numbers of these neurons with different types of activity in the visual discrimination task to the S+ and S− are also shown. S+, Rewarding stimuli; S−, punishing stimuli.
For perirhinal cortex neurons, it was possible to test six in reversal, and of these neurons, three reversed the locations to which they responded (following the reward) and one stopped responding differently to the different locations after reversal.
Object-reward visual discrimination task
The responses of the neuron of Figure 2 are shown for the object-reward visual discrimination task in Figure 2c. There was no differential response in the visual discrimination task, with the firing rates to the reward-related stimulus 14.8 ± 1.8 spikes/s and to the saline-associated stimulus 15.5 ± 1.9 spikes/s (t = 1.99; df = 65; NS).
As shown in Table 2, of the 44 reward-place hippocampal neurons tested in the visual discrimination task, the majority (n = 34) responded equally (typically with a small change from the spontaneous rate of 5.9 spikes/s) to the rewarded stimulus (S+) and the punished stimulus (S-) in the visual discrimination task. The mean response to the S+ was 13.5 ± 1.9 spikes/s and to the S- was 11.5 ± 2.2 spikes/s (paired t = 1.69; df = 33; NS).
An additional 24 hippocampal neurons that were not tested on the place-reward task were tested in the object-reward task (to obtain additional evidence on hippocampal neuronal activity and object-reward associations), and 20 neurons did not discriminate between the S+ and the S-. Three of these additional 24 neurons responded more to the S+, and one more responded more to the S-. For the entire set of 68 hippocampal neurons tested in the visual discrimination task, the mean response to the S+ was 14.2 ± 2.0 spikes/s and to the S- was 13.3 ± 2.2 spikes/s (paired t = 1.67; df = 67; NS).
Recording sites
The recording sites of the neurons with reward-place-related firing are shown in Figure 4. Most were in the anterior hippocampus, the main part of the hippocampus sampled, with 10 neurons in the perirhinal cortex as defined by Suzuki and Amaral (2003).
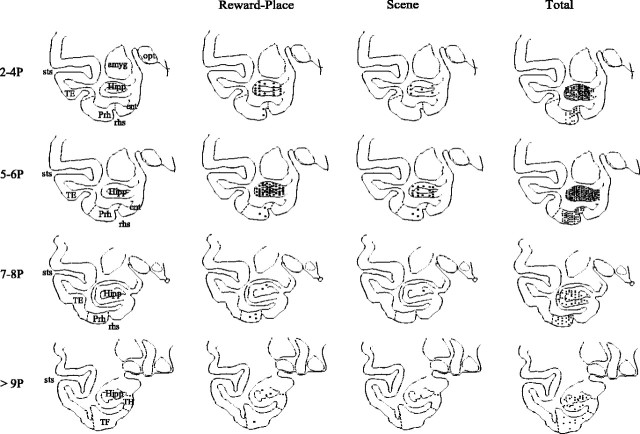
Figure 4.
Recording sites. The hippocampal and perirhinal cortex sites at which reward-place neurons (second column) were recorded are shown. The recording sites of neurons that responded differentially to the different scenes are shown in the third column. The recording sites of all of the neurons are shown in the right column. Coronal sections at different distances in millimeters posterior (P) to the sphenoid reference are shown. amyg, Amygdala; Hipp, hippocampal pyramidal cell field CA3/CA1 and dentate gyrus; opt, optic tract; Prh, perirhinal cortex; rhs, rhinal sulcus; sts, superior temporal sulcus; TE, inferior temporal visual cortex; TF/TH, areas of the parahippocampal gyrus.
Discussion
Because the neurons respond to the location of the same reward in several scenes, these hippocampal neurons code for reward-place associations (see examples in Figs. 1 and 2). Their dependence on the reward associated with a particular place was confirmed by reversing the reward associated with a particular place, which in most cases (80%) leads to reversal of the location in the scene to which a neuron responds.
It is important for understanding memory systems in the brain that these neurons do not just code for reward, in that most do not discriminate between the object stimuli in a standard visual discrimination task in which one visual stimulus (e.g., a triangle) is associated with reward and a different stimulus (e.g., a square) is associated with saline punishment. Thus, these hippocampal neurons encode associations between places and rewards but not between objects and rewards. The hippocampus thus is concerned with arbitrary associations when they involve where, for example, a reward has been seen in a spatial environment, and hippocampal spatial view cells are ideal for this function. We thus conceptually extend the hypothesis that the hippocampus is involved in event or episodic memory exemplified by the ability to form arbitrary associations between places out there and objects (Rolls et al., 1989, 2005), to incorporate, in addition, an analogous role in forming arbitrary associations between places out there and the rewards available at each location.
The primate hippocampus is well suited for this by virtue of its spatial view cells (Rolls, 1999b), by its inputs from “limbic” structures such as the orbitofrontal cortex and amygdala to the anterior hippocampus, and by its CA3 recurrent collateral associative architecture (Rolls and Treves, 1998). Although the reward-place neurons were recorded in the anterior hippocampus (between 2 and 10 mm posterior to the sphenoid reference as shown in Fig. 4), this was the main region sampled. The neurons at least overlap in their distribution with previously described spatial view neurons (which are found at 6-9 mm posterior as well as 12-20 mm posterior) (Rolls et al., 1997a) and with object-place neurons (which were found at 6-9 mm posterior) (Rolls et al., 2005).
The type of space represented by the neurons described here is space out there, in contrast to the place where the animal was located, which was held constant in these experiments. Spatial view neurons are defined as responding to space out there rather than the location where the animal is located, and the space out there can be measured in a number of different ways. One way is by position on a monitor located in front of the monkey, as shown by experiments in which the location of an image on the monitor must be remembered, and spatial view neurons respond to some but not other positions on the monitor (Rolls et al., 1989), often using a local frame of reference in the monitor screen (Feigenbaum and Rolls, 1991). In the experiments described here, an entire scene was presented as an image on the monitor, and it was within each entire scene that different locations were associated with different rewards. This type of spatial scene is very appropriate for use when recording from hippocampal neurons, because monkeys with neurotoxic hippocampal lesions are impaired at a very similar spatial scene memory task in which the location in each of a number of scenes presented on a monitor to touch to obtain reward must be learned (Murray et al., 1998). In addition, spatial view neurons can also be activated when monkeys look at locations in a room (Rolls et al., 1997a, 1998; Robertson et al., 1998; Rolls, 1999b) and remember the places of objects in a room (Rolls et al., 2005). What all of these test situations have in common is that it is space out there that is represented, rather than the place where the animal is located. The neurophysiological investigation described here is the first in which the spatial representation consisted of an entire scene presented on a monitor, and hippocampal neurons with activity related to spatial locations out there were found.
The nature of the encoding by the reward-place neurons described here is now considered. The reward-place neurons clearly do not encode particular spatial responses (i.e., touches to a given position within the frame of the monitor), because reward-place neurons responded in the same way to the different places on the monitor at which in different scenes R1 (or for other neurons R2) was located (see examples in Figs. 1 and 2). Furthermore, the fact that during reversal the majority of the reward-place neurons followed the location of the reward to which they responded when its position was reversed in the scene also shows that the neurons did not encode particular spatial responses, nor particular spatial views, and instead shows that their activity reflected the location in a scene at which a particular reward was found (Table 2). Nor did the reward-place neurons reflect just the fact that any touch response was being made, because the neurons typically fired when the monkey looked at the circled location in a scene, and this firing was independent of whether, on that particular trial, the monkey did decide to reach to touch the screen. Furthermore, the neuronal responses had typical latencies of 200 ms and were stimulus locked (see examples in Fig. 3), yet the touch responses made by the monkey typically had variable latencies of 500-1000 ms. The reward-place neurons did not just represent reward without a spatial component, because the reward-place neurons were not, in general, activated in an object-reward visual discrimination task (Table 2) in which orbitofrontal cortex neurons are differentially activated (Rolls et al., 1996). Thus, the hippocampus may represent place-reward associations, and the orbitofrontal cortex may represent object-reward associations. Some primate hippocampal neurons respond in conditional spatial response tasks in which one nonspatial stimulus requires one spatial response and a different nonspatial stimulus requires a different spatial response (Miyashita et al., 1989), but these neurons are different from the reward-place neurons described here, because conditional response neurons encode the combination of the object and the response, in that they respond to one object-spatial response combination (e.g., image 1 go left) and not to another (e.g., image 2 go right), although the same reward is obtained in these two cases. Thus, conditional object-response neurons (Miyashita et al., 1989) do not encode the places in different scenes where a given reward is obtained and are unlike the reward-place neurons described here. To elucidate further the nature of the neuronal responses described here, an equivalent in the rat might consist of a neuron that responded when tested in different spatial environments to the places in those environments where R1 was located (and for other neurons to the places where R2 was located in the different spatial environments). Moreover, such neurons in the rat, if equivalent to the type of neuron described here, are predicted to alter the locations in an environment to which they respond when the places of the rewards in those environments are reversed. It would be interesting to perform such experiments.
A related possibility to the reward-place association representation discussed above as what is encoded by these primate hippocampal neurons is that they differentially encode reinforced places. A rodent place cell analogy is that more place cells will tend to discharge in places or in conditions that are reinforced. This has been shown in several studies, for example, in the water maze (Hollup et al., 2001), after place-preference training on a rotating arena in rats (Zinyuk et al., 2000), or in a stable environment in mice (Kentros et al., 2004). However, we note that in our macaque study, some neurons respond to the place associated with the less-preferred reward, so it is not just high reward value and place that is encoded. We also note that the reward-place neurons respond consistently differently to places in different scenes, or after reversal, that are associated with particular rewards, so it is not just that the probability of finding a spatial view cell is increased by the presence of a reward in a location (i.e., the neurons code for the same reward in different scenes). Nor is it that the neurons respond just to reward value, because even when the reward-punisher value is more extreme (the preferred reward vs saline), the neurons do not respond differently in a visual discrimination, object-reward task. In summary, the firing of the neurons reflects the reward value (high for some neurons, low for other neurons) of places in a scene and does this consistently across scenes. To fire in this way, the neurons must reflect a particular spatial view to particular reward value associations. In a sense, by implementing this type of association, the firing of the neurons reflects the reward value that is recalled because of the spatial view-reward association.
The hippocampal neurons with place-reward combination tuning could be set up with the CA3 neurons providing a single network where the associatively modifiable recurrent collateral synaptic connections between the neurons could enable arbitrary reward-place associations to be formed, and then for recall of the reward value given the spatial view as a retrieval cue (Rolls, 1989, 1996; Treves and Rolls, 1992; Debanne et al., 1998; Rolls and Treves, 1998; Rolls et al., 2002).
In relation to previous findings, we believe that this is the first investigation to study primate hippocampal neuronal activity in a reward-place memory task, in which the space represented was that relevant to much human event and episodic memory, that is allocentric space out there. The finding that neurons are present in the primate hippocampus that respond to unique combinations of rewards and places out there is what would be expected and required in an associative memory system that forms unique associations between rewards and places. The findings thus provide an important part of the evidence on what is represented in the primate hippocampus and are important in understanding how episodic memories may be formed in humans. It is important to be able to learn where rewards, and punishers, are in the environment, and the type of neuron in the hippocampus described in this study may be important in this type of memory. As shown here, the locations of rewards in a scene can be relearned quite quickly, and when this learning occurs, the responses of hippocampal reward-place neurons generally reverse, showing that this is a flexible, rapid, relearning system. Moreover, this learning system is different from that involved in learning associations between objects [represented in the inferior temporal visual cortex (Rolls and Deco, 2002)] and rewards, which involves neurons in the primate orbitofrontal cortex and lesions of which, in macaques and humans, impair rapid object-reward reversal learning (Rolls, 2004, 2005).
Footnotes
This work was supported by Medical Research Council Grant PG9826510.
Correspondence should be addressed to Prof. Edmund T. Rolls at the above address. E-mail: Edmund.Rolls@psy.ox.ac.uk.
Copyright © 2005 Society for Neuroscience 0270-6474/05/256167-08$15.00/0
References
- Amaral DG, Price JL, Pitkanen A, Carmichael ST (1992) Anatomical organization of the primate amygdaloid complex. In: The amygdala (Aggleton JP, ed), pp 1-66. New York: Wiley.
- Carmichael ST, Clugnet M-C, Price JL (1994) Central olfactory connections in the macaque monkey. J Comp Neurol 346: 403-434. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM (1998) Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol (Lond) 507: 237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Rolls ET (2005) Synaptic and spiking dynamics underlying reward reversal in orbitofrontal cortex. Cereb Cortex 15: 15-30. [DOI] [PubMed] [Google Scholar]
- Feigenbaum JD, Rolls ET (1991) Allocentric and egocentric spatial information processing in the hippocampal formation of the behaving primate. Psychobiology 19: 21-40. [Google Scholar]
- Fisher RA (1932) Statistical methods for research workers. London: Oliver and Boyd.
- Georges-François P, Rolls ET, Robertson RG (1999) Spatial view cells in the primate hippocampus: allocentric view not head direction or eye position or place. Cereb Cortex 9: 197-212. [DOI] [PubMed] [Google Scholar]
- Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI (2001) Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci 21: 1635-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C, Jacob W, Mallot HA (2003) Reward modulates neuronal activity in the hippocampus of the rat. Behav Brain Res 142: 181-191. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vis Res 20: 535-538. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, Verhagen JV (2005) The primate amygdala: neuronal representations of the viscosity, fat texture, grittiness and taste of foods. Neuroscience 132: 33-48. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER (2004) Increased attention to spatial context increases both place field stability and spatial memory. Neuron 42: 283-295. [DOI] [PubMed] [Google Scholar]
- Littell RC, Folks JL (1971) Asymptotic optimality of Fisher's method of combining independent tests. J Am Stat Assoc 66: 802-806. [Google Scholar]
- Marr D (1971) Simple memory: a theory for archiocortex. Philos Trans R Soc Lond B Biol Sci 262: 23-81. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52: 41-49. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Rolls ET, Cahusac PM, Niki H, Feigenbaum JD (1989) Activity of hippocampal formation neurons in the monkey related to a conditional spatial response task. J Neurophysiol 61: 669-678. [DOI] [PubMed] [Google Scholar]
- Murray EA, Baxter MG, Gaffan D (1998) Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci 112: 1291-1303. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Speakman A (1987) Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res 68: 1-27. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW (2001) Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behaviour systems. Brain Res Rev 38: 247-289. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Kelly JL, Amaral DG (2002) Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus 12: 186-205. [DOI] [PubMed] [Google Scholar]
- Robertson RG, Rolls ET, Georges-François P (1998) Spatial view cells in the primate hippocampus: effects of removal of view details. J Neurophysiol 79: 1145-1156. [DOI] [PubMed] [Google Scholar]
- Rolls ET (1989) Functions of neuronal networks in the hippocampus and neocortex in memory. In: Neural models of plasticity: experimental and theoretical approaches (Byrne JH, Berry WO, eds), pp 240-265. San Diego: Academic.
- Rolls ET (1992) Neurophysiology and functions of the primate amygdala. In: The amygdala (Aggleton JP, ed), pp 143-165. New York: Wiley.
- Rolls ET (1996) A theory of hippocampal function in memory. Hippocampus 6: 601-620. [DOI] [PubMed] [Google Scholar]
- Rolls ET (1999a) The brain and emotion. Oxford: Oxford UP.
- Rolls ET (1999b) Spatial view cells and the representation of place in the primate hippocampus. Hippocampus 9: 467-480. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2000) Neurophysiology and functions of the primate amygdala, and the neural basis of emotion. In: The amygdala: a functional analysis, Ed 2 (Aggleton JP, ed), pp 447-478. Oxford: Oxford UP.
- Rolls ET (2004) The functions of the orbitofrontal cortex. Brain Cognit 55: 11-29. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2005) Emotion explained. Oxford: Oxford UP.
- Rolls ET, Deco G (2002) Computational neuroscience of vision. Oxford: Oxford UP.
- Rolls ET, Treves A (1998) Neural networks and brain function. Oxford: Oxford UP.
- Rolls ET, Miyashita Y, Cahusac PMB, Kesner RP, Niki H, Feigenbaum J, Bach L (1989) Hippocampal neurons in the monkey with activity related to the place in which a stimulus is shown. J Neurosci 9: 1835-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Mason R, Wakeman EA (1996) Orbitofrontal cortex neurons: role in olfactory and visual association learning. J Neurophysiol 75: 1970-1981. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Robertson RG, Georges-François P (1997a) Spatial view cells in the primate hippocampus. Eur J Neurosci 9: 1789-1794. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A, Tovee MJ, Panzeri S (1997b) Information in the neuronal representation of individual stimuli in the primate temporal visual cortex. J Comput Neurosci 4: 309-333. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A, Robertson RG, Georges-François P, Panzeri S (1998) Information about spatial view in an ensemble of primate hippocampal cells. J Neurophysiol 79: 1797-1813. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Stringer SM, Trappenberg TP (2002) A unified model of spatial and episodic memory. Proc R Soc Lond B Biol Sci 269: 1087-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Xiang J-Z, Franco L (2005) Object, space and object-space representations in the primate hippocampus. J Neurophysiol, in press. [DOI] [PubMed]
- Stefanacci L, Suzuki WA, Amaral DG (1996) Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol 375: 552-582. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG (1994) Perirhinal and parahippocampal cortices of the macaque monkey - cortical afferents. J Comp Neurol 350: 497-533. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG (2003) Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organisation. J Comp Neurol 463: 67-91. [DOI] [PubMed] [Google Scholar]
- Tabuchi E, Mulder AB, Wiener SI (2003) Reward value invariant place responses and reward site associated activity in hippocampal neurons of behaving rats. Hippocampus 13: 117-132. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S (1983) Neuronal activity in the orbitofrontal cortex of the behaving monkey. Exp Brain Res 49: 93-115. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET (1992) Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2: 189-199. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET (1994) A computational analysis of the role of the hippocampus in memory. Hippocampus 4: 374-391. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices in the monkey. II. Frontal lobe afferents. Brain Res 95: 25-38. [DOI] [PubMed] [Google Scholar]
- Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS (2002) Truncated product method for combining P-values. Genet Epidemiol 22: 170-185. [DOI] [PubMed] [Google Scholar]
- Zinyuk L, Kubik S, Kaminsky Y, Fenton AA, Bures J (2000) Understanding hippocampal activity using purposeful behavior: place navigation induces place cell discharge in both the task-relevant and task-irrelevant spatial reference frames. Proc Natl Acad Sci USA 97: 3771-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]