Abstract
Little is known about activation changes reflecting overlearning, i.e., extensive motor training beyond asymptotic performance. Here we used functional magnetic resonance imaging to trace the neural shifts from an initial to a skilled (learning) and finally overlearned stage (automatization). Scanning occurred before training (PRE) and after 1 (MID) and 2 weeks (POST) of intensive practice on a new bimanual coordination task (>10,500 cycles). Kinematics revealed major improvements between PRE and MID sessions, whereas MID to POST session performance leveled off, indicative of learning and automatization, respectively. Imaging findings showed that activation decreased in bilateral opercular areas, bilateral ventrolateral prefrontal cortex, the right ventral premotor and supramarginal gyrus, and the anterior cingulate sulcus during the learning stage and in the supplementary motor area during the automatization stage. These changes are hypothesized to reflect decreases in attention-demanding sensory processing, as well as suppression of preferred coordination tendencies as a prelude to acquiring new coordination modes. Conversely, learning-related increases were observed in the primary motor cortex (M1), posterior cingulate zone (PCZ), putamen, and right anterior cerebellum. Importantly, both M1 and PCZ activation decreased again to initial level (PRE) during automated performance (POST). Only the putamen and anterior cerebellum remained more activated across both learning and automatization stages, supporting their crucial role in long-term motor memory formation for coordination tasks.
Keywords: fMRI, motor-skill automatization, bimanual coordination, primary motor cortex, cerebellum, basal ganglia, anterior cingulate zone
Introduction
Motor skill acquisition is characterized by three main phases: a highly attention-demanding early phase, an intermediate phase characterized by more established performance levels, and an automatic phase (Fitts and Posner, 1967; Schneider and Shiffrin, 1977; Schmidt and Lee, 1999). Both accuracy and stability of the to-be-learned task improve rapidly across the early phase and then advance more gradually until asymptotic performance is reached. Extended practice beyond this point is defined as the automatization or overlearning phase, which is characterized by feedforward instead of feedback control (Wolpert et al., 1998; Miall et al., 2001), allowing the performer to divert attention to other tasks (Schaffer, 1975; Nissen and Bullemer, 1987; Duncan, 1995; Passingham, 1996).
Functional magnetic resonance imaging (fMRI) has shown that skill acquisition is accompanied by neuroplastic changes associated with a shift in cortical-subcortical interactions (Jueptner et al., 1997a,b; Van Mier, 2000; Doyon and Ungerleider, 2002; Debaere et al., 2004). However, there is still controversy surrounding the precise nature of these shifts in neural activation, which may be attributable to several reasons: types of tasks studied, the amount of practice provided, etc. In this respect, different models have been specified. Doyon and colleagues defined a shift from cortico-cerebellar toward cortico-striatal networks during motor sequence acquisition (Doyon et al., 2002; Penhune and Doyon, 2002), whereas the converse evolution was observed during motor adaptation paradigms in which performers are faced with changing dynamical environments (Shadmehr and Holcomb, 1999; Doyon et al., 2003). Conversely, Hikosaka and colleagues stressed the involvement of two independent cortico-subcortical networks, involving portions of both loops during early as well as later learning (Sakai et al., 1998; Hikosaka et al., 1999a,b, 2002).
Whereas the majority of studies investigated activation changes during initial learning, little is known about the neural basis of the intermediate and overlearning phases. Karni et al. (1995, 1998) studied extended practice up to a performance plateau and showed an increased primary motor cortex representation of the trained sequence. To our knowledge, however, no study has explicitly assessed the whole-brain neural correlates of overlearning beyond asymptotic performance. Furthermore, whereas previous studies focused primarily on unimanual task learning, we investigated whether bimanual learning would activate a distinct neural network. Behavioral studies have shown that the principles governing bimanual coordination are not mere extrapolations of those underlying unimanual performance (Swinnen, 2002). Therefore, we hypothesized a brain network to be specifically related to the acquisition of new coordination patterns.
In the present study, we explored the neural shifts across a complete learning process, i.e., from the initial stage to advanced levels of automaticity beyond asymptotic performance. We used a bimanual coordination task requiring cyclical movements with a 1:2 frequency ratio. Previous behavioral work demonstrated that this task is associated with a high degree of sensorimotor processing and spatiotemporal integration, requiring substantial practice to attain automaticity (Swinnen et al., 1997). Subjects were scanned three times across 2 weeks of intensive practice on the bimanual task, i.e., from an error-prone stage, over a skilled toward a highly automated performance stage. A dual-task paradigm was used to ensure full automatization.
Materials and Methods
Participants
Eleven right-handed (Oldfield, 1971) subjects (five women and six men; 23.9 ± 1.58 years of age) participated in the experiment. All participants were naive with respect to the experimental paradigm. None of them was extensively involved in music or dance courses or had a history of neurological or psychiatric disease. The protocol was approved by the local ethical committee of Katholieke Universiteit Leuven, Belgium. Subjects provided written informed consent before the experiment and were paid for participation.
Experimental task and setup
Participants, lying supine with supported forearms, were instructed to produce a complex bimanual coordination pattern requiring cyclical flexion-extension movements of both wrists simultaneously. The goal was to achieve a perfect 1:2 frequency ratio between both wrists by moving the dominant right wrist exactly twice as fast as the left one. Compared with the sequencing and adaptation tasks that have been used predominantly in previous work, the present coordination task has some unique features: it combines successive as well as simultaneous motor elements into an integrated command structure. This is not a trivial matter, because it has been demonstrated that the principles governing interlimb coordination tasks are not a mere extrapolation from those that have been observed in single-limb tasks (Swinnen, 2002). This refers to constraints that are unique to interlimb coordination and that reflect attempts of the CNS to reduce control complexity.
All movements were metronome paced (Korg DTM-12). Subjects were required to complete an entire movement cycle (flexion-extension-flexion) with the dominant right hand on every beat (2.1 Hz) and with the nondominant limb on every second beat (1.05 Hz). They were instructed to move both wrists continuously throughout each trial at the paced frequency. Wrist-hand orthoses restricted movements to flexion-extension and prevented compensation from adjacent joints. The frictionless axes of the orthoses were aligned to the anatomical wrist axes. Angular displacements were registered by means of non-ferromagnetic high-precision shaft encoders (2048 pulses per revolution, sampling frequency of 100 Hz), fixed to the movement axes of both orthoses.
This new pattern was trained and performed under two conditions: without (Bim) and with (BimVfb) augmented on-line visual feedback. During the Bim condition, no visual information about the movement was made available. During the BimVfb condition, concurrent visual feedback was provided. Normal vision of the hands was prevented in both conditions. In the present paper, we will not consider the visually guided movements but will instead focus on the somatosensory guided conditions.a
All templates during scanning were displayed by means of a liquid crystal display projector (1280 × 1024 pixels; Barco 6300; Barco, Kortrijk, Belgium) and projected onto a mirror, placed in front of the head. During training, a personal computer screen was used for projection.
Procedures
Subjects were scanned before training (PRE), and after a first (MID) and second (POST) week of intensive practice. During each training week, 4 consecutive days of intensive bimanual practice were provided. Table 1 gives an overview of the experimental order.
Table 1.
Schematic overview of the experimental order (for details, see Material and Methods)
|
Scanning |
Training |
Scanning |
Training |
Scanning |
|---|---|---|---|---|
| PRE | Days 1-4 | MID | Days 5-8 | POST |
| Trials 1...40 | Trials 1...40 | |||
| Bimanual trials with and without augmented feedback alternated | Bimanual trials with and without augmented feedback alternated | |||
| 6 conditions | 6 conditions | 6 conditions | ||
| × | × | × | ||
| 3 repetitions per run | Performance test: | 3 repetitions per run | Performance test: | 3 repetitions per run |
| 5 trials: bimanual | 5 trials:bimanual | |||
| 8 runs | 5 trials: two-back | 8 runs | 5 trials: two-back | 8 runs |
|
|
5 trials: dual-task (two-back + bimanual) |
|
5 trials: dual-task (two-back + Bim) |
|
Scanning. The following conditions were performed during scanning: (1) the bimanual 1:2 frequency task (Bim), (2) flexion-extension motions with the nondominant left hand [unilateral left (UniL)] at 1.05 Hz, (3) flexion-extension movements with the dominant right hand [unilateral right (UniR)] at 2.1 Hz, and (4) the baseline rest condition. Two additional conditions were administered, but these will not be discussed further: (5) the bimanual 1:2 frequency task with on-line visual feedback (BimVfb), and (6) observing visual feedback information without producing any movement (VisFb). These will be excluded from all analyses reported below.a
All conditions were paced at 126 beats per minute. In conditions 1-3, subjects had to rely on proprioceptive information about ongoing movements because vision of the hands and arms was prevented at all times. We scanned both the bimanual task and its respective unimanual subtasks to assess the coordination effort (see below).
Each condition lasted 28.5 s and was announced by a template on the screen, remaining visible for 3 s. Subjects were instructed to switch conditions as soon as this template disappeared. To avoid confounding eye movements across conditions, subjects were instructed to keep their eyes open at all times and to fixate a cross, projected in the middle of their visual field. Head movements were restricted by an individually fitted bite bar.
Training. In between the first (PRE) and second (MID) as well as the second and third scan session (POST), the 1:2 coordination pattern was extensively trained with and without visual feedback, alternated from trial to trial.a Each day, subjects completed 45 bimanual trials (duration, 30 s), resulting in >10,500 complete bimanual cycles across the 8 d training period.
To assess attentional demands and degree of automatization of the performed bimanual task, a dual-task paradigm was administered during the training days. This was based on the hypothesis that full automatization is only achieved when a second task, accessing the general pool of mental resources, can be performed simultaneously with the primary (bimanual) task without causing interference (Nissen and Bullemer, 1987; Duncan, 1995; Passingham, 1996). The secondary task consisted of a random sequence of 20 templates, each showing either a horizontal or a vertical line, projected continuously during the duration of the entire trial (30 s) at a rate of 0.66 Hz. Subjects had to compare each projection with the one they had seen two projections earlier (two-back task), and they were to verbalize the outcome of their comparison (same or different). Performance on this two-back task was assessed in isolation (two-back) as well as in combination with the bimanual condition without visual feedback (dual-task). The final five trials of each practice day were performed under the dual-task conditions, whereby subjects were clearly instructed to give priority to the two-back task. As such, shifts in attention between both subtasks were prevented, and learning progress was reflected in the kinematics, which were most sensitive to capture learning-related changes.
Kinematic analysis
The cycle duration (CD), defined as the time elapsing between two successive peak extension positions, was calculated for each limb separately. Target CD values were 476 and 952 ms for the right and left limb, respectively. The cycle duration ratio (CDR) was obtained by dividing the averaged left arm cycle duration by the averaged right arm cycle duration for each trial and condition. Any deviation from a 0.5 ratio reflected deviations from perfect 1:2 frequency locking. Consequently, the absolute deviation from the required CDR (i.e., 0.5) was calculated (CDR error) to assess the accuracy of the produced movement. To obtain a baseline measure of independent performance, free of mutual interference, the CDR error for the unimanual scan conditions was computed.
Subjects were instructed to move with consistent amplitudes (Amp) across sessions, days, and conditions. To reveal possible confounds of amplitude-related brain activation differences between conditions, peak-to-peak amplitude values were calculated per cycle and averaged within and across trials for each of the different scanning conditions.
Repeated-measures ANOVAs were conducted on the training and scanning kinematics. For the training data, the averaged values of the five final trials pertaining to single-task (Bim) and dual-task (Bim + two-back) performance, collected at the end of each day, were used. Statistical analysis for the CDR errors consisted of a 2 × 8 (mode × day) ANOVA. Mode referred to single and dual-task bimanual performance, and day referred to the 8 training days. Errors on the mental task were calculated to control whether attention was paid to the two-back task across dual-task performance. Scores were averaged across each day and subjected to a2 × 8 (mode × day) ANOVA. Mode referred here to performance on the mental task (two-back) and dual-task (Bim + two-back) conditions.
Statistical analysis for the CDR and Amp values during scanning consisted of a 3 × 2 ANOVA with the factors session (PRE, MID, and POST) and condition (Uni and Bim). Both parameter values were averaged for each condition across all repetitions within each session (24 repetitions per condition).
Scan acquisition and imaging analysis
All fMRI measurements were accomplished on a 1.5 T MR scanner (Siemens; Sonata, Erlangen, Germany) using a quadrature head coil. For anatomical details, a three-dimensional high-resolution T1-weighted image was obtained first [MPRAGE (magnetization-prepared rapid gradient echo); repetition time (TR), 11.4 ms; echo time (TE), 4.4 ms; time interval, 300 ms; field of view (FOV), 256 mm; matrix, 256 × 256 mm2; slab thickness, 160 mm; 160 slices). Subjects then performed eight scanning runs, each containing 183 gradient-echo echo-planar T2-weighted functional images (TR, 2840 ms; TE, 50 ms; FOV, 192 mm; matrix, 64 × 64 mm2; slice thickness, 4 mm; 36 sagittal slices). The first three scans from each run were deleted to ensure steady-state magnetization at the start of the task. Eight runs were completed within each session. During one run, three repetitions of the six conditions were performed, each condition containing 10 whole-brain images. The order of the conditions was randomized across runs.
Imaging data were analyzed with SPM99 (http://www.fil.ion.ucl.ac.uk/spm; Wellcome Department of Cognitive Neurology, London, UK). For each subject, functional images were realigned to the first volume of each run to correct for head movements. After coregistering to the anatomical image, the structural images were spatially normalized to the standardized reference frame (Talairach and Tournoux, 1988) by using the representative Montreal Neurological Institute (MNI) template. All functional images were subsampled to a voxel size of 2 × 2 × 2 mm and smoothed with a Gaussian kernel of 8 mm full-width at half-maximum for purposes of group analysis. Condition-specific effects were estimated using a general linear model (Friston et al., 1994, 1995) with a boxcar function, convolved with the standard SPM99 hemodynamic response function. An appropriate high-pass filter (cutoff period at 340 s) was applied to remove low-frequency drifts. Realignment parameters (translation and rotation in x, y, z dimension) were included as covariates of no interest to correct for head movements.
For the first-level analysis, comparisons of interest were calculated as linear contrasts for each subject (n = 11) and run (n = 8) separately, resulting in 88 statistical parametric maps. For the second-level group analysis, mixed-effects analyses were performed. Therefore, the general movement network for bimanual coordination was determined, containing all voxels that were activated in at least one of the three sessions for the bimanual versus rest (Bim-R) comparison. Subsequently, this network was used as an inclusive mask to restrict the main analyses to areas that were activated by the task. To quantify learning-related changes, we were interested in voxels exhibiting (1) a significant between-session change in their response to the bimanual condition, and (2) larger between-session changes in the response to the bimanual than to the unimanual conditions. For this purpose, a conjunction (Price and Friston, 1997) was calculated between the bimanual activation changes [e.g., (Bim-R)pre > (Bim-R)mid] and the bimanual versus unimanual changes [i.e., (Bim-R)pre - (Bim-R)mid > (UniL-R)pre + (UniR-R)pre - (UniL-R)mid + (UniR-R)mid]. This procedure ensures that the identified regions play a critical role in integrating both limb motions into a single movement pattern while preventing modulations, attributable to changes in the unimanual conditions, from being picked up. As such, the extra neural activity required for the bimanual compared with the average of both unimanual performance levels (referred to as the coordination effort) could be extracted across learning. All remaining activation decreases and increases among the three sessions were calculated in a similar way. We expected PRE-MID changes to mainly reflect learning and MID-POST changes to reflect overlearning.
All areas reaching significance on cluster level (p < 0.05), after correction for multiple comparisons, with a cluster level (k) of >10 voxels, will be reported. For large clusters, we also report MNI coordinates of significant activation maxima on voxel level (p < 0.05, after correction for multiple comparisons).
To scrutinize the time course of learning-related changes, we present the estimated hemodynamic response for the Bim-R contrast in the line plots of Figures 2 and 3. Therefore, we estimated the percentage signal change for the peak activation within each significant cluster across all three sessions by the following formula: [β(Bim) - β(Rest)] × 100/β(constant term). Hereby, β represents the condition-specific regressor derived from the general linear model. Note that this procedure yields only a rough estimation of the local percentage signal change of the movement versus rest activation for the most significant voxel.
Figure 2.
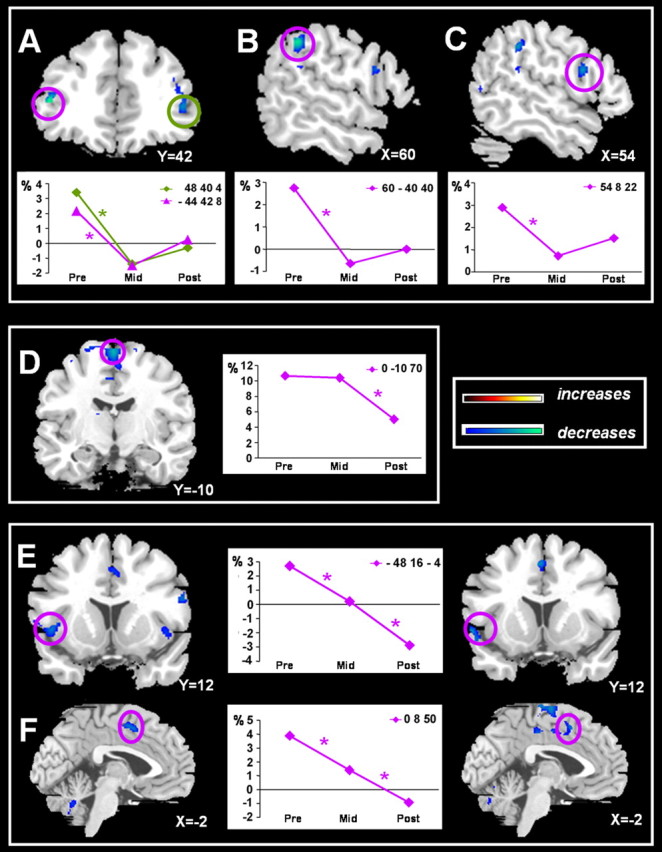
Anatomical localizations of significant coordination effort-related decreases in activation during learning and automatization phases on a representative normalized brain. Exclusively learning-related decreases were found in the bilateral ventrolateral prefrontal cortex (A), right supramarginal gyrus (B), and right ventral premotor area (C). Automatization-related changes were exclusively observed in the SMA-proper (D). Decreases across both phases were found in the left opercular area (E) and anterior cingulate sulcus (F). Each graph represents the estimated signal changes for the most significant voxel in each area for the Bim-R contrast across all three scan sessions. An asterisk indicates a significant decrease for Bim > UniL + UniR contrast at p < 0.05 after correction for multiple comparisons, with k > 10.
Figure 3.
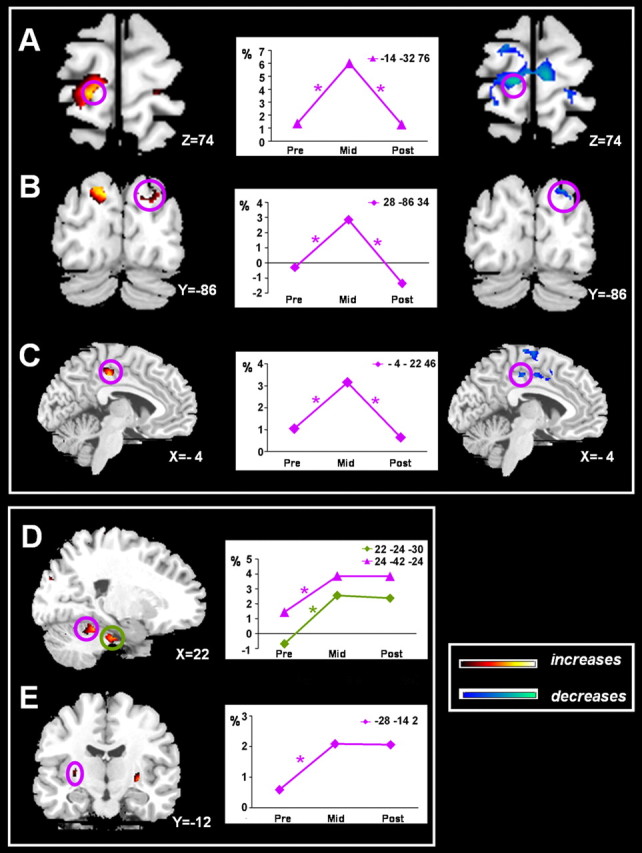
Anatomical localizations of coordination effort-related increases in activation across learning on a representative normalized brain. Temporary increased activation was observed in the left primary motor cortex/dorsal premotor area (A), right superior occipital area (B), and left posterior cingulate sulcus (C). Sustained increases compared with the PRE level were found in the right cerebellar lobule III and V (D) and left putamen/globus pallidus (E). Each graph represents the estimated signal changes for the most significant voxel in each area for the Bim-R contrast across all three scan sessions. An asterisk indicates a significant change for Bim > UniL + UniR at p < 0.05 after correction for multiple comparisons, with k > 10.
Results
Kinematic data
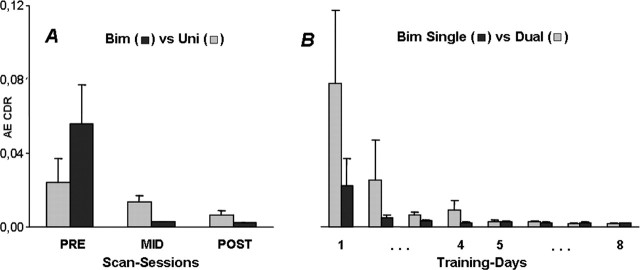
A distinction is made between the kinematics obtained during scanning and during the training sessions, as discussed below. The scanning data (Fig. 1A) show a significant PRE-MID improvement on the bimanual task, whereas small and nonsignificant changes occurred on the unimanual tasks. No additional MID-POST bimanual improvements occurred. Accordingly, statistics revealed a significant session × mode interaction (F(2,16) = 6.16; p = 0.01). The significant performance difference between both conditions during the PRE session disappeared across the MID and POST sessions.
Figure 1.
Changes in mean absolute error (AE) with respect to the required CDR across scanning (A) and training (B) sessions, respectively (see Results). A, Error scores are depicted across the three scanning sessions for bimanual as well as unimanual (control) conditions. B, Each training day is represented by the averaged absolute error of the final five bimanual trials as performed under single-task (bimanual) and dual-task (bimanual + two-back) conditions. Scanning occurred before day 1, after day 4 (first week of training), and after day 8 (second week of training). For additional details, see Materials and Methods.
Peak-to-peak amplitude of unimanual and bimanual trials did not differ from each other (F(1,8) = 0.04; p > 0.05) and remained stable across the three scanning sessions (F(2,16) = 2.84; p > 0.05). Therefore, it can be argued that the observed changes in brain activity reflect learning-related neuroplasticity rather than variations of secondary motor output parameters such as amplitude (Waldvogel et al., 1999) or force (Dettmers et al., 1995).
The kinematic training results (Fig. 1B) supported the performance patterns obtained during scanning. During single-task performance conditions, major improvements were observed across the first days of training; by day 4, a performance plateau was reached. Full automatization was not achieved at this stage, as inferred from the differences in CDR error between the dual-task and single-task conditions. This difference disappeared across the second training block, suggesting that, after 2 weeks of training, an overlearned stage was reached. Statistics, indeed, revealed a significant day × mode interaction (F(7,70) = 2.22; p < 0.05).
To ensure that the kinematic improvements across dual-task performance conditions were not merely attributable to changes in attention allocation between both tasks, errors on the secondary (cognitive) task during both dual-task and single two-back performance conditions were compared. No significant differences occurred across modes. Thus, we can conclude that attention was directed toward the cognitive task during dual-tasking and that the aforementioned CDR error scores truly reflected learning-related differences.
Imaging data
General bimanual network
To determine the general bimanual coordination network, we determined the regions responding more strongly to the bimanual compared with the rest condition across the three scanning sessions (supplemental Table 1, available at www.jneurosci.org as supplemental material). We identified a typical motor network, including the primary sensorimotor hand area along the central sulcus (S1/M1), ventral and dorsal precentral areas, supplementary (SMA) and cingulate motor areas, the lateral prefrontal cortex, frontal and parietal opercular regions, the supramarginal gyrus, the middle temporal cortex (hMT/V5+), (pre)cuneus, thalamus, basal ganglia, and cerebellum. All of these areas were activated bilaterally, with exception of the hMT area, reaching significance only in the right hemisphere. All areas were significantly activated at p < 0.05 with k > 10, after correction for multiple comparisons.
Activation changes related to coordination effort
Within the aforementioned general coordination network (threshold at p < 0.001, uncorrected), coordination effort differences among the scan sessions were calculated. These reflect the neural shifts in activation associated with the decreasing behavioral effort (necessary to produce the coordination task successfully) as was revealed by the kinematic results. In accordance with the behavioral data (Fig. 1), we distinguished between PRE-MID activation differences, reflecting learning-related changes, and MID-POST activation differences, reflecting overlearning-related (automatization) changes. Decreases (PRE > MID, MID > POST) and increases (MID > PRE, POST > MID) will be discussed separately. All areas were significantly activated at p < 0.05 with k > 10, after correction for multiple comparisons, unless stated differently.
Decreases
Three different patterns of activation decreases across pre, mid, and post scanning were distinguished, as discussed below (Table 2).
Table 2.
Locations of activation peaks (MNI coordinates) and Z scores for areas showing coordination effort-related decreases in activation
|
Brain region |
Peak activation coordinates |
BA |
Z values |
Progress |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
|
x
|
y
|
z
|
|
|
|
|||
| Exclusive learning-related decreases (PRE > MID) | |||||||||
| Ventrolateral prefrontal cortex | |||||||||
| R inferior frontal sulcus | 48 | 40 | 4 | BA 45 | 4.91 | PR > MI | |||
| L | −44 | 42 | 8 | BA 45 | 5.61 | PR > MI | |||
| Ventral premotor area | |||||||||
| R inferior precentral sulcus/gyrus | 54 | 8 | 22 | BA 44 | 4.7 | PR > MI | |||
| Inferior parietal cortex | |||||||||
| R supramarginal gyrus | 60 | −40 | 40 | BA 40 | 5.48 | PR > MI | |||
| Exclusive automatization-related decreases (MID > POST) | |||||||||
| Superior frontal cortex | |||||||||
| SMA | 0 | −10 | 70 | BA 6 | 5.18 | MI > PO | |||
| Learning- and automatization-related decreases (PRE > MID and MID > POST) | |||||||||
| Superior frontal cortex | |||||||||
| ACZ (cingulate sulcus)* | 0 | 8 | 50 | BA 24 | 4.34 | PR > MI | |||
| 4.4 | MI > PO | ||||||||
| Opercular/insular area | |||||||||
| L operculum* | −48 | 16 | −4 | 4.35 | PR > MI | ||||
| 4.55 | MI > PO | ||||||||
| R insula |
38 |
20 |
4 |
|
5.77 |
PR > MI |
|||
p < 0.05 after correction for multiple comparisons. L, Left; R, right; PR > MI, learning-related decrease; MI > PO, automatization-related decrease. Asterisks indicate significance for conjunction of both progresses.
Areas showing exclusively learning-related activation decreases (PRE > MID)
Learning-related activation decreases (PRE > MID) were mainly observed in the frontal-precentral region of the right hemisphere. Significant effects were found in the right inferior precentral gyrus (Fig. 2C), corresponding to the ventral premotor area (PMv) (Geyer et al., 2000; Picard and Strick, 2001), the right supramarginal gyrus (Fig. 3B), and the bilateral inferior frontal gyrus (Fig. 2A), corresponding to the ventrolateral prefrontal cortex (VLPFC) (Petrides, 2002). All three areas showed a large response initially, but this effect disappeared as soon as the movement pattern was learned. As such, these areas were particularly recruited when coordination effort was high. As soon as this effort was reduced by training, these regions were no longer activated to an extra extent.
Areas showing exclusively automatization-related activation decreases (MID > POST)
Despite the lack of changes in behavior (CDR error) from MID to POST session, neural activation decreases occurred across the automatization phase in one area, i.e., the SMA-proper (Fig. 2D). Whereas this area was substantially activated during bimanual execution across the novel and learned stage, this activation robustly decreased during automated performance.
Areas showing learning- and automatization-related activation decreases (PRE > MID and MID > POST)
The anterior cingulate zone (ACZ) showed a continuous decrease in activation across learning (PRE > MID) and automatization (MID > POST). The left opercular, insular area showed a similar decrease in activation. For the right opercular, insular area, only a significant PRE-MID decrease in activation was observed.
To assess whether the decreases across both phases were located at the same spot, we calculated a conjunction between the following contrasts: (Bim-R)pre > (Bim-R)mid; (Bim-R)mid > (Bim-R)post; (Bim-R)pre - (Bim-R)mid > (UniL-R)pre + (UniR-R)pre - (UniL-R)mid + (UniR-R)mid; and (Bim-R)mid - (Bim-R)post > (UniL-R)mid + (UniR-R)mid - (UniL-R)post + (UniR-R)post. This analysis confirmed that the left operculum (Fig. 2E) and ACZ (Fig. 2F) exhibited a continuous activation decrease.
Increases
Temporary as well as sustained increases in activation were observed in distinct areas, as discussed below (Table 3).
Table 3.
Locations of activation peaks (MNI coordinates) and Z scores for areas showing coordination effort-related increases in activation
|
|
Peak activation coordinates |
|
|
|
||||
|---|---|---|---|---|---|---|---|---|
| Brain region |
x
|
y
|
z
|
BA |
Z values |
Progress |
||
| Temporary increased activation (MID > PRE and MID > POST) | ||||||||
| Central sulcus; M1 and PMd | ||||||||
| R | 20 | −32 | 76 | BA 4/6 | 4.06** | MI > PR | ||
| 4.36 | MI > PO | |||||||
| L* | −14 | −32 | 76 | BA 4/6 | 5.54 | MI > PR | ||
| 4.51 | MI > PO | |||||||
| Superior occipital area | ||||||||
| R (pre)cuneus* | 28 | −86 | 34 | BA 19 | 4.45 | MI > PR | ||
| 4.5 | MI > PO | |||||||
| L | −10 | −86 | 38 | BA 19 | 4.88 | MI > PR | ||
| 3.24** | MI > PO | |||||||
| Superior frontal cortex | ||||||||
| R PCZ (cingulate sulcus)* | 2 | −24 | 44 | BA 31 | 4.35 | MI > PR | ||
| 3.44** | MI > PO | |||||||
| L | −4 | −22 | 46 | BA 31 | 4.87 | MI > PR | ||
| 4.32 | MI > PO | |||||||
| Sustained increased activation (MID > PRE and POST > PRE) | ||||||||
| Basal ganglia | ||||||||
| R putamen/globus pallidus | 30 | −2 | −8 | 5.08 | MI > PR | |||
| L* | −28 | −14 | 2 | 4.47 | MI > PR | |||
| Cerebellum | ||||||||
| R cerebellar hemisphere: lobule III/IV* | 22 | −24 | −30 | 4.82 | MI > PR | |||
| R cerebellar hemisphere: lobule V*
|
24 |
−42 |
−24 |
|
4.95 |
MI > PR |
||
p < 0.05 after correction for multiple comparisons. L, Left; R, right; MI > PR, learning-related increase; MI > PO, automatization-related decrease. Single asterisks indicate significance for conjunction of both progresses; double asterisks indicate significance on an uncorrected level.
Areas showing temporary increases in activation
The left motor and premotor cortex along the central sulcus [M1 and dorsal premotor area (PMd)] showed a significant learning-related increase (MID > PRE) and a significant automatization-related decrease (MID > POST) in activation (Fig. 3A). This pattern was bilateral, although the learning-related increase of the right M1/PMd reached significance only when a more liberal threshold was applied (p < 0.001, uncorrected). The right (pre-)cuneus (Fig. 3B) and left posterior cingulate zone (PCZ) (Fig. 3C) exhibited a similar significant increase (MID > PRE) and subsequent decrease in activation (MID > POST). However, in the right PCZ and left (pre)cuneus, the automatization-related decreases reached significance only on uncorrected level (p < 0.001).
A conjunction analysis with the following contrasts was performed to test whether the PRE-MID increases and MID-POST decreases were located in the same cluster: (Bim-R)mid - (Bim-R)pre; (Bim-R)mid - (Bim-R)post; (Bim-R)mid - (Bim-R)pre > (UniL-R)mid + (UniR-R)mid - (UniL-R)pre + (UniR-R)pre; and (Bim-R)mid - (Bim-R)post > (UniL-R)mid + (UniR-R)mid - (UniL-R)post + (UniR-R)post. It was confirmed that M1/PMd, PCZ, and the precuneus exhibited only a temporary increase of activity (Fig. 3A-C).
Together, the bilateral M1/PMd, PCZ, and (pre)cuneus showed a strong tendency for temporary increased activation. In contrast to the ACZ (Fig. 2F), the left PCZ showed a robust PRE to MID session increase, followed by a decrease during the automatization phase. As such, both ACZ and PCZ ended up with a similar activation level for automated bimanual performance. Consequently, both areas seem involved in distinct phases during learning, but once the coordination pattern is established, their impact subsides.
Areas showing sustained increases in activation
On subcortical level, major increases from PRE to MID session were observed in right anterior cerebellar lobules III, IV, and V and bilateral putamen, including globus pallidus nuclei. Inspecting the time course of the blood oxygenation level-dependent response (Fig. 3D,E, line plots), it is observed that activity of these subcortical regions remained high during the POST session. To test this response pattern statistically, we performed an additional conjunction analysis between the following contrasts: (Bim-R)mid - (Bim-R)pre; (Bim-R)post - (Bim-R)pre; (Bim-R)mid - (Bim-R)pre > (UniL-R)mid + (UniR-R)mid - (UniL-R)pre + (UniR-R)pre; and (Bim-R)post - (Bim-R)pre > (UniL-R)post + (UniR-R)post - (UniL-R)pre + (UniR-R)pre. This analysis revealed that the right anterior cerebellar lobules III, IV, and V (Fig. 3D) and left putamen, including globus pallidus nuclei (Fig. 3E), were the only regions showing a sustained increased activation across learning. As such, only these regions became significantly more activated during trained bimanual performance.
Activation changes related to unimanual performance
Finally, we checked whether activation differences among sessions occurred for both limb movements separately. The general unimanual network was defined, including all voxels that were activated in at least one of the three sessions for the unimanual left (UniL) versus rest (UniL-R) or unimanual right (UniR) versus rest (UniR-R) comparison. Changes in unimanual performance-related activation across scan sessions were identified by applying a multiple regression analysis, masked with the general unimanual network [e.g., PRE-MID decreases for the UniR condition were defined as (UniR-R)pre > (UniR-R)mid]. All remaining decreases and increases were calculated similarly for the UniL and UniR conditions.
In contrast to the bimanual changes, minor effects were observed. Only for the UniR condition, the medial frontal gyrus [Brodmann area (BA) 46] showed a decrease from PRE to MID session, whereas the inferior temporal gyrus (BA 37) showed a temporary increase from PRE to MID session. As such, both kinematic and imaging results revealed minor changes across unimanual execution, supporting their status as appropriate control conditions for the bimanual coordination effort.
Discussion
We traced the neural changes associated with learning a new bimanual coordination task from an initial to overtrained performance phase. Kinematics revealed a typical learning curve with major improvements reaching asymptotic performance at the end of the first phase (PRE to MID). Additional automatization was obtained across the second phase (MID to POST), as indicated by the performer's increasing capability to successfully combine the bimanual coordination task with an attention-demanding secondary task (i.e., dual-task performance). As such, neural shifts between the PRE and MID session indicated learning-related changes, whereas MID-POST changes reflected overlearning or automatization-related changes. The modulations in cortical and subcortical activations across both stages of bimanual learning will be discussed below.
Changes in cortical activation
The high degree of parietal activation (BA 40) during initial performance decreased across learning and underscores the involvement of this region in sensory processing during visuospatial attention and proprioceptive discrimination (Meyer et al., 1991; Pardo et al., 1991; Jenkins et al., 1994). Both the PMv and VLPFC, densely interconnected with each other (Barbas and Pandya, 1987), showed a similar course. The VLPFC is known to exert a top-down control over sensory information processing by directing attention to the relevant stimuli (Kostopoulos and Petrides, 2002, 2003). These processes were crucial during novel performance to discover the new spatiotemporal relationship between both wrists. As soon as the exact spatiotemporal pattern was established, less spatial attention was required and activation levels dropped considerably.
Activation in left M1 was increased during the learning phase (PRE to MID). Enlarged M1 activation as a result of practice has been observed previously and is hypothesized to reflect the building of a specific motor representation (Karni et al., 1995, 1998; Nudo et al., 1996; Hikosaka et al., 1999a; Ungerleider et al., 2002). We presume that the increased M1 activity in our study was associated with establishing a specific spatiotemporal ordering of muscle commands for both wrists, as required for integrated pattern performance. Surprisingly, overtraining (MID to POST) was associated with bilateral decreases in M1 activation, such that initial (PRE, or even below initial) activation levels were finally obtained in both hemispheres. To our knowledge, such an activation course in M1 across learning has never been observed, presumably attributable to a lack of long-term practice studies with multiple scanning sessions. In this respect, it is noteworthy to bear in mind that >10,500 individual movement cycles were performed by the end of practice. Our results are nonetheless consistent with a decreased primary and secondary motor area activation in professional pianists (comparable with the automated stage) compared with non-musicians (comparable with the novel stage) (Jancke et al., 2000). The present findings imply that automatization is most likely associated with increased neural efficiency, resulting in decreased primary motor cortex activity.
The extra SMA-proper activation during bimanual performance remained high during learning but showed a sharp decrease across automatization. This dynamic change in activation is consistent with the proposed motor-inhibition role of this area (Toma et al., 1999). Indeed, high SMA activation during the initial phases of learning presumably reflected the suppression of preferred coordination modes, characterized by tight bimanual synchronization (Swinnen and Wenderoth, 2004). This was a prerequisite for adopting the new 2:1 frequency-locking mode, free of between-limb interference.
Although the ACZ has rarely been discussed in the context of motor learning studies, we found a continuous drop in activation, from the initial to overlearned stage. ACZ was presumably involved in monitoring the conflicts between preexisting and new coordination modes on a rather planning-related, higher-order level. The rostral part of the ACZ has been shown to fulfill a crucial role in conflict monitoring during cognitive-perceptual tasking, whereas slightly more caudal activation has predominantly been related to motor tasks (Picard and Strick, 1996; Botvinick et al., 1999; Barch et al., 2000; Casey et al., 2000; MacDonald et al., 2000). Here, we extend this conflict-monitoring function of the rostral ACZ from cognitive-perceptual paradigms to pure motor-related task assignments. It is hypothesized that, together with the SMA, the ACZ played a role in suppressing the preexisting preferred movement tendencies, to pave the road for performing new patterns of neural excitation, as was necessary to acquire the present multifrequency task successfully (Swinnen, 2002).
The decreased ACZ activation was initially accompanied by increased PCZ activation. A recent study identified similar shifts (Tracy et al., 2003) but could not resolve unambiguously whether the PCZ was involved in cognitive control or retrieval. The PCZ has indeed been associated with evaluative functions (Vogt et al., 1992), as well as with movement execution (Picard and Strick, 1996). The activation course observed in our study, however, tends to argue against the latter (retrieval) account because the learning-related increase in PCZ activation dropped again with overlearning. It is therefore conceivable that the PCZ was not necessarily involved in the long-term storage of motor skills but rather acted as a temporary evaluation system, monitoring performance at a different learning stage than the ACZ did (Posner and Petersen, 1990). Automated performance with minimal correction during overlearning led therefore to PCZ activation redundancy.
Interestingly, we observed extrastriate activation in the absence of visual feedback signals, confirming the visuospatial memory role of this area. Similar to PCZ, extrastriate activation was increased during the learning phase but diminished with overlearning, presumably for similar reasons. The secondary visual areas have indeed been associated with cognitive processes besides mere visual analysis (Moran and Desimone, 1985; Berman and Colby, 2002; Corbetta et al., 2002). As such, they have been associated with spatial working memory during delayed-response tasking (Postle and D'Esposito, 1999). During initial learning, subjects presumably relied on a visual image of the coordination pattern that was made available as augmented feedback during practice. However, with increasing automaticity, such mental image-supported guidance was no longer warranted.
The insular/opercular activation decreased across learning (bilaterally) and overlearning (left hemisphere). Its pronounced initial involvement can be associated with attention to metronome pacing (Platel et al., 1997; Thaut, 2003). As the pattern became more familiar, movements were triggered more internally, requiring less attention to external pacing via the metronome.
Summarized, it is hypothesized that decreased midline activation mainly reflected decreased inhibitory control at planning- and execution-related levels. Conversely, decreased insular/opercular and extrastriate activation pointed to a shift from external control with extensive sensory processing/integration to internalization of the highly automated coordination pattern. This provides neural evidence for a shift from feedback- toward feedforward-controlled execution.
Changes in subcortical activation
The anterior cerebellum and putamen showed major increases in activation from novel (PRE) to skilled (MID) performance. This learning-related shift from a prefrontal-parietal to a more subcortical control system is consistent with former models, indicating the importance of cortico-striatal and cortico-cerebellar mechanisms during learning (Hikosaka et al., 1999; Doyon and Ungerleider, 2002). However, task differences appear to argue in favor of a differential involvement of these subcortical structures as a function of the learning phase. Doyon et al. (2003) have pointed to an evolution from a cortico-cerebellar to cortico-striatal network during unimanual sequence learning, whereas the reverse takes place during adaptation to environmental perturbations. Here, we used a bimanual coordination task containing sequential as well as simultaneous elements of task performance. Our data showed a substantial and sustained involvement of both subcortical structures during learning and automatization. In fact, the anterior cerebellum and putamen were the only areas remaining substantially more involved during skilled compared with novel execution and are therefore presumed to play a central role in long-term motor memory formation and memory-driven performance involving feedforward control (Wolpert et al., 1998; Miall and Reckess, 2002). Together with cerebellar volumetric results, revealing relatively larger volumes in skilled musicians compared with non-musicians (Hutchinson et al., 2003), our findings reinforce the role of the cerebellum not only in the formation but also in the storage and retrieval of highly skilled movement representations. Additional research is required to assess whether task-related features mediate the differential impact of these subcortical structures as a function of the learning stage.
Conclusions
This is the first study explicitly exploring motor learning-related neural changes across the whole brain that extend beyond behavioral plateau performance. Our results show a gradual shift from cortical to subcortical involvement, associated with a decreasing necessity to inhibit preexisting response tendencies and the development of an internally, feedforward-driven execution mode. The putamen and anterior cerebellum were the only regions exhibiting sustained activation levels beyond behavioral plateau performance related to bimanual coordination effort. This reinforces their presumed involvement in the storage/retrieval of highly skilled movements.
Footnotes
This work was supported by the Research Council of Katholieke Universiteit Leuven (Belgium Contract OT/03/61) and the Research Programme of the Fund for Scientific Research-Flanders (Fonds voor Wetenschappelijk Onder-zoek-Vlaanderen G.0460.04).
Correspondence should be addressed to Stephan P. Swinnen, Laboratory of Motor Control, Group Biomedical Sciences, Katholieke Universiteit Leuven, Tervuurse Vest 101, 3001 Heverlee, Belgium. E-mail: stephan.swinnen@faber.kuleuven.be.
Copyright © 2005 Society for Neuroscience 0270-6474/05/254270-10$15.00/0
During the BimVfb condition, concurrent visual feedback was provided by means of a Lissajous figure, displaying the (slow) left limb displacement on the ordinate and the (fast) right limb displacement on the abscissa. A cursor on the screen showed the orthogonal plot of the displacements of both limbs in real time. When produced correctly, the 1:2 pattern resulted in a mirrored C-configuration. This condition has been shown to be a powerful feedback source, enhancing bimanual learning processes, and was included during training to ascertain optimal learning of the complex movement pattern. During scanning, this condition was included as well as the VisFb condition without motor performance. During the latter condition, the visual signal consisted of a perfect 1:2 Lissajous plot, rotated by 90° (forming an inverted U). As such, the presented signal had the same characteristics (luminance, speed, color, etc.) as during the BimVfb condition, but, to prevent imagery and mental rehearsal, it did not represent an actual movement pattern. Because the focus of the present paper was primarily on learning-related changes, both conditions were excluded from all data analyses mentioned in the present paper.
References
- Barbas H, Pandya DN (1987) Architecture and frontal cortical connections of the premotor cortex (area-6) in the rhesus-monkey. J Comp Neurol 256: 211-228. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC (2000) Anterior cingulate and the monitoring of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci 12: 298-309. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL (2002) Spatial working memory in human extrastriate cortex. Physiol Behav 77: 621-627. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999) Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402: 179-181. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA (2000) Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA 97: 8728-8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL (2002) Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci 14: 508-523. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004) Changes in brain activation during the acquisition of a new bimanual coordination task. Neuropsychologia 42: 855-867. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RSJ (1995) Relation between cerebral-activity and force in the motor areas of the human brain. J Neurophysiol 74: 802-815. [DOI] [PubMed] [Google Scholar]
- Doyon J, Ungerleider LG (2002) Functional anatomy of motor skill learning. In: Neuropsychology of memory (Squire LR, Schacter DL, eds). New York: Guilford.
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG (2002) Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41: 252-262. [DOI] [PubMed] [Google Scholar]
- Duncan J (1995) Attention, intelligence, and the frontal lobes. In: The cognitive neurosciences (Gazzaniga MS, ed), pp 721-733. Cambridge, MA: MIT.
- Fitts PM, Posner MI (1967) Learning and skilled performance in human performance. Belmont, CA: Brock-Cole.
- Friston KJ, Jezzard P, Turner R (1994) Analysis of functional MRI time-series. Hum Brain Mapp 1: 153-171. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1995) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189-210. [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K (2000) Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 202: 443-474. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu XF, Nakamura K, Miyachi S, Doya K (1999a) Parallel neural networks for learning sequential procedures. Trends Neurosci 22: 464-471. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Nakahara H, Lu X, Miyachi S, Nakamura K, Rand MK (1999b) Neural mechanisms for learning of sequential procedures. In: The new cognitive neurosciences (Gazzaniga MS, ed), pp 553-572. Cambridge, MA: MIT. [DOI] [PubMed]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H (2002) Central mechanisms of motor skill learning. Curr Opin Neurobiol 12: 217-222. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Lee LHL, Gaab N, Schlaug G (2003) Cerebellar volume of musicians. Cereb Cortex 13: 943-949. [DOI] [PubMed] [Google Scholar]
- Jancke L, Shah NJ, Peters M (2000) Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Cognit Brain Res 10: 177-183. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RE (1994) Motor sequence learning: a study with positron emission tomography. J Neurosci 14: 3775-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE (1997a) Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 77: 1313-1324. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE (1997b) Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325-1337. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995) Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155-158. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Unger-leider LG (1998) The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulos P, Petrides M (2002) Mid-ventrolateral prefrontal cortex: a region important for visual spatial and visual non-spatial retrieval mechanisms. Int J Psychophysiol 45: 132. [Google Scholar]
- Kostopoulos P, Petrides M (2003) The mid-ventrolateral prefrontal cortex: insights into its role in memory retrieval. Eur J Neurosci 17: 1489-1497. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835-1838. [DOI] [PubMed] [Google Scholar]
- Meyer E, Ferguson SSG, Zatorre RJ, Alivisatos B, Marrett S, Evans AC, Hakim AM (1991) Attention modulates somatosensory cerebral blood-flow response to vibrotactile stimulation as measured by positron emission tomography. Ann Neurol 29: 440-443. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ (2002) The cerebellum and the timing of coordinated eye and hand tracking. Brain Cogn 48: 212-226. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H (2001) The cerebellum coordinates eye and hand tracking movements. Nat Neurosci 4: 638-644. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R (1985) Selective attention gates visual processing in the extrastriate cortex. Science 229: 782-784. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P (1987) Attentional requirements of learning—evidence from performance-measures. Cognit Psychol 19: 1-32. [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM (1996) Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97-113. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991) Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61-64. [DOI] [PubMed] [Google Scholar]
- Passingham RE (1996) Attention to action. Philos Trans R Soc Lond B Biol Sci 351: 1473-1479. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Doyon J (2002) Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. J Neurosci 22: 1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (2002) The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiol Learn Mem 78: 528-538. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996) Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342-353. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001) Imaging the premotor areas. Curr Opin Neurobiol 11: 663-672. [DOI] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RSJ, Lechevalier B, Eustache F (1997) The structural components of music perception—a functional anatomical study. Brain 120: 229-243. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13: 25-42. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M (1999) “What-Then-Where” in visual working memory: an event-related, fMRI study. J Cogn Neurosci 11: 585-597. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1997) Cognitive conjunction: a new approach to brain activation experiments. NeuroImage 5: 261-270. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Putz B (1998) Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci 18: 1827-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer LH (1975) Multiple attention in continuous verbal tasks. In: Attention and performance (Rabbitt PMA, Dornic S, eds), pp 205-213. New York: Academic.
- Schmidt RA, Lee TD (1999) The learning process. In: Motor control and learning. A behavioral emphasis (Schmidt RA, Lee TD, eds), pp 357-383. Champaign, IL: Human Kinetics.
- Schneider W, Shiffrin RM (1977) Controlled and automatic human information-processing. I. Detection, search, and attention. Psychol Rev 84: 1-66. [Google Scholar]
- Shadmehr R, Holcomb HH (1999) Inhibitory control of competing motor memories. Exp Brain Res 126: 235-251. [DOI] [PubMed] [Google Scholar]
- Swinnen SP (2002) Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3: 350-361. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N (2004) Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci 8: 18-25. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Dounskaia N, Walter CB, Serrien DJ (1997) Preferred and induced coordination modes during the acquisition of bimanual movements with a 2:1 frequency ratio. J Exp Psychol Hum Percept Perform 23: 1087-1110. [Google Scholar]
- Talairach J, Tournoux P (1988) Co-planar stereotactic atlas of the human brain. New York: Thieme.
- Thaut MH (2003) Neural basis of rhythmic timing—networks in the human brain. Neurosci Music 999: 364-373. [DOI] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, Nishizawa S, Konishi J, Shibasaki H (1999) Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event-related fMRI study. J Neurosci 19: 3527-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy J, Flanders A, Madi S, Laskas J, Stoddard E, Pyrros A, Natale P, DelVecchio N (2003) Regional brain activation associated with different performance patterns during learning of a complex motor skill. Cereb Cortex 13: 904-910. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A (2002) Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78: 553-564. [DOI] [PubMed] [Google Scholar]
- Van Mier H (2000) Human learning: dynamic systems. In: Brain mapping. The system (Mazziotta JC, Toga AW, Frackowiak RSJ, eds), pp 605-620. San Diego: Academic.
- Vogt BA, Finch DM, Olson CR (1992) Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435-443. [DOI] [PubMed] [Google Scholar]
- Waldvogel D, Hallet M, Ishii K, van Gelderen P (1999) The effect of movement amplitude on activation in functional magnetic resonance imaging studies. J Cereb Blood Flow Metab 19: 1209-1212. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cogn Sci 2: 338-347. [DOI] [PubMed] [Google Scholar]