Abstract
The recollection of emotional autobiographical memories has received little attention in patients with memory disorders. Here, we addressed this topic in amnesic patients with damage to the hippocampus (HC group; n = 8) or the hippocampus, amygdala, and surrounding cortices (HC+ group; n = 2). These patients were asked to recollect emotional events from their lives. HC patients produced recollections that were strikingly similar to those of brain-damaged (n = 10) and healthy (n = 25) comparison participants, in terms of both quantity and quality. In contrast, HC+ patients produced a lower proportion of unpleasant memories compared with the other participants. Specifically, the ratings and words used to describe recollections in the HC+ patients were more affectively positive. All groups produced more memories from between 10 and 30 years of age (the so-called autobiographical memory “bump”) compared with other time periods in their lives. These results suggest that structures surrounding the hippocampus, but not the hippocampus itself, may be necessary for the recollection of highly emotional, unpleasant autobiographical memories. The amygdala and surrounding cortices of the medial temporal lobe may be a necessary component in the neural circuitry necessary for vivid recollection of unpleasant emotional events.
Keywords: amnesia, hippocampus, amygdala, autobiographical memory, emotion, medial temporal lobe
Introduction
Autobiographical memories are often imbued with emotional salience. Events associated with emotional arousal may be remembered more vividly and forgotten more slowly than neutral events (Berntsen and Rubin, 2002; Conway, 2003). The neurobiological systems involved in the acquisition of emotional memories has been the focus of much research (Cahill, 2000; McGaugh, 2003; Buchanan and Adolphs, 2004), but the structures participating in their retrieval are less well understood.
Amnesic patients with medial temporal lobe (MTL) lesions are able to recollect remote autobiographical memories, often in considerable detail (Reed and Squire, 1998; Bayley et al., 2003). For instance, Bayley et al. (2003) demonstrated that patients who were unable to acquire new episodic memories, nonetheless, could describe remote autobiographical memories in as much detail as comparison participants. The cognitive characteristics of these patients' recollections have been well studied (e.g., episodic vs semantic details) (Levine et al., 2002; Bayley et al., 2003), but the emotional characteristics of these patients' autobiographical memories have not been examined.
A related issue in autobiographical memory research is the temporal distribution of memory recollection. Previous research on autobiographical memory in normal individuals has demonstrated three components to the distribution of autobiographical memories: (1) childhood amnesia (reduced number of memories reported from the earliest years of life), (2) a monotonically decreasing retention function that drops quickly at first and then is equivalent for memories from 10 to 20 years ago in older participants, and (3) the autobiographical memory “bump” in which we remember disproportionately more events that occurred between 10 and 30 years of age (Rubin and Schulkind, 1997; Berntsen and Rubin, 2002). The bump has been documented specifically when people are asked to remember their most important memories, happiest memories, and in word-cued memory paradigms, whereas the distribution of saddest, most traumatic, and involuntary memories show different recollection patterns (Rubin and Schulkind, 1997; Berntsen and Rubin, 2002). These reliable patterns of recollection of emotional, real-life events thus provide a means for testing emotional retrieval in amnesic participants. As a result of these patients' memory impairments, the typical patterns of recollection for emotional memories from early adulthood may be disturbed.
Although amnesic patients may be able to produce detailed recollections of the remote past, the question remains as to the emotional nature and temporal distribution of these memories. If the hippocampus is necessary for the rich, vivid recollection of emotional experiences, patients with hippocampal damage should produce a different pattern of recollection (both in terms of quantity and quality) than comparison groups. In contrast, if other medial temporal lobe structures such as the amygdala and parahippocampal cortices are necessary for emotional recollection, patients with damage to these areas should show reduced quantity and quality of recollection for emotional events compared with comparison groups.
We examined the quantity, quality, and temporal distribution of emotional autobiographical memories in 10 amnesic patients. Two of these patients had extensive bilateral medial temporal lobe damage, including the hippocampus, amygdala, and surrounding cortices (HC+ group); the other eight patients had bilateral damage limited to the hippocampus (HC group).
Materials and Methods
Participants
Eight amnesic patients with bilateral damage restricted to the hippocampus (HC group) and two patients with extensive bilateral damage to the medial temporal lobe, including the hippocampus, amygdala, and surrounding cortices (HC+ group), participated in this study (Table 1, patient demographics; Table 2, neuroanatomy). Comparison participants were 10 brain-damaged patients (BDC group), all of whom had focal lesions outside the medial temporal lobe, and 25 healthy comparison participants (NC group) matched to all brain-damaged patients with age and gender distribution (see Table 1 for demographics of all participants). Because autobiographical memory distributions are reported to be changed with advancing age, we attempted to match the groups in terms of the number of participants who were >70 years of age. In the HC group, 3 of 10 patients were >70 years of age, the NC group included eight patients >70 years of age, and the BDC group contained 1 participant who was >70 years of age. All brain-damaged participants were drawn from the Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa.
Table 1.
Demographic characteristics of participants
|
Patient |
Gender |
Education |
Age at onset |
Age at test |
Etiology |
|---|---|---|---|---|---|
| HC + group | |||||
| 1673 | Male | 14 | 62 | 73 | HSE |
| 1951 | Male | 16 | 27 | 51 | HSE |
| HC group | |||||
| 1606 | Male | 12 | 43 | 54 | Anoxia |
| 1794 | Female | 12 | 68 | 78 | Anoxia |
| 1846 | Female | 14 | 30 | 41 | Anoxia |
| 2144 | Female | 12 | 47 | 53 | Anoxia |
| 2363 | Male | 16 | 42 | 45 | Anoxia |
| 2563 | Male | 16 | 43 | 47 | Anoxia |
| 2607 | Female | 14 | 70 | 73 | HSE |
| 2926 | Female | 12 | 62 | 63 | HSE |
| HC summary | 5 females, 3 males | 13.7 ± 1.9 | 47.6 ± 12.9 | 58.4 ± 13.4 | |
| BDC summary | 4 females, 6 males | 14.5 ± 2.5 | 44.8 ± 11.5 | 56.0 ± 10.3 | |
| NC summary |
14 females, 11 males |
15.0 ± 2.5 |
|
58.9 ± 12.9 |
|
Summary entries show mean ± SD. HSE, Herpes simplex encephalitis.
Table 2.
Neuroanatomical data
|
Patient |
HC volume |
HC residual |
Amygdala volume |
Amygdala residual |
|---|---|---|---|---|
| HC + group | ||||
| 1673 | 0 | NA | 0 | NA |
| 1951 | 1125 | −8.10b | 0 | NA |
| HC group | ||||
| 1606 | 4190 | −3.99b | 3010 | −1.18 |
| 1794 | 7189 | 1.13 | 3560 | 1.15 |
| 1846 | 3474 | −4.23b | 2220 | −0.90 |
| 2144 | 3890 | −3.92a | 3080 | −0.22 |
| 2363 | 5110 | −2.64a | 3380 | −0.60 |
| 2563 | NA | NA | NA | NA |
| 2607 | 5605 | −1.19a | 2987 | 0.10 |
| 2926 |
6750 |
−0.09 |
4516 |
3.01 |
HC volume, Bilateral hippocampal volume in mm3; Amygdala volume, bilateral amygdala volume in mm3; HC residual and Amygdala residual, studentized residuals for each region of interest volume compared with age- and gender-matched comparison participants from the study by Allen et al. (2005). NA, Not applicable.
Significant reduction in volume compared with comparison participants at p < 0.05.
Difference at p < 0.01.
Neuroanatomical data
Magnetic resonance (MR) images were obtained from all HC+ and HC patients in a 1.5 T General Electric (Milwaukee, WI) 4096 Plus scanner. The scanning protocol used in this study is identical to that used in previous work from our laboratory (Allen et al., 2002; Buchanan et al., 2004). All brains were reconstructed in three dimensions in Brainvox (Frank et al., 1997), an interactive family of programs designed to reconstruct, segment, and measure brains from MR-acquired images. All regions were traced by hand on contiguous coronal slices of the brain.
The remaining volumes of the amygdala and hippocampus were traced in both hemispheres of each patient. Whole-brain volumes were also determined. Criteria for the boundaries of both the amygdala and hippocampus were derived from the atlas of Duvernoy (1988). Using a method similar to that of Convit et al. (1999) (see also Szabo et al., 2001), point sets tracing the boundaries of the amygdala and hippocampus were first made in parasagittal and axial planes. These point sets were then projected to the coronal slices to guide tracing of the regions of interest.
Data from a normative sample of age- and gender-matched comparison participants described by Allen et al. (2005a) were used to examine reductions in hippocampal and amygdala volumes of the amnesic patients. Volumes of all of the anoxic patients tested in this experiment were included in the study by Allen et al. (2005b). To control for age and gender influences on brain volume, the differences between anoxic brains and comparison brains were converted to studentized residuals (actual value minus expected value) based on equations for model age- and gender-related effects in brain structure (Allen et al., 2005b). Studentized residuals >2.0 were significant at p < 0.05, and >2.66 denotes a difference at p < 0.01 (Allen et al., 2005b). All but one of these anoxic patients (patient 1794) was found to have significantly reduced hippocampal volume compared with comparison subjects. Patients in our sample with damage resulting from encephalitis (both HC+ patients and two in the HC group) were compared with the same normative sample. All but one of the encephalitis patients (patient 2926) showed significant reductions in hippocampal volume compared with the normative group. Importantly, only the two HC+ patients showed a significant reduction in amygdala volume compared with the normative sample. Total volume of left and right hippocampus and amygdala collapsed across sides, and the studentized residual differences in reference to comparison participants are presented in Table 2.
Although patients in the HC group had damage limited to the hippocampus, the patients in the HC+ group had more extensive damage. Patient 1673 had complete bilateral damage to the amygdala and hippocampus. On the right, there was complete damage to the parahippocampal gyrus and damage to the mesial and lateral parts of the temporal pole. On the left, the parahippocampal gyrus was damaged in its anterior and middle parts, but the posterior part was undamaged. Also on the left, there was damage to the mesial anterior temporal region but not to the lateral temporal polar region. Patient 1951 had bilateral damage in the temporal lobes, which included complete damage to the amygdala and near complete damage to the hippocampus. On the right, there was damage in the temporal polar region, the anterior three-fourths of the middle and inferior temporal gyrus, all of the inferior and fifth temporal gyrus including the entorhinal cortex, the anterior one-half of the superior temporal gyrus, and the white matter of the posterior one-half of this gyrus. On the left, there was damage in the mesial one-half of the temporal polar region and the anterior one-half of the fifth temporal gyrus, including the entorhinal cortex.
Neuropsychological data
All patients were individually administered a neuropsychological battery, which included measures of intellect, anterograde verbal and visual memory, retrograde memory, visuoperception, language, and executive functioning (∼3 h) (Tranel, 2005). All HC+ and HC participants had defective anterograde memory, as defined by failure on at least one of four tests of anterograde memory function: the Wechsler memory scale-revised, the Rey auditory verbal learning test, the visual retention test, or complex figure test-delayed recall. As a group, the HC/HC+ patients were significantly inferior to the BDC group on all four memory measures but did not differ from the BDC group in terms of intelligence quotient (IQ) scores. Key indices from the neuropsychology battery are presented in Table 3. There is variability in the extent of HC damage and anterograde amnesia in the HC group; however, every patient endured some damage to the HC, resulting from either anoxia or encephalitis, and was left with at least mild anterograde amnesia.
Table 3.
Neuropsychological data
|
Patient |
WAIS-III |
WMS-5 |
AVLT |
VRT |
CFT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
VIQ |
PIQ |
FSIQ |
GMI |
DRI |
Trial 5 |
DR |
Corr |
Error |
|
|||||
| HC + group | |||||||||||||||
| 1673 | 103 | 91 | 96 | 72 | 52 | 10 | 0 | 4 | 16 | 0 | |||||
| 1951 | 105 | 106 | 106 | 75 | 53 | 9 | 4 | 6 | 5 | 4 | |||||
| HC group | |||||||||||||||
| 1606 | 83 | 80 | 81 | 71 | 52 | 7 | 2 | 3 | 9 | 11 | |||||
| 1794 | 95 | 114 | 100 | 112 | 94 | 8 | 8 | 3 | 10 | 10 | |||||
| 1846 | 89 | 79 | 84 | 57 | 62 | 7 | 2 | 5 | 9 | 6 | |||||
| 2144 | 102 | 94 | 99 | 56 | 57 | 8 | 1 | 5 | 9 | 3 | |||||
| 2363 | 112 | 83 | 98 | 73 | 74 | 8 | 0 | 6 | 5 | 5 | |||||
| 2563 | 98 | 105 | 102 | 75 | 80 | 8 | 2 | 7 | 7 | 7 | |||||
| 2607 | 94 | 81 | 88 | NA | NA | 4 | 0 | 1 | 14 | 0 | |||||
| 2926 | 113 | 111 | 113 | 108 | 97 | 11 | 8 | 5 | 9 | 12 | |||||
| HC summary | 98 ± 11 | 93 ± 14 | 95 ± 11 | 73 ± 19 | 70 ± 17 | 7.6 ± 2 | 2.9 ± 3 | 4.6 ± 2 | 8.9 ± 3 | 6.8 ± 4 | |||||
| BDC summary |
107 ± 19 |
102 ± 18 |
105 ± 18 |
102 ± 8**
|
101 ± 11**
|
11 ± 4*
|
9 ± 4**
|
8 ± 2**
|
4 ± 3**
|
20 ± 5**
|
|||||
WAIS-III, Wechsler adult intelligence scale; VIQ, verbal IQ; PIQ, performance IQ; FSIQ, full-scale IQ; WMS-R, Wechsler memory scale-revised; GMI, general memory index; DRI, delayed recall index; AVLT, auditory-verbal learning test; Trial 5, raw score; DR, delayed recall raw score; VRT, visual retention test; Corr, number correct; Error, number of errors; CFT, complex figure test (delayed recall raw score). Summary scores are means and SDs from the HC and BDC groups. Asterisks indicate a difference between HC and BDC groups (*p < 0.05; **p < 0.01). Scores that are in bold are defective, according to normative values.
Although we did not assess the veracity of recollections from the patients on the emotional autobiographical memory interview, other studies have shown that patients with medial temporal lobe amnesia are not prone to confabulation and are able to produce accurate autobiographical memories (Tranel et al., 2000; Tranel and Jones, 2005). In support of this, both HC+ patients and three of the HC patients completed the Iowa Autobiographical Memory Questionnaire (Jones et al., 1998). This questionnaire consists of questions about episodic and semantic memories from all phases of life (childhood and adolescence, 0-18 years of age; young adulthood, 19-40 years of age; middle adulthood, 41-60 years of age; late adulthood, at least 61 years of age). Performance was scored as a percentage of correct responses that could be corroborated by a family member or friend across each time period. Both HC+ and HC patients were able to produce verifiably accurate memories for the period before brain damage (average percentage correct was 77%, compared with 86% accuracy from a group of brain-damaged patients from a previous study) (Tranel and Jones, 2005).
All participants gave informed consent to participate in these studies, which were approved by the Human Subjects Committee of the University of Iowa.
Procedure
Top-five emotional memory interview. Participants were asked to describe their top-five most emotional memories from throughout their lives and the five most emotional events from any time period in their life. For each recollection, the participant was asked to give the month and year when the event occurred, to the best of their ability. Finally, participants were asked to rate each memory on the following seven-point scales: pleasantness (equal to the most unpleasant memory to the most pleasant memory), intensity (not intense at all to the most intense memory), significance (made no difference in their life to changed their life as much as any event), novelty (totally routine to the most unusual event), vividness (no image to as clear an image as the original), and frequency of rehearsal (never to as often as any event in their life). These instructions, and specifically the rating scales, were modified from the study by Rubin and Schulkind (1997).
Word-cued memory interview. Memories were elicited using a modified Crovitz-Schiffman paradigm (Crovitz and Schiffman, 1974). Thirty nouns were selected from the Affective Norms for Emotional Words database (Bradley and Lang, 1999), including 10 negative (e.g., funeral, cancer), 10 positive (e.g., birthday, kiss), and 10 neutral (e.g., museum, snow) words. The words were read aloud one at a time to the participants, and they were asked to produce one memory in response to each word.
Participants were then instructed to provide the date when the event occurred and give ratings on the same scales as before. For both phases of testing, when a participant failed to produce a memory that was specific to place and time, the participant was prompted by the experimenter to produce a more specific memory. We did not, however, examine differences in memory quantity or quality before and after experimenter prompts (Zola-Morgan et al., 1983).
Testing sessions were conducted over one or two sessions, requiring between 2 and 6 h of testing per participant. Participants always completed the top-five memory testing before continuing with the word-cued phase. Although this static order of testing may have altered performance on one or the other phase of testing as a result of fatigue or another factor, we chose to use a fixed order to reduce the complications of a lengthy experimental procedure.
Scoring
Responses were initially scored as a memory if the response contained a specific narrative that was unique for time and place. For example, an acceptable recollection (e.g., a description of events on a participant's wedding day) including at least time of the year and the year when the event occurred was counted as a memory and included in all subsequent analyses. Unacceptable responses (e.g., in response to the cue word museum: “I don't know when it was, there was an art museum”) were not included in the analyses. This is similar to the binary scoring system described by Zola-Morgan et al. (1983). Because our emphasis in this research is in the emotional characteristics of the memories produced, we chose to use this binary scoring system for a more complete examination of these participants' emotional memories.
To permit comparison with previous work examining autobiographical memories in amnesics, we scored memories from a subset of participants (both HC+ patients, 7 HC participants, 7 BDC participants, and 13 NC participants) using a 0-3 scale to score each memory with respect to how episodic it was based on procedures described by Zola-Morgan et al. (1983). A memory was scored as a 3 if it included specific information from a particular time and place (e.g., “When I was a senior in high school in 1974, in the early summer/late spring, I broke my ankle stealing second base in a high school baseball game”), scored as a 2 if it had some specific details but was not specific to time and place (e.g., in response to the cue word garden, “we always had a garden in the summers”), scored as a 1 for a reference to a fact from the person's life (e.g., in response to the cue word teacher, “my son and daughter-in-law are teachers”), and scored as a 0 for a generic or inappropriate response (e.g., in response to the cue word bird, “Minnesota Blue Jays or something”).
Because we were interested in the emotional characteristics of the participants' recollections, we used each participant's pleasantness ratings to split their memories into pleasant, neutral, and unpleasant categories. This categorization was used in both the top-five memory and the word-cued memory phases. We chose not to categorize word-cued memories by the a priori valence of the word cue, because many of the words prompted memories that were discrepant from their a priori valence category (e.g., the unpleasant cue word “snake” may prompt the recollection of a funny childhood memory rated as pleasant by the participant). Memories with a pleasantness rating of 1 or 2 were classified as unpleasant; ratings of 3, 4, or 5 were classified as neutral; and ratings of 6 or 7 were classified as pleasant. We examined the number of memories in each category separately for the top-five memories and the word-cued memories. Participants' ratings of each pleasantness category of memory on the intensity, significance, novelty, vividness, and frequency of rehearsal scales were averaged between the two phases of testing for analysis to increase reliability of the ratings.
Similarly, for analysis of the role of intensity in the number of memories produced, we classified memories with an intensity rating of 1 or 2 as low intensity; ratings of 3, 4, or 5 were classified as middle intensity; and ratings of 6 or 7 were classified as high intensity.
It is possible that the recollections labeled as pleasant or unpleasant by the HC and HC+ patients may be different from the pleasant or unpleasant memories that comparison participants would produce. To assess this possibility, two independent raters blind to the participants' group membership out of a total group of six raters (mean age, 27.0 ± 2.5 SD; three males, three females) read each transcript of memories of the two HC+ patients, six of the HC patients, and 12 healthy comparison participants. They rated each memory on the same seven-point scale of pleasantness as used by the participants. Inter-rater correlation was high (r = 0.75).
Temporal distribution of memories
The dating of memories was accomplished in several ways. To determine the age of the memory, the number of months between the reported date when the memory occurred and the date of testing was ascertained. To determine the age of the subject at the time of the reported event, the number of months between the participant's date of birth and the reported date of the event's occurrence was determined. To determine the time of the reported event in relation to the date of brain injury, the number of months between the reported date of the event's occurrence and the date of brain injury was ascertained. Very often, participants were unable to recall the day or month when an event occurred but were able to produce the season of the year when the event occurred. When a season of the year was given with the year of an event, this was entered as the first month of the year in which that season began (e.g., Spring, March; Summer, June; Autumn, September; Winter, December). For instance, if the testing took place in October 2000, and a participant reported an event that occurred in Autumn 1990, that memory would be classified as 10 years and 1 month (or 121 months) old. For classification of the age of the subject when the memory occurred, the memories were grouped into the decade in which the memory occurred (e.g., a memory from between 10 and 20 years of age was placed in the second decade group, and so on).
Studies of word-cued memory distribution throughout the lifespan have shown that these memories show a pattern that includes childhood amnesia, a bump in recollections between 10 and 30 years of age, and a recency component described by a monotonically decreasing power function, which is especially good at predicting the most recent 10-20 years (Rubin and Schulkind, 1997). Because we were interested in the bump component of autobiographical memory distributions in word-cued memories, we removed all of the memories from the last 10 years of each participant's life (note that important or top-five memory distributions do not show this retention function, so we examined all memories reported in the top-five memory phase of testing). Four healthy comparison participants and one brain-damaged comparison participant were removed from the word-cued memory data analysis, because they reported very few memories from the remote time period. The number of participants that make up each group at each time period is indicated in Figure 2.
Figure 2.
Distributions of autobiographical memories. A, B, Top-five emotional autobiographical memories (A) and word-cued memories (B). (Note that this figure excludes memories from the last 10 years of each participant's life.) Each decade is labeled by the youngest age included in the study. Tables under each figure indicate the number of participants included within each group at each time point.
Transcript processing
Participants' responses in both memory test phases were audiotaped for later transcription. Transcripts of participants' recollections were analyzed using the computer program Linguistic Inquiry and Word Count 2001 (LIWC2001) (Pennebaker et al., 2001), which counts the total number of words in each sample and computes percentages of words per sample in 74 different categories. We focused on the total word count and affective or emotional process categories. These categories include 615 words drawn from two subcategories: positive emotions (e.g., happy, joy, win) and negative emotions (e.g., hate, enemy, nervous). We compared brain-damaged participants' word use in these subcategories with that of healthy comparison participants separately for the top-five emotional memories and the word-cued memories. Technical errors precluded the proper recording and transcription of several participants' recollections (transcripts from one HC patient, three BDC patients, and three NC patients are missing); appropriate degrees of freedom are reported for each analysis.
In addition to comparing word usage in the autobiographical memory interviews, we included a control task to examine differences in word usage in a task unrelated to memory recollection. Specifically, we examined participants' narrations of the cookie theft picture, drawn from the Boston Diagnostic Aphasia Examination (Goodglass et al., 1983). Participants were instructed to relate what they saw in the picture in verbal form. The narrations of this picture from the patients were tape recorded, transcribed, and analyzed in the same way as their descriptions of the autobiographical recollections.
The proportion of memories rated as pleasant, unpleasant, or neutral and the temporal distribution of the memories across the lifespan were analyzed as quantitative indices of emotional memory. The ratings of the memories and word-use statistics were analyzed as qualitative indices of emotional memory.
Results
Analyses of pleasant and unpleasant memories
The analyses in this section used a three-group (HC, BDC, NC) by three-category (pleasant, unpleasant, neutral) multivariate ANOVA. Post hoc comparisons of group differences were corrected using the Bonferroni procedure. Initial analyses, including gender as a factor, did not reveal any significant effects or interactions (F values <1), and thus these data were collapsed across this factor. One HC patient (2563) completed only the top-five emotional memory phase of testing and not the word-cued memory phase. The HC+ patients were treated as case studies (because we tested only two) and were not included in the group analyses.
Top-five memories
All participants were able to produce five memories. There were no group differences in the proportion of pleasant, unpleasant, or neutral memories (i.e., no group by category interaction; F(4,78) < 1.7; p > 0.18) (Table 4). Across all groups, there was a significant effect of pleasantness (F(2,39) = 3.9; p < 0.03). Both patient groups produced somewhat more pleasant than unpleasant memories, whereas the NC group produced more unpleasant than pleasant memories, although these differences were not significant. Interestingly, neither of the HC+ patients labeled any of their top-five emotional memories as unpleasant. Both patients, however, produced specific episodic memories in this phase of testing (Table 4).
Table 4.
Proportions of neutral, pleasant, and unpleasant memories across both phases of testing
|
Patient group |
Percentage of neutral memories |
Percentage of pleasant memories |
Percentage of unpleasant memories |
|---|---|---|---|
| Top five memories | |||
| Patient 1673 | 80 | 20 | 0 |
| Patient 1951 | 20 | 80 | 0 |
| HC group | 18 ± 35 | 50 ± 34 | 33 ± 30 |
| BDC group | 30 ± 30 | 48 ± 32 | 22 ± 22 |
| NC group | 23 ± 19 | 36 ± 16 | 41 ± 19 |
| Word-cued memories | |||
| Patient 1673 | 50 | 40 | 10 |
| Patient 1951 | 71 | 8 | 21 |
| HC group | 37 ± 25 | 35 ± 13 | 27 ± 17 |
| BDC group | 52 ± 17 | 26 ± 15 | 22 ± 15 |
| NC group |
47 ± 18 |
30 ± 16 |
24 ± 9 |
Entries show means and SDs.
Word-cued memories
There was a significant group difference in the number of word-cued memories (F(2,39) = 4.5; p < 0.02), with the BDC (mean, 26.1 ± 1.1) and HC (mean, 26.86 ± 1.3) groups producing fewer memories than the NC group (mean, 29.6 ± 0.7). Not surprisingly, the two HC+ patients produced even fewer word-cued memories: patient 1951 produced 24, and patient 1673 produced 10.
As in the previous phase of testing, there were no group differences in the proportion of memories across the valence categories (group by category interaction: F(4,76) < 0.6; p > 0.5) (Table 4). There was a significant effect of category across all groups (F(2,38) = 9.5; p < 0.0001). All three groups generated more neutral memories than pleasant and unpleasant memories in this phase of testing (p values <0.05). The two HC+ patients produced proportions of neutral, pleasant, and unpleasant memories similar to those seen for the other patient groups (Table 4). These results demonstrate that HC participants are able to produce a quantity of autobiographical recollections similar to that seen for brain-damaged comparison participants, and the patterns of recollection of pleasant, unpleasant, and neutral memories for both brain-damaged groups were not different from healthy comparison participants.
Analyses of low- and high-intensity memories
Previous research has shown a prominent effect of arousal or intensity in enhancing memory (Bradley et al., 1992) and indicated that this effect may be amygdala dependent (Kensinger and Corkin, 2004). We chose to examine the recollection of memories rated as high intensity compared with those rated as middle or low intensity using the same analysis strategy used for pleasant memories, as described above.
Top-five memories
There was a highly significant main effect of intensity (F(2,39) > 300; p < 0.0001), with more high-intensity than middle- or low-intensity memories produced across all groups. There was no difference in the pattern of high-, middle-, and low-intensity memories across groups (no group by intensity interaction; F(4,78) = 1.2; p = 0.3). Means for number of low-, middle-, and high-intensity memories produced by each group are as follows: BDC mean ± SE, 0.1 ± 0.1, 2.1 ± 0.5, 2.8 ± 0.5; NC mean ± SE, 0.16 ± 0.1, 1.7 ± 0.2, 3.2 ± 0.2; HC mean ± SE, 0.0 ± 0.0, 1.0 ± 0.5, 4.0 ± 0.5, respectively. Interestingly, both HC+ patients produced the same pattern of memory as a function of intensity, and both rated four of their memories in the middle-intensity range and one memory in the high-intensity range. These results show that all groups selectively report fewer low-intensity memories in this task and that HC+ patients show a tendency toward rating their top-five memories in the middle-intensity range.
Word-cued memories
Similar to the top-five memories, there was a significant effect of intensity (F(2,38) > 100; p < 0.0001), with all groups reporting more middle-intensity memories than high- or low-intensity memories. There was no difference in the pattern of intense memories produced in this phase of testing (no group by intensity interaction; F(4,76) = 1.2; p = 0.3). Means for proportion of low-, middle-, and high-intensity memories produced by each group are as follows: BDC mean ± SE, 0.12 ± 0.03, 0.62 ± 0.6, 0.26 ± 0.5; NC mean ± SE, 0.08 ± 0.02, 0.64 ± 0.03, 0.29 ± 0.03; HC mean ± SE, 0.08 ± 0.04, 0.51 ± 0.07, 0.4 ± 0.08, respectively. Patient 1673 from the HC+ group rated 50% of his word-cued memories as high intensity and 50% as low intensity, whereas patient 1951 rated most of his memories (71%) in the middle-intensity range and rated 17 and 13% as low and high intensity, respectively. Middle-intensity, neutral memories were produced with high frequency during this phase of testing across all groups.
Ratings of memories
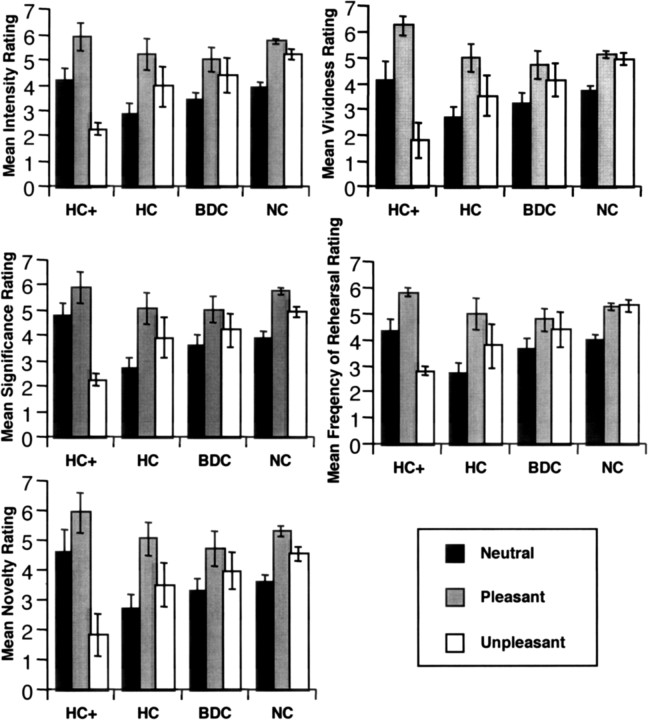
For analyses of memory ratings, data from the top-five emotional memories and word-cued memories were combined. Initial analyses including gender as a factor revealed no main effects or interactions (F values <1.2), and thus these data were collapsed over this factor. Both the amnesic and brain-damaged comparison groups rated pleasant, unpleasant, and neutral memories as less intense than the NC group (p values = 0.003 and 0.03, respectively). The HC group similarly rated memories as less significant and vivid and reported lower frequency of rehearsal than the NC group (p values <0.03) (Fig. 1). There were no group differences in novelty ratings.
Figure 1.
Ratings of intensity, significance, novelty, vividness, and frequency of rehearsal of neutral, pleasant, and unpleasant memories. Error bars indicate SEM.
All three groups showed differential intensity, significance, novelty, vividness, and frequency of rehearsal ratings across the three valence categories of memories, with the pattern unpleasant > pleasant > neutral (significant effects of category for all ratings; F(2,39) > 13; p < 0.0001). Despite the above group differences in the level of ratings, all three groups produced the same pattern of ratings for neutral, pleasant, and unpleasant memories; there were no group by category interactions for any of the ratings (F(4,80) < 1.2; p > 0.3). These data indicate that whereas the HC patients reported lower overall ratings on some of the scales, the pattern of recollection of emotional memories is not qualitatively different from the comparison groups.
Interestingly, the two HC+ patients rated unpleasant memories as less intense, significant, novel, and vivid and reported lower frequency of rehearsal than the other groups (at least 2 SDs lower than the normal comparison group), while rating their pleasant memories as more intense, significant, novel, and vivid and reporting higher frequency of rehearsal for these memories (Fig. 1). These ratings demonstrate that HC+ patients show a positive bias in their rating of pleasant versus unpleasant memories.
Comparison with independent raters
Ratings of pleasantness were not different between the raters and either the amnesic or healthy comparison group (F(1,32) = 2.6; p = 0.12). Importantly, the independent raters did not rate the amnesics' memories as different from the comparison group (no significant group by rater interaction; F(1,32) = 2.3; p > 0.1). There were high positive correlations between the pleasantness ratings of the HC+ patients and the two independent raters (for patient 1673: rater 1, r = 0.88; rater 2, 0.76; for patient 1951: rater 1, r = 0.53; rater 2, 0.53). These data suggest that both HC and HC+ patients were able to categorize their memories as pleasant, unpleasant, or neutral in much the same way as comparison participants and independent raters.
Temporal distribution of memories
We next addressed whether amnesic patients might show a different pattern of recollection of pleasant, unpleasant, and neutral memories from before versus after brain injury. The rationale for this analysis is that if the hippocampus or amygdala is differentially involved in encoding versus retrieval processes, recollections from before brain injury (when these structures were intact) may show a different affective character than those from after brain injury (when these structures were already damaged). Whereas both amnesic and brain-damaged comparison groups recalled more memories from before than after brain damage (F(1,15) = 11.0; p = 0.005; mean percentage of memories from before brain damage: amnesic group, 72 ± 8%; brain-damaged comparison group, 66 ± 7%), there was no difference in the proportion of pleasant, unpleasant, and neutral memories from before versus after brain damage (no group by pleasantness interaction; F(2,14) < 1; p > 0.5). As expected, the recollections of the HC+ patients 1673 and 1951 were predominantly from before the onset of brain damage, with patient 1673 recalling 100% of his memories from before brain damage, whereas patient 1951 produced 91% of his memories from before brain damage, producing only two memories from after amnesia onset. One of these memories was the birth of a nephew, which he rated a 5, or neutral, and the other the death of his grandfather, which he rated a 2, or unpleasant.
Similarly, we chose to next analyze the pattern of memory for high-, middle-, and low-intensity memories from before versus after brain damage. This analysis showed that there was no difference in the proportion of low-, middle-, and high-intensity memories from before versus after brain damage in either group (F(2,14) = 1.3; p > 0.3).
The autobiographical memory bump
We examined the distribution of both top-five emotional memories and word-cued memories in the participants in the present study. In both phases, there was a pronounced bump in recollection in all groups (Fig. 2A,B). The predominant bump for the HC patients in word-cued memories may reflect the amnesia for more recent time periods. There were significant differences in recollection between the bump period and the two decades after the bump across all groups (top-five memories, F(1,40) = 25, p < 0.0001; word-cued memories, F(1,24) = 9.9, p = 0.004) (participants who were <50 years of age were excluded from the analysis of word-cued memories, because the two decades after the bump period, between 31 and 50 years of age, could not be properly analyzed in these participants; appropriate degrees of freedom are reported). Groups showed no significant difference in pattern of recollection over time (no group by decade interactions across either phase of testing; F < 2.5; p > 0.11).
HC+ patients also demonstrated a significant bump in recollections for the time period between 10 and 30 years of age. All of the top-five emotional memories produced by patients 1673 and 1951 were from the time between 9 and 26 years of age. Patient 1951 showed greater recollection in the word-cued phase for memories from before 10 years of age; 41% of his memories came from this time period. Thirty-five percent of his memories came from between 10 and 20 years of age, and 17% came from the time between 21 and 30 years of age. Patient 1673, in contrast, became amnesic at 62 years of age, and he showed a pronounced recollection enhancement for the bump period; 40% of his word-cued memories came from between 10 and 20 years of age, and 50% came from the time between 21 and 30 years of age.
Word use in autobiographical memory descriptions
To further examine the qualitative characteristics of autobiographical recollections, we analyzed the number of words used and the proportion of emotional words further divided into positive and negative emotional words used during each phase of testing. Transcripts from both the top-five memory and word-cued memory phases were combined for these analyses. Table 5 shows the number of words used and proportions of emotional words used. There was not a significant difference in the number of words used in describing memories among the HC, BDC, and NC groups (F(2,32) = 2.8; p = 0.075), although there was a trend toward lower word counts in the BDC group compared with the NC group (p = 0.07). There was no group difference in the proportion of positive and negative emotion between groups (F(2,34) < 1; p > 0.7) nor was there a difference in pattern of emotion word use between groups (no group by emotion interaction; F(2,32) = 1.2; p > 0.3). There was, however a main effect of emotion, such that all groups produced more positive than negative words (F(1,32) = 30; p < 0.0001).
Table 5.
Word usage in autobiographical memory and control task (cookie theft) descriptions
|
Patient group |
Word count |
Percentage of positive words |
Percentage of negative words |
|||
|---|---|---|---|---|---|---|
| Word usage in autobiographical descriptions | ||||||
| Patient 1673 | 1006 | 0.71 | 0.17 | |||
| Patient 1951 | 1364 | 1.55 | 0.30 | |||
| HC group | 3336 ± 1434 | 1.35 ± 0.32 | 0.89 ± 0.35 | |||
| BDC group | 2011 ± 1662 | 1.7 ± 0.80 | 0.7 ± 0.36 | |||
| NC group | 3939 ± 2022 | 1.6 ± 0.56 | 0.90 ± 0.42 | |||
| Word usage in control task (cookie theft picture) | ||||||
| Patient 1673 | 116 | 3.45 | 1.72 | |||
| Patient 1951 | 122 | 0.82 | 0.82 | |||
| HC group | 114 ± 67 | 0.38 ± 0.76 | 1.0 ± 1.3 | |||
| BDC group | 64 ± 21 | 1.18 ± 1.36 | 0.22 ± 0.53 | |||
| NC group |
116 ± 50 |
1.6 ± 2.1 |
0.75 ± 1.3 |
|||
Entries show means and SDs.
Although the HC group showed remarkably similar word use patterns compared with the comparison groups, the HC+ patients used very few negative words to describe their memories, while using an equivalent proportion of positive words in their descriptions. Neither HC+ patient, in fact, used any negative words in the description of their top-five emotional memories. In our control task, affective word use in describing the cookie theft picture was not different among groups (F(2,34) < 1; p > 0.5), demonstrating that the HC+ patients were able to produce affective words normally (Table 5). There was, however, a trend toward group differences in the total number of words used in describing the cookie theft picture (F(1,34) = 2.6; p = 0.08), with the BDC group producing fewer words than the other groups. These results suggest that the HC+ patients do not use negatively affective words in describing their emotional memories, although they are able to do so in their descriptions of visual stimuli.
Episodic recollections
To examine the episodic characteristics of the autobiographical recollections, we analyzed the sum of episodic ratings given to each participants' recollections in both the top-five (e.g., five memories × a possible score of 3 = maximum of 15) and word-cued (30 memories × a possible score of 3 = maximum of 90) phases of testing. For the top-five phase, there was a trend toward a group difference in total episodic rating (F(2,27) = 2.6; p = 0.097). Post hoc tests showed that there were no significant differences among the groups [group means out of a total of 15: HC group mean ± SE, 14.3 ± 0.47; BDC group mean ± SE, 14.7 ± 0.18; NC group mean ± SE, 15.0 ± 0.0). In the HC+ group, patient 1951 had a normal pattern of episodic recollection, scoring the maximum 15, but patient 1673 performed more poorly, achieving a score of 13.
Two participants were excluded from analysis in the word-cued memory phase because of technical errors in the audiotaping, which resulted in unscorable transcripts. In the word-cued memory phase, there was a significant effect of group (F(2,25) = 4.7; p = 0.021), and post hoc tests showed that the HC group (mean ± SE, 72.5 ± 4.5 of a total of 90) had lower performance than the NC group (mean ± SE, 85.0 ± 0.97; p = 0.03); the BDC group (mean ± SE, 76.2 ± 5.8) did not differ significantly from either group. These data show that although HC damage may not affect the characteristics of emotional autobiographical recollection, episodic recollection in general is reduced. Similarly, HC+ patients showed significantly poorer performance in the word-cued phase, with patient 1951 producing a total score of 50 and patient 1673 producing a total score of 23. As documented previously, more extensive MTL damage reduces the ability to produce episodic detail.
Correlations between neuroanatomy and memory
To examine the associations among hippocampal volume, amygdala volume, and memory performance, correlation analyses were conducted. The HC and HC+ groups were combined for this analysis to increase statistical power. We correlated hippocampal and amygdala volumes with the combined proportion of pleasant, neutral, and unpleasant memories from the top-five and word-cued phases of testing. None of the correlations between structure and proportion of emotional memories reached statistical significance. Results demonstrated no consistent association between the volume of either structure and proportion of emotional memories (range of correlations, from r = 0.2 between hippocampal volume and proportion of unpleasant memories to r = 0.5 between hippocampal volume and proportion of pleasant memories). Correlations between amygdala volume and proportion of emotional memories ranged from 0.33 for pleasant to 0.35 for unpleasant. We next correlated hippocampal and amygdala volumes with the total number of memories produced in the word-cued phase of testing. There were significant, positive correlations for both the amygdala (r = 0.76; p = 0.019) and the hippocampus (r = 0.67; p = 0.047).
Discussion
We examined whether amnesics were able to produce detailed autobiographical recollections of emotional events and whether these recollections were dependent on the integrity of the hippocampus. HC patients produced autobiographical memories of emotional events similar in quantity and quality to age-matched normal and brain-damaged comparison participants. In contrast, HC+ patients reported fewer unpleasant autobiographical memories, rated unpleasant memories as less intense, significant, novel, and vivid, and used fewer emotionally negative words in describing their memories than did the other participants. These results suggest that although the hippocampus is not necessary for the vivid recollection of emotional events, other medial temporal lobe structures such as the amygdala and/or surrounding cortices may be necessary for the recollection of unpleasant emotional experiences.
The eight patients with damage limited to the hippocampus displayed memory for emotional autobiographical events that was strikingly similar to comparison participants in both quantity and quality. These results indicate that the recollection of emotional autobiographical memories from the remote past is not dependent on the hippocampus proper. Although the hippocampus may be differentially involved in recollection versus familiarity in the study of recognition memory (Yonelinas et al., 2002) (but see Wixted and Squire, 2004, for a divergent viewpoint), the recollection of remote autobiographical memory is unimpaired after hippocampal damage (Reed and Squire, 1998; Bayley et al., 2003). Results from the current study extend these findings to include the recollection of emotional autobiographical memories. Our findings mirror those from Hamann et al. (1997a,b) in the anterograde domain, demonstrating that hippocampal amnesics show an equivalent enhancement of memory for emotional pictures compared with healthy comparison participants. Interestingly, a recent neuroimaging study examining emotional verbal learning in patients with medial temporal sclerosis has suggested that damage to either the hippocampus or the amygdala impairs the encoding of emotional memories (Richardson et al., 2004). Our results are in contrast to this study, in that there was no difference in the recollection of emotional memories from before versus after damage limited to the hippocampus. Perhaps the developmental course of the unilateral MTL damage of the patients in the study by Richardson et al. (2004), caused by hippocampal sclerosis, has resulted in increased intrahemispheric and interhemispheric communication, which may not be the normal pattern. The acute, adult onset and bilateral nature of our patients' MTL damage, along with methodological differences, make comparison of these groups difficult. Future work focusing on the possible differential roles of the amygdala and hippocampus in encoding versus retrieval may illuminate the issues addressed by this work.
Results examining the temporal distribution of memories demonstrated that although amnesics and brain-damaged comparison participants reported more memories from before brain damage than after, there was not a different pattern of recollection of pleasant versus unpleasant or highversus low-intensity memories from before versus after brain damage. Examination of memories across decades from the HC patients showed a pronounced bump (Rubin et al., 1998) in memories formed between 10 and 30 years of age across both phases of testing. Word-cued testing similarly produced the bump in memories for the range of 10-30 years of age across all groups. These findings suggest a robust recollection of memories from the range of 10-30 years of age despite possible retrograde amnesia caused by HC damage. Future work could more carefully assess the distribution of memories from amnesic patients by specifically cueing memories from specific age ranges and more closely documenting the extent of retrograde amnesia.
The words used by the HC patients in describing their memories were similar in quantity and affective quality compared with the other participant groups. All three groups in fact produced more positive than negative words in their descriptions. These results are similar to previous work examining the qualitative aspects of amnesics' recollections. Reed and Squire (1998) showed that amnesic patients produced the same pattern of recollection of emotional items in their autobiographical memories compared with age-matched comparison participants. These findings provide additional evidence of the successful recollection and description of emotional autobiographical memories in patients with amnesia as a result of hippocampal damage.
Two patients with extensive medial temporal lobe lesions, including the amygdala, hippocampus, and surrounding cortices, showed a marked reduction in the quantity and quality of unpleasant autobiographical memories. Although comparison groups and the HC group were able to produce unpleasant memories when asked to provide their top-five emotional memories, neither HC+ patient was able to do so. Similarly, these patients rated their top-five memories in the middle of the intensity scale, whereas the other groups overwhelmingly rated their top-five memories as high intensity. In the word-cued memory testing, one of the HC+ patients (1951) produced an equivalent proportion of unpleasant memories compared with the other groups (21% compared with 24% for the age-matched comparison group), whereas patient 1673 produced only one unpleasant memory of a total of 10 memories from this phase. It is possible, therefore, for these patients to produce unpleasant recollections, but the quantity of unpleasant autobiographical memories is reduced overall.
These patients' ratings of their memories across the phases of testing again shows impoverished recollections of unpleasant memories. Ratings of the intensity, vividness, significance, novelty, and frequency of rehearsal for unpleasant memories were substantially lower in these patients compared with the other groups (e.g., >2 SDs lower than the NC group). Additionally, the HC+ patients showed a tendency to rate their pleasant memories as higher on all of these scales compared with the other groups.
HC+ patients also used fewer words and a lower proportion of negative affect words to describe their memories compared with the other groups. The number of negative affect words used by the HC+ patients was 1.5 SDs below the mean of the HC group. These results are in line with aging research, which has shown preserved emotional enhancement of memory in healthy older people (Kensinger et al., 2002; Denburg et al., 2003), but older people tend to recollect their past more positively than younger individuals (Kennedy et al., 2004). One study has shown a similar positive bias in patients with early-stage Alzheimer's disease (Hamann et al., 2000). These effects have been linked to reduced amygdala activity in the elderly (Mather et al., 2004) and reduced amygdala volume in Alzheimer's disease (van Hoesen, 1997; Hamann et al., 2000). Together, this work demonstrates that the hippocampus is not necessary either for the enhancement of emotional learning or for the vivid recollection of emotional memories, but that that the amygdala may be an important component in the recollection of unpleasant memories. It is important to note the extent of medial temporal lobe damage in these patients. Both of the patients have at least some damage to the temporal pole and parahippocampal gyrus regions. Damage to these areas most likely contributes to their autobiographical recollection impairment. These areas operate in conjunction with the hippocampus in learning new information, recognizing previously presented stimuli, and retrieval processes (Squire et al., 2004). The role of the amygdala in emotional memory enhancement is well established (Cahill, 2000; Buchanan and Adolphs, 2004); although, damage to other medial temporal lobe areas certainly plays a role in these patients' deficits, the current data along with previous research point to the amygdala as a likely contributor to their positive bias in autobiographical memory.
The time distribution of the HC+ patients' memories shows a pattern similar to that seen for the other brain-damaged participants. Patients 1673 and 1951 both produced the vast majority of their memories from the period before brain damage, and both showed a pronounced bump in memories from before the age of 30. Patient 1951, whose amnesia onset was at the age of 27, produced all but two of his memories from before the age of 25 (the two after age 25 and the only two memories reported from after amnesia onset were the birth of a nephew and the funeral of a grandfather, suggesting that these events were highly emotional and/or often rehearsed by family members). Patient 1673 became amnesic at age 62; all of his memories came from between 13 and 49 years of age. Although we did not specifically address the temporal extent of retrograde amnesia in these patients, these results are consistent with previous work on the time course of retrograde amnesia in postencephalitic patients (Damasio et al., 1985; Reed and Squire, 1998).
The recollections of the HC+ patients were marked by a paucity of detail in terms of the number of memories produced and the number of words used in their description. Patient 1673, in particular, was only able to produce 10 acceptable memories in the word-cued phase of testing. It is difficult to assess whether the recollections produced by these patients are from actual events or confabulations of often-told stories (Reed and Squire, 1998). Independent ratings of the episodic nature of these patients' memories demonstrated reduced episodic production in these patients. Both patients were, however, capable of producing specific episodes in both the top-five and word-cued testing. Although we did not address the veracity of the memories produced in this experiment, both patients were able to produce accurate memories (corroborated by family members) on the Iowa Autobiographical Memory Questionnaire (see Materials and Methods).
In summary, recollections of the emotional past can be accomplished after hippocampal damage, but extensive damage including the amygdala and surrounding cortices may impair the vivid recollection specifically of unpleasant memories. The hippocampus, then, is not necessary for the vivid remembering of emotional events, but the amygdala and surrounding cortices may be necessary for this function. These speculations are based on small numbers of participants, but the consistency with which the HC group recollected their emotional past, the robust positivity bias in both HC+ patients, and consistency with previous research suggest that there are differential roles for the hippocampus and amygdala in the recollection of emotional experience.
Footnotes
This work was supported by a National Research Service Award from the National Institute on Aging to T.W.B., by National Institute of Neurological Disorders and Stroke Program Project Grant P01 19632, and by National Institute of Mental Health Grant R01 067681. We thank Benjamin R. Lewis for assistance with data collection and Joel Bruss, John Allen, and Hanna Damasio (Human Neuroanatomy and Neuroimaging Laboratory, University of Iowa) for the volumetric data presented in this study.
Correspondence should be addressed to Tony Buchanan, Department of Neurology, University of Iowa, 200 Hawkins Drive, Iowa City, IA 52242. E-mail: tony-buchanan@uiowa.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253151-•$15.00/0
References
- Allen JS, Damasio H, Grabowski TJ (2002) Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol 118: 341-358. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H (2005a) Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging, in press. [DOI] [PubMed]
- Allen JS, Tranel D, Bruss J, Damasio H (2005b) Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol, in press. [DOI] [PubMed]
- Bayley PJ, Hopkins RO, Squire LR (2003) Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lesions. Neuron 38: 135-144. [DOI] [PubMed] [Google Scholar]
- Berntsen D, Rubin DC (2002) Emotionally charged autobiographical memories across the life span: the recall of happy, sad, traumatic, and involuntary memories. Psychol Aging 17: 636-652. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ (1999) Affective norms for emotional words. Gainesville, FL: National Institute of Mental Health Center for the Study of Emotion and Attention.
- Bradley MM, Greenwald MK, Petry MC, Lang PJ (1992) Remembering pictures: pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn 18: 379-390. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Adolphs R (2004) The neuroanatomy of emotional memory in humans. In: Memory and emotion (Reisberg D, Hertel P, eds), pp 42-75. New York: Oxford UP.
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C (2004) Circadian regulation of cortisol after hippocampal damage in humans. Biol Psychiatry 56: 651-656. [DOI] [PubMed] [Google Scholar]
- Cahill L (2000) Modulation of long-term memory storage in humans by emotional arousal: adrenergic activation and the amygdala. In: The amygdala (Aggleton J, ed), pp 425-446. Oxford: Oxford UP.
- Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, Roche A, Tsui W (1999) MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res 90: 113-123. [DOI] [PubMed] [Google Scholar]
- Conway MA (2003) Commentary: cognitive-affective mechanisms and processes in autobiographical memory. Memory 11: 217-224. [DOI] [PubMed] [Google Scholar]
- Crovitz H, Schiffman H (1974) Frequency of episodic memories as a function of their age. Bull Psychon Soc 4: 517-518. [Google Scholar]
- Damasio AR, Eslinger PJ, Damasio H, Van Hoesen GW, Cornell S (1985) Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Arch Neurol 42: 252-259. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R (2003) Evidence for preserved emotional memory in normal elderly persons. Emotion 3: 239-253. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (1988) The human hippocampus: an atlas of applied anatomy. New York: Springer.
- Frank RJ, Damasio H, Grabowski TJ (1997) Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. NeuroImage 5: 13-30. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Weintraub W (1983) Boston diagnostic aphasia examination. Philadelphia: Lea and Febiger.
- Hamann SB, Cahill L, Squire LR (1997a) Emotional perception and memory in amnesia. Neuropsychology 11: 104-113. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Cahill L, McGaugh JL, Squire LR (1997b) Intact enhancement of declarative memory for emotional material in amnesia. Learn Mem 4: 301-309. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Monarch ES, Goldstein FC (2000) Memory enhancement for emotional stimuli is impaired in early Alzheimer's disease. Neuropsychology 14: 82-92. [PubMed] [Google Scholar]
- Jones RD, Grabowski T, Tranel D (1998) The neural basis of retrograde memory: evidence from positron emission tomography for the role of non-mesial temporal lobe structures. NeuroCase 4: 471-479. [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL (2004) The role of motivation in the age-related positivity effect in autobiographical memory. Psychol Sci 15: 208-214. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S (2004) Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci USA 101: 3310-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S (2002) Effects of normal aging and Alzheimer's disease on emotional memory. Emotion 2: 118-134. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M (2002) Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging 17: 677-689. [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL (2004) Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci 15: 259-263. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2003) Memory and emotion: the making of lasting memories. New York: Columbia UP.
- Pennebaker JW, Francis ME, Booth RJ (2001) Linquistic inquiry and word count. Mahwah, NJ: Erlbaum.
- Reed JM, Squire LR (1998) Retrograde amnesia for facts and events: findings from four new cases. J Neurosci 18: 3943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ (2004) Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci 7: 278-285. [DOI] [PubMed] [Google Scholar]
- Rubin D, Schulkind M (1997) Distribution of important and word-cued autobiographical memories in 20-, 35-, and 70-year-old adults. Psychol Aging 12: 524-535. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Rahhal TA, Poon LW (1998) Things learned in early adulthood are remembered best. Mem Cognit 26: 3-19. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27: 279-306. [DOI] [PubMed] [Google Scholar]
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P (2001) Amygdalar and hippocampal volumetry in control participants: differences regarding handedness. AJNR Am J Neuroradiol 22: 1342-1345. [PMC free article] [PubMed] [Google Scholar]
- Tranel D (2005) Theories of clinical neuropsychology and brain-behavior relationships: luria and beyond. In: Handbook of clinical neuropsychology (Ricker JH, ed). Amsterdam: Swets and Zeitlinger, in press.
- Tranel D, Jones RD (2005) Knowing what and knowing when. J Clin Exp Neuropsychol, in press. [DOI] [PubMed]
- Tranel D, Damasio H, Damasio AR (2000) Amnesia caused by herpes simplex encephalitis, infarctions in basal forebrain, and anoxia/ischemia. In: Handbook of neuropsychology, Ed 2 (Grafman FBJ, ed), pp 85-110. Amsterdam: Elsevier.
- van Hoesen GW (1997) Ventromedial temporal lobe anatomy, with comments on Alzheimer's disease and temporal injury. J Neuropsychiatry Clin Neurosci 9: 331-341. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR (2004) Recall, recognition, and the hippocampus [reply to Yonelinas et al. (2004)]. Cogn Affect Behav Neurosci 4: 401-406. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauve M-J, Widaman KF, Knight RT (2002) Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci 5: 1236-1241. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Cohen NJ, Squire LR (1983) Recall of remote episodic memory in amnesia. Neuropsychologia 21: 487-500. [DOI] [PubMed] [Google Scholar]