Abstract
Inflammation of the CNS is usually locally limited to avoid devastating consequences. Critical players involved in this immune regulatory process are the resident immune cells of the brain, the microglia. Interactions between the growing family of B7 costimulatory ligands and their receptors are increasingly recognized as important pathways for costimulation and/or inhibition of immune responses.
Human and mouse microglial cells constitutively express B7 homolog 1 (B7-H1) in vitro. However, under inflammatory conditions [presence of interferon-γ (IFN-γ) or T-helper 1 supernatants], a significant upregulation of B7-H1 was detectable. Expression levels of B7-H1 protein on microglial cells were substantially higher compared with astrocytes or splenocytes. Coculture experiments of major histocompatibility complex class II-positive antigen-presenting cells (APC) with syngeneic T cells in the presence of antigen demonstrated the functional consequences of B7-H1 expression on T-cell activation. In the presence of a neutralizing anti-B7-H1 antibody, both the production of inflammatory cytokines (IFN-γ and interleukin-2) and the upregulation of activation markers (inducible costimulatory signal) by T cells were markedly enhanced. Interestingly, this effect was clearly more pronounced when microglial cells were used as APC, compared with astrocytes or splenocytes. Furthermore, B7-H1 was highly upregulated during the course of myelin oligodendrocyte glycoprotein-induced and proteolipid protein-induced experimental allergic encephalomyelitis in vivo. Expression was predominantly localized to areas of strongest inflammation and could be colocalized with microglial cells/macrophages as well as T cells.
Together, our data propose microglial B7-H1 as an important immune inhibitory molecule capable of downregulating T-cell activation in the CNS and thus confining immunopathological damage.
Keywords: encephalomyelitis, glia, immunity, microglia, neuropathology, tolerance, costimulation
Introduction
Antigen presentation in the CNS is thought to play a critical role in the initiation and perpetuation of neuroinflammation. Microglial cells, the resident immune cells, can express major histocompatibility complex (MHC) class II molecules and have the potential to act as effective antigen-presenting cells (APCs) to T cells (Sedgwick et al., 1993). In multiple sclerosis (MS), the prototype autoimmune inflammatory disorder of the CNS, close interactions of MHC class II-positive microglial cells with T cells are visible within the inflammatory lesions (Bo et al., 1994).
A necessary prerequisite for T-cell activation is T-cell receptor engagement by MHC peptides (signal 1) together with a second antigen-independent signal mediated by costimulatory receptors (signal 2) (Lafferty and Woolnough, 1977). Accumulating evidence suggests that during CNS inflammation, activated microglia express costimulatory molecules such as CD80 (B7.1), CD86 (B7.2), and CD40 in addition to certain adhesion molecules (for review, see Aloisi, 2001). The interaction of microglial cells and T cells subsequently leads to either induction of T-cell proliferation, T-effector functions (e.g., cytokine secretion), or both (Becher et al., 2000) (for review, see Aloisi, 2001). The modulation of T-cell function by microglial cells entails an important dichotomy. Microglial cells are capable of secreting high amounts of proinflammatory cytokines [e.g., interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and IL-12] during CNS inflammation. In contrast, they can also participate in the downmodulation of inflammation by producing anti-inflammatory cytokines such as TGF-β, IL-10, or IL-1 receptor antagonist (Kreutzberg, 1996). Along this notion, different model systems involving an inflamed CNS environment have characterized microglia as potent inducers of T-cell cytokine production, whereas their capacity to induce T-cell proliferation is much less evident (Carson et al., 1999; Juedes and Ruddle, 2001).
The discovery of coinhibitory molecules of the B7-CD28 family has brought tremendous advancements in understanding the control of T-cell immunity in different immune compartments (Chen, 2004). Programmed death-1 ligand (PD-L1) or B7 homolog 1 (B7-H1) has been attributed to costimulatory and immune regulatory functions (Kobata et al., 2000; Coyle and Gutierrez-Ramos, 2001; Carreno and Collins, 2002; Liang and Sha, 2002; Sharpe and Freeman, 2002). Previous work described that the costimulation of T cells with B7-H1-Fc fusion protein induces T-cell proliferation and the secretion of IL-10 and interferon-γ (IFN-γ) (Dong et al., 1999). However, more recent data suggest that B7-H1 may also negatively regulate T cells by inhibiting cell cycle progression (Freeman et al., 2000; Carter et al., 2002). B7-H1 exhibits a broader tissue distribution than B7.1/2 (CD80/CD86) (Freeman et al., 2000; Eppihimer et al., 2002; Mazanet and Hughes, 2002; Petroff et al., 2002; Wiendl et al., 2003). B7-H1 is upregulated by IFN-γ and regulates immune responses by interacting with programmed death receptor-1 (PD-1) and another yet unidentified receptor on activated T cells, although not CD28/cytotoxic T-lymphocyte-associated antigen receptor 4 (Coyle and Gutierrez-Ramos, 2001; Carreno and Collins, 2002; Dong et al., 2002; Liang and Sha, 2002; Sharpe and Freeman, 2002; Wang et al., 2003). Interestingly, PD-1-deficient mice suffer from autoimmune disorders resulting from inappropriate activation of B and T cells (Nishimura et al., 1999, 2001), suggesting that PD-1 is an important inhibitory signal that acts to prevent uncontrolled proliferation of autoreactive T cells.
Materials and Methods
Antibodies and reagents. Myelin oligodendrocyte glycoprotein (MOG) peptide 35-MEVGWYRSPFSRVVHLYRNGK-55 was synthesized by MWG Biotech (Ebersberg, Germany). The proteolipid protein 139-151 (PLP139-151) (HCLGKWLGHPDKF) was synthesized using standard g-fluorenylmethoxycarbonyl chemistry. Protein purified derivate (PPD) of Mycobacterium tuberculosis was purchased from Statens Serum Institut (Copenhagen, Denmark). Staphylococcal enterotoxin B (SEB) was provided by Sigma (Deisenhofen, Germany). The following primary antibodies (Abs) were used: anti-mouse B7-H1 (PD-L1), MIH5 (eBioscience, San Diego, CA), 10H5 (L. Chen, Mayo Clinic Rochester, Rochester, MN), BAF1019 (R&D Systems, Minneapolis, MN); anti-mouse inducible costimulatory signal (ICOS), HK5.3 (eBioscience); anti-mouse MHC II, I-A/I-E (BD PharMingen, Heidelberg, Germany); anti-mouse MAC3 (PharMingen); anti-mouse CD45, anti-mouse CD11b, anti-mouse CD3 (all from BD Biosciences, Heidelberg, Germany); anti-mouse F 4/80 (Serotec, Kidlington, UK); and anti-mouse GFAP (DakoCytomation, High Wycombe, UK). Secondary Abs are as follows: goat anti-mouse IgG (H+L) F(ab)2-PE, rat anti-mouse IgG F(ab)2-FITC (Dianova, Hamburg, Germany), anti-rabbit IgG F(ab)2-FITC (Dianova), and donkey anti-rat Cy3 (Jackson ImmunoResearch, West Grove, PA). Mouse IFN-γ and TNF-α were from Sigma. Mouse IL-2 and IFN-γ ELISA were from PharMingen.
Isolation of human microglial cells. The studies were performed in accordance with the guidelines set by the Institutional Review Board of McGill University (Montreal, Canada). Primary adult human glial cells were obtained from surgical resections performed for the treatment of non-tumor-related intractable epilepsy. Tissue was obtained from regions requiring resection to reach the precise epileptic focus and was distant from the main electrically active site. Dissociated cultures of microglia were prepared as described previously (Williams et al., 1992), based on the differential adherence of the glial cells. Briefly, brain tissue was subjected to enzymatic dissociation with trypsin (0.025%) and DNase I (25 μg/ml) (Boehringer Mannheim, Laval, Quebec, Canada) for 30 min at 37°C, followed by mechanical dissociation by passage through a 132 μm nylon mesh (Industrial Fabrics Corporation, Minneapolis, MN). Cells were further separated on a linear 30% Percoll density gradient (Amersham Biosciences, Baie D'Urfé, Quebec, Canada) and centrifuged at 15,000 rpm at 4°C for 30 min. The cells recovered from the interface contained a mixed glial cell population consisting of ∼65% oligodendroglia, 30% microglia, and 5% astrocytes. To enrich for microglia, the mixed cell population was suspended in minimal essential culture medium, supplemented with 5% FCS, 2.5 U/ml penicillin, 2.5 μg/ml streptomycin, 2 mm glutamine, and 0.1% glucose (all from Invitrogen, Burlington, Ontario, Canada), and left overnight in 12.5 cm2 tissue culture flasks (Falcon; Fisher Scientific, Montreal, Quebec, Canada) in a humid atmosphere at 37°C with 5% CO2. The less-adherent oligodendroglia were removed by gentle pipetting, and the remaining adherent cells were allowed to develop morphologically for 3 d. Remaining microglia were of 95% purity as assessed by immunocytochemistry and flow cytometry (Williams et al., 1992; Becher and Antel, 1996). Microglia were cultured for ∼7 d and then harvested by trypsinization (0.25%). T-helper 1 (Th1) as well as Th2 supernatants from T cells were generated as described previously (Kim et al., 2004).
Isolation of human monocytes. For monocyte isolation, blood was obtained by venipuncture, and peripheral blood mononuclear cells were isolated by density gradient centrifugation using lymphocyte separating solution (PAA Laboratories, Linz, Austria). Monocytes were enriched by 1 h of adherence to plastic flasks at 37°C in RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 10% FCS (Biochrom, Berlin, Germany) and penicillin (100 international units (IU)/ml)/streptomycin (10 μg/ml) (Invitrogen). Nonadherent cells were removed. Adherent cells were detached using cell dissociation buffer, and purity was analyzed by flow cytometry (>90% CD14-positive cells). Where indicated, monocytes were stimulated by supernatants from Th1 or Th2 cells as described previously (Kim et al., 2004).
Isolation of murine microglial cells and astrocytes. Mouse microglial cells and astrocytes were isolated from primary mixed brain glial cell cultures using a modification of methods described previously (Giulian and Baker, 1986; Magnus et al., 2001, 2002). In brief, cultures were prepared from the brains of newborn C57BL/6 mice [postnatal day 0 (P0) to P2; Charles River, Sulzfeld, Germany], which were freed of their meninges and minced with scissors under a dissecting microscope (Wild, Heerbrugg, Switzerland). Mixed cell cultures were then grown in basal Eagle's medium supplemented with 10% fetal calf serum (Sigma), 50 IU/ml penicillin, and 50 μg/ml streptomycin at 37°C for 10-14 d. Microglial cells and astrocytes were separated by shaking the culture flasks for 7 h (Primaria; Falcon, Franklin Lakes, NJ). To check the purity of the cell-culture system, we took a small fraction and performed immunocytochemistry with the monoclonal antibodies (mAbs) F 4/80 (1:100) and GFAP (1:100) as described previously (Giulian and Baker, 1986; Zielasek et al., 1992). A total of 95% or more of the cells were either F 4/80 positive (microglial cells) or GFAP positive (astrocytes). All cells used for our studies came from the same primary glial cell preparation.
For the isolation of splenocytes, the spleen was isolated from syngeneic mice and then cell suspensions were generated and used in the experiments (Jung et al., 2001).
Coculture experiments with T cells. Vβ-specific activation of naive T cells by SEB (5 μg/ml) was measured by coculturing naive T cells with irradiated syngeneic APC. T cells were mixed with microglial cells or astrocytes in a ratio of 15:1 to achieve good levels of cytokine secretions by the T cells. In the experiments with splenocytes, the ratio was 1:1. The optimal ratio of microglial cells, astrocytes, or splenocytes and T cells for the maximal T-cell cytokine production had been titrated previously. Microglial cells or astrocytes alone produced no IFN-γ or IL-2, and T cells alone (with or without antigen) secreted rather small amounts of IFN-γ or IL-2. A 10- to 100-fold increase in IFN-γ or IL-2 production could be detected after antigen had been added to the cocultures (data not shown). Supernatants were collected at 24 h, centrifuged to remove particulate debris, and stored in aliquots at -70°C.
For the antigen-specific T-cell experiments, T-cell lines were established from inguinal lymph nodes (LNs) or spleens of immunized mice according to standard procedures (Korn et al., 2003). T-cell lines were restimulated with their respective antigen at least two times before the experiments. For in vitro recall, inguinal LN cells or splenocytes were isolated on day 12 after injection and seeded at 75,000 or 150,000 per microtiter-well in restimulation medium. The T-cell lines were CD4 positive and clearly MHC II restricted. PPD (10 μg/ml) was used for PPD-specific T-cell stimulation. To measure antigen-induced cytokine production by T cells, PPD-specific T cells and microglial cells were cultured in the presence or absence of antigen at a ratio of 5:1.
To achieve higher levels of B7-H1 expression in the blocking experiments, microglial cells, astrocytes, or splenocytes were preincubated with IFN-γ (500 IU/ml) for 24 h, which was thoroughly washed off before the interaction with the T cells. B7-H1 or control antibodies were used at a dilution of 1:100. Concentrations had been titrated and tested previously. Of note, IFN-γ, which was used to stimulate microglial cells, astrocytes, or splenocytes, was undetectable in control cultures.
ELISA. OptEIA ELISA kits from PharMingen were used for the detection of mouse INF-γ and IL-2 in the supernatant.
RNA extraction, cDNA synthesis, and quantitative real-time PCR. Cells were detached by trypsinization, collected and resuspended in 1 ml of TRIzol, and then frozen at -80°C. Total RNA extraction was performed using peqGOLD Tri Fast isolation reagent (peqLab, Erlangen, Germany). For first-strand cDNA synthesis, 2.5 μg of total RNA was dissolved in 21.5 μl of DEPC-treated double-distilled water (H2Odd.). A total of 2 μl of random hexamers (200 ng/μl) was added to each sample before incubation at 70°C for 10 min. Samples were cooled on ice and subjected to a mixture consisting of 5× Moloney murine leukemia virus (M-MLV) reverse-transcription buffer (10 μl/sample; Promega, Madison, WI), deoxyNTPs (dNTPs) (10 mm; 10 μl/sample), RNasin (40 U/μl, 0.25 μl/sample; Promega), M-MLV reverse-transcription buffer (200 U/μl, 1 μl/sample; Promega), and DEPC-treated H2Odd (5.25 μl/sample). This mixture of 26.5 μl was added to each RNA/random hexamer solution. Samples were mixed and incubated for 10 min at room temperature, for 50 min at 42°C, and finally for 15 min at 70°C.
For quantitative real-time (QRT)-PCR, measurement of gene expression was performed using the ABI prism 7700 Sequence Detection system (Applied Biosystems, Foster City, CA). Primers (BioChip Technologies, Freiburg, Germany) were designed when possible to span exon-exon junctions to prevent amplification of genomic DNA and to result in amplicons <150 bp to enhance efficiency of PCR amplification. Relative quantification of specific gene expression was performed by two-step real-time PCR using cDNA as a template, as described previously (Wiendl et al., 2003). Templates were multiplied using PerkinElmer Life Sciences (Emeryville, CA) SYBR Green PCR Master Mix [containing hotstart AmpliTaqGold, SYBR Green PCR buffer (2×), MgCl2, and dNTPs). Reverse-transcription PCR of cDNA specimens was conducted in a total volume of 15 μl with 1× TaqMan Master Mix (PerkinElmer Life Sciences) with primers at optimized concentrations. Thermal cycler parameters were 2 min at 50°C, 10 min at 95°C, and 40 cycles of denaturation at 95°C for 15 s followed by annealing/extension at 60°C for 1 min. The fluorescence resulting from binding of SYBR Green dye to double-stranded DNA was measured directly in the PCR tube. Data were analyzed with the ABI PRISM Detection system using the comparative threshold cycle (CT) method (user bulletin; PerkinElmer Life Sciences). Samples were normalized to 18S rRNA to account for the variability in the initial concentration of the total RNA and conversion efficiency of the RT reaction. Product specificity of the PCR products and quality of primers were confirmed by agarose gel electrophoresis, sequencing of bands, and dissociation curve analysis. The internal reference dye ROX included in the PCR buffer was used to check for fluorescence fluctuations caused by changes in concentration or volume. All PCR assays were performed in duplicate. Real-time monitoring of fluorescent emission from cleavage of sequence-specific probes by the nuclease activity of Taq polymerase allowed definition of the threshold cycle during the exponential phase of amplification. Standard curves were generated for each gene quantitated and were found to have excellent PCR amplification efficiency (90-100%) as determined by the slope of the standard curves. Linear regression analysis of all standard curves was 0.99. Normalization of samples was performed by dividing the copies of the gene of interest by copies of the reference gene, 18S rRNA, to account for the variability in the initial concentration of the total RNA and conversion efficacy of the RT reaction.
Oligonucleotides used in this study are as follows. 18S: 18S-forward (for) (450-469), 5′-CGGCTACCACATCCAAGGAA; 18S-reverse (rev) (636-619), 5′-GCTGGAATTACCGCGGCT. Human, hB7-H1 (PD-L1): B7-H1-for (441-460), 5′-TCAATGCCCCATACAACAAA; B7-H1-rev (560-541), 5′-TGCTTGTCCAGATGACTTCG. hPD-L2: PD-L2-for (360-383), 5′-GTACATAATAGAGCATGGCAGCA; PD-L2-rev (460-439), 5′-C-CACCTTTTGCAAACTGGCTGT. hCD80: CD80-for (23-43), 5′-AGTTAGAAGGGGAAATGTCGC; CD80-rev (133-112), 5′-TCAGGGTAAGACTCCACTTCTG. hCD86: CD86-for (509-530), 5′-ATTCTGAACTGTCAGTGCTTGC; CD86-rev (633-612), 5′CTTCTTAGGTTCTGGGTAACCG. Human leukocyte antigen (HLA)-DR-α: HLA-DR-α-for (274-294), 5′-TGAAGAATTTGGACGATTTGC; HLA-DR-α-rev (400-380), 5′-GGAGGTACATTGGTGATCGG. Murine, mB7.1 (mCD80): mB7.1-for (694-713), 5′-CGCAACCACACCATTAAGTG; mB7.1-rev (843-823), 5′GACGACTGTTATTACTGCGCC. mB7.2 (mCD86): mB7.2-for (368-387), 5′-ACAAAAAAAGCCACCCACAG; mB7.2-rev (507-487), 5′-ACGTGCAGGTCAAATTTATGC. mB7-H1 (PD-L1): mB7-H1-for (579-598), 5′-TGCTTCTCAATGTGACCAGC; mB7-H1-rev (712-693), 5′-ATGTGTTGCAGGCAGTTCTG. mPD-L2: mPD-L2-for (505-524), 5′-AGTACCGTTGCCTGGTCATC; mPD-L2-rev (646-627), 5′-CTAGCCTGGCAGGTAAGCTG.
Mouse strains and induction of experimental allergic encephalomyelitis. For active induction of MOG35-55-experimental allergic encephalomyelitis (EAE), C57BL/6 mice (Charles River) were immunized with 200 μl of an emulsion of equal volumes of MOG35-55 in PBS (2 mg/ml) and Freund's incomplete adjuvant oil (Invitrogen) supplemented with M. tuberculosis H37Ra (2 mg/ml; Difco, Detroit, MI). To enhance the immune response, immunized animals were administered 400 ng of pertussis toxin (Sigma) on the day of immunization and on day 2 after injection. Animals were killed after induction of EAE at times indicated in Results, and tissues were fixed in paraformaldehyde.
For kinetic analysis of B7-H1 expression by flow cytometry, SJL mice (female, 6-12 weeks of age; Harlan Winkelmann, Borchen, Germany) were used according to approved protocols. EAE induction was performed essentially as described previously (Bischof et al., 2004). In brief, 50 nmol of the peptide PLP139-151 in PBS emulsified with an equal amount of CFA containing 200 μg of M. tuberculosis H37RA (Difco) was injected subcutaneously in the back of the foot. In addition, mice received a single intravenous injection of 300 ng of pertussis toxin (List Biologic, Campbell, CA) in PBS. Diseased animals had scores between 2 and 4 on the five-point EAE scale, with a score of 0 being disease free and 5 being moribund or dead.
Flow cytometry. For flow cytometric analysis of inducible surface expression of MHC class II and B7-H1, microglial cells, astrocytes, and splenocytes were preincubated with IFN-γ (500 U/ml) for 48 h. Adherent cells were detached from the surface of the 48-well plastic dishes by incubation with 0.02% EDTA in PBS. Subsequently, samples were treated as described previously (Tabi et al., 1994).
As a marker for T-cell activation in coculture experiments, we measured upregulation of the inducible costimulatory protein ICOS on the PPD-specific T cells after they had been separated from the adherent microglial cells by gentle washing.
For flow cytometric analysis of B7-H1 expression in the CNS in vivo during EAE, cells isolated from the CNS were stained on ice with the indicated antibodies for 20 min and directly before analysis with 1 μg/ml propidium iodide. Flow cytometric analysis was performed on life lymphocytes with a Cyan Cytometer (DakoCytomation) using Summit software (DakoCytomation) for data acquisition and analysis.
Histology. Spinal cords and brains were harvested on days 14-21 after immunization (controls as well as antigen-immunized mice) and snapfrozen in optimal cutting temperature compound. Four- to 10-μm-thick sections were cut, fixed in acetone, and subsequently stained. Single stainings were performed using the avidin-biotin technique (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin. Antibodies used were anti-B7-H1 (MIH5), anti-MAC3, anti-GFAP, and anti-CD3. Isotype-matched control IgG served as a negative control. For triple fluorescence stainings of CNS cells, spinal cords and brains were sectioned at 8 μm and incubated with anti-B7-H1 (MIH5), anti-GFAP, or anti-CD3 and counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Sections were analyzed using the Zeiss (Thornwood, NY) Axio-plan microscope equipped with an image analysis system.
Statistical analysis. Data are representative of experiments performed at least three times with similar results. Significance was assessed by two-sided t test (*p < 0.05; **p < 0.01).
Results
Expression of B7-H1 on human microglial cells: strong upregulation by inflammatory stimuli
Human microglial cells were examined for the expression of B7-H1. We analyzed total RNA from cultured human adult microglial cells for different B7 molecule transcripts and HLA-DR by QRT-PCR. Microglial preparations from two different donors were used (HA376 and HA382). We also studied purified human peripheral blood monocytes, which were previously described to constitutively express B7-H1 mRNA and protein (Selenko-Gebauer et al., 2003; Schreiner et al., 2004). mRNA transcripts of B7-H1 (PD-L1) were detectable on monocytes and microglial cells already before the addition of inflammatory stimuli (Fig. 1). Monocytes and microglia were cultured in the presence of supernatants from Th1 or Th2 T-cell lines to assess whether B7-H1 expression on these cell types can be differentially regulated by exposure to a proinflammatory or an anti-inflammatory environment, respectively. Exposure of microglial cells to either Th1 or Th2 supernatants led to a significant upregulation of B7-H1 mRNA, which was clearly higher with Th1 than with Th2 supernatants [relative increases for Th1, 18.5 (HA376) and 9.4 (HA382); for Th2, 10.1 (HA376) and 2.8 (HA382)]. mRNA transcripts for the classical B7 molecules CD80 (B7.1) and CD86 (B7.2) as well as expression of MHC class II were assessed in parallel. Upregulation of CD80 in response to the proinflammatory Th1 supernatants was stronger in monocytes compared with microglia [relative increases for monocytes, 7.7 and 6.2; relative increases for microglia, 2.0 (HA376) and 3.2 (HA382)] (Fig. 1). CD86 was mildly upregulated by Th2 rather than by Th1 supernatants with no substantial differences between monocytes and microglial cells (Fig. 1). Th1 supernatant exposure results in downregulation of CD86 mRNA in monocytes but not microglia. The pattern of regulation of HLA-DR transcripts by T-cell supernatants was similar for monocytes and microglial cells, in that Th1 supernatants enhanced HLA-DR expression on both cell types, whereas Th2 supernatants had relatively little effect. The induction of HLA-DR mRNA by Th1 supernatants was higher in monocytes than in microglial cells [relative increases for monocytes, 7.3 and 20.9; relative increases for microglia, 4.2 (HA376) and 9.4 (HA382)] (Fig. 1).
Figure 1.
Expression and differential modulation of B7 family members and HLA-DR mRNA in human microglial cells and peripheral blood monocytes. Human microglial cells from two different donors [denominated microglia I (HA376) and microglia II (HA382)] were cultured in the absence or presence of Th1 and Th2 supernatants, harvested after 48 h of induction, and analyzed for the expression of the indicated mRNA by QRT-PCR [B7.1 (CD80), B7.2 (CD86), MHC class II (HLA-DR), B7-H1 (PD-L1)]. For comparison, monocytes from two independent donors (monocytes I and II) were processed under the same conditions. Bars and numbers represent the relative gene expression of indicated molecules calculated in relation to unstimulated microglia (set to 1).
IFN-γ-inducible B7-H1 protein in cultured murine microglial cells: comparison with astrocytes and splenocytes
To validate our data achieved with RNA from human monocytes or microglial cells cultured in the presence or absence of Th1 or Th2 supernatants, we next investigated the expression of B7-H1 and B7 proteins on murine microglial cells in comparison with astrocytes and splenocytes. In the absence of added cytokines, cultured microglial cells expressed B7-H1 protein, which was higher than on astrocytes or splenocytes (Fig. 2). Consistent with our observations with the Th1 supernatants, B7-H1 expression on microglial cells was substantially increased when cells were cultured in the presence of inflammatory stimuli (500 IU/ml IFN-γ). B7-H1 upregulation was visible 12 h after stimulation, and the maximum effect was observed at 72 h (data not shown). In contrast, TNF-α had no effect on B7-H1 expression (data not shown). Similar to the observations in microglia, B7-H1 expression was also inducible by IFN-γ in astrocytes and splenocytes. Relative increases and absolute protein expression levels of B7-H1 were higher in glial cells than in splenocytes (Fig. 2). As a control, we measured MHC II expression, which was induced by IFN-γ in all cells (microglia, astrocytes, splenocytes). Four independent experiments of primary cultured glial cells from different animal preparations were examined.
Figure 2.

B7-H1 protein expression in cultured murine microglial cells, astrocytes, and splenocytes. The expression of B7-H1 and MHC class II (HLA-DR) was assessed by flow cytometry in purified and cultured microglial cells, astrocytes, and splenocytes maintained in the absence or presence of IFN-γ (500 U/ml; 48 h). Histograms show staining with the designated antibodies (open) underlaid with the respective isotype controls (filled). The expression analysis was performed with glial cell cultures from five different animal preparations. A representative experiment is shown.
To corroborate the murine protein data, we analyzed total RNA from microglia, astrocytes, and splenocytes for different B7 molecule transcripts by QRT-PCR. According to the literature, murine microglia expressed CD80 and CD86 mRNA, which was upregulated by IFN-γ (Fig. 3A,B). On astrocytes, expression and regulation was lower (Fig. 3A,B). Corresponding to the protein data (Fig. 2), mRNA for B7-H1 (PD-L1) was expressed in unexposed microglia and was substantially induced after exposure to IFN-γ (factor of 6.3-10.5) (Fig. 3C). Astrocytes expressed lower basal levels, which were also highly upregulated after stimulation with IFN-γ. Of note, low levels of PD-L2 were also detected on microglial cells and were upregulated after treatment with IFN-γ (Fig. 3D).
Figure 3.
mRNA expression of B7 family members in cultured murine microglia and astroglia. Microglia and astrocytes cultured in the absence or presence of IFN-γ (500 U/ml) were harvested after 48 h of induction and analyzed for the expression of the indicated mRNA by QRT-PCR. A, B7.1 (CD80); B, B7.2 (CD86); C, B7-H1 (PD-L1); D, PD-L2. Splenocytes and PHA-stimulated splenocytes were used as controls. Bars and numbers represent the relative gene expression of indicated molecules calculated in relation to unstimulated splenocytes (set to 1). Data represent expression analysis from glial cultures from three different animal preparations (mean ± SEM).
Functional role of B7-H1 on microglial cells: strong inhibition of naive polyclonal T-cell activation
To assess the functional consequences of B7-H1 expression on microglial cells, we used a coculture setting in which microglial cells, astrocytes, or splenocytes were used as APC. APC were induced to upregulate B7-H1 and MHC-II by IFN-γ and subsequently cocultured with naive syngeneic T cells and superantigen (SEB) in the presence of neutralizing anti-B7-H1 antibody (MIH5 or 10B5) or the appropriate isotype control antibody. The release of IFN-γ and IL-2 into the supernatant was measured at the times indicated. Both cytokines are produced primarily by T cells and not by microglial cells or astrocytes (data not shown).
Compared with the isotype control, treatment with anti-B7-H1 mAb strongly augmented the production of IFN-γ and IL-2 by oligoclonally (SEB) stimulated naive T cells (Fig. 4A). In cocultures with microglial cells, in the presence of anti-B7-H1 mAb, the mean amounts of IL-2 and IFN-γ increased from 1789 ± 310 to 2816 ± 776 pg/ml (IL-2) and from 1142 ± 400 to 2110 ± 196 pg/ml (IFN-γ), respectively. For cocultures with astrocytes, IL-2 and IFN-γ were elevated from 1370 ± 60 to 1469 ± 112 pg/ml and from 548 ± 135 to 784 ± 206 pg/ml, respectively, and in the coculture system with splenocytes, IL-2 increased from 2953 ± 324 to 3415 ± 467 pg/ml and IFN-γ from 1285 ± 117 to 1861 ± 349 pg/ml. Albeit in principle, the inhibitory effect of B7-H1 was observed with microglial cells as well as astrocytes and splenocytes, B7-H1 neutralization had its greatest effects in microglial T-cell cocultures, with a mean increase of 57% for IL-2 and 85% for IFN-γ (compared with 7 and 43% in the astrocyte coculture, and 16 and 45% in the splenocyte coculture setting). In general, B7-H1 neutralization had more influence on IFN-γ production than on IL-2 secretion (Fig. 4A). For IL-2 secretion, B7-H1 neutralization only had a significant influence in microglia T-cell cocultures. Together, these data comparing different APC indicate that the inhibitory effect of B7-H1 on T-cell cytokine production is most prominent in microglial T-cell cultures.
Figure 4.

Functional consequences of B7-H1 expression for cytokine expression and T-cell activation. A, Polyclonal T cells were cocultured with microglial cells, astrocytes, or splenocytes under syngeneic conditions. T-cell activation was performed by the addition of SEB. To analyze the function of B7-H1, a neutralizing antibody was compared with an isotype control antibody. Supernatants were taken 24 h after coculture, and cytokines released into the supernatant were measured. Bars show the mean ± SD from three independent experiments, each performed in triplicate. B, PPD-specific T cells were cocultured with syngeneic microglial cells in the presence of PPD with either a B7-H1 blocking antibody or an isotype control antibody. Bars show data from three independent experiments, each performed in triplicate. C, PPD-specific T cells were cocultured with syngeneic microglial as shown in B. After 24 h of coculture, cells were separated and examined for expression of ICOS by flow cytometric analysis. The thick black curve refers to the ICOS expression on PPD-specific T cells after B7-H1 neutralization, whereas the dotted gray curve corresponds to the isotype control antibody setting. Results were reproduced three times; a representative example is shown.
Functional role of B7-H1 on microglial cells: strong inhibition of antigen-specific T-cell activation
Naive, oligoclonally (SEB-) activated T cells may differ considerably from antigen-specific T cells both in their reactivity to TCR-specific stimuli and in their dependence on secondary signals. Therefore, we next assessed the functional significance of B7-H1 expression on the activation of antigen-specific T cells.
Coculture experiments were performed with PPD-specific T-cell lines, and the modulation of T-cell cytokine production by B7-H1 was assessed. As observed with naive T cells, blocking of B7-H1 on microglial cells resulted in a significant increase in both IFN-γ and IL-2 (for IFN-γ, 17.905 ± 1435 vs 23.054 ± 2880 pg/ml, and for IL-2, 165 ± 96 vs 550 ± 91 pg/ml) (Fig. 4B).
The inhibitory effect of B7-H1 was also demonstrated by assessing the expression pattern of T-cell activation markers following coculture in the presence or absence of a neutralizing B7-H1 antibody. Neutralization of B7-H1 led to increased expression levels of ICOS on PPD-specific T cells, thus demonstrating the inhibitory role of microglia-related B7-H1 for T-cell activation (Fig. 4C). The pooled relative mean fluorescence index from three independent experiments showed an overall elevation in ICOS expression of 39% after inhibition of the B7-H1 signal (p < 0.05).
Expression of B7-H1 in the CNS and in the course of EAE
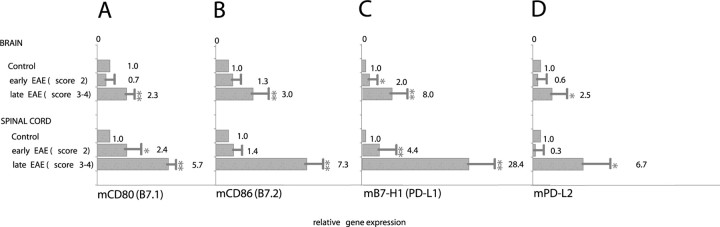
Our in vitro human and murine experiments suggested that microglia and astrocytes are capable of expressing high levels of inhibitory B7-H1 in the presence of inflammatory conditions. Therefore, we next investigated the expression of B7-H1 during CNS inflammation exemplified by MOG- and PLP-induced EAE. Expression of B7 molecules was assessed by QRT-PCR as well as immunohistochemistry at different time points after immunization in brain specimens and in the spinal cord from animals at an early stage of EAE (score 2) and a late stage of EAE (score 3-4). Under control conditions, B7-H1 mRNA was virtually undetectable in the CNS (Fig. 5). In contrast, B7-H1 expression progressively increased during the course of EAE and seemingly correlated with the disease severity (Fig. 5C). mRNA transcripts for PD-L2, the alternative splice variant of B7-H1, were found in late EAE but to a much lesser extent than B7-H1 (Fig. 5D). Expression levels of CD80 and CD86 mRNA in the brain and spinal cord in principle paralleled this pattern (Fig. 5A,B). According to the mRNA data, B7-H1 protein was virtually undetectable in control animals. However, strong immunoreactivity of B7-H1 was detectable in ongoing EAE, and intensity of staining correlated with the severity of EAE as well as the presence of inflammatory infiltrates in the lesions (Fig. 6). Histochemical analysis of the inflamed spinal cord and the CNS revealed B7-H1 coexpression with MAC3 (microglia/macrophages) but not GFAP (astrocytes) (Fig. 6). Interestingly, B7-H1 also costained with CD3 (T cells), corresponding to the notion that T cells are capable of expressing B7-H1 (Dong et al., 2002) (Fig. 6). Thus, our immunohistochemical analysis suggests that B7-H1, although virtually undetectable under physiological conditions, is strongly upregulated in the CNS under inflammatory conditions. As main cellular sources, we found microglia/macrophages as well as invading T cells.
Figure 5.
mRNA expression of B7 family members in the course of EAE. QRT-PCR for B7.1 (CD80) (A), B7.2 (CD86) (B), B7-H1 (PD-L1) (C), and PD-L2 (D) mRNA expression was performed in brain and spinal cord specimens from animals with EAE at different time points after immunization (early EAE, score 2; late EAE, score 3-4). Brain and spinal cord specimens from nonimmunized mice were used as controls. Bars and numbers represent the mean ± SEM of the relative gene expression of indicated molecules calculated in relation to the controls. Three animals of each group were pooled for the analysis (*p < 0.05; **p < 0.01, compared with control).
Figure 6.

Expression of B7-H1 in the CNS. Fluorescent single or multicolor labeling (top) or enzymatic labeling immunohistochemistry (bottom) in CNS specimens of mice with EAE versus control immunized mice is shown, using antibodies for B7-H1, CD3, MAC3, and GFAP, visualized with secondary fluorochrome reagents (top) or peroxidase (bottom). A-C, B7-H1 (red) is strongly expressed in inflammatory infiltrates (overview in A; higher magnification of a single infiltratein C) but is absent in the control CNS (no expression in E). B and D show DAPI for staining nuclei. F-I, Triple labeling of one section for DAPI (blue, F), CD3 (G, green), and B7-H1 (I, red) shows partial colocalization of CD3 with B7-H1 (H, yellow). Triple labeling of another section for DAPI (K, blue), GFAP (L, green), and B7-H1 (N, red) shows basically no colocalization of B7-H1 with GFAP-positive astrocytes (M, overlay). Serial cerebellar cryostat sections from EAE mice were stained with antibodies for B7-H1 (O, Q) and MAC3 (P, R). Strong but not completely overlapping staining patterns for B7-H1 and MAC3 were observed. O and P show an overview (10×); Q and R show a higher magnification focusing on one infiltrate.
To further substantiate our findings on B7-H1 expression and regulation in vivo, we performed serial flow cytometric analysis of B7-H1 on CNS cells during the course of PLP-induced EAE (Fig. 7). Using colabeling with CD45 and CD11b, this method also allows differentiation between (resident) microglia (CD45lowCD11b) and macrophages (CD45highCD11b) as well as lymphocytes (CD45highCD11b-negative) (Becher et al., 2002). We found a significant increase in microglial B7-H1 expression between baseline, day 14, and day 20 (Fig. 7). Importantly, expression of inhibitory B7-H1 on microglia and macrophages correlated well with the recovery phase of the animals, whereas at maximum disease severity (day 14), 37% of microglia cells were positive, and expression increased up to 84% at the time of recovery (day 20) (Fig. 7). Of note, corresponding to our histochemical results, B7-H1 could also be detected on invading T cells but at a much lower extent (data not shown).
Figure 7.
Upregulation of B7-H1 expression by CNS-infiltrating macrophages and CNS resident microglia of mice with EAE. A, EAE was induced by subcutaneous immunization with 50 nmol of PLP139-151 in CFA and intravenous injection of 300 ng of pertussis toxin, and disease score was determined as described in Materials and Methods. Clinical disease symptom speaked at day 14 and subsequently declined until day 20 after disease induction. B, CNS cells were isolated from diseased animals at the peak of clinical disease (day 14) and during remission (day 20). B7-H1 expression by CD45high/CD11b+ macrophages (R1) and CD45low/CD11b+ (R2) microglia was determined by flow cytometry. Data are representative of six individual mice. Error bars indicate SEM. B7-H1 expression is higher during remission (t test; *p < 0.02; **p < 0.01).
Discussion
Our study sheds new light on the immunobiological role of the novel B7-family molecule B7-H1 in the CNS and during neuroinflammation. We found that murine and human microglial cells express high amounts of B7-H1, especially when exposed to inflammatory conditions simulated by the addition of IFN-γ or Th1 supernatants in vitro (Figs. 1, 2, 3). B7-H1 acts as a strong inhibitor of antigen-specific as well as nonspecific polyclonal T-cell activation in that it reduces both the secretion of proinflammatory cytokines (IFN-γ and IL-2) and the expression of T-cell activation markers (ICOS) (Fig. 4). As a “proof of concept,” we provide data showing that B7-H1 is highly upregulated on microglial cells/macrophages during the course of EAE, especially in regions with the strongest inflammatory response (Figs. 5, 6, 7). Thus, our data propose B7-H1, expressed by microglial cells, as a strong immune inhibitory molecule downregulating T-cell activation and thus contributing to the immune homeostasis in the CNS.
The brain had long been considered an immunologically privileged site. This idea is based on the observation that tissue transplants in the CNS are not commonly rejected by the immune system (Medawar, 1948; Barker and Billingham, 1977). An anti-inflammatory and, with regard to invading immune cells, proapoptotic environment in the brain, the limited access of brain-derived antigens to the lymphoid organs, the presence of the blood-brain barrier, low MHC expression in the brain parenchyma, and the absence of dendritic cells were used to explain the lack of an effective immune response to antigens in the brain. However, numerous studies in infectious, autoimmune, and tumor models have challenged this view by showing that potent immune reactions can and do occur in the CNS (Hickey, 2001).
The CNS is constantly patrolled by activated T lymphocytes, which may induce profound damage if they identify their specific or a cross-recognized antigen in the context of appropriate MHC restriction elements. The specialized anatomic barriers like the blood-brain barrier (Fabry et al., 1994) and the peculiarities of the lymphatic drainage do not necessarily guarantee the integrity of this organ. However, limiting the local inflammatory response is crucial for an organ as vulnerable as the CNS. For example, inflammation induces a proapoptotic environment mediated by astrocytes via the CD95 pathway (Bechmann et al., 1999). Together, with the lack of costimulatory molecules (Pender, 1999), this pathway is considered to induce high numbers of apoptotic lymphocytes among infiltrating T cells in autoimmune inflammatory conditions (Gold et al., 1996). Another critical player in the immune homeostasis of the CNS environment is the microglia. Although the exact function of these resident immune cells in the intact CNS remains elusive, early insights from studies of peripheral APC suggest that microglia may have major homeostatic and reparative functions in the normal as well as injured CNS. Considering microglial cells to be the major APC in the CNS, microglia-immune cell interactions may have critical impact on the outcome of brain-derived or brain-directed immune reactions. In this context, costimulatory signals provided by the microglia are key elements in this interface. The lack of costimulatory molecules in the healthy brain changes during the course of an inflammatory response (Aloisi, 2001). Therefore, it seems that a good counterbalance is needed to keep inflammatory situations under control.
Thus far, existing studies have elucidated the relevance of stimulatory second signals on microglial cells (such as CD80, CD86, and CD40), including their contribution to modulate or amplify acute or chronic neuroinflammation (Becher and Antel, 1996; Aloisi et al., 1999; Matyszak et al., 1999; Zehntner et al., 2003). Our study is the first to show the importance of a B7 molecule on microglial cells exerting strong coinhibitory properties, thereby providing novel insights into the complex immunobiology of these CNS APC.
B7-H1, or PD-L1, is a type I transmembrane protein with 20% amino acid identity to B7.1 and 15% amino acid identity to B7.2. Ligation of the B7-H1 leads to diminished proliferation and IL-2 production and induction of cell cycle arrest whereby CD8-T cells appear to be more sensitive to this effect than CD4 cells (Carter et al., 2002). B7-H1 interacts with PD-1 and a yet unidentified non-PD-1 receptor on T cells. B7-H1 is expressed not only on hematopoietic APCs but also on parenchymal cells such as muscle cells, microvascular endothelial cells, renal tubular cells, and cancer cell lines (Dong et al., 1999; Latchman et al., 2001; Eppihimer et al., 2002; Wiendl et al., 2003; Wintterle et al., 2003). In contrast, PD-L2 has a more limited expression, predominantly on cytokine-activated macrophages and dendritic cells (Latchman et al., 2001; Yamazaki et al., 2002). These patterns of (parenchymal) expression may allow for the termination of an immune response in inflamed tissues, limiting organ damage (Wiendl et al., 2003), or in tumors allowing for immune evasion (Dong et al., 2002; Wintterle et al., 2003) (for review, see Chen, 2004). B7-H1 expressed on peripheral professional APC has recently been proposed as a candidate contributing to the maintenance of peripheral tolerance. “Weak” APC, such as monocytes, immature or semimature dendritic cells exhibit tolerogenic rather than immunogenic functions, which negatively control spontaneous autoreactive T-cell activation (Lutz and Schuler, 2002). Accordingly, B7-H1 exerts potent negative regulatory functions for T-cell activation while expressed on these “weak APC” (Brown et al., 2003; Selenko-Gebauer et al., 2003; Schreiner et al., 2004). Following this concept, it is interesting to note that B7-H1 plays a major role in microglial cells (and astrocytes), which are considered to be comparably weak APC in the CNS. In our hands, neither unstimulated microglial cells nor astrocytes were able to induce proliferation in antigen-specific or superantigen-stimulated oligoclonal T cells (data not shown). However, they influence cytokine production and T-cell activation, both features critically influenced by inhibitory B7-H1.
Our data further suggest an involvement of the B7-H1 pathway in the immune regulatory mechanisms controlling autoreactive T-cell responses relevant for the pathogenesis of CNS autoimmunity. This assumption has recently been fueled by the demonstration of the critical role of B7-H1-PD1 interactions in the regulatory mechanisms of experimental autoimmune encephalomyelitis, in which therapeutic interference had critical impact on the onset and severity of the disease (Salama et al., 2003). Similar to our findings in vivo, B7-H1 was found upregulated in the inflamed CNS. Salama et al. (2003) suggested that the B7-H1-PD-1 pathway is particularly important in the induction phase of a MOG-EAE, an assumption that further emphasizes the strong negative regulatory of B7-H1-PD-1 in the periphery.
What might be the role of B7-H1 in the CNS in the regulation of CNS inflammation, respectively, the effector phase of the host immune response? From our data, it could be argued that the proinflammatory activity of T cells might be regulated via a negative feedback loop by IFN-γ-induced B7-H1 expression on microglia (and possibly invading peripheral monocytes/macrophages). This expression of B7-H1 on local APC could force activated Th1 T cells to reduce their cytokine levels, thus diminishing local levels of inflammatory activity. Because microglia cells are capable of quickly migrating to the site of inflammation or injury (Kreutzberg, 1996), they could participate in very early modulation of inflammation. This scenario is strongly supported by a recent report demonstrating the negative regulatory role of B7-H1 (PD-L1) on antigen-presenting cells, T cells, and host tissues by studying PD-L1 knock-out mice (Latchman et al., 2004); experiments using the MOG-EAE model showed that PD-L1 in host tissues and T cells limits responses of self-reactive CD4 T cells in vivo. Importantly, the transfer of encephalitogenic T cells from wild-type mice into PD-L1-/- recipients led to exacerbated disease, demonstrating the importance of host-tissue B7-H1 in negatively regulating the CNS inflammation (Latchman et al., 2004). Because we identified microglial cells as the main cellular source of B7-H1 among the resident CNS cells (Figs. 1, 2, 6, 7), they can be considered key players in mediating the negative regulatory signals in the host tissue, notwithstanding the putative importance of B7-H1 on invading cells (macrophages or T cells). In this view, it is interesting to note that disease was even more severe in PD-L1-/- recipients of PD-L1-/- T cells, therefore suggesting an important negative regulatory role of B7-H1 expressed on invading T cells (Latchman et al., 2004) (Fig. 6). Of note, although we use IFN-γ as a marker of the “Th1 environment,” it is clear that IFN-γ is not the only molecule present within the Th1 milieu that contributes to the observed coinhibitory molecule induction on monocyte/microglia differentiation. It is likely that multiple molecules in the Th1 environment (including but not limited to IFN-γ) are likely to contribute to the modulation of the human monocyte/microglia (Kim et al., 2004).
The idea of local immunosuppression by B7-H1 possibly affecting disease activity and progression is appealing, from both an immunopathogenic and a therapeutic view. Although purely speculative at present, it is possible that B7-H1 could play a role in direct tolerization of autoreactive cells; a recent study reported tolerance induction within the CNS itself rather than within draining lymphoid tissues. Naive MBP-specific T cells of MBP T-cell receptor transgenic mice readily migrated into the CNS without previous activation but were tolerized within the CNS in situ (Brabb et al., 2000). In a model of autoimmune hepatitis, B7-H1 was critical for determining the accumulation and deletion of intrahepatic CD8+ T lymphocytes as shown in B7-H1 knock-out mice (Dong et al., 2004). It remains to be shown in multiple sclerosis (MS) whether an increase in B7-H1 (on APC or T cells), and a parallel decrease in number or function of pathogenic T cells, could possibly account for the phases of relapses and remissions in MS.
Our demonstration of the expression of B7-H1 in areas of strongest inflammation in EAE further emphasizes our assumption on the importance of this regulatory principle in vivo. This negative regulatory feedback loop should be important for keeping the “anti-inflammatory milieu” in the CNS.
Footnotes
This work was supported by grants from the Interdisciplinary Center of Clinical Research Tuebingen (IZKF) to H.W. and F.B., the Gemeinnützige Hertie-Stiftung (1.319.110/02/08 to T.M.; 1.01.1/04/006 to F.B.), and University of Homburg research funds (TG 84). H.W. held a Junior Research Group for Neuroimmunology in Tuebingen (IZKF Tuebingen,1334-0-0). A.B.-O. is supported by the Canadian Institutes of Health Research (CIHR) and a CIHR program grant and is the recipient of the Don Paty Career Scientist Award of the Multiple Sclerosis Society of Canada. B.S. holds a research fellowship from the Deutsche Forschungsgemeinschaft.
Correspondence should be addressed to Dr. Heinz Wiendl, Clinical Research Group for Multiple Sclerosis and Neuroimmunology, Department of Neurology, University of Wuerzburg, Josef-Schneider-Strasse, D-97080 Wuerzburg, Germany. E-mail: heinz.wiendl@klinik.uni-wuerzburg.de.
Copyright © 2005 Society for Neuroscience 0270-6474/05/252537-10$15.00/0
References
- Aloisi F (2001) Immune function of microglia. Glia 36: 165-179. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Polazzi E, Minghetti L, Adorini L (1999) CD40-CD154 interaction and IFN-gamma are required for IL-12 but not prosta-glandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol 162: 1384-1391. [PubMed] [Google Scholar]
- Barker CF, Billingham RE (1977) Immunologically privileged sites. Adv Immunol 25: 1-54. [PubMed] [Google Scholar]
- Becher B, Antel JP (1996) Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18: 1-10. [DOI] [PubMed] [Google Scholar]
- Becher B, Prat A, Antel JP (2000) Brain-immune connection: immunoregulatory properties of CNS-resident cells. Glia 29: 293-304. [PubMed] [Google Scholar]
- Becher B, Durell BG, Noelle RJ (2002) Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 110: 493-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Mor G, Nilsen J, Eliza M, Nitsch R, Naftolin F (1999) FasL (CD95L, Apo1L) is expressed in the normal rat and human brain: evidence for the existence of an immunological brain barrier. Glia 27: 62-74. [DOI] [PubMed] [Google Scholar]
- Bischof F, Hofmann M, Schumacher TN, Vyth-Dreese FA, Weissert R, Schild H, Kruisbeek AM, Melms A (2004) Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. J Immunol 172: 2878-2884. [DOI] [PubMed] [Google Scholar]
- Bo L, Mork S, Kong PA, Nyland H, Pardo CA, Trapp BD (1994) Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol 51: 135-146. [DOI] [PubMed] [Google Scholar]
- Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J (2000) In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med 192: 871-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ (2003) Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170: 1257-1266. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Collins M (2002) The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol 20: 29-53. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG, Campbell IL (1999) Microglia stimulate naive T cell differentiation without stimulating T-cell proliferation. J Neurosci Res 55: 127-134. [DOI] [PubMed] [Google Scholar]
- Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM (2002) PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol 32: 634-643. [DOI] [PubMed] [Google Scholar]
- Chen L (2004) Co-inhibitory molecules of the B7-CD28 family in the control of T cell immunity. Nat Rev Immunol 4: 336-347. [DOI] [PubMed] [Google Scholar]
- Coyle AJ, Gutierrez-Ramos JC (2001) The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol 2: 203-209. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T cell proliferation and interleukin-10 secretion. Nat Med 5: 1365-1369. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793-800. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L (2004) B7-H1 determines accumulation and deletion of intrahepatic CD8(+)T lymphocytes. Immunity 20: 327-336. [DOI] [PubMed] [Google Scholar]
- Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP (2002) Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9: 133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry Z, Raine CS, Hart MN (1994) Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today 15: 218-224. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ (1986) Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6: 2163-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Schmied M, Tontsch U, Hartung HP, Wekerle H, Toyka KV, Lassmann H (1996) Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T cell apoptosis in vivo Brain 119: 651-659. [DOI] [PubMed] [Google Scholar]
- Hickey WF (2001) Basic principles of immunological surveillance of the normal central nervous system. Glia 36: 118-124. [DOI] [PubMed] [Google Scholar]
- Juedes AE, Ruddle NH (2001) Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol 166: 5168-5175. [DOI] [PubMed] [Google Scholar]
- Jung S, Gaupp S, Hartung HP, Toyka KV (2001) Oral tolerance in experimental autoimmune neuritis (EAN) of the Lewis rat. II. Adjuvant effects and bystander suppression in P2 peptide-induced EAN. J Neuroimmunol 116: 21-28. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ifergan I, Antel JP, Seguin R, Duddy M, Lapierre Y, Jalili F, Bar-Or A (2004) Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol 172: 7144-7153. [DOI] [PubMed] [Google Scholar]
- Kobata T, Azuma M, Yagita H, Okumura K (2000) Role of costimulatory molecules in autoimmunity. Rev Immunogenet 2: 74-80. [PubMed] [Google Scholar]
- Korn T, Fischer KD, Girkontaite I, Kollner G, Toyka K, Jung S (2003) Vav1-deficient mice are resistant to MOG-induced experimental autoimmune encephalomyelitis due to impaired antigen priming. J Neuroimmunol 139: 17-26. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19: 312-318. [DOI] [PubMed] [Google Scholar]
- Lafferty KJ, Woolnough J (1977) The origin and mechanism of the allograft reaction. Immunol Rev 35: 231-262. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2: 261-268. [DOI] [PubMed] [Google Scholar]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH (2004) PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA 101: 10691-10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Sha WC (2002) The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol 14: 384-390. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Schuler G (2002) Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23: 445-449. [DOI] [PubMed] [Google Scholar]
- Magnus T, Chan A, Grauer O, Toyka KV, Gold R (2001) Microglial phagocytosis of apoptotic inflammatory T cells leads to down-regulation of microglial immune activation. J Immunol 167: 5004-5010. [DOI] [PubMed] [Google Scholar]
- Magnus T, Chan A, Linker RA, Toyka KV, Gold R (2002) Astrocytes are less efficient in the removal of apoptotic lymphocytes than microglia cells: implications for the role of glial cells in the inflamed central nervous system. J Neuropathol Exp Neurol 61: 760-766. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P (1999) Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. Eur J Immunol 29: 3063-3076. [DOI] [PubMed] [Google Scholar]
- Mazanet MM, Hughes CC (2002) B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol 169: 3581-3588. [DOI] [PubMed] [Google Scholar]
- Medawar PB (1948) Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue and to the anterior chamber of the eye. Br J Exp Pathol 29: 58-69. [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11: 141-151. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291: 319-322. [DOI] [PubMed] [Google Scholar]
- Pender MP (1999) Activation-induced apoptosis of autoreactive and alloreactive T lymphocytes in the target organ as a major mechanism of tolerance. Immunol Cell Biol 77: 216-223. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, Hunt JS (2002) B7 family molecules: novel immunomodulators at the maternal-fetal interface. Placenta 23 [Suppl A]: S95-S101. [DOI] [PubMed] [Google Scholar]
- Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ (2003) Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 198: 71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H (2004) Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol 155: 172-182. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Gregersen R, Dorries R, ter Meulen V (1993) Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med 177: 1145-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J (2003) B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol 170: 3637-3644. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2: 116-126. [DOI] [PubMed] [Google Scholar]
- Tabi Z, McCombe PA, Pender MP (1994) Apoptotic elimination of V beta 8.2+ cells from the central nervous system during recovery from experimental autoimmune encephalomyelitis induced by the passive transfer of V beta 8.2+ encephalitogenic T cells. Eur J Immunol 24: 2609-2617. [DOI] [PubMed] [Google Scholar]
- Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L (2003) Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med 197: 1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl H, Mitsdoerffer M, Schneider D, Chen L, Lochmuller H, Melms A, Weller M (2003) Human muscle cells express a B7-related molecule, B7-H1, with strong negative immune regulatory potential: a novel mechanism of counterbalancing the immune attack in idiopathic inflammatory myopathies. FASEB J 17: 1892-1894. [DOI] [PubMed] [Google Scholar]
- Williams K, Bar-Or A, Ulvestad E, Olivier A, Antel JP, Yong VW (1992) Biology of adult human microglia in culture: comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol 51: 538-549. [DOI] [PubMed] [Google Scholar]
- Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H (2003) Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res 63: 7462-7467. [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H (2002) Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 169: 5538-5545. [DOI] [PubMed] [Google Scholar]
- Zehntner SP, Brisebois M, Tran E, Owens T, Fournier S (2003) Constitutive expression of a costimulatory ligand on antigen-presenting cells in the nervous system drives demyelinating disease. FASEB J 17: 1910-1912. [DOI] [PubMed] [Google Scholar]
- Zielasek J, Tausch M, Toyka KV, Hartung HP (1992) Production of nitrite by neonatal rat microglial cells/brain macrophages. Cell Immunol 141: 111-120. [DOI] [PubMed] [Google Scholar]