Abstract
Hypothalamic hypocretin enhances arousal, similar to the actions of norepinephrine (NE). The physiological actions of NE were examined in hypocretin neurons identified by selective green fluorescent protein expression in transgenic mouse hypothalamic slices using whole-cell recording. NE induced an outward current, inhibited spike frequency, and hyperpolarized hypocretin neurons dose dependently. Similar actions were evoked by the selective α2 adrenergic agonist clonidine. The α2 antagonist idazoxan increased spike frequency, suggesting tonic NE-mediated inhibition. The NE-induced current was inwardly rectified, and the reversal potential was dependent on external potassium concentration; it was blocked by barium in the bath and by GTP-γ-S in the pipette, suggesting activation of a G-protein inward rectifying K+ (GIRK) current. NE and clonidine decreased calcium currents evoked by depolarizing voltage steps. The selective α1 adrenergic agonist phenylephrine had no effect on membrane potential but did increase IPSC frequency; miniature IPSC frequency was also increased, in some cells without any effect on amplitude, suggesting a facilitative presynaptic action at α1 receptors on GABAergic axons that innervate hypocretin neurons. NE therefore inhibits hypocretin neurons directly through two mechanisms: activation of a GIRK current, depression of calcium currents, and indirectly through increased inhibitory GABA input. Similar to NE, dopamine and epinephrine reduced or blocked spikes and, in the presence of TTX, showed direct hyperpolarizing actions. The action of dopamine was blocked by the D2 receptor antagonist eticlopride, whereas a D1/5 antagonist had no effect. These data suggest that catecholamines evoke strong inhibitory actions on hypocretin neurons and suggest negative feedback from catecholamine cells that may be excited by hypocretin.
Keywords: arousal, dopamine, patch clamp, sleep, lateral hypothalamus, norepinephrine, food intake
Introduction
Hypocretin/orexin neurons are found in the lateral hypothalamus/perifornical area. Hypocretin injections into the brain enhance arousal (Hagan et al., 1999). Loss of the peptide or its receptors can result in narcolepsy in rodents, dogs, and humans (Chemelli et al., 1999; Lin et al., 1999; Nishino et al., 2000). Axons from the hypocretin cells project widely throughout the CNS (Peyron et al., 1998; van den Pol, 1999) with a high level of innervation in some select brain regions. One region that receives a dense hypocretin innervation is the locus ceruleus (LC). Most of the LC neurons synthesize norepinephrine (NE) and, similar to the hypocretin neurons, send axons to many regions of the brain including the lateral hypothalamus region where hypocretin cells are located. NE-containing axons are found in apparent contact with hypocretin neurons (Baldo et al., 2003). The hypocretin neurons also send a strong excitatory projection to regions of the brain that synthesize the catecholamines dopamine and epinephrine, which may also play a role in arousal, and these cells in turn project to the hypothalamus (de Lecea et al., 1998; Peyron et al., 1998; Wisor et al., 2001; Kaslin et al., 2004).
Hypocretin-immunoreactive axons make direct synaptic contact with the noradrenergic neurons of the LC (Horvath et al., 1999). At the cellular level, a number of studies have shown a strong excitatory action of hypocretin on LC (Hagan et al., 1999; Horvath et al., 1999; Bourgin et al., 2000; Marcus et al., 2001), in the nucleus of the solitary tract (Smith et al., 2002), another source of noradrenergic projections to the hypothalamus, and in the ventral tegmental area, where dopamine cells are localized (Korotkova et al., 2003). Hypocretin increases the synchronization of firing of individual NE-containing LC neurons (van den Pol et al., 2002), which may enhance cognitive arousal.
The central noradrenergic system is involved in the control of arousal (Foote and Morrison, 1987; Saper et al., 2001; Mallick et al., 2002; Berridge and Waterhouse, 2003). LC neurons fire fastest during wakefulness, slow down with the EEG during non-rapid eye movement (REM) sleep, and nearly stop firing during REM sleep (Aston-Jones et al., 1991). NE is involved in selective attention (Selden et al., 1990a,b), exploratory behavior, and responsiveness to novelty (Booth, 1967; Sara et al., 1995). NE may also play a role in food intake (Booth, 1967; Alexander et al., 1993; Bray, 2000; Wellman, 2000).
Recent reports showed that NE inhibits hypocretin neurons, but no mechanisms of action were elucidated (Li et al., 2002; Yamanaka et al., 2003a). Here, using whole-cell voltage- and current-clamp recording, we studied the direct and indirect mechanisms by which NE and other catecholamines modulate hypocretin neurons.
Materials and Methods
Hypothalamic slices were prepared from postnatal 2- to 9-week-old hypocretin-transgenic mice (kindly provided by Dr. T. Sakurai, University of Tsukuba, Tsukuba, Japan) that expressed green fluorescent protein (GFP) selectively in hypocretin neurons (Li et al., 2002; Yamanaka et al., 2003b); most of the slices used were taken from mice 2-3 weeks of age. Briefly, mice maintained on a 12 hr light/dark cycle were anesthetized with pentobarbital sodium (Nembutal; 80 mg/kg, i.p.) during daylight hours and then decapitated. The brain containing the lateral hypothalamus was rapidly removed and immersed in ice-cold, oxygenated artificial CSF (ACSF) before being trimmed to a hypothalamic block. The hypothalamic block was then glued on the plate of a vibratome, and 220- to 280-μm-thick coronal slices were cut in ice-cold, oxygenated ACSF. Then they were transferred to a chamber filled with oxygenated ACSF and kept at room temperature (22°C) for 1-2 hr before recording.
The slice was placed in the center of a recording chamber on the stage of an upright microscope (BX50WI; Olympus, New Hyde Park, NY) that had infrared differential interference contrast condenser and fluorescence capability. Then it was immobilized with a tungsten screen. The volume of the recording chamber was ∼0.5 ml. Patch pipettes were made of borosilicate glass (World Precision Instrument, Sarasota, FL) by a vertical pipette puller (Narashige PP-83; Narishige, Tokyo, Japan). The resistance of the pipette was 4-6 MΩ after being filled with the pipette solution. All recordings were obtained at 32-33°C. The signals were amplified with the EPC-9 amplifier (Heka, Lambrecht, Germany). Data were sampled at 1 kHz with Pulse 8.5 software (Heka) and only accepted when series resistance was <20 MΩ and changed <15% during recording. Data were analyzed with PulseFit, Axograph 4.5 (Axon Instruments, Union City, CA), and Igor Pro (WaveMetrics, Lake Oswego, OR) software as described previously (Gao and van den Pol, 1999). The frequency of action potentials was measured by using AxoGraph 4.5. The action potentials were detected by a fixed amplitude template, which was moved along the recorded traces to test for a match. Data are expressed as mean ± SEM; t test, ANOVA, and Kolmogorov-Smirnov statistical tests were used. Five concentrations of NE (1, 3, 10, 30, and 100 μm) were used to test the dose-response of NE. To estimate the IC50 value and maximal response, concentration-response curves were fitted with a least square regression using a logistic equation: y = a + b/[1 + exp[(x + c)/d]], where y is the magnitude of effect, a is maximum effect, x is drug concentration, and c is the concentration that inhibits the effects by 50%; b and d are constants.
The bath ACSF solution consisted of (in mm) 124 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.23 NaH2PO4, 26 NaHCO2, and 10 glucose, pH 7.4, with NaOH and was continuously bubbled with 5% CO2 and 95% O2. The pipette solution used for most experiments contained (in mm) 145 KMeSO4 (KCl when recording IPSC), 1 MgCl2, 10 HEPES, 1.1 EGTA, 2 Mg-ATP, and 0.5 Na2-GTP, pH 7.3, with KOH.
In experiments on calcium currents, the recorded neuron was clamped at -80 mV and then given a voltage step to 0 mV. During these experiments, the bath solution contained (in mm) 100 NaCl, 40 TEA-Cl, 2.5 KCl, 5 BaCl2, 10 HEPES, 10 glucose, and 1 μm TTX, pH adjusted to 7.3 with KOH. The pipette solution contained (in mm) 135 CsCl, 1 MgCl2, 10 HEPES, 5 BAPTA-Cs, 4 Mg-ATP, and 0.5 Na2-GTP, pH adjusted to 7.3 with CsOH.
All drugs were given by flow pipette application, unless noted otherwise. Drug solutions were prepared by diluting the appropriate stock solution with ACSF, unless noted otherwise. The following drugs were used: hypocretin-1 (Phoenix Pharmaceuticals, Belmont, CA), hypocretin-2 (Stanford University Peptide Facility, Stanford, CA), TTX (Alomone Labs, Jerusalem, Israel), bicuculline methiodide (BIC), dl-2-amino-5-phosphonovaleric acid (AP-5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), muscimol, norepinephrine bitartrate salt, epinephrine bitartrate salt, dopamine hydrochloride, clonidine hydrochloride, idazoxan hydrochloride, and phenylephrine hydrochloride (Sigma, St. Louis, MO). To protect NE, epinephrine, and dopamine from degradation, all buffers used in an experiment included the antioxidant sodium metabisulfite (10 μm), and solutions were prepared freshly before the experiment and protected from bright light.
Results
Norepinephrine blocks spikes and hyperpolarizes membrane potential
Hypocretin neurons, identified by selective GFP expression (Li et al., 2002; Yamanaka et al., 2003b), were studied in hypothalamic slices from transgenic mice in voltage and current clamp. We first tested the effect of NE (50 μm) in current clamp. Most GFP-expressing hypocretin neurons showed spontaneous spikes with a frequency of ∼2-4 Hz. All hypocretin neurons tested (n = 21) showed a consistent reduction or block of spikes and hyperpolarization by 9.8 ± 1.4 mV (range, 4-16 mV; p < 0.05; ANOVA) in response to NE (50 μm).
To investigate whether non-GFP cells respond to NE similar to the responses of hypocretin cells in current clamp, 17 non-GFP cells were tested. Four of these 17 control cells (23.5%) showed no response to 50 μm NE, as shown in the example in Figure 1B; two control cells were silent and showed no spikes. In 11 of 17 control cells (64.7%), NE (50 μm) blocked spike frequency and hyperpolarized membrane potential (Fig. 1C), similar to the response of hypocretin neurons (Fig. 1A). Two of 17 cells that showed no spontaneous spikes also demonstrated a NE-mediated hyperpolarization (Fig. 1D). In these non-green control neurons, NE application hyperpolarized the membrane potential by 9 ± 1.3 mV (range, 6-16 mV; p < 0.05; ANOVA; n = 11).
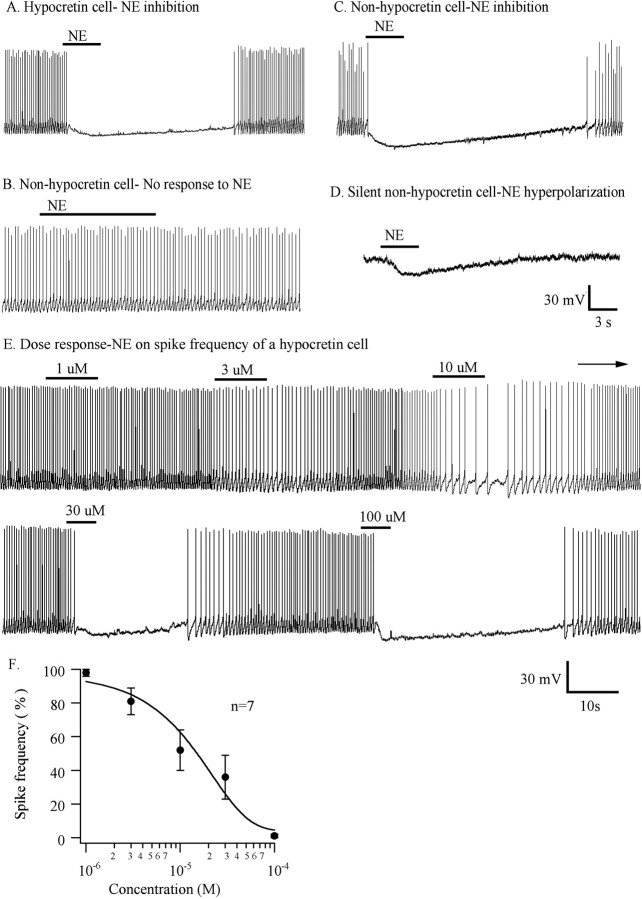
Figure 1.
Inhibitory responses of hypocretin cells and nonhypocretin cells to NE. A, A typical and consistent response of a hypocretin cell to NE (50 μm) during current clamp. B, Some non-GFP control cells showed no response to NE. C, Other non-GFP control neurons (13 of 17 tested) showed an inhibitory response to NE. D, Silent non-GFP cell was hyperpolarized by NE (2 of 17 cells). Drug applications are indicated with horizontal bars. E, A typical hypocretin cell showed a dose-dependent inhibition by NE. F, Dose-response curve for the effect of NE on spike frequency (n = 7).
In hypocretin neurons, the inhibitory effect of NE on spike frequency was dependent on the concentration of NE applied. The neurons were tested with brief application of 1, 3, 10, 30, and 100 μm NE under current clamp. A dose-response curve for the spike frequency produced by NE showed a greater reduction in spike frequency as concentration increased and revealed an IC50 value of 15 μm (n = 7) (Fig. 1F). Figure 1E shows a typical neuron response to five different concentrations of NE.
Receptor type
To determine the adrenoceptor subtypes that mediate the inhibition induced by NE in hypocretin cells, we first examined the actions of a selective α2 adrenoceptor antagonist on the spike frequency under current clamp. Application of 50 μm NE alone blocked spike frequency; spike frequency returned to the control level after washout (Fig. 2A). Then, the neuron was pretreated with the α2 receptor antagonist idazoxan. In the presence of idazoxan, NE failed to reduce the spike frequency. Furthermore, even in the absence of added NE, idazoxan increased spike frequency by 15.4 ± 3.8% (n = 10; p < 0.05; ANOVA) (Fig. 2A), suggesting that the hypocretin neurons may be under ongoing inhibition by endogenous α2 receptor agonists. Next, we tried the selective α2 adrenoceptor agonist clonidine. Clonidine (100 nm) reduced spike frequency and hyperpolarized the membrane potential (4.6 ± 0.7 mV; range, 3-7 mV; n = 5; p < 0.05; ANOVA) (Fig. 2B). When the neuron was pretreated with TTX (0.5 μm), AP-5 (50 μm), CNQX (10 μm), and bicuculline (30 μm), clonidine (100 nm) also hyperpolarized the membrane potential (4.1 ± 0.5 mV; range, 3-6 mV; n = 5; p < 0.05; ANOVA) (Fig. 2C). Together, these data suggest that the α2 adrenoceptor subtype mediates the direct inhibition caused by NE.
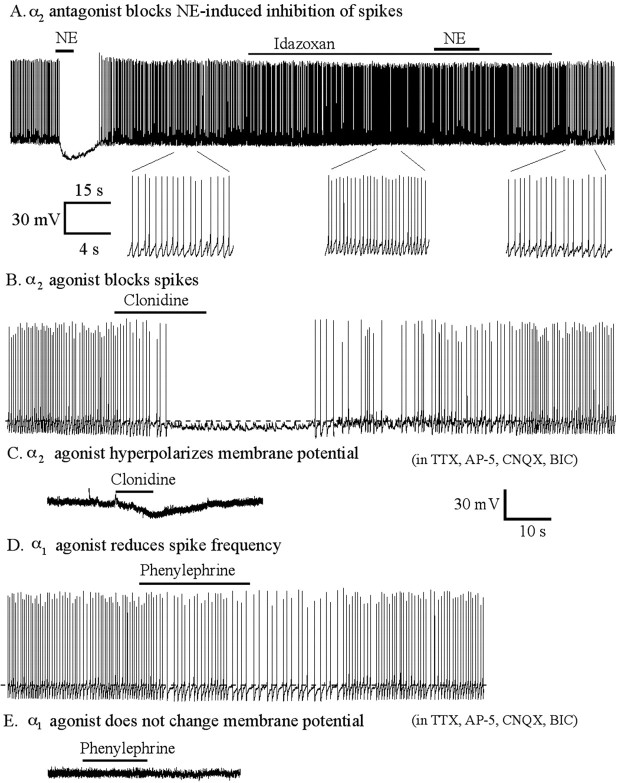
Figure 2.
The α2 receptor mediates direct NE-induced inhibition of hypocretin cells. A, Top, α2 antagonist idazoxan (10 μm) increased spike frequency and blocked NE-induced inhibiton of spikes (n = 7). Bottom, Part of extended traces shown above. B, The α2 agonist clonidine (100 nm) blocked spikes (n = 5). C, Clonidine (100 nm) hyperpolarized the membrane potential in the presence of TTX, AP-5, CNQX, and bicuculline. D, The α1 agonist phenylephrine (25 μm) reduces spike frequency (n = 6). E, Phenylephrine (50 μm) did not change the membrane potential in the presence of TTX (0.5 μm), AP-5 (50 μm), CNQX (10 μm), and bicuculline (30 μm).
To determine which other receptor types mediate inhibition in addition to the α2 receptor, we also studied the effect of the specific α1 adrenoceptor agonist phenylephrine on spike frequency under current clamp. Phenylephrine (25 μm) decreased spike frequency by 30.3 ± 8.4% (n = 6; p < 0.05; ANOVA) (Fig. 2D). Spike frequency returned to the control level after washout of phenylephrine. The membrane potential was not changed by phenylephrine (p > 0.05; ANOVA; n = 6). In the presence of TTX (0.5 μm), AP-5 (50 μm), CNQX (10 μm), and bicuculline (30 μm), phenylephrine (50 μm) exerted no effect on the membrane potential (n = 6; ANOVA; p > 0.05) (Fig. 2E), supporting the view that the α1-mediated inhibition of hypocretin cells was based on indirect mechanisms.
To study β receptors, hypocretin neurons were pretreated with the β receptor antagonist propranolol (20 μm) for 2 min; this caused a small statistically insignificant reduction in spike frequency (11.2 ± 6.8% decrease; n = 7; ANOVA; p > 0.05). In the presence of propranolol, NE (20 μm) still eliminated spikes and hyperpolarized the membrane potential by 8.0 ± 1.2 mV (Fig. 3A); after washout, spike frequency and membrane potential returned to the control level. Subsequently, when NE was applied in the absence of propranolol, spikes were blocked, and membrane potential was hyperpolarized to a similar level of 8.5 ± 1.2 mV. In TTX (0.5 μm), NE (20 μm) hyperpolarized the membrane potential by 9.8 ± 1.4 mV (Fig. 3B), and after washout, the membrane potential returned to the control level. In the presence of propranolol (20 μm), NE still hyperpolarized the membrane potential by 9.2 mV ± 1.6 mV (n = 7). There was no significant difference between NE alone and NE plus propranolol relative to hyperpolarization of the membrane potential (n = 7; ANOVA; p > 0.05).
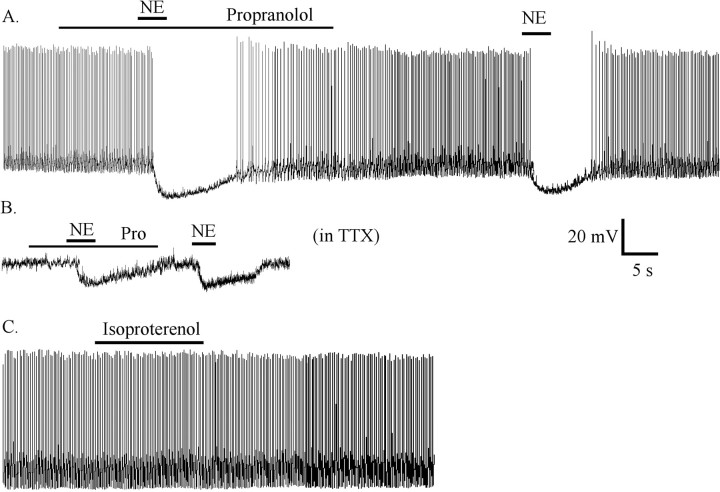
Figure 3.
NE-induced inhibition was not mediated by β receptor. A, After pretreatment and in the presence of the specific β receptor antagonist propranolol (20 μm), NE still blocked spike frequency and hyperpolarized the membrane potential. B, In the presence of TTX (0.5 μm) and propranolol, NE hyperpolarized the membrane potential. C, The β receptor agonist isoproterenol (50 μm) did not change spike frequency or the membrane potential. Pro, propranolol.
To further study possible β receptor involvement, we used the specific β receptor agonist isoproterenol. Isoproterenol (50 μm) had no effect on spike frequency (3 ± 4.3% rise) (Fig. 3C) or membrane potential (-1 ± 1.3 mV; n = 6; ANOVA; p > 0.05). These data suggest that NE-induced inhibition of hypocretin neurons was not mediated by β receptor.
NE-induced GIRK current
In other brain regions (e.g., the LC and the superior cervical ganglion neurons) (Jeong and Ikeda, 1998; Ishimatsu et al., 2002), NE-mediated inhibition can be based on the activation of a G-protein inward rectifying K+ (GIRK) current. We tested the hypothesis that NE activates a GIRK current in hypocretin cells. AP-5 (50 μm), CNQX (10 μm), BIC (30 μm), and TTX (0.5 μm) were used to block synaptic activity in this experiment. Current-voltage curves were obtained using a voltage ramp from -170 to -30 mV. When the concentration of extracellular K+ was 16 mm (145 mm intracellular K+), the NE-induced current reversed at -56 mV and was strongly inwardly rectifying (Fig. 4A1), with an inward current at -130 mV, at least four times greater than the outward current at -40 mV (Fig. 4A2). The α2 adrenoceptor antagonist idazoxan blocked the NE-induced current (Fig. 4A1,A2) (n = 4). With the same voltage ramp from -170 to -30 mV, the specific α2 agonist clonidine (200 nm) mimicked the NE-induced GIRK-type current (n = 6). In contrast, the α1 agonist phenylephrine (25 μm) showed little effect (n = 6). This suggests that the NE-induced inwardly rectified current is mediated by the α2 adrenoceptor.
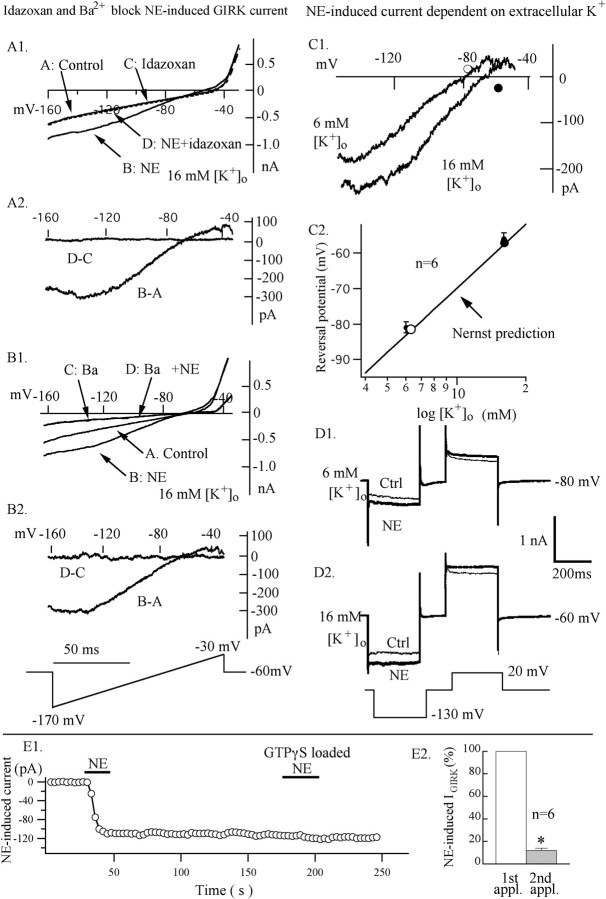
Figure 4.
GIRK current activated by an α2 receptor. The α2 antagonist idazoxan (10 μm) blocked an NE-induced GIRK-type current. AP-5 (50 μm), CNQX (10 μm), BIC (30 μm), and TTX (0.5 μm) were used to block synaptic activity in this experiment. A1, Current was measured in the absence and in the presence of NE (50 μm) with and without idazoxan at voltages from -170 to -30 mV, varied by a voltage ramp lasting 100 msec. Current traces are signal averaged from four traces for control, NE, idazoxan, and NE plus idazoxan, respectively. A2, NE-induced current (B-A) was obtained by subtraction of the trace A from trace B; trace D-C was obtained by subtraction of the trace C from trace D. B, Ba2+ blocked NE-induced GIRK-type current. B1, Current was measured in the absence and the presence of NE (50 μm) with and without Ba2+. Current traces are signal averaged from four traces for control, NE, Ba2+, and NE plus Ba2+, respectively. B2, NE-induced current (B-A) was obtained by subtraction of the trace A from trace B; trace D-C was obtained by subtraction of the trace C from trace D. C1, Dependence on external K+. Current-voltage relationships for the NE-induced current with 6 and 16 mm external K+ are shown. Each trace is the difference between current in NE (50 μm) and control current (signal-averaged 1 NE and 1 control trace from each of 6 neurons). C2, Reversal potential as a function of external K+. Reversal potentials for NE-induced current are -81 mV with 6 mm K+ and -56 mV with 16 mm K+. The solid line is the Nernst potassium equilibrium potential (RT/F)*In([K+]o/[K+]i), with [K+]i = 145 mm and T = 30°C. Bath solution had an equimolar substitution of KCl for NaCl to obtain the desired KCl concentration. D, NE induced outward current at +20 mV and inward current at -130 mV under voltage clamp. When external [K+] was 6 mm, the membrane potential was held at -80 mV; when external [K+] was 16 mm, the membrane potential was held at -60 mV. E, NE initially induced an inwardly rectified K+ current with GTP-γ-S loaded in the pipette. E1, A typical neuron showing that the NE-induced inward current did not recover when the cell was loaded with GTP-γ-S. NE failed to induce the inward current when applied several minutes after cell dialysis. E2, A bar graph illustrates that the response to a second application of NE is significantly reduced (p < 0.05). Asterisks indicate significant statistical differences by ANOVA (p < 0.05).
Previous reports have suggested that the inwardly rectifying potassium current can be blocked with external barium (Takigawa and Alzheimer, 1999). A concentration of 2 mm external Ba2+ blocked the inward current induced by NE (Fig. 4B1,B2) (n = 4).
Figure 4, C and D, shows the dependence of the NE-activated current on external K+ concentration in a typical neuron. As K+ was increased from 6 to 16 mm, the inward current increased dramatically, and the reversal potential shifted as predicted by the Nernst equation for a K+-selective conductance (Fig. 4C1,C2) (n = 6). Figure 4D shows individual current responses obtained before and during NE application at the indicated voltage steps. When the membrane potential was depolarized to +20 mV, NE induced an outward current; when the membrane potential was hyperpolarized to -130 mV, NE induced an inward current (n = 4). These data suggest that NE induces an inwardly rectifying K+ current.
GTP-γ-S is a nonhydrolyzable GTP analog that can activate G-proteins irreversibly. We tested the effect of NE on inwardly rectified current when GTP-γ-S was used in the pipette solution. After whole-cell configuration was achieved, NE could still evoke an inward rectified current for a short time (<3 min). However, as the GTP-γ-S diffused out of the pipette and into the neuron, NE failed to induce an inward-rectified current. Figure 4E1 plots the diminishing effect of NE as GTP-γ-S diffuses into the recorded cell. A second application of NE produced a significantly smaller effect than the first application applied before the GTP analog diffused into the cell (p < 0.05; ANOVA; n = 6) (Fig. 4E2). These data suggest that NE-induced K+ current is G-protein dependent.
α1 Receptor involved in NE-induced enhancement of inhibitory synaptic activity
To determine whether α1 or α2 adrenoceptors are involved in the NE-induced inhibition of spike frequency through changing synaptic activity, we tested clonidine and phenylephrine on IPSCs and EPSCs. The glutamate antagonists AP-5 (50 μm) and CNQX (10 μm) were used to block EPSCs and record IPSCs. Bicuculline (30 μm) was used to block IPSCs to record selectively EPSCs. Clonidine (100 nm) had little effect on the frequency or amplitude of IPSCs or EPSCs. After clonidine was applied, the frequency of EPSCs was 115 ± 21% of control (n = 5; p > 0.05; ANOVA) and then returned to 110 ± 4.5% after washout. Similarly, phenylephrine did not significantly change the frequency or the amplitude of EPSCs (n = 5; p > 0.05; ANOVA for frequency and Kolmogorov-Smirnov for amplitude). In phenylephrine, the frequency of EPSCs was 127 ± 19% of controls (n = 5; p > 0.05; ANOVA) and then returned to 113 ± 18% after washout.
Phenylephrine (25 μm) increased the frequency and amplitude of IPSCs (Fig. 5A). The frequency of IPSCs increased to 469 ± 249% (range, 107-1450%; n = 6; p < 0.05; ANOVA) (Fig. 5B) when phenylephrine was applied, then returned to 125 ± 32% after washout. Figure 5C shows a typical neuron cumulative probability before and during phenylephrine. Phenylephrine increased the amplitude of IPSCs (Kolmogorov-Smirnov test; p < 0.05; n = 5). The mean amplitude in controls was 16.5 ± 0.6 pA, and this increased to 38.2 ± 1.1 pA in phenyephrine and then returned to 22.9 ± 0.5 pA during washout.
Figure 5.
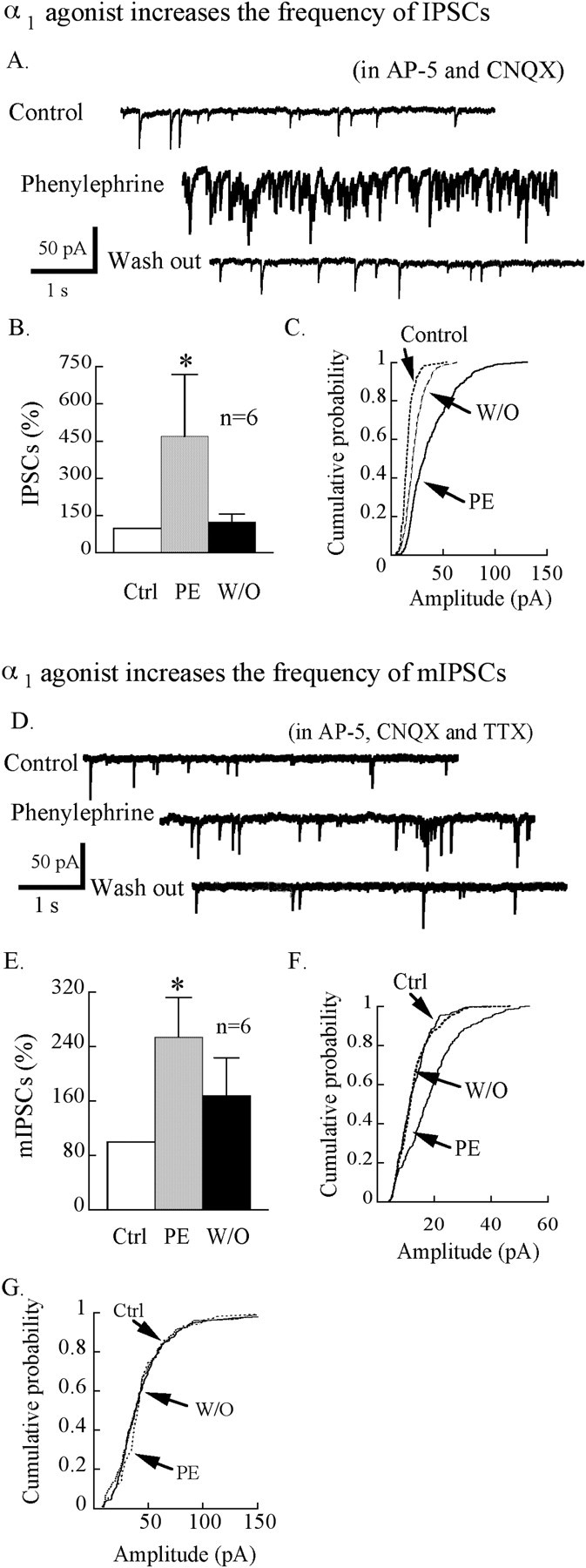
The α1 receptor increases GABA-mediated synaptic tone. The α1 agonist phenylephrine (PE; 25 μm) increased IPSC and mIPSC frequency. Equimolar KCl was used in the pipette solution instead of KMeSO4 when IPSCs were recorded. AP-5 (50 μm) and CNQX (10 μm) were used here to block excitatory synaptic activity. A, An example of IPSCs of hypocrein neurons before, during, and after phenylephrine application (25 μm). B, Bar graph showing the effect of phenylephrine on the mean frequency of spontaneous IPSCs. C, Cumulative probability plot of the amplitude of IPSCs for the cell shown in A. Phenylephrine shifted significantly the amplitude distribution to the right, showing an increase in the amplitude of IPSCs by phenylephrine. D, A typical neuron showing mIPSCs before, during, and after application of the specific α1 agonist phenylephrine (25 μm). E, Bar graph showing that the α1 agonist phenylephrine increased the frequency of mIPSCs. F, An example in which phenylephrine increases frequency and amplitude of mIPSCs. G, Phenylephrine in this cell increases the frequency but not the amplitude of mIPSCs. W/O, Washout; Ctrl, control; PE, phenylephrine.
To determine whether the phenylephrine-induced increase in IPSCs was mediated by presynaptic or postsynaptic actions, we examined miniature IPSCs (mIPSCs) in the presence of TTX (0.5 μm) and glutamate receptor antagonists AP-5 (50 μm) and CNQX (10 μm). Phenylephrine (25 μm) increased the frequency of mIPSCs to 253.7 ± 58.5% of the control level (n = 6; p < 0.05; ANOVA) (Fig. 5E), returning to 167.5 ± 56.0% after washout. Three cells that showed an increased frequency (mean, 277.3 ± 123%) also had an increase in amplitude of mIPSCs in phenylephrine (Kolmogorov-Smirnov test; p < 0.05; n = 3) (Fig. 5F). The other three cells that also showed an increase in mIPSC frequency (mean, 189.2 ± 36.8%) showed no change in amplitude in phenylephrine (Fig. 5G) (Kolmogorov-Smirnov test; p > 0.05; n = 3). Figure 5D shows an example of a neuron before, during, and after phenylephrine. These data showing that some cells show an increase in mIPSC frequency without a change in amplitude suggest that phenylephrine may enhance GABA release by a presynaptic mechanism; in addition, phenylephrine may also increase IPSCs by a postsynaptic effect, as suggested by the change in amplitude in some cells.
NE, phenylephrine, and clonidine decrease Ca2+ current
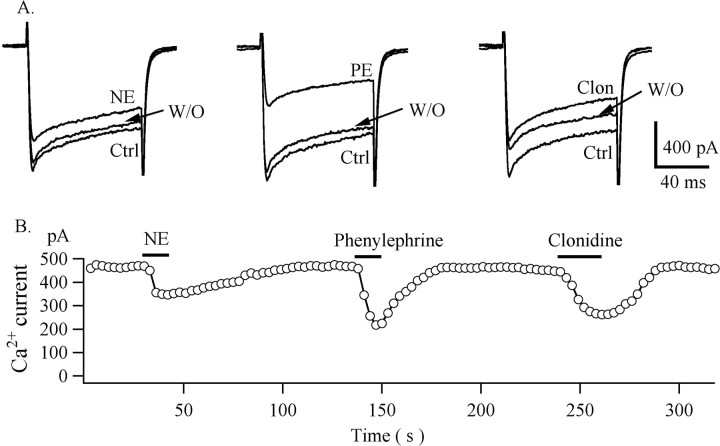
We tested the effect of NE, phenylephrine, and clonidine on Ca2+ currents under whole-cell voltage clamp. AP-5 (50 μm), CNQX (10 μm), bicuculline (30 μm), and TTX (0.5 μm) were used to block synaptic activity. BaCl2 was substituted for CaCl2 in the bath solution to increase the conductance at the calcium channel. Ba2+ currents were evoked by steps from a holding potential of -80 to 0 mV for 100 msec at 3 sec intervals (Fig. 6). Inward currents were completely blocked by the Ca2+ channel blocker Cd2+ (200 μm) (data not shown). The amplitude of sustained Ba2+ current was 814.4 ± 173.4 pA (n = 6) under these conditions. NE (50 μm) decreased the Ba2+ current by 32 ± 4.1% (p < 0.05; ANOVA), which returned to 93 ± 2.6% after washout. Phenylephrine (50 μm) decreased the Ba2+ current by 52.7 ± 2.2% (p < 0.05; ANOVA; n = 5), which returned to 85 ± 5.4% after washout. Clonidine decreased the Ba2+ current by 39 ± 2.5% (p < 0.05; ANOVA; n = 5), returning to 92 ± 6.4% after washout. These experiments suggest that α1 and α2 receptor subtypes can modulate calcium channel activity in hypocretin cells. Attenuation of calcium currents has been previously reported to be mediated by both α1 and α2 receptors in cultured rat median preoptic neurons and hippocampal slices (Kolaj and Renaud, 2001; Calcagnotto and Baraban, 2003).
Figure 6.
NE, phenylephrine, and clonidine decrease Ca2+ currents. A, A single cell responds to NE (50 μm), phenylephrine (50 μm), and clonidine (1 μm). B, Sustained amplitudes of Ca2+ currents were measured during voltage steps from a holding potential of -80 to 0 mV and plotted as a function of time. NE, phenylephrine, and clonidine were applied at the times indicated by horizontal bars. Ctrl, Control; PE, phenylephrine; Clon, clonidine; W/O, washout.
NE-induced response is independent of age
To determine whether there is a different response to NE between young and mature mice, postnatal day 11 (P11) mice and P64 mice were compared. NE (50 μm) was applied directly to the slice by a flow pipette and blocked spike frequency of neurons of P11 mice (n = 6) and P64 mice (n = 8). The resting membrane potential of P11 mice was -61 ± 2 mV. NE (50 μm) hyperpolarized the membrane potential to -72 ± 2 mV, which recovered after washout to -60 ± 2 mV. The resting membrane potential of P64 mice was -63 ± 1.2 mV. NE (50 μm) hyperpolarized the membrane potential to -71 ± 3 mV, which returned to -62 ± 4 mV after washout. There was no significant difference in the NE-induced inhibition between 11-d-old mice and 64-d-old mice (p > 0.05; ANOVA; Newman-Keuls test). To determine whether rapid application by flow pipe was critical, we compared flow pipe to bath application. Bath-applied NE caused hyperpolarization and inhibition of spike frequency (n = 6). There was no significantly different effect between bath application and flow pipe application (p > 0.05; ANOVA), except for the slower response time to bath application.
To ensure that NE is capable of exciting recorded neurons under the conditions used in our slice experiments, the effect of 50 μm NE on supraoptic nucleus magnocellular neurons was tested. NE has previously been shown to excite neurons of the supraoptic nucleus (Armstrong et al., 1986; Boudaba et al., 2003). In contrast to hypocretin cells, supraoptic neurons (n = 3) showed consistent increased spike frequency and depolarization induced by NE, as expected. NE increased spike frequency from 1.1 ± 0.5 Hz to 5.3 ± 0.3 Hz, and then spike frequency returned to 1.5 ± 0.8 Hz after washout. NE also depolarized the membrane potential by 3.2 ± 0.3 mV. The membrane potential returned to control levels after washout.
Epinephrine and dopamine inhibit hypocretin neurons
We also examined the responses of hypocretin neurons to two other catecholamines, epinephrine and dopamine. Epinephrine (50 μm) blocked spikes (Fig. 7A) under current clamp. Epinephrine hyperpolarized the membrane potential by 9 ± 1.0 mV (n = 6; p < 0.05; ANOVA) in normal bath conditions. In the presence of TTX (0.5 μm), epinephrine hyperpolarized the membrane potential by 8 ± 0.6 mV (n = 6; p < 0.05; ANOVA) (Fig. 7B). Similarly, dopamine (50 μm) reduced spike frequency (Fig. 7C) under current clamp. Dopamine hyperpolarized the membrane potential by 8.7 ± 0.7 mV (n = 6; p < 0.05; ANOVA) in normal bath solution. In TTX (0.5 μm), dopamine hyperpolarized the cells by 7.7 ± 0.6 mV (n = 6; p < 0.05; ANOVA) (Fig. 7D). These data show that both epinephrine and dopamine inhibit hypocretin cells and do so by a direct action in the absence of spike-mediated synaptic activity.
Figure 7.
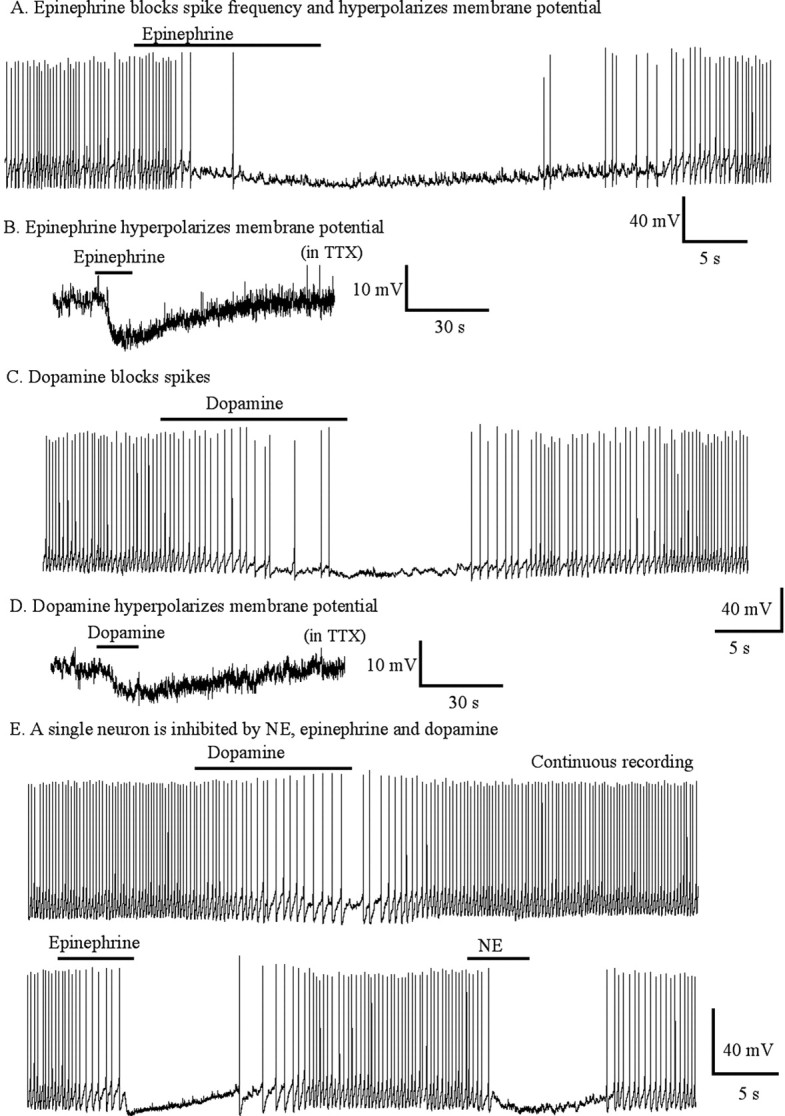
Direct hyperpolarizing actions of epinephrine and dopamine on membrane potential. A, Epinephrine (50 μm) blocked spike frequency and hyperpolarized membrane potential. B, Epinephrine (50 μm) hyperpolarized membrane potential in TTX. C, Dopamine (50 μm) blocked spikes and hyperpolarized membrane potential. D, Dopamine (50 μm) hyperpolarized membrane potential in TTX. E, A single neuron responded to NE (20 μm), epinephrine (20 μm), and dopamine (20 μm).
Single hypocretin neurons were tested to determine whether one cell would respond to all three catecholamines. Figure 7E shows that spikes were depressed by all three transmitters (NE, epinephrine, and dopamine) applied sequentially to a single neuron. All three catecholamines generated dose-dependent inhibition of hypocretin neurons, with greater inhibition produced by higher doses of the transmitters (Fig. 8). NE (20 μm) decreased spike frequency to 20 ± 9.5% of control, epinephrine (20 μm) decreased spike frequency to 29 ± 15% of control, and dopamine (20 μm) decreased spike frequency to 53 ± 17% of control. Spike frequency recovered to baseline after transmitter washout. There was no significant difference between NE- and epinephrine-mediated, NE- and dopamine-mediated, or between epinephrine- and dopamine-mediated inhibition (ANOVA; p > 0.05; n = 6).
Figure 8.
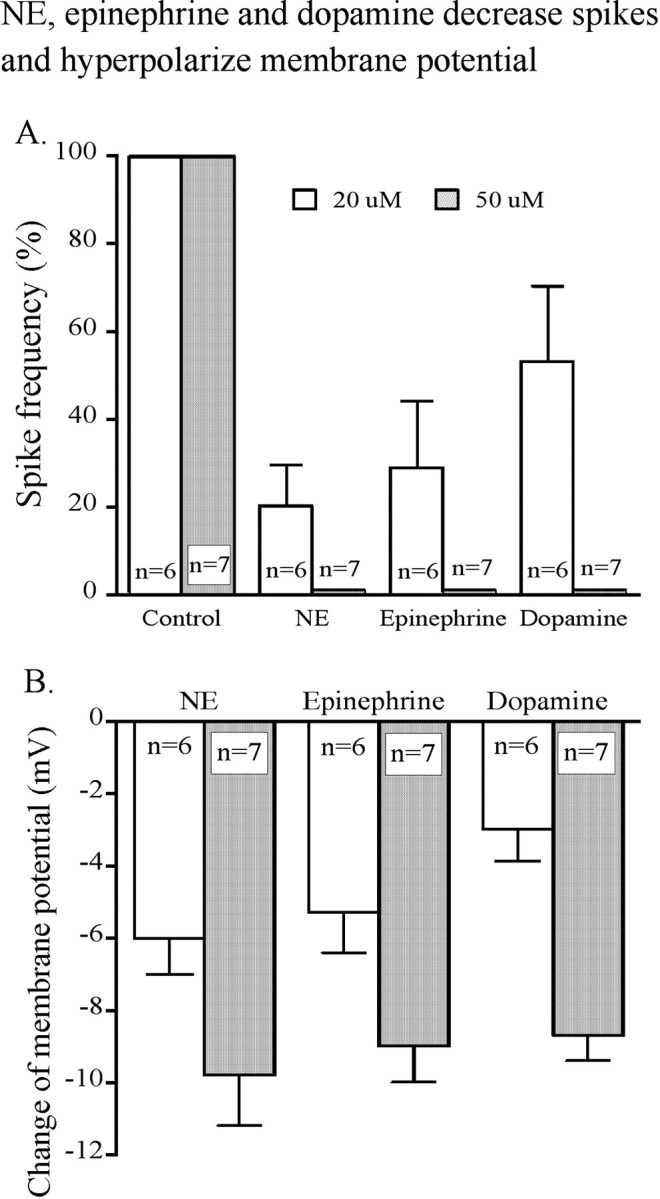
Dose dependence of catecholamine inhibition. The bar graph shows the effect of different concentrations of NE, epinephrine, and dopamine on spike frequency (A) and membrane potential (B).
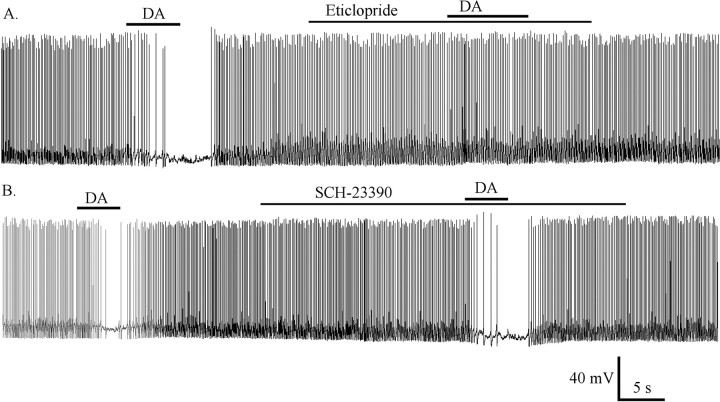
To know which receptor might be involved in dopamine-induced inhibition, we used the dopamine D1/D5 antagonist R(+)-SCH23390 and the D2 antagonist eticlopride in this experiment. Dopamine (50 μm) blocked spike frequency and hyperpolarized the membrane potential by 7.1 ± 0.8 mV (n = 8). After washout, spike frequency and membrane potential returned to the control level; when the neuron was pretreated with the D2 antagonist eticlopride (50 μm), dopamine did not change the spike frequency or membrane potential (Fig. 9A). In contrast, when the neuron was pretreated with the D1/D5 antagonist R(+)-SCH23390 (10 μm), dopamine still blocked spike frequency and hyperpolarized the membrane potential by 10 ± 1.3 mV (n = 6); after washout, the spike frequency and membrane potential returned to the control level. Then, on the same neuron, dopamine alone blocked spike frequency and hyperpolarized the membrane potential by 9.5 ± 1.3 mV (Fig. 9B). The D1/D5 antagonist SCH23390 thus had no effect on the dopamine-mediated hyperpolarization of membrane potential (ANOVA; p > 0.05). These data suggest that the dopamine-induced inhibition of hypocretin neurons was mediated mainly by a D2-type receptor and not by a D1/D5 receptor.
Figure 9.
Dopamine-induced inhibition mediated by the D2 receptor. A, The D2 receptor antagonist epticlopride (50 μm) reversibly blocked the dopamine-mediated inhibition. B, The D1/D5 antagonist SCH23390 (10 μm) had little effect on dopamine-mediated inhibition. DA, Dopamine (50 μm).
Discussion
Hypocretin neurons play an important role in arousal, and loss of hypocretin or its receptors reduces arousal and can lead to narcolepsy. Similarly, NE has also been shown to enhance arousal. NE exerted profound inhibitory actions on hypocretin neurons by three independent mechanisms. In a dose-dependent manner, NE reduced or blocked spontaneous action potentials. In the absence of spike-dependent synaptic input, NE evoked a substantial hyperpolarization of the membrane potential by a mechanism involving activation of a GIRK current through an α2-like receptor. NE reduced calcium currents. NE also exerted an indirect inhibitory effect on hypocretin neurons by increasing the GABA-mediated synaptic activity recorded in hypocretin cells through an α1-like receptor. Hypocretin cells may be under tonic inhibition by NE, because application of the α2 antagonist idazoxan increased spike frequency. Similar to NE, epinephrine and dopamine were also inhibitory to hypocretin neurons.
Mechanisms of NE inhibition of hypocretin neurons
A primary mechanism of inhibition was dependent on activation of a GIRK-type current. The reversal potential for the current was typical of a K+ conductance, and the magnitude of the current was dependent on extracellular K+ levels. The current showed inward rectification and was blocked by extracellular barium. That it is G-protein dependent was demonstrated by the block of NE actions when GTP-γ-S was used to irreversibly activate G-proteins. Together, these characteristics are typical of a GIRK current (Sodickson and Bean, 1996). The NE receptor involved had the characteristics of an α2 receptor, because it could be activated by either NE or the α2 agonist clonidine, and activation of the GIRK current could be blocked by the α2-selective antagonist idazoxan. In contrast, phenylephrine, an α1 agonist, had no effect on the GIRK current. Activation of a GIRK current contributed both to the reduction in spike frequency and to the hyperpolarization of the membrane potential of hypocretin neurons. The β receptor agonist isoproteranol had little effect on hypocretin cells, and the β receptor antagonist proteranol did not block the actions of NE, suggesting the β receptor did not play a prominent role.
NE inhibited Ca2+ conductances, as evaluated with Ba2+ substituted for Ca2+. The Ba2+ conductance could be attenuated both by NE and clonidine, suggesting involvement of the α2 receptor subtype. In addition, phenylephrine attenuated the Ba2+ current, suggesting that both α1 and α2 receptors may underlie the NE-mediated attenuation of calcium conductances. A NE-mediated depression of Ca2+ could secondarily result in an attenuation of many of the cellular processes in which Ca2+ plays a role as second messenger and, if a parallel mechanism exists in the hypocretin axon terminal, would tend to reduce the probability of transmitter release.
Interestingly, we identified a third mechanism of inhibition of the hypocretin neurons. NE caused an increase in the bicuculline-sensitive inhibitory synaptic tone. The increase in synaptic inhibition was consistent with activation of a α1 receptor, because it could be emulated by phenylephrine. The finding that mIPSCs could be increased in frequency but not in amplitude in some cells supports the view that NE can activate an α1 receptor on the terminals of inhibitory neurons that enhances release of GABA onto hypocretin neurons.
Epinephrine and dopamine exerted inhibitory actions that were similar to those found for NE. All three catecholamines reduced spike frequency and hyperpolarized the membrane potential. Epinephrine may act on NE receptors and would therefore probably exert inhibitory effects by mechanisms similar to those delineated for NE (Hoffman and Lefkowitz, 1982). Dopamine acts on its own set of G-protein-coupled receptors. Because the D2 antagonist eticlopride blocked the dopamine-mediated inhibition, but the D1/5 antagonist SCH23390 had no effect, the dopamine D2 receptor is most likely expressed by hypocretin cells and mediates the inhibitory actions of dopamine. Hypocretin neurons send excitatory axons caudally to dopamine- and epinephrine-containing cells, and both epinephrine- and dopamine-containing axons have been detected in the lateral hypothalamus (LH) in the area of hypocretin cells (Hokfelt et al., 1984; van den Pol et al., 1984; Astier et al., 1987; Fallon and Ciofi, 1992; Fadel and Deutch, 2002; Korotkova et al., 2002).
Functional importance of NE inhibition of the hypocretin arousal system
Depending on the receptor subtype expressed, NE has the capacity to either stimulate or depress neuronal activity (Han et al., 2002). For control purposes, we tested the effect of NE on mouse supraoptic nucleus and corroborated previous findings in the rat showing that NE was excitatory and evoked depolarizing actions (Armstrong et al., 1986; Boudaba et al., 2003). This provides additional support for the specificity of robust inhibitory actions of NE on hypocretin cells in mouse brain slices, in contrast to the excitatory actions on other systems related to arousal such as on the serotonin neurons (Brown et al., 2002). NE and hypocretin axons project to a number of common targets and avoid other regions; NE-immunoreactive axons are found throughout the LH and surround the hypocretin neurons (Baldo et al., 2003), demonstrating that the anatomical substrate exists for NE cells to modulate hypocretin neurons. NE in a dose-dependent manner consistently depressed the activity of hypocretin cells. This was true of neonatal and adult hypocretin neurons and was found with both a flow pipe aimed at the recorded cell and with bath application of NE.
In contrast to the inhibitory effect of NE on hypocretin neurons, hypocretin exerts a profound excitatory effect on NE neurons primarily by activating the hypocretin receptor 1 of the rat (Hagan et al., 1999; Horvath et al., 1999; Ivanov and Aston-Jones, 2000) and mouse (van den Pol et al., 2002) LC. In parallel, a high density of hypocretin axons is found in the LC (Peyron et al., 1998), where asymmetrical synapses are made by hypocretin axons onto NE neurons (Horvath et al., 1999).
As loss of hypocretin or its receptors can cause narcolepsy; the symptoms of narcolepsy may be relevant to understand the role of hypocretin in arousal. Narcoleptics have no trouble with directed attention or arousal over the short term but have significant problems with sleep-wake regulation over the long term. Whereas the total sleep per 24 hr may not be increased in narcolepsy, narcolepsy produces a syndrome in which the sleep debt cannot be delayed until night but must be paid every few hours. Hypocretin neurons may play a role in positive energy balance; CNS application of hypocretin has been reported to increase food intake. The arousal mediated by hypocretin neurons may be modulated by the energy state (Yamanaka et al., 2003b). That the two systems may subserve somewhat different functions is supported by analyses of daily rhythms; NE levels are reported to increase at the beginning of the wake period, whereas hypocretin levels increase later in the wake period (Mitome et al., 1994; Mochizuki and Scammell, 2003; Zeitzer et al., 2003), consistent with the view that hypocretin may generally oppose sleepiness during periods of prolonged wakefulness (Yoshida et al., 2001), whereas NE increases rapidly after waking.
One source of NE to the LH may be the LC. Activity of NE-releasing LC neurons correlates with attention and sensory arousal. The synchronized firing rate of LC neurons is positively correlated with cognitive performance (Usher et al., 1999; Rajkowski et al., 2004), possibly mediated by gap junction interneuronal signaling in the LC (Christie et al., 1989). Interestingly, hypocretin enhances the simultaneous firing of LC neurons in slices (van den Pol et al., 2002). NE helps to focus attention on task-relevant behavior by attenuating the influence of distracting stimuli, particularly under conditions of elevated arousal (Robbins, 1984). NE inhibition of hypocretin cells may be part of a negative feedback to inhibit runaway arousal, or to allow a high degree of attention to focus by reducing output of other nonselective arousal-related systems including the hypocretin system. Hypocretin neurons may influence motor control, as suggested by the sleep paralysis and cataplexy found in hypocretin-deficient narcoleptics, by reports of direct activation of motor neurons by hypocretin, and by the reduction in the activity of NE cells during cataplexy; catecholamine feedback inhibition of hypocretin cells may therefore be relevant to the fine-tuning of motor tone (Nishino and Mignot, 1997; Peever et al., 2003; John et al., 2004; Yamuy et al., 2004).
But another possibility is that the NE axons that innervate the hypocretin neurons arise not primarily from the LC but from other NE neurons in the hindbrain, perhaps from A1 or A2, or other axons could arise from adrenergic neurons in C1 that project to the LH (Mason and Fibiger, 1979; Aston-Jones, 2004). These cells have a different function from the LC neurons and may relate more to control of vegetative and homeostatic processes, potentially linking the enteroceptive cells of the gut with the LH. The A2 cells of the nucleus of the solitary tract project to the LH area, and cells of the nucleus of the solitary tract show excitatory responses to hypocretin (Smith et al., 2002; Yang and Ferguson, 2003). Studies combining tract-tracing with immunostaining have shown a very specific innervation of subregions of the hypothalamic paraventricular nucleus by different catecholamine projections (Sawchenko and Swanson, 1982; Cunningham and Sawchenko, 1988). Similar experiments delineating the origin of NE fibers to hypocretin neurons would be helpful in assessing the functional relevance of NE inhibitory actions on the hypocretin cells.
The multiple direct and indirect mechanisms involved, the fact that hypocretin cells appear to be tonically depressed by α2 receptors under baseline conditions, and the finding that dopamine, epinephrine, and NE have similar attenuating actions underscore the importance of catecholamine inhibition of the hypocretin arousal system.
Footnotes
This work was supported by National Institutes of Health Grants NS37788, NS41454, and AI/NS48854. We thank Dr. Claudio Acuna-Goycolea for helpful suggestions on this manuscript.
Correspondence should be addressed to Dr. Anthony N. van den Pol, Department of Neurosurgery, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520. E-mail: Anthony.vandenpol@yale.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250173-11$15.00/0
References
- Alexander JT, Cheung WK, Dietz CB, Leibowitz SF (1993) Meal patterns and macronutrient intake after peripheral and PVN injections of the α2-receptor antagonist idazoxan. Physiol Behav 53: 623-630. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Gallagher MJ, Sladek CD (1986) Noradrenergic stimulation of supraoptic neuronal activity and vasopressin release in vitro: mediation by an α1-receptor. Brain Res 365: 192-197. [DOI] [PubMed] [Google Scholar]
- Astier B, Kitahama K, Denoroy L, Jouvet M, Renaud B (1987) Immunohistochemical evidence for the adrenergic medullary longitudinal bundle as a major ascending pathway to the hypothalamus. Neurosci Lett 78: 241-246. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G (2004) Locus coeruleus, A5 and A7 noradrenergic cell groups. In: The rat nervous system, Ed 3 (Paxinos G, ed), pp 259-294. San Diego: Elsevier Academic.
- Aston-Jones G, Chiang C, Alexinsky T (1991) Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res 88: 501-520. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464: 220-237. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33-84. [DOI] [PubMed] [Google Scholar]
- Booth DA (1967) Localization of the adrenergic feeding system in the rat diencephalon. Science 158: 515-517. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Di S, Tasker JG (2003) Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol 15: 803-810. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L (2000) Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci 20: 7760-7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA (2000) A concise review on the therapeutics of obesity. Nutrition 16: 953-960. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL (2002) Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine, and noradrenaline). J Neurosci 22: 8850-8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Baraban SC (2003) An examination of calcium current function on heterotopic neurons in hippocampal slices from rats exposed to methylazoxymethanol. Epilepsia 44: 315-321. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437-451. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA (1989) Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J Neurosci 9: 3584-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham Jr ET, Sawchenko PE (1988) Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274: 60-76. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY (2002) Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111: 379-387. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Ciofi P (1992) Distribution of monamines within the amygdala. In: The amygdala: neurobiological aspects of emotion memory, and mental dysfunction (Aggleton J, ed), pp 97-144. New York: Wiley.
- Foote SL, Morrison JH (1987) Extrathalamic modulation of cortical function. Annu Rev Neurosci 10: 67-95. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN (1999) Neurotrophin-3 potentiates excitatory GABAergic synaptic transmission in cultured developing hypothalamic neurones of the rat. J Physiol (Lond) 518: 81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N (1999) Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911-10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD (2002) Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol 87: 2287-2296. [DOI] [PubMed] [Google Scholar]
- Hoffman BB, Lefkowitz RJ (1982) Adrenergic receptors in the heart. Annu Rev Physiol 44: 475-484. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Goldstein M (1984) Central catecholamine neurons as revealed by immunohistochemistry with spectial reference to adrenaline neurons. In: Classical transmitters in the CNS (Bjorklund A, Hokfelt T, eds), pp 157-276. New York: Elsevier.
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN (1999) Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415: 145-159. [PubMed] [Google Scholar]
- Ishimatsu M, Kidani Y, Tsuda A, Akasu T (2002) Effects of methylphenidate on the membrane potential and current in neurons of the rat locus coeruleus. J Neurophysiol 87: 1206-1212. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G (2000) Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. NeuroReport 11: 1755-1758. [DOI] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR (1998) G protein α subunit Gαz couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron 21: 1201-1212. [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Boehmer LN, Siegel JM (2004) Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 42: 619-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P (2004) The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci 24: 2678-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Renaud LP (2001) Norepinephrine acts via α(2) adrenergic receptors to suppress N-type calcium channels in dissociated rat median preoptic nucleus neurons. Neuropharmacology 41: 472-479. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Eriksson KS, Haas HL, Brown RE (2002) Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept 104: 83-89. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE (2003) Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 23: 7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN (2002) Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36: 1169-1181. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li H, Kadotani R, Rogers W, Lin X, Qui X, deJong PJ, Nishino S, Mignot E (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98: 365-376. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Majumdar S, Faisal M, Yadav V, Madan V, Pal D (2002) Role of norepinephrine in the regulation of rapid eye movement sleep. J Biosci 27: 539-551. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6-25. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC (1979) Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 187: 703-724. [DOI] [PubMed] [Google Scholar]
- Mitome M, Honma S, Yoshihara T, Honma K (1994) Prefeeding increase in paraventricular NE release is regulated by a feeding-associated rhythm in rats. Am J Physiol 266: E606-E611. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Scammell TE (2003) Orexin/hypocretin: wired for wakefulness. Curr Biol 13: R563-R564. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E (1997) Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol 52: 27-78. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E (2000) Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355: 39-40. [DOI] [PubMed] [Google Scholar]
- Peever JH, Lai YY, Siegel JM (2003) Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol 89: 2591-2600. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996-10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G (2004) Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol 92: 361-371. [DOI] [PubMed] [Google Scholar]
- Robbins TW (1984) Cortical noradrenaline, attention and arousal. Psychol Med 14: 13-21. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE (2001) The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 24: 726-731. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Dyon-Laurent C, Herve A (1995) Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res Cogn Brain Res 2: 181-187. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW (1982) The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257: 275-325. [DOI] [PubMed] [Google Scholar]
- Selden NR, Robbins TW, Everitt BJ (1990a) Enhanced behavioral conditioning to context and impaired behavioral and neuroendocrine responses to conditioned stimuli following ceruleocortical noradrenergic lesions: support for an attentional hypothesis of central noradrenergic function. J Neurosci 10: 531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Cole BJ, Everitt BJ, Robbins TW (1990b) Damage to ceruleocortical noradrenergic projections impairs locally cued but enhances spatially cued water maze acquisition. Behav Brain Res 39: 29-51. [DOI] [PubMed] [Google Scholar]
- Smith BN, Davis SF, van den Pol AN, Xu W (2002) Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience 115: 707-714. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP (1996) GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci 16: 6374-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C (1999) G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol (Lond) 517: 385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G (1999) The role of locus coeruleus in the regulation of cognitive performance. Science 283: 549-554. [DOI] [PubMed] [Google Scholar]
- van den Pol AN (1999) Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 19: 3171-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Herbst RS, Powell JF (1984) Tyrosine hydroxylase-immunoreactive neurons of the hypothalamus: a light and electron microscopic study. Neuroscience 13: 1117-1156. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB (2002) Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol (Lond) 541: 169-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ (2000) Norepinephrine and the control of food intake. Nutrition 16: 837-842. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM (2001) Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21: 1787-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T (2003a) Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun 303: 120-129. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T (2003b) Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38: 701-713. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Chase MH (2004) Hypocretinergic control of spinal cord motoneurons. J Neurosci 24: 5336-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Ferguson AV (2003) Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J Neurophysiol 89: 2167-2175. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S (2001) Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci 14: 1075-1081. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E (2003) Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci 23: 3555-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]