Abstract
The synapses formed by the olfactory nerve (ON) convey sensory information to olfactory glomeruli, the first stage of central odor processing. Morphological and behavioral studies suggest that glomerular odor processing is plastic in neonate rodents. However, long-term synaptic plasticity, a cellular correlate of functional and structural plasticity, has not yet been demonstrated in this system. Here, we report that ON→mitral cell (MC) synapses of 5- to 8-d-old mice express long-term depression (LTD) after brief low-frequency ON stimulation. Pharmacological techniques and imaging of presynaptic calcium signals demonstrate that ON-MC LTD is expressed presynaptically and requires the activation of metabotropic glutamate receptors but does not require fast synaptic transmission. LTD at the ON→ MC synapse is potentially relevant for the establishment, maintenance, and experience-dependent refinement of odor maps in the olfactory bulb.
Keywords: calcium (Ca), imaging, olfactory, patch clamp, metabotropic glutamate receptor, plasticity, LTD
Introduction
The olfactory system detects and discriminates thousands of different odorant molecules in the environment, an ability that is critical for development and survival for many animals. In mammals, odor signals are converted into electrical signals by olfactory receptor neurons in the neuroepithelium of the nasal cavities. These receptor neurons express one or a small number of ∼1000 olfactory receptor proteins (Chess et al., 1994; Serizawa et al., 2003), each of which binds odorants with particular molecular features. The axons of all of the 10,000-20,000 neurons expressing the same receptor protein converge onto a few (one to three) glomeruli (Vassar et al., 1994; Mombaerts et al., 1996), where they form synapses with the distally tufted single primary dendrites of mitral cells (MCs), with dendrites of tufted cells, and with periglomerular cells. The convergence of axons of sensory neurons expressing the same receptor onto topographically defined glomeruli creates a spatial map of the molecular features of the odor in the glomerular layer of the olfactory bulb (Vassar et al., 1994; Mombaerts et al., 1996; Wachowiak and Cohen, 2001). Glomerular “odor images” are reproducible within and across animals and are similar for similar odorant molecular structures (Wachowiak and Cohen, 2001; Takahashi et al., 2004). In rodents, odor maps are present soon after birth (Guthrie and Gall, 2003). Synaptogenesis between olfactory receptor neurons and MCs continues postnatally, suggesting that odor maps are likely refined during early postnatal development and by exposure to odorants from the environment (Guthrie and Gall, 2003). Postnatal synaptogenesis is paralleled by synaptic regression of early formed synaptic contacts as MC dendrites develop a single apical dendrite from multiple immature dendritic processes into several adjacent glomeruli (Lin et al., 2000; Zou et al., 2004). Furthermore, axons from sensory neurons expressing different receptor types can initially intermingle and even mistarget in the glomerular layer, only gradually segregating and retracting to appropriate glomerular positions during the first postnatal days (Zou et al., 2004). Structural plasticity involving synaptic regression and circuit refinement has often been associated with functional plasticity. Indeed, behavioral and functional mapping experiments indicate functional plasticity at the level of olfactory glomeruli both during early odor preference learning and also in adult rodents (Yuan et al., 2002; Fletcher and Wilson, 2003; McLean and Harley, 2004). Despite these data on the system level, there has to date been little evidence for olfactory plasticity at the level of olfactory nerve (ON)→ MC synapses except one report suggesting that ON→ MC synapses can express long-term potentiation (LTP) (Ennis et al., 1998).
Here, we find that ON→ MC synapses can express a long-lasting depression of synaptic efficacy [referred to as ON-MC long-term depression (LTD)]. We use pharmacological and calcium imaging techniques to demonstrate that ON-MC LTD is expressed presynaptically and is mediated by metabotropic glutamate receptors (mGluRs). ON-MC LTD may provide a cellular model for the structural and behavioral plasticity and the dynamic maintenance of odor maps that occur at the level of ON→ MC synapses.
Materials and Methods
Electrophysiology. The olfactory bulb was dissected from 5- to 8-d-old ICR mice. Horizontal slices (300-370 μm thick) were cut and recovered at 32°C for the first hour and then at room temperature (23-25°C). After >1 h of recovery, a slice was transferred into the recording chamber and perfused with artificial CSF (ACSF) containing the following (in mm): 118 NaCl, 25 NaHCO3, 1 NaH2PO4, 3 KCl, 1 MgCl2, 2 CaCl2, and 10 glucose, equilibrated with 95% O2 and 5% CO2. Experiments were performed at room temperature (23-25°C).
Field potentials were recorded in the glomerular layer with glass pipettes (filled with ACSF; 1.0-2.0 MΩ). Patch-clamp recordings were performed in the whole-cell configuration. Glass pipettes (resistance, 3.5-4.5 MΩ) were pulled from borosilicate glass using a two-stage vertical puller (Narishige, Tokyo, Japan). Pipettes contained the following (in mm): 9 KCl, 10 KOH, 120 K-gluconate, 3.48 MgCl2, 4 NaCl, 10 HEPES, 17.5 sucrose, 4 Na2ATP, and 0.4 Na3GTP, pH 7.25. In some recordings, the internal solution contained 3 mm N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX-314) to block action potential generation. A 20 mm BAPTA-containing internal solution was made by replacing 80 mm K-gluconate with 20 mm K4-BAPTA. The osmolarity change was compensated for by addition of sucrose.
Constant-current stimulation pulses (5-100 μA, 300 μs) were applied to the ON layer using glass electrodes (filled with ACSF; 0.5-1.5 MΩ). Test stimuli were delivered every 30 s.
For each cell or slice, the peak amplitude of the evoked EPSPs or field EPSP (fEPSP) component was normalized to the mean response amplitude obtained during the baseline recording period. The amplitude of the NMDA receptor-mediated component of the fEPSP was measured at 20-30 ms after stimulation. Values are reported as percentage change from the mean of the baseline value. Population data are expressed as mean ± SEM, where n represents the number of experiments performed on different MCs (EPSPs) or slices (fEPSPs).
Calcium imaging. Mice [postnatal day 2 (P2)-P5] were anesthetized on ice. Olfactory receptor neurons in nasal cavities were exposed to 2 μl of 0.25% Triton X-100 in physiological saline for 5 min. Subsequently, nasal cavities were injected with 8 μl of 4% Calcium Green-1 dextran (10 kDa; Molecular Probes, Eugene, OR) in physiological saline, and mice were recovered from anesthesia. After 2-6 d, dye-injected mice were used for slice preparation. Fluorescence of Calcium Green-1 was excited by epi-illumination (480 nm), with light provided by a monochromator (Polychrome IV; TILL Photonics, Gräfelfing, Germany) and detected by a cooled 12-bit charge-coupled device under control of TILL Vision (TILL Photonics) software. A filter set consisting of a dichroic beam splitter (DCLP 505 LP; Chroma Technology, Brattleboro, VT) and emission filter (535 ± 25 nm) was used. Fluorescence signals were converted to ΔF/F values, where F is the baseline fluorescence and ΔF is the change in fluorescence. Color-coded maps of ΔF/F were prepared using custom-made macros under Image-Pro Plus (Media Cybernetics, Silver Spring, MD).
Results
LTD at olfactory nerve synapses
Field potential recordings and whole-cell patch-clamp recordings were made in horizontal olfactory bulb slices prepared from young mice (P5-P8). ON→ MC synapses were activated by stimuli delivered via an electrode placed on the ON. fEPSPs were recorded with an electrode placed into an adjacent glomerulus, and EPSPs were recorded from the cell body of MCs with a patch-clamp electrode (Fig. 1A). Single ON stimuli induced glomerular fEPSPs that consisted of two distinct postsynaptic components. The first was pharmacologically identified as a slow NMDA receptor (NMDAR)-mediated component that was blocked by 50 μm d-APV (Fig. 1B1,B2). The second was identified as an AMPA receptor (AMPAR)-mediated component that was blocked by 20 μm 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) (Fig. 1 B1) (Aroniadou-Anderjaska et al., 2000). The AMPAR-mediated fEPSP component consisted of an initial fast spike-like potential that most likely represents action potentials triggered by the ON-MC EPSP and a second slower potential (Fig. 1B2, AMPA1 and AMPA2, respectively). The initial deflection of the fEPSP was resistant to application of NBQX and d-APV but was abolished by TTX and, therefore, represents action potentials of the ON (Fig. 1B1,B2). ON-MC EPSPs exhibited corresponding pharmacological properties: a slow NMDAR-mediated component and a faster AMPAR-mediated component (Fig. 1C) (Aroniadou-Anderjaska et al., 2000).
Figure 1.
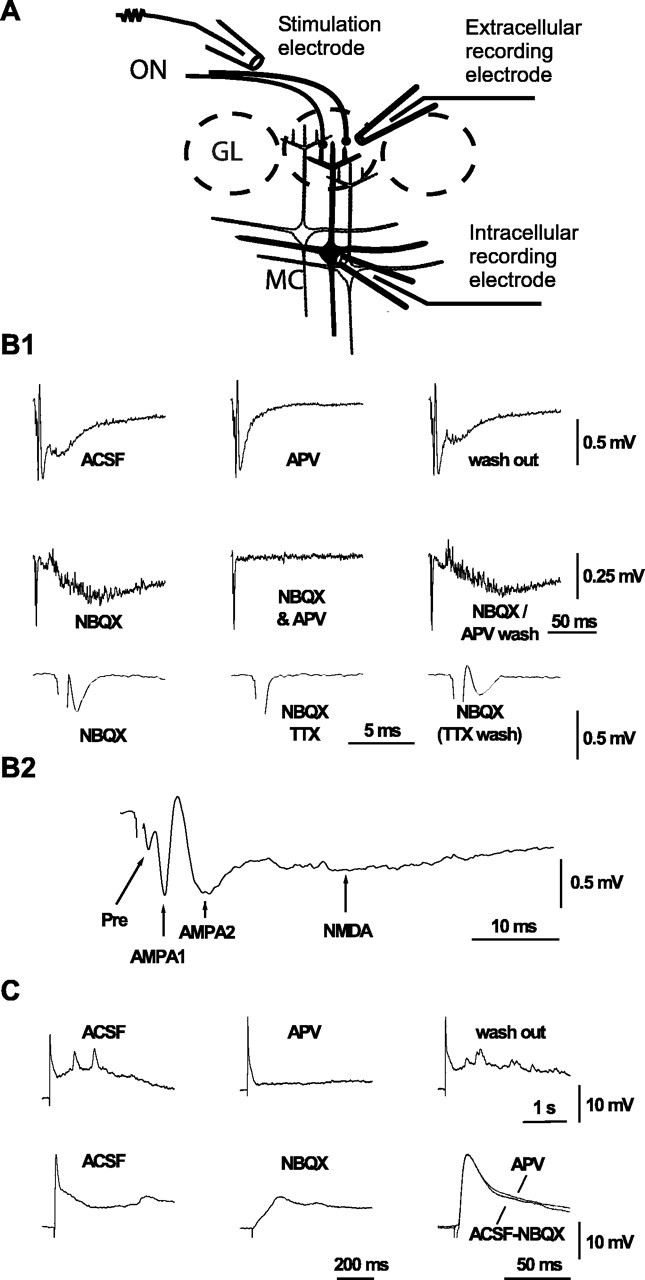
Synaptic responses evoked by ON stimulation in olfactory glomeruli and mitral cells. A, Scheme of the recording configurations for glomerular field potentials, intracellular recordings from mitral cells, and stimulation of the olfactory nerve. GL, Glomerular layer. B, Glomerular field potentials (fEPSPs) evoked by ON stimulation. B1, The first and second rows show successive recordings in control saline (ACSF), after adding 50 μm d-APV (APV), after washout of d-APV, after adding 20 μm NBQX, after supplementing NBQX with d-APV, and after returning to NBQX (NBQX/APV wash). The third row shows the initial fEPSP component (note expanded time scale) in the presence of NBQX, after adding 1 μm TTX, and after washout of TTX. B2, Allocation of fEPSP components to presynaptic action potentials (Pre), two AMPAR-mediated postsynaptic components (AMPA1 and AMPA2), and a slow NMDAR-mediated component (NMDA). C, EPSPs induced by test stimulus to ON and recorded from the soma of MCs. First row, Successive recordings in control saline (ACSF), after adding 50 μm d-APV (APV), and after washout of d-APV. Second row, Recording in control ACSF and after adding 20 μm NBQX (note expanded time scale). The traces to the right show superposition of an AMPAR-mediated EPSP recorded in d-APV and subtraction of the control recording by the recording in NBQX.
After baseline recordings of ON-induced glomerular fEPSPs with test pulses applied every 30 s, 100 stimulation pulses were delivered at 5 Hz and test pulse intensity. Return to the test stimulation frequency revealed a depression of glomerular fEPSPs that initially exceeded 50% and then stabilized to a persistent depression of all postsynaptic fEPSP components (AMPA1 component, 92.3 ± 2%; AMPA2 component, 88.9 ± 3%; NMDA component, 87.1 ± 3% of baseline values at 29-31 min after tetanization; n = 10). In contrast, the presynaptic component of the glomerular fEPSPs remained stable (105 ± 5% of baseline). Matching results were obtained when ON-MC EPSPs were tested (Fig. 2B). In these experiments, the internal solution contained 3 mm QX-314 to block action potential generation in MCs. Tetanization of the ON depressed all components of the ON-MC EPSPs (EPSP peak amplitude, 90.5 ± 4% of baseline at 29-31 min; n = 13).
Figure 2.

Tetanization induces long-term depression at ON synapses. A, Top, Glomerular fEPSPs during baseline recording (a) and 30 min after tetanization at 5 Hz for 20 s (b). Bottom, Time course of glomerular fEPSPs. In this and all following figures, time of tetanization is indicated by a downward arrow. Symbols represent values (mean ± SEM; n = 10) of the presynaptic component (Pre), AMPAR-mediated components (AMPA1 and AMPA2), and NMDAR-mediated component (NMDA). B, Top, ON-MC EPSPs during baseline recording (a) and 30 min after tetanization (b). Bottom, Time course of EPSPs (mean ± SEM; n = 13).
To examine whether the 5 Hz/100 pulse stimulation protocol induced a saturating amount of ON-MC LTD, tetanic stimulation was applied twice with a 30 min interval (Fig. 3). The second tetanic stimulation did not induce further depression, demon-strating that saturation had occurred with the first tetanus. Notably, this experiment, along with the stable presynaptic signals in the fEPSP recordings, also demonstrated that the observed ON-MC LTD was not caused by deterioration of presynaptic or postsynaptic elements during tetanization. The depression of synaptic transmission induced by the 5 Hz/100 pulse stimulation lasted up to at least 2 h, a duration that justifies the term ON-MC LTD.
Figure 3.
ON-MC LTD saturates. Time course of glomerular fEPSPs during two tetanizations (downward arrows) at an interval of 30 min. Open triangles and filled circles represent values of presynaptic and AMPAR-mediated components, respectively (mean ± SEM; n = 5).
LTD at olfactory nerve synapses does not require activation of NMDA receptors or postsynaptic calcium signaling
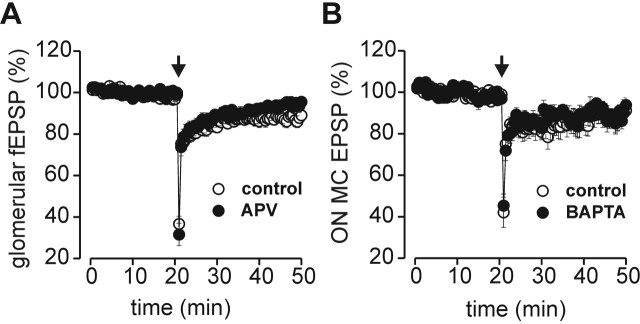
LTD at several synapses depends on the activation of NMDARs leading to postsynaptic Ca2+ elevation (Dudek and Bear, 1992; Lei and McBain, 2004; Liu et al., 2004). To test whether NMDAR activation is necessary for ON-MC LTD, tetanization was performed in the presence of 50 μm d-APV (Fig. 4A). ON-MC LTD was not prevented by blockade of NMDAR (control, 87.3 ± 3%, n = 17; d-APV, 91.9 ± 2%, n = 13; percentage of baseline at t = 20 min after tetanization). Postsynaptic Ca2+ elevation is prevented when the cell is loaded with the Ca2+ chelator BAPTA, a manipulation that blocks several forms of presynaptically and postsynaptically expressed LTD (Sakurai, 1990; Hansel and Linden, 2000; Lei and McBain, 2004). ON-MC LTD did not differ between cells recorded with 20 mm BAPTA-containing pipette solution and controls (control, 90.5 ± 4%, n = 13; BAPTA, 91.3 ± 4%, n = 10; percentage of baseline at t = 30 min) (Fig. 4B). The fact that postsynaptic Ca2+ elevation is not required for the induction of ON-MC LTD points toward a presynaptic mechanism.
Figure 4.
ON-MC LTD is not dependent on NMDAR and postsynaptic calcium signaling. A, Time course of LTD of glomerular fEPSPs in control ACSF (n = 17) and in the presence of 50 μm d-APV (APV; n = 13). B, Time course of ON-MC EPSPs recorded with control patch pipette solution (n = 13) and with 20 mm BAPTA-containing pipettes (n = 10). The arrows represent the time of tetanization. Error bars represent SEM.
LTD at olfactory nerve synapses is expressed presynaptically
One possible mechanism for presynaptically expressed LTD is the downregulation of voltage-dependent calcium channels that mediate glutamate release from ON terminals. To monitor presynaptic calcium signals in the ON, we applied the calcium indicator Calcium Green-1 dextran into the nasal cavities of P2-P5 mice (Wachowiak et al., 2000; Murphy and Isaacson, 2003). Two to six days later, these mice were used to prepare olfactory slices in which the ON fibers were selectively loaded with the calcium indicator. Figure 5A shows a fluorescence image of an indicator-loaded slice with two stimulation electrodes placed onto the ON. Single stimulus to the ON induced calcium signals with amplitudes that were largest within the glomeruli underlying the tip of the activated stimulation electrode, suggesting a higher calcium channel density in olfactory receptor cell terminals than in their axons (Fig. 5A).
Figure 5.
ON-MC LTD reduces calcium transients in ON terminals. A, Calcium signals in olfactory nerve terminals loaded with a calcium indicator. Left, Fluorescence image of a dye-loaded horizontal slice, arrangement of two stimulation electrodes (S1 and S2), and an outline of two regions of interest (ROI 1 and ROI 2). ONL, Olfactory nerve layer; GL, glomerular layer; EPL, external plexiform layer. The traces show calcium signals induced in ROI 1 (blue symbols) and ROI 2 (green symbols) by stimuli delivered via S1, S2, or S1 and S2. Above each trace is a color-coded map of peak calcium signal. B, Experimental setting as above. Color-coded maps show calcium signals evoked by test stimuli delivered to S1 and S2 during baseline recordings (left) and 10-20 min after tetanization of S1 (right). C, Time course of ON Ca2+ signals with test stimuli delivered to S1 and S2 and tetanization delivered to S1 from the experiment illustrated in B. D, Pooled data of ON Ca2+ signals at tetanized (filled symbols; mean ± SEM; n = 9) and control (open symbols; n = 9) ROIs. E, Time course of glomerular fEPSPs acquired during the same set of experiments.
Figure 5B illustrates an experiment in which ON-MC LTD was induced at one site, whereas a second site served as control. Induction of ON-MC LTD resulted in a marked depression of the ON calcium transient at the tetanized site; in contrast, the Ca2+ transient of the control site remained unchanged (Fig. 5B,C). On average, the depression of the calcium transient amounted to 18.6 ± 5.8% of control values at 10-20 min after tetanus (n = 5; controls, 3.5 ± 2.8%, n = 9) (Fig. 5D). Interestingly, not only the amplitude but also the time course of the depression of the calcium transient closely matched that of the fEPSPs recorded in the same set of experiments (Fig. 5E).
These experiments clearly demonstrate a presynaptic mechanism for expression of ON-MC LTD that involves the downregulation of presynaptic calcium influx.
However, presynaptic expression of ON-MC LTD does not exclude the possible participation of postsynaptic activity. To test whether EPSPs are required for induction of ON-MC LTD, we tetanized the calcium-indicator loaded ON when synaptic transmission was also blocked by NBQX (40 μm) and d-APV (50 μm) and monitored the ON calcium signals. LTD of the presynaptic calcium transients was not affected by the blockade of fast synaptic transmission (no tetanization in control ACSF, 95.1 ± 2.2%; no tetanization in NBQX and d-APV control, 99.3 ± 1.5%; tetanization in control ACSF, 84.1 ± 3.8%; tetanization in NBQX and d-APV, 82 ± 2.8% of baseline at 14-18 min after tetanization; n = 6-7) (Fig. 6A).
Figure 6.

ON-MC LTD does not require activation of AMPA or NMDA receptors and is antagonized by the mGluR antagonist MCPG. A, Time course of ON Ca2+ signals in control saline and in the presence of 40 μm NBQX and 50 μm d-APV (APV) with and without tetanization. B, Bath application of 1 mm MCPG (black bar; n = 14) had no effect on test fEPSPs but depressed the expression of ON-MC LTD compared with control (n = 14). C, Bath application of 1 mm MCPG (black bar; n = 14) had no effect on baseline Ca2+ signals but depressed the reduction of the ON Ca2+ signals during ON-MC LTD compared with control (n = 14). The arrows represent the time of tetanization. Error bars represent SEM.
Next, we tested whether mGluRs might be involved in the induction of LTD. LTD of glomerular fEPSPs was antagonized by (S)-a-methyl-4-carboxyphenylglycine (MCPG), a prototypic mGluR antagonist (control, 92.0 ± 2%, n = 17; MCPG, 97.9 ± 1%, n = 14; percentage of baseline at t = 25 min) (Fig. 6B). Importantly, MCPG also antagonized LTD of the presynaptic calcium signals when ionotropic glutamate receptors were blocked by 40 μm NBQX and 50 μm d-APV (control, 83.7 ± 3%, n = 6; MCPG, 95 ± 4.2% of baseline at t = 10-18 min after tetanization, n = 5) (Fig. 6C).
The above sets of experiments demonstrate a presynaptic expression mechanism of ON-MC LTD. Are the mGluRs that mediate induction of ON-MC LTD also localized presynaptically? If so, the mGluR1 that is expressed at high levels in olfactory glomeruli (Ferraguti et al., 1998; Heinbockel et al., 2004) would be the most obvious mGluR subtype to be considered. To test for functional expression of group I mGluRs in the ON terminals, we investigated the effect of (RS)-3,5-dihydroxyphenylglycine (DHPG), a group I mGluR agonist, on ON calcium signals. Bath application of DHPG (50 μm, 5 min) in the presence of 40 μm NBQX and 50 μm d-APV reversibly decreased ON resting calcium levels (by 1.1 ± 0.2% of baseline; n = 11) (Fig. 7A), indicating functional expression of group I mGluR in the ON.
Figure 7.
Induction of ON-MC LTD by direct activation of group I mGluRs and lack of requirement of D2 dopamine receptors. A, Time course of ON resting Ca2+ concentration during bath application of the group I mGluR agonist DHPG in the continuous presence of 40 μm NBQX and 50 μm d-APV (APV). Note that DHPG reversibly decreased resting Ca2+ concentration (n = 11). B, Time course of ON evoked Ca2+ signals (top trace) and baseline Ca2+ concentration (bottom trace) during bath application of DHPG. Note that DHPG-induced long-lasting reduction of synaptically evoked Ca2+ signals and transient reduction of baseline Ca2+ concentration. The inset shows ON Ca2+ signals recorded before (1) and after (2) application of DHPG. C, Bath application of 100 μm sulpiride, a D2 dopamine receptor antagonist (black bar; n = 5), had no effect on the expression of ON-MC LTD compared with control (n = 5). D, ON-MC LTD induced by DHPG. Time course of ON-MC EPSPs (top trace) and change in resting membrane potential (bottom trace) with bath application of DHPG. Note that DHPG-induced long-lasting reduction of ON-MC EPSPs and transient depolarization of the MCs. The inset shows an ON-MC EPSP recorded before (1) and 8 min (2) and 15 min (3) after application of DHPG. Error bars represent SEM.
We next tested DHPG on ON stimulation-induced presynaptic Ca2+ transients (Fig. 7B). Bath application of DHPG (100 μm) reduced ON stimulation-induced Ca2+ transients by 28 ± 2.6% of baseline (n = 8; at t = 30 min after DHPG application) and ON resting calcium levels by 1.3 ± 0.5% of baseline (n = 8; peak response) (Fig. 7B). Importantly, the depression of the evoked presynaptic calcium transient was long lasting, whereas the effect on resting calcium concentration reversed after washout of DHPG. ON stimulation (in the absence of blockers of fast synaptic transmission) or DHPG application may lead to release of GABA and dopamine from juxtaglomerular cells, causing a component of the transient or lasting depression of ON signals. In fact, it is well known that both GABAB and dopamine D2 receptors mediate a (reversible) depression of ON-MC EPSPs via presynaptic mechanisms (Hsia et al., 1999; Ennis et al., 2001). To investigate a possible involvement of these receptors in ON-MC LTD, DHPG-induced depression of ON stimulation-induced presynaptic Ca2+ transients was tested in the presence of the D2 dopamine receptor antagonist sulpiride (100 μm). Blockade of D2 receptors had no effect on the LTD of the presynaptic calcium signals (control, 73 ± 4%, n = 5; sulpiride, 78 ± 4%, n = 5) (Fig. 7C). LTD of ON fEPSP was also not affected in the presence of the GABAB antagonists saclofen (100 μm; n = 10; data not shown) or CGP55845 [(2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid] (5 μm; n = 15; data not shown).
Finally, if DHPG induced LTD of the presynaptic calcium signals, we would expect ON-MC EPSPs to be expressed after direct activation of group I mGluRs. The results illustrated in Figure 7D show that this is in fact the case. Application of DHPG (50 μm, 2 min) resulted in a reversible depolarization (5.6 ± 1 mV; n = 9) of the MCs and a lasting depression of EPSP amplitude (to 78 ± 12% of baseline levels at t = 24 min after DHPG application; n = 4).
Discussion
We found that synapses formed between the ON and MCs can express LTD after a brief tetanic stimulation. We concluded that the expression of ON-MC LTD is presynaptically located based on the depression of presynaptic calcium transients after induction of ON-MC LTD and further substantiated by the ineffectiveness of manipulations that usually affect postsynaptic mechanisms (blockade of NMDA receptors, postsynaptic calcium buffering).
Is ON-MC LTD restricted to synapses at MCs, or do synapses formed between the ON and periglomerular or tufted cells also express LTD? We performed intracellular recordings only from MCs. These other cell types are also activated together with MCs after ON stimulation and may be involved in the generation of the fEPSPs. Because ON-MC LTD is presynaptically expressed, it is likely that ON synapses formed with periglomerular or tufted cells are also affected by ON-MC LTD. In the young animals used in this study, the number of periglomerular cells is smaller than in adults in which synaptic plasticity between the ON and periglomerular cells may be more significant. Indeed, preliminary experiments indicate that glomerular fEPSPs also express LTD in young adult mice (P20-P30; n = 3; data not shown).
We did not systematically investigate whether stimulation protocols other than the 100 pulses at 5 Hz induce plasticity at ON→ MC synapses. We only explored brief high-frequency stimulation (100 pulses at 100 Hz), the most often used protocol for induction of LTP in the hippocampus. Our preliminary exploration (data not shown) suggested that high-frequency stimulation also induces LTD [rather than LTP as suggested in a previous report (Ennis et al., 1998)].
The observation that the degree of depression of presynaptic calcium transients was similar to that of postsynaptic signals was, at first glance, surprising. At many synapses in the CNS, the magnitude of presynaptic calcium transients is highly supra-linearly related to glutamate release following a power law with an exponent between three and four (Sabatini and Regehr, 1997; Murphy et al., 2004). This relationship would predict that 15% depression of the EPSP corresponds to only 4-5% reduction of the presynaptic calcium transient. However, it has been shown at synapses formed by the ON in olfactory glomeruli that the relationship between presynaptic calcium influx and glutamate release is almost linear (cooperativity coefficient, 1.5) (Murphy et al., 2004). High cooperativity increases the steepness of the relationship between the amplitude of the presynaptic calcium transient and transmitter release at calcium concentrations of the apparent dissociation constant. In contrast, relationships with low cooperativity render release probability more sensitive to variations in the amplitude of the calcium transient at calcium values that approach saturating levels under conditions of high cooperativity. The relationship between presynaptic calcium and probability of transmitter release is not known in detail for ON→ MC synapses. The high release probability of ON→ MC synapses (Murphy et al., 2004) suggests, however, that they operate under conditions in which the low cooperativity of Ca2+ for triggering glutamate release may render them particularly sensitive to both short-term (Aroniadou-Anderjaska et al., 2000; Ennis et al., 2001; Murphy and Isaacson, 2003) and long-term (as described here) modulation of presynaptic calcium channel activity.
ON-MC LTD and LTD of evoked ON Ca2+ transients were antagonized by MCPG, an antagonist for mGluRs. Group I mGluRs, in particular the subtype mGluR1, are expressed at high levels in olfactory glomeruli (Ferraguti et al., 1998; Heinbockel et al., 2004), and the specific group I mGluR agonist DHPG induced a long-lasting depression of both evoked ON calcium transients and ON-MC EPSPs. In addition, DHPG induced a reversible reduction of ON resting Ca2+ concentration and a reversible depolarization of MCs. The mGluRs that mediate the transient and the lasting effects of DHPG and induction of ON-MC LTD could be localized at the ON as well as postsynaptically (Heinbockel et al., 2004). Our result that the group I mGluR agonist DHPG decreased ON resting Ca2+ concentration in the absence of fast synaptic transmission (Fig. 7A) and with GABAB and dopamine D2 receptors blocked (Fig. 7C) strongly indicates a functional expression of group I mGluRs at ON terminals. The decrease in presynaptic calcium concentration is in line with previous well documented systems in which group I mGluRs mediate a downregulation of calcium channel activity (Faas et al., 2002; Kitano et al., 2003).
The presence of presynaptic group I mGluRs and a presynaptic expression mechanism do not rule out the possibility that ON-MC LTD is mediated via postsynaptic mGluR and retrograde messengers, but the simplest model that is compatible with our data is that presynaptic mGluR1 involve in induction, whereas presynaptic calcium channels mediate expression of ON-MC plasticity.
How does ON-MC LTD compare with other forms of LTD at central synapses? ON synapses resemble climbing fiber (CF) synapses at Purkinje cells with respect to their high release probability (Dittman and Regehr, 1998; Foster and Regehr, 2004; Murphy et al., 2004). CFs, like ON, express LTD (Hansel and Linden, 2000). CF-Purkinje cell LTD is also mGluR dependent, but, in contrast to ON-MC LTD, it is most likely mediated by postsynaptic changes (Hansel and Linden, 2000; Shen et al., 2002). Although downregulation of presynaptic Ca2+ channels is a classic target of short-term receptor-mediated presynaptic inhibition (Miller, 1990), this mechanism has been linked previously to LTD only at a few synapses such as prelimbic cortex→nucleus accumbens synapses. LTD at these synapses is mediated by group II mGluRs (Robbe et al., 2002), whereas the ON and MCs of the main olfactory bulb express only low levels of group II mGluRs (Sahara et al., 2001), and our pharmacological data demonstrate that LTD of ON presynaptic calcium transients and of ON→ MC synaptic transmission could be induced by direct activation of group I mGluRs. A DHPG-induced and presynaptically expressed form of LTD has been described in the CA1 region of hippocampal slices prepared from juvenile rats (Fitzjohn et al., 2001). However, there are also reports indicating postsynaptic induction and presynaptic expression mechanisms for the hippocampal DHPG-induced form of LTD (Watabe et al., 2002; Hou and Klann, 2004)
ON-MC LTD may be involved in early odor preference learning or central habituation (Wilson, 2000; Fletcher and Wilson, 2003; Yuan et al., 2003; Best and Wilson, 2004; McLean and Harley, 2004), but at present we have no positive evidence that would point in this direction. Electrophysiological mapping of MC activity has demonstrated an MC “odor output map” that differs from the ON “odor input map” in the tuning to specific odors that are represented by different glomeruli (Buonviso and Chaput, 1990; Mori et al., 1992; Yokoi, 1995; Luo and Katz, 2001). Activation of MC glomerular dendritic tufts, and hence MC output, depends on the balance between ON input and synaptic interactions between neighboring glomeruli (e.g., via short axon juxtaglomerular cells). It is therefore conceivable that ON-MC LTD shifts this balance toward a stronger influence of interglomerular interactions and thereby contributes to the difference between ON odor input map and MC odor output map (Buonviso and Chaput, 1990; Mori et al., 1992; Yokoi, 1995; Luo and Katz, 2001).
Additionally, ON-MC LTD may be involved in the establishment and maintenance of receptor cell-specific ON innervation of glomeruli. In young animals, there is considerable regression of ON fibers from aberrant innervations (Zou et al., 2004), and receptor cells are continuously replaced in animals of all ages (Schwob, 2002), a process that likely involves activity-dependent mechanisms.
Finally, whatever the natural conditions are under which ON-MC LTD occurs, plasticity at this synapse would implicate that glomerular odor images are, even at the level of the ON input, somehow less stable than generally assumed.
Footnotes
We thank all members of the Knöpfel laboratory for many helpful discussions and encouragement, as well as Dr. R. Joho for comments on a previous version of this manuscript.
Correspondence should be addressed to Dr. Thomas Knöpfel, Laboratory for Neuronal Circuit Dynamics, Brain Science Institute, The Institute of Physical and Chemical Research (RIKEN), 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan. E-mail: tknopfel@brain.riken.jp.
Copyright © 2005 Society for Neuroscience 0270-6474/05/254252-08$15.00/0
References
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT (2000) Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol 84: 1194-1203. [DOI] [PubMed] [Google Scholar]
- Best AR, Wilson DA (2004) Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci 24: 652-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso N, Chaput MA (1990) Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: eletrophysiological study using simultaneous single-unit recordings. J Neurophysiol 63: 447-454. [DOI] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R (1994) Allelic inactivation regulates olfactory receptor gene expression. Cell 78: 823-834. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG (1998) Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci 18: 6147-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF (1992) Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA 89: 4363-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Linster C, Aroniadou-Anderjaska V, Ciombor K, Shipley MT (1998) Glutamate and synaptic plasticity at mammalian primary olfactory synapses. Ann NY Acad Sci 855: 457-466. [DOI] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT (2001) Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86: 2986-2997. [DOI] [PubMed] [Google Scholar]
- Faas GC, Adwanikar H, Gereau RW, Saggau P (2002) Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: a differential role in acute depression of synaptic transmission and long-term depression. J Neurosci 22: 6885-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knöpfel T (1998) Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol 400: 391-407. [PubMed] [Google Scholar]
- Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SA, Collingridge GL (2001) A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol (Lond) 537: 421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA (2003) Olfactory bulb mitral-tufted cell plasticity: odorant-specific tuning reflects previous odorant exposure. J Neurosci 23: 6946-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Regehr WG (2004) Variance-mean analysis in the presence of a rapid antagonist indicates vesicle depletion underlies depression at the climbing fiber synapse. Neuron 43: 119-131. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall C (2003) Anatomic mapping of neuronal odor responses in the developing rat olfactory bulb. J Comp Neurol 455: 56-71. [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ (2000) Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron 26: 473-482. [DOI] [PubMed] [Google Scholar]
- Heinbockel T, Heyward P, Conquet F, Ennis M (2004) Regulation of main olfactory bulb mitral cell excitability by metabotropic glutamate receptor mGluR1. J Neurophysiol 92: 3085-3096. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E (2004) Activation of the phosphoinositide 3-kinase-Aktmammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24: 6352-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM (1999) Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol 82: 1082-1085. [DOI] [PubMed] [Google Scholar]
- Kitano J, Nishida M, Itsukaichi Y, Minami I, Ogawa M, Hirano T, Mori Y, Nakanishi S (2003) Direct interaction and functional coupling between metabotropic glutamate receptor subtype 1 and voltage-sensitive Cav2.1 Ca2+ channel. J Biol Chem 278: 25101-25108. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ (2004) Two loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci 24: 2112-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DM, Wang F, Lowe G, Gold GH, Axel R, Ngai J, Brunet L (2000) Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity. Neuron 26: 69-80. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304: 1021-1024. [DOI] [PubMed] [Google Scholar]
- Luo M, Katz LC (2001) Response correlation maps of neurons in the mammalian olfactory bulb. Neuron 32: 1165-1179. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW (2004) Olfactory learning in the rat pup: a model that may permit visualization of a mammalian memory trace. NeuroReport 15: 1691-1697. [DOI] [PubMed] [Google Scholar]
- Miller RJ (1990) Receptor-mediated regulation of calcium channels and neurotransmitter release. FASEB J 4: 3291-3299. [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R (1996) Visualizing an olfactory sensory map. Cell 87: 675-686. [DOI] [PubMed] [Google Scholar]
- Mori K, Mataga N, Imamura K (1992) Differential specificities of single mitral cells in rabbit olfactory bulb for a homologous series of fatty acid odor molecules. J Neurophysiol 67: 786-789. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Isaacson JS (2003) Presynaptic cyclic nucleotide-gated ion channels modulate neurotransmission in the mammalian olfactory bulb. Neuron 37: 639-647. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS (2004) Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci 24: 3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ (2002) Role of P/Q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci 22: 4346-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG (1997) Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci 17: 3425-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara Y, Kubota T, Ichikawa M (2001) Cellular localization of metabotropic glutamate receptors mGluR1, 2/3, 5 and 7 in the main and accessory olfactory bulb of the rat. Neurosci Lett 312: 59-62. [DOI] [PubMed] [Google Scholar]
- Sakurai M (1990) Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc Natl Acad Sci USA 87: 3383-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE (2002) Neural regeneration and the peripheral olfactory system. Anat Rec 269: 33-49. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H (2003) Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science 302: 2088-2094. [DOI] [PubMed] [Google Scholar]
- Shen Y, Hansel C, Linden DJ (2002) Glutamate release during LTD at cerebellar climbing fiber-Purkinje cell synapses. Nat Neurosci 5: 725-726. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K (2004) Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol 92: 2413-2427. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R (1994) Topographic organization of sensory projections to the olfactory bulb. Cell 79: 981-991. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB (2001) Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723-735. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Zochowski M, Cohen LB, Falk CX (2000) The spatial representation of odors by olfactory receptor neuron input to the olfactory bulb is concentration invariant. Biol Bull 199: 162-163. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Carlisle HJ, O'Dell TJ (2002) Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol 87: 1395-1403. [DOI] [PubMed] [Google Scholar]
- Wilson DA (2000) Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol 84: 3036-3042. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S (1995) Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92: 3371-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knöpfel T (2002) Optical imaging of odor preference memory in the rat olfactory bulb. J Neurophysiol 87: 3156-3159. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH (2003) Mitral cell beta1 and 5-HT2A receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem 10: 5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S (2004) Postnatal refinement of peripheral olfactory projections. Science 304: 1976-1979. [DOI] [PubMed] [Google Scholar]