Abstract
Trophin-induced synaptic plasticity consists of both presynaptic and postsynaptic processes. The potential interdependence of these mechanisms and their temporal relationships are undefined. The synaptic vesicle protein Rab3A is required for the early, initial 10 min phase but not for the later phase of BDNF-enhanced transmission. We now examine the temporal distinction and mechanistic relationships between these phases of BDNF action. Rab3A mutant cells did not exhibit increased miniature EPSC frequency in response to BDNF in cell culture, indicating an absence of the presynaptic component. In contrast, BDNF enhanced postsynaptic glutamate-induced current in the mutant neurons as in the wild type, indicating that the postsynaptic component of the response was intact. Finally, the postsynaptic NMDA receptor subunit NR2B was phosphorylated at Tyr1472 by BDNF in Rab3A knock-outs, as shown previously in wild type. Our results are the first to demonstrate that presynaptic and postsynaptic components of BDNF-enhanced synaptic activity are independent and temporally distinct.
Keywords: postsynaptic, presynaptic, BDNF, synaptic plasticity, hippocampal, Rab3A
Introduction
BDNF is a major regulator of synaptic activity in the CNS (Thoenen, 2000). Acute modulation of synaptic function by BDNF is attributed to both presynaptic and postsynaptic mechanisms. Several studies have shown changes in presynaptic parameters by BDNF, including an increase in miniature EPSC (mEPSC) frequency within minutes (Lessmann and Heumann, 1998; Li et al., 1998; Schinder et al., 2000; Tyler and Pozzo-Miller, 2001). In addition, several electrophysiological measures of presynaptic function are reduced in BDNF knock-out mice, which also exhibit morphological deficits at the active zone (Pozzo-Miller et al., 1999). Finally, an essential presynaptic contribution by BDNF for the induction of long-term potentiation (LTP) has been demonstrated using CA1- and/or CA3-specific BDNF and tropo-related kinase B (trkB) knock-out mice (Xu et al., 2000; Zakharenko et al., 2003). Collectively, these studies indicate an important contribution of presynaptic elements to BDNF-induced synaptic plasticity.
Postsynaptic components also participate in acute modulation of synaptic efficacy by BDNF. Glutamate-induced current in the postsynaptic cell is enhanced after prolonged BDNF exposure (Crozier et al., 1999), and trkB antagonist in the postsynaptic cell prevents an increase in EPSC amplitude by BDNF (Levine et al., 1995). These electrophysiological studies are supported by biochemical assays demonstrating posttranslational modification of neurotransmission machinery by BDNF. Phosphorylation of the NR2B subunit of the NMDA receptor by BDNF (Lin et al., 1998) may contribute to the increased NMDA channel open probability observed in the presence of BDNF (Levine et al., 1998). Finally, a postsynaptic role for trophin in LTP has been described recently by pairing weak stimulation with BDNF applied locally to dendrites to induce potentiation (Kovalchuk et al., 2002). Although these studies indicate both presynaptic and postsynaptic aspects of BDNF-induced synaptic enhancement, the exact contribution of each of these components remains to be fully defined.
One approach for studying the involvement of presynaptic and postsynaptic machinery is to use systems in which one of the two components is defective such that they can be examined independently. In our previous studies, a gene encoding the presynaptic vesicle protein Rab3A was induced by BDNF (Thakker-Varia et al., 2001). Previous reports on Rab3A knock-out mice demonstrate that although basal synaptic transmission is normal, presynaptic mossy fiber LTP is lacking in the CA3 region (Bean and Scheller, 1997; Castillo et al., 1997). In addition, the knock-out displays increased synaptic depression after short trains of repetitive stimuli, suggesting that Rab3A plays a role in the recruitment of synaptic vesicles to the active zone, possibly at a very late step in the secretion process (Geppert et al., 1997). Using Rab3A knock-out mice, we demonstrated a requirement for this synaptic vesicle protein in the early increase in synaptic charge induced by BDNF (Thakker-Varia et al., 2001). In contrast, a late response of Rab3A mutant cells to BDNF was observed and is temporally similar to that produced by pairing glutamate iontophoresis and BDNF exposure (Crozier et al., 1999). We hypothesize that BDNF manifests a two-component response: an early component that is dependent on Rab3A and a later component that is Rab3A independent.
Materials and Methods
Cell-culture preparation. Time-mated pregnant mice or rats were killed by CO2 asphyxiation in accordance with institutional guidelines for the care and use of animals. Fetuses were removed by cesarean section and transferred to a sterile Petri dish with PBS. Fetal hippocampi were dissected from surrounding brain tissue, and meninges were completely removed. Low-density cultures of dissociated embryonic day 16 wild-type or Rab3A homozygous knock-out mice (The Jackson Laboratory, Bar Harbor, ME) were prepared as described previously (Thakker-Varia et al., 2001). Briefly, pooled tissue from each litter was mechanically dissociated in 7.5% nutrient medium and plated on poly-d-lysine-coated culture dishes at 350,000 cells per dish. Cultures were maintained in serum-free medium (Levine et al., 1995) for 10-13 d.
Electrophysiological recordings. Whole-cell patch-clamp recordings were performed after 10-13 d in culture. Currents were recorded with an Axoclamp 200 (Axon Instruments, Union City, CA) amplifier and digitized with an INDEC interface (INDEC BioSystems, Mountain View, CA). Programs used for data acquisition and analysis were written in house. Internal and external recording solutions have been described previously (Thakker-Varia et al., 2001). The typical range of pipette resistance was 3-5 MΩ. Cell capacitance was 10-20 pF, and access resistance was 7-20 MΩ. The vacuum perfusion system contained BDNF (50 ng/ml; Peprotech, Princeton, NJ) or contained neuron-recording solution (NRS) as a control. To record mESPCs and glutamate-induced currents, NRS and BDNF solutions contained 1 μm tetrodotoxin (TTX) (Sigma, St. Louis, MO). Pyramidal-type cells were recorded in voltage-clamp mode, and the membrane potential was held at -80 mV for mEPSC recordings and at -40 mV for iontophoresis experiments to reduce Mg2+ blockage of NMDA receptors. Five-barrel micropipettes were used for iontophoretic application of glutamate. One barrel, filled with 0.1 m NaCl, was used for current balancing. Other barrels were filled with 0.2m l-glutamate, pH 7.5. Iontophoretic pulses were applied at 10 s intervals with an ejection time sufficient to achieve stable and consistent responses (typically 10-30 ms). Each data point represents a cell from a separate dish, and at least three different platings were used for each condition.
Data analysis. Electrophysiological data were analyzed blind with regard to genotype. Spontaneous synaptic vesicle release was quantified by counting all mEPSCs recorded during 1.4 s sweeps collected every 2 s throughout the recording period. Glutamate-induced currents were analyzed by measuring the peak current for each iontophoretic pulse. Measurements for all sweeps in a 1 min period were averaged (binned). Baseline is considered the mEPSC frequency or glutamate-induced current magnitude during the 2 min period (-2 to 0 min) in NRS immediately before BDNF application. Fold increases were then determined by dividing the mEPSC frequency or glutamate-induced current during BDNF exposure by the baseline. Recordings were rejected if either the 0-5 min binned time period or the 5-10 min binned time period after switching during BDNF exposure was 2× SEM below baseline, indicating “run down.” Student's t test, ANOVA, or Kolmogorov-Smirnov were used for statistical comparisons (unpaired, with p < 0.05 indicating significance).
Preparation of synaptosomes. Adult wild-type and Rab3A knock-out (two animals each) mice were used to prepare synaptosomes according to the first part of the protocol for postsynaptic density preparations (Wu et al., 1986). Fresh cortices were gently homogenized (five strokes at 800 rpm) with a Teflon/glass homogenizer in 5 vol (w/v) in buffer containing protease inhibitors (0.32 m sucrose, 0.5 mm CaCl2, 1 mm MgCl2, and 1 mm NaHCO3). The homogenate was subjected to two successive centrifugations. The supernatants were pooled and centrifuged, and the resulting pellet was resuspended by homogenization in 0.32 m sucrose and 1 mm NaHCO3 containing protease inhibitors. This crude preparation was run on a sucrose gradient. The synaptosomes were recovered as a band, resuspended in 0.32 m sucrose and 1 mm NaHCO3 plus protease inhibitors, pelleted, and resuspended in HEPES buffer containing inhibitors (20 mm HEPES, pH 7.5, containing 110 mm NaCl, 5.3 mm KCl, 1.0 mm MgSO4, 1.8 mm CaCl2, 25 mm glucose, 68.3 mm sucrose, protease inhibitor tablet, 1 mm PMSF, and 0.5 mm vanadate). The synaptosomes were quantitated using BCA protein assay to adjust for equal amounts of protein, equilibrated in HEPES plus NaH2PO4 for 1 h, and treated with BDNF (10 or 50 ng/ml) for 10 min at 37°C. The reaction was stopped by the addition of 4× loading buffer and boiled for 5 min.
SDS-PAGE and Western blot analysis. SDS-PAGE was performed with a 7-12% acrylamide gradient using 100 μg of protein per lane. After transfer to Millipore (Bedford, MA) filters and blocking, antibodies to phospho-Tyr1472-NR2B (1 μg/ml) (a gift from T. Yamamoto, University of Tokyo, Tokyo, Japan) were applied for 1 h. Donkey anti-rabbit secondary antibody (Amersham Biosciences, Piscataway, NJ) was used at 1:5000 for 1 h followed by ECL detection (PerkinElmer Life and Analytical Sciences, Boston, MA). The filters were stripped, and NR2B protein levels were determined using anti-NR2B primary antibody (1:1000) (Up-state Biotechnology, Lake Placid, NY) and donkey anti-rabbit secondary antibody (Amersham Biosciences). Bands were quantitated on a Bio-Rad (Hercules, CA) Gel Doc.
Results
Biphasic response to BDNF
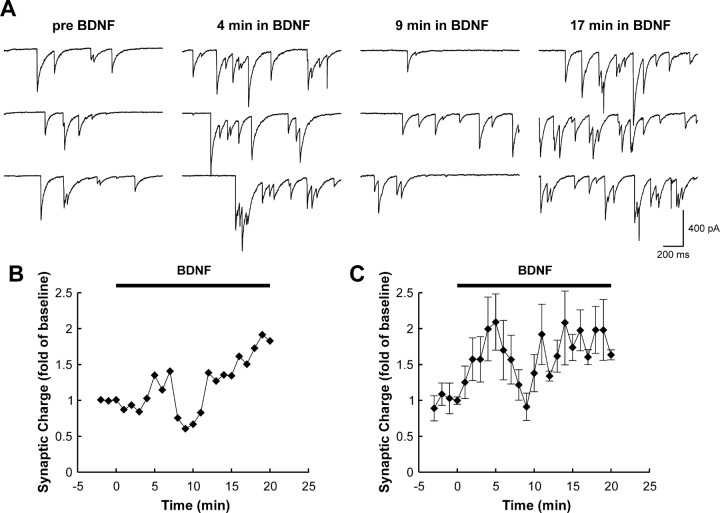
To delineate the temporal profile of the acute synaptic response to BDNF in dissociated hippocampal neurons, whole-cell voltage-clamp recordings were performed for 30 min. For ∼60% of the recordings, a two-component response was elicited. Synaptic charge increased within the first 5 min of BDNF exposure, declined at ∼10 min, and subsequently rose again at ∼15 min. The second peak in activity was maintained for the duration of BDNF treatment (Fig. 1). For the remaining recordings, BDNF produced a maintained elevation in synaptic charge in which two components, although potentially still present, could not be clearly distinguished. These experiments suggest that the majority of pyramidal-like hippocampal neurons show a biphasic response to BDNF. Previously, we defined a requirement for the presynaptic vesicle protein Rab3A in an early phase (within 5 min) of BDNF-mediated synaptic enhancement using Rab3A knock-out mice (Thakker-Varia et al., 2001). These data indicate that the early response to BDNF is presynaptically driven. In contrast, a later response of Rab3A mutant cells to BDNF was observed at 20 min, which is temporally similar to that produced by pairing glutamate iontophoresis and BDNF exposure (Crozier et al., 1999). Therefore, a postsynaptic locus may contribute to this late action of BDNF.
Figure 1.
Prolonged exposure to BDNF evokes a dual-component enhancement of synaptic activity in rat hippocampal cells. A, Sample sweeps taken before and during BDNF exposure at the indicated time points. Note that synaptic activity increases during the first 5 min of exposure and then declines to near-baseline levels but subsequently increases with continued exposure to BDNF. B, Time course of changes in synaptic activity from the experiment shown in A. The two components of the response are evident. C, Group time course of those experiments (13 of 22) that exhibited a two-component response. Points represent average charge ± SEM. In the remaining nine recordings, BDNF produced a persistent elevation in synaptic activity in which a clear separation between an early and late component could not be made.
Effect of BDNF on presynaptic parameters in Rab3A knock-out mice
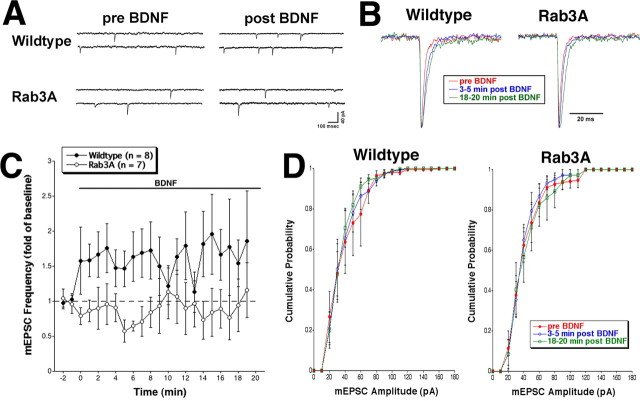
To define the contribution of presynaptic components to the biphasic BDNF response, we studied the effect of trophin on presynaptic parameters. mEPSC frequency was examined in wild-type and Rab3A knock-out cells during application of BDNF. Cells derived from wild-type mice showed a maximal increase of ∼1.7-fold mEPSC frequency within 3 min of BDNF application (average events/s, 0.18 ± 0.05 during -2 to 0 min before BDNF; 0.26 ± 0.08 during 3-5 min after BDNF). However, BDNF did not elicit this enhancement of spontaneous release in mutant cells throughout the 20 min recording. The small decrease from 0.45 ± 0.17 events/s before BDNF to 0.29 ± 0.09 events/s 3-5 min after BDNF was not significant (Fig. 2A,C). For two sets of recordings, the individual mEPSCs were normalized and averaged. Rise time remained constant, but we did note a slight tendency for the mEPSC duration to become prolonged during exposure to BDNF (Fig. 2B). This increase in decay time was similar in both wild-type and Rab3A mutant neurons and was suggestive of a postsynaptic action of BDNF that was not disrupted in Rab3A knock-out mice. This possibility was examined directly with iontophoretic application of glutamate (see below). The distribution of mEPSC amplitudes was not altered by BDNF in Rab3A mutant cells, consistent with observations in wild-type and rat cells (Fig. 2D) (Lessmann and Heumann, 1998). Average mEPSC amplitude was calculated during three time periods: before BDNF (wild type, 38.2 ± 7.4 pA; Rab3A, 41.6 ± 5.1 pA), 3-5 min after BDNF (wild type, 39.9 ± 6.2 pA; Rab3A, 39.8 ± 4.3 pA), and 18-20 min after BDNF (wild type, 35.5 ± 3.8; Rab3A, 42.2 ± 6.7). Therefore, Rab3A is specifically required for the enhanced frequency of vesicle exocytosis observed within the first 5 min of BDNF treatment. These data support the hypothesis that presynaptic mechanisms contribute to the early component of BDNF-induced synaptic plasticity.
Figure 2.
A, Representative traces of mEPSCs in wild-type or Rab3A knock-out cells before and ∼5 min after exposure to BDNF. Whole-cell voltage-clamp recordings (Vhold = -80 mV) were performed in the presence of TTX. An increase in mEPSC frequency was observed within 5 min of BDNF exposure in wild-type cells but not in Rab3A knock-out cells. B, Averaged mEPSCs from before and two time periods after BDNF treatment for wild-type and Rab3A cells. Individual mEPSCs were normalized to the same amplitude and averaged. Rise time was unchanged, but there was a trend toward longer decay times after BDNF treatment for both genotypes. Averaged mEPSCs from a second set of recordings gave similar results. C, Average mEPSC frequency in response to BDNF (bar) over the course of the recording. mEPSC frequency was normalized to baseline (-2 to 0 min). Wild-type cells displayed an immediate increase (∼1.7-fold) in mEPSC frequency in response to BDNF relative to Rab3A knock-out cells (p < 0.05; t test). Rab3A mutant cells did not respond to BDNF over the entire 20 min of recording and actually showed a small but insignificant decrease in mEPSC frequency relative to baseline (p > 0.05; t test). The dashed line indicates baseline. Error bars represent SEM. D, Cumulative mEPSC amplitude probability for wild-type and Rab3A mutant cells (n = 8 and 7, respectively). Errors bars represent SEM. No statistical differences were observed between the amplitudes before and two time periods after BDNF treatment for either wild-type or Rab3A mutant cells (p > 0.05; Kolmogorov-Smirnov test). Recordings were obtained from multiple platings.
Electrophysiological and biochemical analysis of postsynaptic characteristics in the absence of Rab3A
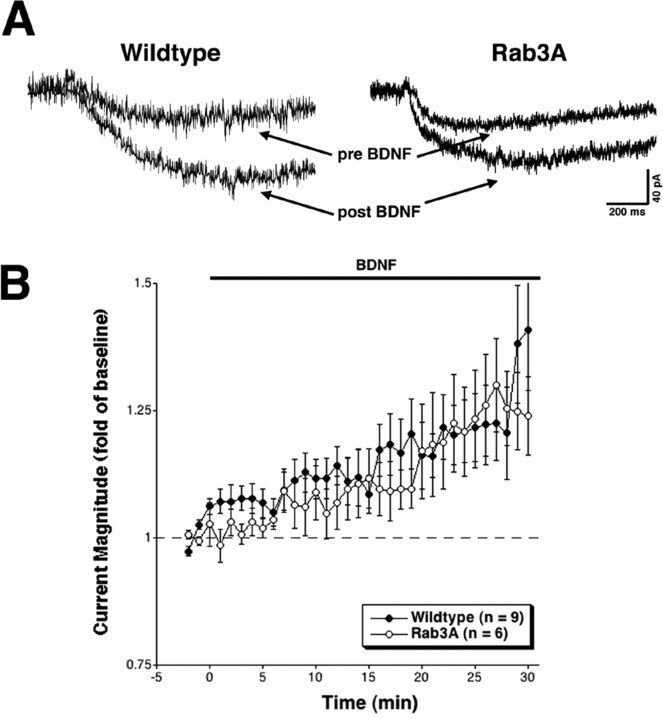
To define postsynaptic components of BDNF-mediated activity, we measured glutamate-induced current in pyramidal-like hippocampal cells in wild-type and Rab3A mutant neurons. Iontophoretic application of glutamate to the base of dendritic processes in cultured embryonic hippocampal neurons evoked a slow inward current, presumably including both synaptic and extrasynaptic neurotransmitter receptors. After establishing stable baseline measurements, BDNF was bath applied while continuing to elicit currents with glutamate iontophoresis. In wild-type cells, the major increase in glutamate-induced current was apparent beginning at 10 min after BDNF treatment and persisted throughout the recording, thus resembling the temporal profile seen previously (before BDNF, 143.7 ± 22.2 pA; 18-20 min after BDNF, 170.2 ± 31.9 pA) (Crozier et al., 1999). Rab3A mutant cells also displayed a similar iontophoretic response to glutamate in the presence of BDNF (before BDNF, 137.4 ± 8.7 pA; 18-20 min after BDNF, 161.5 ± 15.2 pA) (Fig. 3), demonstrating that the postsynaptic component is intact in the absence of Rab3A. The fact that the postsynaptic response to BDNF is not detectable until after 10 min of treatment supports the idea that the second phase of the bimodal BDNF synaptic response has a postsynaptic component.
Figure 3.
A, Example traces showing glutamate-induced current before and ∼20 min after exposure to BDNF. Whole-cell voltage-clamp recordings (Vhold = -40 mV) were obtained in the presence of TTX. The profiles before and after BDNF in wild-type and Rab3A mutant cells are similar. B, Average peak current in response to BDNF (horizontal bar) over the course of the recording. Peak current amplitude was normalized to baseline (-2 to 0 min). Note that the y-axis starts at 0.75, with 1 indicating no response to BDNF. There is an increase in current starting at ∼15 min. Wild-type and Rab3A knock-out cells are not statistically different from each other (p > 0.05; t test). The small, early increase in glutamate-induced current observed in wild-type cells was not statistically significant when compared with Rab3A at individual time points 0-5 min after BDNF (p > 0.05; t test). The dashed line indicates baseline. Error bars represent SEM. Recordings were obtained from multiple platings.
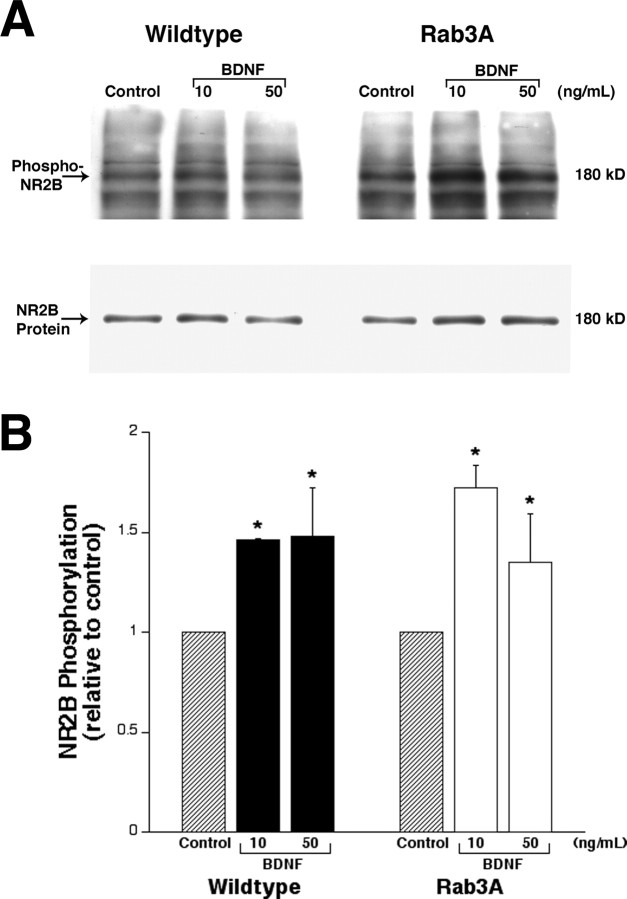
As a check for normal postsynaptic responsiveness to BDNF in Rab3A knock-out mice, a parallel biochemical analysis was performed. Phosphorylation of the NMDA receptor subunit NR2B has been documented in both hippocampal postsynaptic densities treated with BDNF (Lin et al., 1998) and hippocampal slices after LTP induction (Nakazawa et al., 2001). Figure 4 demonstrates a 1.5-fold increase in NR2B phosphorylation at Tyr1472 in both wild-type and Rab3A mutant synaptosomes after 10 min of BDNF. NR2B total protein levels were not altered by BDNF treatment. These results support the concept that the biochemical postsynaptic effects of BDNF are not altered by lack of the Rab3A gene. The fact that Rab3A is not required for either increased glutamate-induced current or enhanced phosphorylation of the NMDA receptor by BDNF after 10 min suggests that the second phase of the bimodal response has a postsynaptic component that is independent of the presynaptic elements.
Figure 4.
A, Western blot of synaptoneurosomal proteins (100 μg) from adult wild-type and Rab3A knock-out mouse cortices. Synaptoneurosomes were incubated in the presence of 10 or 50 ng/ml BDNF for 10 min. Phospho-NR2B was detected using an antibody to Tyr1472, and total NR2B protein was detected on the same membrane. B, Quantitation of NR2B phosphorylation indicates that BDNF enhances NR2B phosphorylation by ∼1.5-fold. No difference in BDNF-induced phosphorylation levels was observed between wild-type and Rab3A knock-out samples. NR2B phosphorylation levels were similar in 10 and 50 ng/ml BDNF treatments. Phospho-NR2B was normalized to total NR2B protein, and data are expressed as a fold of phospho-NR2B relative to control, untreated samples (n = 3). Error bars represent SEM. *p < 0.05, significantly different from control samples (ANOVA).
Discussion
We used Rab3A mutant mice to identify mechanisms underlying the temporally bimodal synaptic response to BDNF. The absence of Rab3A, a synaptic vesicle protein, has specific effects on the presynaptic elements of trophin-induced synaptic enhancement, which are evident within a few minutes of BDNF exposure. The postsynaptic components, in contrast, are manifested after longer BDNF treatment. Both electrophysiological studies on mEPSC frequency and glutamate-induced current as well as biochemical data on NMDA receptor phosphorylation support these findings. Our study is the first to document that the presynaptic and postsynaptic contributions to trophin-mediated synaptic plasticity are autonomous and temporally discrete.
BDNF enhanced the number of vesicles released in wild-type cells, as shown by an increase in mEPSC frequency, confirming previous reports (Lessmann and Heumann, 1998; Li et al., 1998; Schinder et al., 2000; Tyler and Pozzo-Miller, 2001). The lack of effect on mEPSC frequency in Rab3A mutant cells demonstrates that the synaptic vesicle protein is specifically required for the enhanced frequency of vesicle exocytosis by BDNF and is consistent with the proposed role of Rab3A in a late step of vesicle fusion (Geppert et al., 1997). The Rab3A mutant mice also display increased synaptic depression after short trains of repetitive stimuli (Geppert et al., 1994). Finally, a recent study revealed that Rab3A-Rab3D quadruple knock-out mice do not have major alterations in synapse formation or synaptic properties but do have smaller evoked synaptic responses and decreased vesicular release probabilities (Schluter et al., 2004). Therefore, our findings suggest that Rab3A is important in the recruitment of synaptic vesicles during augmented transmission consequent to BDNF exposure. The BDNF-induced increase in mEPSC frequency can be blocked by a dominant-negative trkB in the presynaptic cell (Li et al., 1998), confirming the contribution of presynaptic BDNF signaling to this phenomenon. The association of BDNF with the regulation of synaptic vesicle trafficking has also been demonstrated in BDNF knock-out mice, which have fewer active zone vesicles and reduced levels of vesicle-associated proteins (Pozzo-Miller et al., 1999). BDNF mutant mice exhibit decreased electrophysiological responses, including posttetanic potentiation and paired-pulse facilitation (Pozzo-Miller et al., 1999), both of which are presynaptically driven. In this study, we demonstrate that the presynaptic component occurs early in the bimodal BDNF-mediated synaptic enhancement.
The later phase of the bimodal BDNF response is driven by a postsynaptic component, as evidenced by increased glutamate-activated current in Rab3A mutant cells after 10 min. Therefore, even in the absence of complete presynaptic machinery, the postsynaptic response is intact, suggesting that these components are independent. Our previous electrophysiological studies have defined a role for the NR2B subunit of the NMDA receptor in the postsynaptic BDNF response (Crozier et al., 1999).
These physiological studies are entirely consistent with our biochemical characterization of the actions of BDNF on NMDA receptors in synaptosomes. Similar to our previous studies on rat hippocampus (Lin et al., 1998), BDNF produced rapid tyrosine phosphorylation of the NR2B subunit in wild-type mice. BDNF treatment of Rab3A mutant synaptosomes produced an identical response, indicating that phosphorylation is not dependent on the presynaptic BDNF response and is consistent with observations in isolated postsynaptic densities (Lin et al., 1998). Therefore, the postsynaptic biochemical response to BDNF is independent of the presence of the presynaptic machinery. Fyn kinase is believed to be responsible for tyrosine phosphorylation of the NR2B subunit (Nakazawa et al., 2001). A BDNF-induced increase in synaptic transmission is absent in Fyn kinase knock-outs, suggesting that phosphorylation is essential for the trophin response (I. B. Black, personal communication). Furthermore, Tyr1472 phosphorylation of the NR2B subunit has also been associated with LTP in the CA1 region (Nakazawa et al., 2001), suggesting a role for this mechanism in learning and memory.
Phosphorylation of presynaptic proteins has been shown to regulate BDNF-mediated synaptic enhancement. The vesicle-associated protein synapsin 1 is phosphorylated by BDNF and results in increased glutamate release (Jovanovic et al., 2000). Recent studies demonstrate the association of synapsins with Rab3A and how this interaction regulates the activities of both proteins (Giovedi et al., 2004), which may be relevant to why the absence of Rab3A affects the presynaptic response of the neuron to BDNF. In addition, a Rab3A-associated protein, rabphilin, is phosphorylated within 2 min by activity paradigms via increased intracellular calcium (Foletti et al., 2001). Whether BDNF signaling results in rabphilin phosphorylation remains to be determined. It has already been demonstrated that rabphilin phosphorylation is dependent on the presence of Rab3A. Therefore, one possible explanation for the lack of presynaptic BDNF plasticity in Rab3A mutants may be the insufficient phosphorylation of rabphilin. Phosphorylation is thus emerging as a critical regulator of trophin-induced synaptic plasticity.
In summary, our studies indicate that BDNF-induced plasticity is a dual-component system exhibiting a bimodal profile: an early presynaptic component dependent on Rab3A and a later postsynaptic aspect that is independent of Rab3A. These processes may initiate downstream signals important in LTP, learning, and memory.
Footnotes
This work was supported by grants from the National Institute of Child Health and Human Development and the New Jersey Commission for Science and Technology (I.B.B.) and by National Institute of Neurological Disorders and Stroke Grant NS-041310 (M.R.P.). We thank T. Yamamoto for the phospho-Tyr1472-NR2B antibody.
Correspondence should be addressed to Dr. Ira B. Black, Department of Neuroscience and Cell Biology, Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey, 675 Hoes Lane, Robert Wood Johnson-School of Public Health 363, Piscataway, NJ 08854-5635. E-mail: blackib@umdnj.edu.
R. A. Crozier's present address: Howard Hughes Medical Institute, The Picower Center for Learning and Memory and Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253080-06$15.00/0
J.A. and S.T.-V. contributed equally to this work.
References
- Bean AJ, Scheller RH (1997) Better late than never: a role for rabs late in exocytosis. Neuron 19: 751-754. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA (1997) Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388: 590-593. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Black IB, Plummer MR (1999) Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn Mem 6: 257-266. [PMC free article] [PubMed] [Google Scholar]
- Foletti DL, Blitzer JT, Scheller RH (2001) Physiological modulation of rabphilin phosphorylation. J Neurosci 21: 5473-5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC (1994) The role of Rab3A in neurotransmitter release. Nature 369: 493-497. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Sudhof TC (1997) The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 387: 810-814. [DOI] [PubMed] [Google Scholar]
- Giovedi S, Vaccaro P, Valtorta F, Darchen F, Greengard P, Cesareni G, Benfenati F (2004) Synapsin is a novel Rab3 effector protein on small synaptic vesicles. I. Identification and characterization of the synapsin I-Rab3 interactions in vitro and in intact nerve terminals. J Biol Chem 279: 43760-43768. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS (2000) Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 3: 323-329. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A (2002) Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295: 1729-1734. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Heumann R (1998) Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience 86: 399-413. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR (1995) Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA 92: 8074-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR (1998) Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc Natl Acad Sci USA 95: 10235-10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM (1998) Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci USA 95: 10884-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB (1998) BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res 55: 20-27. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-d-aspartate receptor. J Biol Chem 276: 693-699. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B (1999) Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knock-out mice. J Neurosci 19: 4972-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, Poo M (2000) Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron 25: 151-163. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC (2004) A complete genetic analysis of neuronal Rab3 function. J Neurosci 24: 6629-6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB (2001) Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci 21: 6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H (2000) Neurotrophins and activity-dependent plasticity. Prog Brain Res 128: 183-191. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD (2001) BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Carlin R, Siekevitz P (1986) Binding of l-[3H]glutamate to fresh or frozen synaptic membrane and postsynaptic density fractions isolated from cerebral cortex and cerebellum of fresh or frozen canine brain. J Neurochem 46: 831-841. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF (2000) The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci 20: 6888-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A (2003) Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 39: 975-990. [DOI] [PubMed] [Google Scholar]